Patients with inflammatory bowel disease (IBD) show a higher risk of developing pancreatitis, the main cause being side effects due to medication. Azathioprine (AZA) is a thiopurine immunosuppressant drug indicated for the treatment of this pathology and is one of the active ingredients most associated with acute pancreatitis in those patients. 1 According to Gordon et al, 2 the effect size and morbidity of thiopurine-induced pancreatitis are not known. Studies in adults report an incidence of AZA-induced pancreatitis ranging from 0% to 11%, depending on the type of study (observational vs randomized trial). Small case series report an incidence of up to 6% in pediatric IBD; however, only few prospective controlled studies with a comparison group have been published. Therefore, the absolute and relative risks of AZA-induced acute pancreatitis in children with IBD are unknown yet. 3 We report a case of a pediatric patient who probably had AZA-induced pancreatitis.
An 11-years-old boy, diagnosed with Crohn’s disease (CD) in June 2021, with neither known drug allergies nor other history of interest, was admitted for suffering from epigastric pain of days of evolution, accompanied by nausea and vomiting. He received treatment with exclusive enteral nutrition and oral AZA (50 mg daily), based on determination of thiopurine methyltransferase (TPMT) activity (17.6 U/mL), started 3 weeks before admission.
The abdominal pain was continuous with exacerbations, predominantly at night, and no changes in bowel habits (type 4-6 according to the Bristol Stool Scale) were observed. Physical examination revealed a non-distended, but painful abdomen in the supraumbilical region, with no other findings of interest. A blood test showed normal blood and coagulation parameters, amylase value being 70 UI/L. Abdominal ultrasonography showed subcentimeter adenopathies in the flank and right iliac fossa.
During admission, he was maintained on an absolute diet with intravenous fluid therapy (antiemetics—ondansetron—and gastric protection—ranitidine), without clinical improvement. Due to the persistence of the symptoms, successive analytical controls were requested. Finally, elevation of amylase to 174 UI/L and pancreatic lipase to 397 UI/L were objectifying (Figure 1).
Figure 1.
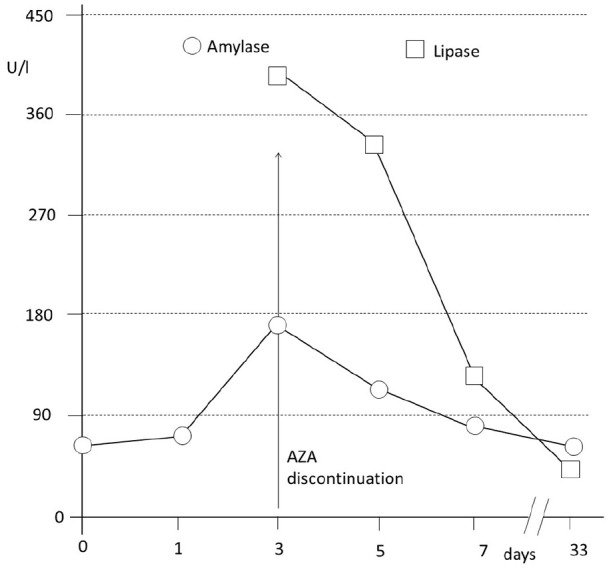
Elevation of amylase and pancreatic lipase secondary to azathioprine, and subsequent normalization of the pancreatic enzyme values after drug discontinuation.
Abbreviation: AZA, azathioprine.
Because drug-induced pancreatitis usually develops after 2-3 weeks from starting medication,4 -6 AZA was considered the possible cause of the pancreatitis. Therefore, AZA was discontinued. A decrease in serum pancreatic enzyme values was observed (Figure 1), and abdominal pain disappeared after withdrawal of AZA. After confirming the diagnosis, clinical course of the patient improved in a short time.
According to the adverse drug reactions probability scale proposed by Naranjo et al, 7 the total score for the present case was 6, indicating that the AZA-induced pancreatitis is probable.
AZA-induced pancreatitis is believed to be idiosyncratic and dose-independent. 8 Nevertheless, determination of TPMT enzyme activity (strongly recommended before initiating therapy with a thiopurine) allows individualized doses of AZA. From data of TPMT (retrospectively collected from 107 patients), Álvarez Beltran et al 9 have established a guide for individual doses of AZA according to the patient’s enzyme phenotype. For instance, in case of TPMT activity lower than 5 U/mL, the administration of AZA is not recommended because of leukocytopenia risks. Other values can be seen in Table 1 (adapted from Álvarez Beltran et al). 9
Table 1.
TPMT enzyme activity and doses of AZA.
| TPMT Activity (U/ml) | Recommended doses of AZA (values in mg/kg/day) |
|---|---|
| <5 | 0 |
| 5.1-13.7 | 0.5 |
| 13.8-18 | 1.5 |
| 18.1-26 | 2.5 |
| 26.1-40 | 3 |
The development of pancreatitis induced by AZA is usually considered a contraindication for its use. 4 Thus, it is important to seek alternative therapeutic options for these patients. Although mercaptopurine (MP) is a metabolite of AZA, there are some studies that support the use of MP, despite AZA-induced pancreatitis in CD children. It has been concluded that AZA-induced pancreatitis should not be considered as an absolute contraindication for future use of MP in these patients. 10
Footnotes
Contributions Statement: Authors have equally contributed to the paper. S.V.G and L.V.G. were responsible of the pharmacology and clinical assessment and diagnosis, respectively.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Silvia Vázquez-Gómez  https://orcid.org/0000-0002-8672-9075
https://orcid.org/0000-0002-8672-9075
References
- 1. Teich N, Mohl W, Bokemeyer B, et al. Azathioprine-induced acute pancreatitis in patients with inflammatory bowel diseases-a prospective study on incidence and severity. J Crohns Colitis. 2016;10(1):61-68. doi: 10.1093/ecco-jcc/jjv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon M, Grafton-Clarke C, Akobeng A, et al. Pancreatitis associated with azathioprine and 6-mercaptopurine use in Crohn’s disease: a systematic review. Frontline Gastroenterol. 2021;12:423-436. doi: 10.1136/flgastro-2020-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aloi M, Cucchiara S. Acute pancreatitis and azathioprine in paediatric inflammatory bowel disease. Lancet Child Adolesc Health. 2019;3:131-132. doi: 10.1016/S2352-4642(19)30019-7. [DOI] [PubMed] [Google Scholar]
- 4. Sturdevant RA, Singleton JW, Deren JL, Law DH, McCleery JL. Azathioprine-related pancreatitis in patients with Crohn’s disease. Gastroenterology. 1979;77(4, pt 2):883-886. doi: 10.1016/0016-5085(79)90387-1. [DOI] [PubMed] [Google Scholar]
- 5. Inoue H, Shiraki K, Okano H, et al. Acute pancreatitis in patients with ulcerative colitis. Dig Dis Sci. 2005;50(6):1064-1067. doi: 10.1007/s10620-005-2705-7. [DOI] [PubMed] [Google Scholar]
- 6. Mallory A, Kern F. Drug-induced pancreatitis: a critical review. Gastroenterology. 1980;78(4):813-820. doi: 10.1016/0016-5085(80)90689-7. [DOI] [PubMed] [Google Scholar]
- 7. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. doi: 10.1038/clpt.1981.15. [DOI] [PubMed] [Google Scholar]
- 8. Chouchana L, Narjoz C, Beaune P, Loriot MA, Roblin X. The benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(1):15-36. doi: 10.1111/j.1365-2036.2011.04905.x. [DOI] [PubMed] [Google Scholar]
- 9. Álvarez Beltran M, Infante Pina D, Tormo Carnicé R, Segarra Cantón O, Redecillas Ferreiro S. Optimising azathioprine treatment: determination of thiopurine methyltransferase activity and thiopurine metabolites. An Pediatr (Barc). 2009;70(2):126-131. doi: 10.1016/j.anpedi.2008.10.01010.1016/j.anpedi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 10. Gallego-Gutiérrez S, Navas-López VM, Kolorz M, et al. Successful mercaptopurine usage despite azathioprine-induced pancreatitis in paediatric Crohn’s disease. J Crohns Colitis. 2015;9(8):676-679. doi: 10.1093/ecco-jcc/jjv086. [DOI] [PubMed] [Google Scholar]


