Understanding the 3-year yield of mt-sDNA for colorectal cancer and advanced precancerous polyps is required to ensure the clinical appropriateness of the 3-year interval and to optimize mt-sDNA's screening effectiveness.
Abstract
Data supporting the clinical utility of multi-target stool DNA (mt-sDNA) at the guideline-recommended 3-year interval have not been reported.
Between April 2015 and July 2016, candidates for colorectal cancer screening whose providers prescribed the mt-sDNA test were enrolled. Participants with a positive baseline test were recommended for colonoscopy and completed the study. Those with a negative baseline test were followed annually for 3 years. In year 3, the mt-sDNA test was repeated and colonoscopy was recommended independent of results. Data were analyzed using the Predictive Summary Index (PSI), a measure of the gain in certainty for dichotomous diagnostic tests (where a positive value indicates a net gain), and by comparing observed versus expected colorectal cancers and advanced precancerous lesions.
Of 2,404 enrolled subjects, 2,044 (85%) had a valid baseline mt-sDNA result [284 (13.9%) positive and 1,760 (86.1%) negative]. Following participant attrition, the year 3 intention to screen cohort included 591 of 1,760 (33.6%) subjects with valid mt-sDNA and colonoscopy results, with no colorectal cancers and 63 advanced precancerous lesions [22 (34.9%) detected by mt-sDNA] and respective PSI values of 0% (P = 1) and 9.3% (P = 0.01). The observed 3-year colorectal cancer yield was lower than expected (one-sided P = 0.09), while that for advanced precancerous lesions was higher than expected (two-sided P = 0.009).
Repeat mt-sDNA screening at a 3-year interval resulted in a statistically significant gain in detection of advanced precancerous lesions. Due to absence of year 3 colorectal cancers, the PSI estimate for colorectal cancer was underpowered and could not be reliably quantified. Larger studies are required to assess the colorectal cancer study findings.
Prevention Relevance:
Understanding the 3-year yield of mt-sDNA for colorectal cancer and advanced precancerous polyps is required to ensure the clinical appropriateness of the 3-year interval and to optimize mt-sDNA's screening effectiveness.
Introduction
Colorectal cancer is the third leading cause of cancer death and third most commonly diagnosed cancer in the United States (1), with an estimated 151,030 incident and 52,580 fatal cases expected in 2022 (2). Screening for colorectal cancer reduces its incidence and mortality (3, 4). Increased participation with effective, acceptable colorectal cancer screening strategies is needed to reduce the public health burden associated with colorectal cancer.
Several professional organizations recommend initiation of average-risk colorectal cancer screening at age 45 with one of several options, including the multi-target stool DNA (mt-sDNA) test (5–7) at an interval of 3 years. In a 2014 pivotal trial with nearly 10,000 average-risk participants age ≥ 50 years, the mt-sDNA test demonstrated 92.3% sensitivity for colorectal cancer and 86.6% specificity (as quantified in participants with non-advanced precancerous lesions or no neoplasia on colonoscopy; ref. 8). The pivotal trial established the performance characteristics of mt-sDNA in the intended use population and determined the test's sensitivity for advanced precancerous lesions at 42.4%. While this and other investigations (9, 10) have demonstrated consistent single application test characteristics, mt-sDNA performance at the guideline-endorsed 3-year testing interval has not been described.
In this prospective, multicenter study (ClinicalTrials.gov Identifier: NCT02419716), we estimated the clinical utility of triennial mt-sDNA screening, using the Predictive Summary Index (PSI), an established measure of information gained from clinical application of a dichotomous test result (i.e., positive or negative), as the primary endpoint (11). Secondary endpoints were the observed versus expected yield of colorectal cancers and advanced precancerous lesions at year 3. From clinical and public health perspectives, the 3-year interval for the mt-sDNA test would be appropriate and considered to have clinical and public health utility if few or no colorectal cancers were identified, especially in advanced stages, and if there were a reasonable yield of advanced precancerous lesions.
Methods
Study design
Between April 2015 and July 2016, eligible, consenting patients were prospectively enrolled at 40 sites within the U.S. (private-practice and academic settings), with completion of all study evaluations by April 2020. The study was conducted in accordance with legal and regulatory requirements, the general principles set forth in the International Ethical Guidelines for Biomedical Research Involving Human Subjects (12, 13), the Declaration of Helsinki (14), and applicable local regulatory requirements and laws. NIH Trial Registration for this study is displayed on ClinicalTrials.gov, via Identifier: NCT02419716, https://clinicaltrials.gov/ct2/show/NCT02419716. We followed the STROBE guidelines for cohort studies in reporting this study.
This was a prospective, longitudinal study designed to assess the clinical impact of repeat testing with the mt-sDNA test (Cologuard; Exact Sciences Corporation, LLC; Madison, WI) at a 3-year interval in average-risk patients. The study sponsor, Exact Sciences, provided Institutional Review Board (IRB)-approved advertising materials for the study sites, although each site was given the opportunity to develop its own materials for dissemination. Sites were not required to advertise for the study; however, each mode of advertisement (radio, flyer, media, etc.) required approval by both the sponsor and the IRB. Exact Sciences obtained approval through the Copernicus Group Independent Review Board and all participants provided written informed consent (ClinicalTrials.gov Identifier: NCT02419716).
Study population
Potential study participants were recruited through advertisement and onsite identification. Asymptomatic persons aged 50 years and older who were considered average risk for colorectal cancer were eligible for enrollment, in accordance with guideline screening age recommendations at the time of study initiation. Similar to the pivotal clinical trial (8), enrollment was weighted toward persons 65 years of age or older to increase the prevalence of advanced neoplasia, such that 35% of enrolled subjects would be ages 50 to 64 years and 65% of enrolled subjects would be ≥ age 65 years.
In addition to meeting the age and average-risk requirements, study inclusion required subjects to have been clinically prescribed the mt-sDNA test for colorectal cancer screening. Subjects were excluded if they had: a personal history of colorectal neoplasia, digestive cancer, or inflammatory bowel disease; undergone colonoscopy within the previous 9 years or a barium enema, computed tomographic colonography, or sigmoidoscopy within the previous 5 years; positive results on fecal occult blood testing within the previous 6 months; undergone colorectal resection for any reason other than sigmoid diverticula; overt rectal bleeding within the previous 30 days; a personal or high-risk family history of colorectal cancer (i.e., two first-degree relatives with colorectal cancer or one first-degree relative diagnosed with colorectal cancer prior to age 60); participated in any interventional clinical study within the previous 30 days; or were unable or unwilling to provide written informed consent.
Study procedures
Participants completed the mt-sDNA test at baseline, and those with a negative baseline mt-sDNA result were also asked to undergo repeat mt-sDNA testing and colonoscopy examination at year 3. Stool collection was to be completed within 90 days of the baseline date and within 90 days of the year 3 date (up to 3 years + 90 days from baseline). The mt-sDNA collection kit was shipped to each enrolled subject per provider prescription, which included detailed instructions that guided participants through stool collection and sample return. As is standard, each provider-ordered mt-sDNA test included access to the built-in navigation program to ensure test completion which assisted participants through reminders, individualized guidance, and multilingual services (15). Clinical procedures are described in the Supplementary Text.
mt-sDNA testing & colonoscopy at baseline and 3 years later
With all participants completing the baseline mt-sDNA test, those with a positive mt-sDNA test were recommended to undergo diagnostic colonoscopy per the standard of care within the specified 90-day time frame and were discontinued from the follow-up part of the study. Participants with a negative baseline mt-sDNA test remained in the study and underwent annual follow-up research visits at year 1 and year 2 to evaluate any changes in medical history. At year 3, participants repeated the mt-sDNA test and were scheduled for a colonoscopy, regardless of test results. Participants contributed to the baseline intention to screen (ITS) analysis population only if they were mt-sDNA positive at baseline and had an evaluable colonoscopy, defined as adequate prep quality and documentation of cecal intubation. Similarly, a participant was included in the year 3 ITS analysis population based on meeting three criteria: mt-sDNA negative test result at baseline, a valid positive or negative mt-sDNA test result at year 3, and a subsequent evaluable colonoscopy. mt-sDNA sample processing and laboratory procedures are described in the Supplementary Text.
Outcomes and measures
At FDA recommendation, the primary endpoint of the study was the PSI at year 3, calculated as PPV3 – (1 – NPV3); where PPV3 is the positive predictive value for colorectal cancer value at year 3 and NPV3 is the negative predictive value for colorectal cancer at year 3 (11). The PSI reflects the total gain in certainty for a screening test; the PSI reciprocal can be used to estimate the number of persons who need to be examined to correctly predict a finding. The calculation was repeated for advanced precancerous lesions, defined as an adenoma with high-grade dysplasia or villous elements; an adenoma >10 mm; or a sessile serrated lesion ≥ 10 mm; Categories 2.1–2.4, Supplementary Table S1). As suggested by the FDA prior to approval of the study protocol, a PSI value significantly greater than zero demonstrates that mt-sDNA testing at year 3 provides additional information about colorectal cancer or advanced precancerous lesions beyond what was provided by a negative test result at baseline, whereas a PSI near zero indicates no additional information gained. The secondary endpoints were the observed versus the expected yield of colorectal cancer and advanced precancerous lesion at year 3. Other predefined outcomes of interest included: subject accountability; mt-sDNA positivity rate at baseline and year 3; probability that a negative mt-sDNA remained negative at year 3; probability that a negative baseline mt-sDNA resulted in absence of colorectal cancer or advanced precancerous lesion through year 3; the distribution of colorectal findings among those with a positive mt-sDNA at baseline versus year 3; and compliance with colonoscopy following a positive mt-sDNA test at baseline versus year 3.
Statistical analysis
Main analysis
Enrollment of 2,173 subjects was estimated for adequate study power, assuming a 16.1% positivity rate at baseline, with 15% per year of the study sample lost to follow-up. The pivotal study (8) results were used to estimate the impact of the baseline mt-sDNA filtering on the year 3 timepoint; there was 90% power to reject the null hypothesis [H0: PPV3 – (1 – NPV3) = 0] against the alternative hypothesis [HA: PPV3 – (1 – NPV3) ≠ 0] assuming 15.3% year 3 PPV, 96.5% NPV to detect 11.7% PSI for study planning purposes. In addition, it was assumed that 15% of the remaining subjects would refuse colonoscopy in year 3. The corresponding two-sided 95% confidence intervals (CI) were calculated (16) with all analyses based on the ITS population.
Secondary analysis
For analysis of secondary endpoints, the observed colorectal cancer incidence rate at year 3 was compared with the corresponding pivotal trial (8) incidence rate as the null hypotheses; namely that the year 3 incidence would be the same as for the pivotal study (e.g., no baseline benefit). The one-sided 95% upper confidence bound for the observed year 3 colorectal cancer incidence was planned using an exact binomial test to rule out the pivotal trial colorectal cancer incidence (8). The one-sided 95% lower confidence bound was similarly planned for the observed year 3 incidence of advanced precancerous lesion to rule out the pivotal trial's incidence of this finding as the null hypothesis.
In addition, we examined the distribution of year 3 findings compared between mt-sDNA negative (two negative mt-sDNA tests 3 years apart) and mt-sDNA positive (mt-sDNA negative converting to a positive 3 years later) using a two-sided exact Kruskal–Wallis test, which was also used to compare the year 3 distribution of advanced precancerous lesion (Categories 2.1–2.4; Supplementary Table S1) against the corresponding pivotal trial's (8) distribution. Sensitivity and robustness analyses are described in the Supplementary Text.
Data availability
Upon request with a clearly stated purpose, study hypothesis, and analysis plan, and approval from Exact Sciences.
Results
Study population
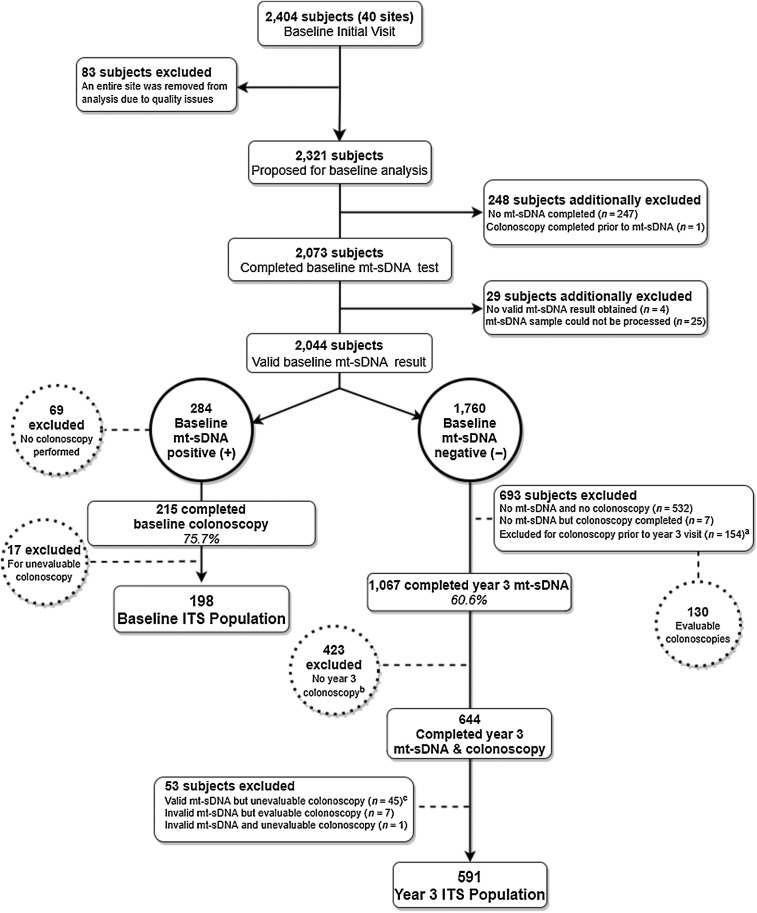
In total, 2,404 participants were enrolled into the study from 40 sites. Due to source verification unavailability, all 83 participants from one site were excluded, resulting in 2,321 participants from the study's onset. An additional 248 participants were excluded due to either not completing the mt-sDNA test (n = 247) or undergoing colonoscopy prior to mt-sDNA testing (n = 1). Moreover, 29 (1.4% of 2,073) participants were excluded due to an invalid mt-sDNA result or an unsuitable sample. Thus, the total number of participants with a valid baseline mt-sDNA test was 2,044 (Fig. 1).
Figure 1.
Study flow diagram. This diagram shows derivation of baseline and analytical cohorts. aOf the 154 participants who had early colonoscopy: colorectal cancer was found (n = 1), along with advanced precancerous lesions (n = 11), while 111 participants had clinically insignificant findings, including non-advanced adenomas (n = 41), no findings on colonoscopy (n = 70), and those that could not be categorized (n = 31). b400/423 had a valid mt-sDNA result. cSubjects excluded were those who did not have documentation or had poor bowel preparation quality (n = 29), those who had a polyp or mass found but no tissue submitted (n = 12), those without documentation of cecal intubation (n = 3), and if a subject had a polyp or mass found but pathology report and verification of lesion could not be retrieved (n = 1).
Baseline results
At baseline, 284 (13.9%) participants were mt-sDNA positive and 1,760 (86.1%) were mt-sDNA negative (Table 1). Of the 284 participants with a positive mt-sDNA test, 215 (75.7%) completed a colonoscopy; 17 participants were excluded from that group for having an unevaluable colonoscopy—3 for no documentation of cecal intubation, 5 for poor bowel prep quality, 8 for no documentation of bowel prep quality, and 1 for no tissue submitted following biopsy of a polyp or mass—resulting in a baseline ITS analysis population of 198 (Fig. 1; Table 1). There were no clinically important baseline differences between the 284 subjects with a positive mt-sDNA and the 198 mt-sDNA positive ITS subjects (Table 1).
Table 1.
Demographic and baseline features of the overall study population and relevant subgroups.
| Baseline | Year 3d | ||||
|---|---|---|---|---|---|
| Baseline test population | mt-sDNA Positive | mt-sDNA and colonoscopy (ITS)b | mt-sDNA negativec | mt-sDNA and colonoscopy (ITS) | |
| N = 2,044a | (N = 284) | (N = 198 of 284) | (N = 1,760) | (N = 591 of 1,760) | |
| Age (years) at enrollment | |||||
| N | 2,044 | 284 | 198 | 1,760 | 591 |
| Mean (SD) | 65.4 (8.54) | 68.9 (7.98) | 68.2 (7.73) | 64.8 (8.49) | 63.6 (8.19) |
| Median | 66.5 | 68.0 | 68.0 | 66.0 | 65.0 |
| Min, Max | 50, 97 | 50, 97 | 50, 97 | 50, 91 | 50, 87 |
| Age Group (years) | |||||
| 50–59 | 516 (25.2) | 33 (11.6) | 26 (13.1) | 483 (27.4) | 182 (30.8) |
| 60–69 | 913 (44.7) | 127 (44.7) | 92 (46.5) | 786 (44.7) | 278 (47.0) |
| 70+ | 615 (30.1) | 124 (43.7) | 80 (40.4) | 491 (27.9) | 131 (22.2) |
| Age Group (years) | |||||
| 50–64 | 682 (33.4) | 48 (16.9) | 34 (17.2) | 634 (36.0) | 236 (39.9) |
| 65+ | 1,362 (66.6) | 236 (83.1) | 164 (82.8) | 1,126 (64.0) | 355 (60.1) |
| Gender, n (%) | |||||
| Male | 928 (45.4) | 147 (51.8) | 101 (51.0) | 781 (44.4) | 270 (45.7) |
| Female | 1,116 (54.6) | 137 (48.2) | 97 (49.0) | 979 (55.6) | 321 (54.3) |
| Race, n (%) | |||||
| White | 1,689 (82.6) | 245 (86.3) | 180 (90.9) | 1,444 (82.0) | 536 (90.7) |
| Black or African American | 189 (9.2) | 19 (6.7) | 10 (5.1) | 170 (9.7) | 31 (5.2) |
| Asian | 102 (5.0) | 10 (3.5) | 4 (2.0) | 92 (5.2) | 16 (2.7) |
| American Indian or Alaska Native | 5 (0.2) | 2 (0.7) | 1 (0.5) | 3 (0.2) | 1 (0.2) |
| Native Hawaiian or Other Pacific Islander | 6 (0.3) | 0 | 0 | 6 (0.3) | 1 (0.2) |
| Other | 51 (2.5) | 8 (2.8) | 3 (1.5) | 43 (2.4) | 6 (1.0) |
| Missing | 2 (0.1) | 2 (0.1) | 0 | ||
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 272 (13.3) | 29 (10.2) | 18 (9.1) | 243 (13.8) | 46 (7.8) |
| Not Hispanic or Latino | 1,769 (86.7) | 254 (89.4) | 180 (90.9) | 1,515 (86.1) | 545 (92.2) |
| Missing | 3 (0.1) | 1 (0.4) | 0 | 2 (0.1) | 0 |
| BMI (kg/m2) at Baseline | |||||
| N | 2,038 | 282 | 197 | 1756 | 590 |
| Mean (SD) | 29.46 (6.86) | 29.32 (6.62) | 29.46 (6.53) | 29.48 (6.90) | 29.42 (6.62) |
| Median | 28.17 | 28.48 | 28.63 | 28.12 | 28.10 |
| Min, Max | 14.9, 63.7 | 16.4, 55.1 | 16.6, 50.8 | 14.9, 63.7 | 16.3, 63.7 |
| Smoking history, n (%) | |||||
| Never smoked | 1,220 (59.7) | 134 (47.2) | 87 (43.9) | 1,086 (61.7) | 371 (62.8) |
| Former smoker | 607 (29.7) | 99 (34.9) | 79 (39.9) | 508 (28.9) | 175 (29.6) |
| Current smoker | 217 (10.6) | 51 (18.0) | 32 (16.2) | 166 (9.4) | 45 (7.6) |
aAll subjects enrolled that completed a baseline mt-sDNA test (29 excluded; seeFig. 1).
bBaseline ITS subjects had both a positive mt-sDNA and an evaluable baseline colonoscopy.
cMt-sDNA negative subjects at baseline were not requested to complete a colonoscopy.
dYear 3 ITS subjects had a negative baseline mt-sDNA test, a valid year 3 mt-sDNA result, and an evaluable 3-year colonoscopy.
The baseline ITS cohort of 198 had a mean age of 68.2 years (SD, 7.73) and was 51.0% male (Table 1). Among the 198 evaluable participants with a positive mt-sDNA test who underwent colonoscopy, colorectal cancer was found in 8 (4%) and advanced precancerous lesions were found in 61 (31%; Table 2); the remaining 129 participants had either non-advanced neoplasia or no neoplastic findings. Mt-sDNA positive subjects ended the study at this point.
Table 2.
Mt-sDNA positive findings and PSI.
| Baseline ITSa | Year 3ITSc | ||||
|---|---|---|---|---|---|
| (N = 198) | (N = 591) | ||||
| mt-sDNA | mt-sDNA | ||||
| Positive results | Negativeb results | Positive results | Negativeb results | PSId (%) | |
| Colonoscopy Finding | (198) | (N/A) | (N = 122) | (N = 469) | 95% CI P |
| Colorectal cancer, N (%) | 8 | N/A | 0 | 0 | 0 |
| (−3.62, 1.03) | |||||
| 1 | |||||
| Advanced precancerous lesions, N (%) | 61 | 22 (34.9) | 41 (65.1) | 9.3 | |
| (1.8,17.6) | |||||
| 0.0124 | |||||
| Negative resultsb: no colorectal cancer, advanced precancerous lesion, or non-advanced lesion, N (%) | 129 | 100 (18.9) | 428 (81.1) | N/A | |
aBaseline ITS subjects had both a positive mt-sDNA and an evaluable colonoscopy at baseline.
bNegative subset is defined as Category 3–6.
cYear 3 ITS subjects had a negative baseline mt-sDNA result, a valid year 3 mt-sDNA result, and an evaluable colonoscopy in year 3.
dYear 3 ITS PSI = [PPV3 − (1−NPV3)] = (22/122) − [1−(428/469)] = 18.03% − 8.74 = 9.29% .
The 1,760 participants who had a negative baseline mt-sDNA result were eligible for study continuation. Comparison of the baseline ITS (N = 198) and mt-sDNA negative (N = 1,760) revealed that the ITS population was older and more likely to be male, White, and current or former smokers (Table 1).
Year 3 results – primary outcome
Of the 1,760 candidate participants eligible for the year 3 analysis, 539 were excluded because they did not submit a year 3 mt-sDNA test and 154 for having a colonoscopy prior to the year 3 visit, leaving 1,067 (60.6%) subjects who submitted the mt-sDNA test in year 3. Of the 154 participants who had early colonoscopy, one had a colorectal cancer at month 22 as previously described; 11 had advanced precancerous lesions; 111 had clinically insignificant findings including 41 with non-advanced adenomas and 70 with no findings on colonoscopy; the remaining 31 could not be categorized. Of the 1,067 participants who submitted the year 3 mt-sDNA test, 644 (60.4%) also completed colonoscopy, with 636 of the 644 (98.8%) having a valid year 3 mt-sDNA result. These 636 participants represent 36% of the year 3 candidate participants. Of those 636 participants, 45 were excluded for having an unevaluable colonoscopy: 3 for no documentation of cecal intubation; 15 for poor bowel prep quality; 14 for no documentation of bowel prep quality; 12 because a polyp or mass was biopsied but no tissue was submitted [polyp not retrieved (n = 5); specimen lost (n = 1); lesion not removed (n = 4); no reason provided (n = 2)]; and 1 for no pathology report provided. Of 7 subjects with an invalid mt-sDNA but evaluable colonoscopy, 3 of whom submitted a second mt-sDNA test that was also invalid, 1 had an advanced precancerous lesion, 3 had non-advanced adenomas, and 3 had no findings on colonoscopy. The year 3 ITS final analysis population included 591 subjects (Fig. 1), of whom 122 (20.6%) were mt-sDNA positive (Supplementary Table S1). Compared with the 1,760 year 3 candidate subjects, the 591 year 3 ITS subjects were more likely to be White, less likely to be of Hispanic/Latino ethnicity and more likely to be lifetime nonsmokers (62.8% vs. 43.9%). The ITS cohort of 591 had a mean age of 63.6 years (SD, 8.19) at baseline, and was 45.7% male (Table 1). There was no clinically significant variation between ITS cohorts at baseline and year 3 in ethnicity or body mass index (BMI). Annual participant follow-up is shown in Supplementary Table S2.
The year 3 ITS population of 591 subjects comprise the cohort on which the PSI was calculated. The year 3 value of the PSI for colorectal cancer was 0% (95% CI, −3.62% to 1.02%), as no cancers were detected at colonoscopy. However, one Stage IV colorectal cancer was detected 22 months after baseline testing due to symptoms that prompted colonoscopy; the subject had a negative mt-sDNA test at baseline and thus did not undergo colonoscopy at that time. Sixty-three (10.7%) participants were found to have an advanced precancerous lesion at colonoscopy (22 of which were true-positives and 41 were false-negatives), resulting in a year 3 PSI measure of 9.3% (95% CI, 1.83–17.63; two-sided P = 0.01; Table 2).
Year 3 results – secondary outcomes
Overall, 83.6% of participants underwent colonoscopy after testing positive in year 3 compared with 75.7% adherence in the baseline mt-sDNA positive cohort. In contrast, 57.6% of participants who were mt-sDNA negative at baseline underwent colonoscopy in year 3 following a second negative mt-sDNA test (two-sided P < 0.0001).
Secondary outcomes of observed versus expected findings in year 3 are shown in Table 3. Expectation was based on the pivotal (8) trial prevalence rates for both colorectal cancer (0.686%) and advanced precancerous lesions (7.60%). The number of observed colorectal cancers was lower than expected (1 vs. 4), which did not reach statistical significance (one-sided P value = 0.09); the one-sided test was prespecified. In contrast, the number of observed advanced precancerous lesions was higher than expected (63 vs. 45; two-sided P value = 0.009). Year 3 mt-sDNA detected 22 (34.9%) of 63 advanced precancerous lesions.
Table 3.
Observed versus expected incidence of colorectal cancer and advanced precancerous lesions.
| Year 3 ITSa | ||
|---|---|---|
| (N = 591) | ||
| Colorectal cancer | Advanced precancerous lesions | |
| Number Observed | 1* | 63 |
| Assumed Incidence (based on pivotal study) (7) | 0.00686 | 0.076 |
| Expected Number | 4 | 45 |
| Observed Incidence | 0.0017 | 0.1066 |
| 95% CI two-sided | (0, 0.00797) | (0.0829, 0.1336) |
| P | 0.087 (one-sided) | 0.0091 (two-sided) |
aYear 3 ITS subjects had a negative baseline T0 mt-sDNA result, a valid year 3 mt-sDNA result, and an evaluable colonoscopy in year 3; Two-sided test used for advanced precancerous lesions because the incidence was higher than expected; One-sided 95% CI prespecified for colorectal cancer.
*Colorectal cancer was identified in month 22 of study and is not included in the year 3.
There was no significant difference in the category distribution of advanced precancerous lesions (categories 2.1–2.4) at baseline versus year 3 among mt-sDNA positives (two-sided P = 0.34; Supplementary Table S1). Further evaluation of this distribution at year 3 for all participants with advanced precancerous lesions (mt-sDNA positive and negative) as compared with all corresponding pivotal trial (8) participants revealed no significant difference in lesion category distribution (P = 0.12).
The findings for a change in mt-sDNA result in year 3 were also evaluated relative to baseline (Supplementary Table S1). When the mt-sDNA result changed from negative at baseline to positive in year 3, the probability of finding advanced precancerous lesions was 18.0% as compared with 8.7% for year 3 mt-sDNA test results that remained negative (two-sided P = 0.005; Supplementary Table S1). As expected, the overall distribution of findings was more advanced for mt-sDNA positive than for mt-sDNA negative results (two-sided Kruskal–Wallis test P < 0.001). Sensitivity and robustness analyses are described in the Supplementary Text.
Discussion
This prospective, multicenter study provides novel data regarding repeat performance of the mt-sDNA tes for average-risk colorectal cancer screening. In 2014, a 3-year rescreening interval for the mt-sDNA test was approved by Centers for Medicare & Medicaid Services (CMS) based on test sensitivity and modeling studies, with no empiric data available at the time regarding programmatic test characteristics (17). In this study, we obtained 3-year interval retesting results in persons whose baseline mt-sDNA test result was negative with the aim of quantifying the findings and determining the clinical utility of this interval. Current colorectal cancer screening guidelines recommend either a 3-year interval or a 1- to 3-year interval (5–7, 18, 19); thus, to assess appropriateness, we measured both the PSI and the difference between observed and expected colorectal cancers and advanced precancerous lesions.
Intuitively, the 3-year interval would seem appropriate if zero or near-zero colorectal cancers were found at year 3 and if there were a reasonable yield of advanced precancerous lesions detected at that time. In this study, we found no colorectal cancers and 63 advanced precancerous lesions among the 591 participants completing both mt-sDNA and colonoscopy in year 3. Because the mt-sDNA test is not designed to identify non-advanced lesions at baseline stage, some lesions may have become advanced during the 3-year study period.
The finding of no colorectal cancers in year 3 resulted in the zero value for the PSI, as no new information was gained because mt-sDNA detected all but one likely colorectal cancer at baseline. Thus, the zero colorectal cancer PSI is consistent with the high colorectal cancer sensitivity of the mt-sDNA test and the natural history of progression from advanced precancerous lesion to colorectal cancer (20). The number of cancers observed in year 3 was numerically lower than expected, likely due to both the high colorectal cancer sensitivity of mt-sDNA and the small sample size in year 3. For advanced precancerous lesions, we expect that some were undetected at baseline due to the previously reported mt-sDNA sensitivity of 42.4% (8), allowing for the year 3 detection of these lesions that were either undetected or not present at baseline, and accounting for the observed number of advanced precancerous lesions relative to what was expected. Year 3 mt-sDNA detected 22 (34.9%) of 63 advanced precancerous lesions. Although the estimate is numerically lower than the 42.4% detection rate noted in the pivotal study, the two 95% CIs overlap. Given the negative mt-sDNA results at baseline, it might be expected that the spectrum of year 3 lesions would be less advanced than those at baseline, and the comparison of baseline and year 3 advanced lesions supports this contention for positive mt-sDNA cases. Taken together, the study findings suggest that repeat mt-sDNA screening at 3 years may be considered clinically appropriate, as evidenced by the reduced number of observed versus expected colorectal cancers, while identifying advanced precancerous lesions at a rate consistent with the pivotal study (8). Last, while not the focus of this study, baseline findings of colorectal cancer sensitivity and positive predictive value for advanced precancerous lesions are consistent with those of the pivotal study (8).
Per the FDA's recommendation, the PSI was the primary outcome, as it indicates the gain in certainty from a dichotomous test, with a value greater than 0% indicating a meaningful gain. The PSI was designed to inform the detection gained from a positive test result beyond what is already known a priori about the disease prevalence, and from a negative test result's ability to further exclude disease. The finding of no colorectal cancers at year 3 contributed to the PSI of 0% (95% CI, −3.62 to 1.03) and the PSI for advanced precancerous lesions of 9.3% (95% CI, 1.8–17.6) consistent with depletion of these lesions after baseline testing. For reference, the pivotal trial (8) PSI (cross-sectional study) was 4.6% for colorectal cancer (two-sided 95% CI, 3.6%–5.9%), while the PSI for advanced precancerous lesions in that trial was 15.5% (95% CI, 13.5%–17.7%). Thus, the baseline PSI for colorectal cancer in this study could not be reliably estimated.
During the follow-up phase of the study, a stage IV colorectal cancer was discovered in 1 participant 22 months after a negative baseline mt-sDNA test. The differential diagnosis for this “interval” cancer includes: (i) the cancer was present at baseline and was missed by mt-sDNA; (ii) the cancer was absent at baseline with no neoplasia present and progressed aggressively over the 22-month interval; (iii) the cancer was absent at baseline, but was a precancerous neoplastic lesion that was missed by mt-sDNA. While we cannot know with certainty, it is more likely that a precancerous lesion was missed at baseline. Had this cancer been discovered by mt-sDNA screening in year 3, the PSI value would have remained nonsignificant (P = 0.63), both clinically and statistically, although with a small number of colorectal cancers in the study.
This study has several limitations. First, the study design is not as rigorous as a clinical trial of retesting at 1 versus 3 years (or some other comparison of intervals) would have been. A clinical trial of different retesting intervals would have been logistically challenging and would have required many more participants. Second, a significant proportion of persons who entered the longitudinal part of the study did not complete it. While dropouts were anticipated, a higher-than-expected proportion of persons who remained mt-sDNA negative at year 3 opted to forego colonoscopy. Given that this was a symptom-free population unlikely to develop on-study symptoms leading to colonoscopy, missing cases were most likely to be missing at random.
Three factors are believed to have contributed to the high dropout rate. First, an implicit “incentive” for study participation was the possibility of deferring colonoscopy for 3 years, provided the baseline results were negative, potentially biasing the study population to those less accepting of screening colonoscopy. For those with an initially negative mt-sDNA test, having a second negative mt-sDNA test 3 years later may have created an even greater disincentive for colonoscopy, with only 57.6% undergoing colonoscopy at year 3. In contrast, 83.6% underwent colonoscopy when their year 3 mt-sDNA result was positive compared with 75.7% colonoscopy adherence for those participants whose baseline mt-sDNA result was positive. A second contributing factor was the onset of the SARS-CoV-2 pandemic, which created a disincentive to undergo colonoscopy and complete the study, as screening colonoscopies were curtailed during the Spring of 2020 due to lack of adequate personal protective equipment, reallocation of healthcare providers, and deferral of elective procedures. Third, while our findings may support a 3-year interval for retesting with mt-sDNA, the finding of no colorectal cancers in year 3 suggests the possibility that a longer interval may be plausible, although extending the screening interval might result in increased risk for missed APLs. Subsequent longitudinal data on repeat testing intervals may clarify this issue and are required to confirm the year 3 colorectal cancer findings.
Missing data notwithstanding, we used several approaches to infer outcomes for those with missing data in year 3. The Test Ignorance Region (Supplemental Text) interrogated the universe of possible outcomes; we confirmed that there was a missing data pattern in contrast to the missing data being at random. In addition, the impact of missing the year 3 colonoscopy was assessed via multiple imputation to provide support for the projection of the increased number of colorectal cancer/advanced precancerous lesions cases among those with a positive year 3 mt-sDNA test. The modeling results provide support for the estimates and conclusions.
A final limitation considered by some is the missed opportunity to obtain follow-up data on the baseline positive subjects, for whom diagnostic colonoscopy was recommended. Obtaining such data would have provided another estimate of the positive predictive value of a baseline positive mt-sDNA test.
While there are many studies quantifying single-application (or cross-sectional) test characteristics for noninvasive colorectal cancer screening tests, there are few studies describing programmatic test characteristics, and even fewer studies on the long-term and most important outcomes of colorectal cancer incidence and mortality. Because mt-sDNA is a recently endorsed stool-based screening test, there are no data on its programmatic test characteristics, and because sufficient time has not yet elapsed from the initial clinical availability of the test, there are no data demonstrating a reduction in colorectal cancer incidence or mortality.
In summary, data from this prospective, multicenter study support a triennial mt-sDNA screening strategy for average-risk persons, as approved by CMS and recommended in national guidelines. More observations of year 3 findings among persons whose index mt-sDNA is negative would be useful to validate the current findings.
Supplementary Material
Supplemental Table S1: Distribution of colorectal lesions among subjects at baseline (T0) and year 3 (T3). Supplemental Table S2: Study subject accountability in the baseline negative population
This Supplemental Text file provides additional information regarding the clinical procedures and sample processing/lab procedures, as well as an explanation of the sensitivity and robustness analyses.
Acknowledgments
This study was funded by Exact Sciences. Exact Sciences was responsible for the design and conduct of the study. Medical writing and editorial support were provided by William K. Johnson, PhD, an employee of Exact Sciences (Madison, WI).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Authors' Disclosures
T.F. Imperiale reports grants from Exact Sciences during the conduct of the study. P.T. Lavin reports grants from Exact Sciences during the conduct of the study. T.N. Marti reports personal fees from Exact Sciences during the conduct of the study. D. Jakubowski reports other support from Exact Sciences during the conduct of the study. S.H. Itzkowitz reports grants and personal fees from Exact Sciences; grants from Freenome; and personal fees from Geneoscopy outside the submitted work. F.P. May reports other support from Exact Sciences during the conduct of the study. P.J. Limburg reports other support from Exact Sciences outside the submitted work. S. Sweetser reports grants from Exact Sciences during the conduct of the study. B.M. Berger reports personal fees from Exact Sciences during the conduct of the study; personal fees from Exact Sciences outside the submitted work. No disclosures were reported by the other author.
Disclaimer
All authors were responsible for interpretation of the data and preparation and review of the manuscript.
Authors' Contributions
T.F. Imperiale: Conceptualization, data curation, supervision, investigation, methodology, writing–original draft, writing–review and editing. P.T. Lavin: Conceptualization, formal analysis, supervision, writing–original draft, writing–review and editing. T.N. Marti: Resources, supervision, writing–original draft, project administration, writing–review and editing. D. Jakubowski: Formal analysis, writing–review and editing. S.H. Itzkowitz: Conceptualization, investigation, writing–review and editing. F.P. May: Writing–review and editing. P.J. Limburg: Conceptualization, supervision, writing–original draft, project administration, writing–review and editing. S. Sweetser: Investigation, writing–review and editing. A. Daghestani: Investigation, writing–review and editing. B.M. Berger: Supervision, writing-review and editing.
References
- 1. American Cancer Society. ACS Facts and Figures 2022. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf.
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 3. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: Has it really made a difference? Dig Dis Sci 2015;60:681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology 2018;155:1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Cancer Society. When should you start getting screened for colorectal cancer? 2021. Available from: https://www.cancer.org/latest-news/american-cancer-society-updates-colorectal-cancer-screening-guideline.html.
- 6. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 7. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for colorectal cancer. JAMA 2021;325:1965. [DOI] [PubMed] [Google Scholar]
- 8. Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multi-target stool DNA testing for colorectal cancer screening. N Engl J Med 2014;370:1287–97. [DOI] [PubMed] [Google Scholar]
- 9. Redwood DG, Asay ED, Blake ID, Sacco PE, Christensen CM, Sacco FD, et al. Stool DNA testing for screening detection of colorectal neoplasia in Alaska native people. Mayo Clin Proc 2016;91:61–70. [DOI] [PubMed] [Google Scholar]
- 10. Cooper GS, Markowitz SD, Chen Z, Tuck M, Willis JE, Berger BM, et al. Performance of multi-target stool DNA testing in African American patients. Cancer 2018;124:3876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linn S, Grunau PD. New patient-oriented summary measure of net total gain in certainty for dichotomous diagnostic tests. Epidemiol Perspect Innov 2006;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Delden JJM, Van Der Graaf R. Revised CIOMS International ethical guidelines for health-related research involving humans. JAMA 2017;317:135–6. [DOI] [PubMed] [Google Scholar]
- 13. Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-Related Research Involving Humans. 2017. Available from:https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf. [PubMed]
- 14. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 15. Exact Sciences Corporation. Support And Resources | Cologuard Patient Site | Risk Info. Available from: https://www.cologuard.com/colon-cancer-screening-support-resources. [Google Scholar]
- 16. Chan I, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999;55:1202–9. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Medicare & Medicaid Services. National Coverage Analysis - Screening for Colorectal Cancer - Stool DNA Testing (CAG-00440N) - Decision Memo. CMS.gov Website. 2014. Available from:https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=277. [Google Scholar]
- 18. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical Guidelines: colorectal cancer screening 2021. Am J Gastroenterol 2021;116:458–79. [DOI] [PubMed] [Google Scholar]
- 19. Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on colorectal cancer. Gastroenterology 2017;153:307–23. [DOI] [PubMed] [Google Scholar]
- 20. Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut 2007;56:1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Distribution of colorectal lesions among subjects at baseline (T0) and year 3 (T3). Supplemental Table S2: Study subject accountability in the baseline negative population
This Supplemental Text file provides additional information regarding the clinical procedures and sample processing/lab procedures, as well as an explanation of the sensitivity and robustness analyses.
Data Availability Statement
Upon request with a clearly stated purpose, study hypothesis, and analysis plan, and approval from Exact Sciences.