Abstract
The eye is an immune-privileged site, with both vasculature and lymphatics absent from the central light path. Unique adaptations have made it possible for immune cells to be recruited to this region of the eye in response to ocular injuries and pathogenic insults. The induction of such immune responses is typically activated by tissue resident immune cells, considered the sentinels of the immune system. We discovered that, despite the absence of an embedded vasculature, the embryonic lens becomes populated by resident immune cells. The paths by which they travel to the lens during development were not known. However, our previous studies show that in response to corneal wounding immune cells travel to the lens from the vascular-rich ciliary body across the zonules that link these two tissues. We now examined whether the zonule fibers provide a path for immune cells to the embryonic lens, and the zonule-associated matrix molecules that could promote immune cell migration. The vitreous also was examined as a potential source of lens resident immune cells. This matrix-rich site in the posterior of the eye harbors hyalocytes, an immune cell type with macrophage-like properties. We found that both the zonules and the vitreous of the embryonic eye contained fibrillin-2-based networks and that migration-promoting matrix proteins like fibronectin and tenascin-C were linked to these fibrils. Immune cells were seen emerging from the ciliary body, migrating along the ciliary zonules to the lens, and invading through the lens capsule at its equator. This is just adjacent to where immune cells take up residence in the embryonic lens. In contrast, the immune cells of the vitreous were not detected in the region of the lens. These results strongly suggest that the ciliary zonules are a primary path of immune cell delivery to the developing lens.
Keywords: Resident immune cells, lens, eye, fibrillin, fibronectin, tenascin-C, ciliary zonules, vitreous, immune privilege
Impact statement
The absence of an embedded vasculature and lymphatics in tissues located in the central light path of the eye is essential to maintaining visual acuity, yet challenging to immune cell trafficking. How tissue resident immune cells are delivered to the lens during its development is unknown. Here, we report the ciliary zonules linking the avascular lens with the highly vascularized ciliary body as a primary migratory pathway of tissue-resident immune cells to the developing lens. Our results also show that the migration-promoting molecules fibronectin and tenascin-C are associated with the fibrillin-2 zonule backbone from early times of development. These matrix proteins could be key factors in directing immune cell migration to the lens along the developing zonule fibrils. Our findings expand current understanding of immune cell delivery during lens development as well as recruitment to the lens in response to eye injury and pathogenesis.
Introduction
Immune cells have essential roles in maintaining tissue homeostasis and in mediating the reparative response to trauma, injury, and pathogenesis.1 –4 This presents a challenge to the tissues of the eye like the lens, whose immune-privileged status is conferred by the absence of a vasculature and lymphatics. This property is essential to maintaining a clear path through which light is focused on the retina. Yet, we and others have shown that the lens activates and recruits immune cells in response to injury, dysgenesis, and pathogenic insults to itself as well as to other regions of the eye.5 –8 Central to understanding the role of the lens in recruiting immune cells was our discovery that this tissue becomes populated by resident immune cells during development.9,10 Tissue-resident immune cells are a property of most tissues,1,11 including other immune-privileged, avascular sites. 12 In the central nervous system (CNS), another immune-privileged site, the function of tissue-resident cells is provided by the microglia. 13 We have shown that resident immune cells are a feature of the lens across species, including in human, mouse, and chick lenses.9,10 They become integrated among the epithelial cells of the lens during development and persist in the adult. 9 Many of the lens-resident immune cells exhibit dendritic morphologies characteristic of professional antigen-presenting cells (APCs), extending major histocompatibility complex II (MHC II+) dendritic processes that wrap around adjacent lens epithelial cells. 9 With a principal function as the sentinels of the immune system, 1 tissue-resident immune cells can be derived from yolk sac erythromyeloid-progenitors with inherent properties of longevity and limited self-renewal, or be sourced from short-lived monocytes in the bone marrow.14,15 In studies with both human pediatric post-cataract surgery and chick mock cataract surgery explants, we have shown that lens-resident immune cells function as immediate responders to lens injury, rapidly populating the wound site. 9
The most likely routes that immune cells could travel to the lens are across the ciliary zonules and the vitreous humor, both matrix-rich compartments that directly contact the lens and reach more peripheral regions of the eye where its vasculature is located. The zonules are tendon-like fibers that link the vascular-rich ciliary body to the lens at its equator. While immune cells of the monocyte/macrophage lineage have been located to the ciliary zonules of the chick embryo eye at E15, by which time resident immune cells have been established among the lens epithelium, 9 it is not yet known whether they migrate to the lens from the ciliary body at earlier stages of development. The vitreous humor is a matrix-rich gel-like substance that fills the posterior chamber of the eye. It is located to a region bordered by the zonules, the posterior lens, and the retina. While both these regions have the potential to provide pathways for immune cells to travel to the lens during the early stages of development, the assembly of their matrix compartments and their acquisition of key molecules that can promote migration have not been closely investigated in the context of their association with immune cells.
The ciliary body, which forms during development from cells located in the peripheral tip of the optic cup and the presumptive ocular mesenchyme, comprises a ciliary epithelium (non-pigmented and pigmented), stromal cells, and muscles. 16 The non-pigmented epithelial cells produce the matrix proteins that form the ciliary zonule fibrils, 17 while the stromal region becomes highly vascularized. During development, the ciliary zonules, like the lens, remain avascular, 9 and extend from the non-pigmented ciliary epithelium lining the inner edge of the developing ciliary body to the equatorial region of the developing lens. 18 Fibrillins form the backbone of the zonule fibrils, with fibrillin-2 predominating during development, and fibrillin-1 in the adult. 18 While fibrillin fibrils have been identified as signal inducers for immune cells, 19 many other matrix proteins and growth factors become linked to these fibrils that have been identified as factors in directing immune cell migration. One is tenascin-C,20,21 which has been identified as a component of the extracapsular region of the lens. 22 During development, tenascin transcripts are expressed by both the ciliary body and the lens. 23 However, the presence of tenascin-C in the developing zonule fibers has not been investigated. While a prominent feature during development, tenascin-C is present only at low levels and at specific sites in adults. However, in the adult, tenascin-C is upregulated in response to tissue damage. Its induction is a danger signal that impacts immune responses, including the triggering of an innate immune response, impacting the adaptive immune response. High levels of tenascin-C have also been associated with chronic inflammation. 24 Similarly, the matrix protein fibronectin is linked to roles in promoting immune cell responses and migration.25 –27 Therefore, the presence of fibronectin and tenascin-C in the zonules, or the vitreous, during development would suggest a potential signaling role in the movement of immune cells to the lens.
Our previous studies reveal that an immune response is induced following corneal debridement wounding in which immune cells migrate from the vasculature-rich ciliary body to the surface of the lens via the ciliary zonule fibrils. 6 However, it is not yet known whether there is a relationship between the development of the ciliary zonules, the migration of immune cells across the zonule fibrils, and the establishment of lens-resident immune cells. In adult eyes, a type of macrophage referred to as hyalocytes is located within the vitreous humor. 28 Their name comes from the hyaloid artery, a transient vasculature that extends across the central vitreous during mammalian eye development. This vasculature regresses before birth, limiting the timing in which it could provide immune cells to the lens. However, in connexin 50/aquaporin-0 double knockout mice in which the posterior lens capsule ruptures through which lens tissue is extruded, and regression of the hyaloid vessels is delayed, immune cells sourced from the retained hyaloid vessels are recruited to repair the damaged region of the lens capsule. 7
Unlike mammalian lenses, the lenses of most non-mammalian vertebrates, including the chick, develop in the absence of a hyaloid artery. 29 In its place, in avian eyes, there is a unique matrix-rich vascular component called the pecten oculi.30,31 It extends through the vitreous outside the central light path, from the posterior of the eye to a region that is both adjacent to the ciliary body and in close proximity to the lens. The pecten oculi persists as a source of immune cells in the adult avian eye. Therefore, the chick embryo eye provides an ideal model for determining the path that resident immune cells travel to the lens in the absence of an associated vasculature, providing relevance to how immune cells populate the lens both during early development and in the adult.
Materials and methods
Animals
All animal studies performed are in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines, approved by Thomas Jefferson University, and with the Association of Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Vision Research. Day 5, 8–10, 12, and 15 chick embryos were used in these studies and the embryonated eggs were obtained from Poultry Futures, Lititz, PA, USA. These studies also included embryonic day 14 and adult mice on the mixed genetic background 129Sv/C57B1/6J.
Sample preparation for cryosectioning
Whole embryonic chick eyes between E8 and E15 were removed after decapitation. Whole heads were used for studies of E5 chick embryo eyes. Samples were fixed in 4% paraformaldehyde overnight at 4°C, cryoprotected in 30% sucrose in Dulbecco’s phosphate-buffered saline (DPBS) with calcium and magnesium for 24 h at 4°C. E14 mouse heads and adult mouse eyes were removed after sacrificing, fixed, and cryoprotected with the same protocol. Samples were then placed in optimal cutting temperature (OCT) freezing media (Polyscience, Niles, IL, USA) and flash frozen using dry ice and 100% ethanol; 30-µm-thick cryosections were cut using a Microm HM 550 Cryostat.
Immunofluorescence labeling of tissue sections
Cryosections were permeabilized in 0.5% Triton-X100 (BP151-100, Fisher) for 1 h and then incubated in block buffer (3% bovine serum albumin [BSA], 5% goat/donkey serum, 0.5% Triton-X100) for 2 h at room temperature prior to incubating with primary antibodies diluted in the block buffer at 37oC overnight. The sections were washed and incubated for 1 h at 37°C in fluorescent-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) diluted in block buffer containing fluorescent-conjugated phalloidin (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI) (Biolegend, 422,801). Sections were washed and mounted in ProLong Diamond Antifade Mountant (Invitrogen, P36970). Primary antibodies used for the embryonic chick studies included fibrillin-2 (JB3, DSHB), tenascin-C (AB19013, Millipore), fibronectin (PA1-23693, ThermoFisher), collagen I (NBP1-300054, Novus), KUL-01 (MCA5770, BioRad), and CD45 (MCA2413GA, BioRad). Primary antibodies used for the mouse studies included thrombospondin (SC-393504, SantaCruz), MAGP1 (gift from Dr Robert Mecham, Washington University School of Medicine in St. Louis), and fibrillin-2 (gift from Dr Robert Mecham).
Antigen retrieval
For studies with the fibronectin antibody the cryosections were treated with an antigen retrieval protocol prior to immunolabeling. The cryosections were brought to room temperature, washed in 1× Tris-buffered saline with 0.1% Tween 20 detergent (TBST) (1× TBS + 0.05% Triton-X100) for 15 min, boiled in sodium citrate buffer (95°C–100°C) for 20 min, and allowed to cool in sodium citrate buffer for 10 min. Sections were blocked in 10% goat serum diluted in 1× TBST for 1 h at room temperature and prior to immunolabeling with the fibronectin antibody diluted in 1% BSA.
RAW 264.7 cell culture
RAW 264.7 (ATCC #TIB-71) is a mouse monocyte/macrophage cell line. They are cultured in complete media (Dulbecco’s modified eagle medium [DMEM] [ATCC #30-2002]) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and l-glutamine in a 37°C incubator with 5% CO2.
Human non-pigmented ciliary epithelial cell culture
Human non-pigmented ciliary epithelial cells (HNPCEpiCs) (ScienCell #6580) were cultured in epithelial cell medium (EpiCM) (ScienCell #4101), supplemented with 2% FBS (#0010), 1% epithelial cell growth supplement (EpiCGS) (#4152), and penicillin/streptomycin (#0503). Primary HNPCEpiCs are received frozen, thawed in a 37°C water bath, gently resuspended, and plated in a T-75 flask coated with 10 mg/mL poly-l-lysine (ScienCell #0413) in complete media in a 37°C incubator with 5% CO2. The cells were grown to 90–100% confluency before passaging and splitting into 35 mm culture dishes (Corning #430165) or six-well culture plates (Corning #3516). Once cells reach 100% confluency, the culture dishes/wells were decellularized using 20 mM ammonia hydroxide (ThermoFisher #390030010) in phosphate-buffered saline (PBS) plus 0.5% Triton-X100 for 5 min at 37°C, leaving behind the ciliary zonule protein-containing extracellular matrix substrates. The matrix substrates were washed twice with DPBS without calcium and magnesium (Corning #21-031-CV), dried down, and dishes stored at 4°C.
Time lapse and cell tracking
RAW 264.7 (1 × 104) cells per well were plated in six-well plates with rehydrated HNPCEpiC-produced matrix substrates or tissue culture plastic alone in complete media (DMEM [ATCC #30-2002] supplemented with 10% FBS, penicillin/streptomycin, and l-glutamine). The cultures were incubated for 2 h at 37°C to allow the RAW 264.7 cells to attach to the HNPCEpiC matrix substrates or the uncoated tissue culture wells. Time-lapse imaging was performed with a Nikon Eclipse Ti microscope with an attached Tokai Hit incubation system that maintains the cultures at 37°C and 5% CO2. Using NIS-Elements AR 5.11.03 64-bit software, a 24-h time-lapse video was acquired. RAW cell tracking and movement parameters were determined from the time-lapse images using Imaris Software version 9.7.0. Cell tracks were evaluated using the “spot” tool and default settings (“Track Spots (over time),” “Classify Spots,” and “Object-Object Statistics”). Various statistical values were determined, including track length, track displacement, mean speed, and straightness of the path of movement. Bar graphs were created using GraphPad Prism 9 and the significance determined comparing the parameters analyzed for RAW 264.7 cell migration on the HNPCEpiC matrix to tissue culture plastic.
Immunofluorescence staining of HNPCEpiC-produced matrix substrates
Decellularized HNPCEpiC culture substrates prepared in 35 mm tissue culture dishes were treated with 0.5% Triton-X100 for 30 min at room temperature and then blocked in 5% goat serum for 30 min at room temperature. The substrates were incubated in primary antibody diluted in 0.1% Tween20 (FisherScientific #BP337-500) overnight at 37°C. Primary antibodies included (tenascin-C [Millipre #AB19013], fibrillin-1 [abcam ab68444], and fibronectin [abcam PA1-23693]). Dishes were then washed two times with PBS, followed by a 1-h incubation at 37°C in secondary antibody diluted in 0.1% Tween20, washed twice in PBS and then mounted in ProLong Diamond Antifade Mountant (Invitrogen, P36970).
Confocal image analysis and Imaris image analysis
Images were acquired using Zeiss LSM800 confocal microscope. Confocal images were obtained at either 5× or 40× and then analyzed using Zeiss Zen Software. To provide an overview of the whole eye, 5× tiles were used. For the studies at 40×, z-stacks were collected, each optical slice at either 0.33 or 0.50 µm. Three-dimensional surface renderings were generated using the Imaris software (version 9.7) 3D View tool. The entire z-stack is generated as a 3D surface structure that can be rotated and zoomed in without distortion of the original image.
Results
Identification of matrix compartments in the developing eye that could guide immune cell migration to the embryonic lens
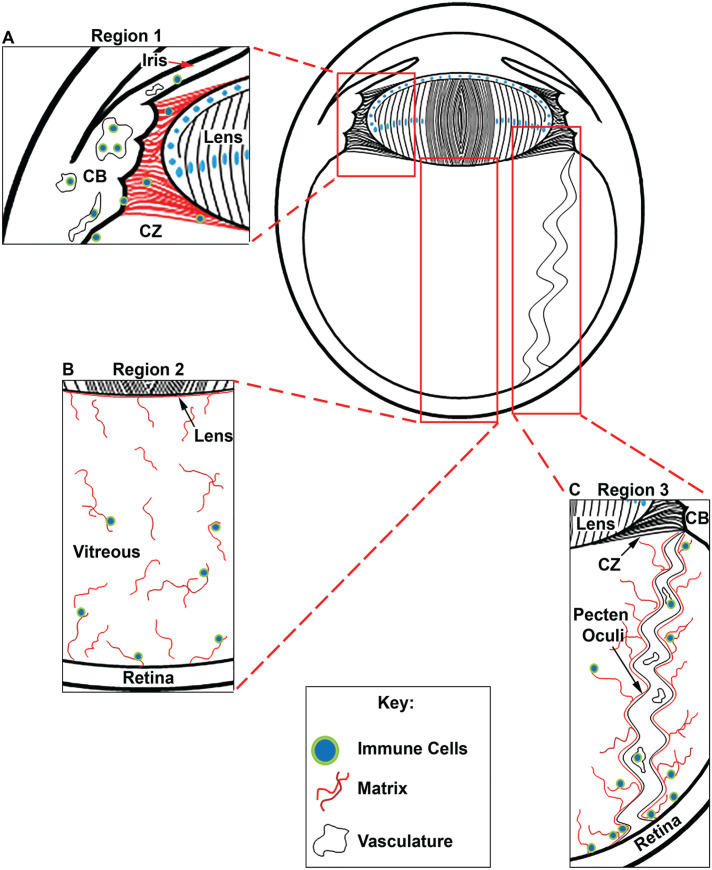
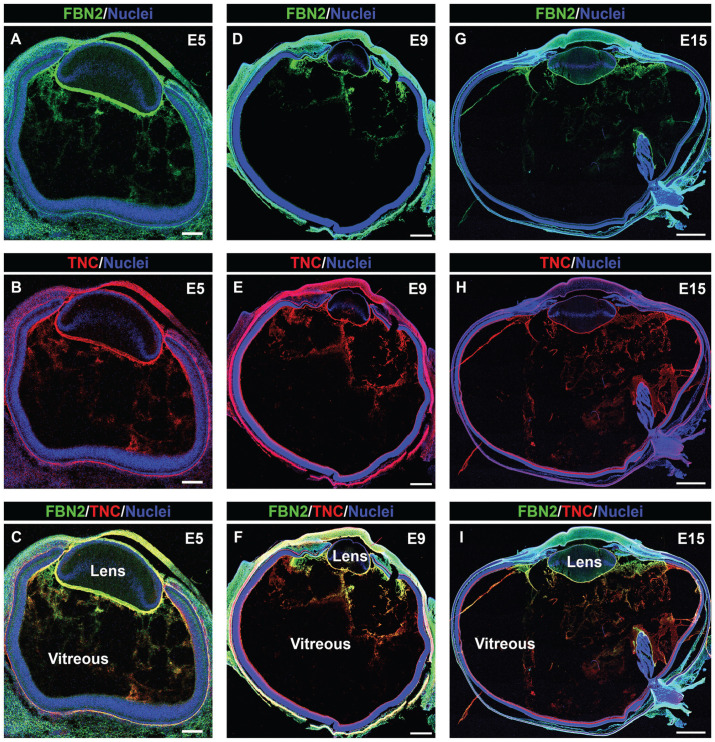
We have examined the development-state-specific expression and organization of cell migration-promoting matrix molecules in three different structures of the chick embryo eye whose proximity to the lens makes them potential paths for the delivery of lens tissue-resident immune cells. They included: region 1—the ciliary zonules that link the highly vascularized ciliary body to the avascular lens; region 2—the vitreous humor that contacts the posterior lens capsule; and region 3—the matrix associated with the pecten oculi, a vascular structure that extends through the vitreous from the posterior retina to the ciliary body, as modeled in Figure 1. Cryosections prepared from chick embryo heads at embryonic day 5 (E5) and chick embryo eyes at both E9 and E15 were co-immunolabeled for fibrillin-2 (Figure 2(A), (C), (D), (F), (G), and (I)) and tenascin-C (Figure 2(B), (C), (E), (F), (H), and (I)). Nuclei were labeled with DAPI, and the sections imaged by confocal microscopy at low magnification. This approach provided an overview of the distribution of fibrillin-2 and tenascin-C matrices across the entire eye during development. The results showed that tenascin-C co-localized with fibrillin-2 in the ciliary zonules forming between the developing ciliary body and lens as early as E5 and were extended posteriorly into the vitreal compartment (Figure 2(A) to (C)). Both fibrillin-2 and tenascin-C remained components of the ciliary zonules and vitreous throughout development (Figure 2(D) to (I)). At E15, they were also highly localized to the matrix associated with the surface of the pecten oculi (Figure 2(G) to (I)). These results showed that fibrillin-2/tenascin-C-rich fibrillar matrices had already begun to organize adjacent to the equatorial and posterior aspects of the lens very early in development, prior to our earliest time point of E5, and continued to expand over time.
Figure 1.
Diagram of the embryonic chick eye, illustrating the three regions of interest that were examined as potential pathways by which resident immune cells could gain access to the avascular lens. (A) Region 1 shows an immune cell path across the ciliary zonules (CZs) that link the highly vascularized ciliary body (CB) to the lens, where we have identified the presence of immune cells in the adult mouse eye. (B) Region 2 identifies the vitreous, a matrix component bounded by the posterior zonules, the posterior lens, and the retina, which is a source of hyalocytes. (C) Region 3 shows the pecten oculi, a vascular-rich structure in avian species that extends from the retina to the ciliary body. (A color version of this figure is available in the online journal.)
Figure 2.
Overview of tenascin-C/fibrillin-2 fibril organization during development of the chick embryo eye; 5× confocal tiles were acquired of cryosections of embryonic day 5 (A to C), 9 (D to F), and 15 (G to I) chick eyes that were co-immunolabeled for fibrillin-2 (green) and tenascin-C (red), and co-labeled for nuclei with DAPI (blue). Mag. bars: E5 = 100 µm, E9 = 500 µm, E15 = 1000 µm. (A color version of this figure is available in the online journal.)
Cell migration–promoting matrix proteins tenascin-C and fibronectin are key molecules of the developing ciliary zonule fibrils linking the ciliary body to the lens
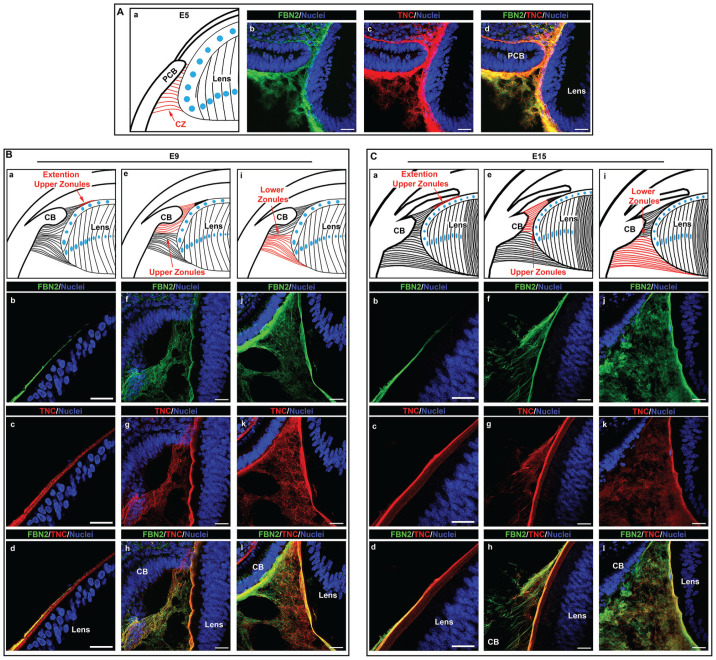
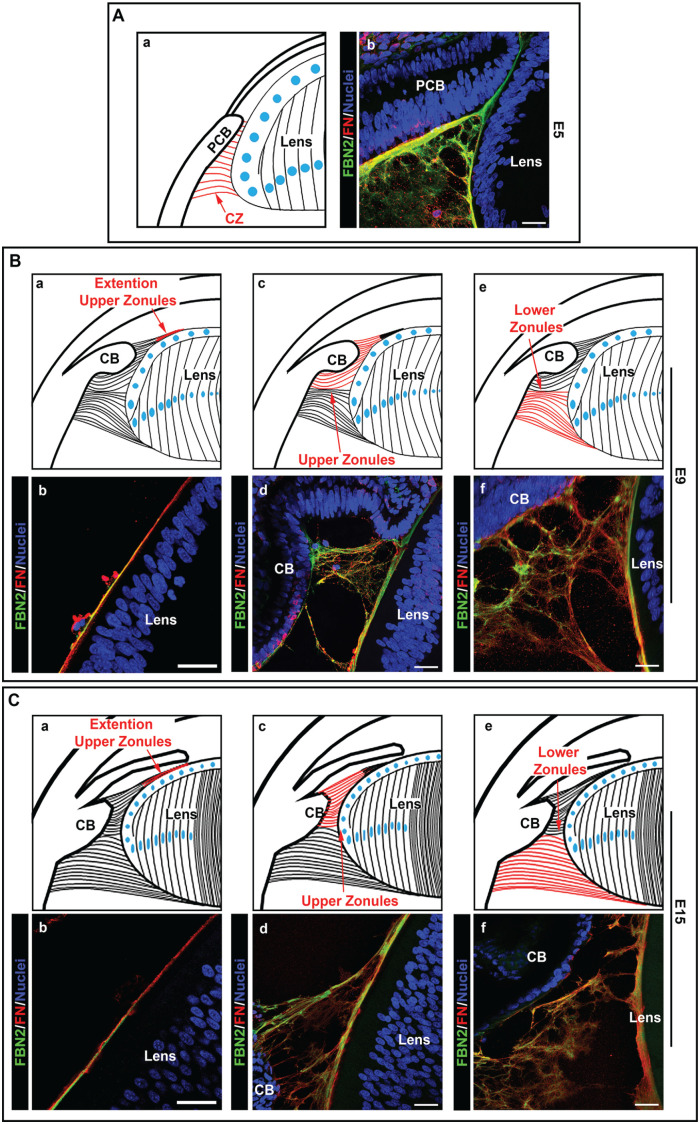
Using high-resolution confocal microscopy, we examined the development-state-specific organization and distribution of migration-promoting matrix molecules associated with the ciliary zonules fibrils. These studies were focused on determining the presence of these molecules in developing zonule fibers and their potential to provide a substrate for the movement of immune cells from the ciliary body to the lens. These questions were examined by immunolabeling cryosections of E5 chick embryo heads, and both E9 and E15 chick embryo eyes for the zonule backbone protein fibrillin-2 and co-immunolabeling for either tenascin-C (Figure 3) or fibronectin (Figure 4), both key molecules in the promotion of cell adhesion and migration.32 –35 Studies using the fibronectin antibody required performing an antigen retrieval protocol prior to immunolabeling, and the results are presented as fibrillin-2/fibronectin overlays (Figure 4), with separate images for each of these matrix molecules included together with the overlays in Supplemental Figure 1. All sections were co-labeled for nuclei with DAPI. Confocal microscopy imaging showed that as early in eye development as E5, at which time the presumptive ciliary body is located in very close proximity to the equator of the developing lens, fibrillin-2-rich zonule fibrils were already present in the region between the ciliary body and the lens. Many of these early zonule fibrils had a fine, feathery appearance (Figure 3(A)), and there was a high degree of co-localization of tenascin-C with fibrillin-2 in this wispy matrix meshwork (Figure 3(Ad)). The use of the antigen retrieval protocol for fibronectin labeling revealed a subset of fibrillin-rich zonules with a more highly developed fibrillar morphology that are co-labeled with fibronectin (Figure 4(A)). Fibronectin was also detected in close vicinity to but not directly associated with the fibrillin-2-labeled fibrils, possibly reflecting the reported role of fibronectin in the assembly of fibrillin. 36
Figure 3.
Tenascin-C is highly co-localized to fibrillin-2-rich zonule fibrils during their developmental-state-specific formation. Cryosections of chick embryo heads at E5 (A), and whole chick embryo eyes at E9 (B) and E15 (C) were co-immunolabeled for the matrix proteins fibrillin-2 (green) and tenascin-C (red). Nuclei were labeled with DAPI (blue); 40× confocal z-stacks were acquired and shown as projection images. At E9 and E15, images were acquired in three distinct regions of the ciliary zonules including where the upper ciliary zonules extend along the anterior region of the lens (Ba–d; Ca–d), the upper zonules that extend anteriorly from the lens equator (Be–h; Ce–h), and the lower zonules that extend posteriorly from the lens equator (Bi–l; Ci–l). Mag. bars = 20 µm. (A color version of this figure is available in the online journal.)
Figure 4.
Fibronectin highly co-localizes to fibrilin-2-rich fibrillar structures of the developing zonules. Cryosections of chick embryo heads at E5 (A), and whole chick embryo eyes at E9 (B) and E15 (C) were co-immunolabeled for the matrix proteins fibrillin-2 (green) and fibronectin (red) following antigen retrieval. Nuclei were labeled with DAPI (blue); 40× confocal z-stacks were acquired and shown as projection images of the overlay between fibrillin-2 and fibronectin. At E9 and E15, images were acquired in three distinct regions of the ciliary zonules, including where the upper ciliary zonules extend along the anterior region of the lens (Ba, b; Ca, b), the upper zonules that extend anteriorly from the lens equator (Bc, d; Cc, d), and the lower zonules that extend posteriorly from the lens equator (Be, f; Ce, f). Mag. bars = 20 µm. (A color version of this figure is available in the online journal.)
As the eye continues to develop, the fibrillin-2-based zonule fibers between the ciliary body and the lens become more extensive and are increasingly organized into thicker, more linear fibrillar structures (Figure 3(Bb, f, j) and (Cb, f, j)). By E9, the three distinct ciliary zonule regions characteristic of adults could be identified. These regions include: (1) the lower zonules that extend posteriorly along the lens equator (Figures 3(Bi), (Ci) and 4(Be), (Ce)); (2) the upper zonules that extend anteriorly along the lens equator (Figures 3(Be), (Ce) and 4(Bc), (Cc)); and (3) a partial extension of the upper zonule fibrils along the anterior surface of the lens (Figures 3(Ba), (Ca) and 4(Ba), (Ca)). Immunolabeling for fibrillin-2 at E9 revealed that the upper zonule fibers no longer had a wispy appearance and had begun to acquire a more distinct fibrillar morphology (Figure 3(Bf)). The fibrils of the upper zonules continued to thicken by E15, their fibrillar structure becoming even more pronounced (Figure 3(Cf)). Also by E9, the upper zonule fibers have extended along the edge of the anterior surface of the lens (Figure 3(Bb)) and were maintained similarly throughout eye development (Figure 3(Cb)). In contrast to the upper zonule fibrils, the morphology of the fibrillin-2-rich lower zonule fibrils appeared finer and less organized at both E9 and E15 (Figure 3(Bj) and (Cj)).
In the studies presented above, fibrillin-2 had been co-immunolabeled with antibody to tenascin-C. This extracellular matrix protein co-localized with fibrillin-2 along most but not all of the upper zonule fibrils at E9 and E15 (Figure 3(Bg, h) and (Cg, h)), and had a high level of coincidence in the ciliary zonule fibrils extended along the anterior surface of the lens (Figure 3(Bb–d) and (Cb–d)). In the fibrillar meshwork that characterized the lower zonule fibrils at these developmental times much of the tenascin-C labeling was also coincident with fibrillin-2 (Figure 3(Bj–l) and (Cj–l)). Note that, in addition to its presence in the ciliary zonules, tenascin-C has been identified as an integral component of the lens capsule. 22 Studies performed after antigen retrieval that were co-immunolabeled for fibronectin and fibrillin-2 highlighted the co-localization of these two matrix proteins in a subset of the upper zonule fibers that had already formed a distinct linear, fibrillar structure (Figure 4(Bd) and (Cd)). Fibronectin, like tenascin-C, localized to the fibrillin-2 containing zonule fibrils extending anteriorly along the lens surface (Figure 4(Bb) and (Cb)), and, as previously shown, also is a component of the lens capsule. 22 Using the antigen retrieval protocol required for immunolabeling with the fibronectin antibody revealed that there is a significant subset of lower zonules that had become organized into more structurally distinct zonule fibrils that were positive for both fibronectin and fibrillin-2 (Figure 4(Bf) and (Cf)). Separate images for each of these matrix molecules included together with the overlays are presented in Supplemental Figure 1.
As a comparison, we examined cryosections of E14 mouse eyes that were co-immunolabeled for fibrillin-2 and thrombospondin-1 (Supplemental Figure 2(B) to (D)) or for the fibrillin binding protein microfibril-associated glycoprotein-1 (MAGP1) and thrombospondin 1 (Supplemental Figure 2(E) to (G)). The pattern of organization of these ciliary zonule molecules in the E14 mouse eye confirmed that the developing ciliary zonules in the embryonic mouse are similar in appearance to those of the developing chick eye. We also performed co-immunolabeling for MAGP1 and thrombospondin-1 in adult mouse eyes (Supplemental Figure 2(I) to (K) and (M) to (O)). The labeling for MAGP1 showed that the zonules were mostly organized as very thick, rope-like structures and that thrombospondin-1 was primarily associated with thinner zonule fibrils still present in the adult (Supplemental Figure 2(E) to (G)).
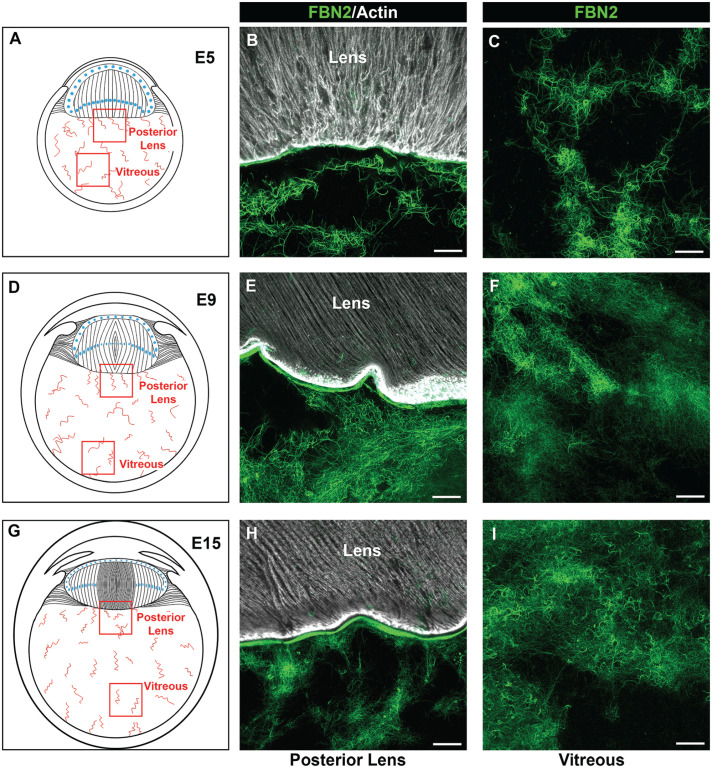
Tenascin-C and fibronectin are components of fibrillin-2 fibrillar network in the vitreous of embryonic eyes
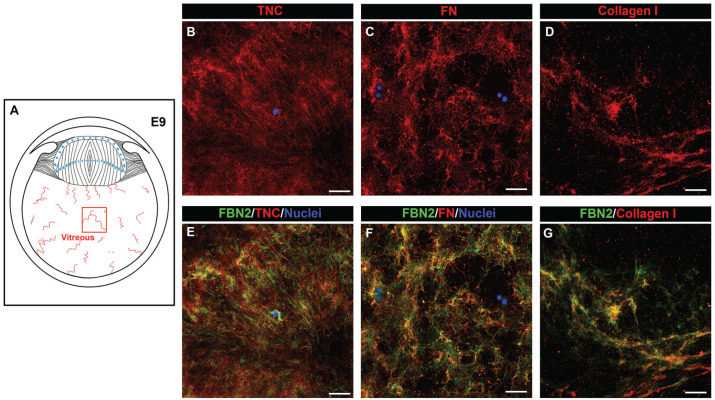
In addition to forming the backbone of the ciliary zonules, fibrillin has been identified as a matrix component of the vitreous. 37 Using high-resolution confocal microscopy, we examined the development-state-specific organization of fibrillin-2 in the vitreous and its association with other matrix components that could support immune cell migration (Figure 5). By E5, a highly interwoven network of structurally fine fibrillin-2 fibrils had been assembled throughout the vitreous (Figure 5(B) and (C)). This fibrillar meshwork extended from a region adjacent to the posterior lens capsule, which had been previously shown to contain fibrillin-2, 22 to the retina. The structure and organization of the fibrillin-rich fibrils of the vitreous remained similar when examined at E9 (Figure 5(E) and (F)) and E15 (Figure 5(H) and (I)). Low-magnification imaging of whole eye cryosections co-immunolabeled for fibrillin-2 and tenascin-C at E9 showed many areas of the vitreous in which these two matrix proteins are coincident, as well as indicated that tenascin-C was present in regions outside of the fibrillin-2 fibrils (Figure 2(D) to (F)). High-magnification, high-resolution confocal imaging of the vitreous in cryosections of E9 eyes co-immunolabeled for tenascin-C and fibrillin-2 support this conclusion (Figure 6(B) and (E)). In this image, a single cell is present that highly expresses fibrillin-2, suggesting that the fibrillin-2 matrix is created by cells localized within the vitreous. We also found that fibronectin is a matrix element of the vitreous in the developing eye (Figure 6(C) and (F)). Like fibrillin-2, its structural organization is fibrillar, with co-immunolabeling for fibronectin and fibrillin-2 showing that the distribution of fibronectin was highly coincident with fibrillin-2 (Figure 6(F)). However, there were also fibrillin-2-rich fibrils that were not also labeled with fibronectin. We also examined the coincidence of fibrillin-2 with collagen I, the latter being a principal component of the adult vitreous. Co-immunolabeling for collagen I and fibrillin-2 revealed that there is a high level of co-localization of these two matrix proteins in the vitreous during development (Figure 6(D) and (G)).
Figure 5.
Fibrilliin-2-rich fibrils extend throughout the vitreous during development. Cryosections of chick embryo heads at E5 (A to C), and whole chick embryo eyes at E9 (D to F) and E15 (G to I) were immunolabeled for the matrix protein fibrillin-2 (green) and co-labeled with fluorescent-conjugated phalloidin (white); 40× confocal z-stacks, each image representing a single optical slice of 0.33 µm, were acquired in the region along the posterior lens capsule (B, E, and H) and in more posterior aspects of the vitreous (C, F, and I), as indicated in the diagrams (A, D, and G). Mag. bars = 20 µm. (A color version of this figure is available in the online journal.)
Figure 6.
Tenascin-C, fibronectin, and collagen I co-localize with the fibrillin-2 fibrils of the developing vitreous. Cryosections of chick embryo eyes at E9 were co-immunolabeled for fibrillin-2 (E to G, green) with either tenascin-C (B, E), fibronectin (C, F), or collagen I (D, G). Nuclei were labeled with DAPI (blue); 40× confocal z-stacks, each image representing a single optical slice of 0.33 µm, were acquired in the center of the vitreous, as indicated in the diagram (A). Mag. bars = 20 µm. (A color version of this figure is available in the online journal.)
The ciliary zonule fibrils provide a path for immune cells to travel from the ciliary body to the lens during development
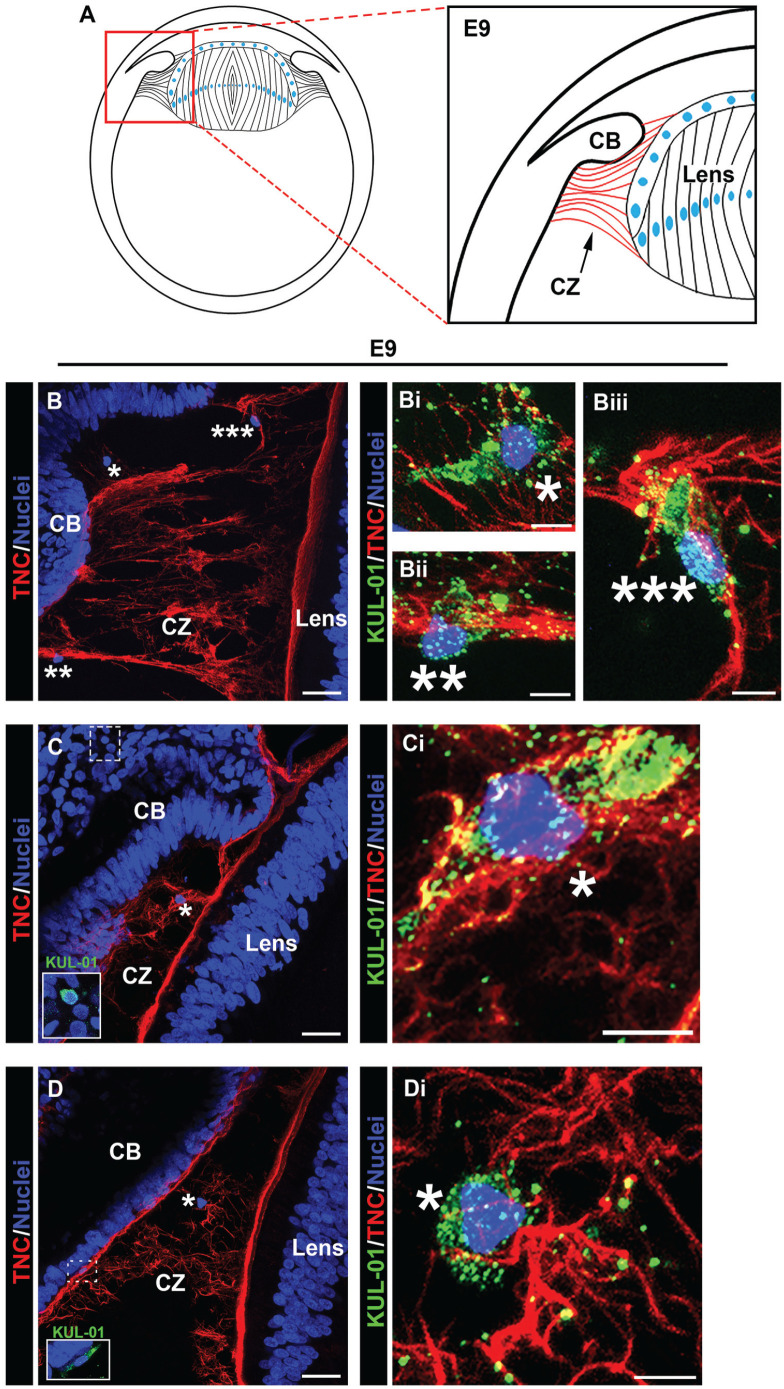
Our findings above show that both fibronectin and tenascin-C are associated with the fibrillin-2-rich zonule fibrils that link the ciliary body to the lens from early stages of eye development. Among their many properties, these matrix proteins play key roles in promoting both the attachment and migration of immune cells.20,21,25 –27 Our previous studies showed the presence of immune cells migrating within the ciliary zonule fibrils at E15. 9 However, by this time in eye development, we also showed that resident immune cells had already populated the lens. 9 We now examined whether immune cells become associated with the zonule fibrils from earlier times of eye development. For these studies, the zonule fibers at E5, E9, and E15 were immunolabeled using antibody to tenascin-C and the sections co-immunolabeled with KUL-01, an antibody specific to chick monocytes and macrophages. Nuclei were labeled using DAPI and the sections imaged by high-resolution confocal microscopy along the zonule fibers (Figures 7 to 9).
Figure 7.
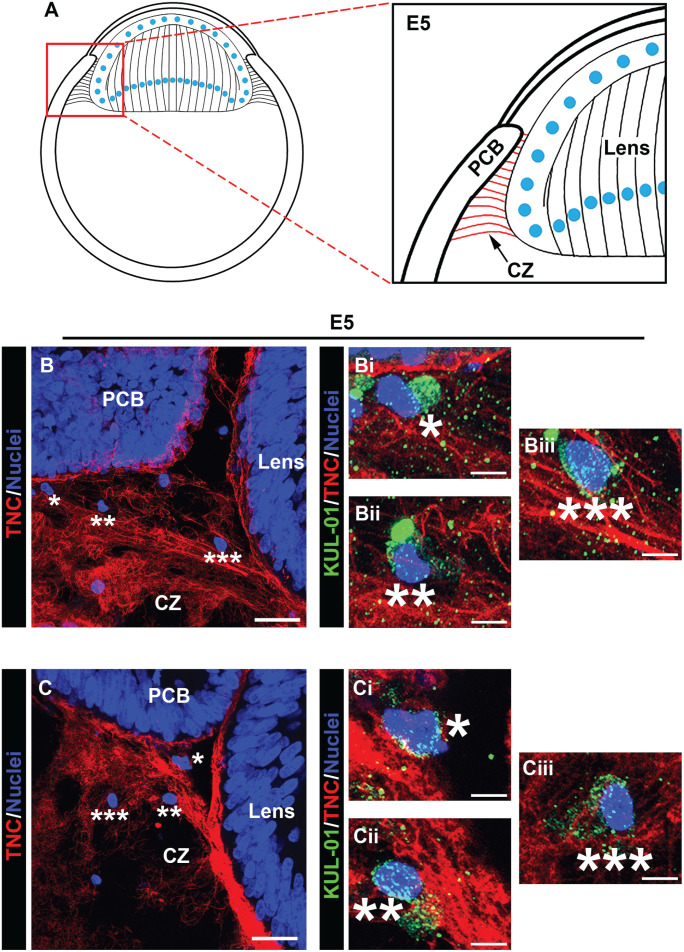
Immune cells populate the ciliary zonules as early as E5. Cryosections of E5 heads were co-immunolabeled for tenascin-C (red) to highlight the ciliary zonules and KUL-01 (green), which is expressed by immune cells of the monocyte/macrophage lineage. Nuclei were labeled with DAPI (blue); 40× confocal z-stacks were acquired of the ciliary zonule region as identified in the diagram (A). Projection images at low magnification are shown with just the tenascin-C and DAPI labels (B, C) and the regions denoted by asterisks shown at high magnification including the KUL-01 labeling for the immune cells (Bi–iii; Ci–iii). Immune cells were identified along the zonule fibers. Mag. bars: (B) and (C) = 20 µm, (Bi–iii) and (Ci–iii) = 5 µm. (A color version of this figure is available in the online journal.)
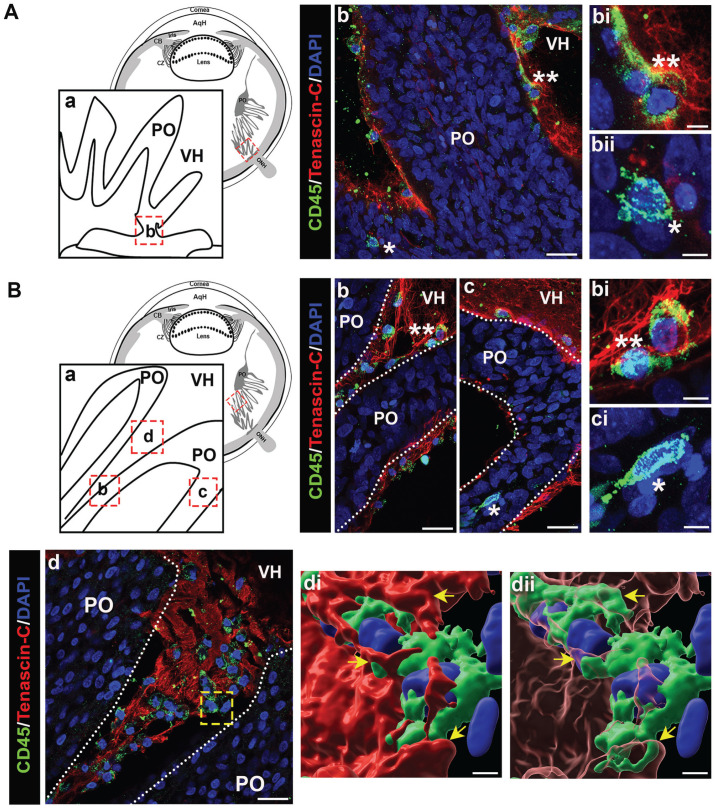
Figure 9.
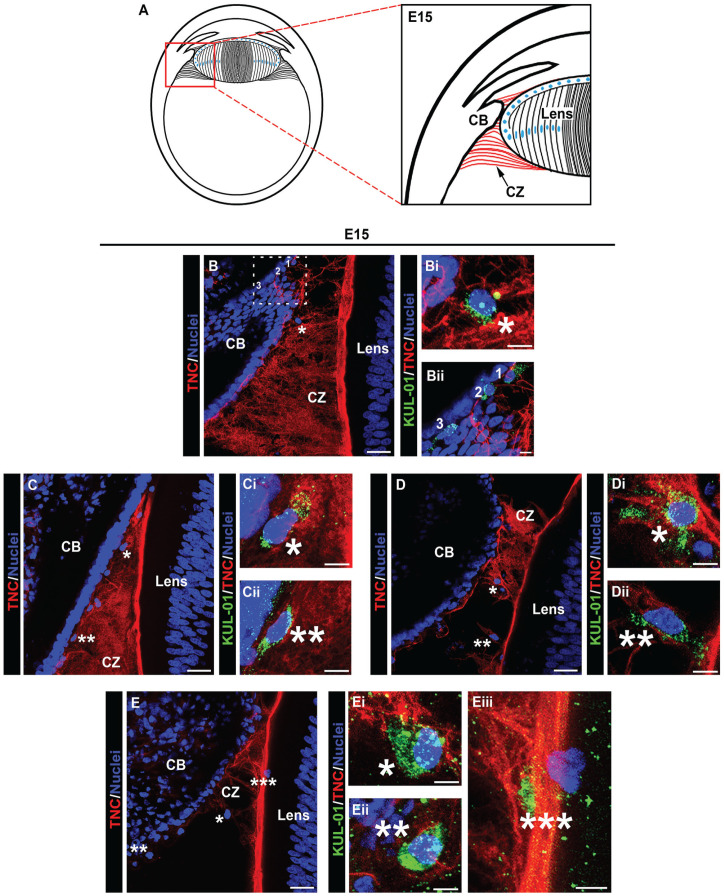
Immune cells emerging from the ciliary body, travel along the ciliary zonules and migrate across the lens capsule during development. Cryosections of whole eyes at E15, diagrammed in (A) were co-immunolabeled for tenascin-C (red) to highlight the ciliary zonules and KUL-01 (green) expressed by immune cells of the monocyte/macrophage lineage (B to E). Nuclei were labeled with DAPI (blue); 40× confocal z-stacks were acquired along different regions of the ciliary zonules and along the matrix capsule that surrounds the lens. Projection images at low magnification are shown with just the tenascin-C and DAPI labels (B to E) and the regions denoted by asterisks are shown at high magnification including the KUL-01 labeling for the immune cells (Bi; Ci, ii; Di, ii, Ei–iii). The dashed line boxed area in (B) is shown at higher magnification and including the KUL-01 label in (Bii), with the individual cells denoted by 1, 2, and 3. At this stage of development, immune cells were identified emerging from the ciliary body, along the zonule fibers, and migrating across the lens capsule. Mag. bars: (B) to (E) = 20 mm and (Bi), (Bii), (Ci), (Cii), (Di), (Dii), and (Ei–iii) = 5 µm. (A color version of this figure is available in the online journal.)
Image analysis of the developing zonules linking the presumptive ciliary body to the lens at E5 (diagramed, Figure 7(A)) revealed that by this early stage of development immune cells have already populated the zonules (Figure 7(B) and (C)). Their localization along the zonules is shown in the low-magnification images by their nuclear label (Figure 7(B) and (C)). The immune cell identity of these zonule-linked cells, denoted by asterisks, is shown in higher magnification images that include the KUL-01 monocyte/macrophage label together with labeling for tenascin-C and DAPI (Figure 7(Bi–iii) and (Ci–iii)). The findings show that immune cells localized all along the zonule fibrils at E5, from positions near the developing ciliary body to where the zonules insert into the lens capsule. These findings provide the first evidence that immune cells traverse the ciliary zonule fibrils at very early stages of lens development. Later in development, E9, when the zonule fibers are thicker, more distinct and more widely distributed, KUL-01+ immune cells are observed migrating along individual zonule fibers (Figure 8(Bii, iii)), and are often located within the zonule fibril networks (Figure 8(Bi), (Ci), and (Di)). The localization of these immune cells along the zonule fibers between the ciliary body and the lens is denoted by the asterisks in the accompanying low-magnification images (Figure 8(B) to (D)). The boxed regions in Figure 8(C) and (D) are shown as inserts within the figures, highlighting the presence of KUL-01+ immune cells that are located both within the ciliary body (Figure 8(C)) and migrating out of the ciliary body to become linked to the zonules (Figure 8(D)). As we previously reported, 9 monocyte/macrophage immune cell populations continued to migrate between the ciliary body and the lens at E15 (Figure 9(A) to (E), low-magnification images showing overlay of tenascin-C and cell nuclei). Here, we show KUL-01+ immune cells in a high-magnification image of the boxed area of Figure 9(A) at three stages of their egress from the ciliary body to the zonules (Figure 9(Bii)), and additional examples of immune cells located within the ciliary zonules just outside of the ciliary body (Figure 9(Bi), (Ci, ii), and (Eii)). In addition to showing immune cells traveling across the ciliary zonule fibers at E15 (Figure 9(Bi), (Di, ii), and (Ei)), we now provide evidence of an immune cell crossing the lens capsule (Figure 9(Eiii)). These results support the conclusion that immune cells originating from the ciliary body migrate across the ciliary zonules to populate the lens beginning at early stages of eye development.
Figure 8.
Immune cells emerging from the ciliary body travel along the ciliary zonules during development. Cryosections of whole eyes at E9, diagrammed in (A) were co-immunolabeled for tenascin-C (red) to highlight the ciliary zonules and KUL-01 (green) expressed by immune cells of the monocyte/macrophage lineage (B to D). Nuclei were labeled with DAPI (blue); 40× confocal z-stacks were acquired along different regions of the ciliary zonules. Projection images at low magnification are shown with just the tenascin-C and DAPI labels (B to D) and the regions denoted by asterisks shown at high magnification including the KUL-01 labeling for the immune cells (Bi to iii; Ci; Di). Dashed line boxed areas in (C) and (D) are shown at higher magnification and including the KUL-01 label in the respective inserts. At this stage of development, immune cells were identified emerging from the ciliary body and along the zonule fibers. Mag. bars: (B) to (D) = 20 µm, and (Bi–iii), (Ci), and (Di) = 5 µm. (A color version of this figure is available in the online journal.)
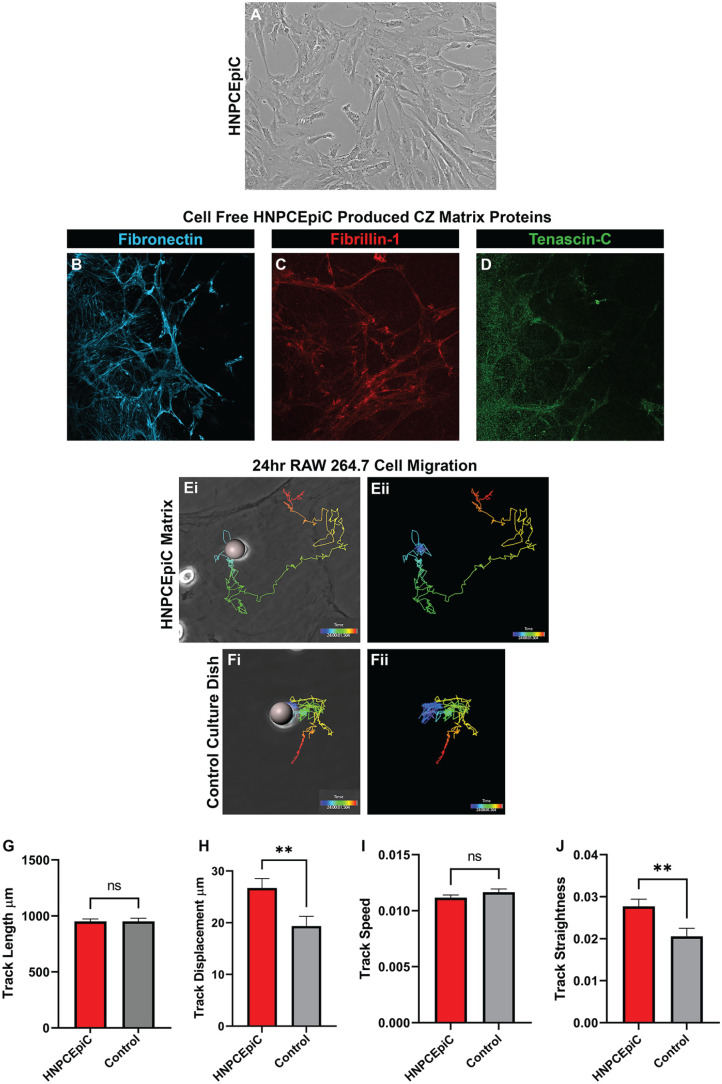
Immune cell migration on ciliary zonule protein matrices was examined by performing time-lapse imaging of RAW 264.7 cell migration, a monocyte/macrophage cell line, on a HNPCEpiC-conditioned substrate that mimics the protein composition and organization of ciliary zonule fibers. 38 Non-pigmented ciliary epithelial cells, which in vivo are located along the inner surface of the ciliary processes, are the source of the ciliary zonule proteins that link the ciliary body to the lens. 17 For these studies, primary HNPCEpiCs are grown to confluence (Figure 10(A)) prior to decellularization. The presence of ciliary zonule matrix molecules in the HNPCEpiC-conditioned matrix on the culture substrate, including those examined in our developmental studies, is confirmed by immunolocalization analysis. Fibrillin, the backbone of the zonule fibrils (Figure 10(C)), fibronectin (Figure 10(B)), and tenascin-C (Figure 10(D)) are all found to be primary components of the HNPCEpiC-conditioned matrix, and to associate with fibrillar matrix structures. RAW 264.7 cells are plated on decellularized HNPCEpiC-conditioned matrix substrates or control tissue culture substrates and time-lapse imaging performed over 24 h in eight spots/well. Imaris imaging software was used to trace the migration track of 126 individual cells on the HNPCEpiC substrate and 114 individual cells on tissue culture plastic. Representative tracks of cells on the HNPCEpiC substrate (Figure 10(E)) and the tissue culture substrate (Figure 10(F)) are shown with purple denoting the time 0 start site and red the cells’ position at 24 h. Imaris image analytics were applied to the tracked cells to quantify the total average distance the cells moved (Figure 10(G), track length), how far the cells moved from their site of origin at time 0 (Figure 10(H), track displacement), how fast the cells moved (Figure 10(I), track speed), and the straightness of the cell migration track (Figure 10(J), track straightness). While the immune cells had a similar speed and overall track length, there were significant differences in how far the cells moved and their ability to move in a directional manner, with the HNPCEpiC-produced matrix substrate found to be an excellent matrix environment for promoting immune cell migration.
Figure 10.
Ciliary zonule-like protein matrix promotes migration of immune cells. (A) Phase contrast image of primary cultures of human non-pigmented ciliary epithelial cells (HNPCEpiCs), the ciliary body cell type that produces the proteins that comprise the ciliary zonules (CZ). (B to D) Confocal microscopy imaging of HNPCEpiC-conditioned matrix substrates immunolabeled for (B) fibronectin, (C) fibrillin-1, or (D) tenascin-C. (E, F) Representative tracks of cells traced with Imaris software from 24-h time-lapse imaging of RAW 264.7 cells migrating on (E) HNPCEpiC-conditioned substrates and (F) tissue culture substrates. (Ei, Fi) phase contrast images of the position of cells at time 0 of the time-lapse study overlaid with the track of the cells with purple denoting the time 0 start site and red the cells’ position at 24 h. (Eii, Fii) Images showing the traced cell paths alone. (G to J) Bar graphs quantifying Imaris image analytics of 126 cells tracked on HNPCEpiC-conditioned substrates as compared to 114 cells tracked on tissue culture plastic to determine (G) track length, (H) track displacement, (I) track speed, and (J) track straightness. While the immune cells had a similar speed and overall track length, there were significant differences in how far the cells moved and their ability to move in a directional manner, with the HNPCEpiC-produced matrix substrate promoting directional immune cell migration. (A color version of this figure is available in the online journal.)
Immune cells associate with the fibrillar matrix of the vitreous during eye development
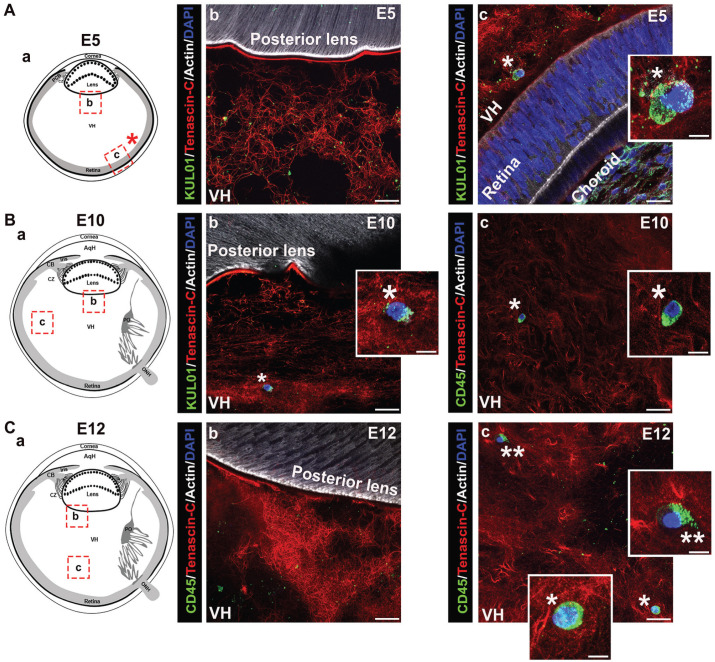
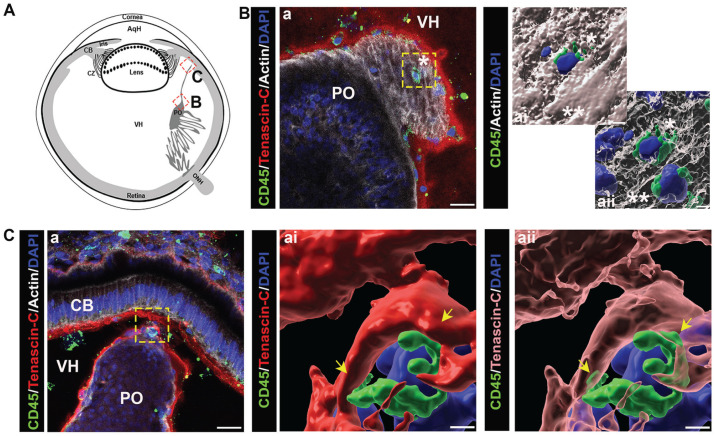
The vitreous has been identified as a site populated by hyalocytes, a monocyte/macrophage cell type with properties of tissue-resident innate immune cells, and which have been linked to both physiological and pathophysiological functions in the eye.28,39 –41 In this study, we examined whether the vitreous might also be a source of the resident immune cells that populate the lens during development. For these studies, cryosections prepared from E5 chick embryo heads, E10 chick embryo eyes, and E12 chick embryo eyes were immunolabeled for tenascin-C and co-immunolabeled for immune cells using either the KUL-01 antibody (Figure 11(A) and (Bb)) or the pan-leukocyte antibody CD45 (Figure 11(Bc) and (C)). The sections were co-labeled with DAPI to detect cell nuclei, and fluorescent-conjugated phalloidin which binds to F-actin. Confocal microscopy images were acquired in different regions of the vitreous as indicted in the diagrams (Figure 11(Aa) to (Ca)). While immune cells were detected among the tenascin-rich fibrils of the vitreous, including regions close to the retina (Figure 11(Ac) to (Cc)), we did not detect immune cells in the region where the vitreous contacts the posterior lens capsule, or in association with or crossing the posterior lens capsule (Figure 11(Ab) to (Cb)). These results do not exclude the possibility that immune cells from the vitreous compartment of the developing eye may provide resident immune cells to the lens at a stage of development we did not examine here. Note that the punctate labeling observed for the KUL-01 and CD45 immune cell labels may represent the ectodomain of these receptors left behind on the matrix as the cells migrate through the vitreous.
Figure 11.
Immune cells associate with the vitreous matrix during eye development. Cryosections of (A) E5 chick embryo heads, (B) E10 chick embryo eyes, and (C) E12 chick embryo eyes were immunolabeled for KUL-01 or CD45 (green), co-immunolabeled for tenascin-C (red), and co-labeled for F-actin with fluorescent-conjugated phalloidin (white) and for nuclei with DAPI (blue).Two regions within the vitreous of the eye were analyzed for the presence of immune cells by confocal microscopy, both in close proximity to the lens (Ab, Bb, and Cb) and further down within the vitreous (Ac, Bc, and Cc) as illustrated in the diagrams (Aa, Ba, and Ca). While at early stages of development, immune cells were identified among the tenascin C–rich fibrils of the vitreous in a region close to the retina, at later stages, immune cells were detected throughout the vitreous matrix. No immune cells were found in direct contact with the posterior lens capsule at any stage analyzed (Ab, Bb, and Cb). Insets represent high magnification of immune cells marked by asterisks in the lower magnification panels. (Ab, c, Cb, c) are projection images; (Bb, c) represent a single optical plane. Mag. bars: (Ab–c), (Bb–c), (Cb–c) = 20 µm and 5 µm for insets. AqH: aqueous humor; CB: ciliary body; CZ: ciliary zonules; ONH: optic nerve head; PCB: presumptive ciliary body; PO: pecten oculi; VH: vitreous humor. (A color version of this figure is available in the online journal.)
Development of the pecten oculi, a vascular structure of the avian eye
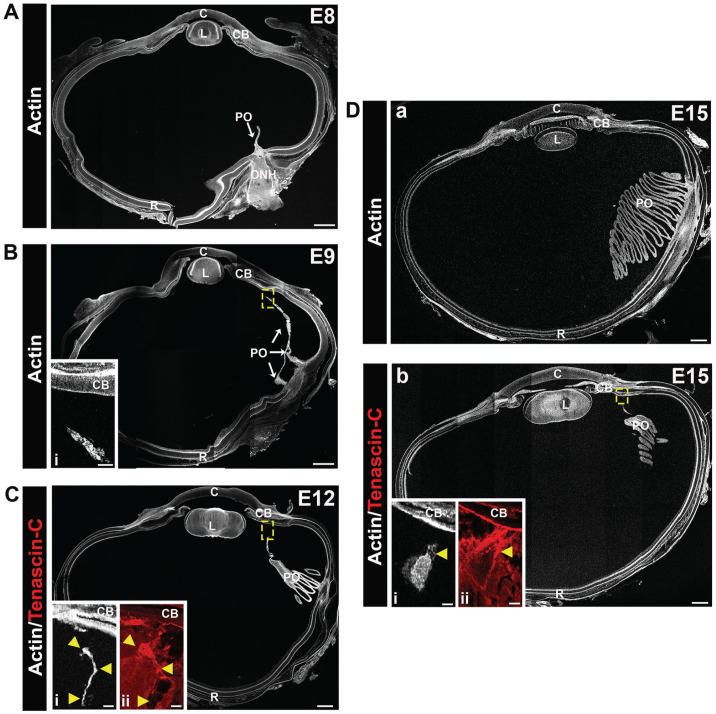
Avian species develop a unique vasculature called the pecten oculi.30,31 It is a pigmented, pleated structure, with the folds of the pleats joined together in a fan-like shape. During development, the pecten oculi begins to form at the head of the optic nerve, extends through the vitreous to a region adjacent to the ciliary body, and persists in the adult. Because of the potential that this vascular structure could be a source of immune cells that populate the developing avian lens, we examined its formation during development by labeling lens cryosections for F-actin with fluorescent-conjugated phalloidin at embryonic days 8, 9, 12, and 15, and imaging the sections by confocal microscopy (Figure 12). To determine whether there was a matrix link between the tip of the pecten oculi and the ciliary body, cryosections were also immunolabeled for tenascin-C and the results shown in higher magnification insets at E12 and E15 (Figure 12(C) and (Db)). The pecten oculi was shown to emerge as a thin projection from the posterior retina into the vitreous by E8 (Figure 12(A)). By E9, the developing pecten oculi was extended anteriorly toward the developing ciliary body (Figure 12(B), inset showing boxed area near the ciliary body at higher magnification). The classic pecten oculi pleats had formed by E12 (Figure 12(C)). Higher magnification at E12 showed that at this time in development the F-actin-labeled cells of the pecten oculi are very close to the ciliary body (Figure 12(Ci), with tenascin-C-rich fibrils providing a link between these two structures (Figure 12(Cii)). By E15, the structure of the pecten oculi was established, with its size and positioning in the vitreous requiring several sections to view its different regions (Figure 12(Da, b)). Higher magnification insets of the boxed region at E15 highlighted the tenascin-rich matrix fibrils that linked the pecten oculi to the ciliary body (Figure 12(Dbi, ii)).
Figure 12.
Tenascin-C matrix fibrils link the developing vasculature of the pecten oculi with the ciliary body. Cryosections of E8, E9, E12, and E15 chick eyes were labeled with fluorescent-conjugated phalloidin (white) to visualize the development of the chick pecten oculi. At E8 (A), the pecten oculi is identified as a thin projection from the optic nerve head (ONH) into the vitreous. By E9 (B), the pecten oculi extends anteriorly through the vitreous to the ciliary body, the region in the dashed box shown at higher magnification as an inset (Bi). At E12 (C), the pleated structure of the pecten has begun to develop, and is more extensive by E15 (Da, b). Higher magnification insets in C and D are from consecutive sections that are also immmunolabeled for tenascin-C (Ci, Di—F-actin [white]; Cii, Dii—tenascin-C [red]). Tenascin C–rich fibrils are shown to provide a link between the pectin oculi and the ciliary body. Arrowheads in Ci and Di indicate the same positions in (Ci) or (Dbi). Mag. bars: (A) to (D) = 500 and 50 µm for the insets. C: cornea; CB: ciliary body; L: lens; PO: pecten oculi; R: retina. (A color version of this figure is available in the online journal.)
Immune cells populate the matrix-rich regions adjacent to the pecten oculi during development
It has been shown previously that hyalocytes, immune cells that are located in the vitreous, accumulate along the surface of the pecten oculi pleats. 42 We have examined the localization of immune cells associated with the pecten oculi during development. For these studies, E12 whole eye cryosections were co-immunolabeled for CD45 and tenascin-C and co-labeled for F-actin and nuclei (Figures 13 and 14). At the posterior aspect of the pecten oculi where it emerges from the retina, CD45+ immune cells were detected both within (Figure 13(Ab, bii)) and along the outside edge of this structure (Figure 13(Ab, bi)). The higher magnification images highlight the different microenvironments of these immune cells (Figure 13(Abi, bii)), and that the immune cell along the outside edge of the pecten oculi is entwined within tenascin-C-rich microfibrils (Figure 13(Abi)). CD45+ immune cells were more abundant in the pleated region (Figure 13(B)). While CD45+ immune cells were detected within the pecten oculi and along its external surfaces (Figure 13(Bc, ci)), immune cells were most highly associated with the vitreal fibrillar matrix that is located between the pleats (Figure 13(Bb, bi, d)). A 3D surface rendering created from a z-stack of the boxed region in Figure 13(Bd) revealed the relationship of these immune cells with the tenascin-C fibrils of the vitreous and their migratory morphology including the extension of processes within the tenascin-C-rich fibrous matrix (Figure 13(Bdi, dii)). We also discovered the presence of immune cells at the apical tip of the pecten oculi at E12 (Figure 14). Here, CD45+ immune cells were located within the pecten oculi tip (Figure 14(Ba, ai, aii)) and associated with the tenascin-C-rich fibrous matrix that lines this tissue’s outer surface and connects the pecten oculi to the ciliary body (Figure 14(Ca, ai, aii)).
Figure 13.
Immune cells are highly concentrated in the vitreous matrix between the pleats of the pecten oculi. Cryosections of E12 chick eyes were co-immunolabeled with the pan-leukocyte marker CD45 (green). Nuclei were labeled with DAPI (blue). Confocal images were obtained in two regions as illustrated in the diagrams including the base of the optic nerve head (ONH) (Aa), and within the pleats (Ba) of the pecten oculi. Boxed in regions illustrate where each image was acquired. Immune cells are detected within the base of the ONH (Ab and Abii) and found entwined with tenascin-C fibrils on the outer edge of this structure (Ab and Abi). Immune cells were found to densely populate the pleated region of the pecten oculi, specifically the inner and outer surface of the folds (Bb, Bbi), in the matrix within the gaps between the folds (Bd), and more rarely within the fold itself (Bc, Bci). Migratory phenotype of immune cells entangled in matrix between folds is highlighted in 3D surface renderings of a confocal Z-stack from which (Bd) was taken with solid (Bdi) and transparent (Bdii) tenascin-C. Asterisks indicate immune cells at higher magnification from selected regions. Mag. Bars: Ab, Bb–d = 20 µm, Abi, Abii, Bbi, Bbii = 5 µm, Bdi, Bdii = 3 µm. PO, pecten oculi; ONH, optic nerve head; VH, vitreous humor. (Ab, Bb–c) Projection image; (Bd) single optical plane; (Bdi, Bdii) 3D surface structure. (A color version of this figure is available in the online journal.)
Figure 14.
The pecten oculi is a likely source of immune delivery to the ciliary body. (A) Diagram of an E12 chick eye with boxed areas indicating the regions imaged, both adjacent to the pleats (B) and at the apex of the pecten (C). E12 eyes were co-immunolabeled for CD45 (green) and tenascin-c (red), and co-labeled for F-actin (white), and nuclei (blue). CD45+ cells were detected within the tissue of the pecten oculi leading to the apex (Ba). Three-dimensional structural renderings were constructed of the boxed area in (Ba) with F-actin both solid (Bai) and transparent (Baii). Immune cells (single and double asterisks) are shown present within the pectin oculi apex and emerging from the apical tip of the pecten oculi associated with the tenascin-C fibrils connecting this structure and the matrix-rich lining of the ciliary body (Ca). Three-dimensional renderings of boxed area in Ca with tenascin-C solid (Cai) and transparent (Caii) highlight processes of the immune cell interacting with the surrounding matrix. (Ba) Single optical plane; (Bai, Baii, Cai, Caii) 3D surface structure and (Ca) projection image. Mag. bars: Ba, Ca = 20 µm, Bai, Baii = 3 µm, Cai, Caii = 2 µm. AqH: aqueous humor; CB: ciliary body; CZ: ciliary zonules; PO: pecten oculi; VH: vitreous humor. (A color version of this figure is available in the online journal.)
Discussion
The eye, like the CNS, is considered immune-privileged. In both, there are unique mechanisms that both prevent and resolve inflammation, which in the eye is essential to preserving clear vision. There are a number of intrinsic features that protect tissues of the eye from inflammation-induced damage. These include the absence of both an embedded vasculature and lymphatics, the induction of immunoprotective factors, and the ability to recruit immunoregulatory cells, including regulatory T-cells, in response to injury or pathogenesis.43,44 The induction of protective immune responses involves the activation of resident immune cells, such as those that populate the lens during development. Tissue-resident immune cells are the first responders to injury or pathogenic insults. 1 They are tasked with protecting tissues by maintaining homeostasis 45 and activating an inflammatory response.1,46 While resident immune cells are a feature of most tissues, there are only a few examples where they must reach and establish their tissue residence in sites of immune privilege like the eye. In the CNS, the microglia serve as its resident immune cells. When activated in response to injury or insult the microglia behave like resident macrophages. 13 Their colonization of the cerebral cortex during development has been shown to involve fibronectin and its receptor α5β1 integrin. 47 This finding suggests that matrix proteins like fibronectin with classical functions in regulating cell adhesion and migration may also be key to directing resident immune cells to the lens.
Similar to the eye, access to immune cells from the systemic circulation is restricted in the CNS, in this case by the blood–brain barrier (BBB).48 –50 Permeability of the BBB and the subsequent migration of leukocytes into the CNS are regulated by endothelial cell junctions and the pericytes surrounding its small blood vessels.48,50,51 In response to inflammation and disease, the trafficking of leukocytes to the CNS is increased through multiple routes.48,52 It is expected that the extravasation of immune cells from the vasculature of the eye involves similar mechanisms to those regulating the trafficking of immune cells from the BBB to the CNS. Indeed, our previous studies show that there is a similar response to an insult to the eye as has been described for the CNS. We found that immune cells are recruited to the surface of the lens, associating directly with the matrix compartment that encapsulates this avascular tissue, following wounding of the cornea. 6 Included among these lens capsule-associated immune cells are GR-1+ cells, 6 characteristic of immune cells with immunoregulatory functions.53,54 In the response to corneal wounding, we have shown that these immune cells travel from the ciliary body to the anterior cornea-facing surface of the lens along the ciliary zonule fibrils that link the lens to the vasculature of the ciliary body. 6
In this study, the matrix structures of the ciliary zonules and the vitreous that surround the lens during its development were examined as the potential pathways by which resident immune cells are delivered to the embryonic lens. Many important regulators of immune cell migration are known to associate with the fibrillin backbone of the ciliary zonules,18,55 including MAGP1, which regulates the bioavailability of transforming growth factor-β (TGFβ). 56 Another is the glycosaminoglycan hyaluronic acid (HA). 57 Immune cell attachment and migration along HA is mediated through their cell surface receptors CD44 and LYVE-1.58 –61 Interestingly, our studies have shown that the ectodomain of LYVE decorates the zonules of adult wild-type mice, likely left behind by migrating immune cells, and is increased along the zonules in N-cadΔlens mice whose lens dysgenesis leads to the immune-privileged lens becoming populated by immune cells. 5
The model for our investigations of the pathways by which resident immune cells could populate the lens during development was the chick embryo. The development of the avian eye differs from that of mammals in that their lens forms in the absence of a surrounding vasculature. Therefore, in the chick embryo eye, the path(s) taken by the resident immune cells to populate the lens during development will not be confounded by the presence of a transient vasculature that is characteristic of the embryonic mammalian eye. In this respect, the avian embryonic eye also serves as a good model for identifying migratory pathways of immune cells to the avascular lens in adult eyes, both avian and mammalian. Our new studies show that at early stages of development, the ciliary zonules are present as thin, wispy fibrillin-2 fibrils restricted to a limited region between the newly forming ciliary body and lens. As the eye continues to develop, these fibrils become more abundant and extensive, and fibrillin-2 is found in thicker fibers that more closely resemble those of the adult eye. We have previously shown that the ciliary zonules insert into the lens capsule just adjacent to where resident immune cells become integrated within the lens epithelium during development. 22 Proteomic analysis of the ciliary zonules from different sources, including human, highlights their molecular complexity, including many different matrix components and signaling molecules associated with the regulation of cell migration.55,62,63 Our studies now reveal that from very early stages of eye development both fibronectin and tenascin-C are highly associated with the fibrillin-2-rich zonule fibrils. Since the primary function of these two matrix molecules is the regulation of cell adhesion and migration,32 –35 this finding indicates that they are key factors in regulating immune cell migration along the early zonule fibrils to the lens.
The vitreous, which is located in the posterior eye and bounded by the zonules, the posterior lens capsule, and the retina, is a matrix-rich region of the eye. Its components have some overlap with those of the ciliary zonules. 55 In the adult eye, its principal matrix components are collagen I and HA, which confer it with gel-like properties.64,65 We find that fibrillin-2-rich fibrils are present throughout the vitreous from very early stages of development. These fibrils appear to interact with the fibrillin-2-rich region located along the surface of the posterior lens capsule. During development, these fibrillin-2 fibrils were highly coincident with collagen I. Many were also decorated with fibronectin and tenascin-C. In addition, there is a tenascin-C matrix network that extends into regions of the vitreous where fibrillin fibrils were not detected. The properties of fibronectin and tenascin-C and their presence along fibrillin-2 fibrils in both the vitreous and the ciliary zonules are consistent with functions in promoting immune cell adhesion and migration. Indeed, our data show that immune cells are associated with the fibrillar matrices of both the ciliary body and the vitreous during eye development.
We have detected immune cells along the matrix fibrils of the ciliary zonules and the vitreous as early as E5 when the primary fiber cells of the lens have formed but secondary fiber cell differentiation has not yet begun. At this early developmental time, the ciliary body is also immature and without its characteristic ciliary processes. The space between the developing ciliary body and lens is small, and as the zonules are relatively short the immune cells populating them appear close to both the ciliary body and the lens. Immune cells were also detected in the vitreous at this early developmental time, but were not found in proximity to the posterior aspects of the lens. The pecten oculi, a vascular structure that is located within the vitreous of avian eyes and is a source of immune cells, 66 has not yet formed. Little changes regarding the presence of immune cells in the vitreous and their absence from the region where the vitreous contacts the lens even after the vasculature of the pecten oculi has developed. In contrast, immune cells are numerous in the vitreous matrix located between the pleats of the pectin oculi. It is likely that these immune cells are hyalocytes sourced from the pecten oculi, similar to the hyalocytes that populate the vitreous from the hyaloid artery in developing mammalian eyes. While the hyaloid artery, located in the central light path, is removed prior to birth, the pecten oculi, located along an outer edge of the vitreous, is maintained in the adult. Our studies reveal that immune cells also localize both within the apical tip of this vascular structure and along tenascin-C-rich matrix fibrils that link the apical tip of the pecten oculi to the ciliary body.
In contrast to the absence of immune cells in the vitreous region that contacts the lens, as early as E9 immune cells are found emerging from the ciliary body onto the zonule fibrils, along the zonules approaching the lens equator, and just adjacent to where the ciliary zonules connect to the equatorial lens capsule. By E15, we also found ciliary zonule-linked immune cells migrating across the lens capsule zone at the lens equator (modeled, Figure 15). While it is most likely that the resident immune cells that populate the embryonic lens from the ciliary zonules are sourced from those immune cells that have emerged from the ciliary body, it cannot be excluded that they also include immune cells sourced, directly or indirectly from the pecten oculi located in the vitreous compartment of the eye. Our data provide strong support for the conclusions that the ciliary zonule fibrils provide a path for migration of resident immune cells to the lens during embryonic development, that these immune cells originate from the vasculature of the ciliary body, and that matrix proteins like fibronectin and tenascin-C likely promote their migration to the lens.
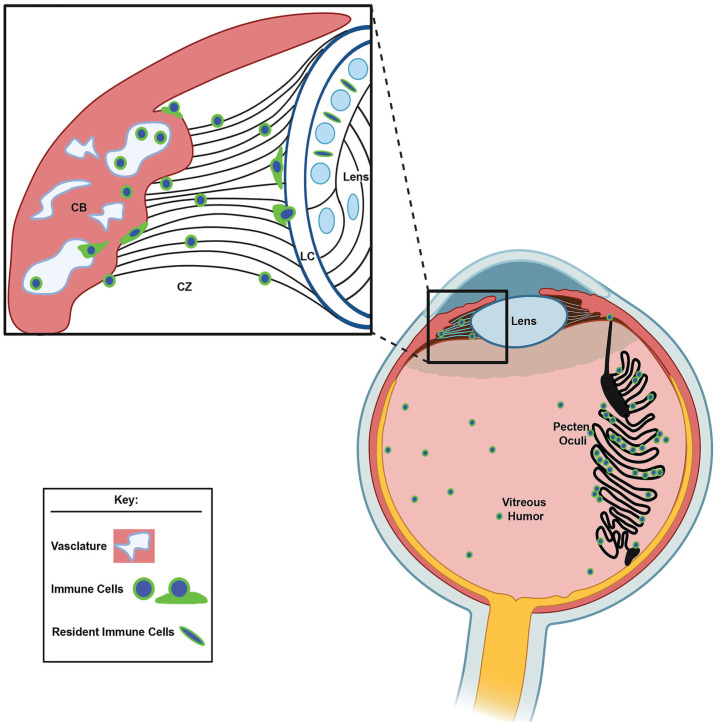
Figure 15.
An illustrated model of the role of the ciliary body in delivering resident immune cells to the lens during development. While our findings provide evidence that immune cells are distributed within the ciliary body, ciliary zonules, pecten oculi, and vitreous throughout development, we discovered that the primary source of immune cells to the embryonic lens is from the highly vascularized ciliary body along the ciliary zonules that link the ciliary body to the lens. The diagram highlights the steps in this process, including the egress of immune cells from the vasculature of the ciliary body, their migration across the zonule fibrils to the lens, and their movement across the equatorial capsule to take residence among the cells of the lens equatorial epithelium (zoomed illustration). CB: ciliary body; CZ: ciliary zonules; LC: lens capsule. Image created with BioRender. (A color version of this figure is available in the online journal.)
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221140411 for The ciliary zonules provide a pathway for immune cells to populate the avascular lens during eye development by JodiRae DeDreu, Phuong M Le and A. Sue Menko in Experimental Biology and Medicine
Acknowledgments
The authors thank Dr Robert Mecham for his gift of the MAGP1 and fibrillin-2 antibodies for the mouse immunolabeling studies.
Footnotes
Authors’ Contributions: ASM designed the research, analyzed the data, and wrote the paper. JD and PML performed the research, analyzed the data, wrote and edited the paper, and created the figures for the paper. All authors approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, National Eye Institute (EY021784 to ASM).
ORCID iD: A. Sue Menko  https://orcid.org/0000-0002-7514-4696
https://orcid.org/0000-0002-7514-4696
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 2013;14:986–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016;17:765–74 [DOI] [PubMed] [Google Scholar]
- 3. Snyder ME, Farber DL. Human lung tissue resident memory T cells in health and disease. Curr Opin Immunol 2019;59:101–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brazil JC, Quiros M, Nusrat A, Parkos CA. Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest 2019;129:2983–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logan CM, Bowen CJ, Menko AS. Induction of immune surveillance of the dysmorphogenic lens. Sci Rep 2017;7:16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeDreu J, Bowen CJ, Logan CM, Pal-Ghosh S, Parlanti P, Stepp MA, Menko AS. An immune response to the avascular lens following wounding of the cornea involves ciliary zonule fibrils. FASEB J 2020;34:9316–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Li Z, Quan Y, Cheng H, Riquelme MA, Li XD, Gu S, Jiang JX. Macrophage recruitment in immune-privileged lens during capsule repair, necrotic fiber removal, and fibrosis. iScience 2021;24:102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou Y, Bennett TM, Shiels A. Mutation of the TRPM3 cation channel underlies progressive cataract development and lens calcification associated with pro-fibrotic and immune cell responses. FASEB J 2021;35: e21288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menko AS, DeDreu J, Logan CM, Paulson H, Levin AV, Walker JL. Resident immune cells of the avascular lens: mediators of the injury and fibrotic response of the lens. FASEB J 2021;35:e21341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker JL, Menko AS. Immune cells in lens injury repair and fibrosis. Exp Eye Res 2021;209:108664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nat Immunol 2019;20:793–801 [DOI] [PubMed] [Google Scholar]
- 12. Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol 2003;74:172–8 [DOI] [PubMed] [Google Scholar]
- 13. Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci 2010;17:6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray JI, Farber DL. Tissue-resident immune cells in humans. Annu Rev Immunol 2022;40:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 2016;44:439–49 [DOI] [PubMed] [Google Scholar]
- 16. Miesfeld JB, Brown NL. Eye organogenesis: a hierarchical view of ocular development. Curr Top Dev Biol 2019;132:351–93 [DOI] [PubMed] [Google Scholar]
- 17. Jones W, Rodriguez J, Bassnett S. Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome. Dis Model Mech 2019;12:dmm037283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Tu Y, De Maria A, Mecham RP, Bassnett S. Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest Ophthalmol Vis Sci 2013;54:2504–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeyer KA, Reinhardt DP. Fibrillin-containing microfibrils are key signal relay stations for cell function. J Cell Commun Signal 2015;9:309–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark RA, Erickson HP, Springer TA. Tenascin supports lymphocyte rolling. J Cell Biol 1997;137:755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abbadi D, Laroumanie F, Bizou M, Pozzo J, Daviaud D, Delage C, Calise D, Gaits-Iacovoni F, Dutaur M, Tortosa F, Renaud-Gabardos E, Douin-Echinard V, Prats AC, Roncalli J, Parini A, Pizzinat N. Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Cardiovasc Res 2018;114:123–37 [DOI] [PubMed] [Google Scholar]
- 22. DeDreu J, Walker JL, Menko AS. Dynamics of the lens basement membrane capsule and its interaction with connective tissue-like extracapsular matrix proteins. Matrix Biol 2021;96:18–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tucker RP. The distribution of J1/tenascin and its transcript during the development of the avian cornea. Differentiation 1991;48:59–66 [DOI] [PubMed] [Google Scholar]
- 24. Giblin SP, Schwenzer A, Midwood KS. Alternative splicing controls cell lineage-specific responses to endogenous innate immune triggers within the extracellular matrix. Matrix Biol 2020;93:95–114 [DOI] [PubMed] [Google Scholar]
- 25. Fernandes NRJ, Reilly NS, Schrock DC, Hocking DC, Oakes PW, Fowell DJ. CD4(+) T cell interstitial migration controlled by fibronectin in the inflamed skin. Front Immunol 2020;11:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Digiacomo G, Tusa I, Bacci M, Cipolleschi MG, Dello Sbarba P, Rovida E. Fibronectin induces macrophage migration through a SFK-FAK/CSF-1R pathway. Cell Adh Migr 2017;11:327–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coito AJ, de Sousa M, Kupiec-Weglinski JW. Fibronectin in immune responses in organ transplant recipients. Dev Immunol 2000;7:239–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vagaja NN, Chinnery HR, Binz N, Kezic JM, Rakoczy EP, McMenamin PG. Changes in murine hyalocytes are valuable early indicators of ocular disease. Invest Ophthalmol Vis Sci 2012;53:1445–51 [DOI] [PubMed] [Google Scholar]
- 29. Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol 2008;19:125–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerhardt H, Liebner S, Wolburg H. The pecten oculi of the chicken as a new in vivo model of the blood-brain barrier. Cell Tissue Res 1996;285:91–100 [DOI] [PubMed] [Google Scholar]
- 31. Pettigrew JD, Wallman J, Wildsoet CF. Saccadic oscillations facilitate ocular perfusion from the avian pecten. Nature 1990;343:362–3 [DOI] [PubMed] [Google Scholar]
- 32. Yamada KM, Aota S, Akiyama SK, LaFlamme SE. Mechanisms of fibronectin and integrin function during cell adhesion and migration. Cold Spring Harb Symp Quant Biol 1992;57:203–12 [DOI] [PubMed] [Google Scholar]
- 33. Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev 1984;3:43–51 [DOI] [PubMed] [Google Scholar]
- 34. Chiquet-Ehrismann R. Tenascins. Int J Biochem Cell Biol 2004;36:986–90 [DOI] [PubMed] [Google Scholar]
- 35. Adams JC, Chiquet-Ehrismann R, Tucker RP. The evolution of tenascins and fibronectin. Cell Adh Migr 2015;9:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell 2009;20:846–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashworth JL, Kielty CM, McLeod D. Fibrillin and the eye. Br J Ophthalmol 2000;84:1312–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shiroto Y, Terashima S, Hosokawa Y, Oka K, Isokawa K, Tsuruga E. The effect of ultraviolet B on fibrillin-1 and fibrillin-2 in human non-pigmented ciliary epithelial cells in vitro. Acta Histochem Cytochem 2017;50:105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakamoto T, Ishibashi T. Hyalocytes: essential cells of the vitreous cavity in vitreoretinal pathophysiology. Retina 2011;31:222–8 [DOI] [PubMed] [Google Scholar]
- 40. Boneva SK, Wolf J, Rosmus DD, Schlecht A, Prinz G, Laich Y, Boeck M, Zhang P, Hilgendorf I, Stahl A, Reinhard T, Bainbridge J, Schlunck G, Agostini H, Wieghofer P, Lange CAK. Transcriptional profiling uncovers human hyalocytes as a unique innate immune cell population. Front Immunol 2020;11:567274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolf J, Boneva S, Rosmus DD, Agostini H, Schlunck G, Wieghofer P, Schlecht A, Lange C. Deciphering the molecular signature of human hyalocytes in relation to other innate immune cell populations. Invest Ophthalmol Vis Sci 2022;63:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Korkmaz D, Kum S. Investigation of the antigen recognition and presentation capacity of pecteneal hyalocytes in the chicken (gallus gallus domesticus). Biotech Histochem 2016;91:212–9 [DOI] [PubMed] [Google Scholar]
- 43. Hong S, Van Kaer L. Immune privilege: keeping an eye on natural killer T cells. J Exp Med 1999;190:1197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol 2003;74:179–85 [DOI] [PubMed] [Google Scholar]
- 45. De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de, Casterlé I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2018;175:400–1513 [DOI] [PubMed] [Google Scholar]
- 46. Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology 2015;144:541–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smolders SM, Swinnen N, Kessels S, Arnauts K, Smolders S, Le Bras B, Rigo JM, Legendre P, Brône B. Age-specific function of α5β1 integrin in microglial migration during early colonization of the developing mouse cortex. Glia 2017;65:1072–88 [DOI] [PubMed] [Google Scholar]
- 48. Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, Engelhardt B, Hallenbeck JM, Lonser RR, Ohlfest JR, Prat A, Scarpa M, Smeyne RJ, Drewes LR, Neuwelt EA. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab 2013;33:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alajangi HK, Kaur M, Sharma A, Rana S, Thakur S, Chatterjee M, Singla N, Jaiswal PK, Singh G, Barnwal RP. Blood-brain barrier: emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mol Brain 2022;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016;19: 771–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 2008;14:1581–93 [DOI] [PubMed] [Google Scholar]
- 52. Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 2011;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 2021;21:485–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19:108–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Maria A, Wilmarth PA, David LL, Bassnett S. Proteomic analysis of the bovine and human ciliary zonule. Invest Ophthalmol Vis Sci 2017;58: 573–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Broekelmann TJ, Bodmer NK, Mecham RP. Identification of the growth factor-binding sequence in the extracellular matrix protein MAGP-1. J Biol Chem 2020;295:2687–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan FL, Choi HL, Underhill CB. Hyaluronan and chondroitin sulfate proteoglycans are colocalized to the ciliary zonule of the rat eye: a histochemical and immunocytochemical study. Histochem Cell Biol 1997;107:289–301 [DOI] [PubMed] [Google Scholar]
- 58. Underhill C. CD44: the hyaluronan receptor. J Cell Sci 1992;103:293–8 [DOI] [PubMed] [Google Scholar]
- 59. Jackson DG. Hyaluronan in the lymphatics: the key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol 2019;78–79:219–35 [DOI] [PubMed] [Google Scholar]
- 60. Maytin EV. Hyaluronan: more than just a wrinkle filler. Glycobiology 2016;26:553–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Johnson LA, Jackson DG. Hyaluronan and its receptors: key mediators of immune cell entry and trafficking in the lymphatic system. Cells 2021; 10:2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bassnett S. Zinn’s zonule. Prog Retin Eye Res 2021;82:100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM. Proteomic analysis of fibrillin-rich microfibrils. Proteomics 2006;6:111–22 [DOI] [PubMed] [Google Scholar]
- 64. Theocharis DA, Skandalis SS, Noulas AV, Papageorgakopoulou N, Theocharis AD, Karamanos NK. Hyaluronan and chondroitin sulfate proteoglycans in the supramolecular organization of the mammalian vitreous body. Connect Tissue Res 2008;49:124–8 [DOI] [PubMed] [Google Scholar]
- 65. Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res 2000;19:323–44 [DOI] [PubMed] [Google Scholar]
- 66. Wolburg H, Liebner S, Reichenbach A, Gerhardt H. The pecten oculi of the chicken: a model system for vascular differentiation and barrier maturation. Int Rev Cytol 1999;187:111–59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221140411 for The ciliary zonules provide a pathway for immune cells to populate the avascular lens during eye development by JodiRae DeDreu, Phuong M Le and A. Sue Menko in Experimental Biology and Medicine