Abstract
Antioxidants are useful for the treatment of oxidative stress mediated liver damage. A naturally occurring antioxidant γ-oryzanol is rapidly hydrolyzed to its active hydrophobic metabolite, ferulic acid, inside the body. Limitations associated with the hydrophobicity of ferulic acid can be overcome by encapsulating in a liposomal formulation. As intravenously administered nanoparticles (including liposomes) can effectively reach the liver, such systems may be suitable drug delivery carriers to treat liver injury. In this study, we prepared a liposomal formulation of ferulic acid (ferulic-lipo) and examined its effects on liver damage induced by CCl4. Ferulic-lipo were ~100 nm in size and drug encapsulation efficiency was about 92%. Ferulic-lipo showed potent scavenging efficacy against hydroxyl radical compared to α-tocopherol liposomes. Ferulic-lipo significantly prevented CCl4-mediated cytotoxicity in human hepatocarcinoma cells. Furthermore, intravenous administration of ferulic-lipo significantly reduced alanine aminotransferase and aspartate amino transferase levels in a rat model of liver injury. CCl4-mediated reactive oxygen species generation in liver was also reduced by intravenous administration of ferulic-lipo. Hepatoprotective effects of ferulic-lipo were demonstrated by histological observation of CCl4-induced liver tissue damage. Therefore, ferulic-lipo exhibit potent antioxidative capacity and were suggested to be an effective formulation for prevention of oxidative damage of liver tissue.
Keywords: ferulic acid, liver toxicity, CCl4, liposome, oxidative stress
Introduction
Liver diseases are the 10th most common cause of death worldwide, with 2 million individuals dying from liver diseases each year.(1) As the liver plays a center role in metabolism, it is the major target of toxicity of various drugs, xenobiotics, and oxidative stress.(2) Oxidative stress is known to be one of the causative agents responsible for liver injury.(3) Reactive oxygen species (ROS) generated by the metabolism of lipids, carbohydrates, proteins, various drugs, and pesticides induce necrosis and apoptosis in the liver by disrupting cellular macromolecules. Although some remarkable drug developments have occurred in the field of medical science in recent years, there remains an unmet need for a dependable synthetic drug for the treatment of the liver injury. Antioxidants are widely used to prevent various diseases mediated by oxidative stress. Dry eye diseases induced by oxidative stress are found to be ameliorated with the treatment of the liposomal formulation containing antioxidant astaxanthin.(4) Antioxidative treatments also seemed to be effective in preventing liver diseases mediated by oxidative stress.(5,6) Vitamin E has previously shown as promising source for the treatment of different liver diseases.(7) In addition, other antioxidants such as vitamin C, N-acetyl cysteine and silymarin are known to act as potent hepatoprotectives agents.(3)
Similarly, γ-oryzanol exhibits antioxidative properties, and is one of the most common natural compounds used for the treatment of various diseases. γ-Oryzanol is insoluble in water and consists of ferulic acid esters of triterpene alcohols. While γ-oryzanol acts as a potent antioxidative therapeutic agent, it has a number of limitations. Specifically, γ-oryzanol is rapidly metabolized inside the body after consumption, which limits its clinical application.(8) Herein, we instead focused our attention on ferulic acid (4-hydroxy-3-methoxy cinnamic acid), which is an active metabolite of γ-oryzanol. Ferulic acid exhibits various therapeutic effects against a number of different diseases, including cancer, diabetes, bacterial infection, hepatic injury, and cardiovascular and neurodegenerative disorders.(9) The broad range of preventive effects associated with ferulic acid are attributed to its potent antioxidative properties. The hydroxyl group attached to phenyl ring of ferulic acid is mainly responsible for its antioxidative properties by donating an electron to scavenge free radicals.(10,11) Ferulic acid is a methoxylated derivative of cinnamic acid that is widely used in the pharmaceutical, food and cosmetics industries because of its low toxicity. Moreover, compounds that exhibit an -OCH3 group in their structure (e.g., ferulic acid) also have a direct effect on the activity of hepatic antioxidant enzymes (e.g., superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and paraoxonase), maintenance of oxidative stress conditions, and inhibition of inflammation leading to apoptosis of liver cells.(12,13) Thus, ferulic acid is expected to be a potent antioxidant and reliable hepatoprotective agent to combat oxidative stress mediated liver injury.
Solubility is of great importance for drug discovery and formulation development.(14) The potential therapeutic applications of ferulic acid are limited by its poor solubility and stability.(15) Encapsulation of active therapeutics into proper formulations (such as liposomes, micelles, dendrimers, and metal-based nanoparticles) is a suitable approach to overcome stability and solubility problems.(16,17) In particular, liposomal formulations are useful for encapsulating hydrophobic drugs into the lipid bilayer to improve stability and solubility of the loaded drugs. Liposomes are suitable carriers for drug delivery because of their structural similarity to cellular membranes and the resultant stability of encapsulated drugs.(18,19) The liver is one of the most targeted organs for nanoparticles following intravenous administration.(20,21) Thus, liposomes are suitable for use as carriers to deliver ferulic acid to injured liver.
In the present study, we employed a CCl4-mediated liver injury model. CCl4 is one of the most commonly used xenobiotics applied in animal model studies to initiate lipid peroxidation mediated tissue injuries. Numerous studies have demonstrated that CCl4 is metabolized in the endoplasmic reticulum of hepatocytes by the phase I cytochrome P450 system to highly reactive trichloromethyl radicals (•CCl3) and peroxy trichloromethyl radicals (•OOCCl3) to induce oxidative damage in the liver.(22) These highly reactive molecules subsequently bind to macromolecules in hepatocytes such as DNA, proteins, and lipids in hepatocytes, leading to lipid peroxidation reactions. The CCl4-induced liver injury model is therefore commonly employed to evaluate the hepatoprotective effects of antioxidative compounds both in vivo and in vitro.(23)
We prepared a liposomal formulation containing ferulic acid as a means to improve the solubility and stability of ferulic acid. We examined the anti-oxidative activity of ferulic acid as a liposomal formulation and its therapeutic potential against ROS induced by CCl4 both in vivo and in vitro.
Materials and Methods
Materials
Ferulic acid (Fig. 1) was purchased from LKT Laboratories, Inc (Tokyo, Japan) and Egg phosphatidyl choline (EPC) was obtained from NOF Corporation (Tokyo, Japan). Amino phenyl fluorescein (APF) was purchased from Goryo Chemical (Sapporo, Japan). CCl4 was brought from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). All reagents used in this study were high grade commercially available products. Seven-week old male Wistar rats (weighing 180 to 200 g) were purchased from Japan SLC, Inc. (Shizuoka, Japan). Ethical permission and approval of the animal experiments was obtained by the Animal and Ethics Review Committee of Tokushima University.
Fig. 1.
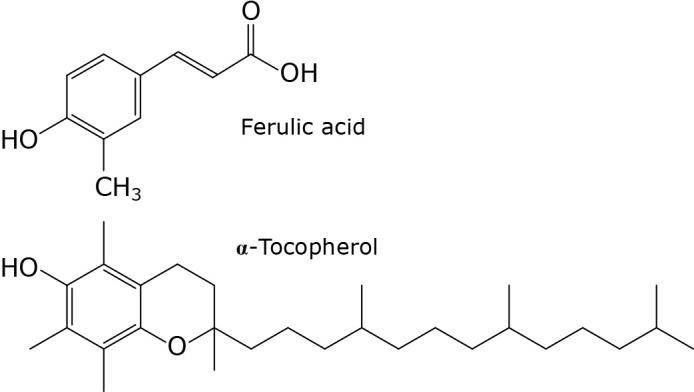
Chemical structures of ferulic acid and α-tocopherol.
Determination of DPPH radical scavenging capacity of ferulic acid
2,2-Diphenyl-1-picrylhydrazil (DPPH) radical scavenging activity of ferulic acid was measured according to an established method with some modifications.(24) Under oxidizing conditions, DPPH radicals form a purple color in ethanol solution, which becomes pale yellow upon reaction with antioxidants. 1 ml of 500 μM ferulic acid (final concentration: 20 μM), 14 ml of 10 mM Tris/HCl (final concentration: 5.6 mM), 10 ml of 125 μM DPPH solution (final concentration: 50 μM) were added to an Erlenmeyer flask. After mixing with a stirrer, the absorbance was measured spectrophotometrically at 517 nm. α-Tocopherol was used as a positive control. DPPH radical scavenging activity (%) was calculated using the following equation:
% DPPH radical scavenging activity = {(A0 − A1)/A0} × 100, where A0 is the absorbance of the control, and A1 is the absorbance of the extractives/standard.
Preparation of liposomes encapsulating ferulic acid
Liposomes were prepared using EPC and ferulic acid by conventional lipid film hydration methods. A chloroform solution containing 100 μl of 200 μM EPC (final concentration: 20 μM) and 10 μl of 20 μM ferulic acid (final concentration: 0.2 μM) was dried using a rotary evaporator (N-1000, EYELA, Tokyo, Japan) to form thin lipid films. Hydration of the dried lipid films was carried out by addition of 1 ml of phosphate-buffered saline (PBS) at room temperature. The liposomal suspension then underwent freeze-thaw treatment for a total of 3 times. A polycarbonate membrane filter with 100 nm pores (Nuclepore, Cambridge, MA) was used to extrude the liposomal suspension. Size (diameter in nm), polydispersity index and zeta potential (mV) of the liposomes (Table 1) were determined using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK).
Table 1.
Physiochemical properties of liposomes
| Sample | Size (nm) |
Polydispersity index |
Zeta potential (mV) |
|---|---|---|---|
| EPC-lipo (control) | 112.5 ± 2.9 | 0.14 ± 0.03 | −10.5 ± 0.35 |
| Ferulic-lipo | 109.3 ± 5.2 | 0.16 ± 0.02 | −7.37 ± 0.67 |
Data are means + SD of three samples prepared on different days.
Measurement of encapsulation efficiency of ferulic acid containing liposomes
Encapsulation efficiency of the ferulic acid containing liposomes (ferulic-lipo) was measured by ultraviolet-visible (UV-Vis) spectrophotometric methods. Ferulic-lipo (0.5 μM/ml) was prepared following the method described above, and the suspension was treated with Triton X-100 (Sigma Science, Albuquerque, NM) to lyse the lipid vesicles. Free ferulic acid was separated from the liposomes by gel filtration using a PD-10 column (GE Healthcare Biosciences KK, Tokyo, Japan). Briefly, ferulic-lipo (1 ml) was loaded onto a PD-10 column and eluted with PBS to obtain the liposomal filtration (1.8 ml). Then, 500 μl of the filtered liposomes solution (final concentration: 0.14 μM), 500 μl of 10% Triton X-100 (final concentration: 1%), and 4 ml PBS were added to a test-tube and incubated for 15 min at 65°C. After vortexing the solution, the absorbance of ferulic acid was measured at 322 nm using a UV-Vis spectrophotometer (DU-800, Beckman Coulter, Brea, CA). A Standard calibration curve was generated by preparing various solutions of ferulic acid at known concentrations. Encapsulation efficiency (EE%) was calculated using the following equation:
Evaluation of scavenging activity of liposomes against hydroxyl radicals
Hydroxyl radicals are generated by a chemical reaction known as the Fenton reaction.(25) As follows:
APF acts as an indicator of reactive oxygen species (ROS) generation. This fluorescein remains non-fluorescent until reaction with a hydroxyl radical; after reacting with a free radical APF becomes fluorescent. The fluorescence intensity of APF was measured to evaluate hydroxyl radical production.(26) To generate hydroxyl radicals, 165 μl of DDW, 30 μl of a 100 μM of APF solution (final concentration: 10 μM), 45 μl of a 10 mM liposomal suspension (final lipid concentration: 1.5 mM), 30 μl of a 10 mM H2O2 solution (final concentration: 1 mM), and 30 μl of a 1 mM FeSO4 solution (final concentration: 100 μM) were added to microplate well and mixed. The fluorescence intensity (excitation: 490 nm; emission: 515 nm) was measured using an Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland) within 10 s.
Estimation of CCl4-mediated cytotoxicity in vitro
HepG2 cells (2 × 105 cells) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C and maintained in 5% atmospheric CO2 for 72 h. After removing the medium, DMEM containing 75 μM of CCl4 was added to the cells. The cells were then incubated with the medium containing CCl4 for 30 min at 37°C. Cells were then further treated with DMEM containing 50 μM, 75 μM, or 100 μM of 50 mM ferulic acid liposomes and incubated at 37°C for 12 h. Following incubation, DMEM was removed and the cells were washed with PBS. After treatment with trypsin, cells were collected and stained with trypan blue (0.4%) and the number of viable cells and total cells were counted.
Preparation of CCl4-induced liver injury rat model
The CCl4-induced liver injury rat model was constructed based on previously published reports with some modifications.(27) Anesthetization of rats was carried out using a small animal anesthetizer (Bio Machinery, Japan). Hepatic injury was induced by intraperitoneal administration of 10% CCl4 (1 ml/kg body weight) diluted in liquid parafilm (1:9 ratio). CCl4-induced liver injured rats were used as a model of acute live injury in this study.
Determination of hepatoprotective effects of ferulic-lipo
To determine the hepatoprotective effects of ferulic-lipo, rats were administered ferulic-lipo intravenously at 30 min after CCl4 administration. The injected dose of EPC and ferulic-lipo were 25 μmol/kg and 2.5 μmol/kg, respectively. After 24 h of liposome administration, rats were sacrificed, and blood was collected from rat hearts under anesthetic conditions. Blood samples were subsequently incubated for 3 h at room temperature and then centrifuged at 1,000 × g for 10 min at 4°C for serum collection. The activities of hepatic marker enzymes aspartate transaminase (AST) and alanine aminotransferase (ALT) were measured using a commercially available kit (transaminase CII-Test Wako).
Histopathological observation of liver damage
Liver tissue samples from sacrificed rats were collected and fixed into optimal cutting temperature (OCT) compound (Sakura Finetek, Tokyo, Japan) and finally stored under frizzing conditions using dry ice/ethanol. The frozen liver tissue samples were cut into 10 μm thick tissue sections using a cryostat (CM3050S; Leica Biosystems, Tokyo, Japan). The sections were then mounted into MAS (strong hydrophilic adhesive)-coated glass slides with Perma Flour Aqueous Mounting Medium (Thermo Fisher Scientific, Waltham, MA). Hematoxylin and eosin (H & E) staining of liver tissues were performed to observe morphological changes in treated rats. Briefly, sections were enclosed with a PAP pen to prepare a hydrophobic membrane. After embedding in 4% paraformaldehyde for 10 min, the sections were washed with PBS. The sections were then stained with Mayer’s Hematoxylin solutions (Fuji Film Wako Pure Chemical, Osaka, Japan) and incubated for 10 min at room temperature, and subsequently stained with 1% eosin (Fuji Film Wako Pure Chemical) for 1 min at room temperature. Ethanol (80% to 100%) was used to dehydrate the sections, which were then cleared with xylene. A hydrophobic mounting agent (Entellan® New; Merck Millipore, Burlington, MA) was used for mounting the sections. Finally, sections were observed using a fluorescence phase contrast microscope (BZ-9000; Keyence, Osaka, Japan).
Observation of CCl4-induced free radical production
Dihydroethidium (DHE) was used as a fluorescent probe to evaluate ROS generation induced by CCl4. Frozen liver tissue sections (10 μm) were used for this experiment, and prepared according to the method described above. Briefly, prepared tissue sections were washed with PBS and then incubated in 50 μM DHE (dissolved in methanol) at 37°C for 30 min under conditions to avoid exposure to light. Slides containing the sections were then washed with PBS and treated with 4% paraformaldehyde for 10 min at room temperature. After washing with PBS, the slides were mounted. The sections were then incubated overnight at room temperature. Finally, fluorescence of DHE in the tissue sections was visualized by confocal laser scanning microscopy (LSM700; Carl Zeiss, Jena, Germany).
DNA fragmentation assay
DNA was isolated from the liver tissue according to the protocol provided by the kit manufacturer (NucleoSpin Tissue Kit; Macherey-Nagel, Germany). Briefly, 25 mg of liver tissue was collected and homogenized using 50 μl of PBS (−). Prior to lysing, 180 μl of Buffer T1 (pre-lyse buffer) and 25 μl of protein kinase was added. After mixing, the solution was incubated at 56°C for 2 h. After vortexing, 200 μl of Buffer B3 (lyse buffer) was added and the solution was further incubated at 70°C for 20 min for lysis. To adjust for DNA binding conditions, 210 μl of ethanol was added to the sample and the sample was then vigorously vortexed. To allow for DNA binding, the sample was transferred onto a tissue column in a collection tube and centrifuged at 11,000 × g for 1 min. After transferring the tissue column to a new collection tube, 500 μl of Buffer BW (wash buffer) was added and the sample was centrifuged at 11,000 × g for 1 min. Another wash was then carried out using 600 μl of Buffer B5 (wash buffer). Residual ethanol was removed by centrifuging the column for 1 min at 11,000 × g. Elution Buffer BE (elution buffer, 100 μl) was then added to elute pure DNA. The DNA concentration was measured using a Nanodrop 8000 (Thermo Fisher Scientific). For gel electrophoresis, 2% agarose gel was used and isolated DNA (600 ng) was applied onto the prepared gel for each of the following groups: control group, CCl4 group, and CCl4 + ferulic acid group. The supplied DNA ladder was used as a marker DNA. Agarose gel electrophoresis was carried out at 100 mA for 40 min. Following electrophoresis, staining was performed using gel red. Finally, the DNA band was observed using a lumino image analyzer (LAS-4000 mini; Fujifilm Life Science, Tokyo, Japan).
Statistical analysis
Statistical analysis was carried out using one-way ANOVA followed by the Tukey post-hoc test. Student’s t test was also used to determine differences between two groups. Data were reported as means ± SD. P values <0.05 were considered to be significant.
Results and Discussion
DPPH radical scavenging potency of ferulic acid
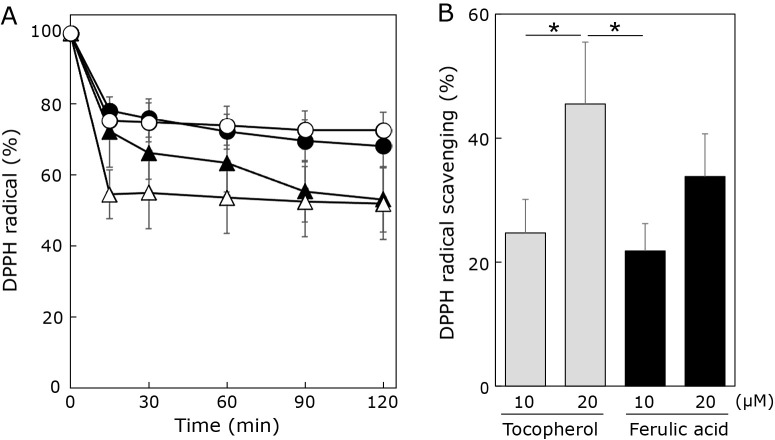
The DPPH radical scavenging potency of ferulic acid in ethanolic solution was measured to assess its antioxidative properties. DPPH radical scavenging activity was evaluated in a time-dependent manner (0 min, 15 min, 30 min, 60 min, 90 min, and 120 min) and compared with the well-known antioxidant α-tocopherol (Fig. 2). Tocopherols react with DPPH radicals by donating a hydrogen ion from the hydroxyl group on the chroman ring.(28) As ferulic acid is a known antioxidant, we expected the scavenging potency for DPPH radicals to be high. As shown in Fig. 2A, the addition of α-tocopherol immediately decreased the absorbance of DPPH radical dose dependently. Contrary to expectations, the absorbance of DPPH radical gradually decreased by addition of ferulic acid. Especially, at 15 min after addition of both compounds, DPPH radical scavenging activity of 20 μM α-tocopherol was higher than that of 20 μM ferulic acid (Fig. 2B). Based on the chemical structures of α-tocopherol and ferulic acid (Fig. 1), the DPPH radical scavenging activity of these two compounds was expected be similar since they both contain the same number of phenolic hydroxyl groups. However, the results of this study suggest that the methoxy group of ferulic acid may result in relatively larger steric hindrance than the methyl group of α-tocopherol while reacting with DPPH radicals. Thus, the lower probability of interaction with DPPH radicals may be responsible for the lower radical scavenging activity of ferulic acid compared to α-tocopherol.
Fig. 2.
Effects of ferulic acid and α-tocopherol on DPPH radicals. (A) Time dependent changes of DPPH radical by addition of ferulic acid at 10 μM (●) and 20 μM (▲) or α-tocopherol at 10 μM (○) and 20 μM (△) were assessed by measuring absorbance at 517 nm. (B) Percentage of DPPH radical scavenging by ferulic acid (black column) and α-tocopherol (grey column) measured at 15 min after addition of the compounds. Data are means ± SD (n = 3). *p<0.05.
Characteristics of liposomal formulations
To overcome the issue of low solubility of ferulic acid in water, we prepared liposomes containing ferulic acid (ferulic-lipo) and evaluated their physiochemical properties. The particle sizes of ferulic-lipo and control liposomes were approximately 100–110 nm (Table 1). The Zeta potential of ferulic-lipo was −7.37 mV (Table 1). The polydispersity index (PDI) values for ferulic-lipo and EPC-lipo were 0.16 and 0.14, respectively (Table 1). The encapsulation efficiency of ferulic acid in liposomes was about 92%. These results demonstrate the successful preparation of a liposomal formulation with a high encapsulation efficiency.
Effect of ferulic-lipo on hydroxyl radical scavenging
After preparing ferulic-lipo, we first checked whether the liposomal formulation exhibited hydroxyl radical scavenging activity. Ferulic-lipo was found to exhibit significant preventive effects (20 μM) compared to EPC-lipo based on fluorescence intensity measurement of hydroxyl radical generation (Fig. 3). Tocopherol-containing liposomes also showed significant preventive effects compared to EPC-lipo. Further, ferulic-lipo showed a greater tendency to scavenge hydroxyl radicals compared to tocopherol-containing liposomes. Based on the chemical structure of α-tocopherol, it is assumed that the active site comprised of a phenolic hydroxyl moiety might exist at the interface between the hydrophobic and hydrophilic regions of the liposomes. On the other hand, the active site of ferulic acid might exist on the surface of the of the liposomes as a result of its two polar hydroxyl groups. The opportunity for liposomal formulations to react with hydroxyl radicals depends on the chemical structure of the target antioxidants as well as its position in the lipid bilayer environment. Therefore, based on the results of the current study, it is suggested that the higher hydroxyl radical scavenging activity observed for ferulic-lipo (compared to α-tocopherol-containing liposomes) is due to the increased opportunity of ferulic acid in the liposomes to interact with hydroxyl radicals generated in aqueous solution, as a result of the location of the active phenolic hydroxyl moiety. Taken together, these results demonstrate that the liposomal formulation of ferulic acid exhibits a relatively higher hydroxyl radical scavenging activity than α-tocopherol.
Fig. 3.
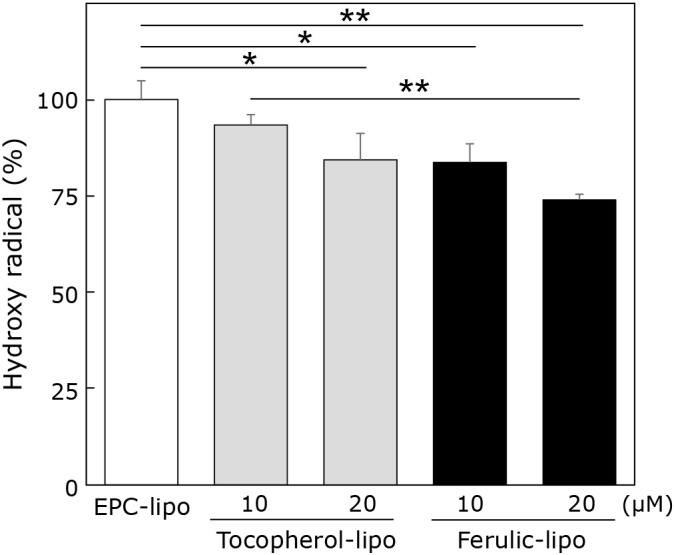
Effects of liposomes encapsulating ferulic acid or α-tocopherol on hydroxyl radicals. Hydroxyl radicals generated via Fenton reaction in the presence of liposomes encapsulating ferulic acid (black column) or α-tocopherol (grey column) were evaluated by the fluorescence intensity of APF. Data are means ± SD (n = 3). *p<0.05, **p<0.01.
Ferulic-lipo protects against CCl4-induced cytotoxicity in HepG2 cells
As ferulic-lipo showed significant antioxidative effects, we examined whether ferulic-lipo also exhibited protective effects against CCl4-induced toxicity in human hepatocarcinoma (HepG2) cells. As shown in Fig. 4, treatment with CCl4 markedly reduced the viability of HepG2 cells (~50%) compared to the control group. Moreover, ferulic-lipo at a concentration of 50 μM did not improve cell viability relative to the CCl4-treated groups. However, at a concentration of 75 μM, ferulic-lipo significantly improved cell viability (by almost 20%). Furthermore, ferulic-lipo at a concentration of 100 μM showed significant improvement in cell viability (~30%). These results demonstrate that following treatment with ferulic-lipo, significant preventive effects against CCl4-induced toxicity were observed in a dose-dependent manner. Thus, ferulic-lipo prevented CCl4-mediated cytotoxicity as a result of its antioxidative capacity.
Fig. 4.
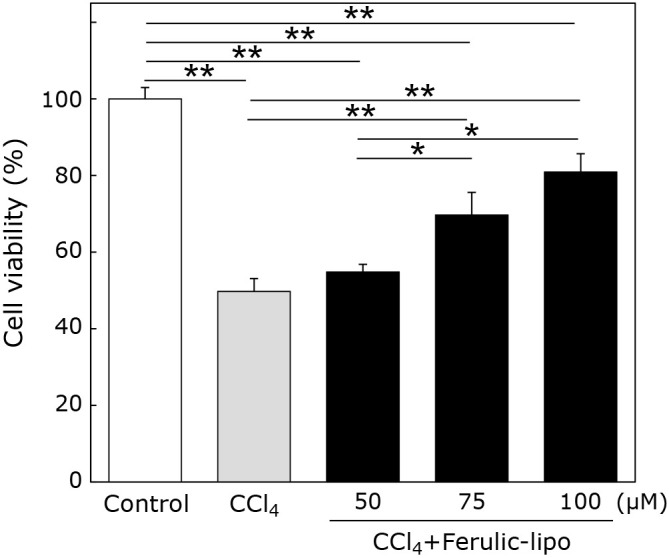
Cell viability of HepG2 treated with CCl4 in the presence of liposomes encapsulating ferulic acid. Cells were treated with 75 μM of CCl4; liposomes were added 30 min after CCl4 treatment. Cell viability was determined after 24 h. Control (white column), CCl4 treatment (grey column) and CCl4 + ferulic-lipo (black column). Data are means ± SD. *p<0.01, **p<0.001.
Effect of ferulic-lipo on CCl4-induced liver injury in vivo
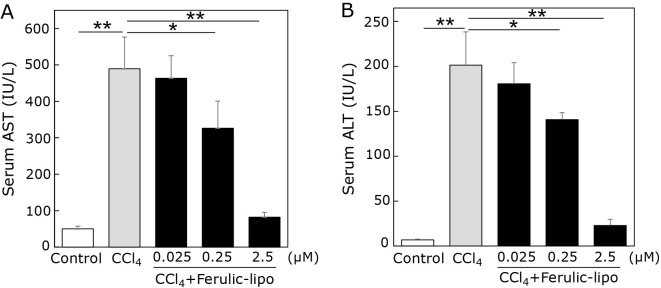
Based on the inhibitory effects of ferulic-lipo against CCl4-mediated cytotoxicity found in HepG2 cells in vitro, we examined the effects of ferulic-lipo in vivo in a CCl4-induced rat model of liver injury. As the extent of liver damage can be evaluated based on leakage of liver enzymes into the blood stream, levels of the hepatic enzymes AST and ALT were measured. After treatment with CCl4, serum AST and ALT levels were greatly increased to approximately 500 IU/L and 200 IU/L (Fig. 5), respectively, indicating severe liver damage. Ferulic-lipo was administrated intravenously to CCl4-treated rats. Contrary to expectations, ferulic-lipo at a concentration of 0.025 μmol/kg did not reduce elevated serum enzymes levels. After increasing the administered amount of ferulic-lipo to 0.25 μmol/kg, we found that serum AST and ALT levels were reduced by 35% and 30%, respectively. However, ferulic-lipo administered at 0.25 μmol/kg was not potent enough to prevent CCl4-induced hepatotoxicity. We increased the amount of administered ferulic-lipo further to 2.5 μmol/kg, which showed very potent preventive effects on CCl4-induced hepatotoxicity. Serum enzyme levels at 2.5 μmol/kg ferulic-lipo were nearly the same as those of the control groups. Taken together, these results suggest that ferulic-lipo possess potent hepatoprotective effects against CCl4-induced hepatotoxicity in vivo as a result of its antioxidative activity.
Fig. 5.
Effect of ferulic-lipo on CCl4-induced liver injury in rats. Liver injury was induced by CCl4. 30 min after CCl4 treatment, ferulic-lipo were intravenously administrated. Serum was collected after 24 h, and the enzymatic activities of AST and ALT were determined. Control (white column), CCl4 treatment (grey column) and CCl4 + ferulic-lipo (black column). Data are means ± SD (n = 3). *p<0.05, **p<0.001.
Effect of ferulic-lipo on oxidative stress induced by CCl4
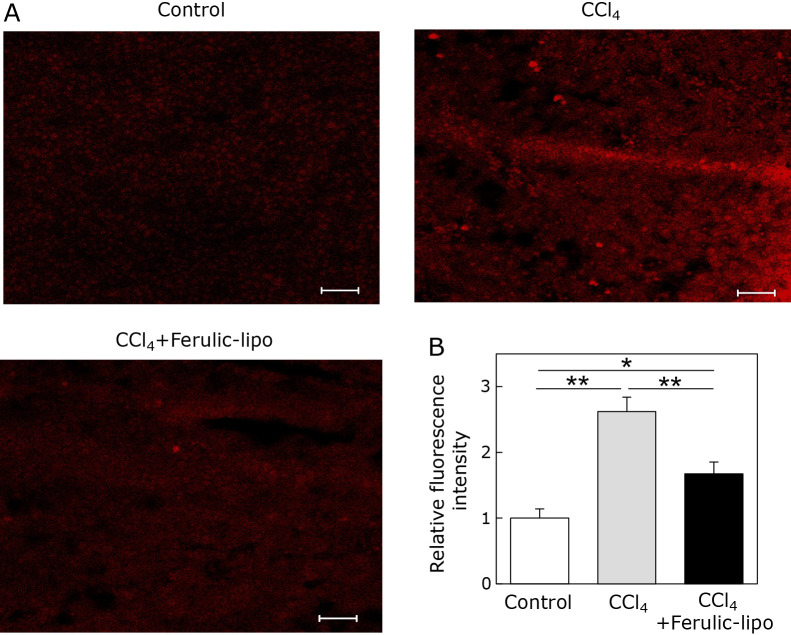
It is well known that CCl4-induced hepatotoxicity depends on production of ROS in the liver.(29) Therefore, we measured hepatocyte ROS generation in the liver after CCl4 administration. As shown in Fig. 6A, fluorescence indicating ROS generation was clearly observed in CCl4 treated group, confirming the presence of CCl4-induced ROS generation in the liver. On the other hand, fluorescence in the liver section after administration of ferulic-lipo was markedly reduced, as shown in CCl4 + Ferulic-lipo treated group. Furthermore, fluorescence intensity, as quantified by image analysis of liver tissue treated with ferulic-lipo was significantly lower (~50%) than that of CCl4-treated liver tissue. These results suggest that intravenous administration of ferulic-lipo can effectively inhibit oxidative stress mediated by CCl4 in the liver.
Fig. 6.
Effect of ferulic-lipo on CCl4-induced ROS generation in the liver. (A) Confocal laser scanning microscopic images of liver sections stained with dihydroethidium (DHE) represented as control liver tissue, CCl4-treated liver tissue and CCl4-treated liver tissue after intravenous administration of ferulic-lipo. (B) Quantified relative fluorescence intensity indicating ROS generation in the liver. Data are means ± SD (n = 3). *p<0.01, **p<0.001. Scale bar = 100 μM.
Effect of ferulic-lipo on CCl4-induced histopathological damage
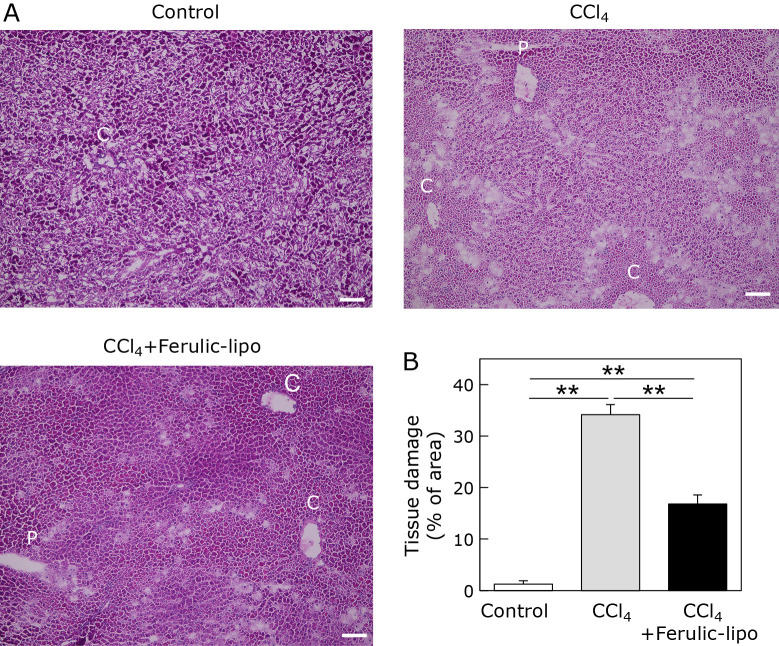
Liver damage was further assessed by histologic observation of liver sections stained with H & E. It has been recognized that CCl4-mediated liver tissue damage is characterized by happening surrounding the central vein region.(30) Hepatocyte damage was clearly observed in CCl4-treated liver tissue near the central vein region (C) compared to the control group (Fig. 7). As shown in Fig. 7A, there was a white section in the liver tissue treated with CCl4, which may represent necrotic cell death according to previously published reports. The white section was significantly decreased (by almost 50%) following treatment with ferulic-lipo. Taken together, these results, suggest that the antioxidant activity of ferulic-lipo results in effective inhibition of CCl4-induced tissue damage in the liver.
Fig. 7.
Effect of ferulic-lipo on CCl4-induced histological changes in liver tissue. (A) Microscopic images of liver sections stained with hematoxylin-eosin (H & E) represented as control liver tissue, CCl4-treated liver tissue, and CCl4-treated liver tissue after intravenous administration of ferulic-lipo. Here, the portal vein and the central vein were denoted by P and C. (B) quantitative estimation of tissue damage in the liver. Data are means ± SD (n = 3). **p<0. 001. Scale bar = 100 μM.
Effect of ferulic-lipo on CCl4-induced DNA fragmentation
It is well known that fragmentation of the double strand of the DNA is a hallmark of apoptosis induced by ROS.(31) Therefore, we assessed CCl4-induced DNA fragmentation in liver tissue using agarose gel electrophoresis. We found that CCl4 treatment induced a high degree of DNA fragmentation in the liver (Fig. 8). On the other hand, DNA fragmentation was significantly reduced following administration of ferulic-lipo. These results suggest that ferulic-lipo treatment can inhibit CCl4-induced apoptosis as a result of its potent antioxidative effects.
Fig. 8.
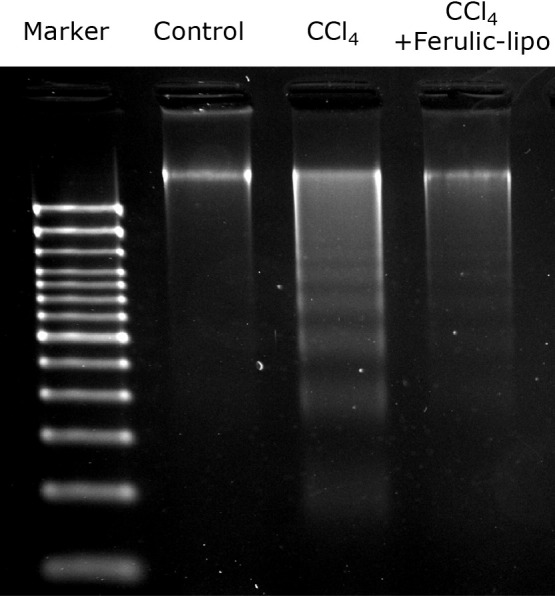
Effect of ferulic-lipo on CCl4-induced DNA fragmentation. DNA extracted from the liver tissue of untreated rats (control), CCl4-treated rats (CCl4), and CCl4-treated rats after intravenous administration of ferulic-lipo (CCl4 + ferulic-lipo) were subjected to agarose gel electrophoresis.
The results of the present study demonstrate that liposomes encapsulating ferulic acid exert potent antioxidative activity. Ferulic-lipo significantly reduced cytotoxicity induced by CCl4 in vitro. Moreover, intravenous administration of ferulic-lipo effectively attenuated CCl4-induced hepatotoxicity, ROS generation, and tissue damage in an in vivo rat model. Results of this study suggest that liposomal formulations containing ferulic acid may offer a promising approach for the treatment of oxidative stress related diseases of the liver.
Author Contributions
TA performed experiments, data analysis and manuscripts writing. SO contributed interpretation and acquisition of data. MH contributed to study concept and drafting the manuscripts. MO revised the manuscripts. KK designed the study, conceived the project, and supervised all experiments.
Acknowledgments
This research was supported by the Research Program for the Development of Intelligent Artificial Exosome (iTEX) from Tokushima University, Japan.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Park JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing Impact of NAFLD. Hepatology 2020; 72: 1605–1616. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci 2002; 65: 166–176. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Tan HY, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 2015; 16: 26087–26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimokawa T, Fukuta T, Inagi T, Kogure K. Protective effect of high-affinity liposomes encapsulating astaxanthin against corneal disorder in the in vivo rat dry eye diseases model. J Clin Biochem Nutr 2020; 66: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients 2014; 6: 6020–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina J, Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs 2005; 65: 2445–2461. [DOI] [PubMed] [Google Scholar]
- 7.Kawanaka M, Mahmood S, Niiyama G, et al. Control of oxidative stress and reduction in biochemical markers by vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot study. Hepatol Res 2004; 29: 39–41. [DOI] [PubMed] [Google Scholar]
- 8.Sawada K, Rahmania H, Matsuki M, et al. Absorption and metabolism of γ-oryzanol, a characteristic functional ingredient in rice bran. J Nutr Sci Vitaminol (Tokyo) 2019; 65 (Supplement): S180–S184. [DOI] [PubMed] [Google Scholar]
- 9.Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 2018; 31: 332–336. [DOI] [PubMed] [Google Scholar]
- 10.Bezerra GSN, Pereira MAV, Ostrosky EA, et al. Compatibility study between ferulic acid and excipients used in cosmetic formulations by TG/DTG, DSC and FTIR. J Therm Anal Calorim 2017; 127: 1683–1691. [Google Scholar]
- 11.Aguilar-Hernández I, Afseth NK, López-Luke T, Contreras-Torres FF, Wold JP, Ornelas-Soto N. Surface enhanced Raman spectroscopy of phenolic antioxidants: a systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vib Spectrosc 2017; 89: 113–122. [Google Scholar]
- 12.Rukkumani R, Aruna K, Suresh Varma P, Padmanabhan Menon V. Hepatoprotective role of ferulic acid: a dose-dependent study. J Med Food 2004; 7: 456–461. [DOI] [PubMed] [Google Scholar]
- 13.Kwon EY, Do GM, Cho YY, Park YB, Jeon SM, Choi MS. Anti-atherogenic property of ferulic acid in apolipoprotein E-deficient mice fed Western diet: comparison with clofibrate. Food Chem Toxicol 2010; 48: 2298–2303. [DOI] [PubMed] [Google Scholar]
- 14.Shakeel F, Salem-Bekhit MM, Haq N, Siddiqui NA. Solubility and thermodynamics of ferulic acid in different neat solvents: measurement, correlation and molecular interactions. J Mol Liq 2017; 236: 144–150. [Google Scholar]
- 15.Zhou Y, Lips A, Nanavaty FS, Bartolone JB. Stabilization of ferulic acid in cosmetic formulations. US Patent 2003; 6,632,444 B1. [Google Scholar]
- 16.Das S, Ng WK, Kanauja P, Kim S, Tan RB. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf B Biointerfaces 2011; 88: 483–489. [DOI] [PubMed] [Google Scholar]
- 17.Das S, Ng WK, Tan RB. Sucrose ester stabilized solid lipid nanoparticles and nanostructured lipid carriers: I. Effect of formulation variables on the physicochemical properties, drug release and stability of clotrimazole-loaded nanoparticles. Nanotechnology 2014; 25: 105101. [DOI] [PubMed] [Google Scholar]
- 18.Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics 2017; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol 2015; 6: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelazeim SA, Shehata NI, Aly HF, Shams SGE. Amelioration of oxidative stress-mediated apoptosis in copper oxide nanoparticles induced liver injury in rats by potent antioxidants. Scientific Reports 2020; 10: 10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie G, Sun J, Zhong G, Shi L, Zhang D. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch. Toxicol 2010; 84: 183–190. [DOI] [PubMed] [Google Scholar]
- 22.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 2005; 38: 698–710. [DOI] [PubMed] [Google Scholar]
- 23.Ko KM, Yick PK, Chiu T, Hui TY, Cheng CHK, Kong YC. Impaired hepatic antioxidant status in carbon tetrachloride intoxicated rats: an in vivo model for screening herbal extracts with antioxidant activities. Fitoterapia 1993; 64: 539–544. [Google Scholar]
- 24.Gangwar M, Gautam MK, Sharma AK, Tripathi YB, Goel RK, Nath G. Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensis fruit extract on human erythrocytes: an in vitro study. ScientificWorldJournal 2014; 2014: 279451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price M, Reiners JJ, Santiago AM, Kessel D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem Photobiol 2009; 85: 1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011; 283: 65–87. [DOI] [PubMed] [Google Scholar]
- 27.Wang CY, Ma FL, Liu JT, Tian JW, Fu FH. Protective effect of salvianic acid a on acute liver injury induced by carbon tetrachloride in rats. Biol Pharm Bull 2007; 30: 44–47. [DOI] [PubMed] [Google Scholar]
- 28.Zeece M. Chapter Five - Vitamins and minerals. Introduction to the Chemistry of Food. Academic Press, 2020; 163–212. [Google Scholar]
- 29.Dutta S, Chakraborty AK, Dey P, et al. Amelioration of CCl4-induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PLoS One 2018; 13: e0196411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josan S, Billingsley K, Orduna J, et al. Assessing inflammatory liver injury in an acute CCl4 model using dynamic 3D metabolic imaging of hyperpolarized [1-13C] pyruvate. NMR Biomed 2015; 28: 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohi K, Mittal N, Hundal MK, Khanduja KL. Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: a Bcl-2 independent mechanism. J Nutr Sci Vitaminol (Tokyo) 2003; 49: 221–227. [DOI] [PubMed] [Google Scholar]