Abstract
A systematic review and meta-analysis were designed to summarize studies conducted on the effects of raspberry and blackcurrant consumption on blood pressure (BP). Eligible studies were detected by searching numerous five online databases including PubMed, Scopus, Web of Science, Cochrane Library, and Google Scholar, until December 17, 2022. We pooled the mean difference and its 95% confidence interval (CI) by applying a random-effects model. Overall, the impact of raspberry and blackcurrant on BP was reported in ten randomized controlled trials (RCTs) (420 subjects). Pooled analysis of six clinical trials revealed that raspberry consumption has no significant reduction in systolic blood pressure (SBP) (weighted mean differences [WMDs], −1.42; 95% CI, −3.27 to 0.87; p = 0.224) and diastolic blood pressure (DBP) (WMD, −0.53; 95% CI, −1.77 to 0.71; p = 0.401), in comparison with placebo. Moreover, pooled analysis of four clinical trials indicated that blackcurrant consumption did not reduce SBP (WMD, −1.46; 95% CI, −6.62 to 3.7; p = 0.579), and DBP (WMD, −2.09; 95% CI, -4.38 to 0.20; p = 0.07). Raspberry and blackcurrant consumption elicited no significant reductions in BP. More accurate RCTs are required to clarify the impact of raspberry and blackcurrant intake on BP.
Keywords: Raspberry, Blackcurrant, Blood pressure
INTRODUCTION
Hypertension increases the risk of cardiovascular disease (CVD) and stroke [1,2]. The linear relationship between blood pressure (BP) levels and the risk of CVD is considered in people at serious risk [2,3]. Therefore, hypertension prevention and treatment are particularly crucial for enhancing the population's quality of life. Because pharmacological treatments always have side effects, nutrition intervention in disease treatment is very noticeable [3].
Therefore, according to the therapeutic and preventive effects of flavonols, flavanols, and anthocyanidins, ingredients for nutraceutical and functional foods, using these dietary supplements is becoming increasingly popular in communities [4]. These components are the subclasses of natural antioxidants’ polyphenols, called flavonoids [5]. Flavonoids are present in berries abundantly [6]. Raspberry and blackcurrant belong to the berry family, which contains large amounts of flavonoids, and have been shown to have antioxidant, anti-inflammatory, and anti-atherosclerotic effects [7]. Several trials have shown the improvement effect of intake of raspberry and blackcurrant on BP, lipid profiles, and cardiovascular function [8]. Indeed, it is evident from this study that consuming flavonoids, whether in the form of food or extracted, dramatically enhances vascular health [9]. Also, they improved vascular endothelial function as a result of inducing nitric oxide (NO) production [10] and reduced brachial BP or central arterial stiffness [11].
A systematic review study evaluated the potential antihypertensive activity of berries in lowering BP [12]. In another study, findings show that raspberry reduces BP after 1 week [13]. In this context, the study of Jeong et al. reported that the changes in systolic blood pressure (SBP) in the raspberry consumption groups were significantly reduced, but no alleviated signs were noted in diastolic blood pressure (DBP) among them in an 8-wk follow-up [14].
Regarding a study, short-term blackcurrant intake reduces central BP in older adults [15]. In contrast, in an intervention study on overweight adults, receiving blackcurrant for 6 weeks showed no effect on BP [16].
However, evidence of the effectiveness of raspberry and blackcurrant on BP has not been conclusive. This study aimed to systematically review and perform meta-analysis on all available human intervention studies to evaluate the potential effects of consumption of raspberry and blackcurrant on BP in randomized controlled trials (RCTs).
MATERIALS AND METHODS
The current review was designed based on the protocols of the Cochrane Handbook for Systematic Reviews and Meta-Analysis (PRISMA) statement [17].
Search strategy
The systematic search was conducted in major databases, including PubMed, Scopus, Web of Science, Cochrane Library, and Google Scholar, from inception to December 17, 2022, with no publication time or language restrictions. Detailed information relating to the search strategy of databases as well as the Medical Subject Heading (MeSH) and non-MeSH keywords used to search the online databases to identify relevant studies are provided in Supplementary Table 1. The reference lists of the relevant literature were also searched manually for any missing potentially eligible trials. We did not include data from unpublished or gray literature, such as conference abstracts, theses, and patents.
Inclusion and exclusion criteria
Relevant studies were selected based on the PICOS framework [18]. Two authors (MRA and AN) independently selected the trials if they met the following criteria: 1) studies that were conducted on adults (≥ 18 years old); 2) received blackcurrant or raspberry consumption compared to a control group 3) reported weighted or standardized mean differences along with 95% confidence intervals (CIs) 4) reported SBP and DBP as outcome measures. We excluded studies with insufficient data or other study design. We also excluded primary trials in the meta-analysis if they: 1) were trials without a control group; 2) blackcurrant or raspberry consumption along with other nutrients.
Data extraction
The first author’s name, country, publication year, number of primary studies, and participant number were extracted and then tabulated. Furthermore, for each primary RCT from included meta-analyses, we also extracted the following required data: duration of intervention, participants’ health status, participant number, mean ± standard deviation (SD) or changes in SBP and DBP, and the dose of consumption if necessary. WebPlotDigitizer software (Copyright 2010–2022, Ankit Rohatgi) was used to estimate the number of measures when they were reported in figures and charts in the original papers. The data extraction was done by two independent authors (MRA and AN), and the possible discrepancies were resolved by discussion with AH.
Quality assessment of studies
A systematic assessment of the risk of bias in the included studies was fulfilled using the Revised Cochrane Risk-of-Bias Tool (RoB 2) [19] and by using the following criteria: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, 6) selective reporting, and 7) other potential threats to validity. Studies were categorized into low risk of bias, high risk of bias, and some concerns, based on Cochrane Handbook recommendations (Table 1).
Table 1. Risk of bias for randomized controlled trials, assessed according to the revised Cochrane risk-of-bias tool for randomized trials (RoB 2).
| Publications | Random sequence generation | Allocation concealment | Blinding of participants, personnel and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other bias |
|---|---|---|---|---|---|---|
| Montanari et al. [20] (2021) | L | S | L | L | L | L |
| Okamoto et al. [15] (2020) | L | S | L | L | L | H |
| Khan et al. [16] (2014) | L | S | L | L | L | L |
| Ohguro et al. [21] (2012) | L | S | H | L | L | L |
| Heneghan et al. [22] (2017) | L | S | H | L | L | L |
| Jeong et al. [14] (2016) | L | S | L | L | L | L |
| Cho et al. [23] (2020) | L | L | L | L | L | L |
| Franck et al. [24] (2020) | L | S | H | L | L | L |
| Jeong et al. [25] (2016) | L | S | L | L | L | L |
| Schell et al. [26] (2019) | L | S | H | L | L | L |
L, low risk of bias; H, high risk of bias; S, some concerns.
Statistical analysis
The estimated effect size was the difference in mean changes SD of SBP and DBP (change in the treatment group/period minus the change in the control group/period) in each of the included studies. If the studies didn’t report mean and SD, we converted the available statistical data into mean and SD by applying the suitable formula: SD difference = square root [(SD pre-treatment)2+(SD post-treatment)2 − (2× R × SD pre-treatment× SD post-treatment)], assuming a correlation coefficient (R) 0.8 as it is a conservative estimate for an expected range of 0–1 [27]. Were used to calculate the SD for mean changes. Weighted mean differences (WMDs) and 95% CIs were calculated for net changes by using the random-effects model, which takes the between-study heterogeneity into account. The between-study heterogeneity was assessed using the Cochrane Q test. Furthermore, to calculate the percentage of total variation explained by the between-study heterogeneity, the I2 statistic (which is an estimate ranging from 0 to 100% with lower values indicating less heterogeneity) was used. The analysis was carried out using Stata software, version 14 (Stata Corp., College Station, TX, USA). The values of p less than 0.05 were regarded to be statistically significant.
RESULTS
Study selection
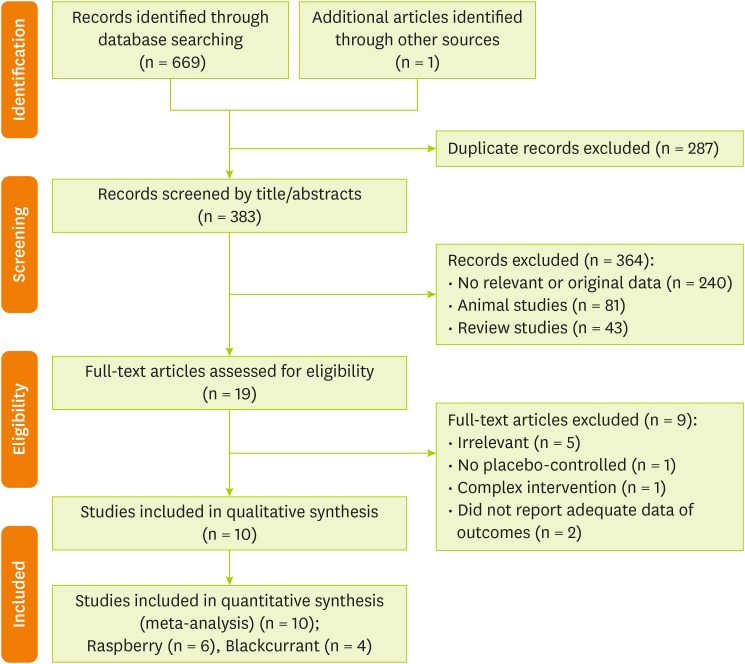
We screened 669 articles according to inclusion and exclusion criteria and, finally, 10 studies [14,15,16,20,21,22,23,24,25,26] were included in the present systematic review (Figure 1).
Figure 1. Flow chart of the number of studies identified and selected into the meta-analysis.
Study characteristics
Characteristics of the eligible studies are reported in Table 2. Studies were conducted in the UK [15,16], USA [26], Turkey [20], Ireland [22], Korea [14,25], and Japan [21]. Of the total ten RCTs that assessed the effect of blackcurrant and raspberry consumption on the DBP and SBP, one study [26] was crossover in design and consisted of 22 participants. Overall, 420 participants, aged 32.19 to 61.7 years, were included in these studies. The duration of the studies ranged from 1 to 24 weeks. Participants were healthy [15,16,22] with metabolic syndrome [25], type 2 diabetes [26], prehypertension [14], slight hyperinsulinemia/hypertriglyceridemia [24], healthy with endurance-trained cyclists [20], patients with open-angle glaucoma [21] and borderline-high cholesterol levels [23]. Of all ten studies, six were supplemented by raspberry [14,22,23,24,25,26], and the other study’s intervention was blackcurrant [15,16,20,21]. Participants’ BMI varied between 21.6 [15] to 35.3 kg/m2 [26] at the study baseline.
Table 2. Demographic characteristics of the included studies.
| Studies | Location | Study design | Health status | Gender | Sample size | Duration (wk) | Mean age (yr) | Baseline BMI (kg/m2) | Intervention | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | ||||||||||
| Montanari et al. [20] (2021) | Turkey | Randomized, double-blind, placebo-controlled, crossover | Healthy, endurance-trained cyclists | Male | 13 | 1 | 39 | 23.6 | 300 mg New Zealand blackcurrant extract | Placebo | SBP/DBP |
| Montanari et al. [20] (2021) | Turkey | Randomized, double-blind, placebo-controlled, crossover | Healthy, endurance-trained cyclists | Male | 13 | 1 | 39 | 23.6 | 600 mg New Zealand blackcurrant extract | Placebo | SBP/DBP |
| Okamoto et al. [15] (2020) | UK | Randomized, double-blind, placebo-controlled, crossover | Healthy | Both | 14 | 1 | 73.3 | 21.6 | 600 mg New Zealand blackcurrant extract | Placebo | SBP/DBP |
| Khan et al. [16] (2014) | UK | Randomized, double-blind, placebo-controlled, parallel trial | Healthy | Both | 32 | 6 | 53 | 28.8 | 250 mL of low blackcurrant juice drink (6.4% juice) four times a day | Placebo | SBP/DBP |
| Khan et al. [16] (2014) | UK | Randomized, double-blind, placebo-controlled, parallel trial | Healthy | Both | 32 | 6 | 53 | 28.8 | 250 mL of high blackcurrant juice drink (20% juice) four times a day | Placebo | SBP/DBP |
| Ohguro et al. [21] (2012) | Japan | Randomized, placebo-controlled, double-blind | Patients with open-angle glaucoma | Both | 38 | 24 | 61.7 | NA | 50 mg black currant anthocyanins | Placebo | SBP/DBP |
| Heneghan et al. [22] (2017) | Ireland | Randomized, placebo-controlled, crossover | Healthy | Both | 80 | 18 | 57.7 | NA | Blackberry polyphenol enriched beverage [total polyphenol content: –700 mg GAE/250 mL serving/d] | Low-dose polyphenol beverage [< 100 mg GAE/250 mL serving/d] | SBP/DBP |
| Jeong et al. [14] (2016) | Korea | Randomized, double-blind, placebo-controlled | Prehypertensive | Both | 22 | 8 | 57.2 | 24.6 | 1,500 mg, moderate dose black raspberry | Placebo | SBP/DBP |
| Jeong et al. [14] (2016) | Korea | Randomized, double-blind, placebo-controlled | Prehypertensive | Both | 23 | 8 | 57.2 | 24.6 | 2,500 mg, high-dose black raspberry | Placebo | SBP/DBP |
| Cho et al. [23] (2020) | Korea | Randomized, double-blind, placebo-controlled | Borderline-high cholesterol levels | Both | 77 | 12 | 47.3 | 23.5 | 600 mg of freeze-dried rubus coreanus extract | Placebo | SBP/DBP |
| Franck et al. [24] (2020) | Canada | Randomized, placebo-controlled, parallel trial | Slight hyperinsulinemia/hypertriglyceridemia | Both | 48 | 8 | 32.19 | 29.9 | 280 g/day of frozen raspberries | Placebo | SBP/DBP |
| Jeong et al. [25] (2016) | Korea | Randomized, placebo-controlled, parallel trial | Metabolic syndrome | Both | 51 | 12 | 58.5 | 25.3 | 750 mg, black raspberry | Placebo | SBP/DBP |
| Schell et al. [26] (2019) | USA | Randomized, crossover | Type 2 diabetes | Both | 22 | 4 | 54 | 35.3 | 250 g frozen red raspberry | Control meal | SBP/DBP |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GAE, gallic acid equivalents.
Effects of raspberry on SBP and DBP
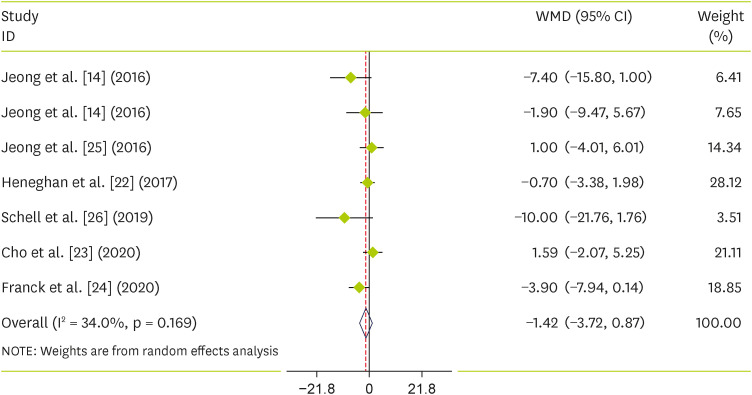
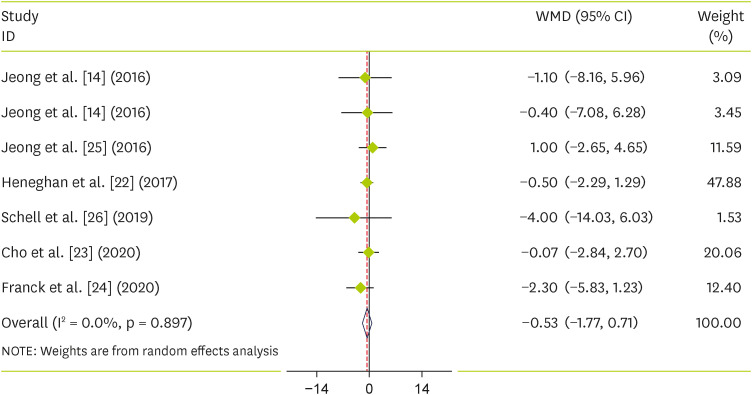
Six primary trials from 10 systematic reviews and meta-analysis evaluated the impact of raspberry on SBP and DBP. We found that raspberry consumption did not reduce SBP compared to the control group (WMD, −1.42; 95% CI, −3.27 to 0.87; p = 0.224) and with no significant between-study heterogeneity (I2= 34%, p = 0.16) (Figure 2). There was also no significant effect on DBP (WMD, −0.53; 95% CI, −1.77 to 0.71; p = 0.401) with significant between-study heterogeneity (I2 = 0.0%, p = 0.897) (Figure 3).
Figure 2. Forest plot detailing weighted mean difference and 95% CIs for the effect of raspberry supplementation on systolic blood pressure.
WMD, weighted mean difference; CI, confidence interval.
Figure 3. Forest plot detailing weighted mean difference and 95% CIs for the effect of raspberry supplementation on diastolic blood pressure.
WMD, weighted mean difference; CI, confidence interval.
Effects of blackcurrant on SBP and DBP
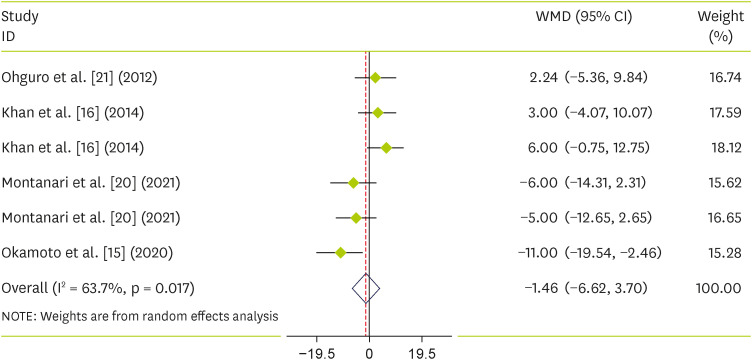
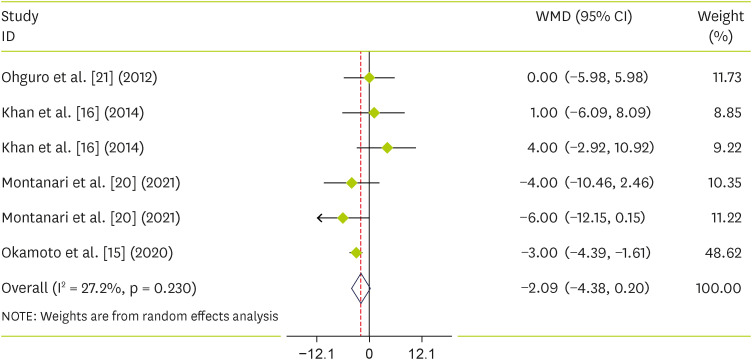
Pooling 6 effect sizes from 4 publications, including 97 participants, we found that blackcurrant did not reduce SBP (WMD, −1.46; 95% CI, −6.62 to 3.7; p = 0.579) (I2 = 34%; p = 0.169) (Figure 4). For DBP, we did not find any reduction along with blackcurrant (WMD, −2.09; 95% CI, −4.38 to 0.20; p = 0.07) (I2 = 27.2%; p = 0.232) (Figure 5).
Figure 4. Forest plot detailing weighted mean difference and 95% CIs for the effect of blackcurrant supplementation on systolic blood pressure.
WMD, weighted mean difference; CI, confidence interval.
Figure 5. Forest plot detailing weighted mean difference and 95% CIs for the effect of blackcurrant supplementation on diastolic blood pressure.
WMD, weighted mean difference; CI, confidence interval.
Sensitivity analysis
To detect the impact of a single trial on the pooled effect sizes, we removed each study from the analysis. The effect sizes for the influence of blackcurrant and raspberry on SBP and DBP were robust in the leave-one-out sensitivity analysis.
Publication bias
Egger’s weighted regression tests were conducted to find the publication bias. The results of Egger’s test showed no publication bias for SBP (p = 0.161), DBP (p = 0.496), SBP (p = 0.06), DBP (p = 0.327) respectively, in the raspberry and blackcurrant groups.
DISCUSSION
To the best of our knowledge, the current systematic review and meta-analysis examined the efficacy of blackcurrant and raspberry on SBP and DBP for the first time. Indeed, in this meta-analysis, a comprehensive assessment of the effect of blackcurrant or blackberry on SBP and DBP was conducted.
Accordingly, we identified a total of 10 RCTs that evaluated the effect of blackcurrant or raspberry on SBP and DBP, and found that neither blackcurrant nor raspberry has beneficial effects on SBP and DBP. This is supported by the observation that high BP increases the risk of CVD as well as all-cause mortality [28,29,30]. Berry fruits are a rich source of nutrients and polyphenols, and there is evidence to support that increased consumption of berries may contribute to the prevention of CVD through effects on BP, blood lipid profiles, and vascular endothelial function [8]. In human-based studies, it has been reported that 600 mg/day of blackcurrant extract for 7 days may improve SBP and DBP in elderly people [15]. Another study showed that 24 months of blackcurrant supplementation with a dose of 50 mg/day improved glaucoma but did not have any significant effect on DBP and SBP in patients with glaucoma [21]. Heneghan et al. found that a 6-week supplementation with 700 mg/day blackberry-derived polyphenol had no significant effect on SBP and DBP [22]. On the other hand, it has been revealed that blackcurrant juice supplementation with a dose of 250 mg/day for 6 weeks has no significant effect on SBP and DBP [16]. Also, the results of another study showed that 600 mg of blackberry extraction consumed for 12 weeks did not significantly reduce SBP and DBP [23]. As well the findings from a study by Jeong et al. [25] revealed that a 12-week supplementation with 750 mg/day black raspberry did not have any significant effect on SBP and DBP. For the inconsistent results observed among studies, differing doses, differences in sample sizes, and periods of intervention need to be addressed. Blackcurrants and raspberries are rich in antioxidants, including anthocyanin and vitamin C. Blackcurrants contain a considerable amount of anthocyanins, which include cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, delphinidin-3-O-glucoside, and delphinidin-3-O-rutinoside [31]. Various biological functions are attributed to blackcurrants, including antihyperlipidemic [32], anti-inflammatory [33,34], and antiatherosclerotic [35] effects.
Blackcurrants and berries improve vascular function via two mechanisms. In one of the mechanisms that are related to the modulation of vascular tone and reactivity, researchers in an in vitro study found polyphenol extract increased NO synthesis by increasing an endothelial nitric oxide synthase (eNOS)-dependent pathway [36]. Enhanced eNOS expression and NO production are found in endothelial cells treated with resveratrol [37]. In another study in vitro, polyphenols also phosphorylated eNOS and activated endothelial-dependent vasodilatation [38]. Another mechanism involved is endothelin-1 inhibition. Polyphenols were found to inhibit the production of endothelin-1, which is derived from the endothelium and is a potent vasoconstrictor [39]. Apart from the anthocyanins present in blackcurrant and berries, vitamin C alone significantly increased the phosphorylation of Akt and eNOS. In a randomized human trial, flavonoids suppressed production of endothelin-1 [40]. Polyphenols enhance endothelial cell plasminogen activator levels, which are relevant to fibrinolysis and thrombosis [41]. Black raspberry regulates BP through the renin-angiotensin system. According to previous in vitro and in vivo studies, black raspberry decreases ACE and renin levels [42]. Isolated anthocyanins, such as delphinidin-3-O-sambubiosides and cyanidin-3-O-sambubiosides, inhibited ACE activity [43]. This evidence demonstrates the importance of blackcurrants and raspberries in preventing hypertension.
Our systematic review in conjunction with meta-analysis has several strengths. We used very precise search terms and a wide range of database searches. Statistical examinations showed no evidence of publication bias in our analyses. Our findings also had several limitations: First, due to the small number of studies, we were not able to better evaluate the effect of blackcurrant and raspberry on SBP and DBP. Second, most of the studies considered showed bias, and thus it is hard to reach a definitive conclusion. Third, the existence of uncontrollable factors in the two comparison groups, such as eating habits and lifestyle, can influence the overall results. Given these limitations, the findings of the present review should be interpreted with caution. Moreover, future intervention studies on SBP and DBP are needed to find the beneficial effect of blackcurrants and raspberries in the prevention of hypertension.
Existing evidence from RCTs in this meta-analysis suggests that treatment with neither blackcurrant nor raspberry is associated with significant changes in SBP and DBP. More studies are necessary to validate the effect of blackcurrant and raspberry supplementation on SBP and DBP.
Footnotes
Funding: This study is related to the project NO. 1400/64926 from Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Amini MR.
- Data curation: Amini MR.
- Formal analysis: Amini MR.
- Methodology: Nikparast A.
- Project administration: Hekmatdoost A.
- Supervision: Hekmatdoost A.
- Writing - original draft: Nikparast A, Tavakoli S.
- Writing - review & editing: Sheikhhossein F, Nikparast A.
SUPPLEMENTARY MATERIAL
Search syntax
References
- 1.Emlek N, Aydin C. The relationship between nondipper hypertension and triglyceride glucose index. Blood Press Monit. 2022;27:384–390. doi: 10.1097/MBP.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens. 2006;24:423–430. doi: 10.1097/01.hjh.0000209973.67746.f0. [DOI] [PubMed] [Google Scholar]
- 3.McInnes GT. Lowering blood pressure for cardiovascular risk reduction. J Hypertens Suppl. 2005;23:S3–S8. doi: 10.1097/01.hjh.0000165622.34192.fd. [DOI] [PubMed] [Google Scholar]
- 4.Ursoniu S, Sahebkar A, Andrica F, Serban C, Banach M. Effects of flaxseed supplements on blood pressure: a systematic review and meta-analysis of controlled clinical trial. Clin Nutr. 2016;35:615–625. doi: 10.1016/j.clnu.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr. 2018;58:1260–1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 6.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathison BD, Kimble LL, Kaspar KL, Khoo C, Chew BP. Consumption of cranberry beverage improved endogenous antioxidant status and protected against bacteria adhesion in healthy humans: a randomized controlled trial. Nutr Res. 2014;34:420–427. doi: 10.1016/j.nutres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Chen G, Liao D, Zhu Y, Xue X. Effects of berries consumption on cardiovascular risk factors: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep. 2016;6:23625. doi: 10.1038/srep23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees A, Dodd GF, Spencer JP. The effects of flavonoids on cardiovascular health: a review of human intervention trials and implications for cerebrovascular function. Nutrients. 2018;10:1852. doi: 10.3390/nu10121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Festa J, Da Boit M, Hussain A, Singh H. Potential benefits of berry anthocyanins on vascular function. Mol Nutr Food Res. 2021;65:e2100170. doi: 10.1002/mnfr.202100170. [DOI] [PubMed] [Google Scholar]
- 11.De Bruyne T, Steenput B, Roth L, De Meyer GR, Dos Santos CN, Valentová K, Dambrova M, Hermans N. Dietary polyphenols targeting arterial stiffness: interplay of contributing mechanisms and gut microbiome-related metabolism. Nutrients. 2019;11:578. doi: 10.3390/nu11030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousefi M, Shadnoush M, Khorshidian N, Mortazavian AM. Insights to potential antihypertensive activity of berry fruits. Phytother Res. 2021;35:846–863. doi: 10.1002/ptr.6877. [DOI] [PubMed] [Google Scholar]
- 13.Jia H, Liu JW, Ufur H, He GS, Liqian H, Chen P. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn Mag. 2011;7:19–24. doi: 10.4103/0973-1296.75885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong HS, Hong SJ, Cho JY, Lee TB, Kwon JW, Joo HJ, Park JH, Yu CW, Lim DS. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: randomized, double-blinded, placebo-controlled clinical trial. Nutrition. 2016;32:461–467. doi: 10.1016/j.nut.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto T, Hashimoto Y, Kobayashi R, Nakazato K, Willems MET. Effects of blackcurrant extract on arterial functions in older adults: a randomized, double-blind, placebo-controlled, crossover trial. Clin Exp Hypertens. 2020;42:640–647. doi: 10.1080/10641963.2020.1764015. [DOI] [PubMed] [Google Scholar]
- 16.Khan F, Ray S, Craigie AM, Kennedy G, Hill A, Barton KL, Broughton J, Belch JJ. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: a randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic Biol Med. 2014;72:232–237. doi: 10.1016/j.freeradbiomed.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–A13. [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG. In: Cochrane handbook for systematic reviews of interventions. Higgins JPT, Churchill R, Chandler J, Cumpston MS, editors. London: Cochrane Collaboration; 2008. Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions; pp. 187–241. [Google Scholar]
- 20.Montanari S, Şahin MA, Lee BJ, Blacker SD, Willems ME. No effects of different doses of New Zealand blackcurrant extract on cardiovascular responses during rest and submaximal exercise across a week in trained male cyclists. Int J Sport Nutr Exerc Metab. 2021;31:66–72. doi: 10.1123/ijsnem.2020-0164. [DOI] [PubMed] [Google Scholar]
- 21.Ohguro H, Ohguro I, Katai M, Tanaka S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica. 2012;228:26–35. doi: 10.1159/000335961. [DOI] [PubMed] [Google Scholar]
- 22.Heneghan C, Kiely M, Manning E, Lucey A. Proceedings of the Nutrition Society. London: Nutrition Society; 2017. Effect of a blackberry-derived polyphenol enriched beverage on blood pressure: a randomized controlled crossover trial; p. 76. [Google Scholar]
- 23.Cho JM, Chae J, Jeong SR, Moon MJ, Ha KC, Kim S, Lee JH. The cholesterol-lowering effect of unripe Rubus coreanus is associated with decreased oxidized LDL and apolipoprotein B levels in subjects with borderline-high cholesterol levels: a randomized controlled trial. Lipids Health Dis. 2020;19:166. doi: 10.1186/s12944-020-01338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franck M, de Toro-Martín J, Garneau V, Guay V, Kearney M, Pilon G, Roy D, Couture P, Couillard C, Marette A, Vohl MC. Effects of daily raspberry consumption on immune-metabolic health in subjects at risk of metabolic syndrome: a randomized controlled trial. Nutrients. 2020;12:3858. doi: 10.3390/nu12123858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong HS, Kim S, Hong SJ, Choi SC, Choi JH, Kim JH, Park CY, Cho JY, Lee TB, Kwon JW, Joo HJ, Park JH, Yu CW, Lim DS. Black raspberry extract increased circulating endothelial progenitor cells and improved arterial stiffness in patients with metabolic syndrome: a randomized controlled trial. J Med Food. 2016;19:346–352. doi: 10.1089/jmf.2015.3563. [DOI] [PubMed] [Google Scholar]
- 26.Schell J, Betts NM, Lyons TJ, Basu A. Raspberries improve postprandial glucose and acute and chronic inflammation in adults with type 2 diabetes. Ann Nutr Metab. 2019;74:165–174. doi: 10.1159/000497226. [DOI] [PubMed] [Google Scholar]
- 27.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. Hoboken (NJ): John Wiley & Sons; 2011. [Google Scholar]
- 28.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 29.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr, Williamson JD, Wright JT., Jr 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Matsumoto H, Morifuji M, Iida H, Takeuchi Y. Development and validation of a liquid chromatography tandem mass spectrometry method for simultaneous determination of four anthocyanins in human plasma after black currant anthocyanins ingestion. J Agric Food Chem. 2010;58:1174–1179. doi: 10.1021/jf9027365. [DOI] [PubMed] [Google Scholar]
- 32.Kim B, Bae M, Park YK, Ma H, Yuan T, Seeram NP, Lee JY. Blackcurrant anthocyanins stimulated cholesterol transport via post-transcriptional induction of LDL receptor in Caco-2 cells. Eur J Nutr. 2018;57:405–415. doi: 10.1007/s00394-017-1506-z. [DOI] [PubMed] [Google Scholar]
- 33.Hirschberg Y, Shackelford A, Mascioli EA, Babayan VK, Bistrian BR, Blackburn GL. The response to endotoxin in guinea pigs after intravenous black currant seed oil. Lipids. 1990;25:491–496. doi: 10.1007/BF02538093. [DOI] [PubMed] [Google Scholar]
- 34.Kumazawa Y, Kawaguchi K, Takimoto H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr Pharm Des. 2006;12:4271–4279. doi: 10.2174/138161206778743565. [DOI] [PubMed] [Google Scholar]
- 35.Huebbe P, Giller K, de Pascual-Teresa S, Arkenau A, Adolphi B, Portius S, Arkenau CN, Rimbach G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br J Nutr. 2012;108:234–244. doi: 10.1017/S0007114511005642. [DOI] [PubMed] [Google Scholar]
- 36.Leikert JF, Räthel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation. 2002;106:1614–1617. doi: 10.1161/01.cir.0000034445.31543.43. [DOI] [PubMed] [Google Scholar]
- 37.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz M, Wessler S, Follmann E, Michaelis W, Düsterhöft T, Baumann G, Stangl K, Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 39.Khan NQ, Lees DM, Douthwaite JA, Carrier MJ, Corder R. Comparison of red wine extract and polyphenol constituents on endothelin-1 synthesis by cultured endothelial cells. Clin Sci (Lond) 2002;103(Suppl 48):72S–75S. doi: 10.1042/CS103S072S. [DOI] [PubMed] [Google Scholar]
- 40.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 41.Abou-Agag LH, Aikens ML, Tabengwa EM, Benza RL, Shows SR, Grenett HE, Booyse FM. Polyphyenolics increase t-PA and u-PA gene transcription in cultured human endothelial cells. Alcohol Clin Exp Res. 2001;25:155–162. [PubMed] [Google Scholar]
- 42.Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Choi HR, Lee SJ, Lee MJ, Ko YJ, Kwon JW, Lee HK, Jeong JT, Lee TB. Blood pressure modulating effects of black raspberry extracts in vitro and in vivo. Korean J Food Sci Technol. 2014;46:375–383. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search syntax