Abstract
Osteochondritis dissecans of the knee is a disease that typically affects skeletally immature patients. Clinically manifested with knee pain, limping, and joint disfunction, this condition has remained misunderstood and undervalued for a long period. Although being a rare condition, its awareness is of utmost clinical interest because of the possible severe consequences it can bring when misrecognized or inadequately treated. Its etiology remains unclear and is still debated. Many theories have been proposed, including inflammation, local ischemia, subchondral ossification abnormalities, genetic factors, and repetitive mechanical microtrauma, with a likely interplay of the same. This review article aims to deliver and discuss current and up-to-date concepts on epidemiology, etiology, and natural history of this pediatric condition.
Level of evidence: level V.
Keywords: Osteochondritis dissecans, knee, children, epidemiology, etiology
Introduction
Osteochondritis dissecans (OCD) is a disease of unknown etiology involving the osteochondral unit of the joint. Sequestration of subchondral bone with possible articular cartilage involvement can occur, leading to osteochondral fragment instability and eventual detachment. 1 Even though the disorder was first described by Konig in 1887, 2 the cause, management, and prognosis of this pathologic condition are far from understood.
The most affected joint is the knee, followed by the ankle, elbow, shoulder, and hip. The condition typically involves a solitary joint; however, some children can develop OCD in several. 3
OCD is classified in juvenile (JOCD) and adult (AOCD), but being a classification based on the status of the growth plate, the terms “open physes” and “closed physes” should be utilized. 4 The “open physis” form affects skeletally immature patients and generally has a better prognosis, while the “closed physis” form affects young adults and is typically characterized by a poorer prognosis.5,6 While OCD is generally considered an idiopathic phenomenon, various etiopathogenetic factors are currently the matter of investigation, including local ischemia, accessory centers of endochondral ossification, repetitive microtrauma, and familiar or genetic predisposition.7–10 The clinical presentation of the OCD of the knee can be rather variable, but usually characterized by knee pain and discomfort that can lead to disability and early osteoarthritis. However, many children can be completely asymptomatic up to the end stages of the disease.
Our knowledge of pathogenesis has recently improved; many issues remain subject to debate, especially for what concerns etiopathogenesis, magnetic resonance imaging (MRI) findings, treatment methods, and feasibility of developing a treatment algorithm. The aim of this article is to address recent updates in the orthopedic literature about the epidemiology, etiology, and natural history of OCD of the knee in skeletally immature patients.
Epidemiology
The exact incidence of OCD of the knee remains still unclear; however, the most recent studies indicate that the incidence ranges between 2.3 and 31.6 cases per 100,000 people with skeletal immaturity, reflecting differences in ethnicity, sex, and age.11–13
Knee OCD rarely presents before the age of 6 years, while it peaks most frequently between 13 and 17 years. 12 In an incidence analysis, Kessler et al. 13 founded that subjects aged from 12 to 19 years old have three times the risk of developing knee OCD compared with subjects aged from 6 to 11 years, regardless of sex and ethnicity.
With regard to gender, males appear to be more affected than females, with a two- to four-fold higher incidence depending on the studies considered.11–13 Nevertheless, some authors14–16 reported a present-day increase in incidence in girls, probably related to the rise in female participation and specialization in high-impact sports. In the ROCK (Research in Osteochondritis Dissecans of the Knee) investigation, female patients were found to have an earlier presentation than males by a year. Although this divergence does not reach statistical significance, it may indicate a clinical relevance due to sex differences in skeletal maturity. 17
In terms of ethnicity and race, Black people presented higher risk of OCD compared with other ethnic groups. 13 However, Whites had 2.6 times a greater risk of OCD than Hispanics, 2.1 times a greater risk than Asians/Pacific Islanders and as much as and 5 times a greater risk of disease than those of mixed ethnicity. The incidence analysis then demonstrated that non-Hispanic Blacks had the highest incidence of knee OCD (31.6/100,000), whereas the lowest incidence was observed in Asians (4.7/100,000), establishing significant interracial differences.
The possible role of sports practice in the incidence of knee OCD has been speculated for decades; interestingly, OCD of the knee was historically referred as “catcher’s knee.” The ROCK study enrolled 1004 knees of both males and female patients. 71.7% and 59.1% were multisport athletes, and almost the entirety of the subjects enrolled considered themselves athletes. 17 Not surprisingly, authors found symptomatic OCD to be more common in young patients participating in sports requiring explosive movements of lower extremities such as basketball and soccer for both sexes. Although these sports were the most practiced in the cohort enrolled, OCD lesions did not appear to be confined to sports that involved cutting tasks or acceleration and deceleration profiles of the lower limbs. Moreover, individuals practicing a primary sport for more than 8 months per year did not have a higher risk of OCD of the knee. This information suggested that frequent sport participation may be associated with the development of knee OCD during the prepubescent transition to adolescence rather than direct trauma. 18
The most classic location for OCD lesions of the knee is the posterolateral aspect of the medial femoral condyle (MFC), which represents a watershed area for vascularity.19,20 To the best of our knowledge, only two multicenter studies examined the location of defects. 17 Up to 77% of osteochondral lesions were in the MFC, 51% of the lesions were on the lateral aspect of the MFC, 19% in the center, and 7% on medial aspect. The lateral femoral condyle (LFC) was involved in 17%–18% of cases, patella in 6%–7%, femoral trochlea in 1%–9.5%, and tibial plateau in 0.2%. Data regarding patellofemoral OCD are deficient and probably underestimate the incidence of the disease in this peculiar location. 21
Etiology
The precise cause of OCD remains unclear, although a number of etiological theories have been proposed. 22 Mechanical, biological, hereditary, and anatomical factors are all speculated to play a role in the development of this disease, whose origin can therefore be considered multifactorial.16,23
Mechanical factors have historically been investigated as primarily responsible for the occurrence of OCD of the knee.24,25 This theory predicts a single major traumatic event or multiple smaller but repeated micro-traumatic events as the main cause. Major trauma or the repetitive stress evokes subchondral reaction that probably interferes with bony trabecular healing, preventing the ability of the bone to recover.
Some authors 8 hypothesize a correlation between the size of the anterior tibial spine (ATS) and the presence of knee OCD. This theory dates back as far as 1957, when Smillie 26 speculated that OCD was related to impingement between the ATS and the lateral chondral aspect of the medial condyle. However, the authors’ statements at that time required further corroborating data.
This concept of impingement between the ATS with the MFC is consistent with the repeated microtrauma etiology proposed by different groups,27,28 although better investigated in other location, such as the elbow joint.29,30 Wechter et al. 31 conducted a study evaluating the anteroposterior and lateral radiographs view of the knee, demonstrating that knees with MFC OCD lesions possessed greater posterior and medial tibial slope in comparison to both normal contralateral knee and controls. However, knees with LFC OCD lesions had no significant difference in lateral tibial slope compared with the contralateral knee or matched controls. Finally, posterior slope was greater in patients with medial OCD lesions than matched controls. An MRI analysis confirmed that patients presenting knee OCD had a more prominent tibial eminence than patients without OCD. 8
Impingement of the tibial eminence on the MFC was also investigated by Chow et al., 32 proving that knees with MFC OCD lesions presented significantly smaller notch width index (NWI) than matched controls. This theory adequately justifies the classic location of OCD of the knee, which is on the posterolateral aspect of the MFC.
In fact, this theory suggests a mechanism of traumatic contact during knee flexion between the most prominent part of the tibia (ATS) and its counterpart on the femur, which turns out to be the posterolateral aspect of the MFC. The finding of OCD typically localized on this area of the femur therefore fits well with this hypothesis.
Other anatomical variants, like a more distal location of the footprint of the posterior cruciate ligament (PCL) as well as the presence of a discoid lateral meniscus (DLM) may further affect the mechanical environment in the knee.4,33,34 Complete discoid menisci were associated with a central OCD, while incomplete discoid menisci were associated with peripheral OCD. 35 Mitsuoka et al. 36 even reported a case of OCD with a complete DLM in a 10-year-old male that completely healed after partial arthroscopic meniscectomy, without specific treatment for the osteochondral lesion of the LFC. The authors suggested that an abnormal repetitive loading on weaker osteochondral structures by a damaged DLM could be one of the main causes of OCD of the LFC.
Still concerning the role of the menisci, some authors recently identified meniscal instability as a biomechanical alteration implicated in the genesis of OCD. In particular, Camathias et al. 37 postulated that hypermobility of the anterior horn could be a contributing factor through repeated impingement of the loose meniscal edge between the articular surfaces. This prolonged mechanical stress in the end may result in damaging the overhanging corresponding femoral osteochondral unit. This postulation was formulated by the authors by observing increased meniscal instability at the anatomical correspondence of the osteochondral lesion in most patients undergoing knee OCD surgery.
MRI is not specific to detect meniscal instability and arthroscopic evaluation is a diagnostic necessary point in case of symptomatic patients. 38 A specific arthroscopic aspiration test was described to detect meniscal instability of the posterior horn of the lateral meniscus. 39
These remarks on meniscal instability are in accordance with what mentioned above about the discoid meniscus. Indeed, in both cases, the chondral articular surfaces are exposed to excessive and repetitive stresses during knee flexion and extension owing to the presence of excessive meniscal encumbrance. Interestingly, Takigami et al. 40 observed that a DLM with a meniscal shift type C according to Ahn’s MRI classifications should be considered as predictive factors for OCD of the LFC.
Many studies20,41 demonstrated a high correlation between OCD location and lower limb mechanical axis deviation. An association was found between varus and valgus axis, respectively, with medial and lateral condyle OCD. 42 Moreover, the convergence of the mechanical axis with the location of the OCD lesion may be considered an associated factor in fragment instability. This convergence is more common in unstable OCD. 41 Other biomechanical factors, including obesity, 43 and soft-tissue instability such as cruciate ligaments hypoplasia 44 have also been implicated.
Knee activity–related positioning has been considered central to the pathogenesis of OCD for a long time. Younger age of OCD presentation and a more posterior location on both femoral condyles were found more typically in baseball catchers than position players (non-catchers). These findings may represent the effects of repetitive and persistent loading of the knees in the hyperflexed position specific to catchers. 45
Local ischemia has been proposed as another causative factor for OCD. The vascular anatomy of subchondral bone is characterized by low arteriole anastomoses predisposing to ischemic insult. Green and Banks 24 were the first to emphasize the role of ischemic subchondral bone. Campbell and Ranawat 46 suggested classifying OCD as idiopathic aseptic necrotic lesions of the epiphysis, showing in their histological examinations a large ischemic bone area beneath the detached fragment. Multiple studies23,47 stated that there is an interplay of vascular and traumatic factors of subchondral bone ischemia, leading to the development of OCD.
This etiologic hypothesis has similarity features with another pediatric orthopedic condition: Legg–Calvè–Perthes disease, which consists of a hip disorder caused by a temporary disruption of blood flow to the femoral head. Deprived of an adequate blood supply, the bone cells die, in a process referred to as “avascular necrosis.” 48
Based on this, some possible genetic associations were investigated, in particular genes involved in heritable thrombophilic risk.49–52 To the best of our knowledge, no similar studies have yet been conducted for what pertains to the genetics of OCD, but it could be a cue for future research.
Mechanical and vascular factors therefore play an important role in the etiopathogenesis of OCD; however, the presence of family cases with high risk of OCD in monozygotic twins suggests that hereditary factors are likewise crucial.10,53 Gornitzky et al. 9 described a positive family history of OCD in 14% of the cohort of patients analyzed, providing preliminary support for a familial inheritance pattern for OCD. Other groups also described familial cases of OCD lesions, in particular in association with short stature and multiple lesion sites.54,55 Preliminary studies identified mutations in candidate genes involved the maintenance of turnover of cartilage as a possible predisposition to develop OCD.56–58
Other biological theories refer to endocrine factors, but the effective role of the intra-articular biochemical environment and endocrine patterns is not still defined.59–61 Vitamin D deficiency was found to statistically correlate with children diagnosed with OCD.62,63 Human growth hormone (hGH) deficiency could lead to atypical ossification nuclei and the subsequent development of OCD lesions. 64
Natural history
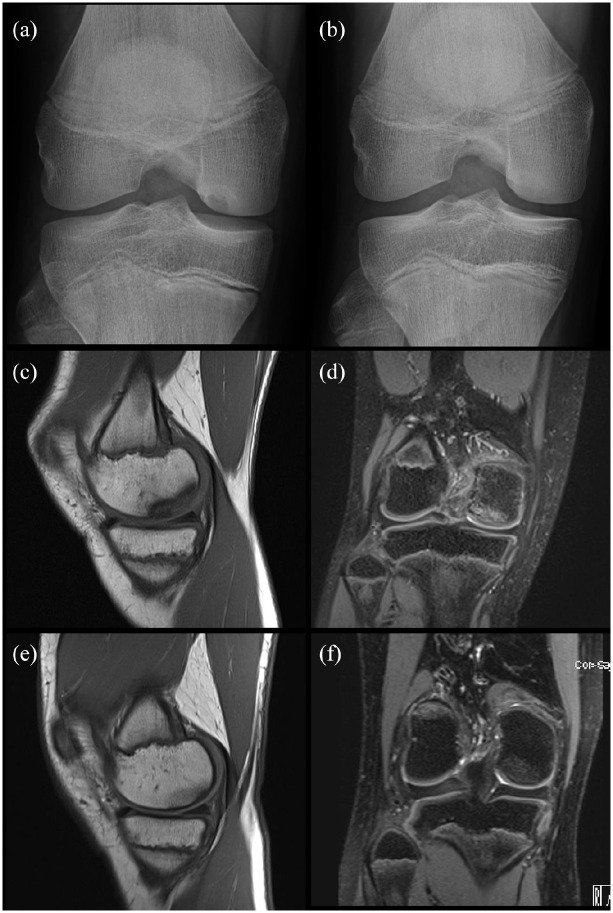
The natural course of OCD of the knee can be characterized by spontaneous healing or worsening over time.4,22 Higher likelihood of healing with sporting activity restriction was found in younger patients with open physes (Figure 1). 65
Figure 1.
Two cases of spontaneous healing of OCD. Case 1: 14 years old boy. X-ray notch view of the right knee (a) shows a stable OCD of the right medial femoral condyle. X-ray (b) after 6 months of sports restriction shows a complete spontaneous healing of OCD. Case 2: 12 years old, female. Detection of OCD on MRI after 18 months of knee pain. Coronal T1-weighted (c) and sagittal T2-weighted (d) MRI images show the area of OCD of the medial lateral condyle with low-signal intensity lesion on T1-images and a signal suggestive of bone marrow edema on T2-images. There is no sign of instability. Coronal T1-weighted (e) and sagittal T2-weighted (f) MRI images after 12 months of sporting activity restriction show OCD healing.
Failure of lesion healing results in intermittent pain, which may last for years until the osteochondral fragment becomes unstable. This event is a tipping point after which sequelae such as premature degenerative joint disease can occur.
Regardless of imaging study findings or possible signs of re-ossification of the osteochondral lesion, a patient can be considered healed when complete resolution of symptoms occurs. However, a patient with a newly diagnosed knee OCD should be closely monitored until radiographic normalization is complete, as symptoms are likely to resume even after years of quiescence. 66
Age is considered a key prognostic factor, specifically skeletal maturity at the time of symptom onset appears to drive clinical outcome, with better prognosis for subjects with open physes.19,67
Another major determinant is the surface area of the lesion, with better outcomes in patients with smaller surface areas. 68 Wall et al. 66 in a prospective study on skeletally immature patients who underwent non-operative treatment found that the mean surface area in the group whose lesions healed was 209 mm2 compared to 288 mm2 in the group with persistent lesions.
Conclusion
OCD of the knee is a common disease of skeletally immature patients. The pathogenesis is poorly understood but seems to be multifactorial in nature with genetic, mechanical, anatomical, and biological predisposing factors. More prospective and research studies are required to increase our knowledge about the factors that play a role in the development of this disease.
Footnotes
Author contributions: F.A., L.R., M.T., S.T., and M.B. contributed to the design of the study. F.M.A., N.N., F.A., L.R., and M.T. contributed to the data collection. M.T., M.B., S.T., F.M.A., N.N., and F.A. contributed to the data analysis and interpretation. F.M.A., N.N., F.A., L.R., M.T., and S.T. contributed to the drafting of the manuscript. M.B., N.N., L.R., M.T., and S.T. contributed to the manuscript final revision. All authors approved the final version of manuscript for submission.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Marco Turati  https://orcid.org/0000-0002-5208-3077
https://orcid.org/0000-0002-5208-3077
Stephane Tercier  https://orcid.org/0000-0002-9355-7937
https://orcid.org/0000-0002-9355-7937
References
- 1. Edmonds EW, Shea KG. Osteochondritis dissecans: editorial comment. Clin Orthop Relat Res 2013; 471(4): 1105–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. König F. The classic: on loose bodies in the joint. Clin Orthop Relat Res 2013; 471(4): 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chau MM, Klimstra MA, Wise KL, et al. Osteochondritis dissecans: current understanding of epidemiology, etiology, management, and outcomes. J Bone Joint Surg Am 2021; 103: 1132–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Accadbled F, Vial J, Sales de, Gauzy J. Osteochondritis dissecans of the knee. Orthop Traumatol Surg Res 2018; 104: S97–S105. [DOI] [PubMed] [Google Scholar]
- 5. Andriolo L, Candrian C, Papio T, et al. Osteochondritis dissecans of the knee—conservative treatment strategies: a systematic review. Cartilage 2019; 10(3): 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cahill BR, Berg BC. 99m-Technetium phosphate compound joint scintigraphy in the management of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med 1983; 11(5): 329–335. [DOI] [PubMed] [Google Scholar]
- 7. Tóth F, Nissi MJ, Ellermann JM, et al. Novel application of magnetic resonance imaging demonstrates characteristic differences in vasculature at predilection sites of osteochondritis dissecans. Am J Sports Med 2015; 43(10): 2522–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavaignac E, Perroncel G, Thépaut M, et al. Relationship between tibial spine size and the occurrence of osteochondritis dissecans: an argument in favour of the impingement theory. Knee Surg Sports Traumatol Arthrosc 2017; 25(8): 2442–2446. [DOI] [PubMed] [Google Scholar]
- 9. Gornitzky AL, Mistovich RJ, Atuahuene B, et al. Osteochondritis dissecans lesions in family members: does a positive family history impact phenotypic potency? Clin Orthop Relat Res 2017; 475: 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gans I, Sarkissian EJ, Grant SF, et al. Identical osteochondritis dissecans lesions of the knee in sets of monozygotic twins. Orthopedics 2013; 36(12): e1559–e1562. [DOI] [PubMed] [Google Scholar]
- 11. Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand 1976; 47(6): 664–667. [DOI] [PubMed] [Google Scholar]
- 12. Shea KG, Ganley TJ. Osteochondritis dissecans. J Bone Joint Surg Am 1996; 78: 641–652. [PubMed] [Google Scholar]
- 13. Kessler JI, Nikizad H, Shea KG, et al. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med 2014; 42(2): 320–326. [DOI] [PubMed] [Google Scholar]
- 14. Kocher MS, Tucker R, Ganley TJ, et al. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med 2006; 34(7): 1181–1191. [DOI] [PubMed] [Google Scholar]
- 15. Cruz AI, Jr, Richmond CG, Tompkins MA, et al. What’s new in pediatric sports conditions of the knee? J Pediatr Orthop 2018; 38(2): e66–e72. [DOI] [PubMed] [Google Scholar]
- 16. Masquijo J, Kothari A. Juvenile osteochondritis dissecans (JOCD) of the knee: current concepts review. EFORT Open Rev 2019; 4(5): 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nissen CW, Albright JC, Anderson CN, et al. Descriptive epidemiology from the research in osteochondritis dissecans of the knee (ROCK) prospective cohort. Am J Sports Med 2022; 50(1): 118–127. [DOI] [PubMed] [Google Scholar]
- 18. Turati M, Boerci L, Piatti M, et al. What’s new about etiopathogenesis of musculoskeletal injuries in adolescent athletes? Minerva Pediatr. Epub ahead of print 27 October 2020. DOI: 10.23736/S0026-4946.20.05944-7. [DOI] [PubMed] [Google Scholar]
- 19. Hefti F, Beguiristain J, Krauspe R, et al. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. https://pubmed.ncbi.nlm.nih.gov/10513356/ (accessed 9 March 2022). [PubMed]
- 20. Brown ML, McCauley JC, Gracitelli GC, et al. Osteochondritis dissecans lesion location is highly concordant with mechanical axis deviation. Am J Sports Med 2020; 48(4): 871–875. [DOI] [PubMed] [Google Scholar]
- 21. Kramer DE, Yen YM, Simoni MK, et al. Surgical management of osteochondritis dissecans lesions of the patella and trochlea in the pediatric and adolescent population. Am J Sports Med 2015; 43(3): 654–662. [DOI] [PubMed] [Google Scholar]
- 22. Bruns J, Werner M, Habermann C. Osteochondritis dissecans: etiology, pathology, and imaging with a special focus on the knee joint. Cartilage 2018; 9(4): 346–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andriolo L, Crawford DC, Reale D, et al. Osteochondritis dissecans of the knee: etiology and pathogenetic mechanisms. A Systematic Review. Cartilage 2020; 11(3): 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Green WT, Banks HH. Osteochondritis dissecans in children. J Bone Joint Surg Am 1953; 35-A: 26–64. [PubMed] [Google Scholar]
- 25. Federico DJ, Lynch JK, Jokl P. Osteochondritis dissecans of the knee: a historical review of etiology and treatment. Arthroscopy 1990; 6(3): 190–197. [DOI] [PubMed] [Google Scholar]
- 26. Smillie IS. Treatment of osteochondritis dissecans. J Bone Joint Surg Br 1957; 39-B: 248–260. [DOI] [PubMed] [Google Scholar]
- 27. Turner MS, Smillie IS. The effect of tibial torsion of the pathology of the knee. J Bone Joint Surg Br 1981; 63-B(3): 396–398. [DOI] [PubMed] [Google Scholar]
- 28. Bramer JA, Maas M, Dallinga RJ, et al. Increased external tibial torsion and osteochondritis dissecans of the knee. Clin Orthop Relat Res 2004; 422: 175–179. [DOI] [PubMed] [Google Scholar]
- 29. Kancherla VK, Caggiano NM, Matullo KS. Elbow injuries in the throwing athlete. Orthop Clin North Am 2014; 45: 571–585. [DOI] [PubMed] [Google Scholar]
- 30. Nissen CW. Osteochondritis dissecans of the elbow. Clin Sports Med 2014; 33: 251–265. [DOI] [PubMed] [Google Scholar]
- 31. Wechter JF, Sikka RS, Alwan M, et al. Proximal tibial morphology and its correlation with osteochondritis dissecans of the knee. Knee Surg Sports Traumatol Arthrosc 2015; 23(12): 3717–3722. [DOI] [PubMed] [Google Scholar]
- 32. Chow RM, Guzman MS, Dao Q. Intercondylar notch width as a risk factor for medial femoral condyle osteochondritis dissecans in skeletally immature patients. J Pediatr Orthop 2016; 36(6): 640–644. [DOI] [PubMed] [Google Scholar]
- 33. Ishikawa M, Adachi N, Yoshikawa M, et al. Unique anatomic feature of the posterior cruciate ligament in knees associated with osteochondritis dissecans. Orthop J Sports Med 2016; 4(5): 2325967116648138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turati M, Anghilieri FM, Accadbled F, et al. Discoid meniscus in human fetuses: a systematic review. Knee 2021; 30: 205–213. [DOI] [PubMed] [Google Scholar]
- 35. Deie M, Ochi M, Sumen Y, et al. Relationship between osteochondritis dissecans of the lateral femoral condyle and lateral menisci types. J Pediatr Orthop 2006; 26(1): 79–82. [DOI] [PubMed] [Google Scholar]
- 36. Mitsuoka T, Shino K, Hamada M, et al. Osteochondritis dissecans of the lateral femoral condyle of the knee joint. Arthroscopy 1999; 15: 20–26. [DOI] [PubMed] [Google Scholar]
- 37. Camathias C, Hirschmann MT, Vavken P, et al. Meniscal suturing versus screw fixation for treatment of osteochondritis dissecans: clinical and magnetic resonance imaging results. Arthroscopy 2014; 30(10): 1269–1279. [DOI] [PubMed] [Google Scholar]
- 38. Simonian PT, Sussmann PS, van Trommel M, et al. Popliteomeniscal fasciculi and lateral meniscal stability. Am J Sports Med 1997; 25(6): 849–853. [DOI] [PubMed] [Google Scholar]
- 39. Jacquet C, Magosch A, Mouton C, et al. The aspiration test: an arthroscopic sign of lateral meniscus posterior horn instability. J Exp Orthop 2021; 8: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takigami J, Hashimoto Y, Tomihara T, et al. Predictive factors for osteochondritis dissecans of the lateral femoral condyle concurrent with a discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc 2018; 26(3): 799–805. [DOI] [PubMed] [Google Scholar]
- 41. Gonzalez-Herranz P, Rodriguez ML, de la Fuente C. Femoral osteochondritis of the knee: prognostic value of the mechanical axis. J Child Orthop 2017; 11(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jacobi M, Wahl P, Bouaicha S, et al. Association between mechanical axis of the leg and osteochondritis dissecans of the knee: radiographic study on 103 knees. Am J Sports Med 2010; 38(7): 1425–1428. [DOI] [PubMed] [Google Scholar]
- 43. Kessler JI, Jacobs JC, Jr, Cannamela PC, et al. Childhood obesity is associated with osteochondritis dissecans of the knee, ankle, and elbow in children and adolescents. J Pediatr Orthop 2018; 38(5): e296–e299. [DOI] [PubMed] [Google Scholar]
- 44. Deroussen F, Hustin C, Moukoko D, et al. Osteochondritis dissecans of the lateral tibial condyle associated with agenesis of both cruciate ligaments. Orthopedics 2014; 37(2): e218–e220. [DOI] [PubMed] [Google Scholar]
- 45. McElroy MJ, Riley PM, Tepolt FA, et al. Catcher’s knee: posterior femoral condyle juvenile osteochondritis dissecans in children and adolescents. J Pediatr Orthop 2018; 38(8): 410–417. [DOI] [PubMed] [Google Scholar]
- 46. Campbell CJ, Ranawat CS. Osteochondritis dissecans: the question of etiology. J Trauma 1966; 6(2): 201–221. [PubMed] [Google Scholar]
- 47. Jans L, Jaremko J, Ditchfield M, et al. Ossification variants of the femoral condyles are not associated with osteochondritis dissecans. Eur J Radiol 2012; 81: 3384–3389. [DOI] [PubMed] [Google Scholar]
- 48. Kim HK. Legg-Calve-Perthes disease: etiology, pathogenesis, and biology. J Pediatr Orthop 2011; 31(2 Suppl.): S141–S146. [DOI] [PubMed] [Google Scholar]
- 49. Kenet G, Ezra E, Wientroub S, et al. Perthes’ disease and the search for genetic associations collagen mutations, Gaucher’s disease and thrombophilia. J Bone Joint Surg Br 2008; 90(11): 1507–1511. [DOI] [PubMed] [Google Scholar]
- 50. López-Franco M, González-Morán G, De Lucas JC, Jr, et al. Legg–Perthes disease and heritable thrombophilia. J Pediatr Orthop 2005; 25(4): 456–459. [DOI] [PubMed] [Google Scholar]
- 51. Woratanarat P, Thaveeratitharm C, Woratanarat T, et al. Meta-analysis of hypercoagulability genetic polymorphisms in Perthes disease. J Orthop Res 2014; 32(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 52. Glueck CJ, Tracy T, Wang P. Legg-Calve-Perthes disease, venous and arterial thrombi, and the factor V leiden mutation in a four-generation kindred. J Pediatr Orthop 2007; 27(7): 834–837. [DOI] [PubMed] [Google Scholar]
- 53. Richie LB, Sytsma MJ. Matching osteochondritis dissecans lesions in identical twin brothers. Orthopedics 2013; 36(9): e1213–e1216. [DOI] [PubMed] [Google Scholar]
- 54. Gorter J, van Raay JJ. A suspected genetic form of bilateral osteochondritis dissecans of the knee in a Dutch family. Knee 2015; 22(6): 677–682. [DOI] [PubMed] [Google Scholar]
- 55. Stattin EL, Tegner Y, Domellöf M, et al. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis Cartilage 2008; 16(8): 890–896. [DOI] [PubMed] [Google Scholar]
- 56. Stattin EL, Wiklund F, Lindblom K, et al. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet 2010; 86: 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jackson GC, Marcus-Soekarman D, Stolte-Dijkstra I, et al. Type IX collagen gene mutations can result in multiple epiphyseal dysplasia that is associated with osteochondritis dissecans and a mild myopathy. Am J Med Genet A 2010; 152A(4): 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yellin JL, Trocle A, Grant SF, et al. Candidate loci are revealed by an initial genome-wide association study of juvenile osteochondritis dissecans. J Pediatr Orthop 2017; 37(1): e32–e36. [DOI] [PubMed] [Google Scholar]
- 59. Turati M, Franchi S, Leone G, et al. Resolvin E1 and cytokines environment in skeletally immature and adult ACL tears. Front Med (Lausanne) 2021; 8: 610866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmal H, Pilz IH, Henkelmann R, et al. Association between intraarticular cytokine levels and clinical parameters of osteochondritis dissecans in the ankle. BMC Musculoskelet Disord 2014; 15: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bigoni M, Turati M, Zatti G, et al. Intra-articular cytokine levels in adolescent patients after anterior cruciate ligament tear. Mediators Inflamm 2018; 2018: 4210593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maier GS, Lazovic D, Maus U, et al. Vitamin D deficiency: the missing etiological factor in the development of juvenile osteochondrosis dissecans? J Pediatr Orthop 2019; 39(1): 51–54. [DOI] [PubMed] [Google Scholar]
- 63. Bruns J, Werner M, Soyka M. Is vitamin D insufficiency or deficiency related to the development of osteochondritis dissecans? Knee Surg Sports Traumatol Arthrosc 2016; 24: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 64. Hussain WM, Hussain HM, Hussain MS, et al. Human growth hormone and the development of osteochondritis dissecans lesions. Knee Surg Sports Traumatol Arthrosc 2011; 19(12): 2108–2110. [DOI] [PubMed] [Google Scholar]
- 65. Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Jt Surg Am 1984; 66: 1340–1348. [PubMed] [Google Scholar]
- 66. Wall EJ, Vourazeris J, Myer GD, et al. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am 2008; 90: 2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Siegall E, Faust JR, Herzog MM, et al. Age predicts disruption of the articular surface of the femoral condyles in knee OCD: can we reduce usage of arthroscopy and MRI? J Pediatr Orthop 2018; 38(3): 176–180. [DOI] [PubMed] [Google Scholar]
- 68. Krause M, Hapfelmeier A, Möller M, et al. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med 2013; 41(10): 2384–2391. [DOI] [PubMed] [Google Scholar]