Abstract
This study aimed to find out the effect of vitamins on respiratory-related viral infections, including coronavirus disease 2019 (COVID-19), through the literature reviews. From January 2000 to June 2021, the studies (cohort studies, cross-sectional studies, case-control studies, randomized control trials) related to vitamins (vitamin A, D, E, C, B6, folate, and B12) and COVID-19/severe acute respiratory syndrome/Middle East respiratory syndrome/cold/influenza were selected from the PubMed, Embase, and Cochrane libraries and analyzed. The relationship between vitamins and virus-related respiratory diseases was identified. Through the review, 39 studies were selected on vitamin D, one study on vitamin E, 11 studies on vitamin C, and 3 studies on folate. Regarding COVID-19, 18 studies on vitamin D, 4 studies on vitamin C, and 2 studies on folate showed significant effects of the intake of these nutrients in preventing COVID-19. Regarding colds and influenza, 3 studies on vitamin D, 1 study on vitamin E, 3 studies on vitamin C, and 1 study on folate demonstrated that the intake of these nutrients significantly prevents these diseases. Therefore, this review suggested the intake of vitamins D, E, C, and folate is important for preventing respiratory diseases related to viruses, such as COVID-19, colds, and influenza. The relationship between these nutrients and virus-related respiratory diseases should be continuously monitored in the future.
Keywords: COVID-19, Vitamins, Respiratory tract infection, Virus
INTRODUCTION
Since the coronavirus disease 2019 (COVID-19) outbreak in 2019, the global death toll has approached 6 million, and the pandemic, now in its third year, is not over [1]. The new coronavirus, the causative agent of COVID-19, is a family of RNA viruses [2]. RNA viruses are considered to have high mutation rates and frequent mutations [3]. Therefore, it is not easy to manufacture COVID-19-related vaccines or therapeutics. Among the top 10 infectious diseases that have caused the most deaths in the world (Spanish flu, Asian flu, Hong Kong flu, 7th cholera epidemic, swine influenza, Ebola, Congo measles, West African meningitis, and severe acute respiratory syndrome [SARS]), excluding cholera and meningitis, the remaining diseases are infectious diseases caused by RNA viruses. Currently, there is no specific treatment for coronavirus infection. In early 2020, the World Health Organization (WHO) declared that SARS-coronavirus 2 had established a pandemic infection and was added to the WHO list of blueprint priority diseases [4].
In 2020, the COVID-19 guidelines published by the WHO, the United Nations Food and Agriculture Organization, the European Food Information Commission, the Centers for Disease Control and Prevention, and the American Nutrition Society identified 4 common nutritional issues. 1) Eat foods that improve immune function, such as vitamins, minerals, dietary fiber, and antioxidants, from fresh, not processed foods. 2) Maintain an adequate intake of minerals (copper, iron, zinc) and vitamins (A, B6, B12, C, D, and folate) directly involved in immune function. 3) Eat whole grains and healthy fats, such as omega-3 fatty acids and nuts. 4) Avoid the intake of high-carbohydrate, high-fat, high-salt foods, alcohol, and frozen foods.
Some nutrients were reported to be actively involved in the proper functioning and strengthening of the immune system, including dietary protein, omega-3 fatty acids, vitamins A, D, E, B1, B6, B12, and C. Supplementation with some of these dietary components was also reported to be effective in improving the health status of patients with viral infections [5]. Viral infections are characterized by compromised immune function and deficient micronutrient stores, particularly vitamins, including vitamins A, B6, B12, C, D, and E [6].
Therefore, based on the studies showing that vitamins are effective in preventing COVID-19, this study attempted to investigate the effects of vitamins on respiratory-related viral infections, including COVID-19, through a review.
MATERIALS AND METHODS
Data extraction
Research searches were performed by 4 independent reviewers, focusing on literature published from January 2000 to June 2021 in the PubMed, Embase, and Cochrane libraries. To identify the publications, individual nutrients and COVID-19/SARS/Middle East respiratory syndrome (MERS)/cold/influenza descriptors were adopted. The articles only written in English, Korean, and Japanese were reviewed. The target nutrients were vitamins A, D, E, C, B6, folate, and B12.
Data selection
The selection criteria for literature in this review were cross-sectional studies, cohort studies, case-controlled studies, and randomized clinical trials (RCTs). The exclusion criteria were: in vitro laboratory research, cell experiments, animal experiments, reviews, systematic literature reviews, meta-analysis studies, and conference proceedings. The lists of bibliographical references of the relevant studies were examined to identify potentially eligible studies. The publications were managed in Rayyan to remove duplicates and apply the inclusion criteria. Whether publications met the selection criteria or not was determined by reviewing the titles and abstracts of the searched papers. When it was difficult to judge a paper based on the title and abstract alone, the text was reviewed to decide whether to select the paper. Through this process, the selected articles were re-examined, and the finally selected papers were included in the review.
RESULTS
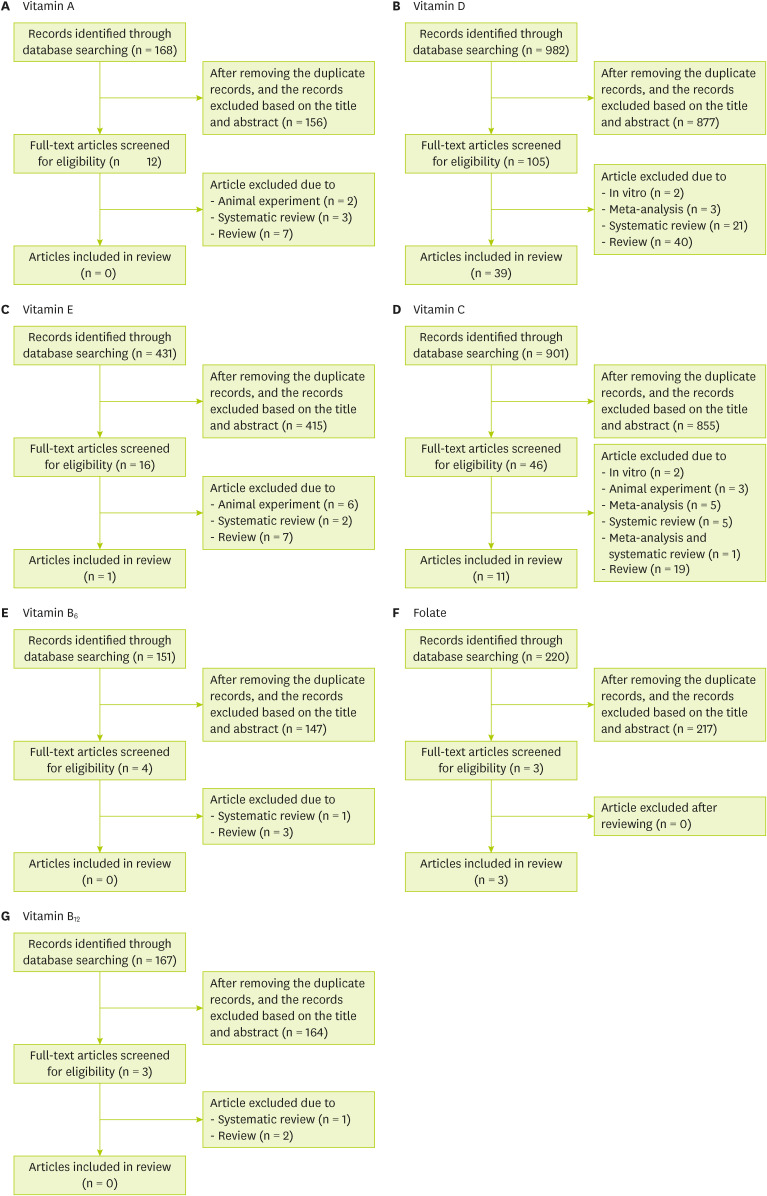
A search for individual nutrients and respiratory-related viral infections in the PubMed, Embase, and Cochrane libraries found 168 papers on vitamin A, 982 papers on vitamin D, 431 papers on vitamin E, 901 papers on vitamin C, 151 papers on vitamin B6, 220 papers on folate, and 167 papers on vitamin B12 (Figure 1). Among these publications, only studies related to cohort studies, cross-sectional studies, case-controlled studies, and RCTs were selected.
Figure 1. Flow diagram of study selection for systematic review of each nutrient.
Vitamin A
Of the total 168 studies on vitamin A, 156 were not related to COVID-19/SARS/MERS/cold/influenza, and 2 animal studies, 3 systematic reviews, and 7 reviews were excluded. Therefore, no cohort studies, cross-sectional studies, case-control studies, or RCTs of vitamin A were found, so a review of vitamin A could not be performed (Figure 1A).
Vitamin D
A total of 982 articles related to vitamin D were found. Among them, 877 articles were excluded by reviewing the title and abstract, and 2 in vitro research studies, 3 meta-analyses, 21 systemic reviews, and 40 reviews were also excluded (Figure 1B). Finally, an article review was conducted on 39 studies, which were classified into 13 cohort studies [7,8,9,10,11,12,13,14,15,16,17,18,19], 11 cross-sectional studies [20,21,22,23,24,25,26,27,28,29,30], 6 case-control studies [31,32,33,34,35,36], and 9 RCTs [37,38,39,40,41,42,43,44,45] (Table 1). The cohort study included 10 COVID-19-related studies and 3 respiratory disease-related studies. The subjects of the study were people infected with COVID-19 in the COVID-19 study and normal people in the study related to respiratory diseases. The cross-sectional study was classified into 9 studies on COVID-19, one study each on influenza, legionella, and pneumonia, and one study each on lung function, respiratory tract infections, and colds. The case-control study consisted of 5 COVID-19-related studies and one influenza-related study. The RCT consisted of 3 studies related to COVID-19 topics, 4 studies related to respiratory diseases, and 2 studies related to influenza. The subjects were divided into a placebo group and an experimental group and took different doses of various forms of vitamin D.
Table 1. Main characteristics of the articles evaluating the association between vitamin D and respiratory-related viral infection.
| Authors | Study design | Sample size | Biomarker | Dose | Result* | Mean or range of age (yr) | Target |
|---|---|---|---|---|---|---|---|
| Baktash et al. (2020) [7] | Cohort | 70 (66.7%), COVID-19-positive group | 25(OH)D | - | + | 81 (79.46–81.16) | COVID-19 |
| 35 (33.3%), COVID-19-negative group | |||||||
| D’Avolio et al. (2020) [8] | Cohort | 107, patients who underwent a nasopharyngeal swab PCR analysis for SARS-CoV-2 and a 25(OH)D measurement | 25(OH)D | - | + | 73 | COVID-19 |
| Meltzer et al. (2020) [9] | Cohort | 489, patients with a 25-hydroxycholecalciferol or 1,25-dihydroxycholecalciferol level measured within 1 year before being tested for COVID-19 from March 3 to April 10, 2020 | 25(OH)D or 1,25(OH)2D | - | + | 49.2 | COVID-19 |
| Cereda et al. (2021) [10] | Cohort | 129, consecutive adult COVID-19 patients hospitalized | 25(OH)D | - | + | 73.6 ± 13.9 | COVID-19 |
| Gavioli et al. (2021) [11] | Cohort | 437, COVID-19 patients | 25(OH)D | - | − | 67 (56–79) | COVID-19 |
| Hastie et al. (2021) [12] | Cohort | 341,184, UK Biobank participants, of which 656 had inpatient confirmed COVID-19 infection and 203 died of COVID-19 infection | 25(OH)D | - | − | 37–73 | COVID-19 |
| Infante et al. (2021) [13] | Cohort | 137, consecutive patients with SARS-CoV-2 infection | 25(OH)D | - | + | 34–89 | COVID-19 |
| Lohia et al. (2021) [14] | Cohort | 270, patients with confirmed COVID-19 | Vitamin D | - | − | 63.81 ± 14.69 | COVID-19 |
| Orchard et al. (2021) [15] | Cohort | 50, SARS-CoV-2 PCR positive hospitalizations | Vitamin D | - | +/− | 60 (51.2–67.0) | COVID-19 |
| Osman et al. (2021) [16] | Cohort | 445, hospitalized patients | Vitamin D | - | + | 50.8 (15–94) | COVID-19 |
| Sabetta et al. (2010) [17] | Cohort | 198, healthy adults | 25(OH)D | - | + | 20–88 | Acute viral respiratory tract infections |
| Berry et al. (2011) [18] | Cohort | 6,789, participants in the nationwide 1958 British birth cohort | 25(OH)D | - | + | 45 | Respiratory infections and lung function |
| Brenner et al. (2020) [19] | Cohort | 9,940, recruited by their general practitioners during a routine health check-up between 2000 and 2002 | 25(OH)D | - | + | 50–75, 62.1 | Respiratory disease |
| Ling et al. (2020) [20] | Cross-sectional | 444, patients had symptoms and signs suggestive of SARS-CoV-2 infection | Cholecalciferol | Approximately ≥ 280,000 IU in a time period of up to 7 wk) | + | 63–83 | COVID-19 |
| Serum 25(OH)D | − | ||||||
| Abdollahi et al. (2021) [21] | Cross-sectional | 118, patients with COVID-19 who were hospitalized in ICU | 25(OH)D | - | + | 65.05 ± 15.75 | COVID-19 |
| De Smet et al. (2021) [22] | Cross-sectional | 186, severe acute respiratory syndrome coronavirus 2 infected individuals hospitalized | 25(OH)D | - | Male, + | 52–80 | COVID-19 |
| Female, − | |||||||
| Katz et al. (2021) [23] | Cross-sectional | 884, patients positively diagnosed with COVID-19 | Vitamin D | - | + | All ages | COVID-19 |
| 31,950, patients had vitamin D deficiency | |||||||
| 87, patients had both vitamin D deficiency and COVID-19 | |||||||
| Luo et al. (2021) [24] | Cross-sectional | 560, individuals who underwent the physical examination program | 25(OH)D | - | + | Control, 49–60 | COVID-19 |
| 335, COVID-19 patients | COVID-19, 43–64 | ||||||
| Nasiri et al. (2021) [25] | Cross-sectional | 329, confirmed cases of COVID-19 | Vitamin D | - | +/− | 64.7 ± 18.5 (15–99) | COVID-19 |
| Meoli et al. (2021) [26] | Cross-sectional | 735, adolescents enrolled during the compulsory military fitness-for-duty evaluation | 25(OH)D | - | − | 18–19 | COVID-19 |
| Pugach and Pugach (2021) [27] | Cross-sectional | 10 countries | 25(OH)D | - | + | 37.8–47.8 | COVID-19 |
| Yadav et al. (2021) [28] | Cross-sectional | 37 countries | Vitamin D | - | + | - | COVID-19 |
| Pletz et al. (2014) [29] | Cross-sectional | 101, control | 25(OH)D, 1,25(OH)2D | - | − | Control, 59.43 | Influenza |
| 50, influenza | + | Influenza, 60.13 | Legionella | ||||
| 49, legionella | Legionella, 62.65 | Streptococcus pneumoniae | |||||
| 100, Streptococcus pneumoniae | Streptococcus pneumoniae, 57.41 | ||||||
| Rafiq et al. (2018) [30] | Cross-sectional | 6,138, participants in the NEO study | 25(OH)D | - | BMI > 30, + | 45–65 | Lung function, airway inflammation, common colds |
| Abdollahi et al. (2021) [31] | Case-control | 201, patients with coronavirus infection | 25(OH)D | - | + | Case, 48 | COVID-19 |
| 201, controls | Control, 46.34 | ||||||
| Al-Daghri et al. (2021) [32] | Case-control | 138, RT-PCR-confirmed SARS-CoV-2 positive | 25(OH)D | - | + | 43 ± 15 | COVID-19 |
| 82, negative controls | |||||||
| Alguwaihes et al. (2021) [33] | Case-control | 150, SARS-CoV-2 (+) | 25(OH)D | - | + | 56.6 ± 16.2 | COVID-19 |
| 72, SARS-CoV-2 (−) | |||||||
| Hernández et al. (2021) [34] | Case-control | 197, COVID-19 with confirmed COVID-19, COVID-19 patients on oral vitamin D supplements for more than 3 mon, 197 control | 25(OH)D | Cholecalciferol, 25,000 IU/monthly in 10 cases, 5,600 IU/weekly in 1, and calcifediol 0.266 mg/monthly in 8 patients were on | + | COVID-19 patients, 61 (47.5–70.0) | COVID-19 |
| COVID-19 patients + vitamin D supplementation, 60 (59.0–75.0) | |||||||
| Controls, 61 (56.0–66.0) | |||||||
| Ye et al. (2021) [35] | Case-control | 80, healthy controls and 62 patients diagnosed with COVID-19 | 25(OH)D | - | + | Control, 42 (31–52) | COVID-19 |
| Case 43, (32–59) | |||||||
| Nanri et al. (2017) [36] | Case-control | 179, cases who reported influenza diagnosis | 25(OH)D | - | − | 37.6 ± 11.6 | Influenza |
| 353, participants who did not reported influenza diagnosis | |||||||
| Rastogi et al. (2020) [37] | RCT | 16, vitamin D supplementation group | 25 (OH)D | 60,000 IU/day, 7 day | + | Intervention group, 50 | COVID-19 |
| 24, control group for asymptomatic and mildly symptomatic SARS-CoV-2 positive individuals | Control group, 47.5 | ||||||
| Ohaegbulam et al. (2020) [38] | RCT | 2, vitamin D-high dose | Vitamin D | 1,000 IU cholecalciferol, 50,000 IU ergocalciferol, 1 mon | + | Vitamin D-high dose, 41, 57 | COVID-19 |
| 2, vitamin D-standard dose | Vitamin D-standard dose, 74, 53 | ||||||
| Murai et al. (2021) [39] | RCT | 119, vitamin D3 group | Mortality rate | 200,000 IU, 4 mon | − | 200,000 IU of vitamin D3 56.5 or placebo 56 | COVID-19 |
| 118, placebo group | |||||||
| Rees et al. (2013) [40] | RCT | 399, vitamin D3 group | Cold, influenza prevalence | Vitamin D3 (1,000 IU/day) + Ca (1,200 mg/day) | − | Vitamin D3 group, 57.9 | Upper respiratory tract |
| 360, placebo group | Placebo group, 57.8 | ||||||
| Aglipay et al. (2017) [41] | RCT | 354, vitamin D dose 400 IU/d | No. of subjects | Vitamin D 400 IU/d or 2,000 IU/d, 4 mon | − | Vitamin D dose 400 IU/d, 2.76 | Viral upper respiratory tract infections |
| 349, vitamin D dose 2,000 IU/d | Vitamin D dose 2,000 IU/d, 2.70 | ||||||
| Shimizu et al. (2018) [42] | RCT | 105, placebo group | 25(OH)D | 10 μg/, 16 wk | Prevalence, − | Placebo, 52.6 ± 6.7 | Upper respiratory tract infection |
| 110, 25(OH)D group | Duration, + | 25(OH)D group, 52.8 ± 6.2 | |||||
| Loeb et al. (2019) [43] | RCT | 650, vitamin D group | 25(OH)D | 14,000 U/wk, 8 mon | − | Vitamin D, 8.6 | Respiratory infections |
| 650, placebo group | Placebo, 8.4 | ||||||
| Urashima et al. (2014) [44] | RCT | 148, vitamin D3 group | Influenza A prevalence | 2,000 IU/day, 2 mon | − | - | Influenza A |
| 99, placebo group | |||||||
| Zhou et al. (2018) [45] | RCT | 168, low dose vitamin D | Person infected with influenza A | 400 IU or 1,200 IU, 4 mon | + | Low dose vitamin D, 7.7 ± 2.5 | Seasonal influenza A |
| 164, high dose vitamin D | High dose vitamin D, 8.0 ± 2.7 |
COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; 25(OH)D, 25-hydroxyvitamin D; ICU, intensive care unit; NEO, Netherlands Epidemiology of Obesity; BMI, body mass index; RT-PCR, reverse transcription polymerase chain reaction; RCT, randomized control trial.
*+: significant effect on the prevention of respiratory-related viral infection; −: no significant effect on the prevention of respiratory-related viral infection.
Vitamin E
We identified 431 studies related to vitamin E in our database. Of these, 415 papers were excluded by reviewing the study titles and abstracts. Only one case was eligible for a literature review, with exclusion of 6 animal experiments, 2 systematic reviews, and 7 reviews (Figure 1C, Table 2). The eligible study was an RCT study to confirm the efficacy of vitamin E in respiratory infections [46].
Table 2. Main characteristics of included article evaluated the association between vitamin E and respiratory-related viral infection.
| Authors | Study design | Sample size | Biomarker | Dose | Result* | Mean or range of age (yr) | Target |
|---|---|---|---|---|---|---|---|
| Meydani et al. (2004) [46] | RCT | 70 (66.7%), COVID-19-positive group | Incidence, No. of subjects and No. of days with respiratory infections (upper and lower), and No. of new antibiotic prescriptions | Vitamin E 200 IU | + | Vitamin E, 84.7 or placebo, 84.3 | Respiratory infection |
| 35 (33.3%), COVID-19-negative group |
COVID-19, coronavirus disease 2019.
*+: significant effect on the prevention of respiratory-related viral infection; −: no significant effect on the prevention of respiratory-related viral infection.
Vitamin C
In vitamin C, 901 papers were searched (Figure 1D), and 855 papers were excluded based on the titles and abstracts. A total of 11 documents were used in the study after excluding 2 in vitro studies, 3 animal experiments, 5 meta-analyses, 5 systematic reviews, one mixed meta-analysis and systematic review, and 19 literature reviews (Table 3). Of these, 3 cross-sectional studies were COVID-19-related studies [47,48,49], and 8 RCTs included 3 studies related to COVID-19 [50,51,52] and 5 studies related to the common cold [53,54,55,56,57].
Table 3. Main characteristics of included article evaluated the association between VC and respiratory-related viral infection.
| Authors | Study design | Sample size | Biomarker | Dose | Result* | Mean or range of age (yr) | Target |
|---|---|---|---|---|---|---|---|
| Xing et al. (2021) [47] | Cross-sectional | 25, COVID-19 patients treated with VC | VC | Patients with COVID-19: IV VC at a dose of 100 mg/kg/day | + | Patients treated with VC (n = 39) | COVID-19 |
| 6, COVID-19 patients | Patients treated without VC (n = 35.63), Healthy (n = 31.42) | ||||||
| 51, healthy volunteers | |||||||
| Zhao et al. (2021) [48] | Cross-sectional | 6, severe | Inflammatory response, immune | Severe, 162.7 mg/kg/days | + | Severe, 56 Critical, 63 | COVID-19 |
| 6, critical | Critical, 178.6 mg/kg/days | ||||||
| Zhao et al. (2021) [49] | Cross-sectional | 55, HDIVC group | No. of patients, duration of systemic inflammatory response syndrome | 100 mg/kg/day | − | HDIVC, 36 | COVID-19 |
| 55, control group | Control, 36 | ||||||
| Zhang et al. (2021) [50] | RCT | 27, severe SARS-CoV-2-related pneumonia. HDIVC group | Mortality, P/F ratio, IL-6 | IV VC 24 g | + | HDIVC, 66.7 | COVID-19 |
| 29, placebo group | Control, 66.3 | ||||||
| Thomas et al. (2021) [51] | RCT | 50, usual care | 50% reduction in symptoms | Ascorbic acid 8,000 mg, Zinc gluconate 50 mg, Ascorbic acid 8,000 mg + Zinc gluconate 50 mg | − | 45.2 | COVID-19 |
| 48, ascorbic acid | Standard of care, 42.0 | ||||||
| 58, zinc gluconate | Ascorbic acid only, 45.6 | ||||||
| 58, ascorbic acid + zinc gluconate | Zinc only, 44.1 | ||||||
| Ascorbic acid with zinc, 48.7 | |||||||
| Kumari et al. (2020) [52] | RCT | 75, intervention (standard of care + IV VC) | No. of days required for treatment, hospital stay | VC 50 mg/kg/day | + | Intervention, 52 | COVID-19 |
| 75, placebo (standard of care) | Placebo, 53 | ||||||
| Audera et al. (2001) [53] | RCT | 42, VC 0.03 g | Daily symptoms, severity | VC 0.03 g | − | VC 0.03 g, 38.6 | Common cold |
| 47, VC 1 g | VC 1 g | VC 1 g, 40.1 | |||||
| 50, VC3 g | VC 3 g | VC 3 g, 39.9 | |||||
| 45, VC 3 g + additives | VC 3 g + bioflavenoids 75 mg, rutin 150 mg, hisperidin 150 mg, rose hip extract 750 mg acerola 150 mg | VC 3 g + additives, 45.1 | |||||
| Van Straten and Josling (2002) [54] | RCT | 84, active treatment | Recorded any common cold infections and symptoms in a daily diary | VC 500 mg | + | Active treatment, 47.7 | Common cold |
| 84, placebo | Placebo, 48.5 | ||||||
| Sasazuki et al. (2006) [55] | RCT | 120, 50 mg of VC | Symptom, cold duration | VC 50/500 mg | − | Low dose, 58.7 | Common cold |
| 144, 500 mg of VC | High dose, 56.3 | ||||||
| Johnston et al. (2014) [56] | RCT | 15, VC | Plasma vitamin, cold episodes, duration, cold duration and severity | VC 1,000 mg | + | VC, 23.0 | Cold incidence |
| 13, placebo | Placebo, 23.2 | ||||||
| Kim et al. (2020) [57] | RCT | 695, VC group | Diagnosis of common colds | VC 6,000 mg | + | < 19, 491 | Common cold |
| 749, placebo group | 20–22, 878 | ||||||
| > 23, 75 |
IV, intravenous; VC, vitamin C; COVID-19, coronavirus disease 2019; HDIVC, high-dose intravenous vitamin C; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; P/F, PaO2/FiO2; IL, interleukin; RCT, randomized control trial.
*+: significant effect on the prevention of respiratory-related viral infection; −: no significant effect on the prevention of respiratory-related viral infection.
Vitamin B6
For vitamin B6, 151 articles were searched, and 147 articles were excluded by reviewing the titles and abstracts. One systematic review and 3 reviews were also excluded (Figure 1E). Thus, no articles were eligible for a literature review.
Folate
A total of 220 articles on folic acid were searched (Figure 1F). As a result of reviewing the titles and abstracts, 217 were excluded. Three papers were classified into 2 cross-sectional studies and one cohort study and underwent literature reviews (Table 4).
Table 4. Main characteristics of included article evaluated the association between folate and respiratory-related viral infection.
| Authors | Study design | Sample size | Biomarker | Dose | Result* | Mean or range of age (yr) | Target |
|---|---|---|---|---|---|---|---|
| Acosta-Elias and Espinosa-Tanguma (2020) [58] | Cross-sectional | 94, pregnant women | The likelihood of requiring hospitalization for SARS-CoV-2 infection | - | + | - | COVID-19 |
| 137, non-pregnant in 2009 A-H1N1 pandemic | A-H1N1 pandemic | ||||||
| 908 of those patients were non-pregnant women in reproductive age | |||||||
| 55, pregnant women in COVID-19 pandemic | |||||||
| Hamer et al. (2009) [59] | Cross-sectional | 352 | Vitamins and minerals concentration | - | + | Men, 75.8 | Respiratory infections |
| Women, 73.7 | |||||||
| Itelman et al. (2020) [60] | Cohort | 162 | Blood folate concentration | - | + | 52 | COVID-19 |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
*+: significant effect on the prevention of respiratory-related viral infection; −: no significant effect on the prevention of respiratory-related viral infection.
The cross-sectional studies consisted of one study on COVID-19 [58] and A-H1N1 and one study on respiratory infections [59]. A cohort study related to COVID-19 was conducted [60].
Vitamin B12
The search yielded 167 studies related to vitamin B12, and among them, 164 papers were excluded by reviewing the titles and abstracts (Figure 1G). No papers underwent a literature review because one systematic review and 2 reviews were excluded.
DISCUSSION
This review was conducted to investigate the effects of vitamins on COVID-19/SARS/MERS/cold/influenza-related viral infections.
Eighteen of the 27 studies related to vitamin D and COVID-19 reported the effect of low levels of vitamin D in the blood on the occurrence of COVID-19. The 25-hydroxyvitamin D (25(OH)D) blood levels of COVID-19-positive patients were reported to be lower than 10–11.1 ng/mL [8,11]. Patients with vitamin D deficiency have seemed more than 5 times higher to be infected with COVID-19 than patients without vitamin D deficiency [23]. In addition, it was reported that for every 1% increase in the prevalence of vitamin D deficiency, the number of deaths from COVID-19 increases by 55 per million [27]. Serum 25(OH)D concentrations in COVID-19-positive patients were 25.95 ± 14.56 ng/mL [20]. Ye et al. [35] identified a potential threshold of serum 25(OH)D of 41.19 nmol/L for COVID-19 prevention. COVID-19-positive patients were hospitalized for a prolonged period if they had low vitamin D levels at the time of admission [25]. It has been reported that the normalization of serum 25(OH)D levels shortened hospital stays and reduced inflammatory biomarkers [37]. Of the 16 subjects who consumed 60,000 IU/d of vitamin D for 7 days, 10 tested negative for COVID-19, and only 5 of the 24 subjects who did not take vitamin D tested negative for COVID-19 [37]. However, 9 studies reported that vitamin D had no significant effect on COVID-19 prevention. Among the 12 studies related to respiratory diseases, 5 studies reported that vitamin D had a significant effect on the prevention of respiratory diseases. Serum 25(OH)D concentrations above 38 ng/mL lowered the risk of developing acute viral respiratory infections by about half [17], and mortality from respiratory diseases increased significantly as 25(OH)D levels fell below 50 nmol/L [19]. The group that consumed 1,200 IU of vitamin D for 4 months (8.0 ± 2.7 years) had a significantly lower number of influenza A infections than the group that consumed 400 IU (7.7 ± 2.5 years) [45].
Vitamin E has not been studied for its effect on COVID-19, and only one study was related to respiratory infections. The number of respiratory infections was significantly lower in the vitamin E intake group (200 IU, 84.7 years) than in the vitamin E non-intake group (84.3 years) [46].
Vitamin C reduced COVID-19-positive diagnoses in 4 out of 6 studies involving COVID-19. The vitamin C concentration in the plasma of COVID-19 patients was 5 times lower than that of healthy people (p < 0.001) [47], and C-reactive protein levels were significantly reduced when 162.7–178.6 mg/kg/day of vitamin C was consumed (p < 0.05), and lymphocyte counts, CD4+ T-cells, and respiratory function were significantly increased (p < 0.05) [48]. The intravenous administration of 24 g of vitamin C reduced COVID-19-induced mortality and interleukin-6 (p = 0.04) levels [50], an inflammatory index. The duration of symptoms and duration of hospitalization were significantly reduced when 50 mg/kg/day of vitamin C was supplied in the same way (p <0.001) [52]. In the studies on vitamin C and colds, 3 out of 5 studies showed the effect of reducing the cold diagnosis rate and the duration of cold symptoms by a vitamin C intake of 500–6,000 mg [54,56,57].
Two studies related to folate and COVID-19 found that folate had a positive effect on COVID-19 prevention. Folate supplementation was a factor in protecting patients from COVID-19 infection [58]. The reason is that folate inhibits furin protease, which is necessary for viruses to enter host cells, and folate inactivates protease 3C-like protease, a protein that the virus needs to replicate [58]. Indeed, it has been reported that blood folate levels were low in patients with severe COVID-19 (p = 0.014) [60]. A study related to folate and respiratory infections also reported that a deficiency in micronutrients, such as folate, was closely related to the occurrence of pneumonia or cold (p < 0.001) [59]. In this study, no publications on the relationship between vitamins and SARS/MERS were found during the study period.
Thus, the results of this study found that maintaining normal blood levels of vitamin D, vitamin E, vitamin C, and folate had a positive effect on the prevention of COVID-19/cold/influenza. This effect is thought to be possible by maintaining normal blood levels through the daily intake of these nutrients. However, due to the limited period of this study, additional research publications related to these vitamins and COVID-19/SARS/MERS/cold/influenza will need to be collected, analyzed and evaluated periodically in the future.
Footnotes
Funding: This work was supported by the Amway corporation.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Park E.
- Funding acquisition: Park E.
- Investigation: Park JH, Lee Y, Choi M.
- Methodology: Lee Y.
- Project administration: Lee Y.
- Supervision: Park EJ.
- Writing - original draft: Park JH, Lee Y, Choi M.
- Writing - review & editing: Park JH.
References
- 1.Rising D. Death toll nears 6 million as pandemic enters its 3rd year [Internet] 2002. [cited 2022 March 7]. Available from https://apnews.com/article/russia-ukraine-coronavirus-pandemic-science-business-health-69e8cbaebb653a0f1cb65ffe33d9afbd .
- 2.Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Cureus. 2020;12:e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16:e3000003. doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui DS, Azhar EI, Memish ZA, Zumla A. In: Encyclopedia of Respiratory Medicine. Janes SM, editor. Amsterdam: Elsevier; 2022. Human coronavirus infections—severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS), and SARS-CoV-2; pp. 146–61. [Google Scholar]
- 5.BourBour F, Mirzaei Dahka S, Gholamalizadeh M, Akbari ME, Shadnoush M, Haghighi M, Taghvaye-Masoumi H, Ashoori N, Doaei S. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch Physiol Biochem. 2020 doi: 10.1080/13813455.2020.1791188. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12082326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van den Abbeele K, Mandal AK, Missouris CG. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2021;97:442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3:e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cereda E, Bogliolo L, Klersy C, Lobascio F, Masi S, Crotti S, De Stefano L, Bruno R, Corsico AG, Di Sabatino A, Perlini S, Montecucco C, Caccialanza R NUTRI-COVID19 IRCCS San Matteo Pavia Collaborative Group. Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin Nutr. 2021;40:2469–2472. doi: 10.1016/j.clnu.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavioli EM, Miyashita H, Hassaneen O, Siau E. An evaluation of serum 25-hydroxy vitamin D levels in patients with COVID-19 in New York city. J Am Nutr Assoc. 2022;41:201–206. doi: 10.1080/07315724.2020.1869626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastie CE, Pell JP, Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2021;60:545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Infante M, Buoso A, Pieri M, Lupisella S, Nuccetelli M, Bernardini S, Fabbri A, Iannetta M, Andreoni M, Colizzi V, Morello M. Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: an Italian retrospective study. J Am Nutr Assoc. 2022;41:250–265. doi: 10.1080/07315724.2021.1877580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohia P, Nguyen P, Patel N, Kapur S. Exploring the link between vitamin D and clinical outcomes in COVID-19. Am J Physiol Endocrinol Metab. 2021;320:E520–E526. doi: 10.1152/ajpendo.00517.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orchard L, Baldry M, Nasim-Mohi M, Monck C, Saeed K, Grocott MP, Ahilanandan D. Vitamin-D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients. Clin Chem Lab Med. 2021;59:1155–1163. doi: 10.1515/cclm-2020-1567. [DOI] [PubMed] [Google Scholar]
- 16.Osman W, Al Fahdi F, Al Salmi I, Al Khalili H, Gokhale A, Khamis F. Serum calcium and vitamin D levels: correlation with severity of COVID-19 in hospitalized patients in Royal Hospital, Oman. Int J Infect Dis. 2021;107:153–163. doi: 10.1016/j.ijid.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry DJ, Hesketh K, Power C, Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 19.Brenner H, Holleczek B, Schöttker B. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients. 2020;12:2488. doi: 10.3390/nu12082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling SF, Broad E, Murphy R, Pappachan JM, Pardesi-Newton S, Kong MF, Jude EB. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020;12:3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdollahi H, Salehinia F, Badeli M, Karimi E, Gandomkar H, Asadollahi A, Sedighiyan M, Abdolahi M. The biochemical parameters and vitamin D Levels in ICU patients with COVID-19: a cross-sectional Study. Endocr Metab Immune Disord Drug Targets. 2021;21:2191–2202. doi: 10.2174/1871530321666210316103403. [DOI] [PubMed] [Google Scholar]
- 22.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2021;155:381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. 2021;84:111106. doi: 10.1016/j.nut.2020.111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people. J Nutr. 2021;151:98–103. doi: 10.1093/jn/nxaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasiri M, Khodadadi J, Molaei S. Does vitamin D serum level affect prognosis of COVID-19 patients? Int J Infect Dis. 2021;107:264–267. doi: 10.1016/j.ijid.2021.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meoli M, Muggli F, Lava SA, Bianchetti MG, Agostoni C, Kocher C, Bührer TW, Ciliberti L, Simonetti GD, Milani GP. Status in adolescents during COVID-19 pandemic: a cross-sectional comparative study. Nutrients. 2021;13:1467. doi: 10.3390/nu13051467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugach IZ, Pugach S. Strong correlation between prevalence of severe vitamin D deficiency and population mortality rate from COVID-19 in Europe. Wien Klin Wochenschr. 2021;133:403–405. doi: 10.1007/s00508-021-01833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav D, Birdi A, Tomo S, Charan J, Bhardwaj P, Sharma P. Association of vitamin D status with COVID-19 infection and mortality in the Asia pacific region: a cross-sectional study. Indian J Clin Biochem. 2021;36:492–497. doi: 10.1007/s12291-020-00950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pletz MW, Terkamp C, Schumacher U, Rohde G, Schütte H, Welte T, Bals R CAPNETZ-Study Group. Vitamin D deficiency in community-acquired pneumonia: low levels of 1,25(OH)2 D are associated with disease severity. Respir Res. 2014;15:53. doi: 10.1186/1465-9921-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafiq R, Thijs W, Prein R, de Jongh RT, Taube C, Hiemstra PS, de Mutsert R, den Heijer M. Associations of serum 25(OH)D concentrations with lung function, airway inflammation and common cold in the general population. Nutrients. 2018;10:35. doi: 10.3390/nu10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdollahi A, Kamali Sarvestani H, Rafat Z, Ghaderkhani S, Mahmoudi-Aliabadi M, Jafarzadeh B, Mehrtash V. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: a case-control study of hospitalized patients in Tehran, Iran. J Med Virol. 2021;93:2359–2364. doi: 10.1002/jmv.26726. [DOI] [PubMed] [Google Scholar]
- 32.Al-Daghri NM, Amer OE, Alotaibi NH, Aldisi DA, Enani MA, Sheshah E, Aljohani NJ, Alshingetti N, Alomar SY, Alfawaz H, Hussain SD, Alnaami AM, Sabico S. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: a multi-centre case-control study. J Transl Med. 2021;19:166. doi: 10.1186/s12967-021-02838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alguwaihes AM, Sabico S, Hasanato R, Al-Sofiani ME, Megdad M, Albader SS, Alsari MH, Alelayan A, Alyusuf EY, Alzahrani SH, Al-Daghri NM, Jammah AA. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: a retrospective case-control study in an Arab Gulf country. Aging Clin Exp Res. 2021;33:1415–1422. doi: 10.1007/s40520-021-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, Muñoz-Cacho P, Olmos JM, Gutiérrez-Cuadra M, Ruiz-Cubillán JJ, Crespo J, Martínez-Taboada VM. Vitamin D status in hospitalized patients with SARS-CoV-2 Infection. J Clin Endocrinol Metab. 2021;106:e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye K, Tang F, Liao X, Shaw BA, Deng M, Huang G, Qin Z, Peng X, Xiao H, Chen C, Liu X, Ning L, Wang B, Tang N, Li M, Xu F, Lin S, Yang J. Does serum vitamin D level affect COVID-19 infection and its severity?-a case-control study. J Am Coll Nutr. 2021;40:724–731. doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
- 36.Nanri A, Nakamoto K, Sakamoto N, Imai T, Akter S, Nonaka D, Mizoue T. Association of serum 25-hydroxyvitamin D with influenza in case-control study nested in a cohort of Japanese employees. Clin Nutr. 2017;36:1288–1293. doi: 10.1016/j.clnu.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri GD, Malhotra P. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med J. 2022;98:87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 38.Ohaegbulam KC, Swalih M, Patel P, Smith MA, Perrin R. Vitamin D supplementation in COVID-19 patients: a clinical case series. Am J Ther. 2020;27:e485–e490. doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CS, Silva CB, Franco AS, Macedo MB, Dalmolin HH, Baggio J, Balbi GG, Reis BZ, Antonangelo L, Caparbo VF, Gualano B, Pereira RM. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rees JR, Hendricks K, Barry EL, Peacock JL, Mott LA, Sandler RS, Bresalier RS, Goodman M, Bostick RM, Baron JA. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384–1392. doi: 10.1093/cid/cit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe K, Chen Y, Laupacis A, Mamdani M, Macarthur C, Hoch JS, Mazzulli T, Maguire JL TARGet Kids! Collaboration. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318:245–254. doi: 10.1001/jama.2017.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu Y, Ito Y, Yui K, Egawa K, Orimo H. Intake of 25-hydroxyvitamin D3 reduces duration and severity of upper respiratory tract infection: a randomized, double-blind, placebo-controlled, parallel group comparison study. J Nutr Health Aging. 2018;22:491–500. doi: 10.1007/s12603-017-0952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeb M, Dang AD, Thiem VD, Thanabalan V, Wang B, Nguyen NB, Tran HT, Luong TM, Singh P, Smieja M, Maguire J, Pullenayegum E. Effect of vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: a randomized controlled trial. Influenza Other Respi Viruses. 2019;13:176–183. doi: 10.1111/irv.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urashima M, Mezawa H, Noya M, Camargo CA., Jr Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: a randomized controlled trial. Food Funct. 2014;5:2365–2370. doi: 10.1039/c4fo00371c. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Du J, Huang L, Wang Y, Shi Y, Lin H. Preventive effects of vitamin D on seasonal influenza A in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J. 2018;37:749–754. doi: 10.1097/INF.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 46.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing Y, Zhao B, Yin L, Guo M, Shi H, Zhu Z, Zhang L, He J, Ling Y, Gao M, Lu H, Mao E, Zhang L. Vitamin C supplementation is necessary for patients with coronavirus disease: an ultra-high-performance liquid chromatography-tandem mass spectrometry finding. J Pharm Biomed Anal. 2021;196:113927. doi: 10.1016/j.jpba.2021.113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao B, Ling Y, Li J, Peng Y, Huang J, Wang Y, Qu H, Gao Y, Li Y, Hu B, Lu S, Lu H, Zhang W, Mao E. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliat Med. 2021;10:1599–1609. doi: 10.21037/apm-20-1387. [DOI] [PubMed] [Google Scholar]
- 49.Zhao B, Liu M, Liu P, Peng Y, Huang J, Li M, Wang Y, Xu L, Sun S, Qi X, Ling Y, Li J, Zhang W, Mao E, Qu J. Qu. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID-19 pneumonia. A retrospective propensity matched before-after study. Front Pharmacol. 2021;12:638556. doi: 10.3389/fphar.2021.638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G, Meng Z, De Backer D, Xiang H, Peng Z. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, Il’Giovine ZJ, Mehra R, McWilliams C, Nissen SE, Desai MY. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical Trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, Sohail H, Kumar B, Memon MK, Rizwan A. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020;12:e11779. doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Audera C, Patulny RV, Sander BH, Douglas RM. Mega-dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Aust. 2001;175:359–362. doi: 10.5694/j.1326-5377.2001.tb143618.x. [DOI] [PubMed] [Google Scholar]
- 54.Van Straten M, Josling P. Preventing the common cold with a vitamin C supplement: a double-blind, placebo-controlled survey. Adv Ther. 2002;19:151–159. doi: 10.1007/BF02850271. [DOI] [PubMed] [Google Scholar]
- 55.Sasazuki S, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Tsugane S. Effect of vitamin C on common cold: randomized controlled trial. Eur J Clin Nutr. 2006;60:9–17. doi: 10.1038/sj.ejcn.1602261. [DOI] [PubMed] [Google Scholar]
- 56.Johnston CS, Barkyoumb GM, Schumacher SS. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin C status: a randomized controlled trial. Nutrients. 2014;6:2572–2583. doi: 10.3390/nu6072572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim TK, Lim HR, Byun JS. Vitamin C supplementation reduces the odds of developing a common cold in Republic of Korea Army recruits: randomised controlled trial. BMJ Mil Health. 2022;168:117–123. doi: 10.1136/bmjmilitary-2019-001384. [DOI] [PubMed] [Google Scholar]
- 58.Acosta-Elias J, Espinosa-Tanguma R. The folate concentration and/or folic acid metabolites in plasma as factor for COVID-19 infection. Front Pharmacol. 2020;11:1062. doi: 10.3389/fphar.2020.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamer DH, Sempértegui F, Estrella B, Tucker KL, Rodríguez A, Egas J, Dallal GE, Selhub J, Griffiths JK, Meydani SN. Micronutrient deficiencies are associated with impaired immune response and higher burden of respiratory infections in elderly Ecuadorians. J Nutr. 2009;139:113–119. doi: 10.3945/jn.108.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itelman E, Wasserstrum Y, Segev A, Avaky C, Negru L, Cohen D, Turpashvili N, Anani S, Zilber E, Lasman N, Athamna A, Segal O, Halevy T, Sabiner Y, Donin Y, Abraham L, Berdugo E, Zarka A, Greidinger D, Agbaria M, Kitany N, Katorza E, Shenhav-Saltzman G, Segal G. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J. 2020;22:271–274. [PubMed] [Google Scholar]