Abstract
While mouse models and two-dimensional (2D) cell culture systems have dominated as research tools for cancer biology, three-dimensional (3D) cultures have gained traction as a new approach that retains features of in vivo biology within an in vitro system. Over time, 3D culture systems have evolved from spheroids and tumorspheres to organoids, and by doing so, they have become more complex and representative of original tissue. Such technological improvements have mostly benefited the study of heterogeneous solid tumors, like those found in breast cancer (BC), by providing an attractive avenue for scalable drug testing and biobank generation. Experimentally, organoids have been used in the BC field to dissect mechanisms related to cellular invasion and metastasis—and through co-culture methods—epithelial interactions with stromal and immune cells. In addition, organoid studies of wild-type mouse models and healthy donor samples have provided insight into the basic developmental cellular and molecular biology of the mammary gland, which may inform one’s understanding of the initial stages of cancer development and progression.
Keywords: Breast cancer, organoids, extracellular matrix, personalized medicine, mammary gland development, high-throughput drug testing
Impact Statement
Given the high rate of breast cancer in women, and its highly heterogeneous nature, the utilization of patient-derived three-dimensional (3D) organoid systems may allow for powerful high-throughput drug testing, which can guide treatment decisions and empower personalized medicine.
Introduction
For most of the history of cancer research, in vitro studies were often restricted to the culture of immortalized cell lines. While the study of these systems was useful and appropriate to answer certain scientific questions, we know that the nature of two-dimensional (2D) cultures introduces many artifacts and prevents full recapitulation of the three-dimensional (3D) structure of the tissue environment. For example, the use of immortalized cell lines in 2D lacks the dynamic spatial and cellular heterogeneity found in tissues, which influences cellular survival and communication.1,2 In addition, the repeated selection for proliferative clones through passaging of cell lines introduces genomic and gene expression alterations, such that key aspects of patient tumors in vivo are lost in 2D. 3
More specifically to the breast, 3D culture originated with the development of spheroids, and later, mammospheres and tumorspheres. 4 These culturing methods were made possible by the discovery of collagenase, an enzyme which allows for digestion of tissues like the mammary gland, for subsequent culture of cellular clusters ex vivo. 5 These multicellular, heterogeneous units were first cultured on floating rat tail collagen gel membranes.6,7 Early efforts revealed the ability of stem-like cells in human mammary tissue to self-propagate and grow as mammospheres on non-adherent plates. These mammary early progenitor cells could be grown at a low density and give rise to differentiated mammary cell types when grown in Matrigel. 8 Mammospheres (also known as spheroids due to their shape) can form branching structures after weeks in culture and have been utilized for the analysis of cancer stem cell (CSC) properties. 9
The inspiration for breast “organoid” culture originates from the approach and culture components first designed for intestinal organoids that were applied and modified for the culture of breast epithelial cells. 10 The terminology has rapidly evolved, and at present, breast organoid culture refers to the culture of groups of cells isolated from mammary tissue that through a combination of self-organization and preservation of in vivo cellular architecture form mammary structures in culture, rather than from existing fragments of ducts or lobules.4,11
Alongside advances in the field of 3D culturing of breast specimens, a few lingering questions regarding their ability to mimic native human breast tissue and tumorigenic potential remained. In response, the field re-optimized conventional techniques, giving rise to what we call today, “patient-derived organoid” (PDO) systems. 12 This striking advancement in 3D culture systems brought about an opportunity to better mimic and interrogate the heterogeneity of breast neoplasia and expanded our understanding of the classic phenotypes of hormone positive and negative BC subtypes. 13 Such information allowed for the formulation of growth conditions which largely preserve this heterogeneity 14 in experimental models, a critical requisite to better characterizing BC and parsing out pathophysiological mechanisms that underlie drug sensitivity and treatment resistance.
The next-generation PDOs also arrived to fill a gap regarding the initial stages of BC development, which have mostly been investigated in mouse models of cancer progression. Genetically engineered mouse models (GEMMs) have revealed underlying molecular mechanisms of BC such as cellular interactions and immune contributions that drive the transition of a normal-like epithelial cell into a transformed cell with an enhanced ability to propagate and metastasize. 15 However, despite the utility of GEMMs in expanding our understanding of cancer biology and disease treatment, several of the existing mouse models of BC only partially recapitulate the human condition at the genetic, cellular, and tissue level (i.e. cellular heterogeneity and the spatiotemporal dynamics of disease progression). To attempt to circumvent this, models have been employed in which human BC tissue is grown in a mouse—however, due to cross-species immune recognition, these animals do not possess intact immunity.
Given the clear evidence that immune cells (such as myeloid derived suppressor cells, tumor-associated macrophages, TAMs, cancer-associated fibroblasts, CAFs) play a critical role in supporting BC development, these models are inadequate to fully understand cancer development, progression, and treatment.16–19 From this perspective, the ability to use normal-like PDO cultures to understand normal breast development in samples from women with high risk of developing BC represents a unique advantage relative to GEMMs. Moreover, the development of PDOs and organoids from GEMMs has allowed for the establishment of highly tunable co-culture systems, providing an opportunity to study the effects of hormone signaling, immune cell cross-talk, and stromal composition on both normal and malignant breast development.20–24 Thus, PDOs provide a robust system for the study of an array of processes contributing to breast development and neoplasia.
In this review, we discuss the history of organoid cultures, their versatility as a tool to study basic mammary gland developmental biology, and their potential to make personalized, precision medicine a reality and ultimately improve patient care and outcomes for BC patients.
The history of breast tissue in a dish
In recent decades, organoid cultures have emerged to meet the need for studying patient biology in vitro. Historically, normal breast epithelium was first cultured on floating collagen gels. 6 In general, in this review, “on” will refer to epithelia cultured on top of a substrate, whereas “in” will be used to describe when cells are grown inside of/embedded in a culture matrix such as Matrigel. Using this approach in Matrigel (discussed in detail in the following section), the Bissell group was able to culture both normal and transformed breast epithelia (from human breast carcinoma cell lines and primary cultures from normal and tumor biopsies) in 3D with the goal of comparing normal breast development and cancer cell biology. 25 When grown in Matrigel—which contains extracellular matrix (ECM) components and growth factors—the epithelium developed structures, and displayed cellular organization consistent with morphologies seen in native breast tissue, as characterized by sphere size immunofluorescence analysis of the epithelial cells grown in 3D.
These initial strategies were adapted and further developed for the culturing of malignant BC tissue as tumorspheres using markers for putative adult mammary stem cells (MaSCs) identified by studies in mammospheres. 26 Mammosphere culture was optimized by the Visvader group and relies on cell sorting of MaSCs followed by culture in Matrigel. Applying this protocol to cells from tumor tissue enables culture of tumorspheres, which has been used to dissect the role of tumor-associated CSCs: also known as tumor-initiating cells.27,28 These cells require further study in BC, and cancer broadly, as they are thought to precede the development of a frank tumor and thus represent an avenue for early targeted therapy before development of a tumor. In addition to culturing in Matrigel, tumorspheres can be cultured on, or within, collagen I gels. 29 Emerging evidence from tumorsphere studies suggest that CSCs may engage a more aggressive transcriptional program to the generally quiescent (unless exposed to pregnancy hormones) MaSCs. 30 Tumorspheres can also be used for drug screening. 31
Modern organoid protocols involve establishing cultures from fresh tissue, rather than cultured cells, as previously discussed. As a result of this effort, there are now many variations for how to isolate and culture breast epithelium as organoids, including distinct chemical and mechanical digestion approaches for tissue preparation and separation.32–34 These differences are subtle but can be tailored to the approach at hand (e.g. culturing tumor vs. normal tissue and mouse vs. human) and should be clearly conveyed in protocols.35,36 Importantly, many of these subtle differences center around disruption of particular components of the ECM, which is made up of proteins that are crucial for tissue structure and organization.
Importance of the culture matrix
The ECM is a non-cellular, structural component of the tissue environment. 37 The stroma is made up of the ECM, stromal cells (namely fibroblasts) and soluble mediators. Within and throughout the ECM, cell-to-cell and cell-to-matrix interactions occur via cell surface proteins like integrins and soluble mediators, such as hormones, cytokines, and chemokines. These interactions provide essential cues for cellular differentiation, communication, and motility: all required for the proper development of the mammary gland and implicated in cancer development. 38 Within the breast, the most abundant components of the mammary ECM are collagen (sub-classified into type I, II, III, IV), laminins, proteoglycans, glycosaminoglycans, and fibronectin.35,36
There is growing appreciation of how the ECM, and cellular perception of stiffness, directs cellular behaviors in disparate processes such as gene expression, cellular differentiation, and tissue organization at homeostasis and including cellular invasion, metastasis, and drug responsiveness of BC cells.39–42 For example, collagen prolyl 4-hydroxylase is upregulated by BC cells during tumor progression and has been shown through organoid studies to be a key mediator of BC cell sensitization to chemotherapies (docetaxel and doxorubicin) which means the dynamic remodeling of the ECM in BC is important for both progression and treatment resistance. 43 Sensation of the ECM by stromal cells is sufficiently precise such that breast carcinoma lines grown in 3D demonstrate invasiveness dependent on collagen matrix alignment. 44 However, this collagen alignment technology has not yet been applied to studying organoids grown in 3D. Overall, better appreciating the role of the ECM in BC is of crucial importance as mammographic density is a known risk factor for the development of BC and the mechanisms behind this connection require further study. 45 The organoid system provides an attractive model for dissecting the role of the ECM and breast tissue density as it relates to BC incidence and progression.
The most commonly used matrix for mammary and breast organoids is Matrigel©: sold by Corning and derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells. 46 Matrigel contains primarily laminin as well as collagen IV, entactin, and fibronectin, which collectively provide a 3D microenvironment which more closely mimics native tissue than 2D culture. Thus, organoids retain higher-order tissue structures similar to those observed histologically in breast tissue. 47 However, and given that Matrigel does not include all components of the breast ECM, such analyses do not fully capture the contribution of components such as proteoglycans and glycosaminoglycans on cellular behaviors. Therefore, and in order to investigate the role of these additional ECM components on organoid cultures, alternative hydrogels should be considered.
In addition to Matrigel, which is a multicomponent hydrogel, organoids can also be grown in gels of specifically collagen I to isolate the role of this ECM component. 48 Such conditions enable the precise study of cellular responses that are not strongly present in Matrigel-based conditions, such as cellular motility and epithelial-to-mesenchymal transition (EMT), due to the axial orientation of collagen I fibers and activation of the ERK pathway by fibronectin provided by the ECM.35,49 In fact, studies utilizing collagen I as the organoid matrix find that this promotes branching morphogenesis and invasiveness, while the Matrigel-based culturing system mainly induces alveologenesis, thus supporting that distinct systems must be considered according to the scientific questions being pursued.50,51 The combination of Matrigel and collagen I for organoid culturing is an additional option. 52 In summary, collagen, and its orientation, is critical for eliciting cellular invasion into the extracellular space compared to culturing in Matrigel, and thus brings to light the utility of combining various culture components to better understand how matrix composition and content both independently and collectively inform mammary epithelial cell behavior relevant to homeostasis and neoplasia.44,53
Given variability across commercially distributed batches of Matrigel and collagen I (such as batch-to-batch variation in composition, concentration, or pH), efforts are ongoing to develop a standardized matrix for organoid culture with the goal of providing greater experimental control across patient-derived systems.54,55 In addition, collagen gels can be engineered to specifically enhance certain ECM features such as alignment. 44 In parallel, synthetic hydrogels are being developed for the culture of stem cells and organoids, including for breast organoids, thus far in hydrogels of glycosaminoglycans.56,57 Advancements in synthetic hydrogels and those designed specifically for breast organoid culture may yield benefits for the field. In summary, the matrix culture conditions are highly biologically relevant and should be chosen with care based on the experimental question.
Normal and neoplastic breast systems
A unique benefit of organoid culture is the ability to grow both neoplastic cells and normal epithelium. When grown under the same conditions, healthy and tumor mammary organoids can be utilized in parallel as a chimeric system to study both cancer and normal biology. In the case of patient-derived tissues, the use of tumor organoids in conjunction with adjacent normal tissue from the same individual serves as an excellent internal control for subsequent study as compared to normal tissue derived from other sources which inevitably introduces variation from differential sample collection, storage time, patient history, and genetic differences (to name a few).
From a developmental biology perspective, organoid culturing preserves cellular and molecular aspects of the breast epithelium, thus providing a platform to understand outstanding biological questions that, until now, could only be properly addressed in tissue from model organisms.14,58 For example, the optimization of culture conditions that allow for the dissection of gene regulation and cellular dynamics during the response to pregnancy hormones, lactation, and involution in rodents may represent a step closer to understanding how normal PDOs respond to the same stimuli.24,59 Given that pregnancy is one of the most important life history factors that modulates BC, such studies may allow for the identification of mechanisms and pathways that support the development of strategies for BC prevention.60–62 Moreover, similar organoid systems could support the study and characterization of BC tissue obtained from pregnant women, thus enabling the investigation of cancer-associated mechanisms ex vivo that were present during in vivo development.
From a cancer perspective, multiple studies have demonstrated the robustness of patient-derived breast cancer organoids (BC PDOs) in terms of preservation of cancer features observed in the primary tumor, and have highlighted them as excellent tools for the assessment and validation of cancer treatments.13,14,63 Crucially, PDO lines can be stably derived from all BC clinical subtypes.13,64 In fact, and in response to its powerful utility, initiatives have been put in place for the establishment of fully characterized BC PDO biobanks that have been made available to the scientific community (Table 1). In addition, PDOs have been shown to be powerful tools for in vivo tumor development as well, representing the next generation of patient-derived xenografts (PDX) when employed as patient-derived organoid xenografts (PDxOs) in mouse models.63,65 Historically, PDX research has provided great insight into mechanisms underlying cancer progression and metastasis initiation as it allows for one to study patient cancer cells in an experimentally tractable model organism. 66 However, a major pitfall of classical PDX studies results from transplantation into an immunodeficient host, which does not recapitulate the microenvironment of a tumor, with the selective pressure of the immune system, in vivo.
Table 1.
Comparison of organoid culture conditions for mouse and human mammary organoids.
| Mouse organoid media components | Human organoid media components | References supplying publicly available PDOs (with necessary MTAs) |
|---|---|---|
| FGF2 a (5 nM) | R-spondin1 | Guillen et al. 65 |
| ITS (insulin/transferrin/selenium, 1X) | Noggin | Sachs et al. 13 |
| FGF7, FGF10, EGF, TGFα, Wnt3a, R-spondin1 | B27 | |
| Conditions to induce pregnancy | Wnt3a | |
| 17-β-Estradiol (40 ng/mL), Progesterone (120 ng/mL),
Prolactin (120 ng/mL) For at least 2 days |
Nicotinamide | |
| Conditions to study involution and lactation | N-acetylcysteine | |
| 1 µg/mL prolactin for 4 days to stimulate lactation, withdrawal of prolactin for 10 days to stimulate involution | Y-27632 (Rock inhibitor—can be removed after first passaging—however, suggested by Sachs et al. to be useful for gene editing protocol) | |
| FGF-7, FGF-10 | ||
| A83-01 | ||
| EGF | ||
| Neuregulin 1 |
-Both human and mouse organoids are cultured in Advanced DMEM F12+++ (5 mM GlutaMax, 5 mM HEPES, 1X penicillin/streptomycin).
-In italics if used by some but not all groups.
-The ATCC is compiling and offering an organoid bank and developing an organoid reagent catalog, however at a charge to investigators.
This component of murine mammary organoid culture should be removed for 24 h before adding in a biologically active component such as pregnancy hormones (a cocktail or estrogen, progesterone, and prolactin) or other reagents such as plasma. 43
The 3D organoid systems were optimized by the Clevers and Rios groups for genetic targeting before transplantation into mice via lentiviral, electroporation, or commercially available reagents (like Lipofectamine 2000). Once modified, PDOs are xenografted to NSG mice and tumor formation takes 2–16 weeks. 64 In this same study, Dekkers et al. show that knockout of p53 and PTEN in normal organoids using lentiviral transduction resulted in organoids with BC features from normal tissue that yielded ER+ tumors after transplantation to mice. This shows how normal organoids combined with genetic manipulation can be employed to study early genetic drivers of BC. This procedure—genetic manipulation of PDOs followed by PDxOs—can be applied to establish tumor xenografts from normal tissue with the selection of any BC relevant gene of interest: exemplifying a powerful merging of techniques (organoids and genetic engineering) to define and explore BC targets. 64
Alongside technical and experimental advantages, PDOs have also been used for basic studies characterizing particular BC patient populations of interest, such as triple-negative breast cancer (TNBC). 63 By deploying single-cell RNA-sequencing analysis of TNBC PDOs, one study discovered populations of luminal progenitor epithelial cells (LPs) that were predominantly enriched in organoid systems derived from TNBC patient specimens, confirming observations previously raised in mouse studies and those from molecular profiling of TNBC primary tumor samples that found elevated luminal progenitor signatures in TNBC tumor tissue, suggesting the LP as the cell of origin for this aggressive BC tumor type.14,63,67
Overall, for culturing normal and tumor breast organoids, the principles are the same for human and mouse. Small differences in plating density, media conditions, frequency of passaging do exist and must be noted (Table 1). Despite any technical differences in protocols, the organoid community has repeatedly demonstrated the cellular and molecular similarity of organoids to their tissue of origin, empowering the utility and interpretability of this system to answer questions relating to homeostasis and cancer of the breast.13,14,47,64
Organoids for the study of metastatic mechanisms and progression
Because of the robust nature of the system, organoids enable detailed and mechanistic studies of a complex and, at times, experimentally intractable problem in cancer biology, the process of BC metastasis.
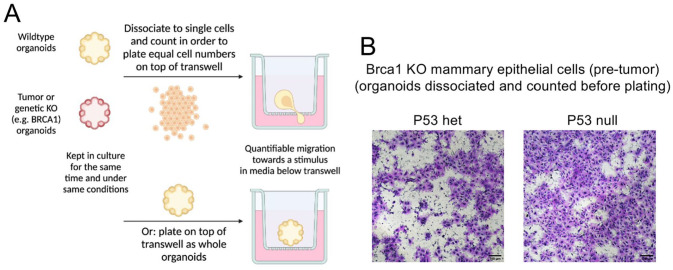
For example, building upon established systems derived from patient specimens and GEMMs, organoids can be assayed for the metastatic ability of epithelium using a transwell migration assay (Figure 1). In this assay, whole organoids or organoids dissociated to single cells are placed on top of a semi-permeable membrane (termed a “transwell”) such that their ability to migrate toward a stimulus placed in the separated culture environment below can be tested. This stimulus can be a particular growth factor, female hormones, conditioned media derived from specific cell types, like fibroblasts or immune cells, or patient-derived biological substances such as plasma. Given that metastasis is the leading cause of cancer-related deaths and represents a major unresolved barrier in the field of BC research, this strategy may allow for defining the physiological alterations and molecular changes which specifically interfere with BC metastasis in patient-derived specimens.
Figure 1.
Transwell migration assay with normal Brca1 KO (knockout) murine mammary organoids. (A) Overall scheme to test migratory properties of organoid cultures. Mammary-derived organoid cultures can either be dissociated into single cells or plated as whole organoids, into transwell chambers. (B) Normal Brca1 KO mammary-derived organoid cultures, either bearing 1 copy or total loss of the p53 gene, were tested for their mobility capacity in transwell chambers, indicating differential migratory capacity based on p53 genotype. Scale bar: 100 µm.
In addition, because it is an optically accessible system, live imaging and confocal microscopy of organoids have been utilized to study and quantify disseminating cells as a proxy for metastasis in cultures derived from patient tumor samples and GEMMs.68,69 More recently, combination with florescent tagging has enabled the identification of specific cell types for the study of EMT processes during the initial stages of metastatic progression in organoids derived from both murine mammary tumors and human BC specimens.48,70 Interestingly, this strategy also allowed for identification of culture conditions better suited for metastasis analysis, with Matrigel conditions considered preferable for tracking tumor growth, while collagen I floating gels are more suitable for the quantification of invasion/dissemination. While this is not a strict dichotomy, it is worth testing hypotheses relating to invasion and metastasis in both culture matrix environments or in varying combinations of the two.
As transplantation assays of mammary carcinoma cell lines into the mammary gland of syngeneic mice have been commonly used as an in vivo metastasis model, replicating this system by transplanting organoids derived from GEMMs could also be used to evaluate the metastatic potential of those cells in a wild-type, immunocompetent host. 71 As these methods are adapted for PDxOs, the clinical relevance of these studies will be further amplified.
Organoid and immune cell co-culture
With the advent of immunotherapies for cancer, many of which target T cells, organoid co-culture with T cells has emerged as a powerful tool in the pancreatic, lung, and gastric cancer fields. 72 This approach has been used to induce and characterize tumor-specific T-cell responses and their specific T-cell receptors.73,74
Because of the paramount importance of the interaction of BC epithelial cells with surrounding stromal and immune cells within the tissue microenvironment, methods have emerged for the study of this interaction using organoid co-culture. Since the stromal and immune compartments can play both pro and antitumor roles during BC development and progression, co-culture systems are an attractive model to parse out these diverse roles and interactions during pathogenesis.
In one study of normal murine mammary organoids, the addition of fibroblasts to the culture was sufficient to induce organoid branching. 20 This finding was confirmed by another group, which showed this interaction requires Wnt signaling by fibroblasts. 21 Other than branching, another study found that CAFs can induce invasiveness and migration in PDOs. 75 In addition to CAFs, TAMs are implicated in promoting BC, a notion supported by organoid experiments showing that the addition of M2-polarized TAMs increased invasiveness of organoids derived from PyMT murine mammary epithelium. 76 Co-culture systems have also been used to show that normal, but not tumor-exposed natural killer (NK) cells are able to restrain organoid size growth and reduce disseminating cells. 77
However, this organoid-stromal and immune co-culture system has yet to be applied widely to study interactions of the adaptive immune system, such as T cells and additional immune cell types, and the epithelium of BC patient-derived systems. As with dissemination, such co-culture application of organoids is well suited and amenable to live imaging studies. Therefore, in addition to the 3D power of organoid culture, it also enables “4D” studies involving a time component in which the cells of the organoid grow over time and/or interact spatially with other key cell types.
From bench to bedside: toward personalized medicine
While the application of organoid technology to developing biobanks of patient tumor and normal culturing systems is useful, it does not fully realize the potential of PDOs in materializing personalized medicine.
Preliminary studies testing drug responses of PDOs to a panel of BC drugs known to inhibit steps of the Her2 signal transduction pathway—comprising afatinib, gefitinib, pictilisib, everolimus, an AKT inhibitor (GDC-0068), and mTORC2 inhibitor (AZD8055)—found that some, but not all, Her2+ PDOs respond to Her2 pathway blocking drugs, thus suggesting intrinsic intratumor heterogeneity that could contribute to the return of untreatable disease. This drug screening was accomplished using cell viability assays after testing over 20 concentrations of each drug. Intriguingly, the same study found that PDOs derived from patient specimens histopathologically classified as Her2 positive did not respond to any of the above-tested Her2-targeting drugs, suggesting that BC patients may benefit from non-traditional BC therapies early in their treatment. 13
In fact, studies have revealed that drugs typically used for other cancer types, such as bortezomib (a proteasome inhibitor commonly utilized for the treatment of multiple myeloma and mantle cell lymphoma), had efficacy against tumor PDOs from a luminal BC that had metastasized to the liver. 78 It is clear that cancer therapies outside of those traditionally used for BC should be considered, especially in the context of metastatic BC, and can now be tested in a high-throughput manner using PDOs. Additional treatment strategies utilizing afatinib, pictilisib, fulvestrant, and others have already been tested in PDOs and PDxOs and have led to the conclusion that, despite classification as the same subtype, there is variability in drug response as well as the dosage required to elicit response.13,65 The study by the Welm group built upon the cell viability assay for drug testing by expanding it to a 384-well format and quantifying canonical readouts of drug response like cell growth and cytotoxicity. 65
Drug testing and repurposing screens using PDOs and PDxOs are not only needed to better prevent disease relapse, but are particularly warranted for advancing the treatment of TNBC, as potentially unknown molecular vulnerabilities may be uncovered to target this particularly aggressive BC subtype. Studies along these lines have investigated the efficacy of PARP inhibitors in Brca1 knockout GEMM-derived mammary organoids and found that diverse mechanisms enable BC cells to acquire resistance to PARP inhibitor therapy. 79 Insights such as these could ultimately guide treatment choices after or even prior to the development of drug resistance. For example, an unbiased screen of TNBC-derived PDOs found that some, but not all, responded to a preclinical compound, birinapant (an Smac mimetic). 65
In an “n of 1” application of PDOs and PDxOs to guiding TNBC clinical care, Guillen et al. followed one stage II TNBC patient with organoid studies using banked tissue throughout their treatment course in order to inform their care. During this study, when liver metastases emerged, PDO characterization of the pretreatment tumor tissue revealed the BC cells were sensitive to eribulin through a comprehensive in vitro drug screen, an observation that was further confirmed in vivo in PDxOs. This drug was then added to the patient’s treatment regimen and led to an improvement in PFS (progression-free survival) and TTNT (time to next systemic therapy) than that typically achieved by the previously prescribed therapy uninformed by organoid studies (a combination of doxorubicin, cyclophosphamide, paclitaxel in this case). 65 Extension of such initiatives to the investigation of a collection of TNBC PDOs in large-scale drug screenings with rapid changes to patient regimens and associated infrastructure prepared in advance may help identify and implement potential new druggable targets. In addition, there is great hope that this application of organoids can inform clinical decisions about outcomes, treatment expectations, and adverse side effects.
Conclusions and future directions
Given our understanding of the value of organoid systems as tools for treatment development and evaluation, an immediate direction the field can take is to determine how aspects of patient-derived BC intrinsic heterogeneity intersect with drug response, immune activation, and disease relapse. As we witness a rapid transformation in the application of 3D organoid systems to addressing outstanding basic and clinical questions, the future advancements of such approaches will show that a single method can empower a synergistic relationship between studies of homeostatic and pathophysiologic biology, lending a new tool to harnessing and understanding patient-specific tumor features that may be exploitable therapeutically.
Indeed, organoid cultures cannot recapitulate all aspects of the tumor microenvironment nor all aspects of the whole-body pathophysiology of cancer. Therefore, studies utilizing such systems should be completed in parallel with in vivo experiments, such as in mouse models. However, at their best, in vivo and in vitro approaches will generate data that inform each other and spark new experiments and lines of questioning that might not have arisen without this interplay of methods and approaches.
Footnotes
Authors’ Contributions: All authors wrote, edited, and reviewed the manuscript. M.K.C. contributed the images for Figure 1.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the CSHL and Northwell Health Affiliation; the Pershing Square Sohn Prize for Cancer Research (awarded to C.O.D.S.); the National Institutes of Health/National Cancer Center [R01CA248158-01] to C.O.D.S.; the National Institutes of Health/National Institute on Aging [R01 AG069727-01] to C.O.D.S.; the National Institutes of Health Medical Scientist Training Program [T32-GM008444] to S.M.L.
ORCID iD: Camila O dos Santos  https://orcid.org/0000-0002-3999-9523
https://orcid.org/0000-0002-3999-9523
References
- 1. Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 2006;7:211–24 [DOI] [PubMed] [Google Scholar]
- 2. Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007;130:601–10 [DOI] [PubMed] [Google Scholar]
- 3. Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007; 8:839–45 [DOI] [PubMed] [Google Scholar]
- 4. Simian M, Bissell MJ. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol 2017;216:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lasfargues EY. Cultivation and behavior in vitro of the normal mammary epithelium of the adult mouse. Anat Rec 1957;127:117–29 [DOI] [PubMed] [Google Scholar]
- 6. Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 1977;13:316–28 [DOI] [PubMed] [Google Scholar]
- 7. Ehrmann RL, Gey GO. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst 1956;16:1375–403 [PubMed] [Google Scholar]
- 8. Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003;17:1253–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw FL, Harrison H, Spence K, Ablett MP, Simões BM, Farnie G, Clarke RB. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia 2012;17:111–7 [DOI] [PubMed] [Google Scholar]
- 10. Sato T, Vries RG, Snippert HJ, Van De Wetering M, Barker N, Stange DE, Van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–5 [DOI] [PubMed] [Google Scholar]
- 11. Labarge MA, Garbe JC, Stampfer MR. Processing of human reduction mammoplasty and mastectomy tissues for cell culture. J Vis Exp 2013; 71:50011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schutgens F, Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol Mech Dis 2020;15:211–34 [DOI] [PubMed] [Google Scholar]
- 13. Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte AA, Lelieveld D, van Hoeck A, Ernst RF, Blokzijl F, Nijman IJ, Hoogstraat M, van de Ven M, Egan DA, Zinzalla V, Moll J, Boj SF, Voest EE, Wessels L, van Diest PJ, Rottenberg S, Vries RGJ, Cuppen E, Clevers H. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 2018;172:373.e10–86.e10 [DOI] [PubMed] [Google Scholar]
- 14. Rosenbluth JM, Schackmann RCJ, Gray GK, Selfors LM, Li CMC, Boedicker M, Kuiken HJ, Richardson A, Brock J, Garber J, Dillon D, Sachs N, Clevers H, Brugge JS. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat Commun 2020;11:1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez-Cuadrado L, Tracey N, Ma R, Qian B, Brunton VG. Mouse models of metastasis: progress and prospects. Dis Model Mech 2017;10:1061–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer 2021;21:345–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Betts CB, Pennock ND, Caruso BP, Ruffell B, Borges VF, Schedin P. Mucosal immunity in the female murine mammary gland. J Immunol 2018;201:734–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cha YJ, Koo JS. Role of tumor-associated myeloid cells in breast cancer. Cells 2020;9:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, Bonneau C, Kondratova M, Kuperstein I, Zinovyev A, Givel AM, Parrini MC, Soumelis V, Vincent-Salomon A, Mechta-Grigoriou F. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018;33:463.e10–79.e10 [DOI] [PubMed] [Google Scholar]
- 20. Koledova Z, Lu P. A 3D fibroblast-epithelium co-culture model for understanding microenvironmental role in branching morphogenesis of the mammary gland. Methods Mol Biol 2017;1501:217–31 [DOI] [PubMed] [Google Scholar]
- 21. Wang J, Song W, Yang R, Li C, Wu T, Dong XB, Zhou B, Guo X, Chen J, Liu Z, Yu QC, Li W, Fu J, Zeng YA. Endothelial Wnts control mammary epithelial patterning via fibroblast signaling. Cell Rep 2021;34:108897. [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee S, Bhat V, Berdnikov A, Liu J, Zhang G, Buchel E, Safneck J, Marshall AJ, Murphy LC, Postovit LM, Raouf A. Paracrine crosstalk between fibroblasts and ER+ breast cancer cells creates an IL1β-enriched niche that promotes tumor growth. iScience 2019;19:388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Adileh M, Martin ML, Klingler S, White J, Ma X, Howe LR, Brown AMC, Kolesnick R. Establishing estrogen-responsive mouse mammary organoids from single Lgr5+ cells. Cell Signal 2017;29:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciccone MF, Trousdell MC, dos Santos CO. Characterization of organoid cultures to study the effects of pregnancy hormones on the epigenome and transcriptional output of mammary epithelial cells. J Mammary Gland Biol Neoplasia 2020;25:351–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A 1992;89:9064–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature 2006;439:84–8 [DOI] [PubMed] [Google Scholar]
- 27. Bajaj J, Diaz E, Reya T. Stem cells in cancer initiation and progression. J Cell Biol 2020;219:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou BBS, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov 2009;8:806–23 [DOI] [PubMed] [Google Scholar]
- 29. Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res 2007;67:8671–81 [DOI] [PubMed] [Google Scholar]
- 30. Sun Q, Wang Y, Officer A, Pecknold B, Lee G, Harismendy O, Desgrosellier JS. Stem-like breast cancer cells in the activated state resist genetic stress via TGFBI-ZEB1. NPJ Breast Cancer 2022;8:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halfter K, Hoffmann O, Ditsch N, Ahne M, Arnold F, Paepke S, Grab D, Bauerfeind I, Mayer B. Testing chemotherapy efficacy in HER2 negative breast cancer using patient-derived spheroids. J Transl Med 2016;14:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001; 128:3117–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 2008;14:570–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development 2006;133:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu J, Xiong G, Trinkle C, Xu R. Integrated extracellular matrix signaling in mammary gland development and breast cancer progression. Histol Histopathol 2014;29:1083–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hynes RO, Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 2012;4:a004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Werb Z, Lu P. The role of stroma in tumor development. Cancer J 2015;21:250–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006;22:287–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020;11:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014;15:802–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joyce MH, Lu C, James ER, Hegab R, Allen SC, Suggs LJ, Brock A. Phenotypic basis for matrix stiffness-dependent chemoresistance of breast cancer cells to doxorubicin. Front Oncol 2018;8:337–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Insua-Rodríguez J, Pein M, Hongu T, Meier J, Descot A, Lowy CM, De Braekeleer E, Sinn HP, Spaich S, Sütterlin M, Schneeweiss A, Oskarsson T. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol Med 2018;10:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM, O’Connor K, Lane AN, Xu R. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat Commun 2018;9:4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ray A, Slama ZM, Morford RK, Madden SA, Provenzano PP. Enhanced directional migration of cancer stem cells in 3D aligned collagen matrices. Biophys J 2017;112:1023–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227–36 [DOI] [PubMed] [Google Scholar]
- 46. Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med 1977;145:204–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buchmann B, Engelbrecht LK, Fernandez P, Hutterer FP, Raich MK, Scheel CH, Bausch AR. Mechanical plasticity of collagen directs branch elongation in human mammary gland organoids. Nat Commun 2021;12:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sirka OK, Shamir ER, Ewald AJ. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J Cell Biol 2018;217:3368–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene 2014; 33:1649–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lo AT, Mori H, Mott J, Bissell MJ. Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J Mammary Gland Biol Neoplasia 2012;17:103–10 [DOI] [PubMed] [Google Scholar]
- 51. Nguyen-Ngoc KV, Ewald AJ. Mammary ductal elongation and myoepithelial migration are regulated by the composition of the extracellular matrix. J Microsc 2013;251:212–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caruso M, Huang S, Mourao L, Scheele CLGJ. A mammary organoid model to study branching morphogenesis. Front Physiol 2022;13:826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ray A, Callaway MK, Rodríguez-Merced NJ, Crampton AL, Carlson M, Emme KB, Ensminger EA, Kinne AA, Schrope JH, Rasmussen HR, Jiang H, DeNardo DG, Wood DK, Provenzano PP. Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. JCI Insight 2022;7:e150330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Calar K, Plesselova S, Bhattacharya S, Jorgensen M, de la Puente P. Human plasma-derived 3D cultures model breast cancer treatment responses and predict clinically effective drug treatment concentrations. Cancers 2020;12:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prince E, Cruickshank J, Ba-Alawi W, Hodgson K, Haight J, Tobin C, Wakeman A, Avoulov A, Topolskaia V, Elliott MJ, McGuigan AP, Berman HK, Haibe-Kains B, Cescon DW, Kumacheva E. Biomimetic hydrogel supports initiation and growth of patient-derived breast tumor organoids. Nat Commun 2022;13:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nowak M, Freudenberg U, Tsurkan MV, Werner C, Levental KR. Modular GAG-matrices to promote mammary epithelial morphogenesis in vitro. Biomaterials 2017;112:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater 2020;5:539–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Signati L, Allevi R, Piccotti F, Albasini S, Villani L, Sevieri M, Bonizzi A, Corsi F, Mazzucchelli S. Ultrastructural analysis of breast cancer patient-derived organoids. Cancer Cell Int 2021;21:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sumbal J, Chiche A, Charifou E, Koledova Z, Li H. Primary mammary organoid model of lactation and involution. Front Cell Dev Biol 2020;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Slepicka PF, Cyrill SL, dos Santos CO. Pregnancy and breast cancer: pathways to understand risk and prevention. Trends Mol Med 2019;25: 866–81 [DOI] [PubMed] [Google Scholar]
- 61. Feigman MJ, Moss MA, Chen C, Cyrill SL, Ciccone MF, Trousdell MC, Yang ST, Frey WD, Wilkinson JE, dos Santos CO. Pregnancy reprograms the epigenome of mammary epithelial cells and blocks the development of premalignant lesions. Nat Commun 2020;11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Somasundara AVH, Moss MA, Feigman MJ, Chen C, Cyrill SL, Ciccone MF, Trousdell MC, Vollbrecht M, Li S, Kendall J, Beyaz S, Wilkinson JE, dos Santos CO. Parity-induced changes to mammary epithelial cells control NKT cell expansion and mammary oncogenesis. Cell Rep 2021;37:110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bhatia S, Kramer M, Russo S, Naik P, Arun G, Brophy K, Andrews P, Fan C, Perou CM, Preall J, Ha T, Plenker D, Tuveson DA, Rishi A, Wilkinson JE, McCombie WR, Kostroff K, Spector DL. Patient-derived triple-negative breast cancer organoids provide robust model systems that recapitulate tumor intrinsic characteristics. Cancer Res 2022;82:1174–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dekkers JF, van Vliet EJ, Sachs N, Rosenbluth JM, Kopper O, Rebel HG, Wehrens EJ, Piani C, Visvader JE, Verissimo CS, Boj SF, Brugge JS, Clevers H, Rios AC. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat Protoc 2021;16:1936–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guillen KP, Fujita M, Butterfield AJ, Scherer SD, Bailey MH, Chu Z, Derose YS, Zhao L, Cortes-Sanchez E, Yang C, Toner J, Wang G, Qiao Y, Huang X, Vahrenkamp JM, Lum DH, Factor RE, Nelson EW, Matsen CB, Poretta JM, Rosenthal R, Beck AC, Buys SS, Vaklavas C, Ward JH, Jensen RL, Jones KB, Li Z, Oesterreich S, Dobrolecki LE, Pathi SS, Woo XY, Berrett KC, Wadsworth ME, Chuang JH, Lewis MT, Marth GT, Gertz J, Varley KE, Welm BE, Welm AL. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer 2022;3:232–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Canc Res 2015;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 2009;15:907–13 [DOI] [PubMed] [Google Scholar]
- 68. Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 2014;15:647–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nguyen-Ngoc KV, Shamir ER, Huebner RJ, Beck JN, Cheung KJ, Ewald AJ. 3D culture assays of murine mammary branching morphogenesis and epithelial invasion. Methods Mol Biol 2015;1189:135–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Padmanaban V, Grasset EM, Neumann NM, Fraser AK, Henriet E, Matsui W, Tran PT, Cheung KJ, Georgess D, Ewald AJ. Organotypic culture assays for murine and human primary and metastatic-site tumors. Nat Protoc 2020;15:2413–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer 2011;11:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN, Voest EE. Tumor organoid–T-cell coculture systems. Nat Protoc 2020;15:15–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL, Kaing S, Kelderman S, van Rooij N, van Leerdam ME, Depla A, Smit EF, Hartemink KJ, de Groot R, Wolkers MC, Sachs N, Snaebjornsson P, Monkhorst K, Haanen J, Clevers H, Schumacher TN, Voest EE. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018;174:1586–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meng Q, Xie S, Gray GK, Dezfulian MH, Li W, Huang L, Akshinthala D, Ferrer E, Conahan C, Perea Del Pino S, Grossman J, Elledge SJ, Hidalgo M, Muthuswamy SK. Empirical identification and validation of tumor-targeting T cell receptors from circulation using autologous pancreatic tumor organoids. J Immunother Cancer 2021;9:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Corsa CAS, Brenot A, Grither WR, Van Hove S, Loza AJ, Zhang K, Ponik SM, Liu Y, DeNardo DG, Eliceiri KW, Keely PJ, Longmore GD. The action of discoidin domain receptor 2 in basal tumor cells and stromal cancer-associated fibroblasts is critical for breast cancer metastasis. Cell Rep 2016;15:2510–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009;16:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chan IS, Knútsdóttir H, Ramakrishnan G, Padmanaban V, Warrier M, Ramirez JC, Dunworth M, Zhang H, Jaffee EM, Bader JS, Ewald AJ. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J Cell Biol 2020;219:e202001134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, Lei JH, Wang L, Bi J, Shao N, Shu D, Wu B, Wu J, Yang Z, Wang H, Wang B, Xiong K, Lu Y, Fu S, Choi TK, Lon NW, Zhang A, Tang D, Quan Y, Meng Y, Miao K, Sun H, Zhao M, Bao J, Zhang L, Xu X, Shi Y, Lin Y, Deng C. Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv Sci 2021;8:e2101176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Duarte AA, Gogola E, Sachs N, Barazas M, Annunziato SR, de Ruiter J, Velds A, Blatter S, Houthuijzen JM, van de Ven M, Clevers H, Borst P, Jonkers J, Rottenberg S. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat Methods 2018; 15:134–40 [DOI] [PubMed] [Google Scholar]