Abstract
Background:
In the Netherlands, lower high-risk human papillomavirus (hrHPV) positivity but higher cervical intraepithelial neoplasia (CIN) 2+ detection were found in self-collected compared with clinician-collected samples. To investigate the possible reason for these differences, we compared sociodemographic and screening characteristics of women and related these to screening outcomes.
Methods:
We extracted data from PALGA on all primary hrHPV screens and associated follow-up tests for 857,866 screened women, invited in 2017 and 2018. We linked these data with sociodemographic data from Statistics Netherlands. Logistic regression was performed for hrHPV positivity and CIN 2+/3+ detection.
Results:
Out of the 857,866 women, 6.8% chose to use a self-sampling device. A higher proportion of self-sampling users was ages 30 to 35 years, was not previously screened, was living in a one-person household, or was the breadwinner in the household. After adjustment for these factors self-sampling had lower hrHPV positivity (aOR, 0.65; 95% CI, 0.63–0.68)) as compared with clinician-collected sampling, as well as lower odds of CIN 2+ (aOR, 0.76; 95% CI, 0.70–0.82) and CIN 3+ (aOR, 0.86; 95% CI, 0.78–0.95) detection.
Conclusions:
It is likely that the observed differences between the two sampling methods are not only related to sociodemographic differences, but related to differences in screening test accuracy and/or background risk.
Impact:
Self-sampling can be used for targeting underscreened women, as a more convenient screening tool. Further investigation is required to evaluate how to implement self-sampling, when it is used as a primary instrument in routine screening.
Introduction
Within the cervical cancer screening programme, primary high-risk human papillomavirus (hrHPV) testing was introduced in the Netherlands at the beginning of 2017, with the possibility for women to choose to either be screened by their general practitioner (GP) or to be screened with a self-sampling device. In the primary invitation letter, self-sampling is suggested as an option for woman experiencing hesitancy with being screened by their GP and for who this would be a potential reason not to participate (1, 2). Self-sampling could potentially reduce barriers to participation as it is less time consuming and less confronting than being screened by a physician (3, 4). In study populations, self-sampling has been shown to increase participation among nonattenders (5). Nonattenders may have a higher risk for cervical intraepithelial neoplasia (CIN) 2+; in the previous Dutch trials, nonattenders who were offered, and subsequently returned, a Self-Sampling Kit were found to have a higher relative risk for CIN 2+/CIN 3+ than women who attended the regular screening programme (6).
Previous studies have also found that self-sampling is a suitable alternative to clinician-collected sampling. Meta-analysis has shown that, using PCR-based hrHPV testing, sensitivity for CIN 2+ and CIN 3+, positive predictive value (PPV) for CIN 2+, and CIN 3+ and test positivity were not significantly different between self-sampling and clinician-collected screening (7). Prior to the implementation of hrHPV-based screening in the Netherlands, a randomized trial (IMPROVE) compared self-sampling and clinician-collected sampling in a population-based screening cohort. IMPROVE found that self-sampling was non-inferior to clinician-collected sampling in CIN 2+ detection and that hrHPV positivity rates were equivalent (8).
Despite evidence that the two sampling methods are equivalent, during the first 2 years of the primary hrHPV cervical cancer screening programme in the Netherlands, a difference in hrHPV positivity and CIN 2+ detection was observed. Self-sampling had significantly lower hrHPV positivity (7.6% in the self-sampling cohort, 9.2% in the clinician-collected cohort) with significantly higher proportion of CIN 2+ lesions compared with clinician-collected sampling among the subgroup of women who were directly referred, attended the gynecologist and had a diagnostic test (9).
There is limited information about which women choose self-sampling, apart from uptake by age; the highest proportion of self-sampling users in the screening programme are in the youngest age group (30 years), followed by women in the oldest age group (60 years; ref. 10). It is not known whether women who choose self-sampling have different sociodemographic characteristics and screening history from those who choose to be screened by their GP. Understanding the profile of women who choose self-sampling will provide in-depth information about the implementation of self-sampling in organized screening.
Given that the Dutch screening programme is the first to offer self-sampling as an alternative for women who would otherwise not attend (albeit via an opt-in system), we first aimed to describe the sociodemographic characteristics and screening history of women who used self-sampling and compare them with women screened by their GP. Second, we aimed to investigate whether personal characteristics explain the differences between self-sampling and clinician-collected sampling in (i) hrHPV positivity and (ii) CIN 2+/3+ detection.
Materials and Methods
Setting
This study was conducted within the Dutch cervical cancer screening programme. Since January 2017, hrHPV testing has been the primary screening modality. Women ages 30 to 60 years are invited every 5 years to take part in cervical cancer screening. Women can either be screened by their GP or request a self-sampling kit at home. Women who do not wish to have a cervical sample taken at their GP can request a self-sampling kit. If requested at primary invitation, in 20117/2018 women were sent the self-sampling kit approximately 4 months after the initial invitation letter. Nonresponders received a reminder letter 4 months after the initial invitation, which also contained information about how to request the self-sampling kit. Women who requested the self-sampling kit after this reminder received it immediately. Women who are hrHPV-negative are re-invited after 5 years; for women ages 40 and 50 with test hrHPV negative, the interval is extended to 10 years. In period of this study, all women ages 45 and 55 years were invited for screening because the hrHPV status of women was not known in the first round of the hrHPV screening programme.
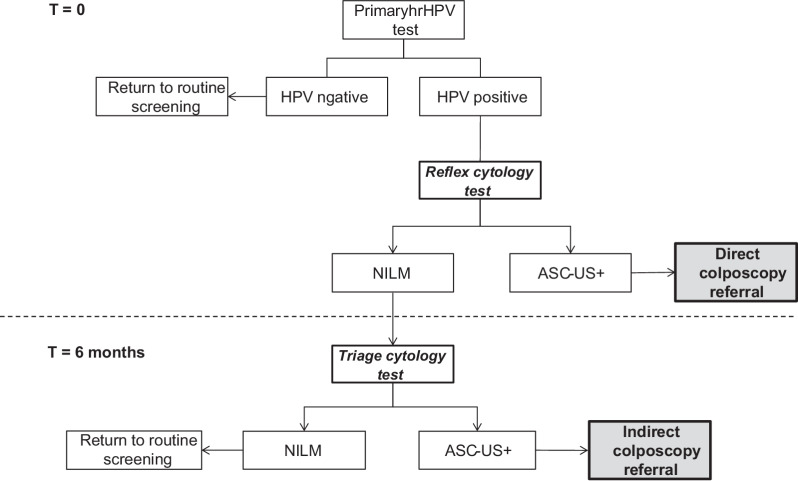
A clinician-collected sample is added to 20 mL of ThinPrep liquid-based cytology medium (PreservCyt Solution; Hologic) while self-sampling is conducted by using the Evalyn brush (Rovers Medical Devices), which is sent directly to the screening laboratory by regular post. Once received by the lab, the dry brushes are processed in 20 mL of ThinPrep liquid-based cytology medium. All samples are then tested with PCR-based cobas 4800 HPV test (Roche Molecular Systems) system. For hrHPV-positive clinician-collected samples, reflex cytology is performed directly on the same sample. Women with hrHPV-positive self-sampling screens are invited to make an appointment with their GP for additional cytology. If abnormal cells are found on cytology, the woman is referred to the gynecologist. The complete referral algorithm for the programme is provided in Fig. 1.
Figure 1.
Referral algorithm for the Dutch primary hrHPV cervical cancer screening programme. hrHPV, high-risk human papillomavirus; NILM, negative for intraepithelial lesion or malignancy; ASC-US+, atypical squamous cells of undetermined significance or higher.
Participants
Women ages 30 to 60 years who were eligible for screening in 2017 and 2018 and who participated in the Dutch cervical cancer screening within 15 months after the beginning of the year of invitation were included in this study (e.g., women eligible for screening in 2018 who were screened between January 1, 2018 and March 31, 2019 were included). Eligibility for invitation was determined by the year of birth of each woman (see Supplementary Table S1).
Data sources
We obtained data from both the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA) and Statistics Netherlands (CBS) for this study. PALGA has nationwide coverage of all pathology laboratories in the Netherlands (11). From PALGA, we received an extract of cervical cytology and histology records, which contained information about all primary screens from the screening programme and the results from follow-up cytology and/or histology after a positive screen. Primary screening results and follow-up examinations belonging to the same woman are combined by use of a pseudonymized identifier based on the first eight letters of a woman's surname (maiden name is used for married women), date of birth and sex, meaning that screening histories can be constructed.
From CBS, we requested data on the personal characteristics of women who participated in the cervical cancer screening programme from cohorts 2017 and 2018 (see Data definitions for exact variables). Participants in the programme had been previously identified as part of another study. The process used to identify participants has been described elsewhere (12). We received both datasets within the secured CBS research environment and linked them based on a combination of CBS ID and date of screening. For 99.6% of the PALGA records, a CBS record could be linked. A summary of the data linkage can be found in Supplementary Fig. S1. The linked dataset contained information of 864,810 screened women.
Data definitions
CIN 2+ and CIN 3+ outcomes, based on diagnostic information recorded in PALGA, were determined by taking the most severe diagnosis within the screening episode. An episode of screening starts with the primary screening test and encompasses any reflex or triage cytology tests and cytology or histology follow-up tests and is completed once a woman is advised to return to regular screening. Follow-up was included up to and including March 31, 2019.
Screening history was determined by the number of previous primary cytology tests (either in the screening programme or testing by medical indication) that a woman underwent previously in between January 1, 2008, and the date of the primary screen selected for inclusion in this study. We categorized these into two groups: (i) no primary cytology tests and (ii) 1+ primary cytology tests.
There are five regional screening organizations in the Netherlands that are responsible for the day-to-day operations of the screening programme and for invitations. Screening region was defined by the residential postcode of each woman registered in PALGA.
Socioeconomic category is determined by CBS based on the income source. If a person has multiple sources of income in a particular year, the income source that contributes the largest amount to a person's income is used to classify this variable into one of 14 categories. We grouped this variable into broader categories: (i) employed; (ii) not employed, social welfare; (iii) not employed, in education; and (iv) no income.
Position in the household is determined by CBS by comparing each household member to the main breadwinner in the household. In our cohort, position in the household was classified into the following categories: (i) breadwinner without partner; (ii) breadwinner with partner; (iii) married partner; (iv) unmarried partner; (v) adult child; and (vi) other household member. If the woman was the breadwinner in the household, she is classified in category 1 or 2, depending on whether she lives in the household with a partner. If the woman was not the breadwinner, she is classified on the basis of her relationship with the breadwinner in the household.
We classified the number of people living in a household into six categories from one person to six or more people.
Standardized household income percentile is calculated by CBS for private households, excluding student houses. We grouped this variable into four categories: (i) 1% to 24%; (ii) 25% to 49%; (iii) 50% to 74%; (iv) 0.75% to 100%.
We used “migration generation” and “country of origin” to define a person's migration background. For migration generation, someone can be classified as “Dutch” (i.e., both parents were born in the Netherlands), “first generation migrant” (i.e., born abroad and has at least one parent who was also born abroad), and “second generation migrant” (i.e., born in the Netherlands with a least one parent born abroad). Country of origin is determined by the country of birth of the person's parents or themselves and is classified into the groups non-Western and Western [i.e., person born in Europe (excluding Turkey), North-America, Oceania (incl Australia), Indonesia and Japan]. On the basis of these two variables, a person's migration background can be (i) Dutch; (ii) non-Western, first generation; (iii) non-Western, second generation; (iv) Western, first generation; (v) Western, second generation.
Data analysis and statistical methods
We used IBM SPSS Statistics for Windows v25 (IBM Corporation) and RStudio (using R v.3.6.2) for data management and analysis. Data linkage was performed using R package dplyr. Pearson chi-square tests were performed to compare differences between proportions. Cases with missing values (N = 6,944) were excluded from statistical analysis, resulting in a total of 857,866 primary screens included in the analysis. Logistic regression was conducted for endpoints hrHPV positivity, CIN 2+ and CIN 3+ detection.
To adjust for loss-to-follow-up in the 14.8% of hrHPV-positive self-sampling users who had no cytology result, we imputed CIN2+/3+ endpoints using a random selection of endpoints from an age- and screening history-matched group of hrHPV-positive self-sampling users who did have a cytology result. We used 10 imputation rounds. R package mitools was used to calculate pooled odds ratios (OR) for CIN2+ and CIN 3+. Because the incidence of hrHPV positivity and CIN 2+/3+ are less than 10%, ORs could be interpreted as relative risks (13).
To control for the influence of screening history on both detection and choice of sampling method, we conducted a separate sensitivity analysis of women aged 35 years and older who had previously been screened.
Ethical approval
This study was conducted as part of the evaluation of the national cervical screening programme, which is legislated under the Population Screening Act in the Netherlands. The Medical Ethics Committee of Erasmus MC University Medical Center reviewed our protocol (MEC-2019–0672) and confirmed that it was not subject to the Dutch Medical Research Involving Human Subjects Act and was therefore exempt from ethical approval. All data owners approved of the study design and gave approval for the use of their data for the purposes of this study.
Data availability
Results of this study are based on our own calculations on publically available data from CBS (dataset name: “Erasmus_MC_BVO_2014_2018_V1_DEF.sav”). This study used a subset of this data from women who were classified as attenders of the screening programme in cohorts 2017 and 2018. This is available upon request to CBS (microdata@cbs.nl). Data from PALGA is available upon request after approval by the Scientific Committee of PALGA.
Results
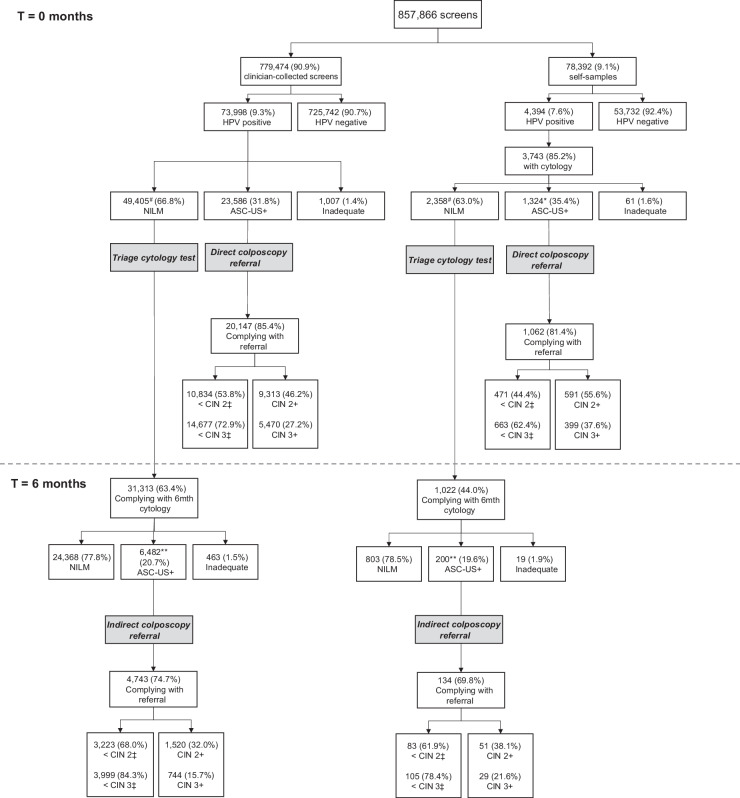
Figure 2 shows the outcomes following primary screening for 857,866 women from cohorts 2017 and 2018. Of these women, 6.8% chose to use self-sampling. The hrHPV positivity rate was higher for the clinician-collected samples (9.3%) than for self-samples (7.6%). Among women who were directly referred for colposcopy and complied with their referral, a significantly higher proportion of women who used self-sampling were diagnosed with a CIN 2+ lesion (55.6% vs. 46.2%; X2 = 35.99, P < 0.001) or CIN 3+ lesion (37.6% vs. 27.2%; X2 = 54.73, P < 0.001). There was no significant difference in the proportion of CIN 2+ or CIN 3+ lesions diagnosed following compliance with indirect referral (CIN 2+: 38.1% for self-sampling vs. 32.0% for clinician-collected sampling, X2 = 2.16, P = 0.14; CIN 3+: 21.6% vs. 15.7%, X2 = 3.47, P = 0.06).
Figure 2.
Outcomes following screening in the Dutch cervical cancer screening programme, cohorts 2017 and 2018 up until March 31, 2019. Cases with missing values for income or screening region are not shown. Approximately 1.5% of CIN 2+/3+ lesions were diagnosed outside of the normal screening pathways (i.e., either after incongruent advice or inadequate cytology). These are not shown on this flowchart but are included in the model. Imputed CIN values for women who used the self-sampling test and had no cytology result are not included. #, There were 56 women given the advice to return to routine screening following a hrHPV+/NILM screening result. Compliance with 6 month cytology is calculated as a proportion of those women receive advice to return for 6 month cytology. *, There were 19 women who used self-sampling given the advice for a repeat cytology test following an hrHPV+/ASC-US screening result. Compliance with referral is calculated as a proportion of those women receive referral advice. **, There were 142 women who received an advice other than referral for colposcopy following a low-grade cytology abnormality. Compliance with referral is calculated as a proportion of those women receive referral advice. ‡, Not all women who complied with referral advice received an histologically confirmed diagnoses (i.e., cytology only). These women are also included in this category. NILM, negative for intraepithelial lesion or malignancy; ASC-US+, atypical squamous cells of undetermined significance or higher.
Table 1 shows the distribution of sociodemographic characteristics and screening history by sampling type. A higher proportion of self-sampling users were ages 30 to 35 compared with women screened by the GP (28.3% vs. 22.0%). In addition, a higher proportion of self-sampling users lived alone (15.8% vs. 11.0%) and had no screening history (29.0% vs. 11.9%) compared with women screened by the GP. Significant differences in the distribution on all other characteristics were also found.
Table 1.
Sociodemographic characteristics of women participating in the Dutch cervical cancer screening programme (N = 857,866), cohorts 2017 and 2018, by test type.
| All participants N = 857,866 | |||
|---|---|---|---|
| Clinician-collected (CC) | Self-sampling (SS) | ||
| N (% of total CC) | N (% of total SS) | P b | |
| Total N (all screened) | 799,740 | 58,126 | — |
| Screening historya | |||
| No cytology tests | 95,343 (11.9%) | 16,831 (29.0%) | <0.01 |
| 1+ cytology tests | 704,397 (88.1%) | 41,295 (71.0%) | |
| Invitation age | |||
| 30 years | 83,970 (10.5%) | 9,054 (15.6%) | <0.01 |
| 35 years | 91,729 (11.5%) | 7,370 (12.7%) | |
| 40 years | 106,364 (13.3%) | 7,008 (12.1%) | |
| 45 years | 125,069 (15.6%) | 7,720 (13.3%) | |
| 50 years | 139,472 (17.4%) | 8,594 (14.8%) | |
| 55 years | 136,968 (17.1%) | 9,398 (16.2%) | |
| 60 years | 116,168 (14.5%) | 8,982 (15.5%) | |
| Migration background | |||
| Dutch | 645,022 (80.7%) | 48,313 (83.1%) | <0.01 |
| Non-western, first generation | 65,903 (8.2%) | 2,549 (4.4%) | |
| Non-western, second generation | 15,419 (1.9%) | 1,636 (2.8%) | |
| Western, first generation | 35,822 (4.5%) | 2,438 (4.2%) | |
| Western, second generation | 37,574 (4.7%) | 3,190 (5.5%) | |
| Socioeconomic category (based on income source) | |||
| Employed | 634,139 (79.3%) | 45,186 (77.7%) | <0.01 |
| Not employed, social welfare | 102,247 (12.8%) | 8,289 (14.3%) | |
| Not employed, in education | 1,959 (0.2%) | 216 (0.4%) | |
| No income | 61,395 (7.7%) | 4,435 (7.6%) | |
| Number of people in the household | |||
| One person | 87,840 (11.0%) | 9,157 (15.8%) | <0.01 |
| Two people | 231,396 (28.9%) | 18,690 (32.2%) | |
| Three people | 172,662 (21.6%) | 11,448 (19.7%) | |
| Four people | 222,656 (27.8%) | 13,355 (23.0%) | |
| Five people | 67,388 (8.4%) | 4,261 (7.3%) | |
| Six or more people | 17,798 (2.2%) | 1,215 (2.1%) | |
| Standardized household income percentile | |||
| 1%–24% | 111,782 (14.0%) | 8,852 (15.2%) | <0.01 |
| 25%–49% | 152,813 (19.1%) | 11,684 (20.1%) | |
| 50%–74% | 229,678 (28.7%) | 16,423 (28.3%) | |
| 75%%–100% | 305,467 (38.2%) | 21,167 (36.4%) | |
| Screening region | |||
| Screening region 1 | 211,458 (26.4%) | 15,990 (27.5%) | <0.01 |
| Screening region 2 | 78,785 (9.9%) | 5,845 (10.1%) | |
| Screening region 3 | 156,603 (19.6%) | 10,846 (18.7%) | |
| Screening region 4 | 175,052 (21.9%) | 12,318 (21.2%) | |
| Screening region 5 | 177,842 (22.2%) | 13,127 (22.6%) | |
| Position in the household | |||
| Breadwinner without partner | 165,700 (20.3%) | 13,841 (23.8%) | <0.01 |
| Breadwinner with partner | 113,610 (14.2%) | 8,490 (14.6%) | |
| Married partner | 400,386 (50.1%) | 25,921 (44.6%) | |
| Unmarried partner | 108,993 (13.6%) | 8,294 (14.3%) | |
| Adult child | 7,907 (1.0%) | 1,066 (1.8%) | |
| Other household member | 6,144 (0.8%) | 514 (0.9%) | |
Note: P values < 0.05 were considered statistically significant.
aNumber of cytology tests (including both those within the programme and those by indication) recorded in PALGA prior to screening, starting from January 1, 2008.
bPearson's chi-square test.
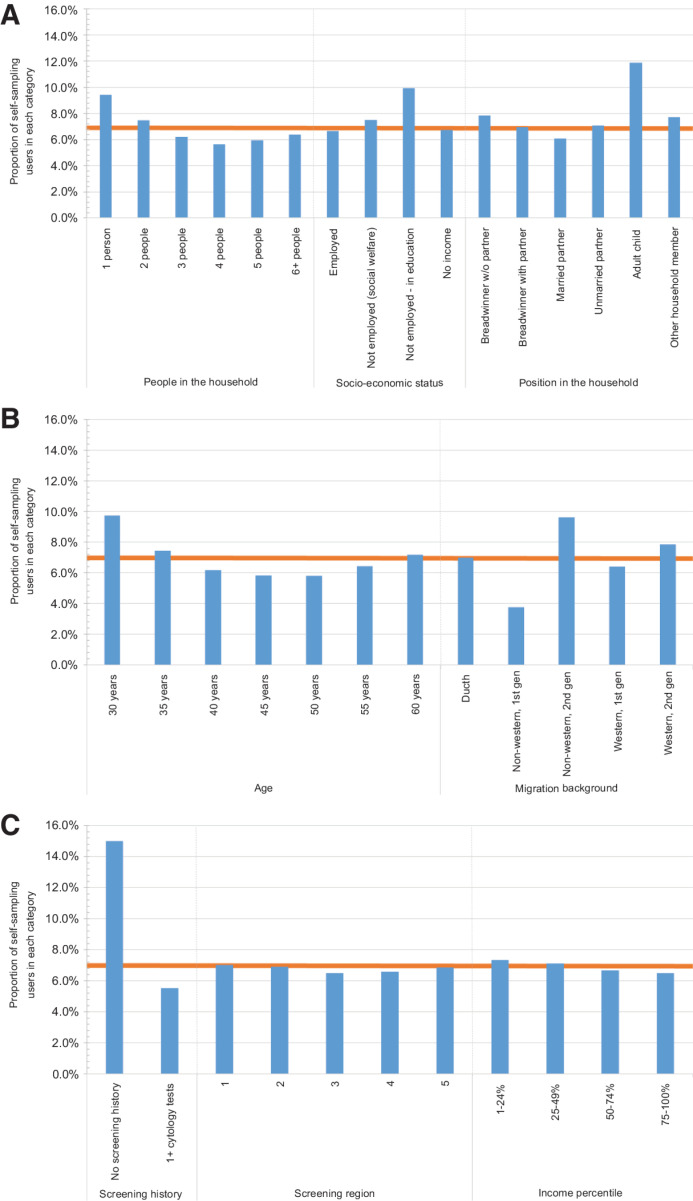
Figure 3A to C show the proportion of self-sampling users within each category of the sociodemographic characteristic variables. Fifteen percent of women with no screening history used self-sampling compared to 5.5% of women who had previously been screened. Compared with the overall proportion of women who chose self-sampling (6.8%), a higher proportion of women aged 30 (9.7%) or 35 years (7.4%) used self-sampling. The proportion of self-sampling users varied considerably between various migration background groups. There was little variation in the proportion of self-sampling users across income groups and screening regions.
Figure 3.
A–C, Proportion of women who used self-sampling within each category, sociodemographic characteristics, and screening history. (A) People in the household, socioeconomic status, and position in the household. (B) Age, and migration background. (C) Screening history, screening region, and income percentile. The orange line denotes the overall proportion of women who used self-sampling across all participants (6.8%). Women are counted once for each variable, that is, the same woman will appear in her respective age, screening history, and sociodemographic variable category. The total in each variable is 857,866 women.
Table 2 shows the results of logistic regression analysis of hrHPV positivity, CIN 2+ and CIN 3+ detection among all screened women. For hrHPV positivity, both unadjusted and adjusted models showed lower odds of hrHPV positivity for self-sampling compared with clinician-collected sampling. For CIN lesions, all CIN detected from direct referrals, indirect referrals and nonstandard programme pathways were included (see Fig. 2) and imputed endpoints were used for hrHPV-positive self-sampling users who had no cytology result. In the model without imputation, the ORs for endpoints CIN2+ and CIN3+ for self-sampling compared with clinician collected are significantly lower compared with the models with imputation. For CIN 2+ detection there was no significant difference in detection in the unadjusted model (pooled OR, 0.96; 95% CI, 0.89–1.03), and for CIN 3+ there was a higher odds found (pooled OR, 1.12; 95% CI, 1.02–1.23). However, after adjustment, there was a lower odds of CIN 2+ (pooled aOR 0.76; 95% CI, 0.70–0.82) and CIN 3+ (pooled aOR, 0.86; 95% CI, 0.78–0.95) detection following self-sampling compared with clinician-collected sampling.
Table 2.
Logistic regression analysis, endpoints hrHPV positivity, CIN 2+ and CIN 3+ (without and with imputation), all screened women.
| Without imputation | With imputation | |||||
|---|---|---|---|---|---|---|
| Unadjusted model | Unadjusted model | Adjusted models | ||||
| Model 1 | Model 1 | Model 2a | Model 3b | Model 4c | Model 5d | |
| (N = 864,810) | (N = 857,866) | (N = 857,866) | (N = 857,866) | (N = 857,866) | (N = 857,866) | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| hrHPV positivity | ||||||
| Clinician-collected | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-sampling | 0.80 (0.78–0.83) | 0.80 (0.78–0.83) | 0.67 (0.65–0.69) | 0.73 (0.70–0.75) | 0.73 (0.70–0.75) | 0.65 (0.63–0.68) |
| CIN 2+ | ||||||
| Clinician-collected | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-sampling | 0.82 (0.76–0.88) | 0.96 (0.89–1.03) | 0.73 (0.68–0.79) | 0.81 (0.75–0.88) | 0.81 (0.75–0.88) | 0.76 (0.70–0.82) |
| CIN 3+ | ||||||
| Clinician-collected | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-sampling | 0.96 (0.87–1.06) | 1.12 (1.02–1.23) | 0.83 (0.76–0.91) | 0.92 (0.84–1.01) | 0.92 (0.84–1.01) | 0.86 (0.78–0.95) |
Note: All cases with missing values for household income and/or screening region (0.8% of total cohort) are excluded from the analyses with imputation presented in this table. Ten rounds of imputation were used to replace missing CIN2+/CIN3+ endpoints for women with a hrHPV-positive self-sampling and no cytology result. Pooled ORs are presented for these endpoints. Pooled ORs with 95% CI not including 1.00 are shown in bold.
aAdjusted for screening history.
bAdjusted for screening history and age.
cAdjusted for screening history, age, and screening region.
dAdjusted for screening history, age, screening region, and sociodemographic characteristics.
Table 3 shows the results of logistic regression analysis of hrHPV positivity, CIN 2+ and CIN 3+ detection among women ages 35+ years who had previously been screened. In this subpopulation, ORs did not differ in the unadjusted models and those adjusted for age and screening region. For CIN 3+, there were no significant differences between the clinician-collected sampling and self-sampling in the models adjusted for age and screening region. Adjusting for sociodemographic characteristics resulted in a decrease in the ORs for both CIN 2+ (pooled aOR 0.73; 95% CI, 0.65–0.83) and CIN 3+ (pooled aOR 0.83; 95% CI, 0.71–0.97).
Table 3.
Logistic regression analysis, endpoints hrHPV positivity, CIN 2+ and CIN 3+ (with imputation), women aged 35+ years who have previously been screened (N = 720,976).
| Unadjusted model | Adjusted models | |||
|---|---|---|---|---|
| Model 1 | Model 2a | Model 3b | Model 4c | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| hrHPV positivity | ||||
| Clinician-collected | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-sampling | 0.73 (0.70–0.76) | 0.73 (0.70–0.77) | 0.73 (0.70–0.76) | 0.66 (0.63–0.69) |
| CIN 2+ | ||||
| Clinician-collected | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-sampling | 0.78 (0.69–0.88) | 0.78 (0.69–0.88) | 0.78 (0.69–0.88) | 0.73 (0.65–0.83) |
| CIN 3+ | ||||
| Clinician-collected | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-sampling | 0.87 (0.75–1.02) | 0.88 (0.75–1.03) | 0.88 (0.75–1.03) | 0.83 (0.71–0.97) |
Note: All cases with missing values for household income and/or screening region (0.8% of total cohort) are excluded from the analyses presented in this table. Ten rounds of imputation were used to replace missing CIN2+/CIN3+ endpoints for women with a hrHPV-positive self-sampling and no cytology result. Pooled ORs are presented for these endpoints. Pooled ORs with 95% CI not including 1.00 are shown in bold.
aAdjusted for age.
bAdjusted for age and screening region.
cAdjusted for age, screening region, and sociodemographic characteristics.
Discussion
Results of our study have shown that, in the first 2 years of primary hrHPV screening in the Netherlands, women who used self-sampling differed from women who chose to be screened by the GP in screening history and sociodemographic characteristics. Women who chose self-sampling were overrepresented in the youngest and oldest age groups and a smaller proportion had a previous cytology test. We also found differences in position in the household and number of people in the household, which are probably related to age. Our results indicate that self-sampling can be used for targeting underscreened women, as a more convenient primary screening tool, which is an important criterion for public health success. Differences in sociodemographic characteristics did make an impact in our logistic regression analyses. When limiting our analysis to women aged 35+ years who had been previously screened, CIN 3+ detection in the self-sampling group only became significantly lower when adjusting for sociodemographic characteristics. Despite a slightly lower risk of CIN 2+/3+ detection, this difference may have limited clinical relevance. A recent Dutch study on the performance of self-sampling compared with clinician-collected sampling found slightly lower sensitivity for CIN 2+/3+ in combination with higher specificity for CIN 2+/3+ in screens taken within the screening programme (14), meaning that self-sampling may result in fewer false-positive screening tests than clinician-collected sampling. Combining these findings, with our results, it seems that self-sampling is good alternative for clinician-collected sampling, especially since it is reaching women at higher risk.
Comparing the results of self-sampling in the Dutch cervical cancer screening programme with other previously published studies is challenging, as self-sampling has been mainly studied in populations of nonresponders. Nonresponders have a higher risk of developing cervical lesions; the majority of cervical cancer diagnoses in the Netherlands are found in women who are not screened (15), demonstrating the success of the Dutch screening programme in preventing cervical cancer diagnoses. One positive finding from our study is that a significantly higher proportion of women who used self-sampling had not been screened within the last ten years (29% vs. 11.9% of women screened by GP), meaning self-sampling is reaching women who are hesitant to be screened. However, most women who used self-sampling had been tested for cervical lesions at least once. Modeling suggests that a gain in health benefits by implementing self-sampling can be achieved when increasing overall participation and limiting the number of “switchers,” that is, women who have previously been screened by the GP (16). This modeling study showed that offering self-sampling will gain health effects if the relative CIN2+ sensitivity of self-sampling (compared with clinician-collected) is high (≥0.95), previously unscreened attendees are recruited, and the total attendance increases (≥6 percentage point). To maximize the benefits of self-sampling in the Dutch screening programme, more active approaches are needed to reach women who are hesitant to be screened. In October 2021, the Health Council of the Netherlands advised that self-sampling kits be sent directly to all women with the primary invitation for screening (17), to increase participation. The expectation is that this will substantially increase the proportion of women choosing for self-sampling.
We found that the hrHPV positivity rate of self-sampling was significantly lower than clinician-collected sampling. Other Dutch studies, namely the IMPROVE trial and the VERA study, showed that hrHPV positivity on self-sampled materials was equivalent to or even higher than clinician-collected sampling. The IMPROVE trial showed hrHPV positivity of self-sampling was not significantly different than clinician-collected sampling (8). It should be noted that the type of hrHPV test used in the IMPROVE trial (GP5+/6+ PCR-EIA) differs from that used in the screening programme (Roche PCR-based cobas 4800). The VERA study tested the concordance between self-samples and clinician-collected samples using the same hrHPV test medium as used in the screening programme. The VERA study showed that self-sampling resulted in higher hrHPV positivity than clinician-collected sampling (4). Despite the study population of the VERA study being comparable with ours, our results show the opposite result. It is possible that the difference lies in the sample processing. In the VERA study, the self-sampling brushes were processed in 4.5 mL of PreservCyt solution, whereas in the screening programme, self-samples are processed in 20 mL of this medium. Recently published research from the Netherlands found that cycle threshold values of the PCR hrHPV test were higher in self-samples than in clinician-collected samples (14). These suggest that dilution with 20 mL of medium may need to be reconsidered going forward, and that the optimal PCR test/dilution volume for use with the self-sampling kit should be validated.
Our study has several strengths. This is the first population-based study that examined a broad range of sociodemographic characteristics of women who use self-sampling and women that choose to go to their GP for a cervical examination. Our dataset contained information about sociodemographic characteristics of attenders, screening history, and the cyto- and histologic results of screening. Both CBS and PALGA provided comprehensive, population-based data on an individual level. Both data sources have national coverage. Given our large sample size, we can conclude that we had sufficient statistical power for our comparisons. By linking data of PALGA and CBS on an individual level, we have been able to evaluate the impact of sociodemographic characteristics, beyond what is normally available for monitoring and evaluation.
Our study also has some limitations. Socioeconomic status is a combination of factors related to income, education, and occupation (18), which we were only able to partly capture in our study. We were, for instance, not able to include information on education level as a covariate in our study. Information about educational status is only complete from registry data for a selection of women in our cohort, namely younger women. For older women, data on education level is usually obtained from population-based surveys and data are weighted, and not available at individual level. As such, we chose not to include this information in our study. Other personal and behavioral factors that we were unable to include in our study [e.g., smoking (19, 20), use of oral contraceptives (21, 22), parity (22, 23)] play a role in the risk of having an hrHPV infection or developing CIN 2+. If these factors differed between groups, it could have introduced confounding into our estimates. However, recent risk prediction modeling has shown that additional lifestyle factors did not improve risk prediction of CIN 2+ over the predictive value of an hrHPV infection (24), so the impact on our estimates may be limited. Follow-up time in our study is limited to follow-up up to and including March 31, 2019. This means that for women in the 2018 cohort who participated at the end of 2018 or in the first 3 months of 2019, limited follow-up time for the CIN 2+/3+ endpoint was available. However, given that this would be the case for both groups, this is unlikely to lead to differences between the groups.
Finally, our study reflects the Dutch situation following invitations for screening in 2017 and 2018, the first cohorts for whom self-sampling was offered as opt-in strategy to all women invited for screening. During those first 2 years of hrHPV-based programme, self-sampling was not widely promoted. When the COVID-19 pandemic disrupted screening in the Netherlands in 2020, one of the strategies adopted by the coordinating organization [Dutch National Institute for Public Health and the Environment (RIVM)] was increasing the prominence of self-sampling in the invitation letter. The result of this has been a recovery in participation rates despite a lower than usual number of clinician-collected screens (25). The disruption due to COVID-19 may expedite the introduction of, or wider availability/uptake of, self-sampling in organized screening programmes (26). This is already being seen in the Netherlands; the proportion of self-sampling use among participants increased from 8.6% in 2019 (10) to 16.3% in 2020 (27). O the basis of advice of the Health Council of the Netherlands (17), self-sampling may eventually become the primary screening modality in the Netherlands. Wider use of self-sampling will likely result in a different profile of self-sampling users that what we have reported in this study, both due to switching and a higher participation rate. Furthermore, our study is only reflective of the specific set-up of the Dutch screening programme in the first screening round. A different combination of collection devices, liquid-based cytology media, HPV test type and laboratory protocols will likely change the relative performance of self-sampling in comparison to clinician-collected sampling. Therefore, it is possible that future hrHPV positivity and detection of CIN 2+/3+ after self-sampling may differ from the results presented in our study.
Women who use self-sampling differ from women who chose to be screened by the GP. The fact that younger women, and with no prior screening, more often chose self-sampling, indicates that self-sampling can be used for targeting underscreened women, as a more convenient primary screening tool. After adjusting for these differences in age, screening history and sociodemographic characteristics, hrHPV positivity and overall CIN 2+/3+ detection remained slightly lower following self-sampling compared with clinician-collected sampling. Our results suggest that these differences between the two collection methods may be caused by factors (e.g., technical or behavioral factors) other than sociodemographic characteristics of screened women. Further investigation is required to evaluate if the differences are related to technical issues in the processing of self-sampling, to know how to implement self-sampling when hrHPV testing on self-collected samples is used as a primary instrument in routine screening.
Supplementary Material
Supplementary Table 1: Birthyears invited for screening in 2017 and 2018. Supplementary Figure 1: Data linkage flowchart, describing how CBS data was linked to PALGA data on an individual level
Acknowledgments
The authors wish to acknowledge the CBS Microdata team for their assistance with managing the data for this study and for the original linkage of the data from PALGA with CBS. C.A. Aitken, F. Inturrisi, S. Kaljouw, J. Berkhof, and I.M.C.M. de Kok received funding from the Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu). The funding source had no involvement in the study design, data collection, data analysis, interpretation of the data, writing of the report or the decision to submit the paper for publication.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Disclosures
C.A. Aitken reports grants from Rijksinstituut voor Volksgezondheid en Milieu during the conduct of the study; grants from Rijksinstituut voor Volksgezondheid en Milieu outside the submitted work. S. Kaljouw reports grants from Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu) during the conduct of the study; grants from Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu) outside the submitted work. H.G. Niesters reports grants from UMC Groningen during the conduct of the study. I.M. de Kok reports grants from The Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu) during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
C.A. Aitken: Formal analysis, investigation, visualization, writing–original draft, project administration. F. Inturrisi: Writing–review and editing. S. Kaljouw: Formal analysis, writing–review and editing. D. Nieboer: Formal analysis, writing–review and editing. A.G. Siebers: Data curation, writing–review and editing. W.J.G. Melchers: Resources, writing-review and editing. A.J.C. van den Brule: Resources, writing–review and editing. A. Molijn: Resources, writing–review and editing. J.W.J. Hinrichs: Resources, writing–review and editing. H.G. Niesters: Resources, writing–review and editing. F.J. van Kemenade: Writing–review and editing. J. Berkhof: Methodology, writing–review and editing. I.M.C.M. de Kok: Conceptualization, supervision, funding acquisition, writing–original draft, writing–review and editing.
References
- 1. Bevolkingsonderzoek Nederland. De uitnodiging. c2020-21 [cited 26 July 2021]. Available from: https://www.bevolkingsonderzoeknederland.nl/baarmoederhalskanker/de-uitnodiging/.
- 2. Rijksinstituut voor Volksgezondheid en Milieu (RIVM). Uitvoeringskader Bevolkingsonderzoek Baarmoederhalskanker. 2017[cited 26 July 2021]. Available from: https://www.rivm.nl/documenten/uitvoeringskader-bevolkingsonderzoek-baarmoederhalskanker.
- 3. Polman NJ, de Haan Y, Veldhuijzen NJ, Heideman DAM, de Vet HCW, Meijer C, et al. Experience with HPV self-sampling and clinician-based sampling in women attending routine cervical screening in the Netherlands. Prev Med 2019;125:5–11. [DOI] [PubMed] [Google Scholar]
- 4. Ketelaars PJW, Bosgraaf RP, Siebers AG, Massuger LFAG, van der Linden JC, Wauters CAP, et al. High-risk human papillomavirus detection in self-sampling compared to physician-taken smear in a responder population of the Dutch cervical screening: results of the VERA study. Preventive Medicine 2017;101:96–101. [DOI] [PubMed] [Google Scholar]
- 5. Gok M, Heideman DA, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JW, et al. HPV testing on self-collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ 2010;340:c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gok M, Heideman DA, van Kemenade FJ, de Vries AL, Berkhof J, Rozendaal L, et al. Offering self-sampling for human papillomavirus testing to non-attendees of the cervical screening programme: Characteristics of the responders. Eur J Cancer 2012;48:1799–808. [DOI] [PubMed] [Google Scholar]
- 7. Arbyn M, Smith SB, Temin S, Sultana F, Castle P, Collaboration on S-S, Testing HPV. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018;363:k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polman NJ, Ebisch RMF, Heideman DAM, Melchers WJG, Bekkers RLM, Molijn AC, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol 2019;20:229–38. [DOI] [PubMed] [Google Scholar]
- 9. Aitken CA, van Agt HME, Siebers AG, van Kemenade FJ, Niesters HGM, Melchers WJG, et al. Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: a population-based cohort study. BMC Med 2019;17:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Netherlands Comprehensive Cancer Organisation (IKNL). Monitor bevolkingsonderzoek baarmoederhalskanker 2019. 2020[cited 20 April 2021]. Available from: https://www.rivm.nl/documenten/monitor-bevolkingsonderzoek-baarmoederhalskanker-2019
- 11. Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aitken CA, Kaljouw S, Siebers AG, Bron M, Morssink A, van Kemenade FJ, et al. Investigating the decrease in participation in the Dutch cervical cancer screening programme: The role of personal and organisational characteristics. Prev Med Rep 2021;22:101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Yu KF.. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- 14. Inturrisi F, Aitken CA, Melchers WJ, van den Brule AJC, Molijn A, Hinrichs JWJ, et al. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: an observational study. The Lancet Regional Health - Europe 2021;11:100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bos AB, Rebolj M, Habbema JD, van Ballegooijen M. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. Int J Cancer 2006;119:2372–5. [DOI] [PubMed] [Google Scholar]
- 16. Rozemeijer K, de Kok IM, Naber SK, van Kemenade FJ, Penning C, van Rosmalen J, et al. Offering self-sampling to non-attendees of organized primary HPV screening: when do harms outweigh the benefits? Cancer Epidemiol Biomarkers Prev 2015;24:773–82. [DOI] [PubMed] [Google Scholar]
- 17. Gezondheidsraad. Verbetermogelijkheden bevolkingsonderzoek baarmoederhalskanker. 2021[cited 5 January 2022]. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2021/10/19/verbetermogelijkheden-bevolkingsonderzoek-baarmoederhalskanker.
- 18. Baker EH. Socioeconomic status, definition. In:Cockerham W.C., Dingwall R., Quah S., editors.The Wiley Blackwell encyclopedia of health, illness, behavior, and societyed. Wiley; 2014:2210–4. [Google Scholar]
- 19. Roura E, Castellsagué X, Pawlita M, Travier N, Waterboer T, Margall N, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer 2014;135:453–66. [DOI] [PubMed] [Google Scholar]
- 20. Vaccarella S, Herrero R, Snijders PJ, Dai M, Thomas JO, Hieu NT, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol 2008;37:536–46. [DOI] [PubMed] [Google Scholar]
- 21. International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet 2007;370:1609–21. [DOI] [PubMed] [Google Scholar]
- 22. Roura E, Travier N, Waterboer T, de Sanjosé S, Bosch FX, Pawlita M, et al. The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: results from the EPIC Cohort. PLoS One 2016;11:e0147029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer 2006;119:1108–24. [DOI] [PubMed] [Google Scholar]
- 24. van der Waal D, Bekkers RLM, Dick S, Lenselink CH, Massuger L, Melchers WJG, et al. Risk prediction of cervical abnormalities: the value of sociodemographic and lifestyle factors in addition to HPV status. Prev Med 2020;130:105927. [DOI] [PubMed] [Google Scholar]
- 25. Castanon A, Rebolj M, Burger EA, de Kok I, Smith MA, Hanley SJB, et al. Cervical screening during the COVID-19 pandemic: optimising recovery strategies. Lancet Public Health 2021;6:e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim AWW. Will COVID-19 be the tipping point for primary HPV self-sampling? Cancer Epidemiol Biomarkers Prev 2021;30:245–7. [DOI] [PubMed] [Google Scholar]
- 27. Netherlands Comprehensive Cancer Organisation (IKNL). Monitor bevolkingsonderzoek baarmoederhalskanker 2020. 2021[cited 15 November 2021]. Available from: https://www.rivm.nl/documenten/monitor-bevolkingsonderzoek-baarmoederhalskanker-2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Birthyears invited for screening in 2017 and 2018. Supplementary Figure 1: Data linkage flowchart, describing how CBS data was linked to PALGA data on an individual level
Data Availability Statement
Results of this study are based on our own calculations on publically available data from CBS (dataset name: “Erasmus_MC_BVO_2014_2018_V1_DEF.sav”). This study used a subset of this data from women who were classified as attenders of the screening programme in cohorts 2017 and 2018. This is available upon request to CBS (microdata@cbs.nl). Data from PALGA is available upon request after approval by the Scientific Committee of PALGA.