Abstract
Apathy is one of the most common neuropsychiatric symptoms (NPS) and is associated with poor clinical outcomes. Research that helps define the apathy phenotype is urgently needed, particularly for clinical and biomarker studies. We used latent class analysis (LCA) with two independent cohorts to understand how apathy and depression symptoms co‐occur statistically. We further explored the relationship between latent class membership, demographics, and the presence of other NPS. The LCA identified a four‐class solution (no symptoms, apathy, depression, and combined apathy/depression), reproducible over both cohorts, providing robust support for an apathy syndrome distinct from depression and confirming that an apathy/depression syndrome exists, supported by the model fit test with the four‐class solution scores evidencing better fitting (Bayesian information criterion adjusted and entropy R 2). Using a data‐driven method, we show distinct and statistically meaningful co‐occurrence of apathy and depressive symptoms. There was evidence that these classes have different clinical associations, which may help inform diagnostic categories for research studies and clinical practice.
Highlights
We found four classes: no symptoms, apathy, depression and apathy/depression.
Apathy conferred a higher probability for agitation.
Apathy diagnostic criteria should include accompanying neuropsychiatric symptoms.
Keywords: apathy, dementia, depression, latent class analysis
1. BACKGROUND
Apathy is one of the most common neuropsychiatric symptoms (NPS) seen in dementia, 1 with a reported prevalence of 43% 2 (though estimates vary widely according to dementia stage and study setting). Characterized by a lack of motivation, decreased initiative, akinesia, and emotional indifference, 3 , 4 , 5 , 6 , 7 apathy is a multidimensional syndrome that can lead to functional impairment, poorer treatment response, and greater mortality. 8 , 9 , 10 Understanding the clinical presentation of such a common and clinically important syndrome is of utmost importance both for the effective targeting of new interventions and for the identification of biomarkers or other research aimed at understanding biological correlates.
One of the key diagnostic challenges in apathy is its relationship with depression. Although clinically distinct, symptoms of apathy and of depression are commonly comorbid and have overlapping features, 3 , 11 thus complicating clinical distinctions between the two. 1 , 7 , 12 Recognizing this, new diagnostic criteria for apathy in neurocognitive disorders were developed in 2021, representing a major step forward in clinical management of apathy and in associated research by providing a standardized framework for assessment. 13 Central to these criteria is that the symptoms of apathy cannot be explained by another psychiatric condition (e.g., depression), but it remains the case that criteria for apathy can, in principle, be met in the presence of heterogeneous depressive symptomatology. This co‐occurrence has the potential to cause a lack of reproducibility, particularly in disease mechanism research in which control groups must be precisely defined. It may also be a barrier to targeting the right interventions to the right people. A data‐driven approach to distinguish apathy and depression symptomatology can support more precision approaches to treatment and augment the application of the clinically informed diagnostic criteria.
Latent class analysis (LCA) is one example of a data‐driven approach. 14 A form of latent variable modeling, LCA describes combinations of generally binary variables in terms of statistically likely patterns that are not directly observed (i.e., latent classes); these patterns are characterized on the basis of the conditional probability of each binary variable within a class.
Thus, using LCA on questionnaire‐based measures of apathy and depression, we aimed to (1) examine how symptoms co‐occur in two independent observational cohort studies and (2) establish whether the latent classes identified were associated with different NPS profiles.
2. METHODS
2.1. Sample descriptions
2.1.1. L‐study
Participants were from the Measuring Long Term Outcomes in People with Dementia in Care Homes (L‐study). This study has been running since 2015 through the King's College London and Maudsley Care Home Research Network. Participants with a diagnosis of dementia living in care homes across the southeast of England were recruited. Consent was obtained from participants or next of kin if participants were unable to consent for themselves. The recruitment and assessments were completed by a trained researcher. The Southampton and South West Hampshire Research Ethics Committee A (formally South Central–Southampton A) approved the study (REC number 13/SC/0265).
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Apathy is a common neuropsychiatric syndrome in dementia, sometimes comorbid with depression, with recent publications describing its clinical aspects and new diagnostic criteria. Relevant research is appropriately cited.
Interpretation: Our findings led to a novel interpretation of the apathy syndrome, with the presentation of a new possible combined apathy/depression syndrome that may be distinct from apathy only.
Future directions: The article proposes a new approach to the study of apathy as follows: (a) depression should be routinely measured and individuals with comorbid symptoms may have to be considered a separate group to those with a distinct apathy syndrome; (b) supporting evidence for future diagnosis iterations, with consideration to be given to depressive and agitation symptoms as accompanying neuropsychiatric features of apathy.
2.1.2. Alzheimer's Disease Neuroimaing Initiative
Additional data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessments can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer's disease (AD). For up‐to‐date information, see www.adni‐info.org.
2.2. Measures
2.2.1. Neuropsychiatric symptoms
NPS were assessed with the Neuropsychiatric Inventory (NPI), completed by a researcher‐led interview with an informed caregiver. 15 The NPI assesses the following 12 NPS, within a reference period of the past 4 weeks: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, sleep and night‐time behavior disorders, and appetite and eating disorders. 16 Each item is rated as present or absent. If a screening question is rated present, subquestions are then completed that cover detailed NPS relating to each domain; each is coded with a yes or no response. If any of these symptoms are rated present, frequency (1–4) and severity (1–3) are rated, with the product of these representing the overall symptom score.
For the L‐study, the NPI Nursing Home (NPI‐NH) version was used as this is the adapted version for institutional settings while the ADNI cohort used the standard NPI. 17
2.2.2. Apathy and depression
Apathy and depression were measured using the subquestions on item G (Apathy) and item D (Depression) of the NPI (see Appendix A for list of questions). For the NPI‐NH there are 15 subquestions while for the standard NPI there are 16, with the standard NPI scale used in ADNI having one fewer subquestion on the Apathy section.
2.2.3. Other neuropsychiatric symptoms
Our second aim was to evaluate the relationship between latent classes of apathy and depression and other NPS. To do this, NPS status (defined as present or absent) was determined for the remaining 10 items on the NPI using binary coding. Participants with a frequency x severity score of ≥1 were considered “symptom present.” The symptom was considered absent if the score was 0.
2.2.4. Dementia severity
The Clinical Dementia Rating (CDR) is a scale for assessing, diagnosing, and staging cognitive impairment. 18 A researcher/clinician conducts a semi‐structured interview with the patient and a reliable informant to rate performance in six domains: memory; orientation; judgement and problem solving; community affairs; homes and hobbies; and personal care. The domains are rated in terms of the impairment level (0 = none, 0.5 = questionable, 1 = mild, 2 = moderate, 3 = severe). A final global rating is then calculated to indicate the level of impairment (0 = normal cognition, 0.5 = questionable or very mild dementia, 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia).
2.3. Data cleaning
Participants with a CDR of 0 or 0.5 were excluded from both cohorts given this would indicate normal cognition or questionable dementia. Data were checked for completeness, and participants with missing age, sex, or CDR were removed from the dataset. Participants with missing NPI/NPI‐NH items D and G were also excluded. A total of 22 participants were removed from the L‐study cohort and a total of 135 participants were removed from the ADNI cohort. Furthermore, within the ADNI cohort only four participants had a CDR of 3 so these were merged with the group scoring 2. See Appendix B for study flow diagram.
2.4. Statistical analysis
To summarize, first we estimated latent classes of the NPI apathy and depression subscale questions with two to six class solutions. This was followed by the bootstrap test to analyze the best‐fitting solution. Then we explored the relationship between latent classes from the best‐fitting LCA model and the presence of other NPI domains. The same steps were performed for the L‐study and ADNI datasets. Further detail is provided below.
We used the Stata LCA plugin version 1.2.1 19 to estimate the latent classes, measured by categorical indicators, within the NPI category D (depression) and G (apathy) sub‐questions. The LCA Bootstrap version 1.0 plugin 20 was then used to evaluate models with two to six latent classes and determine the optimal number of classes using the model‐fit criteria of bootstrapped likelihood ratio test (BLRT), adjusted Bayesian information criterion (aBIC), scaled relative entropy, and log likelihood. A simulation study has shown that BLRT was the best test to identify the correct number of classes, followed by the Bayesian information criterion and the aBIC. 21 Thus, a model with k classes would be considered a better fit than a model with k–1 classes if it had a lower BLRT, P‐value, and aBIC value. In addition, we considered the clinical interpretability of the final model.
After this we used the plugin LCA_Distal_BCH version 1.1 22 to analyze the relationship between the latent classes with other NPI items. This test estimates the association between a latent class variable and an observed distal outcome, that is, other NPI items, using the approach of Bolck et al., 23 as adapted by Vermunt, 24 allowing for the probability of misclassification of classes. Distal probabilities for each NPI item for each latent class are reported along with the Wald test for significance. The Wald test examines the null hypothesis that the probabilities are the same across all latent classes. Pairwise comparisons between latent classes were then performed to determine which latent classes differed with respect to the probabilities of other NPI items.
3. RESULTS
3.1. Participant characteristics
After data cleaning, the L‐study comprised 326 people. Mean age was 87 (standard deviation [SD] 6.99), 73% were female, and median CDR was 2 (see Table 1). The ADNI data comprised 271 people. Mean age was 74 (SD 7.4), 42% were female, with a median CDR of 1 (see Table 1).
TABLE 1.
Cohorts demographics.
| L‐study N = 326 | ADNI N = 271 | |
|---|---|---|
| Care home setting | Community/academic setting | |
| Female | 240 (73%) | 114 (42%) |
| Male | 86 (27%) | 157 (57%) |
| Mean age | 86.88 (SD 6.99) | 74.22 (SD 7.4) |
| CDR (median) | 2 (IQR: 2–3) | 1 (IQR: 1–1) |
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; CDR, Clinical Dementia Rating; IQR, interquartile range; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes; SD, standard deviation.
3.2. Latent class analysis identifies a reproducible 4‐class solution
3.2.1. L‐study
A 4‐class model was considered optimal based on the higher aBIC score (928.52) and higher log likelihood score (–1573.81), P‐value (0.01), and clinical interpretability. Although the 5‐class model had the same P‐value, on balance comparing the other metrics and clinical interpretability the 4‐class model was chosen (see Table 2).
TABLE 2.
Bootstrap likelihood ratio test and scalars for best model fit.
| L‐study | BLRT | |||
|---|---|---|---|---|
| Number of classes | P‐value | BIC (adjusted) | r (EntropyRsqd) | r (loglikelihood) |
| 2 classes | 0.01 | 1119.49 | 0.96 | −1711.13 |
| 3 classes | 0.01 | 977.01 | 0.91 | −1618.97 |
| 4 classes | 0.01 | 928.52 | 0.92 | −1573.81 |
| 5 classes | 0.01 | 898.05 | 0.91 | −1537.65 |
| 6 classes | 0.01 | 907.52 | 0.91 | −1521.46 |
| ADNI | BLRT | |||
|---|---|---|---|---|
| Number of classes | P‐value | BIC (adjusted) | r (EntropyRsqd) | r (loglikelihood) |
| 2 classes | 0.01 | 1064.73 | 0.97 | −1472.78 |
| 3 classes | 0.01 | 941.22 | 0.94 | −1390.36 |
| 4 classes | 0.01 | 852.10 | 0.94 | −1325.13 |
| 5 classes | 0.01 | 845.19 | 0.92 | −1301.01 |
| 6 classes | 0.15 | 852.19 | 0.93 | −1283.84 |
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; BIC, Bayesian information criterion; BLRT, bootstrapped likelihood ratio test; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes.
The four classes were labeled no symptoms, combined apathy/depression, depression, and apathy. Looking at the symptom probability across the different classes (Figure 1), the no symptoms class showed very low or zero probability of apathy and depressive symptoms; the combined apathy/depression class exhibited high probabilities across a range of depressive and apathy symptoms; the depression class comprised mostly depressive symptoms with low probability of some apathy symptoms; the apathy class had high probabilities across apathy symptoms and very low or no probability of depressive symptoms (see Appendix D). The probabilities of participants falling into each class were 42.13%, 18.37%, 9.06%, and 30.42% for the no symptom, combined apathy/depression, depression, and apathy classes respectively (see Appendix C).
FIGURE 1.
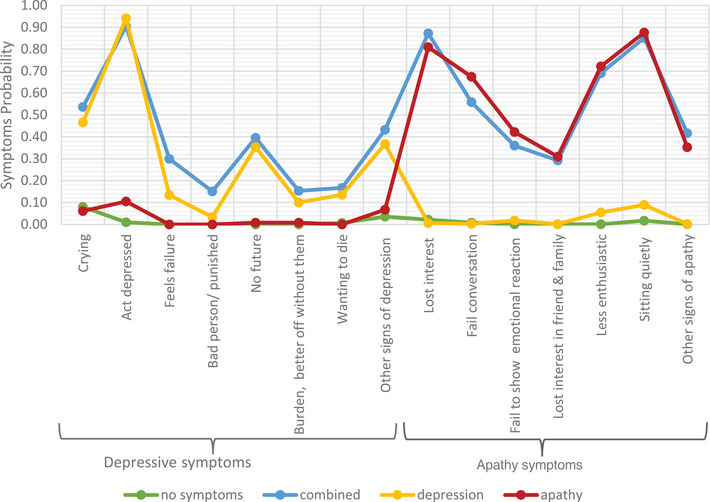
Apathy and depression symptoms probability across the four classes in CHRN, Care Home Research Network.
3.2.2. ADNI
Considering aBIC (852.10) and P‐value (0.01) and clinical interpretability, a 4‐class model was again considered optimal, with a notable similarity to the L‐study across the symptom probabilities (Figure 2). We note that a case could be made for selecting a 5‐class model; however, the additional class in the 5‐class model was very similar to class 2 in the 4‐class model (see Appendix E for more detailed discussion of model selection).
FIGURE 2.
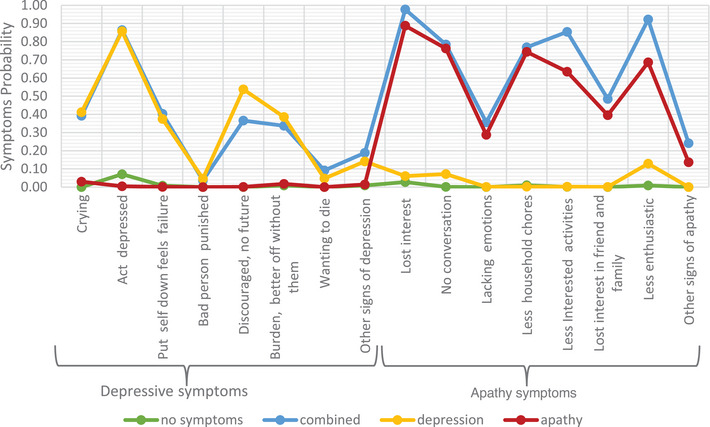
Apathy and depression symptoms probability across the four classes in Alzheimer's Disease Neuroimaging Initiative.
The four classes were given the same labels as the L‐Study, with the same descriptions. The probabilities of participants falling into each class in the 4‐class model were 40.7%, 20.1%, 15.6%, and 23.6% for the no symptoms, combined apathy/depression, depression, and apathy classes, respectively (see Appendix C).
3.3. In individuals with apathy, comorbid depressive symptoms have higher probability of additional NPS burden
Broadly, the latent classes that were characterized by the presence of depressive symptoms (i.e., combined apathy/depression and depression) were associated with a higher burden of comorbid NPS, particularly delusions, anxiety, and irritability, which replicated across the two cohorts. The apathy class (i.e., no depression symptoms) relative to the no symptoms class was associated with a higher probability for agitation in the L‐study, but not in ADNI. This was the only comparison in which apathy conferred a higher probability for any comorbid NPS.
Specific pairwise comparisons between latent classes are described in more detail below. Only NPS domains in which there was evidence of replication across the two cohorts are considered in detail and shown in Table 3. All other results are in Table F.1 in Appendix F.
TABLE 3.
Delusions, anxiety, and irritability probabilities across the four classes, with pairwise comparisons.
| Delusions (symptom probabilities) | Pairwise comparisons (Wald, P) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No symptoms | Combined | Apathy | Depression | Omnibus test (Wald, P) | Combined vs. no symptoms | Depression vs. no symptoms | Apathy vs. no symptoms | Combined vs. depression | Combined vs. apathy | Depression vs. apathy | |
| L‐study | 0.39 | 0.56 | 0.23 | 0.54 | 16.49; P < 0.001 | 4.43; P = 0.04 | 2.08; P = 0.15 | 6.02; P = 0.01 | 0.02; P = 0.89 | 13.55; P < 0.01 | 8.87; P < 0.01 |
| ADNI | 0.14 | 0.25 | 0.04 | 0.20 | 8.30; P = 0.04 | 2.95; P = 0.09 | 0.73; P = 0.39 | 3.24, P = 0.07 | 0.33; P = 0.57 | 7.27; P = 0.01 | 4.97; P = 0.03 |
| Anxiety (symptom probabilities) | Pairwise comparisons (Wald, P) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No symptoms | Combined | Apathy | Depression | Omnibus test (Wald, P) | Combined vs. no symptoms | Depression vs. no symptoms | Apathy vs. no symptoms | Combined vs. depression | Combined vs. apathy | Depression vs. apathy | |
| L‐study | 0.22 | 0.70 | 0.27 | 0.41 | 33.81; P < 0.01 | 32.40; P < 0.01 | 3.95; P = 0.05 | 0.86; P = 0.035 | 5.80; P = 0.02 | 20.00; P < 0.01 | 1.72; P = 0.19 |
| ADNI | 0.16 | 0.59 | 0.29 | 0.30 | 25.74; P < 0.01 | 25.40; P < 0.01 | 2.83; P = 0.09 | 3.48; P = 0.06 | 6.67; P = 0.01 | 9.16; P < 0.01 | 0.01; P = 0.92 |
| Irritability (symptom probabilities) | Pairwise comparisons (Wald, P) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Symptoms | Combined | Apathy | Depression | Omnibus test (Wald, P) | Combined vs. no symptoms | Depression vs. no symptoms | Apathy vs. no symptoms | Combined vs. depression | Combined vs. apathy | Depression vs. apathy | |
| L‐study | 0.38 | 0.69 | 0.68 | 0.47 | 16.92; P < 0.01 | 12.97; P < 0.01 | 6.96; P = 0.01 | 1.43; P = 0.23 | 0.00; P = 0.96 | 5.84; P = 0.02 | 3.61; P = 0.06 |
| ADNI | 0.25 | 0.51 | 0.29 | 0.45 | 13.27; P < 0.01 | 10.92; P < 0.01 | 4.92; P = 0.03 | 0.30; P = 0.58 | 0.43; P = 0.51 | 6.01; P = 0.01 | 2.68; P = 0.10 |
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes.
3.3.1. Delusions
The probability for delusions was highest in the classes characterized by depressive symptoms (combined apathy/depression class in L‐study = 0.56 and in ADNI = 0.25; depression class in L‐study = 0.54 and in ADNI = 0.20) and lowest in the no symptoms and apathy class (no symptoms L‐study = 0.39 ADNI = 0.14; apathy class L‐study = 0.23; ADNI = 0.04), with L‐study Wald test = 16.49, P < 0.001 and ADNI Wald test = 8.3, P = 0.04; see Table 3.
3.3.2. Anxiety
The probability for Anxiety was highest in the classes characterized by depressive symptoms (Combined Apathy/Depression class L‐study = 0.70 and in ADNI = 0.59; Depression class in L‐study = 0.41 and in ADNI = 0.30) and lowest in the No Symptoms and Apathy class (No symptoms in L‐study = 0.22 ADNI = 0.16; Apathy class in L‐study = 0.27 ADNI = 0.29], with L‐study Wald test = 33.81, p < 0.001 and ADNI Wald test = 25.74, p < 0.001 see Table 3.
3.3.3. Irritability
The probability for irritability was highest in the classes characterized by depressive symptoms (combined apathy/depression class L‐study = 0.69 and in ADNI = 0.51; depression class in L‐study = 0.68 and in ADNI = 0.45) and lowest in the no symptoms and apathy class (no symptoms in L‐study = 0.38 and ADNI = 0.25; apathy class in L‐study = 0.47 and ADNI = 0.29), with L‐study Wald test = 16.92, P < 0.001 and ADNI Wald test = 13.27, P = 0.004; see Table 3.
3.3.4. Other associations
Associations that did not replicate across the two datasets are shown in the supporting information, but we highlight here the findings relating to agitation as one of the most clinically interesting of the remaining findings. The combined apathy/depression and apathy classes had a higher probability of agitation relative to the no symptoms class in the L‐study only (probability of agitation for no symptoms, combined apathy/depression, depression, and apathy class was 0.52, 0.80, 0.64, 0.72, respectively, with overall Wald test L‐study = 17.04, P < 0.01); see Table 4. This did not replicate in ADNI (probability of agitation for no symptoms, combined apathy/depression, depression, and apathy class was 0.25, 0.4, 0.4, and 0.39, respectively, with overall Wald test 5.53, P = 0.13); however, the direction of effect was similar.
TABLE 4.
Agitation probabilities across the four classes, with pairwise comparisons.
| Agitation (symptom probabilities) | Pairwise comparisons (Wald, P) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No symptoms | Combined | Apathy | Depression | Omnibus test (Wald, P) | Combined vs. no symptoms | Depression vs. no symptoms | Apathy vs. no symptoms | Combined vs depression | Combined vs. apathy | Depression vs. apathy | |
| L‐study | 0.52 | 0.80 | 0.72 | 0.64 | 17.04; P < 0.01 | 11.53; P < 0.01 | 1.24; P = 0.27 | 8.80; P < 0.01 | 2.32; P = 0.13 | 1.14; P < 029 | 0.59; P = 0.44 |
| ADNI | 0.25 | 0.40 | 0.38 | 0.40 | 5.73; P = 0.13 | 3.82; P = 0.05 | 2.87; P = 0.09 | 3.31; P = 0.07 | 0.00; P = 0.98 | 0.04; P = 0.83 | 0.03; P = 0.87 |
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes.
3.3.5. Clinically significant symptoms
Post hoc, we repeated the analysis using an NPI cut point of ≥4 to examine the association between latent classes and clinically significant other NPS. The pattern of associations was broadly similar; both anxiety and irritability were replicated in ADNI and the L‐study. The association with delusions was however only present in the L‐study, although we note the very small number of ADNI participants, which may have led to our failure to detect an association in this sample (n = 18, 6%). Results from this analysis are included in the supporting information (see Appendix G).
4. DISCUSSION
Here, we used LCA to delineate symptoms of apathy and depression in dementia. The reproducible 4‐class solution provides robust data‐driven validation of an apathy syndrome that is distinct from depression and a combined apathy/depression syndrome. This conclusion is supported by our analysis of neuropsychiatric symptom associations with each of the four classes in which we show that apathy is generally only associated with a more severe NPS profile when comorbid depressive symptoms are present.
The broad implication of these findings is that in apathy research, depression should also be routinely measured and individuals with comorbid symptoms may have to be considered a separate group from those with a distinct apathy syndrome. This could explain some of the heterogeneity observed in NPS associations in other studies of apathy. For example, one study observed that apathy was associated with higher NPI scores across a range of NPS, including delusions, irritability, and anxiety. 25 In light of the findings from the present study, it is possible that the reported associations in this previous study were being driven by the presence of comorbid depressive symptoms in the apathy group. Future studies measuring clinical and biological correlates should consider the possibility of comorbid depressive symptoms as a confounder to produce reproducible research.
The latest diagnostic criteria for apathy stipulate that apathy cannot be due to another illness, disability, or substance abuse but are silent on accompanying neuropsychiatric features. The existence of the combined apathy/depression class suggests further consideration of depressive symptoms (which may not meet criteria for clinical depression) as an accompanying feature of apathy.
In terms of other NPS burden, there was a striking consistency for a higher probability of delusions, anxiety, and irritability in the two classes that captured depressive symptoms (i.e., the depression and combined apathy/depression classes) relative to the no symptoms and apathy classes. This pattern of association points to an affective syndrome with psychotic features, validating both the focus on the relationship between these syndromes in the new International Psychogeriatric Association and International Society to Advance Alzheimer's Research and Treatment criteria for psychosis in neurocognitive disorders, 26 , 27 and results from previous studies reporting the co‐occurrence of both syndromes. 28 , 29 , 30 , 31 Mechanistically, a recent genome‐wide association study observed a positive association between genetic risk for depressive symptoms and Alzheimer's disease with psychosis, while in younger‐aged samples genetic correlations between irritability and depressive symptoms have been observed. 32 , 33 These observations, alongside the current findings, reflect current opinion that comorbid behavioral symptoms may impact response to treatments. 34
Another interesting observation is that there was a higher probability for agitation in the apathy class relative to the no symptoms class in the L‐study. Although only present in one cohort, in which mean age was higher and median dementia severity was slightly greater, this was the only instance in which the apathy class was associated with a higher probability of another NPS. The lack of replication may be due to the more advanced dementia seen in the L‐study, which is a care home study, where we would expect agitation to be more common. 35 Nonetheless, this may be a clinically relevant finding. The association of apathy with comorbid agitation is an important relationship to explore, as these individuals may require additional support and are often referred for specialist consultation. We would argue this is a relationship worth examining more closely in future research to determine whether agitation should be considered an accompanying feature of apathy and incorporated into later revision of the diagnostic criteria (which is currently silent on comorbid NPS). A clear path to take these findings forward is to perform LCA on apathy and depressive questionnaire responses and map the classes identified onto independently rated apathy diagnostic criteria in the same individuals. This analysis would provide the necessary data to evaluate the extent to which application of the criteria captures individuals with depressive symptoms.
A key strength of this study is the replication in two independent cohorts, which confers a high degree of confidence in our findings. This is important as caregiver reports may be influenced by caregiver mood, cultural beliefs, and the educational level of the “caregiver,” 36 although also the caregiver information has the advantage of not being influenced by anosognosia. However, differences in the dementia severity and demographics between the ADNI and the L‐study mean we must acknowledge the possibility that non‐replication of neuropsychiatric symptom associations is due to these differences (as discussed above with respect to agitation). Although we acknowledge the inherent limitation of the NPI in measuring apathy, particularly the relative weighting given to items capturing diminished interest (with a lesser focus on the emotional and initiative dimensions), a recent study showed a considerable overlap with the use of NPI apathy and the diagnostic criteria for apathy. 37 However, if the latent classes captured in this study reflect true unobserved and mutually exclusive groupings, then these should be stable across any measures of depression and apathy, provided appropriate questionnaire items are measured. This is a hypothesis that can readily be tested by future research in other samples with different NPS measurements.
Another limitation of this study is that the NPI subquestions were designed as an aid for the evaluation of each NPI item (i.e., the answering of them is predicated on answering the screening question). We chose to analyze the NPI in this way because of the more granular symptom‐level detail. It is therefore possible that a respondent could answer negatively on the main screening question but would answer positively if they were to have been asked all the subquestions. However, in the original development of the NPI scale, Cummings et al. examined this false‐negative rate to be only at ≈4.5%. 38 Despite this limitation, it is notable that our findings replicated across the two entirely independent samples, showing the same four latent classes.
In summary, this study supports the existence four latent classes (no symptoms, combined apathy/depression, depression, and apathy) replicated in two independent cohorts. In analysis of comorbid NPS burden associated with these classes, we show that the presence of depressive symptoms is the major driver. These findings do not compete with the diagnostic criteria for apathy; rather they should be considered supporting evidence for future iterations, potentially qualifying comorbid NPS symptoms or syndromes. Specifically, we propose that future consideration be given to depressive and agitation symptoms as accompanying neuropsychiatric features of apathy.
CONFLICTS OF INTEREST STATEMENT
Clive Ballard has received grants and personal fees from Acadia pharmaceutical company, grants and personal fees from Lundbeck, personal fees from Roche, personal fees from Otsuka, personal fees from Biogen, personal fees from Eli Lilly, personal fees from Novo Nordisk, personal fees from AARP, personal fees from Addex, personal fees from Enterin, personal fees from GWPharm, personal fees from Janssen, personal fees from Johnson and Johnson, personal fees from Orion, personal fees from Sunovion, personal fees from tauX pharmaceutical company and from Synexus. Zahinoor Ismail has received honoraria/consulting fees from Janssen, Lundbeck, and Otsuka, although not related to this work. Miguel Vasconcelos Da Silva has received payment for presentation from Roche Diagnostics, although not related to this work. All the other authors have nothing to disclose. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
This paper represents independent research partly funded by the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. This study was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
1.
Collaborators
The following are the published list of collaborators associated with ADNI:
Michael Weiner, MD (UC San Francisco, Principal Investigator, Executive Committee); Paul Aisen, MD (UC San Diego, ADCS PI and Director of Coordinating Center Clinical Core, Executive Committee, Clinical Core Leaders); Ronald Petersen, MD, PhD (Mayo Clinic, Rochester, Executive Committee, Clinical Core Leader); Clifford R. Jack, Jr., MD (Mayo Clinic, Rochester, Executive Committee, MRI Core Leader); William Jagust, MD (UC Berkeley, Executive Committee; PET Core Leader); John Q. Trojanowki, MD, PhD (U Pennsylvania, Executive Committee, Biomarkers Core Leader); Arthur W. Toga, PhD (USC, Executive Committee, Informatics Core Leader); Laurel Beckett, PhD (UC Davis, Executive Committee, Biostatistics Core Leader); Robert C. Green, MD, MPH (Brigham and Women's Hospital, Harvard Medical School, Executive Committee and Chair of Data and Publication Committee); Andrew J. Saykin, PsyD (Indiana University, Executive Committee, Genetics Core Leader); John Morris, MD (Washington University St. Louis, Executive Committee, Neuropathology Core Leader); Leslie M. Shaw (University of Pennsylvania, Executive Committee, Biomarkers Core Leader); Enchi Liu, PhD (Janssen Alzheimer Immunotherapy, ADNI 2 Private Partner Scientific Board Chair); Tom Montine, MD, PhD (University of Washington); Ronald G. Thomas, PhD (UC San Diego); Michael Donohue, PhD (UC San Diego); Sarah Walter, MSc (UC San Diego); Devon Gessert (UC San Diego); Tamie Sather, MS (UC San Diego); Gus Jiminez, MBS (UC San Diego); Danielle Harvey, PhD (UC Davis); Michael Donohue, PhD (UC San Diego); Matthew Bernstein, PhD (Mayo Clinic, Rochester); Nick Fox, MD (University of London); Paul Thompson, PhD (USC School of Medicine); Norbert Schuff, PhD (UCSF MRI); Charles DeCArli, MD (UC Davis); Bret Borowski, RT (Mayo Clinic); Jeff Gunter, PhD (Mayo Clinic); Matt Senjem, MS (Mayo Clinic); Prashanthi Vemuri, PhD (Mayo Clinic); David Jones, MD (Mayo Clinic); Kejal Kantarci (Mayo Clinic); Chad Ward (Mayo Clinic); Robert A. Koeppe, PhD (University of Michigan, PET Core Leader); Norm Foster, MD (University of Utah); Eric M. Reiman, MD (Banner Alzheimer's Institute); Kewei Chen, PhD (Banner Alzheimer's Institute); Chet Mathis, MD (University of Pittsburgh); Susan Landau, PhD (UC Berkeley); Nigel J. Cairns, PhD, MRCPath (Washington University St. Louis); Erin Householder (Washington University St. Louis); Lisa Taylor Reinwald, BA, HTL (Washington University St. Louis); Virginia Lee, PhD, MBA (UPenn School of Medicine); Magdalena Korecka, PhD (UPenn School of Medicine); Michal Figurski, PhD (UPenn School of Medicine); Karen Crawford (USC); Scott Neu, PhD (USC); Tatiana M. Foroud, PhD (Indiana University); Steven Potkin, MD UC (UC Irvine); Li Shen, PhD (Indiana University); Faber Kelley, MS, CCRC (Indiana University); Sungeun Kim, PhD (Indiana University); Kwangsik Nho, PhD (Indiana University); Zaven Kachaturian, PhD (Khachaturian, Radebaugh & Associates, Inc and Alzheimer's Association's Ronald and Nancy Reagan's Research Institute); Richard Frank, MD, PhD (General Electric); Peter J. Snyder, PhD (Brown University); Susan Molchan, PhD (National Institute on Aging/National Institutes of Health); Jeffrey Kaye, MD (Oregon Health and Science University); Joseph Quinn, MD (Oregon Health and Science University); Betty Lind, BS (Oregon Health and Science University); Raina Carter, BA (Oregon Health and Science University); Sara Dolen, BS (Oregon Health and Science University); Lon S. Schneider, MD (University of Southern California); Sonia Pawluczyk, MD (University of Southern California); Mauricio Beccera, BS (University of Southern California); Liberty Teodoro, RN (University of Southern California); Bryan M. Spann, DO, PhD (University of Southern California); James Brewer, MD, PhD (University of California San Diego); Helen Vanderswag, RN (University of California San Diego); Adam Fleisher, MD (University of California San Diego); Judith L. Heidebrink, MD, MS (University of Michigan); Joanne L. Lord, LPN, BA, CCRC (University of Michigan); Ronald Petersen, MD, PhD (Mayo Clinic, Rochester); Sara S. Mason, RN (Mayo Clinic, Rochester); Colleen S. Albers, RN (Mayo Clinic, Rochester); David Knopman, MD (Mayo Clinic, Rochester); Kris Johnson, RN (Mayo Clinic, Rochester); Rachelle S. Doody, MD, PhD (Baylor College of Medicine); Javier Villanueva Meyer, MD (Baylor College of Medicine); Munir Chowdhury, MBBS, MS (Baylor College of Medicine); Susan Rountree, MD (Baylor College of Medicine); Mimi Dang, MD (Baylor College of Medicine); Yaakov Stern, PhD (Columbia University Medical Center); Lawrence S. Honig, MD, PhD (Columbia University Medical Center); Karen L. Bell, MD (Columbia University Medical Center); Beau Ances, MD (Washington University, St. Louis); John C. Morris, MD (Washington University, St. Louis); Maria Carroll, RN, MSN (Washington University, St. Louis); Sue Leon, RN, MSN (Washington University, St. Louis); Erin Householder, MS, CCRP (Washington University, St. Louis); Mark A. Mintun, MD (Washington University, St. Louis); Stacy Schneider, APRN, BC, GNP (Washington University, St. Louis); Angela Oliver, RN, BSN, MSG; Daniel Marson, JD, PhD (University of Alabama Birmingham); Randall Griffith, PhD, ABPP (University of Alabama Birmingham); David Clark, MD (University of Alabama Birmingham); David Geldmacher, MD (University of Alabama Birmingham); John Brockington, MD (University of Alabama Birmingham); Erik Roberson, MD (University of Alabama Birmingham); Hillel Grossman, MD (Mount Sinai School of Medicine); Effie Mitsis, PhD (Mount Sinai School of Medicine); Leyla deToledoMorrell, PhD (Rush University Medical Center); Raj C. Shah, MD (Rush University Medical Center); Ranjan Duara, MD (Wien Center); Daniel Varon, MD (Wien Center); Maria T. Greig, HP (Wien Center); Peggy Roberts, CNA (Wien Center); Marilyn Albert, PhD (Johns Hopkins University); Chiadi Onyike, MD (Johns Hopkins University); Daniel D'Agostino II, BS (Johns Hopkins University); Stephanie Kielb, BS (Johns Hopkins University); James E. Galvin, MD, MPH (New York University); Dana M. Pogorelec (New York University); Brittany Cerbone (New York University); Christina A. Michel (New York University); Henry Rusinek, PhD (New York University); Mony J. de Leon, EdD (New York University); Lidia Glodzik, MD, PhD (New York University); Susan De Santi, PhD (New York University); P. Murali Doraiswamy, MD (Duke University Medical Center); Jeffrey R. Petrella, MD (Duke University Medical Center); Terence Z. Wong, MD (Duke University Medical Center); Steven E. Arnold, MD (University of Pennsylvania); Jason H. Karlawish, MD (University of Pennsylvania); David Wolk, MD (University of Pennsylvania); Charles D. Smith, MD (University of Kentucky); Greg Jicha, MD (University of Kentucky); Peter Hardy, PhD (University of Kentucky); Partha Sinha, PhD (University of Kentucky); Elizabeth Oates, MD (University of Kentucky); Gary Conrad, MD (University of Kentucky); Oscar L. Lopez, MD (University of Pittsburgh); MaryAnn Oakley, MA (University of Pittsburgh); Donna M. Simpson, CRNP, MPH (University of Pittsburgh); Anton P. Porsteinsson, MD (University of Rochester Medical Center); Bonnie S. Goldstein, MS, NP (University of Rochester Medical Center); Kim Martin, RN (University of Rochester Medical Center); Kelly M. Makino, BS (University of Rochester Medical Center); M. Saleem Ismail, MD (University of Rochester Medical Center); Connie Brand, RN (University of Rochester Medical Center); Ruth A. Mulnard, DNSc, RN, FAAN (University of California, Irvine); Gaby Thai, MD (University of California, Irvine); Catherine Mc Adams Ortiz, MSN, RN, A/GNP (University of California, Irvine); Kyle Womack, MD (University of Texas Southwestern Medical School); Dana Mathews, MD, PhD (University of Texas Southwestern Medical School); Mary Quiceno, MD (University of Texas Southwestern Medical School); Ramon Diaz Arrastia, MD, PhD (University of Texas Southwestern Medical School); Richard King, MD (University of Texas Southwestern Medical School); Myron Weiner, MD (University of Texas Southwestern Medical School); Kristen Martin Cook, MA (University of Texas Southwestern Medical School); Michael DeVous, PhD (University of Texas Southwestern Medical School); Allan I. Levey, MD, PhD (Emory University); James J. Lah, MD, PhD (Emory University); Janet S. Cellar, DNP, PMHCNS BC (Emory University); Jeffrey M. Burns, MD (University of Kansas, Medical Center); Heather S. Anderson, MD (University of Kansas, Medical Center); Russell H. Swerdlow, MD (University of Kansas, Medical Center); Liana Apostolova, MD (University of California, Los Angeles); Kathleen Tingus, PhD (University of California, Los Angeles); Ellen Woo, PhD (University of California, Los Angeles); Daniel H.S. Silverman, MD, PhD (University of California, Los Angeles); Po H. Lu, PsyD (University of California, Los Angeles); George Bartzokis, MD (University of California, Los Angeles); Neill R. Graff Radford, MBBCH, FRCP (London) (Mayo Clinic, Jacksonville); Francine Parfitt, MSH, CCRC (Mayo Clinic, Jacksonville); Tracy Kendall, BA, CCRP (Mayo Clinic, Jacksonville); Heather Johnson, MLS, CCRP (Mayo Clinic, Jacksonville); Martin R. Farlow, MD (Indiana University); Ann Marie Hake, MD (Indiana University); Brandy R. Matthews, MD (Indiana University); Scott Herring, RN, CCRC (Indiana University); Cynthia Hunt, BS, CCRP (Indiana University); Christopher H. van Dyck, MD (Yale University School of Medicine); Richard E. Carson, PhD (Yale University School of Medicine); Martha G. MacAvoy, PhD (Yale University School of Medicine); Howard Chertkow, MD (McGill Univ., Montreal Jewish General Hospital); Howard Bergman, MD (McGill Univ., Montreal Jewish General Hospital); Chris Hosein, Med (McGill Univ., Montreal Jewish General Hospital); Sandra Black, MD, FRCPC (Sunnybrook Health Sciences, Ontario); Dr Bojana Stefanovic (Sunnybrook Health Sciences, Ontario); Curtis Caldwell, PhD (Sunnybrook Health Sciences, Ontario); Ging Yuek Robin Hsiung, MD, MHSc, FRCPC (U.B.C. Clinic for AD & Related Disorders); Howard Feldman, MD, FRCPC (U.B.C. Clinic for AD & Related Disorders); Benita Mudge, BS (U.B.C. Clinic for AD & Related Disorders); Michele Assaly, MA Past (U.B.C. Clinic for AD & Related Disorders); Andrew Kertesz, MD (Cognitive Neurology St. Joseph's, Ontario); John Rogers, MD (Cognitive Neurology St. Joseph's, Ontario); Dick Trost, PhD (Cognitive Neurology St. Joseph's, Ontario); Charles Bernick, MD (Cleveland Clinic Lou Ruvo Center for Brain Health); Donna Munic, PhD (Cleveland Clinic Lou Ruvo Center for Brain Health); Diana Kerwin, MD (Northwestern University); Marek Marsel Mesulam, MD (Northwestern University); Kristine Lipowski, BA (Northwestern University); Chuang Kuo Wu, MD, PhD (Northwestern University); Nancy Johnson, PhD (Northwestern University); Carl Sadowsky, MD (Premiere Research Inst [Palm Beach Neurology]); Walter Martinez, MD (Premiere Research Inst [Palm Beach Neurology]); Teresa Villena, MD (Premiere Research Inst [Palm Beach Neurology]); Raymond Scott Turner, MD, PhD (Georgetown University Medical Center); Kathleen Johnson, NP (Georgetown University Medical Center); Brigid Reynolds, NP (Georgetown University Medical Center); Reisa A. Sperling, MD (Brigham and Women's Hospital); Keith A. Johnson, MD (Brigham and Women's Hospital); Gad Marshall, MD (Brigham and Women's Hospital); Meghan Frey (Brigham and Women's Hospital); Jerome Yesavage, MD (Stanford University); Joy L. Taylor, PhD (Stanford University); Barton Lane, MD (Stanford University); Allyson Rosen, PhD (Stanford University); Jared Tinklenberg, MD (Stanford University); Marwan N. Sabbagh, MD (Banner Sun Health Research Institute); Christine M. Belden, PsyD (Banner Sun Health Research Institute); Sandra A. Jacobson, MD (Banner Sun Health Research Institute); Sherye A. Sirrel, MS (Banner Sun Health Research Institute); Neil Kowall, MD (Boston University); Ronald Killiany, PhD (Boston University); Andrew E. Budson, MD (Boston University); Alexander Norbash, MD (Boston University); Patricia Lynn Johnson, BA (Boston University); Thomas O. Obisesan, MD, MPH (Howard University); Saba Wolday, MSc (Howard University); Joanne Allard, PhD (Howard University); Alan Lerner, MD (Case Western Reserve University); Paula Ogrocki, PhD (Case Western Reserve University); Leon Hudson, MPH (Case Western Reserve University); Evan Fletcher, PhD (University of California, Davis Sacramento); Owen Carmichael, PhD (University of California, Davis Sacramento); John Olichney, MD (University of California, Davis Sacramento); Charles DeCarli, MD (University of California, Davis Sacramento); Smita Kittur, MD (Neurological Care of CNY); Michael Borrie, MB ChB (Parkwood Hospital); T Y Lee, PhD (Parkwood Hospital); Rob Bartha, PhD (Parkwood Hospital); Sterling Johnson, PhD (University of Wisconsin); Sanjay Asthana, MD (University of Wisconsin); Cynthia M. Carlsson, MD (University of Wisconsin); Steven G. Potkin, MD (University of California, Irvine BIC); Adrian Preda, MD (University of California, Irvine BIC); Dana Nguyen, PhD (University of California, Irvine BIC); Pierre Tariot, MD (Banner Alzheimer's Institute); Adam Fleisher, MD (Banner Alzheimer's Institute); Stephanie Reeder, BA (Banner Alzheimer's Institute); Vernice Bates, MD (Dent Neurologic Institute); Horacio Capote, MD (Dent Neurologic Institute); Michelle Rainka, PharmD, CCRP (Dent Neurologic Institute); Douglas W. Scharre, MD (Ohio State University); Maria Kataki, MD, PhD (Ohio State University); Anahita Adeli, MD (Ohio State University); Earl A. Zimmerman, MD (Albany Medical College); Dzintra Celmins, MD (Albany Medical College); Alice D. Brown, FNP (Albany Medical College); Godfrey D. Pearlson, MD (Hartford Hosp, Olin Neuropsychiatry Research Center); Karen Blank, MD (Hartford Hosp, Olin Neuropsychiatry Research Center); Karen Anderson, RN (Hartford Hosp, Olin Neuropsychiatry Research Center); Robert B. Santulli, MD (Dartmouth Hitchcock Medical Center); Tamar J. Kitzmiller (Dartmouth Hitchcock Medical Center); Eben S. Schwartz, PhD (Dartmouth Hitchcock Medical Center); Kaycee M. Sink, MD, MAS (Wake Forest University Health Sciences); Jeff D. Williamson, MD, MHS (Wake Forest University Health Sciences); Pradeep Garg, PhD (Wake Forest University Health Sciences); Franklin Watkins, MD (Wake Forest University Health Sciences); Brian R. Ott, MD (Rhode Island Hospital); Henry Querfurth, MD (Rhode Island Hospital); Geoffrey Tremont, PhD (Rhode Island Hospital); Stephen Salloway, MD, MS (Butler Hospital); Paul Malloy, PhD (Butler Hospital); Stephen Correia, PhD (Butler Hospital); Howard J. Rosen, MD (UC San Francisco); Bruce L. Miller, MD (UC San Francisco); Jacobo Mintzer, MD, MBA (Medical University South Carolina); Kenneth Spicer, MD, PhD (Medical University South Carolina); David Bachman, MD (Medical University South Carolina); Elizabether Finger, MD (St. Joseph's Health Care); Stephen Pasternak, MD (St. Joseph's Health Care); Irina Rachinsky, MD (St. Joseph's Health Care); John Rogers, MD (St. Joseph's Health Care); Andrew Kertesz, MD (St. Joseph's Health Care); Dick Drost, MD (St. Joseph's Health Care); Nunzio Pomara, MD (Nathan Kline Institute); Raymundo Hernando, MD (Nathan Kline Institute); Antero Sarrael, MD (Nathan Kline Institute); Susan K. Schultz, MD (University of Iowa College of Medicine, Iowa City); Laura L. Boles Ponto, PhD (University of Iowa College of Medicine, Iowa City); Hyungsub Shim, MD (University of Iowa College of Medicine, Iowa City); Karen Elizabeth Smith, RN (University of Iowa College of Medicine, Iowa City); Norman Relkin, MD, PhD (Cornell University); Gloria Chaing, MD (Cornell University); Lisa Raudin, PhD (Cornell University); Amanda Smith, MD (University of South Florida: USF Health Byrd Alzheimer's Institute); Kristin Fargher, MD (University of South Florida: USF Health Byrd Alzheimer's Institute); Balebail Ashok Raj, MD (University of South Florida: USF Health Byrd Alzheimer's Institute)
APPENDIX A.
NPI DEPRESSION AND APATHY DOMAINS QUESTIONS AND DIFFERENCES NOTE TABLE A.1
TABLE A.1.
NPI‐NH and NPI‐12 depression and apathy domains questions.
| NPI‐NH depression domain subquestions | NPI‐NH apathy domain subquestions |
|---|---|
| Does the resident cry at times? | Has the resident lost interest in the world around him/her? |
| Does the resident say, or act like he/she is depressed? | Does the resident fail to start conversation? |
| Does the resident put him/herself down or say that he/she feels like a failure? | Does the resident fail to show emotional reactions that would be expected (happiness over the visit of a friend or family member, interest in the news or sports, etc.)? |
| Does the resident say that he/she is a bad person or deserves to be punished? | Has the resident lost interest in friends and family members? |
| Does the resident seem very discouraged or say that he/she has no future? | Is the resident less enthusiastic about his/her usual interests? |
| Does the resident say he/she is a burden to the family or that the family would be better off without him/her? | Does the resident sit quietly without paying attention to things going on around him/her? |
| Does the resident talk about wanting to die or about killing him/herself? | Does the resident show any other signs that he/she doesn't care about doing new things? |
| Does the resident show any other signs of depression or sadness? | |
| NPI‐12 depression domain subquestions | NPI‐12 apathy domain subquestions |
| Does the patient have periods of tearfulness or sobbing that seem to indicate sadness? | Does the patient seem less spontaneous and less active than usual? |
| Does the patient say or act as if he/she is sad or in low spirits? | Is the patient less likely to initiate a conversation? |
| Does the patient put him/herself down or say that he/she feels like a failure? | Is the patient less affectionate or lacking in emotions compared to his/her usual self? |
| Does the patient say that he/she is a bad person or deserves to be punished? | Does the patient contribute less to household chores? |
| Does the patient seem very discouraged or say that he/she has no future? | Does the patient seem less interested in the activities and plans of others? |
| Does the patient say he/she is a burden to the family or that the family would be better off without him/her? | Has the patient lost interest in friends and family members? |
| Does the patient express a wish for death or talk about killing him/herself? | Is the patient less enthusiastic about his/her usual interests? |
| Does the patient show any other signs of depression or sadness? | Does the patient show any other signs that he/she doesn't care about doing new things? |
The phrasing of questions varies between the NPI‐12 and NPI‐NH to reflect the population/setting. Both versions of the questionnaire have been validated to measure the domains in question with regards with the specific setting/population, enabling the direct comparison of scores.
Abbreviations: NPI‐12, Neuropsychiatric Inventory 12‐item version; NPI‐NH, Neuropsychiatric Inventory Nursing Home version.
APPENDIX B.
B.1.
FIGURE B.1.
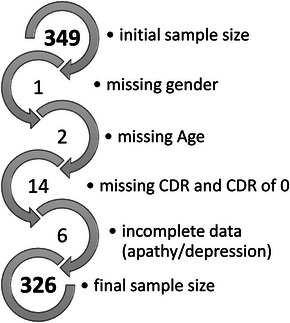
Sample selection for Measuring Long Term Outcomes in People with Dementia in Care Homes (L‐study). CDR, Clinical Dementia Rating.
FIGURE B.2.
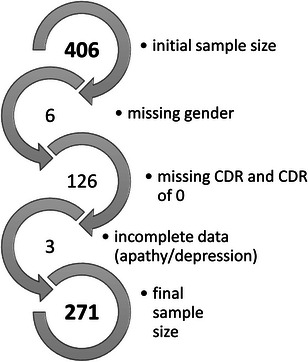
Sample selection for Alzheimer's Disease Neuroimaging Initiative. CDR, Clinical Dementia Rating.
APPENDIX C.
C.1.
Table C.1
TABLE C.1.
Class probabilities in both cohorts.
| No symptoms | Combined apathy/depression | Depression | Apathy | |
|---|---|---|---|---|
| L‐study | 0.42 | 0.18 | 0.09 | 0.30 |
| ADNI | 0.41 | 0.20 | 0.16 | 0.23 |
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes.
APPENDIX D.
D.1.
Tables of symptom probabilities Table D.1 and D.2
TABLE D.1.
Symptom probability across the four classes in L‐study.
| Symptom probability L‐study | No symptoms | Combined apathy/depression | Depression | Apathy |
|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | |
| Cry at times | 0.08 | 0.54 | 0.47 | 0.06 |
| Say, or act like he/she is depressed | 0.01 | 0.90 | 0.94 | 0.10 |
| Put him/herself down or say that feels like a failure | 0.00 | 0.30 | 0.13 | 0.00 |
| Bad person or deserves to be punished? | 0.00 | 0.15 | 0.03 | 0.00 |
| Seem very discourages or say that has no future | 0.00 | 0.40 | 0.35 | 0.01 |
| Is a burden to the family or that the family would be better off without him | 0.00 | 0.15 | 0.10 | 0.01 |
| Talk about wanting to die or about killing himself | 0.01 | 0.17 | 0.14 | 0.00 |
| Show other signs of depression or sadness | 0.04 | 0.43 | 0.37 | 0.07 |
| Lost interest in the world around | 0.02 | 0.87 | 0.01 | 0.81 |
| Fail to start conversation | 0.01 | 0.56 | 0.00 | 0.67 |
| Fail to show emotional reaction that would be expected | 0.00 | 0.36 | 0.02 | 0.42 |
| Lost interest in friends and family members | 0.00 | 0.29 | 0.00 | 0.31 |
| Less enthusiastic about usual interests | 0.00 | 0.69 | 0.05 | 0.72 |
| Sit quietly without paying attention to things going on around | 0.02 | 0.85 | 0.09 | 0.88 |
| Other signs that doesn't care about doing new things | 0.00 | 0.42 | 0.00 | 0.35 |
Abbreviation: L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes.
TABLE D.2.
Symptom probability across the four classes in ADNI study.
| Symptoms probability ADNI | No symptoms | Apathy | Depression | Combined apathy/depression |
|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | |
| Cry at times | 0.00 | 0.03 | 0.41 | 0.39 |
| Say, or act like he/she is depressed | 0.07 | 0.00 | 0.86 | 0.86 |
| Put him/herself down or say that feels like a failure | 0.01 | 0.00 | 0.37 | 0.40 |
| Say that he/she is a bad person or deserves to be punished? | 0.00 | 0.00 | 0.05 | 0.04 |
| Seem very discourages or say that has no future? | 0.00 | 0.00 | 0.54 | 0.37 |
| Is a burden to the family or that the family would be better off without him | 0.01 | 0.02 | 0.39 | 0.34 |
| Talk about wanting to die or about killing himself | 0.00 | 0.00 | 0.05 | 0.09 |
| Show other signs of depression or sadness? | 0.01 | 0.01 | 0.14 | 0.19 |
| Lost interest in the world around | 0.03 | 0.89 | 0.06 | 0.98 |
| Fail to start conversation | 0.00 | 0.76 | 0.07 | 0.78 |
| Less affectionate or lacking in emotions | 0.00 | 0.29 | 0.00 | 0.36 |
| Contribute less to household chores | 0.01 | 0.74 | 0.00 | 0.77 |
| Less Interested in the activities and plans of others | 0.00 | 0.64 | 0.00 | 0.85 |
| Lost interest in friends and family members | 0.00 | 0.40 | 0.00 | 0.49 |
| Less enthusiastic about usual interests | 0.01 | 0.69 | 0.13 | 0.92 |
| Other signs that doesn't care about doing new things | 0.00 | 0.14 | 0.00 | 0.24 |
Abbreviation: ADNI, Alzheimer's Disease Neuroimaging Initiative.
APPENDIX E.
E.1.
Model selection
The 4‐class model within the L‐study has an adjusted BIC and better scaled relative entropy compared to the 5‐class model (see Table 3). Although the adjusted BIC within the ADNI cohort showed that the best model was the 5‐class model, the scaled relative entropy showed a better score for the 4‐class model. Given the minimal score differences between the fitting models, and considering clinical relevance, it was decided that the 4‐class model was more appropriate.
The key difference between the 4‐ and 5‐class model is that the 5‐class model creates an extra apathy group with higher probability of “saying or acting like depressed,” in both cohorts. Furthermore, this extra apathy class has less probability of experiencing “less affectionate or lacking in emotions,” “lost interest in friends and family members” in the ADNI cohort, and “fail to show emotional reaction that would be expected” and “lost interest in friends and family members” and “less enthusiastic about usual interests” in the L‐study cohort. Creating the extra apathy class, seems to be dividing the initial apathy class reducing some of the symptom's probability.
APPENDIX F.
F.1.
Tables of results of analysis of the relationship between the latent classes with other NPI items Table F.1
TABLE F.1.
Of results of relationship between the latent classes with other NPI items.
| NPIA—Delusions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| Distal prob | EST | SE | WALD STATI∼S | P | DISTAL PROB | EST | SE | WALD STATI∼S | TYPE | |
| No symptoms | 0.39 | 0.14 | ||||||||
| Combined apa/dep | 0.56 | 0.25 | ||||||||
| Depression | 0.54 | 0.2 | ||||||||
| Apathy | 0.23 | 0.04 | ||||||||
| Combined vs. no symptoms | 0.7 | 0.33 | 4.43 | 0.04 | 0.74 | 0.43 | 2.95 | 0.09 | ||
| Depression vs. no symptoms | 0.63 | 0.44 | 2.08 | 0.15 | 0.44 | 0.51 | 0.73 | 0.39 | ||
| Apathy vs. no symptoms | −0.77 | 0.31 | 6.02 | 0.01 | −1.31 | 0.73 | 3.24 | 0.07 | ||
| Combined vs. depression | 0.07 | 0.49 | 0.02 | 0.89 | 0.3 | 0.52 | 0.33 | 0.57 | ||
| Combined vs. apathy | 1.47 | 0.4 | 13.55 | <0.01 | 2.05 | 0.76 | 7.27 | 0.01 | ||
| Depression vs. apathy | 1.41 | 0.47 | 8.87 | <0.01 | 1.75 | 0.78 | 4.97 | 0.03 | ||
| Omnibus_Test | 16.49 | <0.01 | 8.3 | 0.04 | ||||||
| NPIB—Hallucinations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.15 | 0.1 | ||||||||
| Combined apa/dep | 0.36 | 0.17 | ||||||||
| Depression | 0.38 | 0.02 | ||||||||
| Apathy | 0.22 | 0.06 | ||||||||
| Combined vs. no symptoms | 1.16 | 0.38 | 9.49 | 0 | 0.51 | 0.5 | 1.04 | 0.31 | ||
| Depression vs. no symptoms | 1.23 | 0.48 | 6.67 | 0.01 | −1.57 | 1.22 | 1.66 | 0.2 | ||
| Apathy vs. no symptoms | 0.48 | 0.35 | 1.87 | 0.17 | −0.55 | 0.64 | 0.75 | 0.39 | ||
| Combined vs. depression | −0.07 | 0.5 | 0.02 | 0.88 | 2.09 | 1.24 | 2.82 | 0.09 | ||
| Combined vs. apathy | 0.68 | 0.4 | 2.82 | 0.09 | 1.06 | 0.68 | 2.43 | 0.12 | ||
| Depression vs. apathy | 0.75 | 0.48 | 2.49 | 0.11 | −1.02 | 1.29 | 0.63 | 0.43 | ||
| Omnibus_Test | 12.28 | 0.01 | 4.36 | 0.22 | ||||||
| NPIC—Agitation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.52 | 0.25 | ||||||||
| Combined apa/dep | 0.8 | 0.4 | ||||||||
| Depression | 0.64 | 0.4 | ||||||||
| Apathy | 0.72 | 0.38 | ||||||||
| Combined vs. no symptoms | 1.34 | 0.39 | 11.53 | 0 | 0.71 | 0.36 | 3.82 | 0.05 | ||
| Depression vs. no symptoms | 0.5 | 0.45 | 1.24 | 0.27 | 0.7 | 0.41 | 2.87 | 0.09 | ||
| Apathy vs. no symptoms | 0.86 | 0.29 | 8.8 | 0 | 0.63 | 0.35 | 3.31 | 0.07 | ||
| Combined vs. depression | 0.83 | 0.55 | 2.32 | 0.13 | 0.01 | 0.44 | 0 | 0.98 | ||
| Combined vs. apathy | 0.47 | 0.44 | 1.14 | 0.29 | 0.08 | 0.39 | 0.04 | 0.83 | ||
| Depression vs. apathy | −0.36 | 0.47 | 0.59 | 0.44 | 0.07 | 0.42 | 0.03 | 0.87 | ||
| Omnibus_Test | 17.04 | 0 | 5.73 | 0.13 | ||||||
| NPIE—Anxiety | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.22 | 0.16 | ||||||||
| Combined apa/dep | 0.7 | 0.59 | ||||||||
| Depression | 0.41 | 0.3 | ||||||||
| Apathy | 0.27 | 0.29 | ||||||||
| Combined vs. no symptoms | 2.14 | 0.38 | 32.4 | 0 | 1.96 | 0.39 | 25.4 | 0 | ||
| Depression vs. no symptoms | 0.91 | 0.46 | 3.95 | 0.05 | 0.77 | 0.46 | 2.83 | 0.09 | ||
| Apathy vs. no symptoms | 0.3 | 0.32 | 0.86 | 0.35 | 0.73 | 0.39 | 3.48 | 0.06 | ||
| Combined vs. depression | 1.23 | 0.51 | 5.8 | 0.02 | 1.19 | 0.46 | 6.67 | 0.01 | ||
| Combined vs. apathy | 1.84 | 0.41 | 20 | 0 | 1.23 | 0.41 | 9.16 | 0 | ||
| Depression vs. apathy | 0.61 | 0.47 | 1.72 | 0.19 | 0.04 | 0.46 | 0.01 | 0.92 | ||
| Omnibus_Test | 33.81 | 0 | 25.74 | 0 | ||||||
| NPIF—Elation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.13 | 0.05 | ||||||||
| Combined apa/dep | 0.19 | 0.04 | ||||||||
| Depression | 0.24 | 0.08 | ||||||||
| Apathy | 0.16 | 0.03 | ||||||||
| Combined vs. no symptoms | 0.43 | 0.44 | 0.95 | 0.33 | −0.32 | 0.85 | 0.14 | 0.71 | ||
| Depression vs. no symptoms | 0.74 | 0.53 | 1.96 | 0.16 | 0.4 | 0.77 | 0.27 | 0.6 | ||
| Apathy vs. no symptoms | 0.24 | 0.39 | 0.39 | 0.53 | −0.55 | 0.86 | 0.41 | 0.52 | ||
| Combined vs. depression | −0.32 | 0.58 | 0.29 | 0.59 | −0.72 | 0.96 | 0.56 | 0.45 | ||
| Combined vs. apathy | 0.19 | 0.48 | 0.15 | 0.7 | 0.24 | 1.06 | 0.05 | 0.82 | ||
| Depression vs. apathy | 0.5 | 0.54 | 0.87 | 0.35 | 0.96 | 0.96 | 0.99 | 0.32 | ||
| Omnibus_Test | 2.32 | 0.51 | 1.18 | 0.76 | ||||||
| NPIH—Disinhibition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.15 | 0.14 | ||||||||
| Combined apa/dep | 0.31 | 0.3 | ||||||||
| Depression | 0.27 | 0.3 | ||||||||
| Apathy | 0.26 | 0.17 | ||||||||
| Combined vs no symptoms | 0.91 | 0.39 | 5.55 | 0.02 | 1.05 | 0.42 | 6.28 | 0.01 | ||
| Depression vs no symptoms | 0.74 | 0.51 | 2.12 | 0.15 | 1.01 | 0.47 | 4.59 | 0.03 | ||
| Apathy vs. no symptoms | 0.68 | 0.34 | 3.9 | 0.05 | 0.24 | 0.45 | 0.29 | 0.59 | ||
| Combined vs. depression | −0.17 | 0.54 | 0.1 | 0.75 | 0.04 | 0.47 | 0.01 | 0.94 | ||
| Combined vs. apathy | −0.23 | 0.4 | 0.33 | 0.57 | 0.81 | 0.47 | 2.99 | 0.08 | ||
| Depression vs. apathy | −0.06 | 0.5 | 0.02 | 0.9 | 0.77 | 0.49 | 2.43 | 0.12 | ||
| Omnibus_Test | 7.08 | 0.07 | 8.51 | 0.04 | ||||||
| NPII—Irritability | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.38 | 0.25 | ||||||||
| Combined apa/dep | 0.69 | 0.51 | ||||||||
| Depression | 0.68 | 0.45 | ||||||||
| Apathy | 0.47 | 0.29 | ||||||||
| Combined vs. no symptoms | 1.27 | 0.35 | 12.97 | 0 | 1.19 | 0.36 | 10.92 | 0 | ||
| Depression vs. no symptoms | 1.24 | 0.47 | 6.96 | 0.01 | 0.91 | 0.41 | 4.92 | 0.03 | ||
| Apathy vs. no symptoms | 0.33 | 0.28 | 1.43 | 0.23 | 0.2 | 0.36 | 0.3 | 0.58 | ||
| Combined vs. depression | 0.03 | 0.53 | 0 | 0.96 | 0.29 | 0.43 | 0.43 | 0.51 | ||
| Combined vs. apathy | 0.93 | 0.39 | 5.84 | 0.02 | 0.99 | 0.4 | 6.01 | 0.01 | ||
| Depression vs. apathy | 0.91 | 0.48 | 3.61 | 0.06 | 0.71 | 0.43 | 2.68 | 0.1 | ||
| Omnibus_Test | 16.92 | 0 | 13.27 | 0 | ||||||
| NPIJ—AMB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.3 | 0.17 | ||||||||
| Combined apa/dep | 0.32 | 0.31 | ||||||||
| Depression | 0.19 | 0.2 | ||||||||
| Apathy | 0.41 | 0.38 | ||||||||
| Combined vs. no symptoms | 0.07 | 0.35 | 0.04 | 0.85 | 1.07 | 0.39 | 7.47 | 0.01 | ||
| Depression vs. no symptoms | −0.61 | 0.55 | 1.23 | 0.27 | 0.21 | 0.5 | 0.18 | 0.67 | ||
| Apathy vs. no symptoms | 0.46 | 0.29 | 2.52 | 0.11 | 0.8 | 0.38 | 4.43 | 0.04 | ||
| Combined vs. depression | 0.68 | 0.59 | 1.3 | 0.25 | 0.86 | 0.51 | 2.84 | 0.09 | ||
| Combined vs. apathy | −0.39 | 0.39 | 1.02 | 0.31 | 0.27 | 0.41 | 0.43 | 0.51 | ||
| Depression vs. apathy | −1.07 | 0.55 | 3.75 | 0.05 | −0.59 | 0.49 | 1.42 | 0.23 | ||
| Omnibus_Test | 4.99 | 0.17 | 9.33 | 0.03 | ||||||
| NPIK—Sleep and night time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.22 | 0.16 | ||||||||
| Combined apa/dep | 0.34 | 0.42 | ||||||||
| Depression | 0.27 | 0.35 | ||||||||
| Apathy | 0.33 | 0.32 | ||||||||
| Combined vs. no symptoms | 0.63 | 0.36 | 3.01 | 0.08 | 1.27 | 0.39 | 10.45 | 0 | ||
| Depression vs. no symptoms | 0.29 | 0.5 | 0.34 | 0.56 | 1.03 | 0.45 | 5.34 | 0.02 | ||
| Apathy vs. no symptoms | 0.6 | 0.31 | 3.72 | 0.05 | 0.91 | 0.38 | 5.58 | 0.02 | ||
| Combined vs. depression | 0.34 | 0.54 | 0.4 | 0.53 | 0.23 | 0.45 | 0.27 | 0.6 | ||
| Combined vs. apathy | 0.03 | 0.39 | 0.01 | 0.94 | 0.36 | 0.4 | 0.81 | 0.37 | ||
| Depression vs. apathy | −0.31 | 0.5 | 0.39 | 0.53 | 0.13 | 0.44 | 0.09 | 0.77 | ||
| Omnibus_Test | 5.05 | 0.17 | 11.93 | 0.01 | ||||||
| NPIL—Eating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L‐study | ADNI | |||||||||
| DISTAL_PROB | EST | SE | WALD_STATI∼S | P | DISTAL_PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.19 | 0.22 | ||||||||
| Combined apa/dep | 0.29 | 0.47 | ||||||||
| Depression | 0.27 | 0.32 | ||||||||
| Apathy | 0.3 | 0.41 | ||||||||
| Combined vs. no symptoms | 0.52 | 0.38 | 1.9 | 0.17 | 1.09 | 0.37 | 8.83 | 0 | ||
| Depression vs. no symptoms | 0.44 | 0.5 | 0.77 | 0.38 | 0.5 | 0.43 | 1.34 | 0.25 | ||
| Apathy vs. no symptoms | 0.6 | 0.32 | 3.52 | 0.06 | 0.89 | 0.35 | 6.55 | 0.01 | ||
| Combined vs. depression | 0.08 | 0.54 | 0.02 | 0.88 | 0.59 | 0.45 | 1.7 | 0.19 | ||
| Combined vs. apathy | −0.08 | 0.4 | 0.04 | 0.84 | 0.19 | 0.39 | 0.25 | 0.62 | ||
| Depression vs. apathy | −0.16 | 0.5 | 0.11 | 0.74 | −0.4 | 0.43 | 0.84 | 0.36 | ||
| Omnibus_Test | 4.13 | 0.25 | 11.11 | 0.01 | ||||||
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes; NPI, Neuropsychiatric Inventory; SE, standard error.
APPENDIX G.
G.1.
Tables of results of analysis of the relationship between the latent classes with other NPI items Table G.1
TABLE G.1.
Of results of relationship between the latent classes with other clinically significant NPI symptoms.
| NPIA—Delusions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.24 | 0.05 | ||||||||
| Combined apa/dep | 0.33 | 0.12 | ||||||||
| Depression | 0.34 | 0.10 | ||||||||
| Apathy | 0.13 | 0.03 | ||||||||
| Combined vs. no symptoms | 0.45 | 0.36 | 1.60 | 0.21 | 0.89 | 0.62 | 2.03 | 0.15 | ||
| Depression vs. no symptoms | 0.48 | 0.47 | 1.07 | 0.30 | 0.72 | 0.72 | 0.99 | 0.32 | ||
| Apathy vs. no symptoms | −0.74 | 0.38 | 3.79 | 0.05 | −0.60 | 0.90 | 0.44 | 0.51 | ||
| Combined vs. depression | −0.03 | 0.51 | 0.00 | 0.95 | 0.17 | 0.95 | 1.92 | 0.81 | ||
| Combined vs. apathy | 1.19 | 0.46 | 6.80 | 0.01 | 1.48 | 0.92 | 2.63 | 0.10 | ||
| Depression vs. apathy | 1.22 | 0.52 | 5.48 | 0.02 | 1.32 | 0.70 | 0.06 | 0.17 | ||
| Omnibus_Test | 8.43 | 0.04 | 3.86 | 0.28 | ||||||
| NPIB—Hallucinations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.08 | 0.01 | ||||||||
| Combined apa/dep | 0.17 | 0.06 | ||||||||
| Depression | 0.28 | 0.02 | ||||||||
| Apathy | 0.09 | 0.03 | ||||||||
| Combined vs. no symptoms | 0.89 | 0.49 | 3.34 | 0.07 | 2.00 | 1.26 | 2.53 | 0.11 | ||
| Depression vs. no symptoms | 1.51 | 0.55 | 7.54 | 0.01 | 1.10 | 1.60 | 0.48 | 0.49 | ||
| Apathy vs. no symptoms | 0.10 | 0.50 | 0.04 | 0.85 | 1.32 | 1.34 | 0.97 | 0.33 | ||
| Combined vs. depression | −0.62 | 0.57 | 1.17 | 0.28 | 0.90 | 1.23 | 0.53 | 0.47 | ||
| Combined vs. apathy | 0.79 | 0.56 | 2.04 | 0.15 | 0.68 | 0.98 | 0.48 | 0.49 | ||
| Depression vs. apathy | 1.41 | 0.58 | 5.98 | 0.01 | −0.22 | 1.30 | 0.03 | 0.87 | ||
| Omnibus_Test | 9.70 | 0.02 | 2.75 | 0.43 | ||||||
| NPIC—Agitation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.28 | 0.06 | ||||||||
| Combined apa/dep | 0.53 | 0.19 | ||||||||
| Depression | 0.33 | 0.10 | ||||||||
| Apathy | 0.44 | 0.07 | ||||||||
| Combined vs. no symptoms | 1.09 | 0.34 | 10.33 | 0.00 | 1.32 | 0.54 | 5.90 | 0.02 | ||
| Depression vs. no symptoms | 0.27 | 0.47 | 0.35 | 0.56 | 0.53 | 0.71 | 0.57 | 0.45 | ||
| Apathy vs. no symptoms | 0.71 | 0.29 | 6.01 | 0.01 | 0.23 | 0.64 | 0.12 | 0.72 | ||
| Combined vs. depression | 0.82 | 0.51 | 2.61 | 0.11 | 0.79 | 0.66 | 1.42 | 0.23 | ||
| Combined vs. apathy | 0.38 | 0.37 | 1.09 | 0.30 | 1.09 | 0.62 | 3.12 | 0.08 | ||
| Depression vs. apathy | −0.43 | 0.47 | 0.86 | 0.35 | 0.31 | 0.74 | 0.17 | 0.68 | ||
| Omnibus_Test | 12.86 | 0.00 | 6.74 | 0.08 | ||||||
| NPIE—Anxiety | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.13 | 0.04 | ||||||||
| Combined apa/dep | 0.46 | 0.25 | ||||||||
| Depression | 0.20 | 0.20 | ||||||||
| Apathy | 0.18 | 0.04 | ||||||||
| Combined vs. no symptoms | 1.72 | 0.38 | 20.65 | 0.00 | 2.11 | 0.62 | 11.58 | 0.00 | ||
| Depression vs. no symptoms | 0.52 | 0.56 | 0.86 | 0.35 | 1.81 | 0.70 | 6.73 | 0.01 | ||
| Apathy vs. no symptoms | 0.37 | 0.38 | 0.93 | 0.34 | 0.07 | 0.86 | 0.01 | 0.94 | ||
| Combined vs. depression | 1.20 | 0.57 | 4.52 | 0.03 | 0.30 | 0.52 | 0.32 | 0.57 | ||
| Combined vs. apathy | 1.35 | 0.42 | 10.58 | 0.00 | 2.04 | 0.76 | 7.28 | 0.01 | ||
| Depression vs. apathy | 0.15 | 0.56 | 0.07 | 0.79 | 1.75 | 0.78 | 5.00 | 0.03 | ||
| Omnibus_Test | 21.70 | 0.00 | 16.04 | 0.00 | ||||||
| NPIF—Elation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.08 | 0.04 | ||||||||
| Combined apa/dep | 0.03 | 0.00 | ||||||||
| Depression | 0.07 | 0.00 | ||||||||
| Apathy | 0.04 | 0.00 | ||||||||
| Combined vs. no symptoms | −0.94 | 0.85 | 1.23 | 0.27 | −10.80 | 0.17 | 3963.30 | 0.00 | ||
| Depression vs. no symptoms | −0.21 | 0.87 | 0.06 | 0.81 | −31.26 | ### | 0.00 | 1.00 | ||
| Apathy vs. no symptoms | −0.74 | 0.62 | 1.40 | 0.24 | −31.26 | ### | 0.00 | 1.00 | ||
| Combined vs. depression | −0.72 | 1.13 | 0.41 | 0.52 | 20.46 | ### | 0.00 | 1.00 | ||
| Combined vs. apathy | −0.20 | 0.99 | 0.04 | 0.84 | 20.46 | ### | 0.00 | 1.00 | ||
| Depression vs. apathy | 0.53 | 0.96 | 0.31 | 0.58 | 0.00 | ### | 0.00 | 1.00 | ||
| Omnibus_Test | 2.37 | 0.50 | 3963.30 | 0.00 | ||||||
| NPIH—Disinhibition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.06 | 0.03 | ||||||||
| Combined apa/dep | 0.15 | 0.06 | ||||||||
| Depression | 0.21 | 0.13 | ||||||||
| Apathy | 0.14 | 0.01 | ||||||||
| Combined vs. no symptoms | 0.96 | 0.53 | 3.36 | 0.07 | 0.61 | 0.83 | 0.54 | 0.46 | ||
| Depression vs. no symptoms | 1.34 | 0.61 | 4.92 | 0.03 | 1.47 | 0.78 | 3.52 | 0.06 | ||
| Apathy vs. no symptoms | 0.86 | 0.47 | 3.32 | 0.07 | −0.83 | 1.24 | 0.44 | 0.51 | ||
| Combined vs. depression | −0.38 | 0.62 | 0.38 | 0.54 | −0.86 | 0.78 | 1.23 | 0.27 | ||
| Combined vs. apathy | 0.10 | 0.51 | 0.04 | 0.84 | 1.43 | 1.28 | 1.26 | 0.26 | ||
| Depression vs. apathy | 0.49 | 0.57 | 0.74 | 0.39 | 2.29 | 1.20 | 3.63 | 0.06 | ||
| Omnibus_Test | 6.27 | 0.10 | 5.64 | 0.13 | ||||||
| NPII—Irritability | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.21 | 0.05 | ||||||||
| Combined apa/dep | 0.40 | 0.21 | ||||||||
| Depression | 0.41 | 0.28 | ||||||||
| Apathy | 0.24 | 0.15 | ||||||||
| Combined vs. no symptoms | 0.91 | 0.35 | 6.56 | 0.01 | 1.54 | 0.57 | 7.33 | 0.01 | ||
| Depression vs. no symptoms | 0.97 | 0.46 | 4.46 | 0.03 | 1.89 | 0.61 | 9.77 | 0.00 | ||
| Apathy vs. no symptoms | 0.19 | 0.33 | 0.34 | 0.56 | 1.14 | 0.58 | 3.89 | 0.05 | ||
| Combined vs. depression | −0.06 | 0.49 | 0.01 | 0.90 | −0.36 | 0.50 | 0.50 | 0.48 | ||
| Combined vs. apathy | 0.72 | 0.39 | 3.30 | 0.07 | 0.40 | 0.50 | 0.63 | 0.43 | ||
| Depression vs. apathy | 0.78 | 0.47 | 2.75 | 0.10 | 0.76 | 0.51 | 2.22 | 0.14 | ||
| Omnibus_Test | 9.10 | 0.03 | 10.78 | 0.01 | ||||||
| NPIJ—AMB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.24 | 0.08 | ||||||||
| Combined apa/dep | 0.32 | 0.25 | ||||||||
| Depression | 0.12 | 0.15 | ||||||||
| Apathy | 0.31 | 0.20 | ||||||||
| Combined vs. no symptoms | 0.41 | 0.36 | 1.32 | 0.25 | 1.39 | 0.49 | 8.05 | 0.00 | ||
| Depression vs. no symptoms | −0.81 | 0.65 | 1.52 | 0.22 | 0.74 | 0.61 | 1.49 | 0.22 | ||
| Apathy vs. no symptoms | 0.34 | 0.31 | 1.27 | 0.26 | 1.10 | 0.49 | 5.02 | 0.03 | ||
| Combined vs. depression | 1.22 | 0.69 | 3.16 | 0.08 | 0.65 | 0.57 | 1.32 | 0.25 | ||
| Combined vs. apathy | 0.07 | 0.39 | 0.03 | 0.86 | 0.30 | 0.46 | 0.41 | 0.52 | ||
| Depression vs. apathy | −1.15 | 0.65 | 3.09 | 0.08 | −0.35 | 0.56 | 0.40 | 0.53 | ||
| Omnibus_Test | 4.68 | 0.20 | 8.89 | 0.03 | ||||||
| NPIK—Sleep and night time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.12 | 0.06 | ||||||||
| Combined apa/dep | 0.30 | 0.27 | ||||||||
| Depression | 0.17 | 0.25 | ||||||||
| Apathy | 0.18 | 0.23 | ||||||||
| Combined vs. no symptoms | 1.09 | 0.40 | 7.38 | 0.01 | 1.84 | 0.55 | 11.32 | 0.00 | ||
| Depression vs. no symptoms | 0.36 | 0.60 | 0.36 | 0.55 | 1.74 | 0.61 | 8.18 | 0.00 | ||
| Apathy vs. no symptoms | 0.41 | 0.39 | 1.13 | 0.29 | 1.63 | 0.54 | 9.07 | 0.00 | ||
| Combined vs. depression | 0.73 | 0.61 | 1.44 | 0.23 | 0.10 | 0.49 | 0.04 | 0.84 | ||
| Combined vs. apathy | 0.68 | 0.43 | 2.47 | 0.12 | 0.21 | 0.44 | 0.22 | 0.64 | ||
| Depression vs. apathy | −0.05 | 0.59 | 0.01 | 0.93 | 0.11 | 0.48 | 0.05 | 0.82 | ||
| Omnibus_Test | 7.43 | 0.06 | 12.68 | 0.01 | ||||||
| NPIL—eating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRN | ADNI | |||||||||
| DISTAL PROB | EST | SE | WALD_STATI∼S | P | DISTAL PROB | EST | SE | WALD_STATI∼S | P | |
| No symptoms | 0.14 | 0.17 | ||||||||
| Combined apa/dep | 0.22 | 0.35 | ||||||||
| Depression | 0.13 | 0.25 | ||||||||
| Apathy | 0.25 | 0.35 | ||||||||
| Combined vs. no symptoms | 0.56 | 0.42 | 1.79 | 0.18 | 0.93 | 0.39 | 5.64 | 0.02 | ||
| Depression vs. no symptoms | −0.06 | 0.64 | 0.01 | 0.93 | 0.46 | 0.47 | 0.96 | 0.33 | ||
| Apathy vs. no symptoms | 0.74 | 0.35 | 4.47 | 0.03 | 0.97 | 0.37 | 6.76 | 0.01 | ||
| Combined vs. depression | 0.62 | 0.68 | 0.83 | 0.36 | 0.47 | 0.48 | 0.95 | 0.33 | ||
| Combined vs. apathy | −0.18 | 0.43 | 0.17 | 0.68 | −0.04 | 0.40 | 0.01 | 0.93 | ||
| Depression vs. apathy | −0.80 | 0.63 | 1.59 | 0.21 | −0.51 | 0.46 | 1.21 | 0.27 | ||
| Omnibus_Test | 5.56 | 0.14 | 8.83 | 0.03 | ||||||
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; CHRN, Care Home Research Network; L‐study, Measuring Long Term Outcomes in People with Dementia in Care Homes; NPI, Neuropsychiatric Inventory; SE, standard error.
Vasconcelos Da Silva M, Melendez‐Torres GJ, Ismail Z, Testad I, Ballard C, Creese B. A data‐driven examination of apathy and depressive symptoms in dementia with independent replication. Alzheimer's Dement. 2023;15:e12398. 10.1002/dad2.12398
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found in the Appendix and at: http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Contributor Information
Miguel Vasconcelos Da Silva, Email: Miguel.1.dasilva@kcl.ac.uk.
for the Alzheimer's Disease Neuroimaging Initiative:
Michael Weiner, Paul Aisen, Ronald Petersen, Clifford R. Jack, Jr., William Jagust, John Q. Trojanowki, Arthur W. Toga, Laurel Beckett, Robert C. Green, Andrew J. Saykin, John Morris, Leslie M. Shaw, Enchi Liu, Tom Montine, Ronald G. Thomas, Michael Donohue, Sarah Walter, Devon Gessert, Tamie Sather, Gus Jiminez, Danielle Harvey, Michael Donohue, Matthew Bernstein, Nick Fox, Paul Thompson, Norbert Schuff, Charles DeCArli, Bret Borowski, Jeff Gunter, Matt Senjem, Prashanthi Vemuri, David Jones, Kejal Kantarci, Chad Ward, Robert A. Koeppe, Norm Foster, Eric M. Reiman, Kewei Chen, Chet Mathis, Susan Landau, Nigel J. Cairns, Erin Householder, Lisa Taylor Reinwald, Virginia Lee, Magdalena Korecka, Michal Figurski, Karen Crawford, Scott Neu, Tatiana M. Foroud, Steven Potkin, Li Shen, Faber Kelley, Sungeun Kim, Kwangsik Nho, Zaven Kachaturian, Richard Frank, Peter J. Snyder, Susan Molchan, Jeffrey Kaye, Joseph Quinn, Betty Lind, Raina Carter, Sara Dolen, Lon S. Schneider, Sonia Pawluczyk, Mauricio Beccera, Liberty Teodoro, Bryan M. Spann, James Brewer, Helen Van der Swag, Adam Fleisher, Judith L. Heidebrink, Joanne L. Lord, Ronald Petersen, Sara S. Mason, Colleen S. Albers, David Knopman, Kris Johnson, Rachelle S. Doody, Javier Villanueva Meyer, Munir Chowdhury, Susan Rountree, Mimi Dang, Yaakov Stern, Lawrence S. Honig, Karen L. Bell, Beau Ances, John C. Morris, Maria Carroll, Sue Leon, Erin Householder, Mark A. Mintun, Stacy Schneider, Angela Oliver, Daniel Marson, Randall Griffith, David Clark, David Geldmacher, John Brockington, Erik Roberson, Hillel Grossman, Effie Mitsis, Leyla deToledoMorrell, Raj C. Shah, Ranjan Duara, Daniel Varon, Maria T. Greig, Peggy Roberts, Marilyn Albert, Chiadi Onyike, Daniel D'Agostino, II, Stephanie Kielb, James E. Galvin, Dana M. Pogorelec, Brittany Cerbone, Christina A. Michel, Henry Rusinek, Mony J. de Leon, Lidia Glodzik, Susan De Santi, P. Murali Doraiswamy, Jeffrey R. Petrella, Terence Z. Wong, Steven E. Arnold, Jason H. Karlawish, David Wolk, Charles D. Smith, Greg Jicha, Peter Hardy, Partha Sinha, Elizabeth Oates, Gary Conrad, Oscar L. Lopez, MaryAnn Oakley, Donna M. Simpson, Anton P. Porsteinsson, Bonnie S. Goldstein, Kim Martin, Kelly M. Makino, M. Saleem Ismail, Connie Brand, Ruth A. Mulnard, Gaby Thai, Catherine Mc Adams Ortiz, Kyle Womack, Dana Mathews, Mary Quiceno, Ramon Diaz Arrastia, Richard King, Myron Weiner, Kristen Martin Cook, Michael DeVous, Allan I. Levey, James J. Lah, Janet S. Cellar, Jeffrey M. Burns, Heather S. Anderson, Russell H. Swerdlow, Liana Apostolova, Kathleen Tingus, Ellen Woo, Daniel H.S. Silverman, Po H. Lu, George Bartzokis, Neill R. Graff Radford, Francine Parfitt, Tracy Kendall, Heather Johnson, Martin R. Farlow, Ann Marie Hake, Brandy R. Matthews, Scott Herring, Cynthia Hunt, Christopher H. van Dyck, Richard E. Carson, Martha G. MacAvoy, Howard Chertkow, Howard Bergman, Chris Hosein, Sandra Black, Bojana Stefanovic, Curtis Caldwell, Ging Yuek Robin Hsiung, Howard Feldman, Benita Mudge, Michele Assaly, Andrew Kertesz, John Rogers, Dick Trost, Charles Bernick, Donna Munic, Diana Kerwin, Marek Marsel Mesulam, Kristine Lipowski, Chuang Kuo Wu, Nancy Johnson, Carl Sadowsky, Walter Martinez, Teresa Villena, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Reisa A. Sperling, Keith A. Johnson, Gad Marshall, Meghan Frey, Jerome Yesavage, Joy L. Taylor, Barton Lane, Allyson Rosen, Jared Tinklenberg, Marwan N. Sabbagh, Christine M. Belden, Sandra A. Jacobson, Sherye A. Sirrel, Neil Kowall, Ronald Killiany, Andrew E. Budson, Alexander Norbash, Patricia Lynn Johnson, Thomas O. Obisesan, Saba Wolday, Joanne Allard, Alan Lerner, Paula Ogrocki, Leon Hudson, Evan Fletcher, Owen Carmichael, John Olichney, Charles DeCarli, Smita Kittur, Michael Borrie, T Y Lee, Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M. Carlsson, Steven G. Potkin, Adrian Preda, Dana Nguyen, Pierre Tariot, Adam Fleisher, Stephanie Reeder, Vernice Bates, Horacio Capote, Michelle Rainka, Douglas W. Scharre, Maria Kataki, Anahita Adeli, Earl A. Zimmerman, Dzintra Celmins, Alice D. Brown, Godfrey D. Pearlson, Karen Blank, Karen Anderson, Robert B. Santulli, Tamar J. Kitzmiller, Eben S. Schwartz, Kaycee M. Sink, Jeff D. Williamson, Pradeep Garg, Franklin Watkins, Brian R. Ott, Henry Querfurth, Geoffrey Tremont, Stephen Salloway, Paul Malloy, Stephen Correia, Howard J. Rosen, Bruce L. Miller, Jacobo Mintzer, Kenneth Spicer, David Bachman, Elizabether Finger, Stephen Pasternak, Irina Rachinsky, John Rogers, Andrew Kertesz, Dick Drost, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Susan K. Schultz, Laura L. Boles Ponto, Hyungsub Shim, Karen Elizabeth Smith, Norman Relkin, Gloria Chaing, Lisa Raudin, Amanda Smith, Kristin Fargher, and Balebail Ashok Raj
REFERENCES
- 1. Tagariello P, Girardi P, Amore M. Depression and apathy in dementia: same syndrome or different constructs? A critical review. Arch Gerontol Geriatr. 2009;49:246‐249. [DOI] [PubMed] [Google Scholar]
- 2. Leung DKY, Chan WC, Spector A, Wong GHY. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36:1330‐1344. [DOI] [PubMed] [Google Scholar]
- 3. Lanctot KL, Amatniek J, Ancoli‐Israel S, et al. Neuropsychiatric signs and symptoms of Alzheimer's disease: new treatment paradigms. Alzheimers Dement (N Y). 2017;3:440‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Reekum R, Stuss DT, L Ostrander. Apathy: why care? J Neuropsychiatry Clin Neurosci. 2005;17:7‐19. [DOI] [PubMed] [Google Scholar]
- 5. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30‐33. [Google Scholar]
- 6. R Marin, S. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243‐254. [DOI] [PubMed] [Google Scholar]
- 7. Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer's disease. Am J Psychiatry. 2001;158:872‐877. [DOI] [PubMed] [Google Scholar]
- 8. Rajkumar AP, Ballard C, Fossey J, et al. Apathy and Its Response to Antipsychotic Review and Nonpharmacological Interventions in People With Dementia Living in Nursing Homes: wHELD, a Factorial Cluster Randomized Controlled Trial. J Am Med Dir Assoc. 2016;17:741‐747. [DOI] [PubMed] [Google Scholar]
- 9. Ishii S, Weintraub N, Mervis JR. Apathy: a common psychiatric syndrome in the elderly. J Am Med Dir Assoc. 2009;10:381‐393. [DOI] [PubMed] [Google Scholar]
- 10. Lanctot KL, Aguera‐Ortiz L, Brodaty H, et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement. 2017;13:84‐100. [DOI] [PubMed] [Google Scholar]
- 11. Benoit M, Berrut G, Doussaint J, et al. Apathy and depression in mild Alzheimer's disease: a cross‐sectional study using diagnostic criteria. J Alzheimers Dis. 2012;31:325‐334. [DOI] [PubMed] [Google Scholar]
- 12. Selbaek G, Engedal K, Bergh S. The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: a systematic review. J Am Med Dir Assoc. 2013;14:161‐169. [DOI] [PubMed] [Google Scholar]
- 13. Miller DS, Robert P, Ereshefsky L, et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 2021;17:1892‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weller BE, Bowen NK, Faubert SJ. Latent Class Analysis: a Guide to Best Practice. Journal of Black Psychology. 2020;46:287‐311. [Google Scholar]
- 15. Cummings J. The Neuropsychiatric Inventory: development and Applications. J Geriatr Psychiatry Neurol. 2020;33:73‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10‐6. [DOI] [PubMed] [Google Scholar]
- 17. Wood S, Cummings JL, Hsu M‐A, et al. The Use of the Neuropsychiatric Inventory in Nursing Home Residents: characterization and Measurement. Am J Geriatr Psychiatry. 2000;8:75‐83. [DOI] [PubMed] [Google Scholar]
- 18. Morris JC, McKeel DW Jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Ann Neurol. 1988;24:17‐22. [DOI] [PubMed] [Google Scholar]
- 19. Lanza ST, Dziak JJ, Huang L, Wagner AT, Collins LM. LCA Stata Plugin Users' Guide Version 1.2.1. University Park: The Methodology Center. Penn State; 2018. [Google Scholar]
- 20. Huang L, Dziak JJ, Wagner AT, Lanza ST. LCA Bootstrap Stata function users’ guide (Version 1.0). University park: the methodology center. Penn State; 2016. [Google Scholar]
- 21. Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: a Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14:535‐569. [Google Scholar]
- 22. Huang L, Dziak JJ, Bray BC, Wagner AT, LCA_Distal_BCH Stata function Users’ Guide (Version 1.1). 2017.
- 23. Bolck A, Croon M, Hagenaars J. Estimating latent structure models with categorical variables: one‐step versus three‐step estimators. Political analysis. 2004;12:3‐27. [Google Scholar]
- 24. Vermunt JK. Latent class modeling with covariates: two improved three‐step approaches. Political analysis. 2010;18:450‐469. [Google Scholar]
- 25. Clarke DE, van Reekum R, Simard M, et al. Apathy in Dementia: clinical and Sociodemographic Correlates. J Neuropsychiatry Clin Neurosci. 2008;20:337‐347. [DOI] [PubMed] [Google Scholar]
- 26. Fischer CE, Ismail Z, Youakim JM, et al. Revisiting Criteria for Psychosis in Alzheimer's Disease and Related Dementias: toward Better Phenotypic Classification and Biomarker Research. J Alzheimers Dis. 2020;73:1143‐1156. [DOI] [PubMed] [Google Scholar]
- 27. Cummings J, Pinto LC, Cruz M, et al. Criteria for Psychosis in Major and Mild Neurocognitive Disorders: international Psychogeriatric Association (IPA) Consensus Clinical and Research Definition. Am J Geriatr Psychiatry. 2020;28:1256‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sweet RA, Bennett DA, Graff‐Radford NR, Mayeux R. Assessment and familial aggregation of psychosis in Alzheimer's disease from the National Institute on Aging Late Onset Alzheimer's Disease Family Study. Brain. 2010;133:1155‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043‐1053. [DOI] [PubMed] [Google Scholar]
- 30. Wilkosz PA, Miyahara S, Lopez OL, Dekosky ST, Sweet RA. Prediction of psychosis onset in Alzheimer disease: the role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. Am J Geriatr Psychiatry. 2006;14:352‐360. [DOI] [PubMed] [Google Scholar]
- 31. Ismail Z, Creese B, Aarsland D, et al. Psychosis in Alzheimer disease — mechanisms, genetics and therapeutic opportunities. Nat Rev Neurol. 2022;18:131‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeMichele‐Sweet MAA, Klei L, Creese B, et al. Genome‐wide association identifies the first risk loci for psychosis in Alzheimer disease. Mol Psychiatry. 2021;26:5797‐5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stringaris A, Zavos H, Leibenluft E, Maughan B, Eley TC. Adolescent irritability: phenotypic associations and genetic links with depressed mood. Am J Psychiatry. 2012;169:47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agüera‐Ortiz L, Babulal GM, Bruneau M‐A, et al. Psychosis as a Treatment Target in Dementia: a Roadmap for Designing Interventions. J Alzheimers Dis. 2022;88(4):1203‐1228. 1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anatchkova M, Brooks A, Swett L, et al. Agitation in patients with dementia: a systematic review of epidemiology and association with severity and course. Int Psychogeriatr. 2019;31:1305‐1318. [DOI] [PubMed] [Google Scholar]
- 36. de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory‐Clinician rating scale (NPI‐C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22:984‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lanctôt KL, Scherer RW, Li A, et al. Measuring Apathy in Alzheimer's Disease in the Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): a Comparison of Instruments. Am J Geriatr Psychiatry. 2021;29:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


