Abstract
Visual display terminal work has increased rapidly in recent years. Loss of visual acuity is an unfortunate associated effect. Here, we performed a randomized, placebo-controlled study in 60 healthy adults. Participants received a diet containing astaxanthin (9 mg/day) or placebo for 6 weeks. Visual acuity, functional visual acuity, and pupil constriction rate were measured before and after visual display terminal work. In participants aged ≥40 years, corrected visual acuity of the dominant eye after visual display terminal work at 6 weeks after intake demonstrated a higher protective effect of astaxanthin in the astaxanthin group vs the control group (p<0.05). In participants aged <40 years, no significant difference was seen between the astaxanthin and control groups. Moreover, no significant difference was found in functional visual acuity and pupil constriction rate between the astaxanthin and control groups. These results suggest astaxanthin reduces oxidative stress caused by visual display terminal work. Age-related reduction in ciliary muscle strength is likely the main detractor of visual acuity. Correspondingly, astaxanthin reduced visual display terminal work-induced visual stress in the middle-aged and elderly. This study was registered in the UMIN-CTR database (UMIN000043089).
Keywords: astaxanthin, visual display terminals, visual acuity, regulatory function, oxidative stress
Introduction
Due to the increase in work on electronic displays, which is known as visual display terminal (VDT) work, cases of visual disturbances are on the rise.(1–3) Subjective visual disturbances with various nonspecific symptoms (eye fatigue, hyperemia, pain, etc.) that are frequently observed in those who conduct prolonged use of VDT have drawn attention as a serious occupational health problem.(4–7) In addition, the widespread adoption of smartphones has contributed to these effects. Accommodation disorder caused by staring at a smartphone screen is now called smartphone presbyopia.(8) Suzuki et al.(9) examined the duration of smartphone usage in university students and found reduced accommodation and an increase in near point distance in participants with longer duration of smartphone usage. Therefore, daily VDT work may reduce visual-function related quality of life (QOL).
Astaxanthin in Haematococcus extract is a carotenoid. Carotenoids can be classified structurally into carotenes (e.g., β carotene) and xanthophylls (e.g., astaxanthin and lutein). The main characteristic of xanthophylls is the presence of terminal hydroxyl groups.(10) In particular, astaxanthin has more hydroxyl groups than other xanthophylls. This characteristic of astaxanthin is thought to be responsible for its excellent antioxidant effects and various profound effects on human health. It has been reported that astaxanthin has various positive effects such as an improvement in visual regulatory function,(11) the prevention of arteriosclerosis,(12) and antioxidant effects.(13–15) Astaxanthin has also been reported to prevent brain aging and improve memory by its antioxidant effects as it can cross the blood-brain barrier.(16,17) In terms of ophthalmology, astaxanthin has been shown to influence levels of oxidation-related parameters in the aqueous humor.(14) This is particularly important when considering the role of oxidative reactions in various eye disorders.(15) Astaxanthin available in the market can be classified into the following two types based on the structure: fatty-acid esterified astaxanthin and free astaxanthin. Astaxanthin extracted from Haematococcus and krill is esterified. Astaxanthin extract derived from the yeast Phaffia rhodozyma and Paracoccus carotinifaciens is free astaxanthin.(18–20) The effects of free astaxanthin (12 mg/day, 8 weeks) on stress and sleep conditions were compared in a group administered astaxanthin derived from Paracoccus carotinifaciens and a placebo group, and the results indicated that ingestion of astaxanthin may improve sleep in people who have a higher tendency for depression.(21) A study of mice receiving food containing a mixture of the same amount of Haematococcus-derived esterified astaxanthin, Phaffia rhodozyma-derived free astaxanthin, or free astaxanthin showed the highest plasma and hepatic concentrations of astaxanthin in the Haematococcus-derived astaxanthin group.(19)
The antioxidant effects of astaxanthin reduce oxidative damage through various mechanisms such as radical scavenging and singlet oxygen quenching (which reduce oxidative stress), reduction of lipid peroxidation, and regulation of gene expression related to oxidative stress.(22,23) In addition, a previous in vitro study showed neuroprotective effects (and possibly antioxidant effects) of astaxanthin on amyloid-β peptide toxicity.(24) Furthermore, previous clinical trials demonstrated improved antioxidant capacity(25) and oxidation status of red blood cells(26) after the intake of astaxanthin. One study in rats showed unique characteristics of astaxanthin such as absorption into the blood and crossing the blood-brain barrier.(27) Furthermore, it has been suggested that astaxanthin prevents various neurological brain disorders that are caused by reactive oxygen species (ROS).(28–30) These findings show that astaxanthin is effective in the prevention of cognitive decline. Furthermore, it has been confirmed that astaxanthin prevents nitric oxide degradation involved in vasodilation.(31) Previous clinical trials showed that astaxanthin improves blood flow(32) and significantly increases retinal capillary blood flow around the optic papilla.(33) It is expected that astaxanthin recovers the function of the ciliary body and improves accommodation, eye fatigue, and shoulder stiffness by improving blood circulation.
Acuity is an important aspect of visual function. Previous studies have reported that diet can improve visual acuity. According to Nakamura et al.,(34) in a trial of healthy individuals aged 40 years and older who received placebo or astaxanthin (2, 4, and 12 mg/day for 28 days), the 4 mg and 12 mg astaxanthin groups showed improved visual acuity at a distance (5 m) after intake of astaxanthin compared with the baseline. In a study by Ishigaki et al.,(35) the effects of 30-day intake of docosahexaenoic acid (DHA) (1,500 mg/day) or placebo on visual acuity in 32 members of a university track and field club and a university fencing club showed that the DHA group had significantly improved visual acuity compared with the baseline. Meanwhile, a study by Yanai et al.,(36) in which 60 healthy adults received either β carotene (microalgae) extracted from Dunaliella bardawil or placebo, the Dunaliella bardawil-derived β carotene group showed significantly improved visual acuity. Furthermore, there are some studies on the prevention of VDT-work related visual impairment. In Kizawa et al.,(37) 44 healthy Japanese individuals received anthocyanin + astaxanthin + lutein, or placebo for 6 weeks. The anthocyanin + astaxanthin + lutein group showed improved visual function after VDT work.
These studies show the positive effects that diet can have on visual acuity. However, to our knowledge, Nakamura et al.’s(34) study is the only one that addresses the positive effects of a diet containing astaxanthin as an active ingredient on visual acuity in humans. Nakamura et al.(34) also demonstrated improved visual acuity compared with baseline in non-VDT workers who received astaxanthin for 28 days. However, their study was not a well-controlled study, and improvement was only observed for uncorrected visual acuity.
Thus, our present double-blind, placebo-controlled trial examined the effects of Haematococcus-derived astaxanthin on visual acuity in healthy Japanese men and women who regularly performed VDT work and reported frequent eye fatigue. The duration of oral administration of astaxanthin was 42 days (6 weeks).
Study Participants and Methods
Study design
This was a randomized, double-blinded, placebo-controlled, parallel study. The assignment rate was 1:1. The trial was performed from January to April 2021. The trial was approved by the Ethics Committee of Miura Clinic, Medical Corporation Kanonkai, prior to its implementation (approval number: R2013). Then, the trial protocol was registered in the UMIN-CTR (UMIN registration number: UMIN000043089). The trial was performed according to the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects after a thorough ethics review. The tests were performed in the Miura Clinic, Medical Corporation Kanonkai (Osaka, Japan).
Participants
The inclusion criteria for this trial were as follows: (1) men and women between 20 years and 64 years of age; (2) Japanese individuals with a high risk of eye fatigue who regularly perform VDT work; (3) those with corrected binocular visual acuity of 1.0; (4) those who will not change contact lenses, glasses, etc. that are for correction during the study; and (5) individuals with the ability to provide informed consent and who provided written informed consent to participate in this trial. Meanwhile, the exclusion criteria were as follows: (a) those with a history of diabetes; liver, renal, gastrointestinal, heart, and respiratory diseases; peripheral vascular disorder; disease that may affect the results of this trial or surgery; (b) those with a history of eye disease or surgery; (c) those receiving ophthalmological treatment and those using ophthalmic drugs; (d) those with ametropia; (e) those with abnormal values of liver and renal function tests; (f) patients on treatment; (g) those with food and drug allergies; (h) anemic individuals; (i) those who take part in active sports and those on diet; (j) those with irregular lifestyle; (k) those who cannot stop taking health foods (including foods for specified health use and foods with functional claims) or designated quasi-drugs; (l) those using medical products (including over-the-counter and prescription drugs) and on continuous treatment; (m) those with excessive alcohol intake; (n) those who cannot abstain from alcohol within the 24 h preceding the trial; (o) pregnant women and women of childbearing potential during the clinical trials; (p) current or planned participation in another clinical trial or participation in another clinical trial within the past 4 weeks at the start of the present clinical trial; and (q) other individuals deemed ineligible by the principal investigator. Participant recruitment was performed by the volunteers in the Medical Corporation Kanonkai. Those who wanted to participate in the trial provided written informed consent. No sponsor or sponsoring company member was among the participants.
Selection, randomization, and blinding
The participant schedule is shown in Table 1. Sixty out of 120 individuals who agreed to participate in this trial were deemed eligible by the principal investigator and were included. The participants were randomly assigned to two groups [test group (n = 30) and placebo group (n = 30)] by stratified block randomization. Sex, age, uncorrected visual acuity, and pupil constriction rate were used as assignment factors at test before screening. Astaxanthin intake was assigned using a computer-generated randomization list by a member of staff in charge of participant allocation who was not directly involved in the trial. Everyone involved in the trial, such as participants, interventionalists, and evaluators, were blinded to the treatment assignment. The assignment table was sealed and stored in a locker by a member of staff in charge of participant allocation until unlocking after the case finalization.
Table 1.
Participant schedule
| Test Period | ||||
|---|---|---|---|---|
| Registration | Allocation | |||
| Scr (Baseline) | Start of intake | 6 weeks | ||
| Registration | ||||
| Obtaining consent | × | |||
| Selection | × | |||
| Intervention | ||||
| Astaxanthin group |

|
|||
| Placebo group |

|
|||
| Evaluation item | ||||
| Background questionnaire | × | |||
| Interview | × | × | ||
| Physical measurements | × | × | ||
| Physiological tests | × | × | ||
| Laboratory tests | × | × | ||
| VDT test | × | × | ||
| Visual function tests | × | × | ||
| Analysis of subjective measures | × | × | ||
| Diary entry |

|
|||
VDT, visual display terminal.
Intervention
The test diet used in this trial was a soft capsule containing astaxanthin (9 mg) in a 180 mg/capsule with Haematococcus pigments (containing astaxanthin) (BGG Japan Co., Ltd., Tokyo, Japan) as the principal component. The placebo soft capsule used in this trial contained safflower oil instead of astaxanthin. The participants received the test diet or placebo diet (one capsule once a day) with water or lukewarm water during or after breakfast. The intervention period was 6 weeks. During the ethics review before the trial, it was confirmed that the test diet and placebo could not be differentiated by color, smell, or flavor.
Endpoints
Test was performed before screening or astaxanthin intake (Scr) and at 6 weeks after intake (6w). During test at Scr and 6w, participants were asked to play a video game (Tetris) as VDT work on a portable gaming device (Nintendo DS) for 60 min. Visual data before and after VDT work during test at Scr and 6w were collected to calculate changes in pre- and post-VDT work. Values for items of vision were calculated for both eyes (average across both eyes), dominant eye, and non-dominant eye. The primary outcome was visual acuity. The secondary outcomes were all data obtained for the evaluation of the pupil constriction rate, functional visual acuity, and subjective measures [visual analogues scale (VAS)], except for the primary outcome. Pupil constriction rate was evaluated using the TriIRIS C9000 (Hamamatsu Photonics K.K., Shizuoka, Japan).
Sixty-second changes in visual acuity were measured using a functional visual acuity tester, Kowa AS-28 (Kowa, Nagoya, Japan), to evaluate functional visual acuity. A Landolt ring equivalent to the previously measured best visual acuity was displayed at the beginning of the test. The size of the subsequent visual target on the screen varied depending on a correct or incorrect response. Specifically, a correct response led to a smaller visual target on the screen, whereas an incorrect response led to a larger visual target. In addition, if the participant was unable to respond within 2 s, it was counted as incorrect. Functional visual acuity was calculated as the mean of 60-s changes in visual acuity.
Statistical analysis
All outcomes in this trial were measured at test before screening and astaxanthin intake (Scr) and at 6 weeks after intake. Measurement data at Scr were used as baseline data. Changes (Δ6w) were calculated as the difference between baseline and measurement values 6 weeks after intake. Group comparisons of participant characteristics (i.e., sex, age, and physical features) were performed using Student’s t test. All decimal visual acuity data were converted into logMAR visual acuity data for statistical analysis. In addition, data averaged across both eyes and data of the dominant eye were evaluated. The actual measurement data of visual acuity (uncorrected and corrected), the pupil constriction rate, and functional visual acuity were analyzed using analysis of variance (ANCOVA) with actual measurement data at week 0 as covariates. VAS assessment was performed using Wilcoxon’s signed rank test.
Two-tailed tests were used for all statistical analyses. P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 27.0 for Windows (IBM Japan, Tokyo, Japan). Since the analysis did not consider multiple items or redundancy due to the repeated sampling, the significance level was not adjusted.
Results
Participants
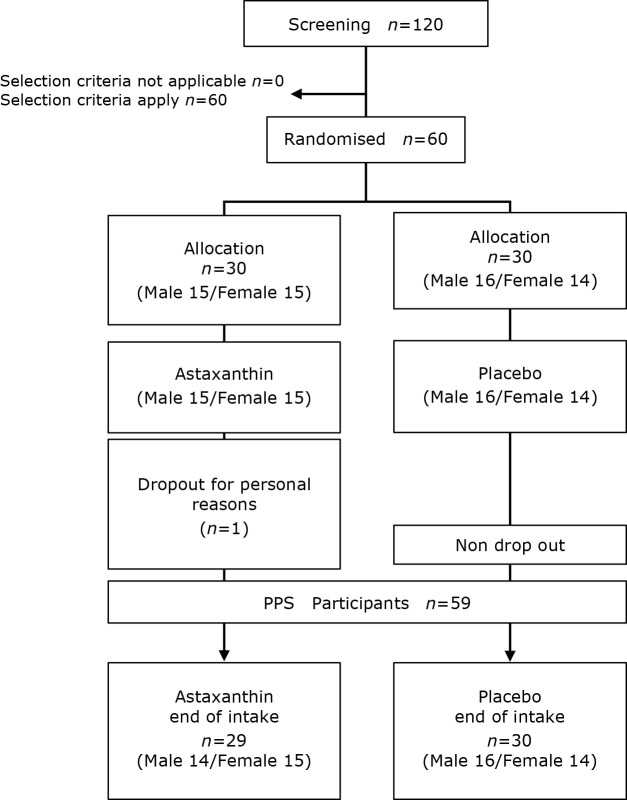
Participant follow-up flow chart is shown in Fig. 1. The implementation period of the trial was between January 25, 2021, and April 30, 2021. All participants were recruited in January 2021. Healthy Japanese men and women with corrected binocular visual acuity of 1.0 and above (without contact lenses) or who can switch to glasses during the study were included. Those with frequent eye fatigue who regularly perform VDT work were excluded from this study. Of 120 individuals who agreed to participate in the trial, 60 individuals met the inclusion criteria and were included in the trial. The participants were assigned to two groups: test diet group (n = 30) and placebo group (n = 30). Before the case conference, one participant withdrew for personal reasons. As a result, 59 participants completed the trial and were included in the analysis. Therefore, the participants ultimately included in the efficacy analysis (per protocol set) consisted of 29 participants in the test diet group (14 men and 15 women) and 30 participants in the placebo group (16 men and 14 women).
Fig. 1.
Participant flow chart.
Characteristics and age distribution of the participants are shown in Table 2. There were no significant between-group differences in any items on background characteristics. Stratified analysis of participants in the trial (<40 years and ≥40 years) was performed.
Table 2.
Participant background
| Astaxanthin group | Placebo group | |
|---|---|---|
| All participants | ||
| n (male/female) | 29 (14/15) | 30 (15/15) |
| Age* | 39.2 ± 11.9 | 39 ± 11.5 |
| ≥40 years | ||
| n (male/female) | 12 (6/6) | 10 (6/4) |
| Age* | 50.3 ± 8.6 | 52 ± 7.0 |
| <40 years | ||
| n (male/female) | 17 (8/9) | 20 (10/10) |
| Age* | 31.4 ± 6.1 | 32.0 ± 5.8 |
*Mean ± SD.
Near-vision visual acuity before and after VDT work
The results of visual acuity are shown in Table 3 and changes in visual acuity (≥40 years; after VDT work; dominant eye, corrected) is shown in Fig. 2. Analysis of those aged 40 years and older showed no significant difference in visual data of the dominant eye (corrected) between the astaxanthin and placebo groups after VDT work at week 0 (−0.100 ± 0.074 vs −0.084 ± 0.072). However, there was a significant difference between the astaxanthin and placebo groups in visual data after VDT work at week 6 (−0.126 ± 0.094 vs −0.038 ± 0.086, p<0.05). In addition, changes in near-vision visual acuity between baseline and 6 weeks after intake (hereafter, Δ week 6) were significantly lower in the test diet group than in the placebo group (p<0.05). In addition, changes in visual data before and after VDT work at week 0 (post-VDT work–pre-VDT work) were significantly higher in the astaxanthin group than in the placebo group. There was a significant difference in near point visual acuity between the two groups at week 0 but not at week 6.
Table 3.
Changes in visual acuity
| group | n | 0 week | 6 weeks | Δ6 weeks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Changes | Before | After | Changes | Before | After | Changes | |||||||
| ≥40 years | Uncorrected eye (Right) |
Astaxanthin group | 12 | Mean | 0.380 | 0.385 | 0.005 | 0.331 | 0.300 | −0.031 | −0.049 | −0.085 | −0.036 | ||
| SD | 0.296 | 0.294 | 0.172 | 0.336 | 0.240 | 0.189 | 0.138 | 0.174 | 0.164 | ||||||
| Placebo group | 10 | Mean | 0.579 | 0.601 | 0.023 | 0.496 | 0.564 | 0.068 | −0.082 | −0.037 | 0.045 | ||||
| SD | 0.344 | 0.339 | 0.090 | 0.337 | 0.373 | 0.161 | 0.113 | 0.131 | 0.126 | ||||||
|
p value (between group) |
0.1689 | 0.1313 | 0.7581 | 0.6294 | 0.2655 | 0.1899 | 0.5438 | 0.4695 | 0.2051 | ||||||
| Uncorrected (Left) |
Astaxanthin group | 12 | Mean | 0.402 | 0.409 | 0.006 | 0.370 | 0.346 | −0.024 | −0.032 | −0.063 | −0.030 | |||
| SD | 0.310 | 0.304 | 0.082 | 0.250 | 0.317 | 0.147 | 0.118 | 0.130 | 0.211 | ||||||
| Placebo group | 10 | Mean | 0.571 | 0.573 | 0.003 | 0.623 | 0.572 | −0.050 | 0.052 | −0.001 | −0.053 | ||||
| SD | 0.337 | 0.339 | 0.040 | 0.343 | 0.318 | 0.184 | 0.181 | 0.115 | 0.209 | ||||||
|
p value (between group) |
0.2411 | 0.2495 | 0.8905 | 0.0784† | 0.1845 | 0.5985 | 0.2265 | 0.2526 | 0.8048 | ||||||
| Uncorrected (Average) |
Astaxanthin group | 12 | Mean | 0.391 | 0.397 | 0.006 | 0.351 | 0.323 | −0.028 | −0.041 | −0.074 | −0.033 | |||
| SD | 0.285 | 0.287 | 0.089 | 0.267 | 0.264 | 0.129 | 0.100 | 0.126 | 0.150 | ||||||
| Placebo group | 10 | Mean | 0.575 | 0.587 | 0.013 | 0.560 | 0.568 | 0.009 | −0.015 | −0.019 | −0.004 | ||||
| SD | 0.327 | 0.320 | 0.043 | 0.330 | 0.339 | 0.154 | 0.133 | 0.065 | 0.144 | ||||||
|
p value (between group) |
0.1814 | 0.1618 | 0.8100 | 0.4125 | 0.1659 | 0.5847 | 0.6244 | 0.2065 | 0.6460 | ||||||
| Corrected (Right) |
Astaxanthin group | 12 | Mean | −0.146 | −0.106 | 0.040 | −0.148 | −0.119 | 0.029 | −0.002 | −0.013 | −0.010 | |||
| SD | 0.099 | 0.068 | 0.060 | 0.084 | 0.115 | 0.111 | 0.114 | 0.097 | 0.143 | ||||||
| Placebo group | 10 | Mean | −0.088 | −0.075 | 0.013 | −0.077 | −0.057 | 0.019 | 0.011 | 0.018 | 0.006 | ||||
| SD | 0.074 | 0.064 | 0.042 | 0.077 | 0.084 | 0.055 | 0.060 | 0.085 | 0.063 | ||||||
|
p value (between group) |
0.1299 | 0.2778 | 0.2355 | 0.1592 | 0.3496 | 0.6086 | 0.7239 | 0.4448 | 0.7204 | ||||||
| Corrected (Left) |
Astaxanthin group | 12 | Mean | −0.127 | −0.065 | 0.062 | −0.118 | −0.109 | 0.010 | 0.009 | −0.044 | −0.053 | |||
| SD | 0.101 | 0.098 | 0.114 | 0.094 | 0.111 | 0.112 | 0.052 | 0.093 | 0.123 | ||||||
| Placebo group | 10 | Mean | −0.092 | −0.083 | 0.010 | −0.115 | −0.087 | 0.027 | −0.022 | −0.005 | 0.018 | ||||
| SD | 0.066 | 0.059 | 0.031 | 0.121 | 0.111 | 0.084 | 0.098 | 0.085 | 0.061 | ||||||
|
p value (between group) |
0.3429 | 0.6102 | 0.1506 | 0.4424 | 0.3684 | 0.3023 | 0.3845 | 0.3152 | 0.1014 | ||||||
| Corrected (Average) |
Astaxanthin group | 12 | Mean | −0.137 | −0.086 | 0.051 | −0.133 | −0.114 | 0.019 | 0.003 | −0.028 | −0.031 | |||
| SD | 0.091 | 0.076 | 0.061 | 0.060 | 0.104 | 0.081 | 0.059 | 0.075 | 0.093 | ||||||
| Placebo group | 10 | Mean | −0.090 | −0.079 | 0.011 | −0.096 | −0.072 | 0.023 | −0.005 | 0.007 | 0.012 | ||||
| SD | 0.066 | 0.053 | 0.025 | 0.086 | 0.067 | 0.058 | 0.058 | 0.039 | 0.051 | ||||||
|
p value (between group) |
0.1798 | 0.8023 | 0.0566† | 0.7468 | 0.2125 | 0.6257 | 0.7295 | 0.1810 | 0.1832 | ||||||
| Dominant eye (uncorrected) |
Astaxanthin group | 12 | Mean | 0.409 | 0.395 | −0.014 | 0.387 | 0.326 | −0.060 | −0.023 | −0.069 | −0.046 | |||
| SD | 0.305 | 0.321 | 0.164 | 0.334 | 0.278 | 0.166 | 0.121 | 0.174 | 0.159 | ||||||
| Placebo group | 10 | Mean | 0.559 | 0.559 | −0.001 | 0.535 | 0.529 | −0.006 | −0.024 | −0.029 | −0.005 | ||||
| SD | 0.372 | 0.387 | 0.026 | 0.405 | 0.374 | 0.139 | 0.180 | 0.140 | 0.154 | ||||||
|
p value (between group) |
0.3224 | 0.3025 | 0.7844 | 0.9954 | 0.3135 | 0.4559 | 0.9840 | 0.5615 | 0.5484 | ||||||
| Dominant eye (corrected) |
Astaxanthin group | 12 | Mean | −0.146 | −0.100 | 0.046 | −0.148 | −0.126 | 0.023 | −0.002 | −0.026 | −0.024 | |||
| SD | 0.099 | 0.074 | 0.059 | 0.084 | 0.094 | 0.103 | 0.114 | 0.076 | 0.130 | ||||||
| Placebo group | 10 | Mean | −0.088 | −0.084 | 0.003 | −0.080 | −0.038 | 0.042 | 0.008 | 0.047 | 0.039 | ||||
| SD | 0.074 | 0.072 | 0.030 | 0.079 | 0.086 | 0.046 | 0.056 | 0.064 | 0.045 | ||||||
|
p value (between group) |
0.1299 | 0.6310 | 0.0429* | 0.1996 | 0.0233* | 0.8074 | 0.7870 | 0.0251* | 0.1428 | ||||||
Mean ± SD (*p<0.05, †p<0.10).
Fig. 2.
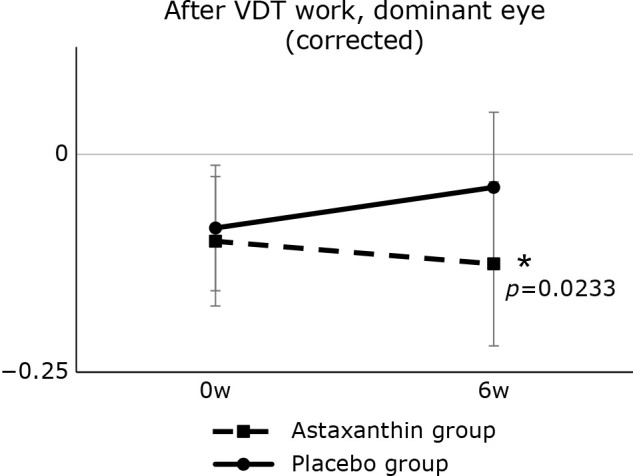
Changes in visual acuity (≥40 years) after VDT work, dominant eye (corrected). Data show the actual measured values. *p<0.05 vs placebo group.
There was no significant difference in near-point visual acuity of uncorrected (right), uncorrected (mean), corrected (right), corrected (left), and the dominant eye (uncorrected) between the two groups. In addition, in those under 40 years of age, there was no significant difference in any measurement of near-point visual acuity between the two groups.
Pupil constriction rate
In those aged 40 years and older, there was no significant changes in pupil constriction rate. In those under 40 years of age, changes (after VDT work–before VDT work) in the mean pupil constriction rate of the dominant eye at week 0 were higher in the astaxanthin group than in the placebo group, but this was not significant (0.010 ± 0.055 vs −0.020 ± 0.042, p = 0.007). Whereas those at Δ week 6 (after VDT work–before VDT work) were significantly lower in the astaxanthin group than in the placebo group (0.023 ± 0.085 vs 0.033 ± 0.069, p<0.05).
Functional visual acuity
In those aged 40 years and older, the visual acuity of the dominant eye before VDT work was 0.855 ± 0.377 and 0.882 ± 0.304 at week 0 and 0.964 ± 0.289 and 0.952 ± 0.228 at week 6 in the astaxanthin and placebo groups, respectively. The visual acuity averaged across both eyes before VDT work was 0.879 ± 0.322 and 0.897 ± 0.268 at week 0 and 0.963 ± 0.265 and 0.987 ± 0.175 at week 6 in the astaxanthin and placebo groups, respectively. In those aged 40 years and older, the visual acuity of the dominant eye after VDT work was 0.836 ± 0.344 and 0.908 ± 0.315 at week 0 and 0.855 ± 0.269 and 0.884 ± 0.293 at week 6 in the astaxanthin and placebo groups, respectively. The visual acuity averaged across both eyes after VDT work was 0.846 ± 0.315 and 0.924 ± 0.293 at week 0 and 0.835 ± 0.207 and 0.940 ± 0.220 at week 6 in the astaxanthin and placebo groups, respectively. There was no significant difference between the two groups in any of the above parameters.
Subjective evaluation using the VAS and functional visual acuity
There was no significant difference in any measurement between those aged 40 years and older and those under 40 years of age (data not shown).
Results of laboratory tests
The results of laboratory tests are shown in Table 4. Although some items showed significant differences in changes in visual acuity between the astaxanthin and placebo groups, all were within the range of physiological changes, and there were no safety problems with the use of astaxanthin.
Table 4.
Changes in laboratory test results
| Items | 0 week | 6 weeks | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Astaxanthin | Placebo | Astaxanthin | Placebo | Astaxanthin | Placebo | |||
| Total bilirubin (mg/dl) | 0.83 | 0.97 | 0.84 | 0.98 | 0.8131 | 0.8175 | ||
| AST (GOT) (U/L) | 18.83 | 20.67 | 19.03 | 19.27 | 0.679 | 0.0263 | ||
| ALT (GPT) (U/L) | 15.76 | 20.67 | 14.72 | 17.30 | 0.2307 | 0.0053 | ||
| γ-GT (U/L) | 18.14 | 30.40 | 17.62 | 27.67 | 0.3935 | 0.151 | ||
| CK (U/L) | 89.86 | 110.20 | 96.86 | 116.83 | 0.051 | 0.4518 | ||
| Total protein (g/dl) | 7.27 | 7.28 | 7.17 | 7.19 | 0.0711 | 0.1109 | ||
| Creatinine (mg/dl) | 0.74 | 0.75 | 0.73 | 0.75 | 0.5212 | 0.9428 | ||
| Urea nitrogen (mg/dl) | 12.88 | 12.54 | 12.84 | 12.50 | 0.9555 | 0.9453 | ||
| Uric acid (mg/dl) | 4.94 | 5.16 | 4.78 | 5.37 | 0.1714 | 0.2011 | ||
| Total cholesterol (mg/dl) | 209.45 | 215.50 | 207.66 | 207.80 | 0.5353 | 0.0412 | ||
| TG (triglycerides) (mg/dl) | 88.90 | 89.53 | 77.72 | 94.03 | 0.0832 | 0.6179 | ||
| Sodium (mEq/L) | 140.90 | 140.97 | 140.93 | 140.53 | 0.9168 | 0.1411 | ||
| Potassium (mEq/L) | 4.16 | 4.28 | 4.11 | 4.18 | 0.3598 | 0.1042 | ||
| Chloride (mEq/L) | 102.90 | 102.53 | 104.14 | 102.80 | 0.0062 | 0.4185 | ||
| Calcium (mg/dl) | 9.47 | 9.49 | 9.30 | 9.36 | 0.0028 | 0.0073 | ||
| Magnesium (mg/dl) | 2.13 | 2.08 | 2.13 | 2.10 | 0.7452 | 0.5142 | ||
| Serum iron (μg/dl) | 100.41 | 116.33 | 105.66 | 119.27 | 0.6112 | 0.7754 | ||
| Serum amylase (U/L) | 85.52 | 77.07 | 83.48 | 75.53 | 0.3619 | 0.3803 | ||
| HDL cholesterol (mg/dl) | 66.48 | 68.97 | 69.28 | 67.80 | 0.021 | 0.4153 | ||
| ALB: modified BCP method (g/dl) | 4.56 | 4.61 | 4.49 | 4.53 | 0.0392 | 0.0671 | ||
| White blood cell count (/μl) | 5,496.55 | 5,666.67 | 5,662.07 | 5,610.00 | 0.3973 | 0.8166 | ||
| Red blood cell count (×104/μl) | 464.34 | 469.60 | 456.14 | 467.87 | 0.0338 | 0.7059 | ||
| Hemoglobin (g/dl) | 14.02 | 14.30 | 13.61 | 14.08 | 0.0011 | 0.1156 | ||
| Hematocrit (%) | 43.62 | 44.12 | 43.07 | 44.14 | 0.1155 | 0.9708 | ||
| MCV (fl) | 94.00 | 94.10 | 94.48 | 94.43 | 0.1094 | 0.3255 | ||
| MCH (pg) | 30.17 | 30.45 | 29.81 | 30.11 | 0.0026 | 0.0052 | ||
| MCHC (%) | 32.08 | 32.37 | 31.55 | 31.87 | 0.0008 | 0.0006 | ||
| Patelet count (×104/μl) | 26.68 | 28.25 | 26.22 | 27.13 | 0.284 | 0.0346 | ||
| Glucose (mg/dl) | 85.10 | 87.53 | 85.17 | 87.50 | 0.9622 | 0.9733 | ||
| LDL cholesterol (mg/dl) | 120.52 | 124.37 | 120.14 | 119.23 | 0.8715 | 0.0511 | ||
| HbA1c/NGSP (%) | 5.22 | 5.28 | — | — | — | — | ||
| ALP (U/L) | 60.66 | 62.40 | 63.21 | 64.63 | 0.0026 | 0.0324 | ||
| LD (LDH) (U/L) | 174.69 | 178.53 | 175.79 | 176.17 | 0.5808 | 0.4304 | ||
γ-GT, gamma-glutamyltransferase; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCP, bromocresol purple; CK, creatine kinase; GOT, glutamic-oxaloacetic transaminase; GPT, glutamic pyruvate transaminase; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; LD (LDH), lactate dehydrogenase; LDL, low density lipoprotein; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NGSP, National Glycohemoglobin Standardization Program; TG, triglycerides. Each number in the 0-week and 6-week groups represents the mean.
Discussion
This was a randomized, placebo-controlled, parallel study of the effects of 6-week astaxanthin intake on visual acuity and function in 60 healthy individuals between 20 and 64 years of age. One participant withdrew for personal reasons. As a result, 59 participants were included in the analysis. Analysis of all participants showed no significant difference in any measurement between the astaxanthin and placebo groups. However, in individuals aged 40 years and older, the actual measurement value and changes in visual acuity of the dominant eye (corrected) after VDT work at 6 weeks after intake showed significant difference between the astaxanthin and placebo groups. VDT work can cause visual burden. In other words, comparison of visual acuity between pre- and post-VDT work at 6 weeks after astaxanthin intake showed lower visual acuity after VDT work compared with that before VDT work in both groups. However, the results showed that astaxanthin prevented the reduction in VDT-work related visual acuity. Nagaki et al.(33) reported that astaxanthin increased peripapillary blood flow, suggesting that astaxanthin prevents reduction in visual acuity by maintaining blood flow. In addition, other studies of the effects of astaxanthin on visual acuity showed significantly improved visual acuity of the dominant eye (corrected). A study reported a higher rate of right eye dominance in the Japanese population (overall, 70–75%; men, 70.73%; and women, 75%).(38) In fact, the rate of right eye dominance in the present trial was 71.9%. One study of the Japanese general population reported reduced visual acuity (about 0.09 to 0.12) after VDT work. In addition, smaller changes in uncorrected visual acuity may be responsible for the significant difference in corrected but not in uncorrected visual acuity. Furthermore, VDT work requires not only constant near-point accommodation but also looking into the distance in the course of maintaining a safe awareness of one’s surroundings. Therefore, most VDT workers use a device for hyperopia correction when looking into the distance and a device for myopia correction when viewing a near target, leading to a high tension of the ciliary muscles. Tension of the ciliary muscles may lead to reduced accommodation ability, resulting in asthenopia and reduced visual acuity.(39,40) Astaxanthin has been reported to have antioxidant effects(14,15,27) and has been shown to cross the blood-brain barrier,(27) suggesting its involvement in the mechanism. In a clinical study, 16 male patients (mean age, 71.3 years) and 19 female patients (mean age, 70.6 years) who underwent bilateral cataract surgery received oral Haematococcus-derived astaxanthin (6 mg/day) for 2 weeks; astaxanthin affected O2•− scavenging activity in the aqueous humor of the female patients, suggesting that it may be involved in changes of vascular endothelial growth factor (VEGF) levels in the anterior eye.(14) Hashimoto et al. reported that the total hydroperoxide level was similar in younger patients (<70 years old) and older patients (≥70 years old) before taking astaxanthin but decreased significantly in younger patients after intake.(15) In one experiment performed on rats, astaxanthin was detected in the hippocampus and cerebral cortex after a single dose.(27) This suggests that the antioxidant capacity of astaxanthin reduces brain ROS from stress induced by VDT work, leading to reduced blood ROS, resulting in reduced tension of the ciliary muscle. The gradual progression of hyperopia, which is known as presbyopia, begins in the 40s. This is due to age-related reduction in the regulation of ciliary muscle. Prolonged near work, such as VDT work, is a significant burden on vision (e.g., focusing ability). This may explain the positive effects of astaxanthin as shown in the stratified analysis of participants aged 40 years and older.
The stratified analysis of changes in the pupil constriction rate averaged across both eyes and those of the dominant eye before and after VDT work at 6 weeks after intake showed a significant difference between the astaxanthin and placebo groups in participants under 40 years of age but not in those in their 40s and older. Unlike other trials with the pupil constriction rate as the primary outcome, the present trial used visual acuity as the primary outcome. This may explain the nonsignificant results of the pupil constriction rate in our participants aged 40 years and older. Some studies showed significantly improved pupil constriction rate after the intake of astaxanthin, whereas the present results for pupil constriction rate in participants aged 40 years and older were not significant. This may be due to the differences in participant backgrounds.
In summary, the stratified analysis showed positive effects of astaxanthin intake for 6 weeks or more on visual acuity loss in participants aged 40 years or older. No study has examined the effects of astaxanthin on visual acuity. However, for example, Kizawa et al.(37) reported that a mixed diet containing anthocyanin derived from bilberry (72 mg/day), lutein (10 mg/day), and astaxanthin (6 mg/day) improves visual acuity after VDT work. As was found by Kizawa et al.,(37) age-stratified analysis in this trial showed that dietary functional components could prevent loss of visual acuity. In addition, the dose of astaxanthin (9 mg/day) used in the present trial was equivalent to that used in a study by Nakamura et al.(34) which compared visual acuity between pre-and post-intake of astaxanthin alone (4 mg or 12 mg/day) to show the significant positive effect. In Nakamura et al.,(34) participants were not asked to perform VDT work. In the present study, the period of intake was 6 weeks as compared with that of 28 days in Nakamura et al.’s study.(34)
Our study has several limitations. Since a questionnaire survey of genetic and environmental factors in subjects with low uncorrected visual acuity was not included in this study, it was not possible to examine factors that improved visual acuity except for the pupil constriction rate. Furthermore, because tests for presbyopia and astigmatism in these subjects were not performed in this study, it was not possible to evaluate the effects of presbyopia and astigmatism on visual acuity after VDT work. Therefore, the mechanisms of the effects of astaxanthin on the prevention of a temporary loss of visual acuity are unknown. Future studies should examine the issue.
The present findings showed that the intake of astaxanthin (9 mg/day) for 6 weeks could prevent VDT work-related visual acuity loss. In addition, the nonsignificant relationship between corrected visual acuity and the pupil constriction rate revealed the absence of the direct effects of improved pupil constriction rate on improvement in visual acuity.
Author Contributions
TS, YK, and YL designed the trial. NM designed and conducted the trial. TS and YK wrote the manuscript. NM was responsible for the interpretation of the results of the trial. All authors reviewed and accepted the content of the final manuscript.
Acknowledgments
We would like to express our sincere gratitude to the participants and measurement staff who contributed to this trial.
Abbreviations
- DHA
docosahexaenoic acid
- QOL
quality of life
- ROS
reactive oxygen species
- VAS
visual analogue scale
- VDT
visual display terminal
Conflict of Interest
This trial was sponsored by BGG Japan Co., Ltd., which provided all costs for the implementation of the trial. TS and YK are employees of BGG Japan Co., Ltd. YL is an employee of Beijing Gingko-Group Biological Technology Co., Ltd., which belongs to the same company group as BGG Japan Co., Ltd. NM received funding from BGG Japan Co., Ltd. to provide medical services to the trial participants.
References
- 1.Tyrrell RA, Leibowitz HW. The relation of vergence effort to reports of visual fatigue following prolonged near work. Hum Factors 1990; 32: 341–357. [DOI] [PubMed] [Google Scholar]
- 2.Murata K, Araki S, Yokoyama K, Yamashita K, Okumatsu T, Sakou S. Accumulation of VDT work-related visual fatigue assessed by visual evoked potential, near-point distance and critical flicker fusion. Ind Health 1996; 34: 61–69. [DOI] [PubMed] [Google Scholar]
- 3.Notice of the Director-General of the Labour Standards Bureau. Occupational health guidelines for VDT work. Jpn J Ophthalmol 1986; 57: 379–384. (in Japanese) [Google Scholar]
- 4.Matula RA. Effects of visual display units on the eyes: a bibliography (1972–1980). Hum Factors 1981; 23: 581–586. [DOI] [PubMed] [Google Scholar]
- 5.Knave BG, Wibom RI, Voss M, Hedström LD, Bergqvist UO. Work with video display terminals among office employees. I. Subjective symptoms and discomfort. Scand J Work Environ Health 1985; 11: 457–466. [DOI] [PubMed] [Google Scholar]
- 6.Word Health Organization. Visual Display Terminals and Workers’ Health (offset publication 99). Geneva: World Health Organization, 1987. [PubMed] [Google Scholar]
- 7.Bergqvist U. Possible health effects of working with VDUs. Br J Ind Med 1989; 46: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onozato N, Hara N. The role of near response in modern society. Neuro-Ophthalmology Japan 2019; 36: 397–403. (in Japanese) [Google Scholar]
- 9.Suzuki Y, Maeda F, Tatara S. Does mobile device usage reduce visual acuity in the elderly? Niigata J Health & Welfare 2019; 19: 67. (in Japanese) [Google Scholar]
- 10.Capelli B, Bagchi D, Cysewski GR. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013; 12: 145–152. [Google Scholar]
- 11.Nagaki Y, Hayasaka S, Yamada T, Hayasaka Y, Sanada M, Uonomi T. Effects of astaxanthin on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J Trad Med 2002; 19: 170–173. [Google Scholar]
- 12.Ryu SK, King TJ, Fujioka K, Pattison J, Pashkow FJ, Tsimikas S. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in LDLR(−/−) and ApoE(−/−) mice. Atherosclerosis 2012; 222: 99–105. [DOI] [PubMed] [Google Scholar]
- 13.Choi HD, Kim JH, Chang MJ, Kyu-Youn Y, Shin WG. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res 2011; 25: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto H, Arai K, Takahashi J, Chikuda M. Effects of astaxanthin on VEGF level and antioxidation in human aqueous humor: difference by sex. J Clin Biochem Nutr 2019; 65: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto H, Arai K, Takahashi J, Chikuda M. The effect of aging on the antioxidative activity of astaxanthin in human aqueous humor. J Clin Biochem Nutr 2021; 68: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekikawa T, Kizawa Y, Li Y, Takara T. Cognitive function improvement with astaxanthin intake: a randomized, double-blind, placebo-controlled study. Pharmacometrics 2019; 97: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekikawa T, Kizawa Y, Li Y, Takara T. Cognitive function improvement with astaxanthin and tocotrienol intake: a randomized, double-blind, placebo-controlled study. J Clin Biochem Nutr 2020; 67: 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K, Sugimoto N, Yamada T, Maitani T. Studies on optical isomerism of astaxanthin in natural food colors and principal pigment in phaffia color. Food Hyg Saf Sci (Shokuhin Eiseigaku Zasshi) 2000; 41: 44–47. (in Japanese) [Google Scholar]
- 19.Aoi W, Maoka T, Abe R, Fujishita M, Tominaga K. Comparison of the effect of non-esterified and esterified astaxanthins on endurance performance in mice. J Clin Biochem Nutr 2018; 62: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi M, Ishibashi T, Maoka T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on cognitive function in middle-aged and older individuals. J Clin Biochem Nutr 2018; 62: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi M, Kawamura M, Kawashima Y, Uemura T, Maoka T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on the status of stress and sleep in adults. J Clin Biochem Nutr 2020; 66: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galasso C, Corinaldesi C, Sansone C. Carotenoids from marine organisms: biological functions and industrial applications. Antioxidants (Basel) 2017; 6: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dose J, Matsugo S, Yokokawa H, et al. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int J Mol Sci 2016; 17: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CH, Chen CY, Chiou JY, Peng RY, Peng CH. Astaxanthine secured apoptotic death of PC12 cells induced by beta-amyloid peptide 25-35: its molecular action targets. J Med Food 2010; 13: 548–556. [DOI] [PubMed] [Google Scholar]
- 25.Iwabayashi M, Fujioka N, Nomoto K, et al. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti-aging Medicine 2009; 6: 15–21. [Google Scholar]
- 26.Nakagawa K, Kiko T, Miyazawa T, et al. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr 2011; 105: 1563–1571. [DOI] [PubMed] [Google Scholar]
- 27.Tso MOM, Lam TK. Method of retarding and ameliorating central nervous system and eye damage. U.S. Patent 5527533; 1996. [Google Scholar]
- 28.Wu H, Niu H, Shao A, et al. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar Drugs 2015; 13: 5750–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 2017; 39: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galasso C, Orefice I, Pellone P, et al. On the neuroprotective role of astaxanthin: new perspectives? Mar Drugs 2018; 16: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussein G, Nakamura M, Zhao Q, et al. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull 2005; 28: 47–52. [DOI] [PubMed] [Google Scholar]
- 32.Miyawaki H, Takahashi J, Tsukahara H, Takehara I. Effects of astaxanthin on human blood rheology. J Clin Biochem Nutr 2008; 43: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagaki Y, Mihara M, Takahashi J, et al. The effect of astaxanthin on retinal capillary blood flow in normal volunteers. J Clin Therap Med 2005; 21: 537–542. (in Japanese) [Google Scholar]
- 34.Nakamura A, Isobe R, Otaka Y, et al. Changes in visual function following peroral astaxanthin. Jpn J Clin Ophthalmol 2004; 58: 1051–1054. (in Japanese) [Google Scholar]
- 35.Ishigaki H, Mashimo I, Morishige U, Shudo N. The effect of one month intake of DHA on visual functions of the athlete. Bull Aichi Inst Technol 2006; 41-B: 185–188. (in Japanese) [Google Scholar]
- 36.Yanai K, Ehara T, Sakaguchi E, Osami S. Effects of microalgae Dunaliella bardawil-derived β-carotene on visual function in Japanese: a randomized, double-blinded, placebo-controlled study. Jpn Pharmacol Ther 2018; 46: 1579–1590. (in Japanese) [Google Scholar]
- 37.Kizawa Y, Sekikawa T, Kageyama M, Tomobe H, Kobashi R, Yamada T. Effects of anthocyanin, astaxanthin, and lutein on eye functions: a randomized, double-blind, placebo-controlled study. J Clin Biochem Nutr 2021; 69: 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda T. A study of dominant hand (dominant leg, eye, and ear). Kyushu Neuropsychiatry 1983; 29: 242–249. (in Japanese) [Google Scholar]
- 39.Namba T, Fukahori A, Morikawa A, et al. Reduction of natural vision control function by visual display terminal (VDT) work and recovery effect of circumocular thermotherapy. Jpn Pharmacol Ther 2008; 17: 363–371. (in Japanese) [Google Scholar]
- 40.Katsuda T, Kamoshita I, Kubo A. A trial of the effect of a bilberry extract supplemented diet on visual function: A randomized, double-blinded, placebo-controlled, parallel study. Jpn Pharmacol Ther 2018; 46: 1013–1021. (in Japanese) [Google Scholar]