Abstract
Objectives
Spondyloarthritis (SpA) is known as series of immune-mediated inflammatory disease of the axial and peripheral joints. Human leucocyte antigen (HLA)-B27 is a genetic risk factor for SpA. Recent evidence suggests that the interleukin −17 (IL17) axis strongly contributes to SpA. This study aimed to assess the efficacy of an IL17A peptide-based vaccine on SpA manifestations in model rats.
Methods
HLA-B27/human β2-microglobulin (hβ2M) transgenic rats were immunised with heat-inactivated Mycobacterium tuberculosis (MT) to develop spondylitis and arthritis as an experimental SpA model after immunisation with a keyhole limpet hemocyanin-conjugated IL17A peptide-based vaccine with an alum adjuvant three times. The IL17A antibody titre was assessed using ELISA, and arthritis score and joint thickness were monitored two times a week. Enzyme-linked immunospot (ELISpot) assays for IL4- and interferon-γ-secreting splenocytes were conducted to evaluate IL17A-specific T cell activation. We also evaluated the effect of IL17A vaccine in SpA therapeutic model.
Results
The IL17A peptide-based vaccine with alum adjuvant successfully induced antibody production and suppressed the arthritis score and joint thickness. X-ray and histological analyses showed that enthesitis, bone destruction and new bone formation were inhibited by the IL17A vaccine. The ELISpot assay showed that the IL17A peptide-based vaccine did not elicit any IL17A-reactive T cell responses. IL17A vaccine tends to mitigate, but not significant, in SpA treatment model. These data showed that the peptide-based vaccine targeting IL17A alleviated the SpA phenotype in a heat-inactivated MT-induced SpA model in HLA-B27/hβ2M transgenic rats.
Conclusions
IL17A peptide-based vaccine may be a therapeutic option for SpA treatment.
Keywords: Spondylitis, Ankylosing; Vaccination; Autoimmunity
What is already known about this subject?
Interleukin-17A (IL17A) is known to have a pivotal role in inflammation and bone metabolism in spondyloarthritis (SpA). Antibody therapy against IL17A is effective but expensive in the clinical setting.
What does this study add?
IL17A vaccine has a beneficial effect on SpA model rats without induction of auto-reactive T cell against IL17A.
How might this impact on clinical practice?
IL17A vaccine might be an additional therapeutic approach for SpA treatment.
Introduction
Spondyloarthritis (SpA), including ankylosing spondylitis, is triggered at the tendons and enthesis in sacroiliac joints of the spine via an immune-mediated inflammatory mechanism, resulting in ankylosis with bone erosion and new bone formation.1 Many studies suggested that major histocompatibility complex class I antigen B27 (human leucocyte antigen; HLA-B27) is associated with axial SpA or ankylosing spondylitis.2–8 Transgenic rats with HLA-B27 and human β2-microglobulin (hβ2M) have been established and analysed to verify the relationship between HLA-B27 and the manifestations of SpA, including inflammation in peripheral and axial joints and other tissues.9 10 To date, the precise mechanism(s) of articular inflammation leading to SpA remains unknown.
In addition to HLA-B27, recent evidence suggests that the interleukin (IL)−23/IL-17 (IL23/IL17) axis strongly contributes to the pathological mechanism that triggers SpA.11–13 IL17A is involved in arthritogenic inflammation via the regulation of Wnt and receptor activator of nuclear factor-κB ligand pathways and new bone formation via the Janus kinase/signal transducer and activator of transcription pathway at the joints and entheses.14 Based on these studies, some biological therapies using neutralising antibodies targeting IL17A, such as secukinumab (a fully human monoclonal antibody) and ixekizumab (a humanised monoclonal antibody), have been evaluated in clinical studies.15–18
As the efficacy of these biological antibodies has been approved, more therapeutic options are available for SpA treatment. However, biological therapy is expensive and burdensome for patients, even with medical insurance support.19 To address this issue, we have focused on a therapeutic vaccine through which individual therapeutic antibodies can be produced.20 We have previously demonstrated the effectiveness of potential therapeutic vaccines for hypertension, diabetes mellitus and stroke.21–25 In this study, we investigated the efficacy of an IL17 vaccine against SpA manifestations in HLA-B27/hβ2M transgenic rats.
In this study, we developed a peptide-based vaccine for IL17A based on our previous findings and evaluated its efficacy against the manifestations of SpA in a rat model.
Methods
HLA-B27 transgenic rats, induction of SpA-like phenotype and scoring of arthritis
Lewis HLA-B27/hβ2M transgenic rats were obtained from the University of Texas Southwestern Medical Center by crossing two transgenic strains, as previously described.9 Namely, the 21–3 (male) and 283–2 (female) transgenic rats were crossed, and the tails of their littermates were analysed for transgenes via genotyping. All rats were maintained with light/dark cycles every 12 hours. All animal experiments were approved by the Animal Experiment Committee of Osaka University.
To induce the spondylitis and arthritis phenotype, heat-inactivated Mycobacterium tuberculosis (MT) (90 µg) mixed with the incomplete Freund’s adjuvant (total amount: 100 µL) was subcutaneously injected 3 weeks after the first IL17A vaccination.10 After MT injection, the body weight, joint thickness and spondylarthritis severity were monitored every 2–3 days. The paw joint thickness was measured using an electric calliper. The arthritis score was calculated based on the swelling scores of each finger and tail (none=0; mild swelling=1; severe swelling=2). MT-treated HLA-B27 tg rats were used as positive control (N=5). As negative control, HLA-B27-negative rats were used (N=1–2). For SpA treatment model, MT was administrated, followed by IL17A vaccine injection three times at 2 weeks interval. MT-treated HLA-B27 tg rats were used as positive control (N=6). As negative control, HLA-B27 negative rats were used (N=2).
Peptide preparation and immunisation
The IL17A epitope peptide was synthesised by the Peptide Institute (Osaka, Japan). The amino acid sequences from 65aa to 72aa were selected as epitopes for IL17A.24 The synthesised peptide was conjugated with keyhole limpet hemocyanin (KLH; Enzo Life Sciences) as a carrier protein and mixed with an aluminium adjuvant (InVivoGen). The KLH-conjugated vaccine or KLH-only as a control (100 µg/body) was subcutaneously injected into the rats three times at 0, 2 and 4 weeks (N=4–6).
Antibody titre determination via ELISA
Serum samples from the tail vein at 0, 2, 4, 6 and 9 weeks were evaluated via ELISA for antibody titres, as described in a previous study. Briefly, bovine serum albumin (Peptide Institute)-conjugated epitope (10 µg/mL) or recombinant IL17A (R&D Systems) was coated on a 96-well plate on the first day. On the second day, the wells were blocked with the blocking buffer (PBS-T (Tween-20, 0.05%) containing 5% skim milk) for 2 hours at room temperature. The sera were diluted 10-fold to 31 250-fold in a blocking buffer and incubated overnight at 4℃. The following day, the wells were washed and incubated with horseradish peroxidase (HRP)-conjugated anti-IgG antibody (GE Healthcare) for 3 hours at room temperature. HRP-conjugated antisubclass IgG antibodies (IgG1: Abcam; IgG2a: Abcam) were used for IgG subclass analysis. After washing with PBS-T, the wells were incubated with the peroxidase chromogenic substrate, 3,3’−5,5’-tetramethyl benzidine (Sigma-Aldrich), for 30 min at room temperature, and the reaction was halted with 0.5 N sulfuric acid. The absorbance of the wells was measured immediately at 450 nm using a microplate reader (Bio-Rad). The antibody titre was shown as the serum dilution with half-maximal binding (optical density: 50%). The half-maximal antibody titre of each sample was calculated from the highest absorbance in the dilution range using the Prism V.8 software.
Enzyme-Linked immunospot (ELISpot) assay
Cellular immune responses of splenocytes after vaccination were evaluated via an ELISpot assay (UCT Biosciences), according to the manufacturer’s instructions. The polyvinylidene fluoride membrane-coated plates were incubated with IL4 or interferon (IFN)γ capture antibodies at 4℃ overnight. The wells were washed with PBS and blocked with the blocking solution (UCT Biosciences) for 2 hours at room temperature. Splenocytes were isolated from vaccinated spleens and adjusted to 2×105 cells/well. The cells were stimulated with recombinant IL17A (R&D Systems), KLH (Enzo Life Sciences) or phorbol 12-myristate 13-acetate (PMA; Sigma) plus ionomycin (IO; Sigma) at 37 ℃ for 48 hours. The washed wells were incubated with a biotinylated polyclonal antibody against rat IL4 or IFNγ for 2 hours at 4℃. After the wells were washed with PBS-T, they were incubated with the streptavidin-HRP conjugate for 1 hour at room temperature. HRP was developed using a substrate solution (UCT Biosciences). IL4 or IFNγ spots were counted using a dissecting microscope (LMD6500; Leica).
Histological analysis
For histological analysis, tissues (joint, tail, kidney, liver and lungs) were collected 9 weeks after the first vaccination and fixed in 10% neutral buffered formalin. Fixed tissues were embedded in paraffin, cut into 5 µm-thick sections and stained with H&E.
For immunohistological analysis, paraffin-embedded tissues were cut into 3 µm-thick sections and stained with CD68 (Bio-Rad), CD14 (Bioss antibodies) and alkaline phosphatase (ALP; Santa Cruz). Stained tissue sections were observed under a BZ-X810 microscope (Keyence).
Radiologic analysis (X-ray analysis)
Rats were imaged with X-rays at the end of the experiments using the MX-20 specimen radiography system, as previously described.26
Statistical analysis
All values are presented as the mean±SE of the mean. Student’s t test and one-way analysis of variance followed by Tukey’s post hoc multiple test were used to assess the significant differences in each experiment using Prism V.8 software (GraphPad Software). Differences were considered significant when the p value was less than 0.05.
Results
Peptide-based IL17A vaccine induces IL17A-specific antibody production in HLA-B27/hβ2M transgenic rats
IL17A functions as a homodimer or heterodimer with IL17A or IL17F.27 The interface for the dimer, which is an amino acid sequence, was chosen as the epitope for the vaccine in this study, which consists of amino acid sequences, RPSDYLNR from 65aa to 72aa (figure 1A). This epitope was conjugated at the N-terminus with KLH as a carrier protein. The KLH-conjugated vaccine (100 µg peptide/rat) in combination with an alum adjuvant was subcutaneously injected into rats three times every 2 weeks (figure 1B). Heat-inactivated MT (90 µg/rat) was administered to rats to induce SpA 3 weeks after the first vaccine23 (figure 1B). The antibody titre was measured at 0, 2, 4, 6 and 9 weeks using ELISA. The antibody titre increased from week 2 to its highest value at week 6 (figure 1C and online supplemental figure 1). The antibody level was not increased by the KLH-control vaccine. Next, we examined whether the IL17A vaccine-induced antibodies recognised and bound to recombinant IL17A using ELISA. The IL17A vaccine-induced antibody at 9 weeks recognised recombinant IL17A but not IL17F (figure 1D). To assess the Th1:Th2 balance induced by the alum-adjuvanted IL17A vaccine, IgG subclass analysis was performed using ELISA. The IgG1 antibody titre was found to be dominant (figure 1E) compared with the IgG2a antibody titre. The IgG2a/IgG1 ratio showed that the IL17A vaccine with alum adjuvant shifted the IL17A immune response towards Th2 (figure 1F). These data suggest that the IL17A vaccine successfully induces Th2-shifted IL17A-specific antibody production in the SpA model in HLA-B27/hβ2M transgenic rats.
Figure 1.
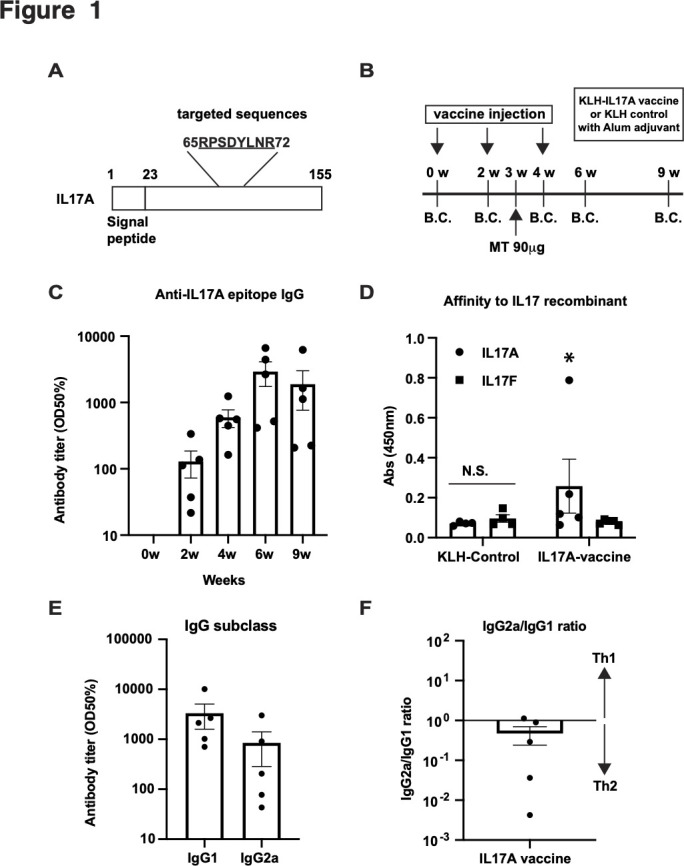
Interleukin (IL)−17A (IL17A) vaccine induces antibody production in a spondyloarthritis (SpA) model in human leucocyte antigen (HLA)-B27 transgenic rats. (A) Targeted sequences of IL17A vaccine are shown. (B) Experimental protocol for IL17A vaccination of the inactivated Mycobacterium Tuberculosis (MT)-induced SpA model in HLA-B27 transgenic rats. The epitope was conjugated with keyhole limpet hemocyanin (KLH). KLH-conjugated IL17A vaccine was injected with an alum adjuvant. HLA-B27 transgenic rats were immunised with the IL17A vaccine with KLH or KLH only intracutaneously thrice every 2 weeks. Inactivated MT was administrated to induced SpA. Blood was collected at 0, 2,4, 6 and 9 weeks to evaluate the antibody titre. Joint, tail and other tissues were collected at 9 weeks. The arthritis score and joint thickness were evaluated every few days. B.C.: blood collection. (C) Antibody titre for IL17A epitope. (D) Binding affinity of the vaccine-induced antibody for recombinant IL17A analysed via ELISA. Antibody of immunised serum at 9 weeks showed specific binding to recombinant IL17A, but not recombinant IL17F. (E) Antibody titre of IL17A epitope-specific IgG subclasses, IgG1 (Th2) and IgG2a (Th1). (F) The balance of Th1/Th2 was evaluated by the IgG2a/IgG1 ratio. IgG2a antibody titre was individually divided by the IgG1 antibody titre. See also online supplemental figure 1.
rmdopen-2022-002851supp001.pdf (1.3MB, pdf)
Manifestations of SpA are significantly alleviated by the peptide-based IL17A vaccine in HLA-B27/hβ2M transgenic rats
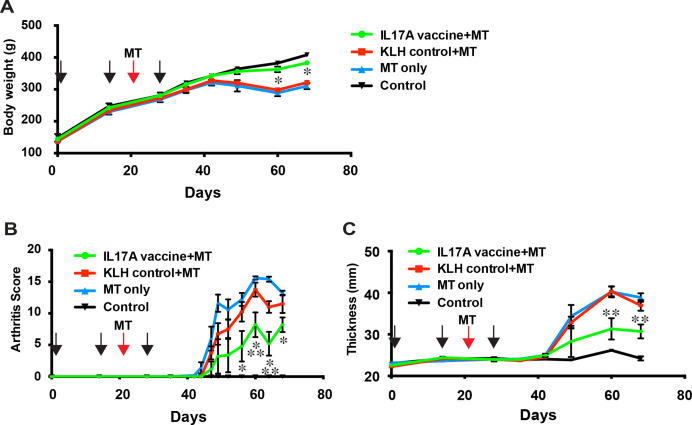
To test the effect of the IL17A vaccine on SpA, we evaluated the arthritis score and joint thickness of rats administered IL17A or KLH-control vaccine every few days after MT injection. We monitored the body weight and clinical scoring at the joints and tail macroscopically. Bodyweight decreased in the KLH control+MT vaccine and MT-only groups (figure 2A). We confirmed that arthritis severity and swelling at the joint developed around 20 days after MT immunisation in the control-KLH vaccine (KLH control+MT) and no-treatment (MT only) groups. The severity of arthritis score and joint swelling induced by MT immunisation was significantly alleviated (figure 2B, C). These results suggest that the IL17A vaccine successfully attenuates the SpA phenotype.
Figure 2.
IL17A vaccine alleviates Mycobacterium tuberculosis (MT)-induced spondyloarthritis (SpA) in HLA-B27 transgenic rats. (A) Body weight was monitored after IL17A vaccination. (B) Arthritis score and (C) joint thickness were analysed every 2–3 d to determine the arthritis severity. Data are shown as the mean ± SE error of the mean (SEM). *p < 0.05 versus MT only, **p < 0.05 versus MT only, and KLH-control+MT, respectively.
IL17A vaccine reduces the axial and peripheral inflammation in HLA-B27 transgenic rats
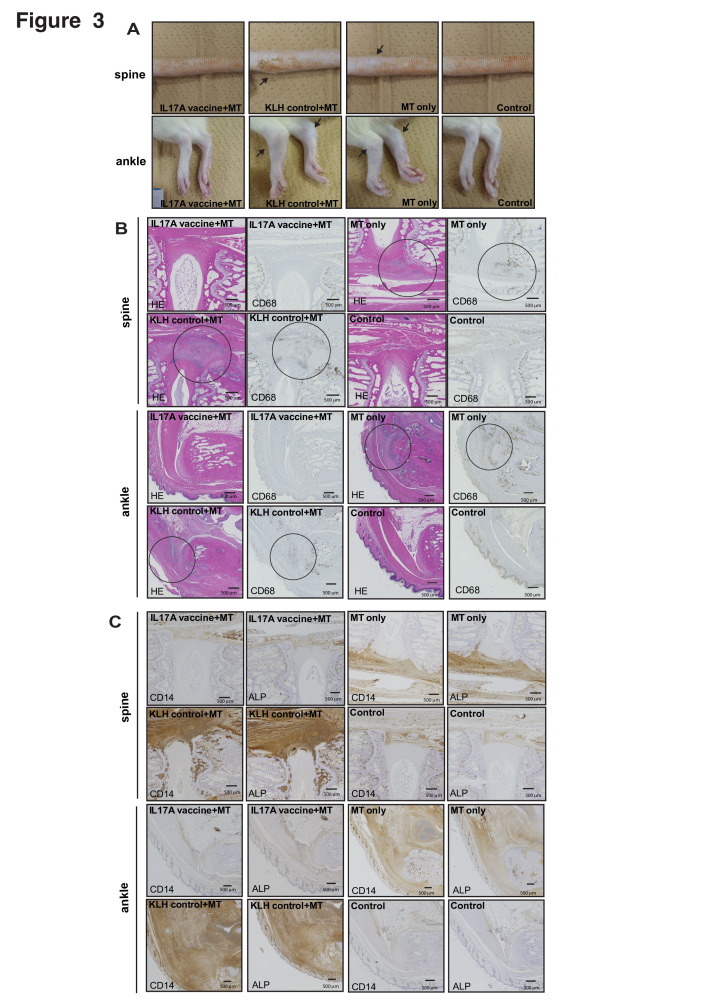
Inhibition of SpA-related manifestations in the spine joints, as shown in figure 2, suggests that the IL17A vaccine suppresses inflammation. Six weeks after MT immunisation, swelling of axial (spine) and peripheral (ankle) joints was observed in the KLH-control (KLH control+MT) and no treatment (MT only) groups but to a lesser extent in the IL17A vaccine group (figure 3A). Bone destruction and bone formation were detected via X-ray analysis in ankle joint but not in spine (online supplemental figure 2). Next, we stained the spinal and peripheral joint sections with HE staining as well as markers of inflammation (CD68) and bone formation (CD14 and ALP). We detected immune cell invasion and CD68 staining in the KLH-control (KLH control+MT) and no treatment (MT only) groups. However, the IL17A vaccine suppressed cell invasion and CD68-positive inflammatory cells during enthesis (figure 3B and online supplemental figure 3A). We also evaluated bone formation via CD14 and ALP staining. Similar to the results for inflammatory markers, CD14-positive and ALP-positive cells were increased in the KLH-control (KLH control+MT) and the no-treatment (MT only) groups compared with the IL17A vaccine (IL17A vaccine+MT) and control groups (figure 3C and online supplemental figure 3B). These results suggest that the IL17A vaccine suppresses enthesitis and bone destruction/formation in the joints of HLA-B27 transgenic rats.
Figure 3.
IL17A vaccine suppresses enthesitis and bone remodelling in the joints of HLA-B27 transgenic rats. (A) Representative pictures of spine and ankle. Arrows indicate swelling. (B) Immunohistological analysis (HE and CD68 staining) of the joint of spine and ankle. Circle indicates invasion of immune cells. (C) Immunohistological analysis (CD14 and alkaline phosphatase (ALP) staining) of the joint of spine and ankle at 9 weeks. See also online supplemental figure 2 and 3.
IL17A peptide-based vaccine does not induce IL17A-specific T cell activation
Next, we evaluated the safety of the IL17A vaccine. Vaccine-elicited autoimmune T cell reactions to endogenous IL17A protein pose a major safety concern. To assess this, the cellular immune response was evaluated via an ELISpot assay using immunised splenocytes. KLH stimulation increased the number of IL4 spots compared with IL17A stimulation or the control. IL4 spots after IL17A stimulation were comparable to those without stimulation (control) (figure 4A, B). The number of IFNγ-producing splenocytes increased with KLH stimulation but not with IL17A stimulation (figure 4C, D), suggesting that the IL17A-autoreactive T cells were not produced by the IL17A vaccine in this study.
Figure 4.
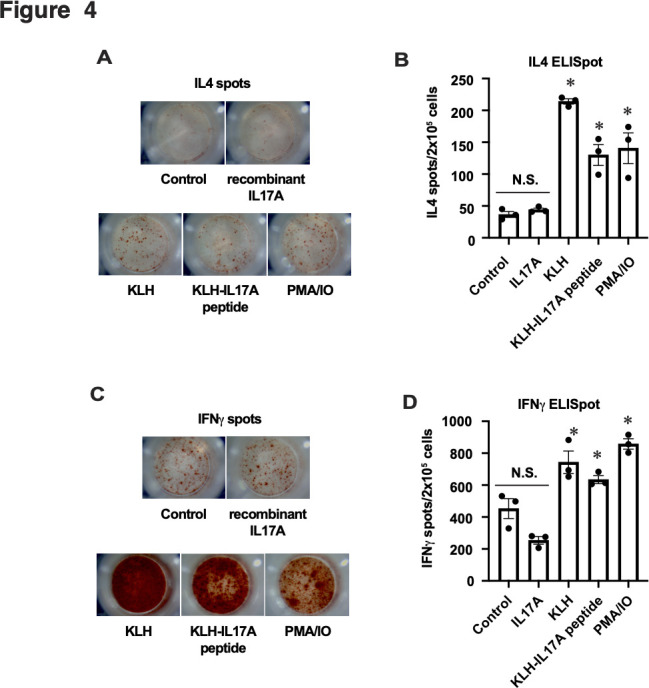
IL17A peptide vaccine does not activate IL17A-specific T cell reaction. Representative images of (A) IL4 or (C) interferon (IFN)γ spots obtained via the enzyme-linked immunospot (ELISpot) assay. Splenocytes from immunised HLA-B27 transgenic rats were stimulated with recombinant IL17A, KLH or KLH-IL17 peptide (vaccine), or phorbol 12-myristate 13-acetate (PMA) plus ionomycin (IO) (PMA/IO) as a positive control. The graph shows the number of (B) IL4 or (D) IFNγ spots per 2 x 105 cells. Data are shown as the mean ± SEM. *p < 0.05 versus Control and IL17A, respectively.
IL17A peptide vaccine tend to have therapeutic effect in MT-induced SpA treatment model, but not significant
Finally, we evaluated whether IL17A vaccine has a beneficial effect on MT-induced SpA in therapeutic model. HLA-B27 transgenic rats were immunised with MT before IL17A vaccine administration,28 followed by three doses of IL17A vaccine at 2-week interval. We evaluated the arthritis score and joint thickness every few days (figure 5A). IL17A epitope-specific antibody titre was increased, measured by ELISA (figure 5B and online supplemental figure 4). Body weight was not significantly different among groups (figure 5C). Arthritis score and joint thickness were not significantly different in time window in this study (figure 5D, E). However, arthritis score and joint thickness tend to relieve from 40 days to 44 days since MT injection in IL17A vaccine group compared with KLH control group. Collectively, IL17A vaccine did not show the therapeutic effect in 10 weeks in therapeutic model.
Figure 5.
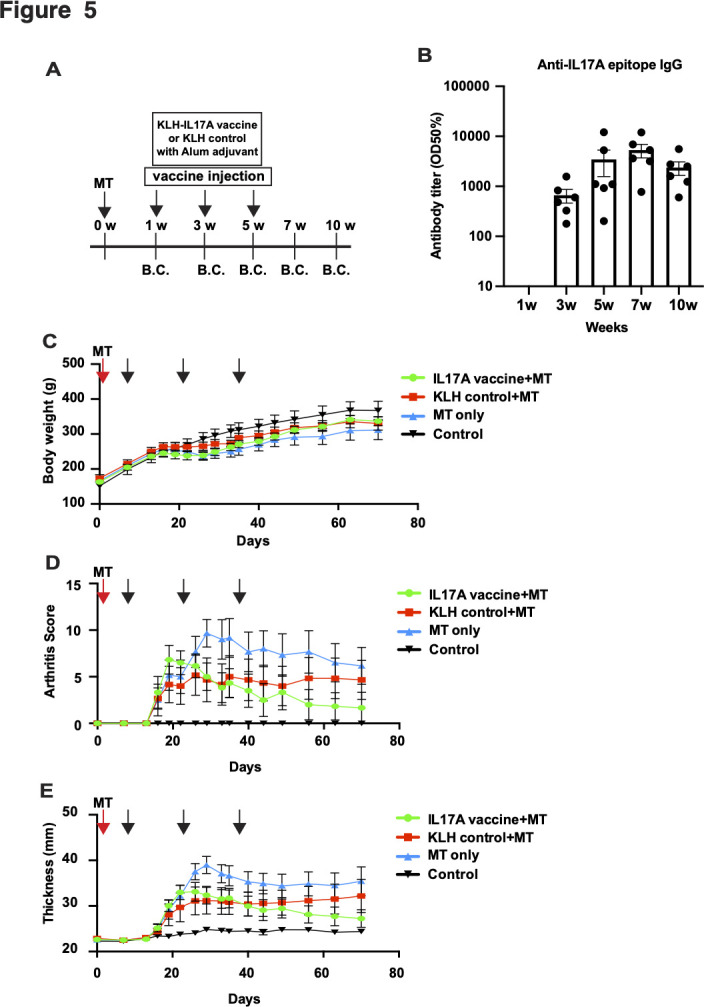
IL17A peptide vaccine does not mitigate MT-induced SpA in treatment model. (A) Experimental protocol for IL17A vaccination of MT-induced SpA treatment model in HLA-B27 transgenic rats. MT was administered to induce SpA at a week before administration of IL17A vaccine. IL17A vaccine or KLH control was administrated thrice at 2 weeks interval. Blood was collected at 1, 3, 5, 7 and 10 weeks to evaluate the antibody titre. The arthritis score and joint thickness were evaluated every few days. B.C.: blood collection. (B) Antibody titre for IL17A epitope. (C) Body weight was monitored after MT administration. (D) Arthritis score and (E) joint thickness were analysed every 2–3 days to determine the arthritis severity. Data are shown as the mean ± SE error of the mean (SEM). See also online supplemental figure 4.
Discussion
In the present study, we showed that the IL17A vaccine successfully induced antibody production by specifically binding to IL17A but not IL17F. The arthritis score and thickness of the hind limb paw were strongly suppressed after the SpA-like phenotype was induced by heat-inactivated MT. Histological analysis revealed that articular inflammation and new bone formation were inhibited at the joints and spine. Moreover, the IL17A vaccine did not cause IL17A-specific T cell activation as well as any tissue damage in the lungs, kidneys and liver. These data suggest that therapeutic vaccines targeting IL17A protect against SpA manifestations in HLA-B27/β2M transgenic rats, without causing any adverse effects.
IL17A vaccine has been used to treat IL17A-related autoimmune disorders.29–32 IL17A vaccine was first developed to prevent experimental autoimmune encephalomyelitis (EAE) in an animal model in 2006. In that study, mice immunised with recombinant ovalbumin-conjugated mouse IL17A with Gerbu 100 adjuvant produced an IL17A-specific antibody, which suppressed IL17A bioactivity. The IL17A vaccine successfully inhibited the symptoms of proteolipid protein peptide 139–151-induced EAE in Swiss Jim Lambert (SJL) mice.32 IL17A peptide-based vaccine has also been used to treat psoriasis. That study revealed that immunisation with the Q11 self-assembling nanofiber system using multiple IL17A peptides and the universal T-cell epitope, Pan DR T-Helper Epitope (PADRE), increased the antibody titre in mice. Alum-adjuvanted Q11-IL17A vaccine prevents psoriasis in imiquimod-induced model mice by shifting antibody production towards IgG1 vs IgG2b.31 In addition to the peptide-based vaccine, our previous study showed the beneficial effects of the IL17A DNA vaccine for systemic lupus erythematosus (SLE). A DNA vaccine encoding a hepatitis B core (HBc)-IL17A epitope fusion protein successfully induced anti-IL17A IgG production via intramuscular administration with electroporation and protected the SLE model mice (NZBWF1 or MRL/lpr mice) from pathological symptoms, such as macrophage infiltration and renal damage.30 Virus-like particle-based IL17A vaccine also induces IL17A antibodies, which worsens chronic colitis in a 2,4,6-trinitrobenzene sulfonic acid-induced colitis model in mice.29 Indeed, basic and clinical studies using IL17A targeting monoclonal antibodies to treat inflammatory bowel disease (IBD), such as Crohn’s disease, showed unfavourable results.33–36 This suggests that the IL17A vaccine may be applicable for IL17A-related pathologies, such as SpA, psoriasis, EAE, and SLE but not IBD.
In this study, the IL17A epitope peptide-based vaccine-suppressed SpA manifestations in SpA model rats without any toxic effects, such as body weight loss (figure 2A). Our previous study using DNA vaccine encoding the same IL17A epitope, RPSDYLNR, showed that the splenocytes from immunised mice were not activated by the IL17A epitope or recombinant IL17A, suggesting that the IL17A vaccine does not induce autoreactive T cells.30 In our previous studies, KLH-conjugated vaccines targeting endogenous molecules did not induce autoimmune T cells21–24 because shorter B cell epitopes were chosen not to be presented by the major histocompatibility complex.37 In this study, the IL17A-peptide vaccine did not induce IL17A-autoreactive T cell activation (figure 4). These results indicate that the IL17A epitope-based vaccine safely exerts its therapeutic effects on IL17A-related pathologies in animal models.
The IL23/IL17 axis has been targeted as a pathogenic signalling pathway for psoriatic arthritis and SpA.38 Monoclonal antibodies targeting IL17A, such as secukinumab, ixekizumab and netakimab, have shown beneficial effects in clinical studies.16–18 39 40 From a basic standpoint, prophylactic IL17A blockade by monoclonal antibody effectively inhibits inflammation as well as bone destruction and formation in the axial and peripheral joints of HLA-B27 transgenic animals as experimental SpA models. Therapeutic IL17 inhibition with monoclonal antibodies also significantly mitigates SpA manifestations.28 Importantly, the therapeutic use of IL23 antibody does not alleviate the SpA phenotype in animal models,41 suggesting that IL17A is a suitable molecular target for the treatment of SpA as a therapeutic vaccine.
We also evaluated the therapeutic effect of IL17A in therapeutic MT-induced SpA model. The symptoms of SpA (arthritis score and joint thickness) was not improved in 20 days since onset of SpA symptoms. However, the symptoms tend to be mitigated, but not significant, in IL17A vaccine group, compared with KLH-control group (figure 5D and E). IL17A-specific antibody titre was higher at 7 weeks (49 days) since MT treatment, suggesting that the therapeutic effect of IL17A vaccine needs the certain level of antibody titre. Further longitudinal investigation need to be performed to evaluate the therapeutic effect of IL17A vaccine. In previous study, treatment with IL17A blockade with antibody has an therapeutic effect in MT-induced SpA model in HLA-B27 tg rats.28 SpA symptoms started to be improved after more than 10 days since IL17A antibody treatment. We speculated that the effect of therapeutic effect of IL17A vaccine may be active in much later compared with antibody therapy due to IL17A antibody induction time. These data led us to propose that combinatory treatment such as antibody therapy (fast acting) and vaccine therapy (long-term acting) might be more effective in SpA treatment.
In this study, our results showed that the IL17A peptide-based vaccine-induced IL17A-specific antibodies to effectively inhibit SpA manifestations in a rat SpA model. Therefore, IL17A antibodies induced via active immunisation may be additional therapeutic options for SpA treatment in the future.
Study limitation
As a weak point of this study, we performed experiment with minimum number of animals (4–6 rats) in each group due to our experimental circumstances.
Acknowledgments
We would like to thank Dr. Joel D. Taurog, Dr. Robert E. Hammer, and The University of Texas Southwestern Medical Centre for the 21-3 and 283-2 rat lines. We utilized 21-3 and 283-2 rat lines under non-exclusive technology license agreements with the University of Texas Southwestern Medical Centre. We would like to thank all members of the Department of Health Development and Medicine, Osaka University Graduate School of Medicine, for supporting this project, especially Ms. Satoe Kitabata for secretarial support.
Footnotes
Contributors: HH, TT, HN designed the research study; HH, JS, YY conducted the experiments and acquired the data; HH, SY, SB, AT, MT, SK, MS, HT, MS, TT, HN, RM, HR analysed the data, and HH, TT, JS, RM, HN contributed to writing and editing the manuscript. HN is the guarantor for this manuscript.
Funding: This work was supported by a grant from the Japan Agency for Medical Research and Development (20ek0109312h0003).
Competing interests: The Department of Health Development and Medicine is an endowed department that is supported by AnGes, Daicel, and FunPep. The Department of Clinical Gene Therapy is financially supported by Novartis, AnGes, Shionogi, Boeringher, Fancl, Saisei Mirai Clinics, Rohto, and FunPep. HN is a scientific advisor of Funpep. AT, MT, SK, TE, RA, MS, HT and RM are employers and stockholders of Funpep. The funder provided support in the form of salaries for authors but did not have any additional role in the study design, data analysis, decision to publish, or preparation of this manuscript. All other authors declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data related to this study for analysis are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All procedures of this study were approved by the Animal Experiment Committee of Osaka University.
References
- 1.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. 10.1056/NEJMra1406182 [DOI] [PubMed] [Google Scholar]
- 2.Fahed H, Mauro D, Ciccia F, et al. What does human leukocyte antigen B27 have to do with spondyloarthritis? Rheum Dis Clin North Am 2020;46:225–39. 10.1016/j.rdc.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 3.Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6. 10.1007/s00296-002-0237-4 [DOI] [PubMed] [Google Scholar]
- 4.International Genetics of Ankylosing Spondylitis Consortium (IGAS), Cortes A, Hadler J, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013;45:730–8. 10.1038/ng.2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiltz U, Baraliakos X, Karakostas P, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:1415–22. 10.1002/acr.21688 [DOI] [PubMed] [Google Scholar]
- 6.Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum 2009;60:717–27. 10.1002/art.24483 [DOI] [PubMed] [Google Scholar]
- 7.Ziade N, Abi Karam G, Merheb G, et al. Hla-B27 prevalence in axial spondyloarthritis patients and in blood donors in a Lebanese population: results from a nationwide study. Int J Rheum Dis 2019;22:708–14. 10.1111/1756-185X.13487 [DOI] [PubMed] [Google Scholar]
- 8.Ziade NR. Hla B27 antigen in middle Eastern and Arab countries: systematic review of the strength of association with axial spondyloarthritis and methodological gaps. BMC Musculoskelet Disord 2017;18:280.:280. 10.1186/s12891-017-1639-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taurog JD, Rival C, van Duivenvoorde LM, et al. Autoimmune epididymoorchitis is essential to the pathogenesis of male-specific spondylarthritis in HLA-B27-transgenic rats. Arthritis Rheum 2012;64:2518–28. 10.1002/art.34480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tok MN, Satumtira N, Dorris M, et al. Innate immune activation can trigger experimental spondyloarthritis in HLA-B27/huβ2m transgenic rats. Front Immunol 2017;8:920.:920. 10.3389/fimmu.2017.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutet M-A, Nerviani A, Gallo Afflitto G, et al. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci 2018;19:530. 10.3390/ijms19020530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravallese EM, Schett G. Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 2018;14:631–40. 10.1038/s41584-018-0091-8 [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan V, Gracey E, Brown MA, et al. Pathogenesis of ankylosing spondylitis-recent advances and future directions. Nat Rev Rheumatol 2017;13:359–67. 10.1038/nrrheum.2017.56 [DOI] [PubMed] [Google Scholar]
- 14.McGonagle DG, McInnes IB, Kirkham BW, et al. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis 2019;78:1167–78. 10.1136/annrheumdis-2019-215356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:1705–13. 10.1016/S0140-6736(13)61134-4 [DOI] [PubMed] [Google Scholar]
- 16.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 17.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- 18.Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung J. Special issue on therapeutic antibodies and biopharmaceuticals. Exp Mol Med 2017;49:e304. 10.1038/emm.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagami H, Morishita R. Recent advances in therapeutic vaccines to treat hypertension. Hypertension 2018;72:1031–6. 10.1161/HYPERTENSIONAHA.118.11084 [DOI] [PubMed] [Google Scholar]
- 21.Kawakami R, Nozato Y, Nakagami H, et al. Development of vaccine for dyslipidemia targeted to a proprotein convertase subtilisin/kexin type 9 (PCSK9) epitope in mice. PLoS One 2018;13:e0191895. 10.1371/journal.pone.0191895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawano T, Shimamura M, Nakagami H, et al. Therapeutic vaccine against S100A9 (S100 calcium-binding protein A9) inhibits thrombosis without increasing the risk of bleeding in ischemic stroke in mice. Hypertension 2018;72:1355–64. 10.1161/HYPERTENSIONAHA.118.11316 [DOI] [PubMed] [Google Scholar]
- 23.Nakagami F, Koriyama H, Nakagami H, et al. Decrease in blood pressure and regression of cardiovascular complications by angiotensin II vaccine in mice. PLoS One 2013;8:e60493. 10.1371/journal.pone.0060493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang Z, Nakagami H, Osako MK, et al. Therapeutic vaccine against DPP4 improves glucose metabolism in mice. Proc Natl Acad Sci U S A 2014;111:E1256–63. 10.1073/pnas.1322009111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe R, Suzuki J-I, Wakayama K, et al. A peptide vaccine targeting angiotensin II attenuates the cardiac dysfunction induced by myocardial infarction. Sci Rep 2017;7:43920.:43920. 10.1038/srep43920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikawa M, Myoui A, Tomita T, et al. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum 2003;48:2670–81. 10.1002/art.11227 [DOI] [PubMed] [Google Scholar]
- 27.Amatya N, Garg AV, Gaffen SL. IL-17 signaling: the yin and the yang. Trends Immunol 2017;38:310–22. 10.1016/j.it.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Tok MN, van Duivenvoorde LM, Kramer I, et al. Interleukin-17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis Rheumatol 2019;71:612–25. 10.1002/art.40770 [DOI] [PubMed] [Google Scholar]
- 29.Guan Q, Weiss CR, Qing G, et al. An IL-17 peptide-based and virus-like particle vaccine enhances the bioactivity of IL-17 in vitro and in vivo. Immunotherapy 2012;4:1799–807. 10.2217/imt.12.129 [DOI] [PubMed] [Google Scholar]
- 30.Koriyama H, Ikeda Y, Nakagami H, et al. Development of an IL-17A DNA vaccine to treat systemic lupus erythematosus in mice. Vaccines (Basel) 2020;8:83. 10.3390/vaccines8010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shores LS, Kelly SH, Hainline KM, et al. Multifactorial design of a supramolecular peptide anti-IL-17 vaccine toward the treatment of psoriasis. Front Immunol 2020;11:1855.:1855. 10.3389/fimmu.2020.01855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uyttenhove C, Van Snick J. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur J Immunol 2006;36:2868–74. 10.1002/eji.200636662 [DOI] [PubMed] [Google Scholar]
- 33.Fauny M, Moulin D, D’Amico F, et al. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis 2020;79:1132–8. 10.1136/annrheumdis-2020-217927 [DOI] [PubMed] [Google Scholar]
- 34.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa A, Andoh A, Araki Y, et al. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 2004;110:55–62. 10.1016/j.clim.2003.09.013 [DOI] [PubMed] [Google Scholar]
- 36.Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol 2016;111:1599–607. 10.1038/ajg.2016.298 [DOI] [PubMed] [Google Scholar]
- 37.Nakamaru R, Nakagami H, Rakugi H, et al. Future directions of therapeutic vaccines for chronic diseases. Circ J 2020;84:1895–902. 10.1253/circj.CJ-20-0703 [DOI] [PubMed] [Google Scholar]
- 38.Ceribelli A, Motta F, Vecellio M, et al. Clinical trials supporting the role of the IL-17/IL-23 axis in axial spondyloarthritis. Front Immunol 2021;12:622770. 10.3389/fimmu.2021.622770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdes S, Nasonov E, Kunder E, et al. Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin Exp Rheumatol 2020;38:27–34. [PubMed] [Google Scholar]
- 40.van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018;392:2441–51. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- 41.van Tok MN, Na S, Lao CR, et al. The initiation, but not the persistence, of experimental spondyloarthritis is dependent on interleukin-23 signaling. Front Immunol 2018;9:1550. 10.3389/fimmu.2018.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002851supp001.pdf (1.3MB, pdf)
Data Availability Statement
All data related to this study for analysis are available upon reasonable request.