Abstract
Purpose
Treatment of Menière’s Disease (MD) comprises an array of both non-destructive and destructive treatment options. In patients who are therapy–refractory to non-destructive medical treatment, endolymphatic mastoid shunt surgery (EMSS) is both recommended and debated controversially. The aim of this study was to investigate safety in terms of hearing, vestibular function, complication rate, and efficacy with regards to vertigo control of EMSS in patients with MD according to the current diagnostic criteria of 2015.
Methods
Retrospective analysis of 47 consecutive patients with definite or probable MD with description of demographic parameters, pre- and postoperative MD treatment, pre- and postoperative audiometric (pure tone audiometry) and vestibular (caloric testing) results. The parameters were compared between patients with and without postoperative vertigo control.
Results
31/47 patients (66.0%) had improved vertigo control postoperatively. Postoperative hearing and vestibular preservation were predominantly stable. No significant differences between patients with improved vertigo control and patients with no change or worse vertigo episodes were found. In the treatment refractory group, 4 patients required a revision EMSS and 6 a destructive MD treatment (5 gentamicin intratympanically, 1 labyrinthectomy). No peri- or postsurgical complications were reported.
Conclusions
EMSS was found to be beneficial in two thirds of the patients with definite or probable Morbus Menière and a safe procedure regarding hearing and vestibular preservation with no postoperative complications. Therefore, EMSS should be considered before inducing destructive treatment options, such as intratympanic gentamicin application or labyrinthectomy.
Keywords: Endolymphatic sac surgery, Endolymphatic mastoid shunt surgery, EMSS, Menière’s disease, MD
Introduction
Meniere’s disease (MD) is a diverse disorder defined by three core symptoms: episodic vertigo, tinnitus, and sensorineural hearing loss. Both intrinsic, e.g., genetics, and extrinsic factors, e.g., influences from environment, may play a role in the development of MD [1]. The underlying pathomechanism of MD is well-discussed, though still unknown. Histopathological studies from the late 1980s hypothesize an association with an endolymph hydrops causing this fluctuating disease pattern [2]. The current diagnostic criteria published in 2015 define MD as a clinical syndrome characterized by recurrent, spontaneous vertigo attacks and fluctuating aural symptoms (sensorineural hearing loss, tinnitus, and aural fullness) [3]. MD patients suffer from an impaired quality of life [4, 5] and, therefore, require profound education about the available treatment options, which include both medical and surgical techniques, as well as non-destructive and destructive methods. Depending on the presented constellation of symptoms, the treatment concept should be tailored to the patient individually. An international team of MD experts of 4 different continents recommended the staged consideration of treatment options, based on a literature review and their own experiences [6] and presented an algorithm for the different forms of MD following five steps: the first step should consist of a conservative medical treatment including lifestyle adjustments, as in adequate sleeping [7], investigation of sleep apnea [8], decreased stress, avoidance of caffeine, alcohol, and tobacco, and adaption of a low salt diet. Vestibular rehabilitation and psychotherapy are recommended, as well [9–11]. In addition to those lifestyle changes, a medical treatment with betahistine can be initiated [12, 13]. As a second step and also non-destructive treatment, the intratympanic application of corticosteroids (dexamethasone or methylprednisolone) is recommended [14]. Since the treatment with intratympanic corticosteroid has shown satisfactory results, a decline in non-destructive surgical treatment with endolymphatic sac surgery has been observed. Therefore, it is recommended as a third-line treatment. It has long been criticized as a placebo-surgery, but a recent systematic review concluded low evident effect in improving symptoms in MD [15, 16]. Manipulation on the inner ear to decompress the endolymphatic sac evolved in the 1920s, when Portmann drew parallels between glaucoma [17] which was later expanded by William House in the 1960s by inserting material for a permanent shunt into the subarachnoid space or mastoid [18]. A recent review from Kersbergen and Ward thoroughly depicts the history regarding surgical manipulation of the inner ear treating MD [19]. Furthermore, four types of surgical techniques have been introduced, ranging from the most minimal invasive option, endolymphatic sac decompression, over endolymphatic sac incision, endolymphatic–mastoid shunt to the most invasive technique, the endolymphatic–subarachnoid shunt. The fourth step is a destructive medical technique with application of the ototoxic agent gentamicin intratympanically, which is stated as an effective method to eradicate vertigo in MD [20, 21]. The downside of this technique is the ototoxic effect in the cochlea that leads to permanent hearing impairment in some cases. As a fifth and final step the surgical destruction of the vestibular organ is seen as ultima ratio. Literature on vestibular neurectomy or labyrinthectomy is scarce. Older studies from the 1990s or 2000s revealed a more efficient treatment of vertigo attacks with vestibular neurectomy than with intratympanic gentamicin injection [22, 23]. Alongside with the labyrinthectomy a simultaneous cochlear implantation is recommended [6].
In the present study we focus on the well-debated efficacy of non-destructive endolymphatic sac surgery (endolymphatic mastoid shunt surgery—EMSS) in patients fulfilling the current diagnostic criteria for MD and investigating the preservation of postoperative hearing and vestibular function.
Materials and methods
Patient selection and ethical considerations
The retrospective data analysis was approved on April 11th 2019 by the Institutional Review Board of the University Hospital, LMU Munich (Ethikkommission der Medizinischen Fakultät der LMU München), reference number 19-086. Data collection was performed using the electronical clinical patient registry, including surgical reports, medical history, course of treatment, audiometric, and caloric testing pre- and postoperatively. Demographic data for each patient included sex, age, date of surgery, and date of last visit. Our study revealed 72 consecutive patients with MD at our academic tertiary referral center, who underwent EMSS between 2004 and 2019. A subset of 15 patients received EMSS and CI simultaneously as a first line surgical treatment and were not included to this study due to lack of follow-up of audiometric and caloric testing. Another 10 patients were excluded due to lack of data to categorize them along the diagnostic criteria of 2015 [3]. The remaining 47 patients with definitive or probable MD were divided into two groups: patients who reported of reduced or complete subsidence of vertigo attacks post-surgery, while patients in the second group had no benefit or worsening of symptoms after surgery.
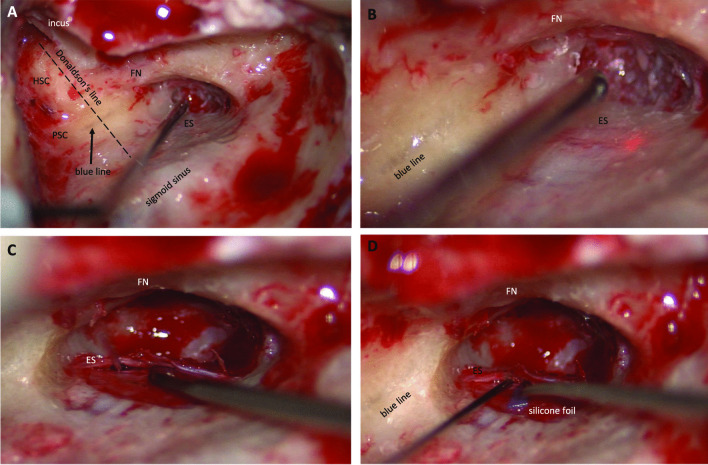
Surgical procedure—endolymphatic mastoid shunt surgery (Fig. 1)
Fig. 1.
Endolymphatic–mastoid shunt surgery. Depiction of the endolymphatic–mastoid shunt surgery with A dissection of the landmarks: skull base to the middle fossa, sigmoid sinus, antrum with short process of the incus, facial nerve in the mastoid segment, horizontal, and posterior semicircular canal (“blue line”). The Donaldson’s line (dashed line) is drawn along the horizontal semicircular canal (HSC) and cuts the PSC perpendicularly. B Endolymphatic sac is identified lying inferior-posteriorly to the “blue line” of the posterior semicircular canal (PSC) as a duplicature of the dura mater (C) and incised. D Small triangular silicone foil (edge length 2 × 3 mm) is inserted as a permanent shunt. ES endolymphatic sac, FN facial nerve, HSC horizontal semicircular canal, PSC posterior semicircular canal
All patients received a standard EMSS procedure with application of a silicone foil as drainage. The procedure includes a mastoidectomy and dissecting the following landmarks: skull base to the middle fossa, sigmoid sinus, antrum with short process of the incus, facial nerve in the mastoid segment, horizontal, and posterior semicircular canal. The posterior semicircular canal was thinned out until the semicircular canal is visible as a blue line (Fig. 1A). The endolymphatic sac was identified lying inferior-posteriorly to the posterior semicircular canal as a duplicature of the dura mater. After thinning and removal of the bone superficial to the endolymphatic sac (= endolymphatic sac decompression; Fig. 1A, B), it was opened (Fig. 1C) and a small triangular silicone foil (edge length 2 × 3 mm) was inserted as a shunt (Fig. 1D). Postoperatively all patients received regular otologic follow-up treatment including audiometric testing of bone conduction, as well as monitoring for facial nerve impairment or nystagmus.
Audiometry
Audiometric tests were performed as a pure tone audiometry with testing for each ear at 0.5, 1, 2, 3, 4, 6, and 8 kHz via headphone and with air and bone conduction thresholds between − 10 dB and 120 dB hearing level (dB HL) for each ear separately. Aided air conduction was measured with warble tones in free-field. Thresholds exceeding 120 dB HL were recorded as 130 dB HL for statistical purposes.
Caloric testing
To evaluate the vestibular function, caloric testing on electronystagmography (ENG) was performed pre- and postoperatively using cold (30 °C) and warm (44 °C) water. To calculate for unilateral weakness the Jongkees-formula was applied [24].
Data analysis
For preoperative analysis the worst audiometric result within 6 months prior to EMSS was considered. Regarding postoperative analysis, the first bone and air conduction audiometry after surgery, and for long-term results the last documented audiometric test was used. As per applicability to the study, this report was generated according to the Strengthening the Reporting of Observational studies in Epidemiology statement [25]. Summary data of audiometric tests was calculated according to the guidelines of the Committee on Hearing and Equilibrium of the American Academy of Otolaryngology–Head and Neck Surgery with the pure tone average for the air conducted frequencies 0.5, 1, 2, and 3 kHz (AC-PTA4CHE) [26]. In addition, bone conduction stability was analyzed with pure tone average for the bone conducted frequencies 0.5, 1, 2, and 4 kHz (BC-PTA).
Statistical analysis
Statistical analysis was performed using SPSS (version 24) software (SPSS Inc, Chicago, IL). Shapiro–Walk test was used to test for normative distribution. Since the cohort is rather small, only descriptive statistical analysis was performed. All figures were created with Microsoft Excel version 1906.
Results
Demography and course of MD treatment
Of all included 47 patients, 31 (66.0%) reported of reduced or complete subsidence of vertigo attacks post-surgery. 16 patients (34.0%) accounted for the group of patients, who reported of no benefit or worsening of symptoms after surgery. No postsurgical complications (immediate postoperative vertigo, significant drop of bone conduction threshold, deafness, wound healing issues, postoperative infection, scarring issues) arose in both groups. The mean duration of first consultation until last follow-up was 4.6 ± 3.8 years and slightly shorter in the group with improved symptoms. The mean of age, sex, and side of the involved ear was evenly distributed in both groups. In the improved group 58.1% (n = 18) patients were diagnosed with definitive MD and 62.5% (n = 10) in the treatment refractory group. Regarding preoperative treatment, the majority of the patients (n = 38, 80.9%) in both groups were unsuccessfully treated with betahistine prior to EMSS. An ear tube was received by 25.5% (n = 12) of all patients. Intratympanic gentamicin injection was performed in 2 patients (4.3%) and intratympanic steroid injection in 2 patients of the improved group (6.5%).
Concerning postoperative treatment, the average postoperative follow-up was considerably longer in treatment refractory group (improved group: 15.9 ± 13.1 months vs. treatment refractory group: 23.5 ± 21.3 months). Despite reported improvement or subsidence of symptoms, a subset of 7 patients (22.5%) in the improved group continued with medication of betahistine. Due to lack of benefit or worsening of MD symptoms, 56.3% (n = 9) of the treatment refractory group received postoperative medical MD treatment. A destructive MD treatment was performed in 5 patients (31.3%) with 5 patients receiving gentamicin and 1 of those patients a labyrinthectomy. All data are depicted in Table 1.
Table 1.
Patients’ characteristics
| Improved group | No benefit group | Total | |
|---|---|---|---|
| Sex [n (%)] | |||
| Female | 13 (41.9) | 10 (62.5) | 23 (48.9) |
| Male | 18 (58.1) | 6 (37.5) | 24 (51.1) |
| Age [years ± SD] | 58.2 ± 12.2 | 50.9 ± 13.4 | 56.2 ± 13.0 |
| Side [n (%)] | |||
| Right | 16 (51.6) | 9 (56.3) | 25 (53.2) |
| Left | 19 (48.4) | 7 (43.8) | 26 (55.3) |
| Duration of symptoms [years ± SD (n)] | 4.3 ± 3.4 (30) | 4.8 ± 3.7 (14) | 4.6 ± 3.8 (44) |
| Previous treatments [n (%)] | |||
| Betahistine | 27 (87.1) | 11 (68.8) | 38 (80.9) |
| IT gentamicin | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Ear tube | 6 (19.4) | 6 (37.5) | 12 (25.5) |
| IT dexamethasone | 2 (6.5) | 0 (0) | 2 (4.3) |
| Disease categorya [n (%)] | |||
| Definitive Morbus Menière | 18 (58.1) | 10 (62.5) | 28 (59.6) |
| Probable Morbus Menière | 13 (41.9) | 6 (37.5) | 19 (40.4) |
| Postoperative treatment | |||
| No therapy [n (%)] | 27 (77.4) | 4 (25.0) | 31 (66.0) |
| Second line therapy [n (%)] | |||
| Betahistine | 7 (22.5) | 4 (25.0) | 11 (23.4) |
| IT gentamicin | 0 (0) | 5 (31.3) | 5 (10.6) |
| IT dexamethasone | 0 (0) | 1 (6.3) | 1 (2.1) |
| Revision EMSS | 0 (0) | 4 (25.0) | 4 (8.5) |
| Labyrinthectomy | 0 (0) | 1 (6.3) | 1 (2.1) |
| Total [n] | 31 | 16 | 47 |
Patient’s characteristics including pre- and postoperative additional Meniére specific treatment
EMSS endolymphatic sac surgery, IT intratympanic application, n number; SD standard deviation, yr years
aAccording to Lopez-Escamez et al. [3]
Audiometric results
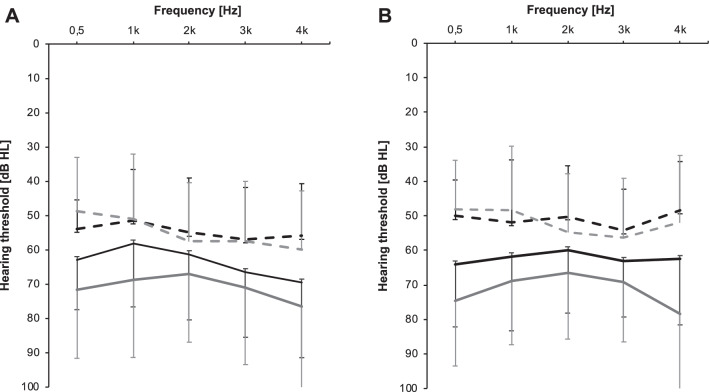
All patients exhibited hearing impairment preoperatively according to WHO criteria (AC-PTA4WHO—air conduction of the frequencies 0.5, 1, 2, and 4 kHz) with no differences between both groups (Table 2). Bone conduction thresholds of the treated ears remained stable in both groups after surgery, air conduction seemed to have improved postoperatively (Fig. 2A, B). Postoperative bone conduction was stable in both groups as depicted by scattergrams (BC-PTA—bone conduction of the frequencies 0.5, 1, 2, and 4 kHz), with 87.1% (n = 27) in the improved group and 93.8% (n = 15) in the treatment refractory group exhibited preserved hearing (all 89.4%, n = 42). Long-term results were available in 21/31 of the improved patients and 9/16 treatment refractory patients, showing a slight decrease in hearing preservation (improved group: 90.5%, n = 19; treatment refractory group: 77.8%, n = 7; all 86.7%, n = 26; Fig. 3A, B).
Table 2.
Hearing loss
| Improved group | No benefit group | Total | |
|---|---|---|---|
| Follow-up audiogram preoperative (months ± SD) | 1.5 ± 1.5 | 1.2 ± 0.9 | 1.4 ± 1.2 |
| AC-PTA of hearing loss preoperative (dB ± SD) | 63.7 ± 18.6 | 62.3 ± 18.6 | 63.0 ± 18.6 |
| Grade of hearing loss preoperative [n (%)]a | n = 31 | n = 16 | n = 47 |
| No impairment | 0 (0) | 0 (0) | 0 (0) |
| Slight impairment | 2 (6.5) | 1 (6.3) | 3 (6.4) |
| Moderate impairment | 14 (45.2) | 5 (31.3) | 19 (40.4) |
| Severe impairment | 11 (35.5) | 3 (18.8) | 14 (29.8) |
| Profound impairment or deafness | 4 (12.9) | 7 (43.8) | 11 (23.4) |
| Follow-up audiogram postoperative (months ± SD) | 1.6 ± 2.3 | 1.3 ± 1.5 | 1.5 ± 1.9 |
| AC-PTA of hearing loss postoperative (dB ± SD) | 70.9 ± 23.7 | 65.4 ± 19.4 | 68.2 ± 21.5 |
| Grade of hearing loss postoperative [n (%)]a | n = 31 | n = 15 | n = 46 |
| No impairment | 0 (0) | 0 (0) | 0 (0) |
| Slight impairment | 4 (10.8) | 1 (6.6) | 5 (10.9) |
| Moderate impairment | 4 (16.2) | 4 (26.7) | 8 (17.4) |
| Severe impairment | 12 (32.4) | 7 (46.7) | 19 (41.3) |
| Profound impairment or deafness | 11 (40.5) | 3 (20.0) | 14 (30.4) |
| Follow-up audiogram postoperative, long-term (months ± SD) | 15.8 ± 13.1 | 23.5 ± 21.3 | 19.7 ± 17.2 |
| AC-PTA of hearing loss postoperative, long-term (dB ± SD) | 68.3 ± 21.4 | 71.6 ± 19.1 | 70.0 ± 20.3 |
| Grade of hearing loss postoperative, long-term [n (%)]a | n = 22 | n = 10 | n = 32 |
| No impairment | 0 (0) | 0 (0) | 0 (0) |
| Slight impairment | 3 (10.7) | 1 (10.0) | 4 (12.5) |
| Moderate impairment | 7 (21.6) | 1 (10.0) | 8 (25.0) |
| Severe impairment | 7 (32.1) | 5 (50.0) | 12 (37.5) |
| Profound impairment or deafness | 5 (21.6) | 3 (30.0) | 8 (25.0) |
Audiometry results with the grade of hearing loss in dB hearing level including the time of testing
n number, AC-PTA pure tone average (air conduction frequencies: 0,5, 1, 2, 3, and 4 kHz)
aAccording to WHO
Fig. 2.
Pure tone audiometry pre- and postoperative. Pure tone audiometry pre- (black) and postoperatively (grey) in A all 31 patients who reported of better vertigo control after endolymphatic mastoid shunt surgery and B all 16 patients who showed no improvement after endolymphatic mastoid shunt surgery. Bone conduction is depicted as dotted lines and air conduction as solid lines. Standard deviation is indicated by whiskers
Fig. 3.
Scattergram of pre- vs. postoperative and preoperative vs. long-term bone conduction pure tone average. Change in bone conduction pure tone average of the frequencies 0.5, 1, 2, and 3 kHz of those patients who reported of better vertigo control after endolymphatic mastoid shunt surgery (marked as a circle) and those who showed no improvement after endolymphatic mastoid shunt surgery (marked as a cross). A Depicts the immediate postoperative results from 31 improved and 16 patients without benefit from the procedure; B shows the long-term results of 22 improved and 10 no-benefit patients. The solid line indicates no alterations; the area between the dotted lines indicates unaltered thresholds within a ± 15 dB HL range. BC: bone conduction; HL: hearing level; PTACHE 1995: pure tone average as indicated by the Committee on Hearing and Equilibrium (CHE) [26]
Vestibular function
Of all 47 patients in a subset of 22 patient both pre- and postoperative caloric testing results were available for analysis (improved group: 15/31 patients; treatment refractory group: 7/16 patients). The postoperative ENG was performed within 13.8 ± 11.7 months after surgery. Pre- vs. postoperative caloric testing results showed comparable values in both groups. In the improved group 5 patients (33.3%, 5/15) changed from a normal to an abnormal response in caloric testing on the operated side. In the treatment refractory group, 1 patient (14.3%, 1/7) had an abnormal result postoperatively. The mean peripheral vestibular response to caloric stimulation was stable in both groups. In none of the patients a complete loss of vestibular function was observed (Table 3).
Table 3.
Caloric testing of vestibular function
| Improved group | No benefit group | Total | |
|---|---|---|---|
| Mean time preoperative [months ± SD] | 6.2 ± 6.7 | 5.0 ± 5.5 | 5.8 ± 6.4 |
| Mean follow-up postoperative [months ± SD] | 13.8 ± 11.7 | 13.7 ± 13.6 | 13.8 ± 12.6 |
| Abnormal [n (%)] | |||
| Preoperative | 4 (26.7) | 2 (28.6) | 6 (27.3) |
| Postoperative | 7 (46.7) | 2 (28.6) | 9 (40.9) |
| Change in pre-/postoperative vestibular excitability [n (%)] | |||
| Remained normal | 6 (40.0) | 4 (57.1) | 10 (45.5) |
| Abnormal/normal | 2 (13.3) | 1 (14.3) | 3 (13.6) |
| Remained abnormal | 2 (13.3) | 1 (14.3) | 3 (13.6) |
| Normal/abnormal | 5 (33.3) | 1 (14.3) | 6 (27.3) |
| Mean peripheral vestibular excitability [% ± SD] | |||
| Preoperative | 46.2 ± 20.5 | 37.7 ± 32.4 | 40.7 ± 24.3 |
| Postoperative | 43.1 ± 22.8 | 36.0 ± 31.1 | 40.0 ± 25.0 |
| Mean vestibular excitability [°/s ± SD] | |||
| Preoperative | 10.7 ± 5.6 | 11.6 ± 9.9 | 11.0 ± 7.0 |
| Postoperative | 7.7 ± 4.4 | 10.2 ± 6.5 | 8.5 ± 5.1 |
| Number of patients whose mean peripheral vestibular excitability [n (%)] | |||
| Decreased > 10% | 2 (13.3) | 1 (14.3) | 3 (13.6) |
| Decreased > 20% | 2 (13.3) | 1 (14.3) | 3 (13.6) |
| Increased > 25% | 3 (20.0) | 1 (14.3) | 4 (18.2) |
| Total (n) | 15 | 7 | 22 |
Caloric testing of periphery vestibular function including time of testing
EMSS endolymphatic sac surgery, mo months, n number, RVR reduced vestibular response, SD standard deviation
Discussion
The efficacy of endolymphatic sac surgery is well-debated and literature on it is broad (Table 4). The surgical techniques differ and range from a simple decompression of the endolymphatic sac to the invasive procedure of creating a subarachnoidal shunt. The most common technique reported in the literature is endolymphatic mastoid shunt surgery (EMSS) which can be performed with the mere incision of the endolymphatic sac or with creating a permanent shunt by applying a small silicone foil to the incision, which was performed in the study at hand.
Table 4.
Literature on endolymphatic sac surgery
| Year | Author | No. of patients | Form of endolymphatic sac surgery | Follow-up | Vertigo control | Hearing preservation | Version of diagnostic criteria | Audiometic results (stage of MD/PTA) | Preoperative audiometric testing | Preoperative vestibular testing | Postoperative audiometric testing | Postoperative vestibular testing | Vestibular migraine/other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1977 | Arendberg et al. [38] | 35 | EMSS with silicone | n.g | 88.9% | 85.2% | 1985 | Y | N | N | N | N | n.g./n.g |
| 1979 | Arendberg et al. [39] | 66 | EMSS with silicone | 10.3 mo | 90.8% | n.g | 1985 | Y | N | N | N | N | n.g./n.g |
| 1981 | Thomsen et al. [32] | 15 | EMSS with silicone | 12 mo | 87% | 87% | 1972 | Y | N | N | N | N | n.g./Y |
| 1983 | Brown et al. [40] | 245/328 | EMSS vs. ES decompression | n.g | n.g | n.g | n.g | N | n.g | n.g | n.g | n.g | n.g./n.g |
| 1983 | Goldenberg et al. [41] | 48 | EMSS with silicone | 1–5 yrs | 81% | n.g | n.g | N | n.g | n.g | n.g | n.g | n.g./n.g |
| 1983 | Miller et al. [42] | 24 | EMSS with silicone | > 12 mo | 87% | 79% | n.g | Y | N | n.g | N | n.g | n.g./n.g |
| 1983 | Spector et al. [43] | 122 | EMSS with silicone | 3 yrs | 83% | 63% | 1972 | Y | N | Y | N | Y | n.g./n.g |
| 1983 | Thomsen et al. [33] | 13 | EMSS with silicone | 36 mo | 69% | 69% | 1972 | Y | N | N | N | N | n.g./Y |
| 1984 | Bretlau et al. [36] | 13 vs. placebo | EMSS | 36 mo | 70% | 61% | 1972 | Y | N | N | N | N | n.g./n.g |
| 1984 | Glasscock et al. [44] | 310 | ES subarachnoidal shunt | 1–11 yrs | 66% | 65% | 1972 | N | N | N | N | N | n.g./n.g |
| EMSS | 1–11 yrs | 50% | 57% | 1972 | N | N | N | N | N | n.g./n.g | |||
| Arendberg valve procedure | 1–11 yrs | 49% | 53% | 1972 | N | N | N | N | N | n.g./n.g | |||
| 1985 | Huang et al. [45] | 339 | EMSS with/ without silicone | n.g | 80% | n.g | n.g | Y | N | n.g | N | n.g | n.g./n.g |
| 1986 | Smyth et al. [46] | 21 | ES decompression | 12 mo | 86% | 86% | n.g | Y | N | N | N | N | n.g./Y |
| 21 | ES decompression | 8–10 yrs | 71% | 52% | n.g | Y | N | N | N | N | n.g./Y | ||
| 1986 | Thomsen et al. [34] | 12 | EMSS with silicone | 84 mo | 75% | 42% | 1972 | Y | N | N | N | N | n.g./Y |
| 1987 | Arendberg et al. [47] | 214 | EMSS with silicone | 35.1 mo | 73.9% | 60.3% worse | 1985 | n/a | Y | N | Y | N | n.g./n.g |
| 1987 | Brackmann et al. [48] | 169 | EMSS with silicone | > 12 mo | 71.1% | 62.2% | 1985 | N | N | N | N | N | n.g./n.g |
| 1987 | Kitahara et al. [49] | 140 | EMSS without silicone | 24 mo | 94% | 60.7% | 1985 | Y | N | N | N | N | n.g./n.g |
| 1988 | Luetje et al. [50] | 29/189 | ES subarachnoid shunt | < 24 mo | 76% | 74% | 1972 | Y | N | N | N | N | n.g./n.g |
| 4/189 | ES tube | < 24 mo | 75% | 95% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 6/189 | EMSS | < 24 mo | 83% | 95% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 10/189 | ES marsupialization | < 24 mo | 80% | 95% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 4/189 | EMSS with silicone | < 24 mo | 100% | 95% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 68/189 | ES subarachnoid shunt | > 24 mo | 91% | 67% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 48/189 | ES tube | > 24 mo | 87% | 73% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 6/189 | EMSS | > 24 mo | 83% | 80% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 3/189 | ES marsupialization | > 24 mo | 33% | 100% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 3/189 | EMSS with silicone | > 24 mo | 100% | 100% | 1972 | Y | N | N | N | N | n.g./n.g | ||
| 1988 | Monsell et al. [51] | 63 | EMSS with silicone | 24 mo | 90% | 41% | 1972/1985 | Y | N | N | N | N | n.g./n.g |
| 1989 | Bretlau et al. [52] | 11 vs. placebo | EMSS | 108 mo | 90.9% | n.g | 1972 | Y | N | N | N | N | n.g./n.g |
| 1993 | Telischi et al. [53] | 234 | ES subarachnoidal shunt | > 10 yrs | 63% | n.g | n.g | N | N | N | N | N | n.g./n.g |
| 1994 | Moffat et al. [54] | 100 | EMSS | n.g | 81% | 74% | 1985 | Y | N | N | N | N | n.g./Y |
| 1996 | Welling et al. [55] | 10 | EMSS with silicone | > 24 mo | 60% | 66% | 1985 | Y | N | N | N | N | n.g./n.g |
| 1997 | Quaranta et al. [56] | 20 | EMSS with silicone | > 5 yrs | 85% | 65% | n.g | Y | N | N | N | N | n.g./n.g |
| 1998 | Gianoli et al. [57] | 37 | ES decompression | 12 mo | 85% | 86% | 1985 | Y | N | Y | N | N | n.g./Y |
| 24 mo | 100% | 85% | 1985 | Y | N | Y | N | N | n.g./Y | ||||
| 1998 | Pensak et al. [58] | 96 | EMSS with silicone | 5 yrs | 91% | n.g | 1995 | N | N | N | N | N | n.g./n.g |
| 1998 | Quaranta et al. [59] | 20 | EMSS | 6 yrs | 85% | 45% | 1985 | Y | N | Y | N | N | n.g./n.g |
| 1998 | Sajjadi et al. [60] | 27 | ES decompression | 1–7 yrs | 85% | 70% | 1972 | Y | N | N | N | N | n.g./Y |
| 1998 | Thomsen et al. [61] | 15 | EMSS with silicone | No change 6 mo–12 mo | 86% | 86% | 1995 | Y | N | N | N | N | n.g./Y |
| 1999 | Huang et al. [62] | 51 | EMSS with silicone | n.g | 94.1% | 88,20% | 1985 | Y | N | N | N | N | n.g./n.g |
| 2001 | Quaranta et al. [63] | 15/45 | ES decompression | n.g | n.g | 67% | n.g | Y | N | N | N | N | n.g./n.g |
| 15/45 | EMSS with silicone | n.g | n.g | 87% | n.g | Y | N | N | N | N | n.g./n.g | ||
| 2001 | Sennaroglu et al. [64] | 25 | ES decompression | n.g | 52% | 72% | 1985 | Y | N | n.g | N | n.g | n.g./Y |
| 2003 | Ostrowski et al. [65] | 68 | ES decompression | 55 mo | 72% | 82% | 1995 | Y | N | Y | N | N | n.g./Y |
| 2005 | Kaylie et al. [66] | 74 | EMSS | 18–24 mo | 72,8 | 72% | 1995 | Y | N | N | N | N | n.g./n.g |
| 2006 | Convert et al. [67] | 21/59 | EMSS with silicone | 24 mo | 71.9% | 71.1% | 1995 | Y | N | Y | N | n.g | n.g./Y |
| 38/59 | ES decompression | 24 mo | 71.9% | 71.1% | 1995 | Y | N | Y | N | n.g | n.g./Y | ||
| 2007 | Brinson et al. [68] | 108/196 | ES decompression | 18–24 mo | 75% | 54% | 1995 | Y | N | n.g | N | n.g | n.g./Y |
| 88/196 | EMSS with silicone | 18–24 mo | 75% | 62% | 1995 | Y | N | n.g | N | n.g | n.g./Y | ||
| 2008 | Lee et al. [69] | 226 | EMSS | > 15 yrs | 87% | n.g | 1995 | N | N | n.g | N | n.g | n.g./n.g |
| 2008 | Wetmore et al. [70] | 51 | EMSS with silicone | n.g | 78% | n.g | 1995 | Y | N | N | N | N | n.g./n.g |
| 2010 | Derebery et al. [71] | 183 | EMSS with silicone | 12–24 mo | 86% | 71% | 1995 | Y | N | n.g | N | n.g | n.g./n.g |
| 2012 | Kim et al. [37] | 16 | EMSS with silicone | 1–18 mo | 94% | 100% | 1995 | Y | N | Y | N | Y | Y/Y |
| 2017 | Wick et al. [72] | 6/53 | EMSS with silicone + without steroid | 90 mo (35–112) | 66% | n.g | 1995 | Y | Y | N | Y | N | n.g./n.g |
| 20/53 | EMSS with silicone + steroids intratympanic | 90 mo (35–112) | 83% | n.g | 1995 | Y | Y | N | Y | N | n.g./n.g | ||
| 27/53 | EMSS with silicone + steroids intratympanic + intravenous | 90 mo (35–112) | 66% | n.g | 1995 | Y | Y | N | Y | N | n.g./n.g | ||
| 2021 | Gendre et al. [27] | 73 | ES decompression/EMSS | > 2 mo | 67% (50% vs. 75%) | n.g | 1995/2015 | Y | Y | Y | Y | Y | n.g./n.g |
Caloric testing of periphery vestibular function including time of testing
EMSS endolymphatic sac surgery, ES endolymphatic sac, MD Menière’s disease, mo months, N no, n.g. not given, no. number, PTA pure tone average, vs. versus, Y yes, yrs years
The study at hand shows that EMSS is a safe and non-destructive method, that results in improved symptom control in a fair share—around 2 thirds—of patients with MD, when other non-destructive methods such as betahistine or intratympanic application of corticosteroids failed. Not a single patient in the reported cohort exhibited postoperative complications and bone conduction thresholds remained stable. To our knowledge, this is the second study investigating the effect of endolymphatic sac surgery on patients diagnosed with MD according to the current diagnostic criteria from 2015 [27]. All patients received a detailed history and diagnostic workup ruling out vestibular migraine, vestibular neurinoma, vestibular paroxysm, autoimmune inner ear disorder, and other differential diagnoses. All other available studies in the literature applied older versions of the diagnostic criteria and information on differential diagnosis seem to be lacking (Table 4). Moreover, the study at hand depicts detailed pre- and postoperative audiometric and vestibular results showing the safety of this non-destructive treatment option for MD patients. All patients received the same type of endolymphatic sac surgery—a endolymphatic mastoid shunt with application of a triangular silicone foil which seems to be the most common technique according to the literature [28–30] (Table 4). The most crucial novel aspect of the study at hand is the comparison of results between both study subgroups: patients who seem to have benefitted from the procedure and those who have not. The focus has never been laid on categorizing patients regarding the treatment effect before. However, this might be essential to further investigate the disease and how those patients can be effectively treated. Comparing those two patient groups regarding demographic characteristics, distribution of gender and side, age, and disease category according to the current diagnostic criteria were similar. In addition, with regard to hearing, the results between both patient groups were similar, as well as results from peripheral vestibular testing with regard to pre- and postoperative values. Interestingly, patients who benefitted from the procedure, had slightly better values regarding the peripheral vestibular function (side difference around 10%) than those who did not benefit from the procedure, which might illustrate the potential stop of the disease progress by performing the EMSS. Nevertheless, the grade of vestibular excitability was similar. Due to the small sub cohort numbers (group 1: n = 15; group 2: n = 7) no sound conclusions can be drawn and analysis was solely descriptive. Therefore, larger numbers and a prospective study setting would be necessary to find correlations or even predicators to estimate preoperatively a treatment success. The limitations of the study lie in the retrospective nature of the study: the cohort is relatively small and results regarding vestibular excitability was only available in 22 patients. In addition, due to the retrospective character of the study, thorough documentation to categorize the patients on the basis of the current diagnostic criteria for MD was not sufficient in 10 patients who were excluded prior to analysis. Another limitation is the relatively short follow-up results. Most of the other studies in the literature have longer follow-up with up to 9 years (Table 4).
Investigations on the effect of endolymphatic sac surgery go back to the 1950s. To date, about 40 studies assessed endolymphatic sac surgery, predominately within a retrospective setting [31] (Table 4). The without doubt most striking study on endolymphatic sac surgery comes from Denmark and was conducted as a double-blind prospective study comparing the efficacy between EMSS with a silicone shunt and mastoidectomy as placebo surgery [32]. This group has published recurrently multiple follow-up results up to 9 years on their study and showed vertigo control rates between 69 and 87% with no significant difference between both study and placebo group [32–36]. However, the authors did not specify the extent of the mastoidectomy. Therefore, we do not know, how extensive the mastoidectomies were performed, which could have the same effect as an endolymphatic decompression surgery. However, since publication of these results, the efficacy of endolymphatic sac surgery has been called into doubt repeatedly and its benefit is still debated [31]. Nevertheless, vertigo control rate was reported in the literature between 49 and 100% (Table 4). Whether this effect indicates the genuine efficacy of EMSS or is solely accounted for a placebo effect [32–36] or even due to the characteristic phenomenon, that MD patients consult their physician at the climax of symptom severity, can only be answered by future blinded prospective placebo-controlled studies.
All in all, the follow-up duration of the study at hand (19.7 ± 17.2 months) is on the lower range of reports from the literature, where follow-up results from 12 months to 9 years are given (Table 4). Gender was similarly distributed as in most studies [27–30]. More than half of the studies focused on hearing preservation, as well, without presenting detailed information on the audiograms of the investigated cohort. Hearing preservation was very satisfactory postoperatively in the study at hand (80.9%), also with similar long-term results (68.8%) compared to studies in the literature, ranging from 41 to 100% (Table 4), which underlines the safety and non-destructive character of this MD treatment option. Decreased long-term hearing results might be influenced by patients with a therapy–refractory progressive MD and resulting hearing impairment. This can also be observed in the difference of results between the both groups. Only two other recent publications presented pre- and postoperative results from caloric testing. Kim et al. investigated the peripheral vestibular function via caloric testing and found no significant of changes pre- to postoperative values [37], as well as the researchers around Gendre et al. who in addition observed no postoperative value changes in vestibular evoked myogenic potentials and video head impulse test [27].
Conclusions
The treatment effect of EMSS was found beneficial in two thirds of the patients with definite or probable MD. It is a safe procedure regarding hearing and vestibular preservation with a low complication rate. Therefore, EMSS should be considered before inducing destructive treatment options, such as intratympanic gentamicin application or labyrinthectomy. Regarding the classification of patients who benefit from EMSS and those who do not, no sound correlation with clinical or functional parameters were found. For further insights prospective studies with larger power are needed.
Acknowledgements
The authors would like to thank Beatrice Lentge for expert technical assistance. This work is part of the doctoral thesis of Ivelina Stoycheva. Preliminary results of this study were presented at the 91st annual conference of the German Society of Oto-Rhino-Laryngology in 2020 in Berlin, Germany.
Author contributions
JLS designed the experiments, analyzed data, and wrote the paper; IS collected and analyzed data; BGW, MB, TR, and MC provided critical revision; FI designed the experiments, analyzed data, and provided critical revision. All authors discussed the results and implications and commented on the manuscript at all stages.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Federal Ministry of Education and Health (BMBF) in the context of the foundation of the German Center for Vertigo and Balance Disorders (DSGZ) (Grant Number 01 EO 0901).
Availability of data and materials
Original data are available on demand.
Code availability
Statistical analysis was performed using SPSS (version 24) software (SPSS Inc, Chicago, IL). Shapiro–Walk test was used to test for normative distribution. All figures were created with Microsoft Excel version 1906.
Declarations
Conflict of interest
JLS received travel expenses from MED EL GmbH, Innsbruck, Austria and Cochlear Deutschland GmbH & Co. KG.
Ethical approval
Study protocol was performed according to ethical guidelines of the 2002 Declaration of Helsinki, and carried out after approval by the Institutional Review Board and Ethics Committee of the Ludwig-Maximilians-Universität München, Munich, Germany (Ethikkommission der LMU München) (reference number 19-086).
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jennifer L. Spiegel, Email: jennifer.spiegel@med.uni-muenchen.de
Ivelina Stoycheva, Email: stoycheva.ivelina@gmail.com.
Bernhard G. Weiss, Email: bernhard.weiss@med.uni-muenchen.de
Mattis Bertlich, Email: mattis.bertlich@med.uni-muenchen.de.
Tobias Rader, Email: tobias.rader@med.uni-muenchen.de.
Martin Canis, Email: martin.canis@med.uni-muenchen.de.
Friedrich Ihler, Email: friedrich.ihler@med.uni-muenchen.de.
References
- 1.Roman-Naranjo P, Gallego-Martinez A, Soto-Varela A, Aran I, Moleon MDC, Espinosa-Sanchez JM, Amor-Dorado JC, Batuecas-Caletrio A, Perez-Vazquez P, Lopez-Escamez JA. Burden of rare variants in the OTOG gene in familial Meniere’s disease. Ear Hear. 2020;41(6):1598–1605. doi: 10.1097/AUD.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 2.Gurkov R, Pyyko I, Zou J, Kentala E. What is Meniere’s disease? A contemporary re-evaluation of endolymphatic hydrops. J Neurol. 2016;263(Suppl 1):S71–S81. doi: 10.1007/s00415-015-7930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandala M, Newman-Toker DE, Strupp M, Suzuki M, Trabalzini F, Bisdorff A, Classification Committee of the Barany S. Japan Society for Equilibrium R. European Academy of O. Neurotology, Equilibrium Committee of the American Academy of O-H. Neck S, Korean Balance S Diagnostic criteria for Meniere’s disease. J Vestib Res. 2015;25(1):1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JP, Harris JP. Impact of Meniere’s disease on quality of life. Otol Neurotol. 2001;22(6):888–894. doi: 10.1097/00129492-200111000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Yardley L, Dibb B, Osborne G. Factors associated with quality of life in Meniere’s disease. Clin Otolaryngol Allied Sci. 2003;28:436–441. doi: 10.1046/j.1365-2273.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 6.Nevoux J, Barbara M, Dornhoffer J, Gibson W, Kitahara T, Darrouzet V. International consensus (ICON) on treatment of Meniere’s disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1S):S29–S32. doi: 10.1016/j.anorl.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Holgers KM, Finizia C. Health profiles for patients with Meniere’s disease. Noise Health. 2001;4(13):71–80. [PubMed] [Google Scholar]
- 8.Nakayama M, Masuda A, Ando KB, Arima S, Kabaya K, Inagaki A, Nakamura Y, Suzuki M, Brodie H, Diaz RC, Murakami S. A Pilot study on the efficacy of continuous positive airway pressure on the manifestations of Meniere’s disease in patients with concomitant obstructive sleep apnea syndrome. J Clin Sleep Med. 2015;11(10):1101–1107. doi: 10.5664/jcsm.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Esch BF, van der Scheer-Horst ES, van der Zaag-Loonen HJ, Bruintjes TD, van Benthem PP. The effect of vestibular rehabilitation in patients with Meniere’s disease. Otolaryngol Head Neck Surg. 2017;156(3):426–434. doi: 10.1177/0194599816678386. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;1:CD005397. doi: 10.1002/14651858.CD005397.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman S, Mahoney AE, Cremer PD. Cognitive behavior therapy for chronic subjective dizziness: a randomized, controlled trial. Am J Otolaryngol. 2012;33(4):395–401. doi: 10.1016/j.amjoto.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Strupp M, Zwergal A, Feil K, Bremova T, Brandt T. Pharmacotherapy of vestibular and cerebellar disorders and downbeat nystagmus: translational and back-translational research. Ann N Y Acad Sci. 2015;1343:27–36. doi: 10.1111/nyas.12774. [DOI] [PubMed] [Google Scholar]
- 13.van Esch BF, van Wensen E, van der Zaag-Loonen HJ, Benthem P, van Leeuwen RB. Clinical characteristics of benign recurrent vestibulopathy: clearly distinctive from vestibular migraine and Meniere’s disease? Otol Neurotol. 2017;38(9):e357–e363. doi: 10.1097/MAO.0000000000001553. [DOI] [PubMed] [Google Scholar]
- 14.Patel M, Agarwal K, Arshad Q, Hariri M, Rea P, Seemungal BM, Golding JF, Harcourt JP, Bronstein AM. Intratympanic methylprednisolone versus gentamicin in patients with unilateral Meniere’s disease: a randomised, double-blind, comparative effectiveness trial. Lancet. 2016;388(10061):2753–2762. doi: 10.1016/S0140-6736(16)31461-1. [DOI] [PubMed] [Google Scholar]
- 15.Devantier L, Schmidt JH, Djurhuus BD, Hougaard DD, Handel MN, Liviu-AdelinGuldfred F, Edemann-Callesen H. Current state of evidence for endolymphatic sac surgery in Meniere’s disease: a systematic review. Acta Otolaryngol. 2019;139(11):953–958. doi: 10.1080/00016489.2019.1657240. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MW, Kaylie DM. Is endolymphatic sac surgery beneficial for Meniere’s disease? Laryngoscope. 2020;130(12):2738–2739. doi: 10.1002/lary.28647. [DOI] [PubMed] [Google Scholar]
- 17.Portmann G. The saccus endolymphaticus and an operation for draining for the relief of vertigo. Proc R Soc Med. 1927;20(12):1862–1867. doi: 10.1177/003591572702001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.House WF. Subarachnoid shunt for drainage of endolymphatic hydrops. A preliminary report. Laryngoscope. 1962;72:713–729. doi: 10.1288/00005537-196206000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kersbergen CJ, Ward BK. A historical perspective on surgical manipulation of the membranous labyrinth for treatment of Meniere’s disease. Front Neurol. 2021;12:794741. doi: 10.3389/fneur.2021.794741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullens B, van Benthem PP. Intratympanic gentamicin for Meniere’s disease or syndrome. Cochrane Database Syst Rev. 2011;3:CD008234. doi: 10.1002/14651858.CD008234.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Quaglieri S, Gatti O, Rebecchi E, Manfrin M, Tinelli C, Mira E, Benazzo M. Intratympanic gentamicin treatment ‘as needed’ for Meniere’s disease. Long-term analysis using the Kaplan–Meier method. Eur Arch Otorhinolaryngol. 2014;271(6):1443–1449. doi: 10.1007/s00405-013-2597-7. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein H, Wanamaker H, Flanzer J, Rosenberg S. Vestibular neurectomy in the United States–1990. Am J Otol. 1992;13(1):23–30. [PubMed] [Google Scholar]
- 23.Morel N, Dumas G, Nguyen DQ, Mohr E, Hitter A, Schmerber S. Vestibular neurotomy versus chemical labyrinthectomy for disabling Meniere disease. Ann Otolaryngol Chir Cervicofac. 2005;122(6):271–280. doi: 10.1016/s0003-438x(05)82361-8. [DOI] [PubMed] [Google Scholar]
- 24.Jongkees LB, Maas JP, Philipszoon AJ. Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel) 1962;24:65–93. [PubMed] [Google Scholar]
- 25.Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative“ S, The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Monsell EM. New and revised reporting guidelines from the committee on hearing and equilibrium. American Academy of Otolaryngology-Head and Neck Surgery Foundation Inc. Otolaryngol Head Neck Surg. 1995;113(3):176–178. doi: 10.1016/S0194-5998(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 27.Gendre A, Bourget-Aguilar K, Calais C, Espitalier F, Bordure P, Michel G. Evaluation of vestibular function following endolymphatic sac surgery. Eur Arch Otorhinolaryngol. 2021 doi: 10.1007/s00405-021-06743-3. [DOI] [PubMed] [Google Scholar]
- 28.Flores Garcia ML, Llata Segura C, Cisneros Lesser JC, Pane Pianese C. Endolymphatic sac surgery for Meniere’s disease—current opinion and literature review. Int Arch Otorhinolaryngol. 2017;21(2):179–183. doi: 10.1055/s-0037-1599276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim MY, Zhang M, Yuen HW, Leong JL. Current evidence for endolymphatic sac surgery in the treatment of Meniere’s disease: a systematic review. Singap Med J. 2015;56(11):593–598. doi: 10.11622/smedj.2015166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sood AJ, Lambert PR, Nguyen SA, Meyer TA. Endolymphatic sac surgery for Meniere’s disease: a systematic review and meta-analysis. Otol Neurotol. 2014;35(6):1033–1045. doi: 10.1097/MAO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 31.Pullens B, Verschuur HP, van Benthem PP. Surgery for Meniere’s disease. Cochrane Database Syst Rev. 2013;2:CD005395. doi: 10.1002/14651858.CD005395.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomsen J, Bretlau P, Tos M, Johnsen NJ. Placebo effect in surgery for Meniere’s disease. A double-blind, placebo-controlled study on endolymphatic sac shunt surgery. Arch Otolaryngol. 1981;107(5):271–277. doi: 10.1001/archotol.1981.00790410009002. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen J, Bretlau P, Tos M, Johnsen NJ. Placebo effect in surgery for Meniere’s disease: three-year follow-up. Otolaryngol Head Neck Surg. 1983;91(2):183–186. doi: 10.1177/019459988309100213. [DOI] [PubMed] [Google Scholar]
- 34.Thomsen J, Bretlau P, Tos M, Johnsen NJ. Endolymphatic sac-mastoid shunt surgery. A nonspecific treatment modality? Ann Otol Rhinol Laryngol. 1986;95(1 Pt 1):32–35. doi: 10.1177/000348948609500107. [DOI] [PubMed] [Google Scholar]
- 35.Bretlau P, Thomsen J, Tos M, Johnsen NJ. Placebo effect in surgery for Meniere’s disease. A double-blind placebo-controlled study on endolymphatic sac shunt surgery. Adv Otorhinolaryngol. 1982;28:139–146. [PubMed] [Google Scholar]
- 36.Bretlau P, Thomsen J, Tos M, Johnsen NJ. Placebo effect in surgery for Meniere’s disease: a three-year follow-up study of patients in a double blind placebo controlled study on endolymphatic sac shunt surgery. Am J Otol. 1984;5(6):558–561. [PubMed] [Google Scholar]
- 37.Kim SH, Ko SH, Ahn SH, Hong JM, Lee WS. Significance of the development of the inner ear third window effect after endolymphatic sac surgery in Meniere disease patients. Laryngoscope. 2012;122(8):1838–1843. doi: 10.1002/lary.23332. [DOI] [PubMed] [Google Scholar]
- 38.Arenberg IK, Spector GJ. Endolymphatic sac surgery for hearing conservation in Meniere disease. Arch Otolaryngol. 1977;103(5):268–270. doi: 10.1001/archotol.1977.00780220062005. [DOI] [PubMed] [Google Scholar]
- 39.Arenberg IK. Endolymphatic sac valve implant surgery. III. Preliminary results in 61 cases (66 ears) Laryngoscope. 1979;89(7 Pt 2 Suppl 17):26–39. doi: 10.1002/lary.5540890703. [DOI] [PubMed] [Google Scholar]
- 40.Brown JS. A ten year statistical follow-up of 245 consecutive cases of endolymphatic shunt and decompression with 328 consecutive cases of labyrinthectomy. Laryngoscope. 1983;93(11 Pt 1):1419–1424. [PubMed] [Google Scholar]
- 41.Goldenberg RA, Justus MA. Endolymphatic mastoid shunt for treatment of Meniere’s disease: a five year study. Laryngoscope. 1983;93(11 Pt 1):1425–1429. [PubMed] [Google Scholar]
- 42.Miller GW, Welsh RL. Surgical management of vestibular Meniere’s disease with endolymphatic mastoid shunt. Laryngoscope. 1983;93(11 Pt 1):1430–1440. [PubMed] [Google Scholar]
- 43.Spector GJ, Smith PG. Endolymphatic sac surgery for Meniere’s disease. Ann Otol Rhinol Laryngol. 1983;92(2 Pt 1):113–118. doi: 10.1177/000348948309200203. [DOI] [PubMed] [Google Scholar]
- 44.Glasscock ME, 3rd, Kveton JF, Christiansen SG. Current status of surgery for Meniere’s disease. Otolaryngol Head Neck Surg. 1984;92(1):67–72. doi: 10.1177/019459988409200115. [DOI] [PubMed] [Google Scholar]
- 45.Huang TS, Lin CC. Endolymphatic sac surgery for Meniere’s disease: a composite study of 339 cases. Laryngoscope. 1985;95(9 Pt 1):1082–1086. [PubMed] [Google Scholar]
- 46.Smyth GD, Kerr AG, Primrose W. Operations on the saccus endolymphaticus and vestibular nerve: have they fulfilled their promise? Otolaryngol Head Neck Surg. 1986;94(5):594–600. doi: 10.1177/019459988609400511. [DOI] [PubMed] [Google Scholar]
- 47.Arenberg IK. Results of endolymphatic sac to mastoid shunt surgery for Meniere’s disease refractory to medical therapy. Am J Otol. 1987;8(4):335–344. [PubMed] [Google Scholar]
- 48.Brackmann DE, Nissen RL. Meniere’s disease: results of treatment with the endolymphatic subarachnoid shunt compared with the endolymphatic mastoid shunt. Am J Otol. 1987;8(4):275–282. [PubMed] [Google Scholar]
- 49.Kitahara M, Kitajima K, Yazawa Y, Uchida K. Endolymphatic sac surgery for Meniere’s disease: eighteen years’ experience with the Kitahara sac operation. Am J Otol. 1987;8(4):283–286. [PubMed] [Google Scholar]
- 50.Luetje CM. A critical comparison of results of endolymphatic subarachnoid shunt and endolymphatic sac incision operations. Am J Otol. 1988;9(2):95–101. [PubMed] [Google Scholar]
- 51.Monsell EM, Wiet RJ. Endolymphatic sac surgery: methods of study and results. Am J Otol. 1988;9(5):396–402. [PubMed] [Google Scholar]
- 52.Bretlau P, Thomsen J, Tos M, Johnsen NJ. Placebo effect in surgery for Meniere’s disease: nine-year follow-up. Am J Otol. 1989;10(4):259–261. doi: 10.1097/00129492-198907000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Telischi FF, Luxford WM. Long-term efficacy of endolymphatic sac surgery for vertigo in Meniere’s disease. Otolaryngol Head Neck Surg. 1993;109(1):83–87. doi: 10.1177/019459989310900115. [DOI] [PubMed] [Google Scholar]
- 54.Moffat DA. Endolymphatic sac surgery: analysis of 100 operations. Clin Otolaryngol Allied Sci. 1994;19(3):261–266. doi: 10.1111/j.1365-2273.1994.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 55.Welling DB, Pasha R, Roth LJ, Barin K. The effect of endolymphatic sac excision in Meniere disease. Am J Otol. 1996;17(2):278–282. [PubMed] [Google Scholar]
- 56.Quaranta A, Onofri M, Sallustio V, Iurato S. Comparison of long-term hearing results after vestibular neurectomy, endolymphatic mastoid shunt, and medical therapy. Am J Otol. 1997;18(4):444–448. [PubMed] [Google Scholar]
- 57.Gianoli GJ, Larouere MJ, Kartush JM, Wayman J. Sac-vein decompression for intractable Meniere's disease: two-year treatment results. Otolaryngol Head Neck Surg. 1998;118(1):22–29. doi: 10.1016/S0194-5998(98)70370-5. [DOI] [PubMed] [Google Scholar]
- 58.Pensak ML, Friedman RA. The role of endolymphatic mastoid shunt surgery in the managed care era. Am J Otol. 1998;19(3):337–340. [PubMed] [Google Scholar]
- 59.Quaranta A, Marini F, Sallustio V. Long-term outcome of Meniere’s disease: endolymphatic mastoid shunt versus natural history. Audiol Neurootol. 1998;3(1):54–60. doi: 10.1159/000013781. [DOI] [PubMed] [Google Scholar]
- 60.Sajjadi H, Paparella MM, Williams T. Endolymphatic sac enhancement surgery in elderly patients with Meniere’s disease. Ear Nose Throat J. 1998;77(12):975–982. doi: 10.1177/014556139807701209. [DOI] [PubMed] [Google Scholar]
- 61.Thomsen J, Bonding P, Becker B, Stage J, Tos M. The non-specific effect of endolymphatic sac surgery in treatment of Meniere’s disease: a prospective, randomized controlled study comparing “classic” endolymphatic sac surgery with the insertion of a ventilating tube in the tympanic membrane. Acta Otolaryngol. 1998;118(6):769–773. doi: 10.1080/00016489850182413. [DOI] [PubMed] [Google Scholar]
- 62.Huang TS. Three new surgeries for treatment of intractable Meniere’s disease. Am J Otol. 1999;20(2):233–237. [PubMed] [Google Scholar]
- 63.Quaranta N, De Thomasis G, Gandolfi A, Piazza F, Zini C, Quaranta A. Long-term audition results in patients with chronic endolymphatic hydrops after selective vestibular neurotomy and endolymphatic sac surgery. Acta Otorhinolaryngol Ital. 2001;21(3):131–137. [PubMed] [Google Scholar]
- 64.Sennaroglu L, Sennaroglu G, Gursel B, Dini FM. Intratympanic dexamethasone, intratympanic gentamicin, and endolymphatic sac surgery for intractable vertigo in Meniere’s disease. Otolaryngol Head Neck Surg. 2001;125(5):537–543. doi: 10.1067/mhn.2001.119485. [DOI] [PubMed] [Google Scholar]
- 65.Ostrowski VB, Kartush JM. Endolymphatic sac-vein decompression for intractable Meniere’s disease: long term treatment results. Otolaryngol Head Neck Surg. 2003;128(4):550–559. doi: 10.1016/s0194-5998(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 66.Kaylie DM, Jackson CG, Gardner EK. Surgical management of Meniere’s disease in the era of gentamicin. Otolaryngol Head Neck Surg. 2005;132(3):443–450. doi: 10.1016/j.otohns.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Convert C, Franco-Vidal V, Bebear JP, Darrouzet V. Outcome-based assessment of endolymphatic sac decompression for Meniere’s disease using the Meniere’s disease outcome questionnaire: a review of 90 patients. Otol Neurotol. 2006;27(5):687–696. doi: 10.1097/01.mao.0000227661.52760.f1. [DOI] [PubMed] [Google Scholar]
- 68.Brinson GM, Chen DA, Arriaga MA. Endolymphatic mastoid shunt versus endolymphatic sac decompression for Meniere’s disease. Otolaryngol Head Neck Surg. 2007;136(3):415–421. doi: 10.1016/j.otohns.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 69.Lee L, Pensak ML. Contemporary role of endolymphatic mastoid shunt surgery in the era of transtympanic perfusion strategies. Ann Otol Rhinol Laryngol. 2008;117(12):871–875. doi: 10.1177/000348940811701201. [DOI] [PubMed] [Google Scholar]
- 70.Wetmore SJ. Endolymphatic sac surgery for Meniere’s disease: long-term results after primary and revision surgery. Arch Otolaryngol Head Neck Surg. 2008;134(11):1144–1148. doi: 10.1001/archotol.134.11.1144. [DOI] [PubMed] [Google Scholar]
- 71.Derebery MJ, Fisher LM, Berliner K, Chung J, Green K. Outcomes of endolymphatic shunt surgery for Meniere’s disease: comparison with intratympanic gentamicin on vertigo control and hearing loss. Otol Neurotol. 2010;31(4):649–655. doi: 10.1097/MAO.0b013e3181dd13ac. [DOI] [PubMed] [Google Scholar]
- 72.Wick CC, Manzoor NF, McKenna C, Semaan MT, Megerian CA. Long-term outcomes of endolymphatic sac shunting with local steroids for Meniere’s disease. Am J Otolaryngol. 2017;38(3):285–290. doi: 10.1016/j.amjoto.2017.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data are available on demand.
Statistical analysis was performed using SPSS (version 24) software (SPSS Inc, Chicago, IL). Shapiro–Walk test was used to test for normative distribution. All figures were created with Microsoft Excel version 1906.