Abstract
Aims: We previously reported that glucagon-like peptide-1 receptor agonists (GLP-1RAs) reduced serum low-density lipoprotein cholesterol (LDL-C) levels in patients with type 2 diabetes mellitus receiving statins, which increased LDL receptor (LDLR) expression. Nevertheless, it remains unclear how much LDLR expression contributes to the LDL-C-lowering effect of GLP-1RAs. We examined the effect of a GLP-1RA, namely, exendin-4, on serum LDL-C levels and its mechanism inLdlr−/− and C57BL/6J mice.
Methods: Ten-week-oldLdlr−/− and C57BL/6J mice received exendin-4 or saline for 5 days, and serum lipid profiles and hepatic lipid levels were examined. Cholesterol metabolism-related gene expression and protein levels in the liver and ileum and the fecal bile acid (BA) composition were also examined.
Results: Exendin-4 treatment significantly decreased serum very-low-density lipoprotein cholesterol (VLDL-C) and LDL-C levels and mature hepatic SREBP2 levels and increased hepaticInsig1/2 mRNA expression in both mouse strains. InLdlr−/− mice, exendin-4 treatment also significantly decreased hepatic cholesterol levels and fecal BA excretion, decreased hepaticCyp7a1 mRNA expression, and increased small intestinalFgf15 mRNA expression. In C57BL/6J mice, exendin-4 treatment significantly decreased small intestinal NPC1L1 levels.
Conclusions: Our findings demonstrate that exendin-4 treatment decreased serum VLDL-C and LDL-C levels in a manner that was independent of LDLR. Exendin-4 treatment might decrease serum cholesterol levels by lowering hepatic SREBP2 levels and cholesterol absorption inLdlr−/− and C57BL/6J mice. Exendin-4 treatment might decrease cholesterol absorption by different mechanisms inLdlr−/− and C57BL/6J mice.
Keywords: GLP-1 receptor agonist, VLDL-cholesterol, LDL-cholesterol, Cholesterol absorption, Ldlr-deficient mice, SREBP2
Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that stimulates glucose-dependent insulin secretion, inhibits glucagon secretion in the pancreas, and controls blood glucose levels 1 , 2) . Hence, the administration of GLP-1 receptor agonists (GLP-1RAs) has been proposed as an efficacious and safe therapeutic approach for managing type 2 diabetes mellitus (T2DM). In addition, GLP-1RAs have been reported to decrease plasma triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) levels in patients with T2DM 3 - 6) . Thus, GLP-1RAs are suitable therapeutic options for T2DM with dyslipidemia. However, the effect of GLP-1RAs on serum cholesterol profiles has not yet been completely determined.
We previously reported that GLP-1RAs reduced serum LDL-C levels independent of the percent reduction in body mass index in obese Japanese T2DM patients treated with statins, which increased LDL receptor (LDLR) expression 7) . Liraglutide (200 µg/kg/day, twice daily), a GLP-1RA, decreased serum total cholesterol (TC), LDL-C and TG levels by downregulating hepatic LDLR and PCSK9 expression in diabetic (db/db) mice over 7 weeks of treatment 8) . Semaglutide (4 or 12 µg/kg/day), a GLP-1RA, had no significant effects on TC levels; however, at the highest dose (60 µg/kg/day), the TC level was reduced by 25% (not reaching significance) in Ldlr−/− mice fed with a high-fat diet after 17 weeks of treatment 9) . Therefore, it remains unclear how much LDLR expression contributes to the LDL-C-lowering effect of GLP-1RAs. To elucidate the role of LDLR in LDL-C-lowering effect of GLP-1RAs, we analyzed the effect of short-term peripheral GLP-1RAs on serum cholesterol profiles using LDLR-deficient (Ldlr−/−) and C57BL/6J mice. Exendin-4, a GLP-1RA, is a 39-amino-acid peptide that shares 53% sequence homology with native GLP-1 10) and was administered for 5 days to characterize acute effects on serum cholesterol levels. Expression levels of genes and proteins involved in cholesterol metabolism were analyzed in the liver and small intestine, together with fecal bile acid (BA) composition in Ldlr−/− and C57BL/6J mice.
Methods
Animal Experiment
Nine-week-old male Ldlr−/− mice were bred on a C57BL/6J background at the National Cerebral and Cardiovascular Center (NCVC) Research Institute. Nine-week-old male C57BL/6J mice were obtained from Japan SLC (Shizuoka, Japan) and acclimated to laboratory conditions for 1 week. They were fed regular chow (CE-2; crude fat: 4.8%, crude protein: 25.1%, total calories: 3.43 kcal/g; CLEA Japan, Tokyo, Japan). They were allowed free access to chow and water, and their environment was maintained at a constant temperature with a 12 h light–dark cycle. When the mice were 10 weeks old, we randomly divided them into two groups (Ldlr−/−: n=10 or 11 mice/group and C57BL/6J: n=13 mice/group). The mice in one of the groups were injected intraperitoneally with 10-nmol/kg body weight exendin-4 in saline (Phoenix Pharmaceuticals, Burlingame, CA, USA) every 24 h for 5 days, and those in the other group received saline as a vehicle control. Body weight and food intake were measured every day. Food intake (g/mouse/day) was determined for 5 days by subtracting the amount of food remaining from the previous day, divided by the number of mice in each cage.
On the day after the last injection, all mice were sacrificed under anesthesia. Blood samples were collected from the inferior vena cava. At autopsy, the liver and small intestine were carefully examined macroscopically. The liver and ileum of the small intestine were immediately frozen in liquid nitrogen and stored at −80℃ until RNA extraction. Parts of the livers were then fixed in 10% neutral buffered formalin solution (pH 7.4; FUJIFILM Wako Pure Chemical, Osaka, Japan). The experimental protocol was performed according to the NCVC Research Institute’s and Nagoya University’s Guidelines for Animal Experiments and was approved by the Institutional Ethics Review Committee for Animal Experimentation.
Biochemical Examination of Ldlr−/− and C57BL/6J Mice
Serum was collected from peripheral blood obtained from the aorta of each mouse at autopsy and then centrifuged. The blood glucose levels of the mice were measured using an automatic blood glucose meter (Medisafe-Mini GR-102; Terumo, Tokyo, Japan). The serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using a FUJI DRI-CHEM 7000V system (Fuji Film, Tokyo, Japan). The levels of cholesterol and TG in the serum lipoprotein fractions were measured using gel filtration high-performance liquid chromatography and an enzymatic method (LipoSEARCH) by Skylight Biotech Inc. (Akita, Japan). Cholesterol and TG profiles were measured for a total of 20 lipoprotein subfractions: two chylomicron fractions; five VLDL fractions (large, medium, and small VLDL subclasses); six LDL fractions (large, medium, small, and very small LDL subclasses); and seven HDL fractions (very large, large, medium, small, and very small subclasses), which were divided by particle diameters. Hepatic lipids were extracted according to a protocol described by Folch et al. 11) with slight modifications and analyzed by Skylight Biotech Inc. Serum levels of lathosterol, campesterol, and β-sitosterol were measured via gas chromatography by SRL Inc. (Tokyo, Japan).
Histological Analysis with Oil Red O Staining
For the detection of lipids in the liver, part of the fixed liver was frozen. The frozen tissue was serially sectioned into 10-µm-thick sections. The sections were stained with Oil red O. Digital photographs of the stained sections were taken with a BZ-9000 microscope.
Total RNA Extraction and RT-PCR
Total RNA was extracted from the liver tissues of mice with TRIzol reagent (Life Technologies, Carlsbad, CA, USA) combined with RNase-free DNase I (Life Technologies) and purified using RNA Clean & Concentrator (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. After RNA purification, aliquots of total RNA were subjected to reverse transcription using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). Real-time polymerase chain reaction (RT-PCR) analysis was performed in a StepOnePlus Real-Time PCR System (Applied Biosystems) with SYBR Green (Applied Biosystems) using the primers shown in Supplemental Table 1 . The mm99999915_g1 (mouse glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and mm00433278_m1 (mouse fibroblast growth factor (FGF) 15 (Fgf15)) TaqMan probes (Applied Biosystems) were used. The levels of mRNA transcripts were calculated by normalizing the comparative 2−∆∆Ct values of specific mouse genes to that of Gapdh or hypoxanthine phosphoribosyltransferase (Hprt).
Supplemental Table 1. Primer sequences for quantitative RT–PCR.
| Sequence (5’ to 3’) | ||
|---|---|---|
| Fgfr4 | F | GAACCCCATGCCTACCATCC |
| R | ATGTGTATGTGCCACGGTCC | |
| Insig1 | F | GGAATGTCACGCTCTTCCCC |
| R | AACTTGTGTGGTTCTCCCAGG | |
| Insig2 | F | GGACTAGCTTGCTTTCCTGACA |
| R | TCCCAGTGGAAAAGGTGAACTG | |
| Cyp7a1 | F | CCTTGGGACGTTTTCCTGCT |
| R | CCCGTTGTCCAAAGGAGGTT | |
| Shp | F | GGGCACGATCCTCTTCAACC |
| R | GGCTCCAAGACTTCACACAGT | |
| Hmgcr | F | TTGAACTCCCCATCGAGCCA |
| R | AGCTGGGATATGCTTGGCATT | |
| Cyp8b1 | F | GCTCCCCATAAGACGCCATC |
| R | GTGTGGGTGAGCCATCAGTT | |
| Fxr | F | AGGAGTACGCTCTGCTCACA |
| R | TGTAGCACATCAAGCAGGGG | |
| Npc1l1 | F | TCCTAAAGGGGGCCTAGCAG |
| R | GTTCCGTAAGGGCTTGTGGT | |
| Abcg5 | F | GTCCTGCTGAGGCGAGTAAC |
| R | CGCCCTTTAGCGTGTTGTTC | |
| Abcg8 | F | GGGGCGCTCATTCCTTTCAA |
| R | ACAGTGCTCCGGCAATTCTC | |
| Mttp | F | CGAAGATCGCCCCACTGAAA |
| R | AGCCAGTAGTTCCTCTCCAGAT | |
| Srebf2 | F | GTCACCTTCTGGAGACACCG |
| R | CCAGCACGGATAAGCAGGTT | |
| Ldlr | F | AAGCTAAGGATGAGCACCGC |
| R | TCGTTCCTGCTGCATGAGTC | |
| Pcsk9 | F | GGTGGATCCAGCTGTAAGGC |
| R | GGGTAAGGTGCGGTAAGTCC | |
| Hmgcs | F | GCAAAAAGATCCGTGCCCAG |
| R | CGAGCTAGAGATTTCTGCACCA | |
| Lxra | F | CCCCTTGGCCTTTTCCTACATT |
| R | CAAGGACATCTCTTCCTGGAGC | |
| Apob | F | AGCAATGTGACGGCTTCCAG |
| R | GTACTGGCAAGTTTGGCTGC | |
| Abca1 | F | AGAACCCGAGCCAGTATGGA |
| R | CAGGGACAAAGGACATCGCA | |
| Apoa1 | F | GAACGAGTACCACACCAGGG |
| R | CTGGCCTTGTCGATCACACT | |
| Fasn | F | AATGGGAGAAGCCATGTGGG |
| R | GAGCAGGGACAGGACAAGAC | |
| Srebf1 | F | GAACTGGACACAGCGGTTTTG |
| R | CAGCATAGGGGGCGTCAAACA | |
| Acaca | F | AAGAAGCTCCTGCTGCGATT |
| R | GAAGCTTCCATCCTGGCTGT | |
| Scd1 | F | TGCGATACACTCTGGTGCTC |
| R | TAGTCGAAGGGGAAGGTGTG | |
| Ctnnb1 | F | AGCACATCAGGACACCCAAC |
| R | CCGAGCAAGGATGTGGAGAG | |
| Gapdh | F | CATCACCATCTTCCAGGAGCG |
| R | GATGATGACCCTTTTGGCTCC | |
| Hprt | F | TGCCGAGGATTTGGAAAAAGTGT |
| R | GTGATGGCCTCCCATCTCCT |
Western Blotting
Mouse livers (50 mg) were homogenized in RIPA buffer (Sigma Aldrich, St. Louis, MO, USA) with a protease inhibitor cocktail (Sigma Aldrich) at 4℃. After a 30 min incubation on ice, the protein was obtained by centrifugation at 12,000 rpm for 20 min at 4℃. Twenty micrograms of protein were separated on 4%–12% Bis-Tris Nupage gels (Life Technologies) and transferred to polyvinylidene difluoride membranes. After transfer, the membranes were incubated overnight at 4℃ with antibodies against SREBP2 (NB100-74543, Novus Biological LLC, Centennial, CO), LXRɑ (ab176323; Abcam, Cambridge, UK), NPC1L1 (sc-166802; Santa Cruz Biotechnology, Inc), microsomal triglyceride transfer protein (MTP) (sc-135994; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GAPDH (#2118; Cell Signaling Technology, Beverly, MA, USA), and HPRT (ab133242; Abcam). The membranes were incubated with a secondary antibody (goat antirabbit IgG-HRP; #7074, Cell Signaling Technology or m-IgGκ BP-HRP; sc-516102, Santa Cruz Biotechnology, Inc.) and visualized using the ECL Prime detection kit (Cytiva, Tokyo, JAPAN) and a ChemDoc Touch imager (Bio-Rad Laboratories Inc., Hercules, CA, USA). Image Lab 6.1 software (Bio-Rad Laboratories Inc.) was used for the densitometry analysis. The protein levels in the liver and small intestine were normalized to the protein level of GAPDH and HPRT, respectively.
Fecal bile Acid Composition
Ldlr−/− and C57BL/6J mouse feces were collected at the time of sacrifice. The levels of BAs, including primary BAs, namely, cholic acid (CA), taurocholic acid, taurodeoxycholic acid (TDCA), α-muricholic acid (MCA), β-MCA, tauro-α (Tα)-MCA, and tauro-β (Tβ)-MCA, and secondary BAs, namely, deoxycholic acid (DCA), hyodeoxycholic acid (HDCA), ω-MCA, and 7-oxo-DCA, in mouse feces (50 mg) were measured using liquid chromatography–hybrid quadrupole–time-of-flight mass spectrometry (LC-Q-TOF/MS) at the Technosuruga Laboratory Co., Ltd. (Shizuoka, Japan).
Statistical Analysis
Continuous variable data are shown as the mean±SD or mean±SE. The significance of the differences between the exendin-4 and control groups was analyzed using the unpaired Student’s t-test or Wilcoxon’s rank-sum test. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Chicago, IL, USA), and the differences were considered significant at a p-value of <0.05.
Results
Exendin-4 Reduced Serum VLDL-C and LDL-C Levels in Ldlr−/− and C57BL/6J Mice
We treated 10-week-old Ldlr−/− and C57BL/6J mice with 10-nmol/kg exendin-4 or saline as a control for 5 days to elucidate whether exendin-4 treatment reduces serum LDL-C levels in Ldlr−/− and C57BL/6J mice. Exendin-4 treatment tended to decrease the average food intake in Ldlr−/− mice and significantly decreased it in C57BL/6J mice ( Fig.1A and Supplemental Fig.1 ) ; however, there was no significant difference in body weight or random blood glucose levels between the two groups of Ldlr−/− and C57BL/6J mice ( Fig.1B, C ) . Exendin-4 treatment significantly decreased serum TC, very-low-density lipoprotein cholesterol (VLDL-C), and LDL-C levels in Ldlr−/− and C57BL/6J mice ( Fig.1D-G and Supplemental Fig.2 ) . Serum high-density lipoprotein cholesterol levels were significantly increased in exendin-4-treated Ldlr−/− mice compared to control Ldlr−/− mice. A subclass analysis of each lipoprotein via HPLC was performed. The cholesterol levels in large, medium, and small VLDL and large, medium, and small LDL subfractions decreased and the TG levels in small and very small LDL subfractions increased in the exendin-4-treated Ldlr−/− mice compared with the control Ldlr−/− mice ( Supplemental Fig.2 ) . The cholesterol levels in large and medium VLDL subfractions and medium, small, and very small LDL subfractions decreased in the exendin-4-treated C57BL/6J mice compared to the control C57BL/6J mice. The levels of serum AST and ALT also did not differ between the two groups in Ldlr−/− and C57BL/6J mice ( Fig.1H, I ) . Serum lathosterol, campesterol, and β-sitosterol levels in exendin-4-treated Ldlr−/− mice were lower than those in saline-treated Ldlr−/− mice ( Fig.1J-L ) . Serum campesterol levels were decreased by exendin-4 treatment, although the serum lathosterol level was not detected in C57BL/6J mice.
Fig.1. Characteristics and serum lipid profiles in saline (control)- and exendin-4-treated Ldlr−/− and C57BL/6J mice .
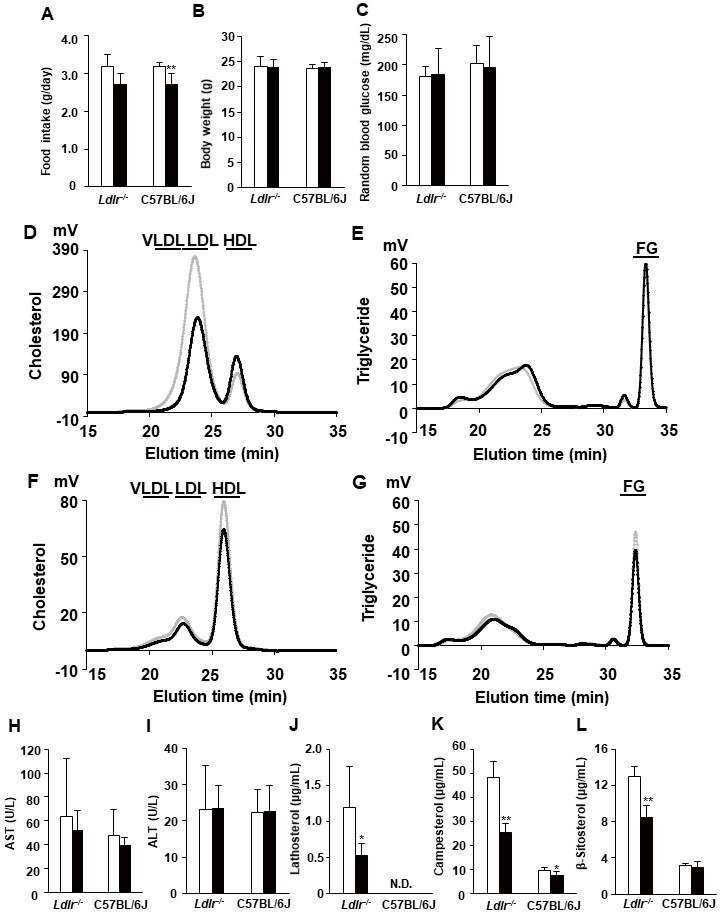
The average food intake (A) and body weight (B) and random blood glucose levels (C) in exendin-4-treated (n=10 Ldlr−/− mice and n=13 C57BL/6J mice; black columns) and saline-treated (n=11 Ldlr−/− mice and n=13 C57BL/6J mice; white columns) mice. Cholesterol and triglyceride profiles of serum lipoproteins by gel filtration HPLC in exendin-4-treated (n=7; black line) and saline-treated (n=7; gray line) Ldlr−/− mice (D, E) and exendin-4-treated (n=4; black line) and saline-treated (n=4; gray line) C57BL/6J mice (F, G). Serum AST (H) and ALT (I) levels were assessed in exendin-4-treated (n=10 Ldlr−/− mice and n=13 C57BL/6J mice; black columns) and saline-treated (n=9 Ldlr−/− mice and n=13 C57BL/6J mice; white columns) mice. Serum concentrations of lathosterol (J), campesterol (K), and β-sitosterol (L) in exendin-4-treated (n=5; black columns) and saline-treated (n=5; white columns) Ldlr−/− and C57BL/6J mice. The values (D-G) are the means of four or seven animals per group. *p<0.05 and **p<0.01 vs. the control group as calculated by unpaired t-test. AST, aspartate aminotransferase; ALT, alanine aminotransferase. VLDL, very-low-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; FG, free glycerol.
Supplemental Fig.1.
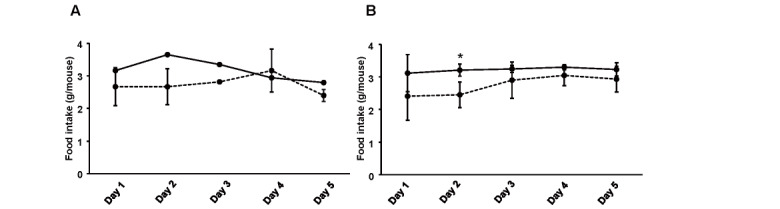
Food intake for 5 days in Ldlr-/- (A) and C57BL/6J (B) mice
Supplemental Fig.2. Cholesterol and triglyceride distributions among serum lipoprotein subclasses in Ldlr-/- and C57BL/6J mice.
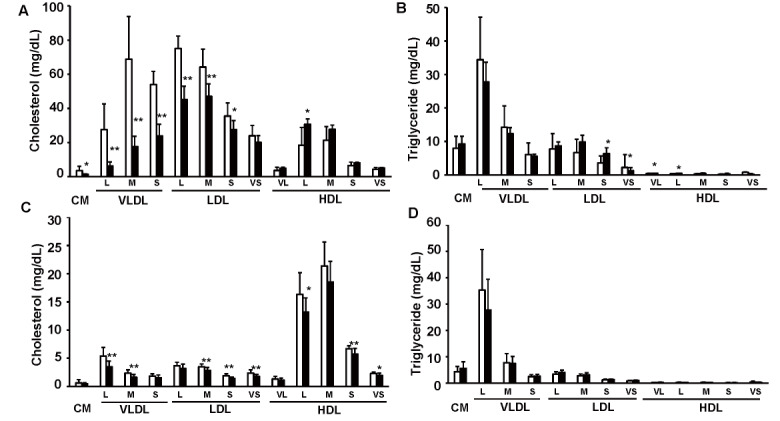
Cholesterol and triglyceride profiles in serum lipoproteins in exendin-4-treated (n=7; black columns) and saline-treated (n=7; white columns) Ldlr-/- mice (A, B) and exendin-4- treated (n=13; black columns) and saline-treated (n=13; white columns) C57BL/6J mice (C, D). *p<0.05 and **p<0.01 vs. the control group as calculated by unpaired t test. These values are the mean±SD. CM: chylomicron; VLDL: very-low-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; HDL: high-density lipoprotein cholesterol. VL: very large; L: large; M: medium; S: small; VS: very small
Exendin-4 Reduced Hepatic Cholesterol Levels in Ldlr−/− Mice
We examined TC, free cholesterol (FC), phospholipid (PL), and TG levels in the livers of Ldlr−/− and C57BL/6J mice ( Fig.2A-D ) . In Ldlr−/− mice, hepatic TC, FC, and PL levels were significantly decreased in the exendin-4 group compared to the control group although they were not significantly different between the two groups of C57BL/6J mice. Hepatic histopathological analysis with Oil red O staining was negative in both the exendin-4 and control groups, and no histopathological changes due to exendin-4 treatment were observed in Ldlr−/− and C57BL/6J mice ( Fig.2E-H ) .
Fig.2. Hepatic levels of lipids and histological sections in Ldlr−/− and C57BL/6J mice .
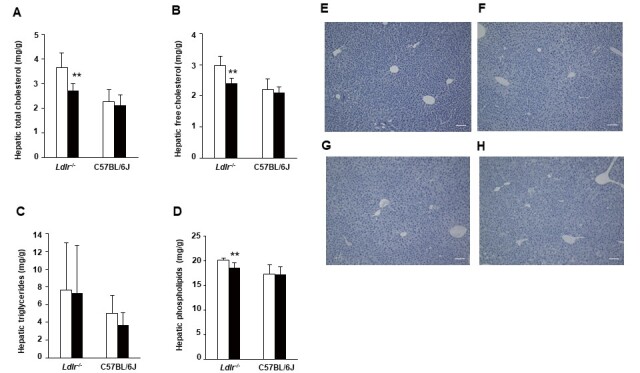
Hepatic levels of total cholesterol (A), free cholesterol (B), triglycerides (C), and phospholipids (D) in exendin-4-treated (n=7 Ldlr−/− mice and n=4 C57BL/6J mice; black columns) and saline-treated (n=7 Ldlr−/− mice and n=4 C57BL/6J mice; white columns) mice. Representative sections of mouse livers stained with Oil Red O in control-treated (E) and exendin-4-treated (F) Ldlr−/− mice and saline-treated (G) and exendin-4-treated (H) C57BL/6J mice. The values of hepatic lipids levels are the mean±SD. **p<0.01 vs. the control group as calculated using unpaired t-test.
Exendin-4 Treatment Decreased Hepatic Mature SREBP2 Levels and Increased Insig1/2 mRNA Expression in Ldlr−/− and C57BL/6J Mice and Decreased Cyp7a1 mRNA Expression in Ldlr−/− Mice
To clarify whether exendin-4 treatment decreases hepatic cholesterol synthesis and catabolism in Ldlr−/− and C57BL/6J mice, we investigated the mRNA expression and protein levels in the livers of Ldlr−/− and C57BL/6J mice using quantitative RT-PCR and western blotting ( Fig.3A-C ) . The expression of hydroxymethylglutaryl-CoA reductase/synthase (Hmgcr/s), associated with cholesterol synthesis, is regulated by sterol regulatory element-binding protein 2 (SREBP2), encoded by Srebf2 and acts as a transcription factor to regulate the level of cellular cholesterol. The mRNA expression of insulin-inducible signal 1/2 (Insig1/2) encodes a protein that degrades HMGCR and SREBP. The hepatic mRNA expression levels of Insig2 and Insig1 were significantly increased in the exendin-4 group compared with the control group in Ldlr−/− and C57BL/6J mice, respectively ( Fig.3A ) . The hepatic mature SREBP2 protein level was significantly decreased in the exendin-4 group compared with the control group in both types of mice ( Fig.3B, C ) . The mRNA expression levels of ATP-binding cassette transporter G5/8 (Abcg5/8), which are associated with cholesterol excretion, were significantly decreased in the exendin-4 group compared with the control group in Ldlr−/− and C57BL/6J mice. The protein level of LXRα, which induces cholesterol 7α-hydroxylase (Cyp7a1), a rate-limiting enzyme of the catabolic pathway by which cholesterol is converted to BAs, and Abcg5/8 transcription in the liver did not change in the exendin-4 group compared to the control group in Ldlr−/− and C57BL/6J mice ( Fig.3B, C ) .
Fig.3. Levels of hepatic mRNA expression and protein associated with cholesterol synthesis, catabolism, and excretion in Ldlr−/− and C57BL/6J mice .
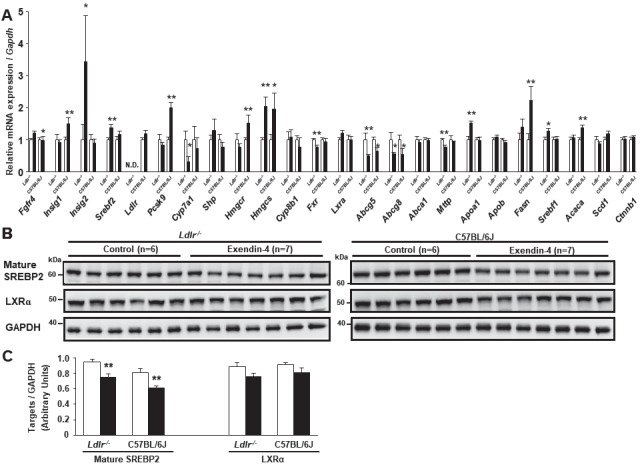
Hepatic mRNA expression in exendin-4-treated (n=11 Ldlr−/− mice and n=8 C57BL/6J mice; black columns) and saline (control)-treated (n=10 Ldlr−/− mice and n=8 C57BL/6J mice; white columns) mice (A). Hepatic expression of Srebf2, Pcsk9, Hmgcs, Lxra, Abcg5, Abcg8, Abca1, Mttp, Apob, Fasn, and Srebf1 was assessed in seven control and seven exendin-4-treated Ldlr−/− mice. Western blots for hepatic mature SREBP2 and LXRα were performed in Ldlr−/− and C57BL/6J mice (B, C). Each protein level was assessed via western blotting normalized to the level of GAPDH in Ldlr−/− and C57BL/6J mice treated with exendin-4 (n=7; black columns) or saline (n=6; white columns). These values are the mean±SE. *p<0.05 and **p<0.01 vs. the control group as calculated by unpaired t-test. Similar results were obtained in two or three independent experiments (B, C). N.D., not detected.
In the liver of Ldlr−/− mice, the mRNA expression levels of Cyp7a1 and farnesoid X receptor (Fxr), which is a nuclear hormone receptor that is activated by BAs, were significantly decreased in the exendin-4 group compared to the control group. The mRNA level of FGF receptor 4 (Fgfr4), a BA receptor, was significantly increased in the exendin-4 group compared with the control group in Ldlr−/− mice.
Additionally, hepatic expression of lipogenic genes, such as Srebf1, stearoyl-CoA desaturase 1 (Scd1), fatty acid synthase (Fasn), acetyl-CoA carboxylase (Acaca), and beta-catenin (Ctnnb1), was examined. Exendin-4 treatment increased Srebf1 mRNA expression in Ldlr−/− mice and increased Fasn and Acaca mRNA expression in C57BL/6J mice.
Exendin-4 Treatment Increased Fgf15 mRNA Expression in the Small Intestine of Ldlr−/− Mice and Decreased NPC1L1 Protein Levels in the Small Intestine of C57BL/6J Mice
We investigated the mRNA expression and protein levels of genes related to small intestinal cholesterol absorption in Ldlr−/− and C57BL/6J mice ( Fig.4A-D ) . The mRNA expression of Fgf15, which is known to be a direct target of FXR, was significantly increased in the exendin-4 group compared to the control group in Ldlr−/− mice but not in C57BL/6J mice ( Fig.4B ) . The protein level of Niemann-Pick C1-like 1 (Npc1l1), associated with intestinal cholesterol absorption, significantly decreased in C57BL/6J mice ( Fig.4C, D ) . The protein levels of LXRα and MTP did not change in the exendin-4 group compared with the control group in Ldlr−/− or C57BL/6J mice.
Fig.4. Levels of small intestinal mRNA expression and protein associated with cholesterol absorption in Ldlr−/− and C57BL/6J mice .
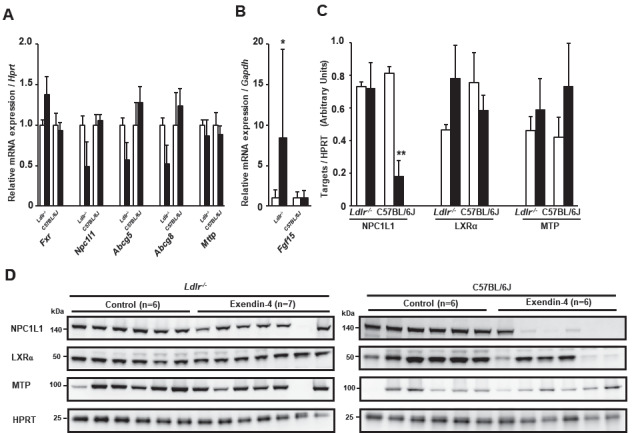
Small intestinal mRNA expression in exendin-4-treated (n=11 Ldlr−/− mice and n=8 C57BL/6J mice; black columns) and saline (control)-treated (n=10 in Ldlr−/− mice and n=8 in C57BL/6J mice; white columns) mice (A, B). Western blots for small intestinal NPC1L1, LXRα, and MTP were performed in Ldlr−/− and C57BL/6J mice (C, D). Protein levels of small intestinal NPC1L1, MTP, and LXRα were assessed by western blotting normalized to the level of HPRT in Ldlr−/− and C57BL/6J mice treated with exendin-4 (n=6 or 7; black columns) or saline (n=6; white columns). These values are the mean±SE. Similar results were obtained in two independent experiments (C, D). *p<0.05 and **p<0.01 vs. the control group in A and C, as calculated by unpaired t-test, and in B, as calculated by the Wilcoxon rank-sum test.
Exendin-4 Decreased Fecal BA Excretion in Ldlr−/− Mice
BA composition in the feces of Ldlr−/− and C57BL/6J mice was measured via LC-QTOF/MS to evaluate fecal BA excretion ( Fig.5A-D ) . The levels of total BAs in the feces of exendin-4-treated Ldlr−/− mice were significantly decreased compared with those in the feces of saline-treated Ldlr−/− mice ( Fig.5A ) . The BAs in the feces of Ldlr−/− mice were mainly the secondary BAs DCA (27.6% of total BAs in the exendin-4 group and 42.1% of total BAs in the control group) and ω-MCA (34.3% of total BAs in the exendin-4 group and 30.7% of total BAs in the control group) ( Fig.5C ) . The level of DCA was significantly reduced in the feces of exendin-4-treated Ldlr−/− mice. The proportions of the primary BAs: CA and α-MCA in the feces of Ldlr−/− mice were increased by exendin-4 treatment, whereas the proportion of DCA was decreased by this treatment. The secondary BA HDCA was not detected in Ldlr−/− mice treated with exendin-4. Changes in fecal BA excretion in Ldlr−/− mice treated with exendin-4 were not observed in C57BL/6J mice treated with exendin-4 ( Fig.5B, D ) .
Fig.5. Bile acid profile of the feces of Ldlr−/− and C57BL/6J mice treated with saline (control) or exendin-4 .
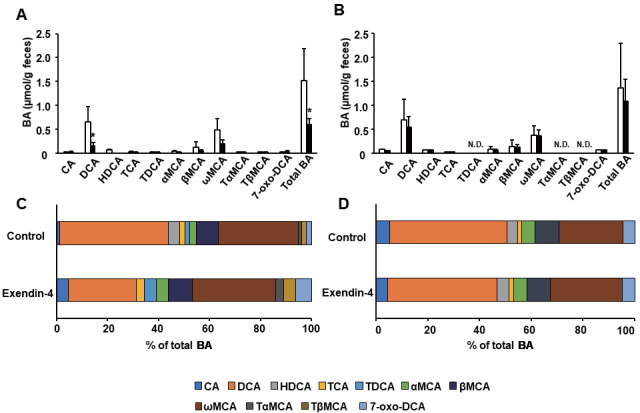
The bile acid (BA) composition was determined via LC-Q-TOF/MS. The BA composition in (A) exendin-4-treated (n=4; black columns) and saline-treated (n=4; white columns) Ldlr−/− mice, and (B) exendin-4-treated (n=5; black columns) and saline-treated (n=5; white columns) C57BL/6J mice. Total BA levels were calculated as the sum of the level of each BA. These values are the mean±SD. (A, B) The level of each BA is shown as the percentage of the total BA level (C, D). *p<0.05 vs. the control group, as calculated by unpaired t-test. Some BAs were below the detection limit. N.D., not detected.
Discussion
In the present study, exendin-4 administration for 5 days significantly decreased serum VLDL-C and LDL-C levels in Ldlr−/− and C57BL/6J mice. We previously reported that GLP-1RAs reduced the serum LDL-C levels in Japanese T2DM patients treated with statins 7) . Statins inhibit cholesterol synthesis, thereby increasing LDLR. In the present study, however, exendin-4 treatment decreased serum VLDL-C and LDL-C levels in Ldlr−/− mice, suggesting that it regulated serum cholesterol levels independent of LDLR. Exendin-4 treatment significantly decreased hepatic mature SREBP2 levels in Ldlr−/− and C57BL/6J mice. The hepatic mRNA expression level of Insig2 and Insig1 was significantly increased in the exendin-4 group compared with the control group in Ldlr−/− and C57BL/6J mice, respectively. INSIG1 and INSIG2 block the processing of SREBP2 in the Golgi at the posttranslational level by the binding to SCAP (SREBP cleavage activating protein) 12) . Transcriptional regulation of Insig1 and Insig2 genes is reported to be different. FXR and HIF-1α have been shown to activate hepatic Insig2 expression 13 , 14) . Insig1 is a direct PPARδ target gene in hepatocytes 15) . The mechanism by which the binding of SCAP to INSIG1 or INSIG2 leads to its retention in the endoplasmic reticulum is not clear. Therefore, exendin-4 treatment appears to have decreased hepatic mature SREBP2 level by increasing hepatic Insig1/2 mRNA levels in Ldlr−/− mice and C57BL/6J mice. Nevertheless, the hepatic mRNA levels of Hmgcr and Pcsk9, which are regulated by SREBP2, were significantly increased in exendin-4-treated C57BL/6J mice compared with saline-treated C57BL/6J mice, although they did not differ between exendin-4- and saline-treated Ldlr−/− mice. Transcriptional regulation of Hmgcs and Hmgcr by acyl-CoA binding protein 16) and regulation of Pcsk9 by HNF-1α 8) have been reported. SREBP2-related hepatic mRNA expressions may also be affected by transcription factors other than SREBP2 in the present study. Therefore, we could not elucidate whether exendin-4 treatment decreased hepatic cholesterol synthesis via decreasing hepatic mature SREBP2 level in Ldlr−/− mice in C57BL/6J mice.
In Ldlr−/− mice, exendin-4 treatment significantly decreased hepatic cholesterol levels and fecal BA excretion with decreased hepatic Cyp7a1 mRNA expression and increased small intestinal Fgf15 and hepatic Fgfr4 mRNA expression. Cholesterol is converted to BAs in the liver, and BAs act as detergents to promote the absorption of dietary cholesterol in the small intestine. Most BAs are absorbed in the distal ileum and returned to the liver, but during this process, up to 5% are excreted into the feces. The BA pool is constantly maintained; hence, the amount of BA excretion is almost equal to the amount that is newly synthesized in the liver. FGF15 has been reported to activate its receptor, FGFR4, in hepatocytes, which leads to inhibition of Cyp7a1 and Cyp8b1 mRNA expression via an SHP-dependent or SHP-independent mechanism 17 , 18) . Gardes et al. reported that FXR agonists decreased the BA pool size and the absorption of cholesterol by increasing intestinal Fgf15 expression in Ldlr−/− mice 19) . Inagaki et al. reported that FXR stimulated Fgf15 expression in the small intestine and suppressed Cyp7a1 expression in the liver via FGFR4, which led to decreased fecal BA excretion 18) . Thus, one possible mechanism is that exendin-4 activates FXR via unknown mechanisms, and FXR then stimulates Fgf15 expression in the small intestine; Cyp7a1 expression was suppressed via FGFR4 in the liver, thereby decreasing fecal BA excretion in Ldlr−/− mice. Decreased hepatic Cyp7a1 expression and fecal BA excretion in exendin-4-treated Ldlr−/− mice may also be due to the decrease in hepatic mature SREBP2 levels. Further in vitro analyses investigating the underlying mechanism are needed in the future. Exendin-4 treatment also altered the BA composition and significantly reduced DCA levels in the feces of Ldlr−/− mice in the present study. Because BA and DCA are associated with cholesterol absorption in the intestine, the decrease in DCA may explain the decrease in cholesterol absorption in Ldlr−/− mice. Recently, liraglutide was shown to suppress caloric intake, promote marked weight loss, improve glucose tolerance, and reduce plasma cholesterol levels by altering the microbiome in diet-induced obese (DIO) mice 20) . Therefore, intestinal bacteria may be changed in Ldlr−/− mice by exendin-4 treatment. In C57BL/6J mice, exendin-4 treatment significantly decreased small intestinal NPC1L1 levels in the present study. NPC1L1 is a transporter for cholesterol absorption, and it mediates the absorption of dietary and biliary cholesterol from the intestinal lumen. Therefore, it was suggested that exendin-4 decreased serum cholesterol levels by decreasing cholesterol absorption from the intestine in C57BL/6J mice. Exendin-4 treatment might decrease cholesterol absorption by different mechanisms in Ldlr−/− and C57BL/6J mice.
Exendin-4 treatment for 5 days significantly decreased the average food intake in C57BL/6J mice and tended to decrease it in Ldlr−/− mice. There was no significant difference in serum TG levels, which were affected by food intake in Ldlr−/− and C57BL/6J mice. GLP-1 analogs are known to delay gastric emptying and to inhibit food intake. Indeed, the rate of gastric emptying has been shown to influence dietary cholesterol absorption efficiency in mice 21) . Therefore, a delay in gastric emptying may partially affect cholesterol absorption in Ldlr−/− and C57BL/6J mice.
It was previously reported that 50 µg/kg/day (almost 12 nmol/kg/day) exendin-4 prevented hepatic VLDL overproduction and hepatic steatosis induced by a high-fat diet in APOE*3-Leiden mice 22) . In this study, we used almost the same dose of exendin-4 (10 nmol/kg/day). Lee et al. reported that 1-nmol/kg/day exendin-4 administered via intraperitoneal injection every other day for 10 weeks decreased serum free fatty acids and TG levels and hepatic lipid accumulation and improved hepatic inflammation in DIO C57BL/6J mice 23) . Yin et al. reported that 1-nmol/kg/day exendin-4 via intraperitoneal injection for 8 weeks decreased TC and LDL-C levels in diabetic Apoe−/− mice 24) . Taher et al. reported that plasma cholesterol levels decreased in hamsters that received twice daily intraperitoneal injections of 20 µg/kg (4.8 nmol/kg) exendin-4 for 7 days 25) . Our results suggest that intraperitoneal single bolus injections of exendin-4 (10 nmol/kg/day) for 5 days achieved sufficient plasma levels to produce pharmacological effects.
There is no evidence that GLP-1 signaling is mediated by LDLR and LDLR deficiency was associated with the lack of GLP-1 signaling. The expression of GLP-1R on hepatocytes and intestinal epithelial cells remains controversial 26) . GLP-1 may signal via a neural circuit originating in the hepatic-portal area 27) . GLP-1 may bind to GLP-1Rs on neuronal fibers in the liver and small intestine. Khound et al. reported that exendin-4 and palmitic acid inhibited de novo lipogenesis in mouse primary hepatocytes 28) . These results suggest an unidentified GLP-1R on hepatocytes or that exendin-4 may act on other hormone receptors.
This study has some limitations. First, this is a short-term experiment for the pharmacological effects of exendin-4. The experimental results may differ between short- and long-term experiments. To evaluate the LDL-C-lowering effects of GLP-1RA properly, it is necessary to use mice fed with a high-fat diet for a couple of weeks and administered with exendin-4 for at least 4 weeks in future studies. Second, Ldlr−/− mice fed with a normal diet were used in this study. We used Ldlr−/− mice fed with a normal diet to eliminate the effect of exendin-4 on TG metabolism due to high-fat diet feeding. If the LDL-C-lowering effects for patients with homozygous familial hypercholesterolemia are to be examined, exendin-4 treatment must be examined in Ldlr−/− mice fed with a high-fat diet as a model of homozygous familial hypercholesterolemia. Finally, we failed to explore the mechanism of GLP-1 signaling on cholesterol synthesis and absorption in Ldlr−/− and C57BL/6J mice. Further in vitro analyses investigating the underlying mechanism are needed in the future.
In conclusion, our findings demonstrate that exendin-4 treatment decreased serum VLDL-C and LDL-C levels in a manner that was independent of LDLR. Exendin-4 treatment decreased hepatic mature SREBP2 levels and increased hepatic Insig1/2 mRNA expression in Ldlr−/− and C57BL/6J mice. Exendin-4 treatment might decrease cholesterol absorption by different mechanisms in Ldlr−/− and C57BL/6J mice. Hence, GLP-1RAs may be reasonable therapeutic options for patients with T2DM and familial hypercholesterolemia with defective LDLR activity.
Acknowledgments
We thank Ms. Rie Oishi and Ms. Megumu Morimoto for providing technical assistance with the mouse breeding and Ms. Hitomi Komai and Ms. Masumi Sakata for providing technical assistance with the molecular biology experiments in NCVC. We thank Sapporo General Pathology Laboratory Co., Ltd. (Sapporo, Japan) for the technical assistance with the preparation of the histopathological sections and Oil red O staining of the liver in C57BL/6J and Ldlr−/− mice, and CARE at Nagoya University for the technical support for the animal experiments.
Sources of Funding
This work was supported by grants from the Japanese Ministry of Health, Labor, and Welfare of Japan (H26-nanji-ippan-056, H30-nanji-ippan-003); JSPS KAKENHI (JP17K08681); the Intramural Research Fund for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center (25-2-5, 27-6-24, 29-6-11); the Japan Research Foundation for Clinical Pharmacology and the Hoansha Foundation.
Disclosure
The authors have no conflicts of interest to disclose.
Data Availability
All data generated or analyzed during this study are included in this article or in the data repositories listed in the References.
References
- 1).MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE and Wheeler MB: The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes, 2002; 51 Suppl 3: S434-442 [DOI] [PubMed] [Google Scholar]
- 2).Yamato E, Noma Y, Tahara Y, Ikegami H, Yamamoto Y, Cha T, Yoneda H, Ogihara T, Ohboshi C, Hirota M and Shima K: Suppression of synthesis and release of glucagon by glucagon-like peptide-1 (7-36 amide) without affect on mRNA level in isolated rat islets. Biochem Biophys Res Commun, 1990; 167: 431-437 [DOI] [PubMed] [Google Scholar]
- 3).Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P and Dandona P: Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract, 2007; 13: 444-450 [DOI] [PubMed] [Google Scholar]
- 4).Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME and Maggs DG: Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin, 2008; 24: 275-286 [DOI] [PubMed] [Google Scholar]
- 5).Horton ES, Silberman C, Davis KL and Berria R: Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care, 2010; 33: 1759-1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC and Reaven PD: Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis, 2010; 212: 217-222 [DOI] [PubMed] [Google Scholar]
- 7).Hasegawa Y, Hori M, Nakagami T, Harada-Shiba M, Uchigata Y: Glucagon-like peptide-1 receptor agonists reduced the LDL-cholesterol in Japanese patients with type 2 diabetes mellitus treated with statins. J Clin Lipidol, 2018; 12: 62-69 [DOI] [PubMed] [Google Scholar]
- 8).Yang SH, Xu RX, Cui CJ, Wang Y, Du Y, Chen ZG, Yao YH, Ma CY, Zhu CG, Guo YL, Wu NQ, Sun J, Chen BX and Li JJ: Liraglutide downregulates hepatic LDL receptor and PCSK9 expression in HepG2 cells and db/db mice through a HNF-1a dependent mechanism. Cardiovasc Diabetol, 2018; 17: 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, Hecksher-Sørensen J, Ingvorsen C, Polex-Wolf J and Knudsen LB: The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE(-/-) and LDLr(-/-) Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl Sci, 2018; 3: 844-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Eng J, Kleinman WA, Singh L, Singh G and Raufman JP: Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem, 1992; 267: 7402-7405 [PubMed] [Google Scholar]
- 11).Folch J, Lees M and Sloane Stanley GH: A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem, 1957; 226: 497-509 [PubMed] [Google Scholar]
- 12).Yabe D, Brown MS and Goldstein JL: Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci U S A, 2002; 99: 12753-12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Hubbert ML, Zhang Y, Lee FY and Edwards PA: Regulation of hepatic Insig-2 by the farnesoid X receptor. Mol Endocrinol, 2007; 21: 1359-1369 [DOI] [PubMed] [Google Scholar]
- 14).Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J and DeBose-Boyd RA: Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem, 2017; 292: 9382-9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Qin X, Xie X, Fan Y, Tian J, Guan Y, Wang X, Zhu Y and Wang N: Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology, 2008; 48: 432-441 [DOI] [PubMed] [Google Scholar]
- 16).Vock C, Döring F and Nitz I: Transcriptional regulation of HMG-CoA synthase and HMG-CoA reductase genes by human ACBP. Cell Physiol Biochem, 2008; 22: 515-524 [DOI] [PubMed] [Google Scholar]
- 17).Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ and Moschetta A: FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res, 2007; 48: 2693-2700 [DOI] [PubMed] [Google Scholar]
- 18).Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ and Kliewer SA: Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab, 2005; 2: 217-225 [DOI] [PubMed] [Google Scholar]
- 19).Gardes C, Chaput E, Staempfli A, Blum D, Richter H and Benson GM: Differential regulation of bile acid and cholesterol metabolism by the farnesoid X receptor in Ldlr -/- mice versus hamsters. J Lipid Res, 2013; 54: 1283-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Madsen MSA, Holm JB, Pallejà A, Wismann P, Fabricius K, Rigbolt K, Mikkelsen M, Sommer M, Jelsing J, Nielsen HB, Vrang N and Hansen HH: Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep, 2019; 9: 15582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Kirby RJ, Howles PN and Hui DY: Rate of gastric emptying influences dietary cholesterol absorption efficiency in selected inbred strains of mice. J Lipid Res, 2004; 45: 89-98 [DOI] [PubMed] [Google Scholar]
- 22). Parlevliet ET, Wang Y, Geerling JJ, Schroder-Van der Elst JP, Picha K, O’Neil K, Stojanovic-Susulic V, Ort T, Havekes LM, Romijn JA, Pijl H and Rensen PC: GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One, 2012; 7: e49152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW and Lee WY: Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One, 2012; 7: e31394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Yin QH, Zhang R, Li L, Wang YT, Liu JP, Zhang J, Bai L, Cheng JQ, Fu P and Liu F: Exendin-4 Ameliorates Lipotoxicity-induced Glomerular Endothelial Cell Injury by Improving ABC Transporter A1-mediated Cholesterol Efflux in Diabetic apoE Knockout Mice. J Biol Chem, 2016; 291: 26487-26501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Taher J, Baker CL, Cuizon C, Masoudpour H, Zhang R, Farr S, Naples M, Bourdon C, Pausova Z and Adeli K: GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol Metab, 2014; 3: 823-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, Streutker C, Holland D, Cao X, Baggio LL and Drucker DJ: GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology, 2013; 154: 127-139 [DOI] [PubMed] [Google Scholar]
- 27).Ionut V, Hucking K, Liberty IF and Bergman RN: Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia, 2005; 48: 967-975 [DOI] [PubMed] [Google Scholar]
- 28).Khound R, Taher J, Baker C, Adeli K and Su Q: GLP-1 Elicits an Intrinsic Gut-Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance. Arterioscler Thromb Vasc Biol, 2017; 37: 2252-2259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article or in the data repositories listed in the References.


