Abstract
Soil and irrigation water salinity are major limiting factor to citrus industry in arid environments.
The objective of this study was to assess the effects of different salt stress levels on growth and ion uptake of three-month-old citrus rootstocks; sour orange (Citrus aurantium) and Volkamer lemon (Citrus volkameriana). Six levels of NaCl-salinity were used, 0.7 (control), 2, 4, 8, 12 and 15 dS m−1. Salinity increment from 2.0 to 15.0 dS m−1 significantly reduced seedlings height, stem diameter, leaf area, root dry weight, leaf relative water content, chlorophyll content index and chlorophyll fluorescence by one to three folds. In addition, leaf and root N concentration reduced by 10%–50%, P 6%–50%, K 8%–47%, Ca+2 7%–51% and Mg+2 7%–50% when salt stress in the irrigation water increased from 2.0 to 15.0 dS m−1. Conversely, salt stress increment (2.0–15.0 dS m−1) increased leaf stomatal resistance (5 folds), proline concentration (1 fold), Na+ and Cl− in the leaf (10 fold) and root (4 fold) when compared to control (0.7 dS m−1). In term of rootstock, Volkamer had higher seedling height, stem diameter, and root constituents (length, fresh and dry weight) than sour orange. While sour orange had higher leaf Cl−, Ca+2 and Mg+2, Volkamer lemon had higher N, Na+, K+, and P. However, root nutrient (N, Na+, Cl−, P and Mg+2) from Volkamer had consistently higher concentration compared to sour orange at 4.0, 8.0, 12.0 and 15.0 dS m−1. Therefore, we believe that the Volkamer rootstock is more tolerant to salt stress than sour orange.
Keywords: Salt stress, Lemon, Sour orange, NaCl, Ions uptake
1. Introduction
Soil salinity is considered a major threat to the citrus industry in many parts of the world, particularly in semi-arid and arid regions where saline water mostly is used for irrigation [1]. Saline water contains inorganic dissolved salts including cations [sodium (Na+), calcium (Ca+2) and magnesium (Mg+2)] and anions, [chloride (Cl−), sulfate (SO4−2) and bicarbonate (HCO3−)] [2]. These ions generate an electrical field in the solution so that the concentration of salts correlates linearly with electrical conductivity (EC) of the water [2]. High EC (saline conditions >4 dS m−1) disrupt root membrane integrity, reduce nitrate (NO3−) uptake by Cl− (compete with NO3− during uptake and translocation to leaf), alter the activities of nitrogen (N) assimilating enzymes, and reduce relative growth rate [3]. Plant respond to salt stress by increasing root/canopy ratio and chlorophyll content and inducing changes in the leaf anatomy to prevent leaf ion toxicity, maintain leaf water, turgor and photosynthesis [4].
Citrus spp. (grapefruit, lemons, and oranges) are among the most salinity-sensitive of all agricultural crops [5]. Previous old records of threshold for EC (saturated-soil, ECs) in citrus was about 1.4 dS m−1where fruit yields decreased by about 13% for each 1.0 dS m−1 increase in ECs above this value [5]. More recent findings indicated that EC values over 3 dS m−1 and sodium adsorption ratio (SAR) over 9 in saturated soil extract are characterized as critical for the survival of citriculture cultivation [6]. Similarly, Anjum et al. [7] indicated than EC values up to 2 dS m−1 have no negative effects on citrus seedlings. However, salt stress negatively affect relative growth rate, net assimilation rate, and nutrient uptake and utilization in citrus [[7], [8], [9]]. The highest growth rate, standing leaf area and survival rate of grafted citrus trees decreased with increasing tissue Na+ and Cl− concentrations [10]. Growing mandarin (Citrus reticulate) seedlings (1 year) in sandy soil that was fertigated with saline nutrient solution (50 mM NaCl) for ten weeks showed a significant reduction in leaf growth compared to control (5 mM NaCl) [11]. This was due partially to higher leaf water potential (more negative). In trifoliate orange seedlings, saline growing medium (100 mM NaCl) reduced plant height, stem diameter, leaf number and root component; length, surface area, diameter and volume [12]. Although salt stress (20–100 mmol L−1-NaCl or NaCl + CaCl2) did not affect the leaf water potential of sour orange seedlings, it significantly increased the shoot to root ratio (on a fresh weight basis) [13].
The gradual and progressive injury becomes more acute over time because trees tend to accumulate salts in their woody tissues over the years before toxic symptoms appear [2]. Soil salinity (NaCl) creates low nutrient-ion activities environment and extreme ratios of Na+/Ca+2, Na+/K+, Ca+2/Mg+2 and Cl−/NO3− resulting in nutritional imbalances and crop growth reduction [14]. Excess ion accumulation (Cl− and Na+) in citrus tissues can cause specific ion toxicities [5,15]. In addition, salt stress in the growing medium reduces nutrient levels (N, P, K+, Ca+2, and Mg+2) [16].
Plants adapt to saline conditions through the maintenance of their cell turgor. Maintenance of cellular turgidity requires the lowering of tissue osmotic potential through the accumulation of compatible solutes (organic and inorganic) in response to osmotic stress [17]. Osmotic adjustment (Cl− uptake) was the main tolerance mechanism of mature citrus trees [13 year old, ‘Fino 49’ (Citrus limon); grafted on Citrus macrophylla rootstock] for maintenance of turgor under salinity condition [18]. In addition, some citrus rootstocks have been identified for their capacity to exclude Cl− and Na+ and therefore is widely used during the selection of salt tolerant citrus [1].
Availability of sufficient supplies of fresh water is the main limiting factor for productive agriculture in countries arid and semi-arid regions [19]. The use of saline water for agriculture is considered as one of alternative sources of irrigation water in Jordan that suffers from shortage of freshwater resources [20]. Major sources of low-quality water are found in Jordan River, Wades, wells springs and drains with salinity level ranging from 3 to 11 dS m−1 [19,21]. Sour orange (Citrus aurantium L.) and Volkamer Lemon (Citrus volkameriana) are two multiple use species that are generally grown as rootstock for sweet oranges. They comprise over 70% of the rootstocks used in Jordan for various citrus scions. Although citrus is highly sensitive to salinity, we hypothesize that rootstocks response to salt stress is different. This study was undertaken to evaluate salt tolerance level of two important rootstock and to identify their potential use in improving salinity tolerance in citrus. Seedling growth and ion and nutrients uptake and partitioning as well as physiological responses such as chlorophyll content and stomatal resistance and their impact on growth and development were also evaluated under greenhouse conditions.
2. Materials and methods
2.1. Site description and plant material
The experiment was conducted in a greenhouse at The University of Jordan, Amman, Jordan (lat. 32° 0′ 40.4316″ N, long. 35° 52′ 20.3628″ E). Seeds of two citrus rootstocks ‘‘sour orange” and “Volkamer lemon” were obtained from National Center for Agricultural Research, Jordan, and were germinated in plastic trays filled with peatmoss and grown under greenhouse conditions at average of 30/20 ± 3 °C (day/night temperature), 14–15 h daylength and 40–65% relative humidity. When seedlings were 3 months old, uniform sized seedlings were selected and transplanted into 5 L (plastic container-height 18 cm, top diameter 22 cm, and bottom diameter 13 cm). Containers filled with 3 peat moss (Pindstrup Mosebrug, Ryomgaard, Denmark): 1perlite (Agra Perlite, Tuincentrum, Holland) (by volume) growing substrate. Seedlings of both rootstocks were irrigated once a week with Hoagland's No.2 basal salt (Caisson Labs, Smithfield, UT, USA) that contains 565.4 mg L−1 (Ca(NO3)2.4H2O), 606 mg L−1 (KNO3), 240.8 mg L−1 (MgSO4), 15.03 mg L−1 (NH4H2PO4), 2.86 mg L−1 (H3BO3), 5.32 mg L−1 (Cu12Fe2H12O8), 1.81 mg L−1 (MnCl2.4H2O), 220 μg L−1 (Zn(NO3)2.6H2O), 20 μg L−1 (MoO3), 80 μg L−1 (CuSO4.5H2O).
2.2. Treatments
Two weeks after transplanting, six levels of NaCl salinity were used to irrigate seedling twice a week. Treatments of 0.7 (control), 2, 4, 8, 12 and 15 dS m−1 which is equivalent to 5, 17, 34, 68, 103 and 136 mM-NaCl, respectively were used. Tap water (control, 0.7 dS m−1) was used to prepare saline water treatments. Salinity levels were gradually increased to avoid osmotic shock and pot moisture content was maintained above 70% of its field capacity using the specified EC value).
2.3. Physiological measurements
Plants physiological responses: chlorophyll content index (SPAD), chlorophyll fluorescence (Fv/Fm), stomatal resistance, leaf relative water content (RWC) and proline concentration) were determined at the end of the experimental period (4 months after the initiation of salinity treatments). Leaf area was measured using leaf area meter (AM 300, Bio-scientific Ltd, Hoddesdon, UK). Root length were measured following the procedure of Al-Satari et al. [22] using a Regent STD 1600 scanner accompanied with WinRhizo Pro 2005 software (Regent Instruments, Quebec). Shoot and root dry weights were determined after drying at 70 °C for 48 h. Leaf-level physiology was determined on three fully mature leaves. Chlorophyll content index was measured using a hand-held chlorophyll meter (SPAD-502 Plus, Minolta, Japan). Leaf chlorophyll fluorescence was determined following the procedure of Othman et al. [23] and using a Pulse-Modulated Fluorometer (OS1-FL modulated chlorophyll fluorometer, ADC Bio Scientific Ltd., Hertford, UK). Stomatal resistance to CO2 was measured using steady state porometer (AP4 model, Delta T devices, Burwell, UK). For RWC, leaf fresh weight (FW) was determined directly after sampling and then turgid leaf weight (TW) was recorded after leaves were placed on distilled water to rehydrate for 24 h. Leaves were then dried for 24 h at 70 °C to determine leaf dry weight (DW). Relative water content was calculated using the following equation:
| RWC = (FW - DW)/ (TW - DW)* | 100 |
Proline content was determined in fresh leaf samples following the procedure of Bates et al. [24]. A fully expanded fresh and mature leaf was excised. Then a 0.5 g leaf sample was placed in a mortar and ground using a pestle and liquid nitrogen. Ten ml of 3% (5 M) 5-salfosalislic acid was added to the ground samples and the mixture was filtered using two layers of Whatman paper (no. 41). Then, 2 ml of the supernatant was mixed with 2 ml of acid ninhydrin solution (1.25 g ninhydrin, 30 ml of glacial acetic acid, 20 ml of 6 M phosphoric acid), and 2 ml of glacial acetic acid and left for 1 h. The mixture then was heated in a water bath at 100 °C for 1 h to allow for color development. One ml of toluene was added to each sample and mixed. Sample absorbance at 520 nm was measured using a spectrophotometer (SmartSpec TM Plus Spectrophotometer, Bio-Rad, CA, USA).
2.4. Morphological measurements
Plants were sampled 4 months after initiation of salinity treatments and the following growth parameters were measured: plant height, stem diameter, total leaf area, and leaves, stem and root dry weight. Plant height of the seedlings was recorded in (cm) from the plant tip to the crown while stem diameter of the seedling was measured in (mm ± 0.01) at 3 cm above soil surface using vernier caliper. Three representative leaves from each seedling were taken to measure average leaf area that will be used to estimate total leaf area per plant by using Leaf Area Meter (AM 300), Bio-scientific Ltd, UK. Leaves, stems and roots dry weight were measured after drying at 70 °C for 48 h.
2.5. Plants mineral analysis
Leaf and root mineral analyses were determined following the procedures of Mehlich [25] and Al-Ajlouni et al. [26]. Leaf and root samples were collected, oven dried for 2 d at 70 °C, then total N was determined using a Discrete Analyzer (Easy Chem. Plus, Analytical Technologies, Anagni, Italy). Leaf and root P, K+, Ca+2 and Mg+2 extracts were prepared using the Mehlich [25] procedure and elements then were quantified using inductively coupled plasma mass spectrometry (ELAN ICP-MS, PerkinElmer, Waltham, MA).
2.6. Statistical analysis
The study was designed using randomized complete block design with two factors, six salinity levels and two rootstocks. There were four replicates per treatment, and each replicate presented a mean of two seedlings. The analysis of variance (ANOVA) and the least significant difference test (P < 0.05) in SAS (Version 9.1 for Windows; SAS Institute, Cary, NC) were used to determine differences between salinity levels, rootstocks and their interactions.
3. Results and discussion
3.1. Rootstock morphology and physiology
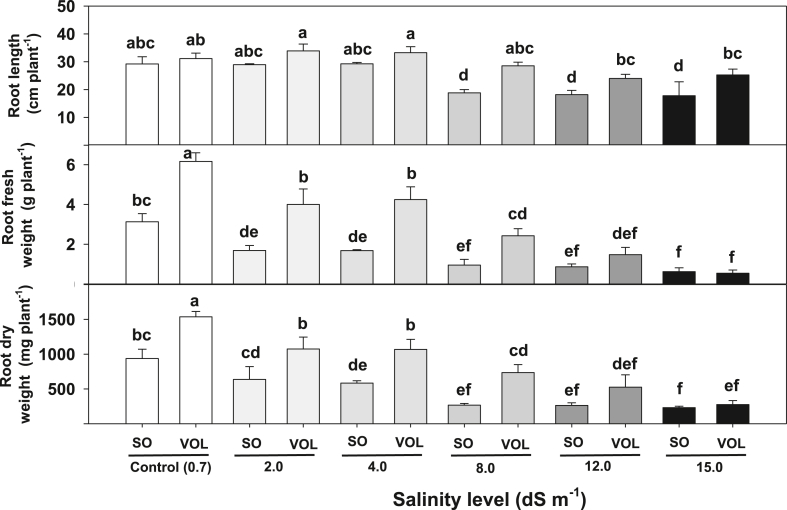
Salt stress reduced the shoot (plant height, stem diameter, leaf area) and root growth (length, fresh and dry weight) of both rootstocks seedlings (Table 1). Under salt stress (2.0–4.0 dS m−1), the reduction in the shoot and root components (except root length) was between 4 and 39% when compared to control (0.7 dS m−1). In addition, the reduction in shoot and root component was higher than 40% when salt stress levels were greater than 8.0 dS m−1 (except for root length). Salt stress reduced citrus root growth and predisposes trees to biotic attack by root rot, nematodes and bacterial disease [27]. Our results (specifically on sour orange) was in agreement with Zekri and Parsons [28] who found that exposure to 40 mM of NaCl (∼5.0 dS m−1) for four months reduced sour orange root and shoot dry weight by about 30% (with no leaf necrosis) compared to plants grown in 5 mM-NaCl (∼0.7 dS m−1) added to half-strength Hoagland's solution. However, salt stress of 2 and 4 dS m−1 (17, 34 mM-NaCl) slightly increased (not significant) root length, specifically at Volkamer rootstock (Fig. 1, Fig. 2). Elongation of plant roots is attributed to cell division and expansion in the root apical meristem [29]. The slight elongation of roots at moderate salt stress (2 and 4 dS m−1) might be because salt stress alter root morphology and plasticity and promote cell division and expansion [29].
Table 1.
Main effects of rootstock (sour orange (SO) and Volkamer lemon (Vol)) and NaCl salinity on plant height, stem diameter, leaf area, shoot and root fresh and dry weight.
| Rootstock | Salinity (dS m−1) | Plant height (cm) | Stem diameter (mm) | Leaf area (cm2· plant−1) | Shoot |
Root |
|||
|---|---|---|---|---|---|---|---|---|---|
| FW (g·plant−1) | DW (g·plant−1) | Length (cm·plant−1) | FW (g·plant−1) | DW (g·plant−1) | |||||
| SO | 18.4 b | 2.3 b | 50.1 a | 4.4 | 1.11 | 23.7 b | 1.5 b | 0.49 b | |
| Vol | 29.8 a | 3.5 a | 44.7 b | 4.7 | 1.40 | 29.3 a | 3.1 a | 0.87 a | |
| 0.7 | 38.0 a | 4.0 a | 70.1 a | 8.1 a | 2.34 a | 30.2 a | 4.6 a | 1.24 a | |
| 2.0 | 26.6 b | 3.7 a | 55.2 b | 6.5 b | 1.70 b | 31.4 a | 2.8 b | 0.86 b | |
| 4.0 | 26.0 bc | 3.6 a | 47.8 bc | 6.1 b | 1.57 b | 31.3 a | 3.0 b | 0.83 b | |
| 8.0 | 22.0 bc | 2.4 b | 35.2 d | 3.5 c | 0.92 c | 23.7 b | 1.7 c | 0.50 c | |
| 12.0 | 19.9 c | 2.2 b | 28.8 e | 2.4 c | 0.69 cd | 21.1 b | 1.2 cd | 0.39 c | |
| 15.0 | 12.3 d | 1.5 c | Na | 0.8 d | 0.30 d | 21.5 b | 0.6 d | 0.25 c | |
| p-value | Rootstock (R) | <0.0001 | <0.0001 | 0.01 | 0.99 | 0.12 | <0.0001 | <0.0001 | <0.0001 |
| Salinity (S) | <0.0001 | <0.0001 | 0.03 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| R × S | 0.06 | 0.36 | 0.22 | 0.83 | 0.89 | 0.04 | 0.01 | 0.02 | |
Values in columns followed by different letters indicate significant differences between treatments at p < 0.05. Data represents mean of 24 seedlings for each rootstock and 8 seedlings for each salinity level.
Fig. 1.
Effect of different salinity levels (0.7 (control, 2.0, 4.0, 8, 12, 15 dS m−1) on shoot and root of (A) Volkamer lemon and (B) sour orange rootstocks. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Root length, fresh and dry weight of sour orange (SO) and Volkamer lemon (Vol) under different salinity levels. Different letters indicate significant differences between treatments at p < 0.05. Data represents mean of 4 seedlings.
A greenhouse study on six-month-old citrus rootstocks (Pomelo, Cleopatra Mandarin, and Calamansi) showed that irrigation with saline water (NaCl, 6, 9, 12 and 16 dS m−1) for 3 months significantly reduced plant growth (height, leaf area, shoot fresh and dry weight), leaf chlorophyll content and increased proline concentration [15]. At 16 dS m−1, Cleopatra Mandarin accumulated less Na+ and Cl−, while Calamansi had the highest leaf Na+ and Cl concentration. Conversely, Cleopatra Mandarin had higher leaf proline content than pomelo and Calamansi [15]. In this study, stem diameter and root length were less affected by salinity compared to the other studied morphological variables. The reduction in stem diameter at the highest salinity level (15.0 dS m−1) was 63% (4.0 vs. 1.5 cm) and was 29% (30.2 vs. 21.5 cm) for root length. In fact, total root length increased by about 4% when salt stress ranged from 2.0 to 4.0 dS m−1 (Table 1). Conversely, leaf area and root fresh weight were the most variables negatively affected by the gradual increment of salt stress (2.0–15 dS m−1). Given that leaf area and root fresh weight were the most sensitive morphological component, we believe that both parameters are suitable indicator traits for salinity stress studies.
Volkamer lemon had higher plant height, stem diameter, and root component (length, fresh and dry weight) than sour orange when grown in saline environment (Table 1). Volkamer root fresh and dry weight were significantly higher than sour orange at control (0.7), 2.0, 4.0 dS m−1 and slightly numerically higher (not significant) at 12.0 and 15.0 dS m−1 (Fig. 1, Fig. 2). Although both species had similar total root length at control (0.7), 2.0 and 4.0 dS m−1, Volkamer lemon had higher values at greater salt stress level, 8.0–15.0 dS m−1 (Fig. 2). However, total leaf area from sour orange was significantly higher than those from Volkamer (Table 1).
The overall ability of citrus rootstocks to tolerate salt stress depends in their capacity to exclude or sequester excess toxic ions [30]. Mahmoud et al. [9] found that the tolerance citrus to salt stress was associated with its ability to restrict absorption and partitioning of major elements such as Na+ and Cl−. Leaf sensitivity to Na+ and Cl− in various species of citrus is a detrimental factor in their tolerance of elevated salinity in both soil and water [31]. Generally, salt tolerant citrus rootstocks have higher osmolytes (proline and glycine betaine) levels, shoot biomass, total chlorophyll content, photosynthesis rate, stomatal conductance (inverse of stomatal resistance), and internal CO2 concentration [30]. In addition, those tolerant rootstocks accumulate lower amounts of Na+ and Cl− as well as higher levels of reactive oxygen species in their shoots compared to the salt-sensitive ones [30]. Bleda et al. [32] suggested that chlorophyll fluorescence can be used as non-destructive variable to assess the physiological responses of citrus to salinity and degrees of salt stress. In fact, chlorophyll fluorescence (Fv/Fm) can provide critical information about the impact of stress on photosystem II [33]. Salinity (50 mmol l−1, NaCl) reduced chlorophyll fluorescence (Fv/Fm) by about 16% (0.82 vs. 0.69) in citrus rootstocks [32]. When ‘Olinda’ Valencia trees budded to three rootstocks (sour orange, C22, and C146) and the un-grafted rootstocks were irrigated for 6 months with saline water (1, 3, 5, and 10 dS m−1), chlorophyll fluorescence (Fv/Fm) and content (SPAD) and stomatal conductance decreased with increased salt level, while electrolyte leakage increased [34]. In addition to chlorophyll fluorescence, SPAD value has been used as a non-destructive technique to estimate chlorophyll content which is essential component of photosynthesis and play key role in plant growth and productivity [35,36]. Xiong et al. [37] found a significant relationship between SPAD value and chlorophyll content in the leaf. In Eugenia uniflora, a high positive exponential relationships (p ≤ 0.01) were fund between SPAD values and Chlorophyll (a+b), Chlorophyll a, and Chlorophyll b contents [35]. In this study, a saline environment reduced leaf RWC, chlorophyll content index (SPAD) and fluorescence (Fv/Fm) and increased stomatal resistance as well as proline content (Table 2). The gradual increase in salt stress (2.0 to 15.0 dS m−1) reduced RWC by 46%, chlorophyll content index by 92%, and chlorophyll fluorescence (Fv/Fm) by 13%. Conversely, stomatal resistance increased by 427% and proline concentration by 100% (Table 2). In terms of rootstock, sour orange had higher chlorophyll content index (SPAD) and RWC than Volkamer lemon across salt levels, 2.0–15 dS m−1 (Table 2). Conversely, Volkamer lemon grown in saline conditions had higher stomatal resistance, proline and chlorophyll fluorescence (Fv/Fm) compared to Volkamer.
Table 2.
Main effects of rootstock (sour orange (SO) and Volkamer lemon (Vol)) and NaCl salinity on leaf relative water content (RWC), chlorophyll content index (SPAD), stomatal resistant, proline content and chlorophyll fluorescence (Fv/Fm).
| Rootstock | Salinity (dS·m−1) | RWC (%) | SPAD | Stomatal resistance (s·cm−1) | Proline (μmole·g−1 FW) | Fv/Fm |
|---|---|---|---|---|---|---|
| SO | 74.6 a | 48.0 a | 8.2 b | 9.20 b | 0.44 b | |
| Vol | 69.6 b | 38.6 b | 12.9 a | 11.0 a | 0.53 a | |
| 0.7 | 90.6 a | 77.5 a | 3.7 d | 6.80 de | 0.53 a | |
| 2.0 | 85.2 ab | 63.9 a | 4.2 d | 7.80 d | 0.51 ab | |
| 4.0 | 78.2 b | 47.2 b | 6.4 d | 10.9 c | 0.49 b | |
| 8.0 | 69.4 c | 30.1 c | 12.4 c | 11.5 b | 0.46 c | |
| 12.0 | 60.0 d | 18.8 cd | 24.8 a | 13.6 a | 0.46 c | |
| 15.0 | 49.3 e | 6.2 d | 19.5 b | na | Na | |
| p-value | Rootstock (R) | 0.004 | 0.002 | <0.0001 | <0.0001 | 0.03 |
| Salinity (S) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.04 | |
| R × S | 0.38 | 0.32 | 0.11 | 0.21 | 0.43 |
Values in columns followed by different letters indicate significant differences between treatments at p < 0.05. Data represents mean of 24 seedlings for each rootstock and 8 seedlings for each salinity level.
Anjum [7] reported that salinity below 2 dS m−1 have no potential impact on citrus seedling growth. However, In this study, irrigation with moderate level of saline water (2.0 dS m−1) reduced plant height (compared to control, 0.7 dS m−1) by 30%, stem diameter by 8%, leaf area by 22%, shoot fresh and dry weight by 20%–27% and root fresh and dry weight by 31%–39%. Given that the reduction in most morphological variables was between 20% and 39% when irrigated with 2.0 dS m−1 water, we believe that the EC of irrigation water for citrus nurseries should clearly be below 2.0 dS m−1 to guarantee acceptable growth rate for young citrus rootstocks.
3.2. Leaf and root nutrient concentration
The response of citrus to salinity can be classified into osmotic and specific ion effects [30]. Osmotic effects (water stress) occur when the concentration of salts increase in the soil solution regardless of the salts that are present. Specific ion effects when specific ions (e.g. Na+ and Cl−) that accumulate in the plant tissues cause specific toxicity symptoms [2]. However, it is difficult to attribute that the growth suppression is mainly due to either osmotic or specific ion effects [2]. Salinity interrupts plant nutrient uptake in two ways; (1) the ionic potential of the substrate can influence nutrient acquisition, and (2) the disruption of plant mineral relations because the interference of major ions (i.e. Na+ and Cl−) in the substrate reduce nutrient availability. This competition mostly alters the nutritional balance; Na+-induced Ca2+ and/or K+ deficiencies and Ca+2-induced Mg+2 deficiencies [14,38,39]. In our study, irrigation with saline water significantly and consistently affected leaf and root nutrient concentration (Table 3, Table 4). The gradual increment of salt stress reduced N, K+, Ca+2, P and Mg+2 and increased Na+ and Cl− concentration in both leaf (Table 3) and root tissues (Table 4). Compared to control (0.7 dS m−1), the reduction in leaf N across the salt levels (2.0–15.0 dS m−1) ranged from 10% to 38%, K from 8% to 47%, Ca+2 from 13% to 51%, P from 6% to 31% and Mg+2 from 26% to 44%. Leaf K content in non-stressed citrus was 67% higher (2.60% vs. 1.55% of the dry weight) than rootstocks grown in saline substrate (50-mmol·L−1, NaCl) [32]. Nutrient reduction (N, P, K+, Ca+2 and Mg+2) could be attributed to higher leaf Na+ and Cl− under salt stress (Table 3). Leaf Na+ at 15.0 dS m−1 treatment increased by 1001% and Cl− by 871% compared to control (0.7 dS m−1) (Table 3). Our results show that Na+ and Cl− accumulate excessively in the tissues of these two rootstocks when there are abundant in the irrigation solution. Excess Na + accumulation in Citrus tissues caused chlorosis and necrosis along the tips and margins of older leaves and Ca+2 and Mg+2 imbalance [2].
Table 3.
Main effects of rootstock (sour orange (SO) and Volkamer lemon (Vol)) and NaCl salinity on leaf nutrient concentration.
| Rootstock | Salinity (dS·m−1) | (N) (%) | Na+ |
Cl− |
K+ |
Ca+2 |
P |
Mg+2 |
|---|---|---|---|---|---|---|---|---|
| (mg·L−1) | ||||||||
| SO | 2.1 b | 16285 b | 24906 a | 20653 b | 10849 a | 4439 b | 5060 a | |
| Vol | 2.6 a | 17183 a | 22470 b | 22779 a | 9820 b | 5124 a | 4958 b | |
| 0.7 | 2.9 a | 2522 f | 3893 f | 27980 a | 14127 a | 5661 a | 7120 a | |
| 2.0 | 2.6 b | 8830 e | 13083 e | 25835 b | 12245 b | 5316 b | 5311 b | |
| 4.0 | 2.5 c | 16135 d | 23314 d | 23845 c | 11066 c | 4858 c | 4746 c | |
| 8.0 | 2.2 d | 20037 c | 29231 c | 20335 d | 9517 d | 4626 d | 4602 d | |
| 12.0 | 2.0 e | 25106 b | 34797 b | 17463 e | 8132 e | 4333 e | 4301 e | |
| 15.0 | 1.8 f | 27774 a | 37809 a | 14839 f | 6920 f | 3895 f | 3974 f | |
| p-value | Rootstock (R) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Salinity (S) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| R × S | <0.0001 | 0.03 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Values in columns followed by different letters indicate significant differences between treatments at p < 0.05. Data represents mean of 24 seedlings for each rootstock and 8 seedlings for each salinity level.
Table 4.
Main effects of rootstock (sour orange (SO) and Volkamer lemon (Vol)) and NaCl salinity on root nutrient concentration.
| Rootstock | Salinity (dS·m−1) | (N) (%) | Na+ |
Cl− |
K+ |
Ca+2 |
P |
Mg+2 |
|---|---|---|---|---|---|---|---|---|
| (mg·L−1) | ||||||||
| SO | 1.3 b | 16729 b | 25502 b | 24048 | 22864 a | 5588 b | 6572 b | |
| Vol | 1.7 a | 19886 a | 30618 a | 23333 | 15652 b | 6119 a | 7416 a | |
| 0.7 | 2.0 a | 7239 e | 11173 e | 29958 a | 19736 a | 7170 a | 9392 a | |
| 2.0 | 1.7 b | 12783 d | 19835 d | 26378 b | 18455 b | 6688 b | 8786 b | |
| 4.0 | 1.6 c | 18428 c | 27758 c | 24575 c | 16956 c | 6224 c | 7607 c | |
| 8.0 | 1.3 d | 22915 b | 35422 b | 23113 d | 15763 d | 5751 d | 6566 d | |
| 12.0 | 1.2 e | 25978 a | 39556 a | 20439 e | 14472 e | 4839 e | 4881 e | |
| 15.0 | 1.0 f | 25968 a | 40084 a | 17680 f | 13166 f | 4449 f | 4730 f | |
| p-value | Rootstock (R) | <0.0001 | <0.0001 | <0.0001 | 0.15 | 0.02 | <0.0001 | 0.005 |
| Salinity (S) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| R × S | 0.174 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Values in columns followed by different letters indicate significant differences between treatments at p < 0.05.Data represents mean of 24 seedlings for each rootstock and 8 seedlings for each salinity level.
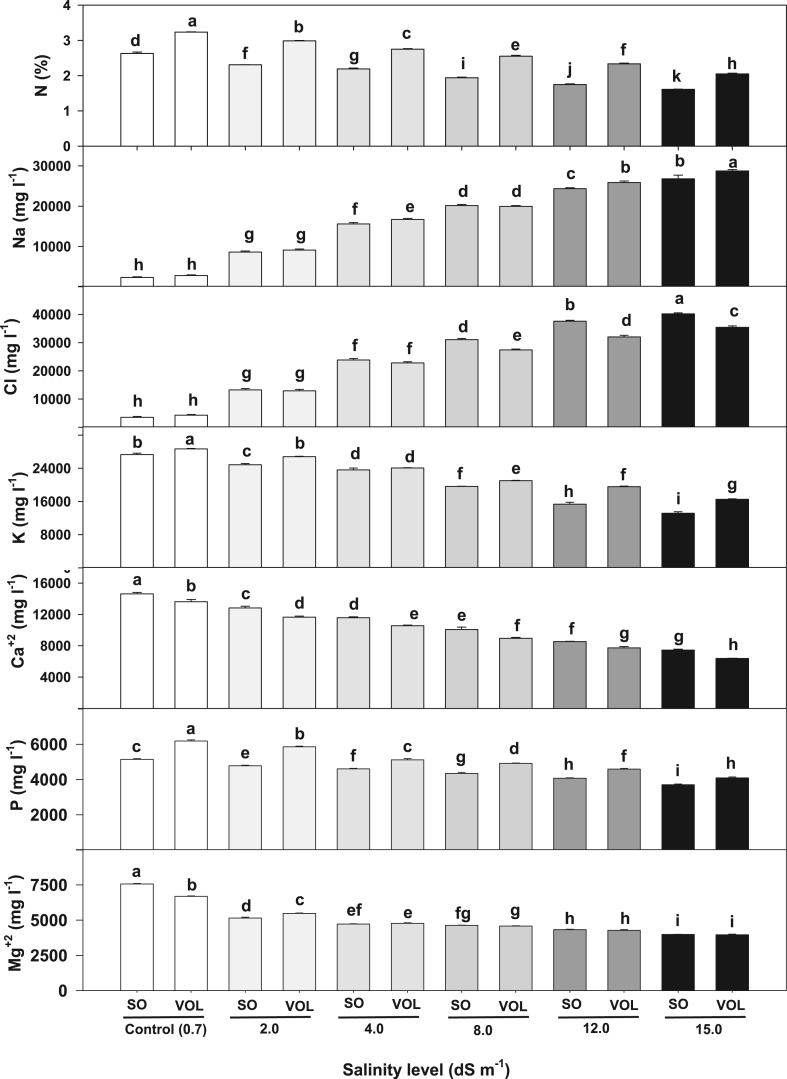
Rootstock leaf nutrients concentrations differed significantly in response to salt stress treatment (Table 3). Across salinity level, sour orange had higher leaf concentration of Cl−, Ca+2 and Mg+2 and lower N, Na+, K+, and P compared to Volkamer lemon (Table 3). There was a significant species and salinity interaction for root nutrient analysis (Fig. 3). The interaction was caused more by the differential ion accumulation (specifically, Na+ and Cl−) at the higher levels of salinity. The partitioning of that interaction indicated that control treatment had higher N, K+, Ca+2, P and Mg+2 and lower Na+ and Cl− in both species and across the salinity levels. Sour orange had higher Ca+2 and Cl (8.0, 12.0, 15.0 dS m−1) concentration over each salinity level when compared to Volkamer. Conversely, Volkamer N, Na+, K+ and P leaf concentration at each salinity level were higher than in sour orange (Fig. 3).
Fig. 3.
Leaf nutrient concentration of sour orange (SO) and Volkamer lemon (Vol) under different salinity levels. Data represents mean of 4 seedlings.
Fernandez and Ballester et al. [40] assessed the contribution of inorganic and organic solutes in osmotic adjustment of sour orange and alemow (Citrus macrophylla Wester). Both sour orange and alemow were exposed to four solutions of different ionic composition (40 mM Na+ without Cl−, 40 mM Cl− without Na+, 40 mM NaCl, and 3.5 times the concentrated macronutrients of the base solution). They found that inorganic solutes accounted for 65 to 85% of total solutes associated with the osmotic adjustment. Organic solutes contribution ranged from 15 to 35%, depending on the genotype and the isosmotic treatment. Carbohydrates (especially, glucose) were the major organic components (6–24%). Hence, inorganic solute level in shoot tissues is a key factor to screen citrus rootstock for salinity tolerance. The tolerance level of citrus trees to salt stress is associated with its capacity to restrict the uptake and transport of excess ions including Na+, Cl− and boron into roots and shoots [1]. In addition, the tolerance mechanisms in citrus rootstocks normally include the ability to limit the accumulation of Cl− in leaves [41,42]. This exclusion process in citrus leaves was associated with transport processes in the root [41,43,44]. In this study, the accumulation of nutrients in leaves of sour orange and Volkamer lemon in response to salt stress was inconsistent. While sour orange had higher leaf Cl−, Ca+2 and Mg+2, Volkamer lemon had higher N+, Na, K+, and P (Table 3).
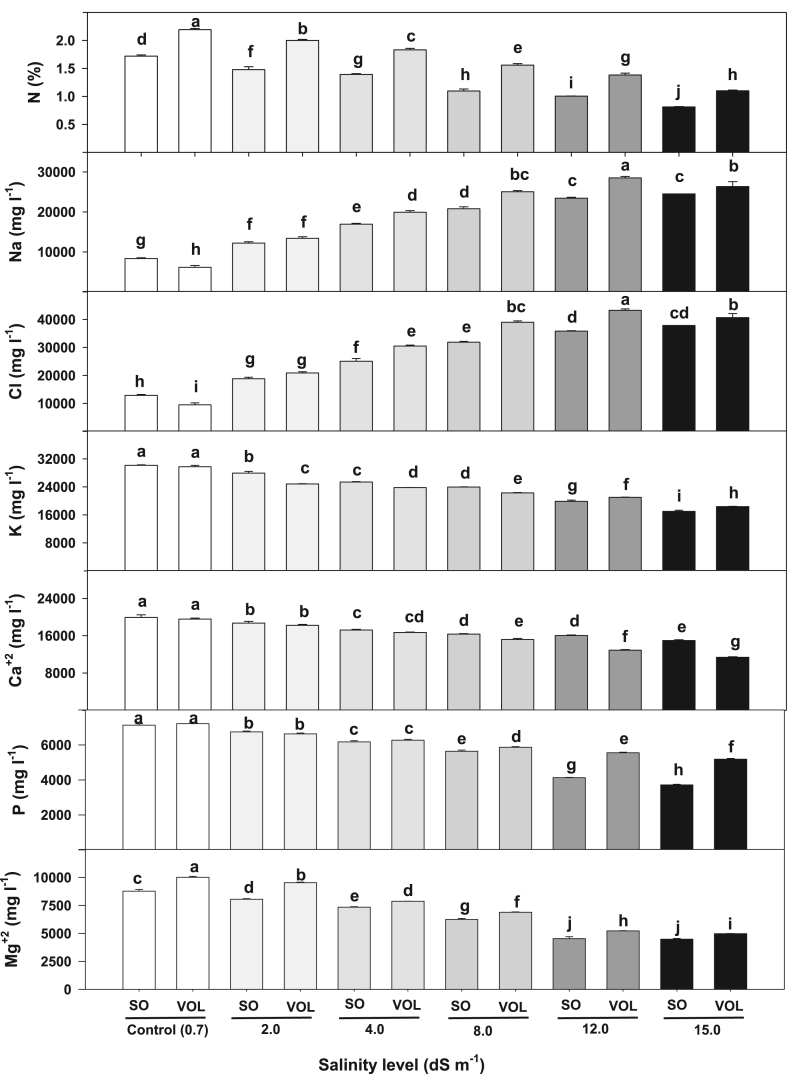
For roots, N concentration decreased by 15%–50%, K+ by 12%–41%, Ca+2 by 7%–33%, P by 7%–38% and Mg+2 by 7%–50% when salt stress in the irrigation water increased from 2.0 to 15.0 dS m−1 (Table 4). Concurrently, Na+ concentration in the roots increased by 77% (12783 mg L−1) to 259% (25968 mg L−1) and Cl− increased by 78% (19835 mg L−1) to 259% (40084 mg L−1) when compared to control (Na+, 7239 mg L−1; Cl−, 11173 mg L−1). Except for Ca+2, Volkamer lemon had higher nutrient concentration (N, Na+, Cl−, Ca+2, P and Mg+2) than sour orange under salinity stress (Table 4). Finding a rootstock that can limit excessive salt accumulation, especially Na+ and Cl−, is critical for selecting salt tolerance citrus. In a study conducted by Khankahdani et al. [45], Volkamer lemon was judged to be the best rootstock for ‘IAC’, ‘Tahiti’, ‘Deperse’ and ‘Persian’ lime scions. When these scions were grafted on Volkamer rootstock, they had higher concentration of Fe, Zn, Mn and lower concentration of Na+ than on ‘Bakraei’, ‘Mexican lime’ and ‘Sour orange’. In this study, the percentage increase of Na and Cl in the shoots was higher than in the roots (Table 4). For example, at 2.0 dS m−1, the percentage increase (compared to control) in Na+ and Cl− in the leaf was 250% and 236% while Na+ and Cl− in the root increased by 77% and 78%, respectively. The species and salt stress treatment interactions revealed that, root nutrient (N, Na+, Cl−, P and Mg+2) from Volkamer had consistently higher concentration compared to sour orange at 4.0, 8.0, 12.0 and 15.0 dS m−1 (Fig. 4).
Fig. 4.
Root nutrient concentration of sour orange (SO) and Volkamer lemon (Vol) under different salinity levels. Data represents mean of 4 seedlings.
4. Conclusions
We studied the influence of different salt stress (control 0.7, 2.0, 4.0, 8.0, 12.0 and 15 dS m−1) on sour orange and Volkamer lemon rootstocks. The gradual increased in salinity level from 2.0 to 15 dS m−1 significantly reduced leaf physiological component (leaf chlorophyll content fluorescence (Fv/Fm) and RWC). As a result, shoot (plant height, stem diameter, leaf area) and root (fresh and dry weight) significantly decreased. Interestingly, Volkamer accumulated more proline in its tissues and had higher seedling height, stem diameter, and root component (length, fresh and dry weight) than sour orange. While sour orange had higher leaf Cl−, Ca+2 and Mg+2, Volkamer lemon had higher N, Na+, K+, and P. However, root nutrient (N, Na+, Cl−, P and Mg+2) from Volkamer had consistently higher concentration compared to sour orange at 4.0, 8.0, 12.0 and 16.0 dS m−1. Therefore, we believe that Volkamer is more tolerant to salt stress than sour orange. Because the reduction in most morphological variables (plant height, leaf area shoot fresh and dry weight and root fresh and dry weight) was between 20% and 39% when irrigated with 2.0 dS m−1, we conclude that both citrus rootstocks are sensitive to even a moderate salinity level (2.0 dS m−1).
Author contribution statement
Yahia Othman, Ph.D.: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Muayyad Bany Hani, Dr.: Performed the experiments; Analyzed and interpreted the data.
Jamal Y. Ayad, Prof.: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Rolston St. Hilaire, Prof.: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data associated with this study has been deposited at Zenodo.org: https://doi.org/10.5281/zenodo.7515348
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Srivastava A.K., Singh S. Citrus decline: soil fertility and plant nutrition. J. Plant Nutr. 2009;32(2):197–245. doi: 10.1080/01904160802592706. [DOI] [Google Scholar]
- 2.Grattan S.R., Diaz F.J., Pedrero F., Vivaldi G.A. Assessing the suitability of saline wastewaters for irrigation of Citrus spp.: emphasis on boron and specific-ion interactions. Agric. Water Manag. 2015;157:48–58. doi: 10.1016/j.agwat.2015.01.002. [DOI] [Google Scholar]
- 3.Ashraf M., Shahzad S., Imtiaz M., Rizwan M. Salinity effects on nitrogen metabolism in plants – focusing on the activities of nitrogen metabolizing enzymes: a review. J. Plant Nutr. 2018;41(8):1065–1081. doi: 10.1080/01904167.2018.1431670. [DOI] [Google Scholar]
- 4.Acosta-Motos J.R., Ortuño M.F., Bernal-Vicente A., Diaz-Vivancos P., Sanchez-Blanco M.J., Hernandez J.A. Plant responses to salt stress: adaptive mechanisms. Agron. 2017;7:18. doi: 10.3390/agronomy7010018. [DOI] [Google Scholar]
- 5.Maas E.V. Salinity and citriculture. Tree Physiol. 1993;12:195–216. doi: 10.1093/treephys/12.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Ziogas V., Tanou G., Morianou G., Kourgialas N. Drought and salinity in citriculture: optimal practices to alleviate salinity and water stress. Agron. 2021;11:1283. doi: 10.3390/agronomy11071283. [DOI] [Google Scholar]
- 7.Anjum M.A. Effect of NaCl concentrations in irrigation water on growth and polyamine metabolism in two citrus rootstocks with different levels of salinity tolerance. Acta Physiol. Plant. 2008;30:43–52. [Google Scholar]
- 8.Ruiz D., Martínez V., Cerdá A. Citrus response to salinity: growth and nutrient uptake. Tree Physiol. 1997;17:141–150. doi: 10.1093/treephys/17.3.141. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud L.M., Dutt M., Vincent C.I., Grosser J.W. Salinity-induced physiological responses of three putative salt tolerant citrus rootstocks. Horticulture. 2020;6:90. doi: 10.3390/horticulturae6040090. [DOI] [Google Scholar]
- 10.Simpson C.R., Nelson S.D., Melgar J.C., Jifon J., King S.R., Schuster G., Volder A. Growth response of grafted and ungrafted citrus trees to saline irrigation. Sci. Hortic. 2014;169:199–205. [Google Scholar]
- 11.Cámara J.M., García-Sánchez F., Nieve s M., Cerdá A. Effect of interstock (‘Salustiano’ orange) on growth, leaf mineral composition and water relations of one year old citrus under saline conditions. J. Hortic. Sci. Biotechnol. 2003;78(2):161–167. doi: 10.1080/14620316.2003.11511600. [DOI] [Google Scholar]
- 12.Wu Q.S., Zou Y.N. Mycorrhizal symbiosis alters root H+ effluxes and root system architecture of trifoliate orange seedlings under salt stress. J. Anim. Plant Sci. 2013;23:143–148. [Google Scholar]
- 13.Chatzissavvidis C., Papadakis I., Therios I. Effect of calcium on the ion status and growth performance of a citrus rootstock grown under NaCl stress. Soil Sci. Plant Nutr. 2008;54:910–915. [Google Scholar]
- 14.Grattan S.R., Grieve C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992;38:275–300. [Google Scholar]
- 15.Alam A., Ullah H., Attia A., Datta A. Effects of salinity stress on growth, mineral nutrient accumulation and biochemical parameters of seedlings of three citrus rootstocks. Int. J. Fruit Sci. 2020;20:786–804. [Google Scholar]
- 16.Forner-Giner M.A., Legaz F., Primo-Millo E., Forner J. Nutritional responses of citrus rootstocks to salinity: performance of new hybrids Forner-Alcaide 5 and Forner-Alcaide 13. J. Plant Nutr. 2011;34:1437–1452. [Google Scholar]
- 17.Turner N. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018;69:3223–3233. doi: 10.1093/jxb/ery181. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Pérez J.G., Robles J.M., Tovar J.C., Botía P. Response to drought and salt stress of lemon ‘Fino 49’ under field conditions: water relations, osmotic adjustment and gas exchange. Sci. Hortic. 2009;122(1):83–90. [Google Scholar]
- 19.Al-Kharabsheh A. Challenges to sustainable water management in Jordan. Jordan J. Earth Environ. Sci. 2020;11:38–48. [Google Scholar]
- 20.Hussain M., Muscolo A., Farooq M., Ahmad W. Sustainable use and management of non-conventional water resources for rehabilitation of marginal lands in arid and semiarid environments. Agric. Water Manag. 2019;221:462–476. [Google Scholar]
- 21.Mohsen M.S. Water strategies and potential of desalination in Jordan. Desalination. 2007;203:27–46. [Google Scholar]
- 22.Al-Satari Y., Al-Ramamneh E., Ayad J., Dalbouh M., Amayreh I., Khreisat Z. Impact of seedling age on the survival and productivity of Atriplex halimus shrubs in drought-affected rangelands of Jordan. Rangel. J. 2018;40(3):287–296. doi: 10.1071/RJ17102. . [DOI] [Google Scholar]
- 23.Othman Y., Steele C., VanLeeuwen D., Heerema R., Bawazir S., StHilaire R. Remote sensing used to detect moisture status of pecan orchards grown in a desert environment. Int. J. Rem. Sens. 2014;35(3):949–966. [Google Scholar]
- 24.Bates L.S., Waldren R.P., Tears I.D. Rapid determination of free proline in water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- 25.Mehlich A. New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese, and zinc. Commun. Soil Sci. Plant Anal. 1978;9(6):477–492. doi: 10.1080/00103627809366824. [DOI] [Google Scholar]
- 26.Al-Ajlouni M., Ayad J., Othman Y. Increasing nutrient levels promote growth and flower quality in lilies grown under soilless culture. Hortic. Sci. (HORTSCI) 2017;44(4):171–177. [Google Scholar]
- 27.Syvertsen J., Garcia-Sanchez F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014;103:128–137. [Google Scholar]
- 28.Zekri M., Parsons L.R. Calcium influences growth and leaf mineral concentration of citrus under saline conditions. Hortic. Sci. (HORTSCI) 1990;25:784–786. [Google Scholar]
- 29.Arif M., Islam M., Robin A. Salinity stress alters root morphology and root hair traits in Brassica napus. Plants. 2019;8:192. doi: 10.3390/plants8070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahid M.A., Balal R.M., Khan N., Simón-Grao S., Alfosea-Simón M., Cámara-Zapata J.M., Mattson N.S., Garcia-Sanchez F. Rootstocks influence the salt tolerance of Kinnow Mandarin trees by altering the antioxidant defense system, osmolyte concentration, and toxic ion accumulation. Sci. Hortic. 2019;250:1–11. [Google Scholar]
- 31.Ferguson L., Grattan S.R. How salinity damages citrus: osmotic effects and specific ion toxicities. HortTechnology. 2005;15:95–99. [Google Scholar]
- 32.Bleda J.F., Madrid R., Garcia-Torres A.L., Garcia-Lidon A., Porras I. Chlorophyll fluorescence and mineral nutrition in citrus leaves under salinity stress. J. Plant Nutr. 2011;34:1579–1592. [Google Scholar]
- 33.Smethurst C.F., Shabala S. Screening methods for waterlogging tolerance in lucerne: comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 2003;30:335–343. doi: 10.1071/FP02192. [DOI] [PubMed] [Google Scholar]
- 34.Simpson C.R., Nelson S.D., Melgar J.C., Jifon J., Schuster G., Volder A. Effects of salinity on physiological parameters of grafted and ungrafted citrus trees. Sci. Hortic. 2015;197:483–489. [Google Scholar]
- 35.Mielke M., Schaffer B., Li C. Use of a SPAD meter to estimate chlorophyll content in Eugenia uniflora L. leaves as affected by contrasting light environments and soil flooding. Photosynthetica. 2010;48:332–338. doi: 10.1007/s11099-010-0043-2. [DOI] [Google Scholar]
- 36.Khasawneh A., Alsmairat N., Othman Y., Ayad J., Al-Hajaj H., Qrunfleh I. Controlled-release nitrogen fertilizers for improving yield and fruit quality of young apricot trees. Sci. Hortic. 2022;303 doi: 10.1016/j.scienta.2022.111233. [DOI] [Google Scholar]
- 37.Xiong D., Chen J., Yu T., Gao W., Ling X., Li Y., Peng S., Huang J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015;5 doi: 10.1038/srep13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fageria V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001;24:1269–1290. [Google Scholar]
- 39.Waraich E.A., Ahmad R., Ashraf M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop. Sci. 2011;5:764–777. [Google Scholar]
- 40.Fernandez‐Ballester G., Martinez V., Ruiz D., Cerdá A. Changes in inorganic and organic solutes in citrus growing under saline stresses. J. Plant Nutr. 1998;21:2497–2514. doi: 10.1080/01904169809365582. [DOI] [Google Scholar]
- 41.Storey R., Walker R. Citrus and salinity. Sci. Hortic. 1999;78:39–81. [Google Scholar]
- 42.Hussain S., Luro F., Costantino G., Ollitrault P., Morillon R. Physiological analysis of salt stress behaviour of citrus species and genera: low chloride accumulation as an indicator of salt tolerance. South Afr. J. Bot. 2012;81:103–112. [Google Scholar]
- 43.Teakle N.L., Tyerman S.D. Mechanisms of Cl‐transport contributing to salt tolerance. Plant Cell Environ. 2010;33:566–589. doi: 10.1111/j.1365-3040.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 44.Henderson S.W., Baumann U., Blackmore D.H., Walker A.R., Walker R.R., Gilliham M. Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biol. 2014;14:1–18. doi: 10.1186/s12870-014-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khankahdani H., Rastegar S., Golein B., Golmohammadi M., Jahromi A. Effect of rootstock on vegetative growth and mineral elements in scion of different Persian lime (Citrus latifolia Tanaka) genotypes. Sci. Hortic. 2019;246:136–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at Zenodo.org: https://doi.org/10.5281/zenodo.7515348