Abstract
Endospores of Bacillus spp., especially Bacillus subtilis, have served as experimental models for exploring the molecular mechanisms underlying the incredible longevity of spores and their resistance to environmental insults. In this review we summarize the molecular laboratory model of spore resistance mechanisms and attempt to use the model as a basis for exploration of the resistance of spores to environmental extremes both on Earth and during postulated interplanetary transfer through space as a result of natural impact processes.
In the latter half of the 19th century Tyndall, Cohn, and Koch (25, 84, 211a) independently discovered that certain species of bacteria spend at least part of their lives as dormant cellular structures now known as endospores (hereafter called simply spores for convenience). Spores have since been recognized as the hardiest known form of life on Earth, and considerable effort has been invested in understanding the molecular mechanisms responsible for the almost unbelievable resistance of spores to environments which exist at (and beyond) the physical extremes which can support terrestrial life. Examples of sporeforming bacteria are rather widespread within the low-G+C subdivision of the gram-positive bacteria and represent inhabitants of diverse habitats, such as aerobic heterotrophs (Bacillus and Sporosarcina spp.), halophiles (Sporosarcina halophila and the gram-negative Sporohalobacter spp.), microaerophilic lactate fermenters (Sporolactobacillus spp.), anaerobes (Clostridium and Anaerobacter spp.), sufate reducers (Desulfotomaculum spp.), and even phototrophs (Heliobacterium and Heliophilum spp.).
Despite the diversity exhibited by sporeforming bacterial species, the sporeformers about which we have gleaned the most detailed molecular information are common rod-shaped soil inhabitants belonging to the genera Bacillus and Clostridium, and among this restricted subset, most work has concentrated upon the descendants of a single strain of Bacillus subtilis called strain 168. Consequently, most of this review will concentrate upon spore resistance in B. subtilis 168 and its close relatives, from which we have gained several valuable (and hopefully universal) insights into spore resistance mechanisms. However, we can easily imagine that the spore resistance mechanisms uncovered through study of B. subtilis and closely related species may not be entirely applicable to sporeformers as phylogenetically and ecologically diverse as the gram-negative homoacetogen Sporomusa or to bacteria which do not form true endospores but form aerial spore-bearing mycelia (such as Streptomyces spp.) or fruiting structures (such as Myxobacter and Myxococcus spp.). This caveat has been most eloquently expressed by Slepecky and Leadbetter (200).
According to our current understanding, the developmental pathway leading from a vegetatively growing bacterial cell to a spore is triggered by depletion from the bacterium's local environment of a readily metabolized form of carbon, nitrogen, or phosphate. (For recent reviews of the molecular details of this surprisingly complex and fascinating differentiation process, see references 38, 46, 57, 65, 150, 190, 203, and 206.) In the dormant state, spores undergo no detectable metabolism and exhibit a higher degree of resistance to inactivation by various physical insults, including (but not limited to) wet and dry heat, UV and gamma radiation, extreme desiccation (including vacuum), and oxidizing agents. Despite their metabolic inactivity, however, spores are still capable of continually monitoring the nutritional status of their surroundings, and they respond rapidly to the presence of appropriate nutrients by germinating and resuming vegetative growth. Spore formation thus represents a strategy by which the bacterial cell escapes temporally from nutritionally unfavorable local conditions via dormancy. In addition to temporal escape, spores can also be relocated spatially via wind, water, living hosts, etc., to environments potentially favorable for germination and resumption of vegetative growth. As a result, bacterial spores can be found in environmental samples obtained from all parts of the Earth, both above and below the surface, and as such represent a highly successful strategy for the survival and widespread dispersal of microbial life.
Dormant spores exhibit incredible longevity and can be found in virtually every type of environment on Earth, even in geographical locations obviously removed spatially from their point of origin (for example, spores of strictly thermophilic Bacillus spp. can be isolated from cold lake sediments) (155, 156). Reliable reports exist of the recovery and revival of spores from environmental samples as old as 105 years (54, 81, 154), and there recently appeared a somewhat more controversial report that viable Bacillus sphaericus spores were recovered from the gut of a bee fossilized in Dominican amber for an estimated 25 to 40 million years (20)!
It becomes apparent from studying the process of spore formation, the ubiquitous global distribution of spores, and the environmental record of spore longevity that a sporulating bacterium cannot predict beforehand how long or in what environment it will spend its dormant state. Therefore the sporulating cell must “prepare for the worst” each time it undergoes differentiation. How does the spore achieve such hardiness? The molecular mechanisms underlying spore resistance properties were until recently relatively refractory to experimental dissection. However, as the result of decades of elegant genetic, molecular biological, and biochemical studies, molecular models have emerged which describe how spores of bacteria such as B. subtilis resist exposure to germicidal agents such as heat, UV, and oxidative damage in the laboratory (reviewed in references 52 and 190). From these laboratory studies it is clear that spore resistance mechanisms during dormancy rely on diverse physiological events which occur during all stages of the life cycle: growth, sporulation, and germination. However, in spite of the vast amount of data that has been collected in the laboratory, we have very little idea of the possible degree to which our experimental models accurately reflect the life history of sporeforming bacteria in their native habitats. In order to maintain the potential for viability, the dormant spore must either (i) prevent damage which would inactivate critical cellular components needed for successful germination and resumption of growth or (ii) repair or replace those damaged critical components during germination, before their inactivation results in cell death. In the terrestrial (soil) environment where many sporeforming bacteria are found, such potentially lethal extreme conditions can include cycles of heat and cold, including freezing-thawing, physical abrasion, extreme dessiccation, exposure to corrosive chemicals, attack by other organisms and their extracellular degradative enzymes, and prolonged exposure to solar radiation (133).
Because of their notorious resistance and longevity, bacterial spores have also been studied as possible candidates for transfer of life between the planets as a result of impact processes (66, 107). The natural processes by which spores could be transported through interplanetary space would expose them to an entirely new set of environmental stresses, including extreme shock, acceleration, vacuum, and bombardment by UV and ionizing radiation as well as heavy high-energy atomic (HZE) particles (109). In this review, we will attempt for the first time to summarize recent advances in our understanding of how bacterial spores resist inactivation by stresses imposed by both terrestrial and extraterrestrial environments and to explore how the current laboratory models of spore resistance can serve as important intellectual scaffolds from which we can construct new environmental spore resistance models.
SPORE RESISTANCE IN THE LABORATORY
Studies of the resistance of Bacillus and Clostridium spores to a variety of treatments in the laboratory have identified a number of factors important in determining the level of spore resistance. These factors include the genetic makeup of the sporulating species, the precise sporulation conditions, particularly the temperature, the spore coats, the relative impermeability of the spore core, the low water content of the hydrated spore's core, the high level of minerals in the spore core, the saturation of spore DNA with α/β-type small, acid-soluble proteins (SASP), and repair of damage to macromolecules during spore germination and outgrowth. While all of these factors are important in at least one or more spore resistance property, their relative importance varies considerably both for the same resistance property in spores of different species and for different resistance properties within the same species (Table 1).
TABLE 1.
Role of various factors in resistance of spores of Bacillus species to different treatmentsa
| Treatment | Effect on resistance
|
|||||
|---|---|---|---|---|---|---|
| α/β-Type SASP | Core water | Core minerals | Repair | Spore coats | Sporulation conditions | |
| Desiccation | ++ | − | ? | +/−b | ? | ? |
| Dry heat | ++ | − | +/− | + | ? | ? |
| Wet heat | + | ++ | + | − | +/− | + |
| γ-Radiation | − | (++)c | ? | ++ | ? | ? |
| UV radiation | ++ | −d | ? | ++ | −e | − |
| Alkylating agentsf | ++ | ? | ? | ++ | − | ? |
| Formaldehydef | ++ | ? | ? | ++ | − | ? |
| Glutaraldehydef | − | ? | ? | − | ++ | +/− |
| Iodinef | − | ? | ? | − | ++ | +/− |
| Peroxidesfg | ++ | ++ | ? | − | + | ? |
Assignment of the importance of resistance factors is largely based on work with B. subtilis, but there are also data on the importance of core water and minerals for spores of other species. Symbols: ++, major importance; +, some importance but not a major factor; +/−, minor importance but has some effect; −, no effect; ?, no data available. See references 10, 12, 15, 26, 38, 39, 42, 45, 52, 56, 93, 98, 99, 102, 113, 124, 125, 133, 135, 153, 165, 168, 169, 175, 176, 179, 180, 181, 182, 183, 185, 190, 191, 209a, and 230.
DNA repair is likely important in resistance to killing at very high vacuum.
This is likely important in γ-radiation resistance, but this has not been shown by experiment.
A low core water content may be important in ensuring that DNA is saturated with α/β-type SASP.
Spore coats may play a minor role in resistance to UV-B and UV-A wavelengths (165).
The relative impermeability of the spore core undoubtedly plays a role in resistance to all chemicals.
Enzymes that detoxify peroxides are not important in spore peroxide resistance (21).
Parameters Contributing to Spore Resistance
Genetic makeup of the sporulating species.
While mutations in a number of individual genes alter specific factors involved in spore resistance (181, 190, 191), it is also clear that in wild-type organisms the overall genomic information is extremely important in determining levels of spore resistance (52). This is seen most notably with wet heat resistance, as spores of thermophiles are more resistant than spores of mesophiles, which in turn are more resistant than spores of psychrophiles (52). At least a part of the increased wet-heat resistance of spores of thermophiles is due to their decreased core water content (see below). However, this does not explain fully these spores' increased wet-heat resistance, which may be due simply to the generally increased thermostability of the proteins of thermophiles.
Sporulation conditions.
Many studies have shown that within a single species, modulation of the sporulation conditions has a significant effect on spore resistance (26, 52, 169). Parameters that have been varied include metal ion concentrations and temperature, and most often only spore wet-heat resistance has been analyzed. In general, the interpretation of changes in spore resistance with variation of the mineral ion content of sporulation media has been difficult on a mechanistic basis. However, sporulation at an elevated temperature invariably results in spores with increased heat resistance. This effect is mediated at least in part by a decrease in core water content in spores prepared at higher temperature. However, the mechanism(s) controlling spore core water content is not known.
Spore coats.
The spore coats appear to play some role in spore resistance, especially in preventing the access of peptidoglycan-lytic enzymes to the spore cortex, and also likely plays a role in spore resistance to some chemicals, such as hydrogen peroxide (38, 102, 165, 169, 191). A B. subtilis mutant completely lacking the spore coat layers has been constructed (38). Spores of this mutant are highly sensitive to lysozyme and 5% H2O2 but exhibit normal resistance to wet heat and 254-nm UV light (165), indicating that resistance to wet heat and laboratory-generated 254-nm UV-C are probably not functions of the spore coat. However, the precise role(s) of individual coat proteins in these resistance properties is not clear.
Core permeability.
The spore core exhibits relatively low permeability to hydrophilic molecules greater than approximately 200 Da (53). Since the spore has two membranes, either or both could be the permeability barrier restricting entry into the spore core. Although it is not clear if the outer membrane is an intact membrane in the mature dormant spore, some data are consistent with its being a functional membrane. The inner membrane is clearly an intact membrane, and while the reason(s) for its decreased permeability is not clear, it is significantly compressed in the dormant spore (205).
Core water content.
While the water content of the cortex, coat, and exosporium regions of a spore suspended in water is similar to that in growing cells (75 to 80% of wet weight), the water content of the spore core is much lower (28 to 50% of wet weight) (52). While the precise mechanism by which the core's water content is reduced during sporulation is not clear, this event does involve the function of the spore cortex (4, 39, 52, 151, 152, 191). There is abundant evidence that core water content is inversely related to spore wet-heat resistance (52).
Spore mineral content.
Spores have very high levels of divalent ions, in particular Ca2+, with the great majority of these cations being present in the spore core (128, 189). Both the amounts and identities of the major cations in spores can be varied, either by alterations in the metal ion content of sporulation medium (199) or by removal of spore metal ions by titration to low pH and then back-titration to pH 7 with appropriate metal ion hydroxide (12). Using these procedures, spores with extremely low divalent cation levels can be generated (H+, Na+, or K+ spores), as can spores with high levels of any of a variety of divalent cations (Ca2+, Mg2+, and Mn2+). Upon analyses of spore wet-heat resistance, Ca2+ spores are the most resistant, with resistance similar to that of native spores, while H+ spores are the least resistant; in general, divalent cation-loaded spores are more resistant than monovalent cation-loaded spores (12, 52, 98, 99). While studies are not as extensive, increased spore core mineralization is also associated with increased resistance to oxidizing agents (98) and, at least in spores of Bacillus stearothermophilus, with increased resistance to dry heat (1). Not all the reasons for the effects of spore core mineral content on spore resistance are known. However, increased core mineralization is often associated with decreased core water content, and this may contribute to increased spore resistance to wet heat.
In contrast to spore core minerals, which play a clear role in spore resistance, the role of dipicolinic acid (DPA), with which much of the spore's divalent cations are likely chelated, in spore resistance is less clear. Studies in B. subtilis have shown that spores lacking DPA due to a specific mutation in the spoVFA or spoVFB locus (also called dpaA and dpaB), which encode the two subunits of DPA synthetase (33), have significantly increased spore core water and decreased heat and H2O2 resistance (7; M. Paidhungat, B. Setlow, and P. Setlow, unpublished).
However, these DPA-less spores exhibit no decrease in UV resistance and are actually more UV resistant than spores of their wild-type parents (Paidhungat et al., unpublished). This finding is not unexpected, as DPA has been shown to be a photosensitizer in spores (190, 191). Spores of Bacillus cereus that lack DPA have also been isolated, although the specific mutation causing loss of DPA has not been identified; as expected, these DPA-less spores are heat sensitive (60, 231). However, secondary mutations which restored much of the spore's heat resistance but which did not restore DPA production were identified in the DPA-less strain (60), suggesting that DPA is not essential for full spore heat resistance. Unfortunately, the elevated heat resistance phenotype of these DPA-less B. cereus spores was extremely unstable, and these strains have never been studied further. A B. subtilis mutant that produces DPA-less spores that retain heat resistance has also been isolated (231). However, the identity of the mutation(s) generating spores with the DPA-less yet heat-resistant phenotype has not been determined, and again these mutant spores have not been studied further.
α/β-Type SASP.
Spore DNA is saturated with a group of unique proteins called α/β-type SASP (184, 186, 189–191). These proteins are synthesized only during sporulation in the developing spore and are degraded beginning early in spore germination. These proteins bind to DNA largely on the outside of the DNA helix and straighten and stiffen the DNA while changing the DNA to an A-like helix. DNA properties in vitro are also dramatically changed when DNA is bound by α/β-type SASP, and the DNA's reactivity with a variety of chemicals decreases dramatically. The properties of a DNA–α/β-type SASP complex in vitro appear to be duplicated in vivo, so much so that DNA does not appear to be the target for spore killing by wet heat or a number of potentially genotoxic or mutagenic chemicals. However, spore killing by dry heat and radiation occurs in large part (and possibly completely) through DNA damage, and any deficiency in α/β-type SASP in the spore results in spores that are more sensitive to a variety of treatments than are the corresponding wild-type parental spores and killed in large part by damage to DNA, even by treatments (e.g., wet heat) that do not kill wild-type spores by DNA damage.
Repair of damage to macromolecules.
As noted above, spores are killed by some treatments at least in part by damage to DNA. Consequently, it is not surprising that DNA repair during spore germination and outgrowth plays a role in resistance of spores to these treatments (133, 190, 191). Spores appear to contain at least some of the enzymes found in growing cells for repair of DNA damage, and DNA damage accumulated in the dormant spore will also often induce synthesis of DNA repair proteins upon subsequent spore germination (182). In addition, at least one protein is uniquely present in spores which is dedicated to the repair during germination and outgrowth of the major lesion caused by UV irradiation of spores, the thyminyl-thymine adduct termed the spore photoproduct (SP) (45, 185, 191). Not surprisingly, spores of species with mutations eliminating particular DNA repair pathways are invariably more sensitive to killing by agents which generate DNA damage repaired by the eliminated pathway than are spores of their wild-type parents (182, 185).
Since some of the treatments used to kill spores (e.g., wet heat and oxidizing agents) can damage proteins as well as DNA, it is possible that repair of protein damage during spore germination and outgrowth might also be important in determining spore survival after various treatments. Enzymes that might be involved in “repairing” protein damage include aspartate-O-methyltransferase, methionine sulfoxide reductase, and various heat shock proteins. However, aspartate-O-methyltransferase appears to be absent from at least B. subtilis (63), and methionine sulfoxide reductase plays no role in spore resistance to wet heat or oxidizing agents (62). Many workers have noted that spore recovery after a killing treatment (usually wet heat) is often much greater on rich media than on poor media, and this difference has been ascribed to the need for some type of protein “repair” in order for spore outgrowth and eventual colony formation on a poor medium (168, 169). Indeed, the heat shock proteins, which play a major role in the resistance of growing cells to heat stress (64a), have been suggested to play a role in spore heat resistance (174), but more recent work has indicated that this is not the case (112a; E. Melly and P. Setlow, unpublished).
Laboratory Spore Resistance Models
There have been an enormous number of studies of resistance of spores of Bacillus and Clostridium spp. under many different conditions. However, many fewer studies have analyzed the effect of one or more key variables on spore heat resistance. The following discussion will focus on these studies, as these have given us the best insight into specific factors contributing to particular spore resistance properties. As mentioned above, many of these studies have been carried out with spores of B. subtilis, as the ease of genetic manipulation as well as the availability of the complete genomic sequence (86) have greatly facilitated studies with this organism. However, analyses of spores of other species have also provided valuable information. Several general conclusions that have come from these studies are that the causes of spore resistance are multifactorial and that the importance of these multiple factors varies both between species and for different resistance properties. The current state of our understanding of the importance of various factors in spore resistance to different treatments, primarily in B. subtilis, is summarized in Table 1.
Heat resistance.
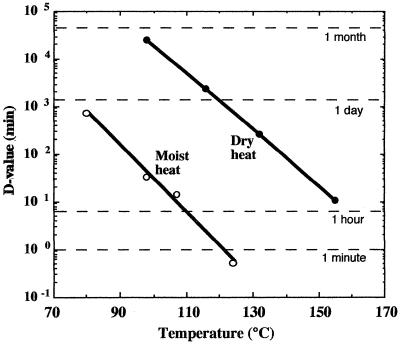
The hallmark property of bacterial spores is their remarkable resistance to heat. B. subtilis spores can survive moist heat (100°C at atmospheric pressure) with a D value (decimal reduction time, the time required to lower viability by a factor of 10) of 20 to 30 min (47) (Fig. 1). Moreover, spores survive approximately 1,000-fold longer in dry heat than in moist heat (47) (Fig. 1).
FIG. 1.
Survival times (D values; decimal reduction time) for spores of B. subtilis strain 5230 exposed to wet and dry heat. (Data are modified from reference 47.)
(i) Wet-heat resistance.
Wet-heat resistance is one of the most striking properties of spores of Bacillus and Clostridium species, as these spores require incubation at temperatures 30 to 40°C higher to achieve inactivation equivalent to that of growing cells of the same organisms (52). The target for spore killing by wet heat is not clear but is almost certainly not spore DNA (190, 191), and there is evidence that the target is a spore protein (11, 52, 191). However, the identity of this protein(s) is by no means clear. Multiple factors cause spore resistance to wet heat, with at least four factors identified to date, including sporulation temperature, protection of spore DNA by α/β-type SASP, spore core mineralization, and spore core dehydration (52, 191). However, the proteins of the heat shock response, which can play a major role in protecting growing cells of Bacillus species from heat stress (64a), play no significant role in spore heat resistance (112a; Melly and Setlow, unpublished).
Sporulation will take place at temperatures above those optimum for growth, although for an individual strain the maximum temperature at which sporulation will take place is generally a few degrees below the maximum temperature for growth. A number of studies have shown that both within and across species, spores prepared at higher temperatures are generally more heat resistant than spores prepared at lower temperatures (26, 52). A major reason for this effect is that in general spore core water content goes down as the sporulation temperature increases, and as discussed below, there is a good inverse correlation between core dehydration and spore wet-heat resistance. However, it also seems likely that other factors are important in this effect, in particular the greater heat stability of proteins of thermophilic spore formers than of those of mesophiles.
As noted above, spore killing by wet heat does not occur through DNA damage, such as depurination, that might be expected at elevated temperatures. Indeed, spore DNA is well protected against DNA damage caused by wet heat, including depurination, by the saturation of the spore DNA with α/β-type SASP (180, 191). However, in mutant B. subtilis strains lacking the majority of α/β-type SASP due to deletion of appropriate coding genes, the resultant α−β− spores are significantly more sensitive to wet heat and are killed in large part (if not completely) by DNA damage, including abasic sites presumably generated as a result of depurination (180, 191).
As noted above, the spore core contains an extremely high level of divalent mineral ions, predominantly Ca2+, Mg2+, and, to a lesser extent, Mn2+ (128). While the majority (≥75%) of these ions are associated with the spore's depot of DPA, some are also associated with other core anions. In general, the higher the level of core mineral ions, the more wet-heat resistant are the spores (52). This effect appears to be due in part to a decrease in core water with increasing core mineralization, but core minerals are also likely to have more specific effects on protein stability. The ability to remove core minerals by exchange with H+ and then back-titration with metal ion hydroxides has also allowed demonstration that the identity of the core mineral ion influences spore wet-heat resistance, as Ca2+ and Mg2+ spores are most resistant, with K+ and Na+ spores being less resistant and H+ spores being the least resistant (12, 52). However, the precise reason for these effects is not at present clear.
While the factors cited above have significant effects on spore wet-heat resistance, several of these factors exert their effects indirectly through modulation of spore core water content. This is clearly the major factor determining spore wet-heat resistance, as over a rather wide range of core water contents in spores of different species, there is a good inverse correlation between spore wet-heat resistance and core water content (10, 52). However, it is not precisely clear how a lower core water content results in increased spore wet-heat resistance. It is thought that reduced core water reduces the amount of water associated with spore proteins, thus stabilizing them to thermal denaturation. Unfortunately, there are no good data on the precise level of free water in the spore core, as this knowledge would allow detailed examination of the effects of this level of free water on protein heat resistance in vitro. In addition, although it is clear that achievement of a reduced spore core water content requires the action of the developing spore's peptidoglycan cortex, exactly how this structure functions in modulating spore core water content is not clear (39).
(ii) Dry-heat resistance.
In contrast to the situation with wet heat, the killing of spores by dry heat does appear to proceed in large part via DNA damage, as spores exposed to dry heat acquire both DNA damage and mutations (140, 181, 231). In addition, spores of DNA repair mutants are more sensitive to dry heat than are spores of their wild-type parents, and during germination of dry-heat-treated spores, genes encoding DNA repair proteins are greatly induced (182). Consequently, DNA repair capacity is an important parameter in determining spore dry-heat resistance. Again in contrast to the situation with spore wet-heat resistance, spores of thermophiles do not have higher resistance to dry heat than spores of mesophiles (1). In addition to DNA repair capacity, two other factors have been identified which affect spore dry heat resistance, spore core mineralization and DNA protection by α/β-type SASP.
Although there have been studies with spores of only a few species, in at least one species (B. stearothermophilus), variations in spore core mineralization affect spore resistance to dry heat, with demineralized spores having significantly lower resistance (1). However, the mechanism of this effect is not understood, and it has not been seen with spores of all species examined.
As noted above, spore killing by dry heat is accompanied by DNA damage which is likely to be due to base loss, largely through depurination (181). B. subtilis spores lacking α/β-type SASP are much less resistant than wild-type spores to dry heat, and killing of α−β− spores is also accompanied by DNA damage (181). Since α/β-type SASP protect DNA in vitro against depurination caused by dry heat, these data suggest that α/β-type SASP binding to DNA in spores is a major factor in spore dry-heat resistance.
Desiccation resistance.
Spores are clearly much more resistant than their growing counterparts to extended desiccation and multiple cycles of freeze-drying with freezing at −78°C. When typical laboratory vacuum systems are used for desiccation or freeze-drying, wild-type spores often exhibit no detectable killing after extended desiccation or multiple cycles of freeze-drying and rehydration (56, 167, 191). A major reason for spore resistance to these processes is protection of spore DNA by α/β-type SASP, as α−β− spores are much more sensitive to freeze-drying, and possibly extended desiccation, and killing by these processes is accompanied by DNA damage (190, 191). It has been reported that extreme desiccation (10−6 Pa at 77 K for 24 h) resulted in complete killing of Escherichia coli and Halobacterium halobium cells, but spores of Clostridium mangenoti and B. subtilis survived to 55 and 75%, respectively (85). Under prolonged desiccation in high vacuum (<10−4 Pa for 80 h), spores of a repair-deficient (rec uvr spl) B. subtilis strain were inactivated to less than 10−4 survival. Lethality for wild-type spores was not observed under this condition, but the survivors exhibited significant mutagenesis (127). However, neither the mechanism for this DNA protection by α/β-type SASP nor the DNA damage caused by desiccation is known.
Chemical resistance.
Spores are generally significantly more resistant than growing cells to a wide variety of toxic chemicals, including acids, bases, phenols, aldehydes, alkylating agents, and oxidizing agents (15, 102, 168, 169, 191, 209a). In many cases the reasons for spore resistance to these types of agents are not known, and for many chemicals (e.g., acids, bases, aldehydes, and oxidizing agents), the target for spore killing is not known, although there are some data implicating protein damage in killing by oxidizing agents (145, 146). However, for other agents (e.g., alkylating agents), it is clear that the target for spore killing is spore DNA (183).
Four factors important in spore resistance to at least some chemicals have been identified and/or suggested, including the presence of spore coats, the impermeability of the spore core to hydrophilic chemicals, low spore core water content, and protection of spore DNA by α/β-type SASP (15, 169, 191, 209a). However, in contrast to the situation in growing cells, in which specific enzymes sometimes detoxify chemical poisons, this appears not to be a factor in spore resistance to chemicals, presumably because of the inactivity of enzymes in the spore core (6, 21, 189).
The various layers of proteinaceous spore coats (and possibly the outer spore membrane) which surround the spore cortex certainly protect the spore from attack by very large molecules such as lytic enzymes that can hydrolyze the spore cortex. There are also data indicating that the coat and outer spore membrane protect spores against killing by some smaller chemical agents, including glutaraldehyde, iodine, and some oxidizing agents (15, 38, 102, 165, 169, 209a). The mechanism of this effect is not clear; possibly the coat or outer membrane is a permeability barrier to some chemicals, or the toxic chemicals may simply react with the spore coats, thus reducing the amount of toxic agent which can attack more-central spore molecules such as enzymes or DNA in the spore core. However, for some toxic chemicals (e.g., alkylating agents), the spore coats appear to play no role in spore resistance (38, 183).
Pioneering work by Gerhardt and his coworkers showed that the spore, in particular the spore core, is relatively impermeable to small hydrophilic molecules larger than about 200 Da (53), and even smaller molecules may penetrate the spore core only very slowly (178). It seems very likely that this low spore core permeability must play a significant role in spore resistance to toxic chemicals. However, there are no data bearing directly on this point, as there is no way known to readily modulate spore core permeability.
Since most of the toxic chemicals used to kill spores are water soluble and carry out reactions in water, it is reasonable to suppose that a low spore core water content might slow reactions of toxic chemicals with targets in the spore core. While one study found a decrease in spore resistance to H2O2 with increasing core water content (153), further study on the importance of this factor is needed.
For several types of toxic chemicals (e.g., formaldehyde and peroxides), there is strong evidence that one factor in spore resistance is protection of spore DNA from attack by the binding of α/β-type SASP (93, 175, 191). Oxidizing agents do not appear to kill wild-type spores by DNA damage, while α−β− spores lose resistance to these agents and are killed by DNA damage (175, 190, 191). Formaldehyde does kill wild-type spores by DNA damage, and α−β− spores are much more sensitive to formaldehyde killing (93). However, for some other chemicals such as alkylating agents, DNA protection by α/β-type SASP binding plays no role in spore resistance, even though these chemicals kill spores by DNA damage (183, 191).
UV radiation resistance.
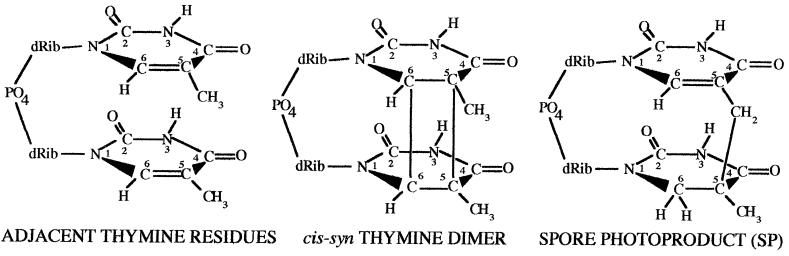
Depending on the species analyzed, spores are 10 to 50 times more resistant than growing cells to UV radiation at 254 nm in water (185). However, the magnitude of the difference in UV resistance between spores and growing cells can be different at different wavelengths. There are two main reasons for the increased UV resistance of spores: a difference in the UV photochemistry of DNA in spores and the efficient and relatively error-free repair of the novel photoproduct formed by UV light in spore DNA. While the major UV photoproduct formed in DNA of growing cells is a cis,syn-cyclobutane-type thymine dimer, this photoproduct is not generated by 254-nm UV irradiation of spores in water (34, 185, 190, 191). Rather, the major UV photoproduct formed in spores is the unique thymine adduct 5-thyminyl-5,6-dihydrothymine (130, 215), which is called SP (Fig. 2).
FIG. 2.
Structures of a single pair of adjacent thymines on the same DNA strand (left), a cis-syn cyclobutyl thymine-thymine dimer (center), and SP (right). dRib, d-ribose.
The major and possibly only cause of the altered UV photochemistry of DNA in spores is the saturation of spore DNA with α/β-type SASP. Indeed, α/β-type SASP binding to DNA in vitro suppresses formation of all cyclobutane pyrimidine dimers upon UV irradiation and promotes SP formation and also blocks formation of various 6-4 addition products (135, 190, 191). Repair of SP and other types of DNA damage will be discussed below.
Spores are also more resistant than growing cells to UV irradiation in the dry state, and wild-type B. subtilis spores treated with 254-nm UV in the lab show similar inactivation rates whether irradiated as air-dried monolayers (90% lethal dose [LD90] = 114 J/m2) (227) or in aqueous suspension (LD90 = 156 J/m2) (C. Salazar and W. L. Nicholson, unpublished). However, UV irradiation of spores rendered severely anhydrous under high vacuum generates significant amounts of photoproducts other than SP (89); as yet, the identities of these additional photoproducts are not known, nor is the reason for their generation.
In addition to the elevated resistance of dormant spores to 254-nm UV, germinating spores undergo a transient period of UV resistance even higher than that of spores before they return to the UV resistance characteristic of vegetative cells (207; reviewed in reference 185). An explanation for the transient elevated UV resistance seen in germinating spores comes from the observation that this period coincides with an overall lowering of the photoreactivity of DNA within the germinating spore (78, 177, 204). It is thought that during return of spore DNA from the A to the B conformation during germination, it passes through a transitional conformation which is geometrically unfavorable to the production of either SP or cyclobutane-type dimers. Support for this hypothesis comes from the observation that germinating spores of mutants lacking α/β-type SASP, whose DNA does not appear to be in an A-like conformation within the dormant spore (136, 176), do not exhibit this transient period of ultrahigh UV resistance (177). Another related but separate developmental phenomenon is high UV resistance exhibited by the fully germinated spores of repair-deficient (splB uvr) mutants. This phenomenon has been attributed to a specific germinative excision repair of pyrimidine dimers distinct from the nucleotide excision repair (114, 218), although direct genetic or biochemical evidence for this postulated pathway is lacking.
γ-Radiation resistance.
In addition to UV resistance, spores are often significantly more resistant than growing cells to γ-radiation (56, 167). It has been suggested that one factor which may result in increased spore γ-radiation resistance is the decreased level of spore core water, which may reduce the amount of hydroxyl radicals formed by γ-irradiation. However, this suggestion has not yet been tested directly. In contrast to the situation with spore UV resistance, in which α/β-type SASP play a predominant role, these proteins appear to play no significant role in spore γ-radiation resistance (190, 191). Although it appears certain that DNA repair during spore germination will be an important element in determining the level of spore γ-radiation resistance, there again has been relatively little work on the role of specific DNA repair systems or on the nature of the DNA damage caused by γ-irradiation of spores.
Resistance to ultrahigh hydrostatic pressure.
Extremophilic archaea and bacteria which have been isolated near deep-sea thermal vents from greater than 2 km below the ocean surface not only survive but grow at in situ pressures of 200 atm or greater (79) (1 atm = 1.013 bar or 101.3 kPa). Application of even higher hydrostatic pressure is currently being explored as a method for decreasing the numbers of vegetative bacterial and spore contaminants in a number of different types of food (94). Destruction of vegetative bacteria by pressure consists of two apparently distinct behaviors: (i) a step change in the number of survivors with the application of a pressure pulse and (ii) a first-order rate drop in the number of survivors during the ensuing pressure hold period (129, 160). The rate and degree of bacterial cell inactivation by ultrahigh pressure vary widely in different experiments and depend on a number of variables, including (i) the magnitude, rate, and duration of compression, (ii) the rate of decompression, (iii) the particular bacterium tested, (iv) the medium in which the bacteria are suspended, (v) the temperature, and (vi) the degree to which cells are allowed to resuscitate before viability is tested (196). Various mesophilic bacteria and fungi treated with 3,000 to 4,000 atm at 5°C demonstrated D values of 7.5 to 15 min (3). The effect of temperature can be quite dramatic; cells of Listeria innocua survived 4,400 atm at 25 to 26°C with a D value of 7.4 min (160), while Listeria monocytogenes cells were inactivated much more rapidly by application of less pressure (3,700 atm) but applied at a higher temperature of 45°C, exhibiting a D value of 1.1 min under these conditions (198).
Spores are extremely resistant to killing by ultrahigh hydrostatic pressures, generally more so than the corresponding growing cells (29, 168). Analysis of spore killing by hydrostatic pressure has shown that spore killing rises as the pressure increases to some maximum level of killing and then decreases as the pressure increases further (168, 224). Not all the reasons for the latter observation are known. However, it appears most likely that spore killing by hydrostatic pressure is due to the induction of spore germination by this treatment, with the germinated spores then being killed rapidly by pressure (139, 143, 161, 168, 224). With B. subtilis spores, the mechanism by which spore germination is triggered by moderate pressure (100 MPa or 987 atm) appears to involve the normal germinant receptors, but at higher pressures (600 MPa or 5,923 atm), triggering of spore germination does not require the germinant receptors (168, 225). In addition, spores germinated at lower pressures (100 MPa) become sensitive to UV and oxidizing agents, while spores germinated at higher pressures (600 MPa) are much more resistant (224). Thus, while spores are germinated by both high and lower pressures, spores germinated at high pressures remain significantly resistant, presumably even to killing by pressure itself. The sensitivity of lower-pressure (100 MPa)-germinated B. subtilis spores to UV and oxidizing agents appears to be due to the degradation of α/β-type SASP accompanying the spore germination induced by these pressures; in contrast, while higher pressures induce spore germination, degradation of α/β-type SASP is not induced (224). Although the reason for the lack of α/β-type SASP degradation accompanying spore germination induced by high pressure is not known, this observation as well as other data indicate that α/β-type SASP play an important role in the resistance of B. subtilis spores to extremely high pressure (224).
SPORE DNA REPAIR MECHANISMS
Dormant but viable spores can persist in the environment over millennial time spans (see above) in a metabolically inactive state. Therefore, environmental damage accumulates unrepaired in spore cellular components during dormancy. As discussed in the preceding section, the cellular target of many sporicidal treatments is DNA, and spore resistance to DNA-damaging treatments is due in part to protective mechanisms which either (i) prevent or dramatically slow the rate of formation of certain types of DNA damage (e.g., damage induced by oxidizing agents, dry heat, or desiccation) or (ii) alter the type of damage formed in spore DNA (e.g., 254-nm UV light inducing formation in spore DNA of SP rather than cyclobutane pyrimidine dimers) (187, 190, 191). Despite these protective mechanisms, potentially lethal and mutagenic damage nonetheless does accumulate in spore DNA during long-term storage of spores in the laboratory (190) and during exposure of spores to environmental stresses, particularly solar radiation (201, 212) (see below). Therefore, another major determinant of the degree of spore resistance to extreme environments is the speed and accuracy with which spore DNA damage can be repaired during germination. In the laboratory, the critical time frame for DNA repair during germination can be quite short. For example, in germinating spores of Bacillus megaterium, de novo RNA and protein synthesis begins well within the first 5 min of germination, using entirely endogenous reserves of precursors and energy (192–194). Abasic sites, helix-distorting lesions such as SP, or breaks in the phosphodiester backbone of spore DNA could exert potentially lethal effects early in germination by blocking the progress of RNA polymerase, thus halting expression of any number of critical pathways leading to replicative DNA synthesis and outgrowth. In addition, unrepaired lesions in spore DNA itself would physically block replication, leading to both lethal and mutagenic consequences (reviewed in reference 51). We have not yet obtained a complete catalog either of the identities of all types of damage incurred in the DNA of spores exposed to extreme environments or of all DNA repair systems involved in spore DNA repair. Below is a summary of what we know to date from laboratory studies.
General DNA Repair Systems
NER.
Nucleotide excision repair (NER) in B. subtilis closely resembles the analogous system in Escherichia coli, which has been extremely well characterized (87; reviewed in references 50, 51, and 88). Although the molecular details of NER in B. subtilis have not been elucidated to the same degree as NER in E. coli, several lines of evidence suggest that the two processes are essentially similar. First, the B. subtilis homologs of the genes encoding the UvrB and UvrC subunits of the E. coli excinuclease have been identified, mapped (115), cloned, and sequenced (22, 23) for some time, and the B. subtilis homolog of the UvrA protein has recently been identified as part of the B. subtilis genomic sequencing project (86). The deduced amino acid sequences of all three of these proteins show a high degree of similarity to their E. coli counterparts. Second, functional Uvr(A)BC excinuclease activity was obtained in vitro by mixing the purified B. subtilis homolog of UvrC protein with purified E. coli UvrA and UvrB proteins (87).
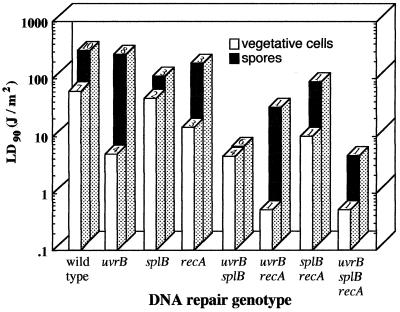
Regulation of expression of some genes for the B. subtilis NER pathway has been studied using uvr-lacZ fusions. The NER genes are expressed constitutively at a low level during vegetative growth (23) and during sporulation (P. J. Riesenman and W. L. Nicholson, unpublished). Expression of uvr-lacZ fusions is inducible by DNA damage both during vegetative growth (23) and during the outgrowth phase of germination of spores treated with 254-nm UV or dry heat (182). B. subtilis strains carrying mutations of genes in the NER pathway make UV-sensitive vegetative cells, but their spores are only slightly more UV sensitive than wild-type spores (115, 122) (Fig. 3).
FIG. 3.
UV resistance of spores and vegetative cells of B. subtilis DNA repair mutants. All strains were derivatives of the prototype laboratory strain 168. The average UV dose required to kill 90% of the population (LD90 value) is given for vegetative cells (open bars) and spores (solid bars). LD90 values are averages of values reported in the literature; the number of reports from which each value was derived is listed on the top of each bar. Data are from references 45, 58, 59, 113, 122, 126, 131, 132, 134, 179, 182, 185, 186 and 227 and from P. Fajardo-Cavazos (unpublished data).
Recombination-mediated repair.
Munakata and Rupert (126) reported that in B. subtilis, spores of a recA single mutant were no more sensitive to 254-nm UV than were wild-type spores. However, when combined with mutations inactivating either NER (uvrB) or SP lyase (splB), mutation in recA significantly enhanced spore sensitivity to 254-nm UV, thus implicating the Rec pathway in spore UV resistance (126). More recently, it has been reported that recA single mutants of B. subtilis produce spores which are about twofold more UV sensitive than wild-type spores (59, 182). Expression of a recA-lacZ fusion is inducible by DNA damage and entrance of cells into the competent state (reviewed in reference 228). Levels of RecA protein were found to be quite low in dormant spores, and expression of a recA-lacZ fusion was induced during the outgrowth phase of germination of spores previously treated with 254-nm UV or dry heat (182). Thus, it appears that expression of the NER and Rec pathways in response to DNA damage is controlled by classic DNA damage-inducible (Din) circuitry (228).
Other general repair systems.
In the recently completed sequence of the B. subtilis genome, a number of open reading frames presumably encoding components of various base excision repair and mismatch repair systems have been identified (86). To date, however, virtually no information exists regarding the role of these additional general DNA repair systems in spore resistance to extreme environmental conditions.
SP Lyase, an SP-Specific DNA Repair System
Unlike the generalized NER and Rec systems, which operate in both vegetative cells and outgrowing spores on a variety of types of DNA damages (182, 190), SP lyase is specifically dedicated to the repair of SP which has accumulated in dormant spores exposed to UV. Elegant genetic, biochemical, and physiological experiments performed in the late 1960s and 1970s indicated that SP lyase was produced during sporulation, packaged in the dormant spore, and activated during early germination to monomerize SP in situ back to two thymines (124, 125, 219). This broad scheme of repair has in large part been substantiated and explored in greater detail during the 1990s using a number of molecular approaches. DNA from B. subtilis strain 168 which could rescue a mutant lacking SP lyase activity (the original splB1 mutation isolated by Munakata [113]) was cloned, mapped genetically to the pts-kinA region of the chromosome, and sequenced (45). From the nucleotide sequence of the region, it was observed that the SP lyase (spl) locus was organized as a bicistronic operon, consisting of splA, encoding a protein of 79 amino acids (a.a.) and 9.2 kDa of unknown function, and splB, encoding a 40-kDa protein which exhibited limited regional homology to the DNA photolyase/6-4 photolyase/blue-light photoreceptor family of proteins (45, 133). Mutational inactivation and complementation experiments indicated that the splB cistron encoded the information missing in the SP lyase-deficient splB1 mutant (43, 45, 132).
Regulation of SP lyase expression during sporulation.
Expression of the splAB operon during B. subtilis growth and development has been studied by constructing a translational fusion between splB and the E. coli lacZ gene (148). In these experiments it was observed that the splAB operon is expressed as part of the EςG regulon of forespore-specific genes, by the following criteria: (i) the splB-lacZ fusion was expressed in the forespore at stage III of sporulation, (ii) expression of the splB-lacZ fusion was abolished in a sigGΔ1 mutant strain lacking sigma-G, (iii) expression of the splB-lacZ fusion could be activated during vegetative growth by artificially inducing expression in trans of the sigG gene encoded on an extrachromosomal plasmid, and (iv) the splAB operon was efficiently transcribed in vitro by purified EςG RNA polymerase from a major sigma G-type promoter preceding the operon called P1 (148). Unlike DNA repair genes such as uvr and rec (182, 228), expression of the splB-lacZ fusion was not DNA damage inducible during vegetative growth, nor was expression induced by growing cells under conditions which induce genetic competence (148). Interestingly, the SASP themselves are also expressed in the developing forespore at stage III of sporulation as part of the EςG regulon (138, 208; reviewed in reference 188).
A database search was at first unable to identify a protein homolog for the putative 79-a.a. protein encoded by the splA cistron preceding splB. However, genetic experiments hinted that the splAB operon contained unexplored regulatory features. First, deletions extending from upstream removing P1 and part of splA did not inactivate operon expression (45). Second, primer extension mapping of in vivo splAB mRNA revealed a second transcript arising from the intercistronic region between splA and splB, apparently from another EςG-dependent promoter called P3 (148). Third, in vitro mutations which inactivated the major P1 promoter and an in-frame deletion of splA had the effect of increasing the expression levels but not the timing, forespore compartmentalization, or EςG dependence of expression of splB-lacZ fusions integrated at the prophage SPβ locus (149). cis/trans analysis of partial diploids for splA indicated that the SplA gene product is a trans-acting negative regulator of splB-lacZ expression and apparently acts by modulating the level of transcription initiating from P1 versus P3 (44). Although neither the molecular mechanism of this regulatory circuit nor its potential function during sporulation has yet been elucidated, it is interesting to note that the deduced SplA amino acid sequence is similar to that of another small regulatory protein, the trp RNA-binding attenuation protein (TRAP) of B. subtilis. By analogy, this observation opens the possibility that SplA may operate at the level of splAB mRNA by a TRAP-like mechanism (44; reviewed recently in reference 5).
Genetics and biochemistry of SP lyase.
Soon after cloning of the splAB operon, it was demonstrated that the splB cistron encoded SP lyase activity, from evidence that (i) only subclones of splAB containing wild-type splB DNA could rescue Munakata's splB1 mutation (43, 45) and (ii) knockout mutations of the splB cistron but not the splA cistron resulted in spore UV sensitivity (45, 132). The first clue to the enzymatic mechanism of SP lyase came from examination of the deduced amino acid sequence of the B. subtilis SplB protein. The 342-a.a. sequence of SplB was observed to contain only four cysteines, three of which were tightly clustered at residues 91, 95, and 98 and the fourth at residue 141 (45). The SplB sequence surrounding residues C91, C95, and C98 was found through sequence database searching to be highly similar to the amino acid signature for the [4Fe-4S] clusters of a family of S-adenosylmethionine (SAM)-dependent, radical-utilizing enzymes represented by anaerobic (type III) ribonucleotide reductase, pyruvate-formate lyase, lysine-2,3-aminomutase, biotin synthases (BioB), and lipoic acid synthetase (LipA) (132) (Fig. 4). All members of the family of SAM-dependent, radical-utilizing enzymes have three features in common: (i) they contain oxygen-labile [4Fe-4S] clusters and hence require anaerobic conditions for their activity; (ii) they utilize SAM as a cofactor; and (iii) they operate by a radical mechanism, in which the [4Fe-4S] cluster splits SAM to generate methionine and a 5′-adenosyl radical, which then proceeds to participate either directly or indirectly in catalysis (reviewed in references 163 and 223).
FIG. 4.
Comparison of amino acid regions forming [4Fe-4S] clusters in the SplB amino acid sequences from Bacillus anthracis (B.an), B. amyloliquefaciens (B.am), B. stearothermophilus (B.st), B. subtilis (B.su), Clostridium acetobutylicum (C.ac), and C. difficile (C.di) with the [4Fe-4S] clusters of: the activating subunits of ribonucleotide reductase (NrdG) and pyruvate-formate lyase (Act) from Escherichia coli (E.co), phage T4 (T4), and Haemophilus influenzae (H.in) and the [4Fe-4S] clusters from lysine-2,3-aminomutase (KamA) from Clostridium subterminale (C.su), biotin synthase (BioB) from B. subtilis, and the probable lipoic acid synthase (YutB) from B. subtilis. Highly conserved residues are in bold, and invariant cysteines are marked with an asterisk.
The evidence is mounting that SP lyase belongs to the family of SAM-dependent, radical-utilizing enzymes. First, iron was shown to be associated with SP lyase activity, as survival of UV-irradiated spores of a B. subtilis strain relying only upon SP lyase for DNA repair was lower when the spores were germinated on solid growth medium lacking iron (132). Second, SplB protein carrying an amino-terminal histidine tag was overexpressed and purified from E. coli; the purified protein exhibited a reddish-brown color characteristic of iron-containing proteins (162). Third, the presence of a [4Fe-4S] cluster in the active form of SP lyase was inferred by chemical assay for iron and acid-labile sulfide and by UV-visible spectroscopy (162) and most recently has been confirmed by electron paramagnetic resonance spectroscopy (R. Rebeil and W. L. Nicholson, unpublished observations). Fourth, in vitro SP lyase activity was obtained from purified SplB protein only when reactions were carried out under anaerobic conditions, and activity was shown to be absolutely dependent on SAM (162). Whereas many of the molecular details of the SP cleavage reaction remain to be elucidated, it is clear from the evidence gathered to date that SP lyase is indeed a member of the SAM-dependent, radical-utilizing enzyme family and is unique in being the first and only DNA repair protein discovered to date which utilizes this novel mechanism of catalysis.
As noted above for spore protective mechanisms, the evidence collected to date indicates that repair of DNA damage in the spore during germination is also the result of a number of general and specific DNA repair mechanisms acting in concert. The net result of the relative contributions to the known DNA repair systems in determining the resistance of B. subtilis spores versus vegetative cells to 254-nm UV is summarized in Fig. 3.
SPORE RESISTANCE TO EXTREME TERRESTRIAL ENVIRONMENTS
Differences between the Laboratory and the Environment
As discussed in the preceding sections, a fairly detailed molecular picture of spore resistance properties in the laboratory is emerging, which appears to be a combination of (i) mechanisms for protection of critical spore components, particularly DNA, during dormancy and (ii) mechanisms for rapid and accurate repair of cellular damage during germination. A fundamental biological question inevitably arises from these studies: Do the models which have been developed to explain spore resistance in the laboratory provide an adequate description of the phenomenon as it occurs in the environment? Although considerable effort has been directed towards investigation of spore UV resistance and DNA repair under a number of environmental conditions (see below), the gulf between the laboratory and the environment is only beginning to be bridged. In an attempt to answer the question of biological relevance, it is perhaps instructive to first compare some important parameters which differ between the laboratory and the field. We will use the spore UV resistance model as an example, as it is this model which has been advanced from laboratory studies in the greatest detail.
Source of spores.
Most but not all of the laboratory model of spore UV resistance has been derived directly from studies on a single organism, Bacillus subtilis. This situation has arisen for the sensible reason that B. subtilis is by far the best-characterized spore-forming microorganism because it is amenable to highly refined genetic and molecular biological manipulation (61, 86, 203). In addition, there is a body of experimental evidence gathered from other spore-forming microorganisms which supports the B. subtilis model describing the role of α/β-type SASP in spore DNA protection and UV photochemistry (reviewed in references 184 and 190). Studies on spore DNA repair mechanisms, in contrast, have focused almost exclusively on B. subtilis because of the early isolation of mutations affecting NER and SP lyase in this species (113, 122); the sole exception in this case is the Bacillus amyloliquefaciens homolog of SP lyase, which has been cloned, completely sequenced, and shown to function in B. subtilis (133). In addition, SP lyase homologs have been cloned and partially sequenced from Clostridium perfringens (211) and identified in the sequenced genomes of Bacillus anthracis, B. stearothermophilus, Clostridium acetobutylicum, C. difficile, and Streptomyces coelicolor. Therefore, the evidence to date indicates that SP lyases, like SASP, are widely conserved among the spore-forming eubacteria.
Both laboratory and environmental studies of spore resistance mechanisms have until recently been conducted almost exclusively using “tame” laboratory strains which have not experienced either growth or sporulation under natural environmental conditions in several decades. Conversely, until very recently we had no data regarding the UV resistance properties of spores isolated from natural environmental sources or from extreme environments. Recently it was shown that populations of dormant Bacillus spp. spores isolated and purified directly from soils in the Sonoran desert were significantly more UV resistant than the benchmark laboratory B. subtilis strain, 168 (134). One round of growth and sporulation of these Sonoran desert isolates on standard laboratory media lowered the UV resistance of their spores to levels essentially identical to that of B. subtilis 168, suggesting that spore UV resistance is determined at least in part by the environment in which a bacterium sporulates (134).
Growth, sporulation, dormancy, and germination conditions.
In the laboratory, B. subtilis spores are usually prepared by cultivating the bacterium at 37°C in a liquid nutrient broth-based sporulation medium at a high growth rate and to high cell density until some essential nutrient (usually the carbon source) is exhausted from the medium (171). After 1 to 2 days of aerobic incubation, spores are purified from vegetative cells, the pasteurized spore suspension is air dried or diluted in buffer and exposed to the extreme condition being studied, and survival is quantitated by plating the spores on nutrient medium and counting colonies arising after incubation at 37°C (reviewed in reference 137). Although very little is known about the growth or sporulation of bacteria in their natural habitats, it is not difficult to envision that this process probably bears little resemblance to the manner in which spores are prepared and assayed in the laboratory. Growth of spore-forming bacteria in their natural environments (e.g., soil, decaying organic matter, plant surfaces, and insect and mammalian guts) (i) is almost certainly slower, (ii) probably takes place in microcolonies on and within a solid substrate (aggregated soil particles, e.g.), (iii) at the very least is subject to wide variations in temperature, UV flux, nutrient, water, and oxygen availability, and (iv) probably occurs in direct competition with other micro- and macroorganisms. To date, we know almost nothing about how spore-forming bacteria grow and sporulate under these diverse conditions or of the properties of the resulting spores.
Solar Radiation as Primary Source
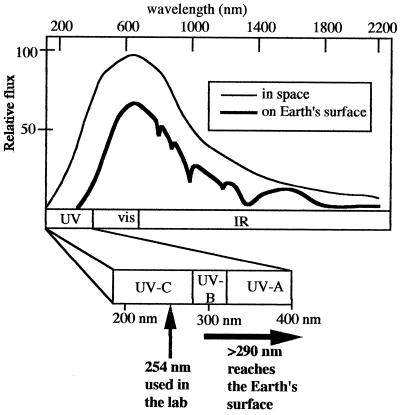
Historically, most laboratory studies of spore UV photochemistry and DNA repair have been performed using monochromatic 254-nm UV light (UV-C) due to two technical expedients: (i) 254-nm UV is relatively cheap and simple to generate using a low-pressure mercury arc lamp, and (ii) 254 nm coincides well with the absorption maximum of DNA, so that biological and photochemical effects can be observed at relatively low fluences. However, sunlight reaching the Earth's surface is not monochromatic 254-nm radiation but a mixture of UV, visible, and infrared radiation, the UV portion spanning approximately 290 to 400 nm (the so-called UV-B and UV-A portions of the UV spectrum) (214) (Fig. 5). The laboratory model of spore UV resistance has therefore been constructed largely using a wavelength of UV radiation not normally experienced on the Earth's surface, even though ample evidence exists that both DNA photochemistry (195) and cellular responses to UV (213) are strongly wavelength dependent.
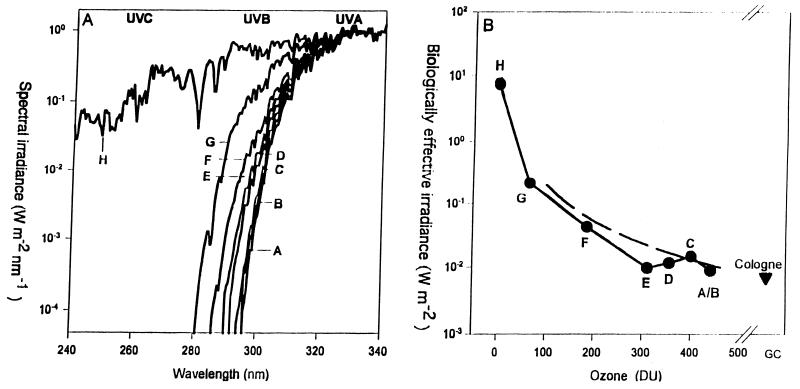
FIG. 5.
Solar spectrum in space (thin line) and on Earth's surface (thick line). Below the spectrum is an expansion of the UV portion, showing the approximate boundaries between UV-C, UV-B, and UV-A. Also shown is the UV wavelength commonly used in the laboratory (254 nm) and the UV wavelengths incident on the Earth's surface (290 nm and longer). vis, visible; IR, infrared.
Radiation from the sun drives essentially all life processes at the Earth's surface, but paradoxically is also a major source of lethal damage to spore cellular components and both lethal and mutagenic damage to spore DNA. The physical effects of solar radiation can be divided into direct and indirect effects.
Direct solar effects.
Solar UV radiation can cause lethal and mutagenic damage to spores by direct interaction between photons and DNA (116, 212). As mentioned above, the best-characterized UV damage in spore DNA is the unique thymine dimer 5-thyminyl-5,6-dihydrothymine, commonly called SP, as well as a number of less-abundant unidentified photoproducts (34), one of which may be the (6-4) photoproduct (90). SP has been detected in spore DNA irradiated at a number of UV wavelengths extending through the UV-C, UV-B, and UV-A portions of the spectrum, using either artificial UV sources or sunlight (212). The presence of additional photoproducts in spore DNA exposed to solar UV has long been inferred (212, 227), but the identity of some of these photoproducts has only recently begun to be elucidated (201) (see below). Although much attention has focused on the UV-B portion of sunlight, since it is a major cause of DNA damage, recent research has clearly demonstrated that UV-A, especially wavelengths in the range from 320 to 365 nm, can also induce production of pyrimidine dimers besides producing other kinds of DNA and cellular damage (201, 212, 213).
Indirect solar effects.
Solar radiation exerts a number of indirect, albeit important secondary effects which have in some cases been mimicked in the laboratory, including heating of spores, desiccation of spores through evaporation, and generation of reactive oxygen species in spores (213).
As noted above from laboratory studies (Fig. 1), spores can withstand heating for extended periods at temperatures well above those normally prevailing in most environments at the Earth's surface. This observation has been confirmed experimentally using spores of several different B. subtilis strains exposed as air-dried films to solar heating at temperatures exceeding 70°C but shielded from primary UV effects (165, 201, 226, 227). The net result of these studies is that spores suffered little or no detectable loss in viability after exposures of up to 30 h under these conditions.
The effects of extreme desiccation on B. subtilis spores and spore DNA have been studied using vacuum generated either in the laboratory (13, 36, 37, 42, 127) (see above) or in space orbit (66) (see below). Current evidence indicates that extreme desiccation under vacuum leads to predominantly single-strand breaks, DNA-protein cross-links, and other uncharacterized DNA damage in spores (13, 42); to date, vacuum-induced damage to non-DNA spore components has not been reported.
It is almost certain that the desiccation induced by extreme vacuum in the laboratory is far greater than the levels of desiccation found in terrestrial environments. Although no systematic studies of desiccation resistance of spores in terrestrial environments have been undertaken, it is interesting to note that viable spores have been recovered from 300-year-old air-dried herbarium soils and from 10-meter-deep permafrost soils at least 10,000 years old (reviewed in reference 154).
Several lines of evidence indicate that absorption of solar UV-B and UV-A by a variety of compounds within the cell can result in the generation of reactive oxygen intermediates, such as hydrogen peroxide and superoxide anion. These activated oxygen species target several cellular components in addition to DNA, causing enzyme photoinactivation and lipid peroxidation (reviewed in reference 213). These additional types of solar damage to non-DNA spore cellular components have not been examined to date.
We have some idea of how each of the above secondary solar effects impacts spores in the laboratory, but how do each of these physical parameters affect spore DNA in the environment? Spores in the environment are not exposed to single, isolated stresses as studied in the laboratory. Rather, solar exposure creates some or all of these effects simultaneously. Factors such as the constantly shifting solar angle resulting from Earth's rotation and tilt combined with ever-changing atmospheric conditions cause complex cyclic variations in total radiation flux and spectrum, particularly at the UV-B extreme (214). In addition, spores can undergo untold numbers of cycles of desiccation-hydration, heating-cooling, and freezing-thawing during dormancy. Even spores buried in soil, whose DNA is well shielded from the primary effects of solar UV, are subjected to these indirect stresses.
Protection of Spores from Lethal Solar UV Damage
What structures or features of the spore have been identified in laboratory studies which could protect the spore from lethal solar UV damage? As mentioned above, SASP binding to spore DNA in the spore core protects it both from 254-nm UV-C damage and from damage due to oxidizing agents (see above). These results imply that SASP may also protect spore DNA from the direct and indirect effects of solar UV-B and UV-A, although this notion has not to date been tested experimentally. Spores of Bacillus subtilis possess a thick protein coat organized as an electron-dense outer coat layer and a lamella-like inner coat layer (Fig. 6) (reviewed in reference 38). The spore coats have been shown to protect the spore from lethal oxidative damage in the laboratory but not from lethal damage inflicted by exposure to 254-nm UV-C (202). In order to test if the spore coat layers conferred resistance to environmentally relevant UV, spore coat-defective mutants of B. subtilis (containing the gerE36 mutation and lacking the inner coat and/or the cotE::cat mutation and lacking the outer coat) were used to study the relative contribution of spore coat layers to spore resistance to hydrogen peroxide and various artificial and solar UV treatments (165). Spores of strains carrying mutations in gerE and/or cotE were very sensitive to lysozyme and to 5% H2O2, as were chemically decoated spores of the wild-type parental strain (165). However, spores of all coat-defective strains were as resistant to 254-nm UV-C as wild-type spores. Spores possessing the gerE36 mutation were approximately twofold more sensitive than wild-type spores to artificial UV-B, full-spectrum sunlight, and UV-A sunlight, and spores of strains carrying both the gerE36 and cotE::cat mutations, completely lacking spore coats, behaved as single gerE36 mutant spores in their pattern of UV sensitivity. Although the differences observed were not dramatic, they were reproducible and statistically significant and suggest that the spore coats, particularly the inner coat layer, play some role in spore resistance to environmentally relevant UV wavelengths. In contrast, spores of strains possessing only the cotE::cat mutation were about two fold more resistant than wild-type spores to all UV treatments used; the reason for this anomalous observation is not at present known (165).
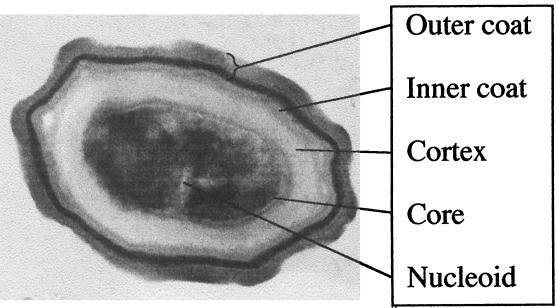
FIG. 6.
Cross-section of a spore of B. subtilis. The DNA is contained in the nucleoid (electron-light regions) within the spore core. The core is surrounded by the protective cortex and the lamellar inner spore coat and electron-dense outer spore coat. The long axis of the spore is 1.2 μm; the core area is 0.25 μm2. (The electron micrograph was kindly provided by S. Pankratz.)
DNA Photochemistry of Spores Exposed to Sunlight
It has long been established that spore DNA is a target of solar radiation, as sunlight was shown to exert both lethal and mutagenic effects on spores (116, 117, 212). As noted earlier, SP is clearly the principal DNA photoproduct formed in bacterial spores irradiated with UV-C (34, 215). Working with spores in aqueous suspension of a B. subtilis strain lacking both NER and SP lyase, Tyrrell (212) showed that, as in the laboratory, SP was also the major photoproduct identified in spore DNA exposed to sunlight. However, when Tyrrell (212) measured SP formation and spore lethality as a function of UV dose at a number of different UV wavelengths, including polychromatic sunlight, he calculated that spore DNA accumulated 823 and 862 SP per lethal event per genome when irradiated at 254 nm (UV-C) and 313 nm (UV-B), respectively. In contrast, he found that spores accumulated only half as many SP per lethal event per genome (448 and 447, respectively) when irradiated with 365 nm (UV-A) or with full-spectrum sunlight, respectively. From these data, Tyrrell (212) first inferred that spore DNA exposed to sunlight, especially to UV-A wavelengths, appeared to accumulate some type(s) of lethal damage which was not SP in nature.
Evidence supporting this notion was first obtained indirectly by exposing spores of isogenic strains of B. subtilis single mutants lacking either NER or SP lyase to radiation at a number of UV wavelengths, including laboratory UV-C (254 nm) and UV-B (290–320 nm), full-spectrum sunlight (290 to 400 nm), and sunlight from which the UV-B portion had been filtered (UV-A sunlight; 325 to 400 nm) and calculating their survival relative to that of spores of a strain lacking both DNA repair systems (227). It was observed that in spores exposed to UV-C in the laboratory, SP lyase contributed more to spore survival than NER, but in response to UV-B or full-spectrum sunlight, NER and SP lyase contributed equally to spore survival. The relative increase in importance of the general NER system to spore survival at environmentally relevant UV wavelengths again implied that spore DNA exposed to sunlight was accumulating some type(s) of damage in addition to SP which was preferentially repaired by the less specific NER pathway (227). Furthermore, it was noted that at longer UV wavelengths wild-type spores decreased in their UV resistance from being 33-fold (UV-C) to 30-fold (UV-B) to 12-fold (full-spectrum sunlight) to 6-fold (UV-A sunlight) more resistant than mutants lacking both NER and SP lyase, suggesting that at longer UV wavelengths spores were inactivated by damage other than SP (227). What are the cellular targets in spores for lethal cellular damage by the UV-A component of sunlight? Two broad possibilities can be envisioned: (i) DNA damage which is recognized neither by NER nor by SP lyase, such as strand breaks, abasic sites, or modified bases, and (ii) damage to other, non-DNA cellular targets.
(i) In addition to SP, what is the identity of a postulated DNA photoproduct(s) produced in spores exposed to sunlight? This particular question has been addressed by exposing B. subtilis spores to either full-spectrum sunlight or UV-A sunlight, isolating spore DNA, and probing it for DNA damage using a combination of enzymes which cleave the DNA phosphodiester backbone at cyclobutane pyrimidine dimers (endonuclease V) or abasic sites (endonuclease IV) and denaturing agarose gel electrophoresis to assess the sizes of the single-stranded DNA fragments resulting from the nicked molecules (201). Cyclobutane dimers were generated in spore DNA at physiologically relevant levels by exposure of spores to artificial UV-B and full-spectrum sunlight but not to UV-A sunlight, whereas single-strand and double-strand breaks were induced in spore DNA exposed to full-spectrum sunlight and UV-A sunlight but not artificial UV-B. From these observations it was concluded that cyclobutane pyrimidine dimers are induced in spore DNA by the UV-B portion of sunlight and that phosphodiester backbone breaks are induced in spore DNA by the UV-A wavelengths present in sunlight. In contrast, abasic sites were not detected in spore DNA under any exposure condition tested (201).
(ii) Regarding non-DNA cellular damage, it has been well established that UV-A can generate free oxygen radicals within cells and that bacteria possess overlapping inducible stress regulons which respond to both oxidative damage and UV-A exposure (reviewed in reference 213), although almost nothing is yet known concerning the identity of these postulated cellular damages in spores exposed to sunlight.
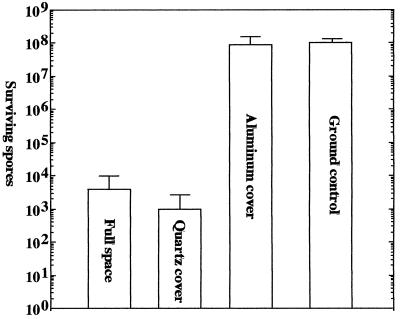
B. subtilis Spores as Biological Solar Dosimeters
The late 1970s and early 1980s were marked by an increased scientific interest in devising methods to measure the postulated increase in the biologically effective solar UV flux impacting the Earth's surface resulting from global stratospheric ozone depletion (116, 212). B. subtilis spores were found to possess several properties rendering them particularly suitable for use as biological solar dosimeters, including their simplicity and ease of use and transport to and from environmental testing sites; their stability upon prolonged storage, both before and after exposure; and the reproducibility of their UV inactivation response (212). Tyrrell (212) first showed that spores of a B. subtilis strain lacking both NER and SP lyase were inactivated rapidly by sunlight; an exposure to 25 min of noontime sunlight in Rio de Janeiro, Brazil, was sufficient to inactivate 99.9% of DNA repair-deficient spores, while spores of an isogenic wild-type strain retained essentially full viability over the same time period. These results were in agreement with the relative sensitivity of spores of the two strains in the laboratory to monochromatic UV of 254, 313, 334, or 365 nm, indicating that in combination NER and SP lyase are important determinants of spore resistance to solar UV as well as to laboratory-generated UV (212). Tyrrell's (212) original dosimetric system consisted of spore suspensions contained within a stoppered quartz cuvette and agitated by magnetic stirring throughout the sunlight exposure period. Subsequent refinements in portability and ease of sampling resulted in the increased use of B. subtilis spores as field dosimeters. Such improvements included application of spores as dried films onto membrane filters (116) or glass microscope slides (217); use of strains containing auxotrophic markers to quantitate mutagenesis by solar UV using a reversion assay (116); improved methods for recovery and quantitation of spores from the dried films on the dosimeter (89, 217); and inclusion in the same sample of both wild-type and double mutant spores, distinguishable upon plating by different genetic markers (217). Using a series of cutoff filters, two independent studies have concluded that the most effective portion of the solar spectrum for inactivation of spore dosimeters consisted of UV-B radiation in the 305- to 325-nm range (117, 217). A recent experiment simultaneously compared the response of the spore dosimeter with that of a Brewer sunlight spectrophotometer at the same location and over the same time periods and found good correlation between the two methods, indicating that the spore dosimetric system was indeed a relevant method for monitoring biologically active solar UV (123).
Monitoring the Ozone Layer with Spore Dosimeters
The stratospheric ozone layer constitutes a protective atmospheric filter against biologically harmful solar UV radiation. Satellite-based ozone measurements conducted since 1979 have revealed statistically significant downward trends in the ozone column on a global scale, except in the tropics (48). As a consequence of this progressive ozone depletion, increased levels of UV-B radiation have been measured in the southern as well as the northern hemisphere (104). An increased UV-B level of solar radiation will have many harmful effects on the biosphere and on human health (229). A quantitative assessment of the implications of progressive ozone depletion for life on Earth has been attempted theoretically, by applying radiative transfer models for different total ozone columns, where the resulting solar spectrum is weighted with biological action spectra (96), or attempting to correlate the inactivation rate of biological dosimeters exposed to sunlight with simultaneous measurements of ozone column thickness and spectral irradiance (120, 123, 159), or experimentally by applying UV radiation suitable for the simulation of a certain degree of ozone depletion (14). Solar UV dosimetry based on spore inactivation is probably the most sensitive measure of the change in the ozone concentration because of the high relative sensitivity to the shorter-wavelength portion of solar spectrum.
Solar UV monitoring studies using variations of the spore dosimetry system first described by Tyrrell (212) have been carried out by a number of groups at various global sites, including Rio de Janeiro, Brazil (212); Tokyo, Japan (117, 118); Dallas, Tex. (217); Indonesia (121); and Antarctica (157). The longest-running use of spore dosimetry to measure biologically relevant UV flux from sunlight is the ongoing study by Munakata (116–120), which has collected dosimetry and meteorologic data at Tokyo since 1979 to calculate both daily and seasonal variations in the sporocidal and mutagenic effects of sunlight. Analysis of data summarized from this study led to the conclusions that (i) an increase in the ratio of spore inactivation dose to total solar irradiance (global insolation) has been detected in Tokyo by the spore dosimeter between 1980 and 1993 and (ii) this increase is only partially explained by a decrease in the stratospheric ozone concentration measured from nearby Tsukuba aerological observatory, suggesting that changes in other atmospheric factors occurring over this time period have also contributed to the observed increase in dosimeter response (119). Further attempts to extend this spore dosimetry include the comparisons of dose-rate profiles at several sites in the northern hemisphere (120) and continuous monthly biomonitoring at several sites, including the tropics (121). More recently, a dosimetric system based on measuring protein synthesis during germination of UV-irradiated B. subtilis spores immobilized in a biofilm and subsequent growth has been developed (158) and was used to measure the biologically harmful effects of the increase in UV flux accompanying the seasonal appearance of the “ozone hole” over Antarctica. This study demonstrated that spore dosimetry can indeed detect changes in the depth of the ozone layer, but again a simple response of the dosimeter to changes in ozone column thickness was insufficient to explain all of the phenomena observed (159).
Using extraterrestrial sunlight as a natural radiation source and optical filters in an experiment on board the German Spacelab D2 mission, the terrestrial UV radiation climate was simulated at different ozone concentrations down to very low ozone values (75). Biologically effective irradiances as a function of the simulated ozone column thickness were measured with B. subtilis spores immobilized in a biofilm (158) and compared to expected irradiances, using radiative transfer calculations and the biofilm action spectrum. After the mission, the biofilms were incubated and stained, and optical densities indicative of the biological activity were determined for each exposure condition by image analysis. With decreasing (simulated) ozone concentrations the biologically effective solar UV irradiance strongly increased (Fig. 7). The full spectrum of extraterrestrial solar radiation increases the biologically effective irradiance by nearly 1,000-fold compared to the solar spectrum at the surface of the Earth for average total ozone columns.
FIG. 7.
Increase in biologically effective UV irradiance (biologically effective dose) with decreasing simulated ozone column thickness as measured by the spore biofilm technique in a space experiment. (A) Extraterrestrial solar spectral irradiances filtered through a quartz (7 mm) plate (H) or additionally through cutoff filters simulating different ozone column thicknesses with a progressive simulated ozone depletion from curves A to G. (B) Biofilm data of biologically effective solar irradiance for the different ozone column thicknesses, given in Dobson units (DU) (A to G), the extraterrestrial UV spectrum (H), and ground control (GC, Cologne). The dashed line shows the corresponding curve for DNA damage calculated by Madronich (95). (Data are modified from reference 75.)
SPORE RESISTANCE TO EXTRATERRESTRIAL ENVIRONMENTS
The high resistance of bacterial spores to environmental extremes such as desiccation, high and low temperature, and radiation makes them ideal candidates with which to study the biological responses to the harsh environment of space (reviewed in references 66 and 209). Questions to be tackled include the biological effects of exposure to the space environment and the likelihood of interplanetary transfer of life. Experiments have been carried out, first on balloons and rockets (91, 92) and later on spacecraft in low Earth orbit (Spacelab 1, Spacelab D2, the Exobiology Radiation Assembly [ERA] on the European Retrievable Carrier [EURECA], the National Aeronautics and Space Administration's Long Duration Exposure Facility [LDEF], and Biopan on the Russian satellite Foton) and outside the Earth's magnetic field (Apollo missions 16 and 17) (reviewed in references 70 and 71).
Space
In order to understand the physical parameters imposed on spores, it is first necessary to understand the space environment itself. The environment in Earth orbit is characterized by a high vacuum, an intense radiation climate, and extreme temperatures (Table 2).
TABLE 2.
Physical conditions prevailing in the interplanetary space environment and in low Earth orbit
| Parameter | Interplanetary space | Low Earth orbit (≤500 km) |
|---|---|---|
| Space vacuum | ||
| Pressure (Pa) | 10−14 | 10−6–10−4a |
| Residual gas (particles/cm3)a | 1 H | 105 H, 2 × 106 He, 105 N, 3 × 107 O, spacecraft atmosphere |
| Solar electromagnetic radiation | ||
| Irradiance (W/m2) | —b | 1,360 |
| Spectral range (nm) | Continuum | Continuum |
| Cosmic ionizing radiation | ||
| Annual dose rate (Gy/yr) | ≤0.1c | 400–10,000d |
| Temperature (K) | >4b | Wide rangeb |
| Microgravity (g) | <10−6 | 10−3–10−6 |
Values in Earth orbit depend on outgassing of the spacecraft. Sources of contamination include waste dumping (H2O and organics) and thruster firing (H2O, N2O, and NO).
Values differ depending on orientation and distance to the Sun.
Depending on shielding; highest values at mass shielding of 0.15 g/cm2.
Depending on altitude, orbit, and shielding; highest values at high altitudes and mass shielding of 0.15 g/cm2.
Vacuum.
In free interplanetary space, pressures down to 10−14 Pa prevail. Within the vicinity of a body, the pressure may increase significantly due to outgassing. In a low Earth orbit, pressure reaches 10−4 to 10−6 Pa. The major constituents of this environment are molecular oxygen and nitrogen as well as highly reactive oxygen and nitrogen atoms. In the vicinity of a spacecraft, the pressure increases further, depending on the degree of outgassing. For example, in the shuttle cargo bay, a pressure of 3 × 10−5 Pa was measured.
Radiation.
The radiation environment of our solar system is governed by components of galactic and solar origin. The galactic cosmic radiation entering our solar system is composed of protons (85%), electrons, α-particles (14%), and the so-called HZE particles (1%), which are defined as cosmic ray primary ions with charges of Z >2 and energies high enough to penetrate at least 1 mm of spacecraft or spacesuit shielding (56a). The solar particle radiation, emitted in solar wind and during solar flares, is composed of 90 to 95% protons, 5 to 10% α-particles, and a relatively small number of heavier ions. In the vicinity of the Earth, in the radiation belts, protons and electrons are trapped by the geomagnetic field. The spectrum of solar electromagnetic radiation spans several orders of magnitude, from short-wavelength X-rays to radio frequencies. At the distance of the Earth from the sun (1 astronomical unit), solar irradiance amounts to 1,360 W m−2, the solar constant. Of this radiation, 45% is attributed to the infrared fraction, 48% to the visible fraction, and only 7% to the UV range. The extraterrestrial solar spectral UV irradiance has been measured during several space missions, such as Spacelab 1 and EURECA.
Temperature.
The temperature of a body in space, which is determined by the absorption and emission of energy, depends on its position with respect to the sun and other orbiting bodies and also its surface, size, mass, and albedo (reflectivity). In Earth orbit, the energy sources include solar radiation (1,360 W m−2), the Earth albedo (480 W m−2), and terrestrial radiation (230 W m−2). Periodically, an orbiting object can be shaded from the sun as it passes on the Earth's night side. Therefore, in Earth orbit, the temperature of a body can reach both extremely high and extremely low values.
Interplanetary Transfer of Spores
The idea of interplanetary transfer of life was originally put forward independently by Richter (164), Lord Kelvin (210), and Arrhenius (2). The theory of panspermia, as postulated by Arrhenius (2), holds that reproductive bodies of living organisms can exist throughout the universe and develop wherever the environment is favorable. The theory implies that during the evolution of the universe, conditions favorable to the development of life have prevailed at different locations and at different times. For example, early in the development of our solar system, at a time when conditions on the young Earth may have been hostile to the development of life, conditions conducive to the development of living systems may have existed on other bodies either within our solar system (such as Mars, Venus, Europa, or Titan) or in other solar systems. The panspermia theory does not set a precondition for terrestrial life having arisen on Earth; living organisms could well have arisen elsewhere in the solar system (or universe) and been transported to Earth, where they encountered environmental conditions favorable to growth, proliferation, and eventual evolution into more complex forms. Once established, life on Earth would then be subject to transfer to other celestial bodies. Since its formulation, this theory has been subjected to several criticisms, with arguments such as that it cannot be experimentally tested, that it shunts aside the question of the origin of life, and that living organisms will not survive long exposure to the hostile environment of space, especially vacuum and radiation. However, recent experimental evidence, summarized below, has led to a reexamination of the feasibility of the notion of interplanetary transfer of living material, particularly microorganisms.
The scenario of interplanetary transfer of life involves three basic hypothetical steps: (i) the escape process, i.e., removal to space of biological material which has survived being lifted from the surface to high altitudes; (ii) interim state in space, i.e., survival of the biological material over time scales comparable with interplanetary or interstellar passage; (iii) the entry process, i.e., nondestructive deposition of the biological material on another planet. We do not know with certainty whether panspermia is likely to have occurred in the history of the solar system or whether it is feasible at all, but additional support to the idea of panspermia is given by a variety of recent discoveries. These are (i) the discovery of meteorites, some of lunar and some of Martian origin, on the Earth's surface (82); (ii) the detection of organics in two Martian meteorites (221a) and the currently controversial supposition of hints of former microbial life in one of the Martian meteorites (103), although it is still highly debatable whether a putative Martian biology was somehow involved in the phenomena observed; (iii) the probability of small particles between 0.5 μm and 1 cm in diameter (112) or even boulder-sized rocks reaching escape velocities by the impact of large meteorites on a planet, e.g., on Earth (105–107) or Mars (142, 216); (iv) the ability of bacterial spores to survive the shock waves of a simulated meteorite impact (71); (v) the high UV resistance of microorganisms at the low temperatures of deep space (220); (vi) the high survival of bacterial spores over extended periods in space (66, 72); (vii) the paleogeochemical evidence of a very early appearance of life on Earth in the form of metabolically advanced microbial prokaryotic ecosystems, leaving not more than approximately 0.4 Ga for the evolution of life from the simple precursor molecules to the level of the prokaryotic, photoautotrophic cell (173); (viii) the biochemical evidence of a common ancestor for all life forms on Earth (222), which might have been already a prokaryotic cell not fundamentally different from present-day prokaryotes (55a); and (ix) the likelihood of accidental or intentional transport by probes sent to other planets (30). Concerning this last observation, what appears to be an actual case of accidental transport of a bacterium from the Earth to the moon and back has been reported; after spending 2.5 years on the lunar surface, the common human oral bacterium Streptococcus mitis was recovered from the interior case of a camera carried aboard the lunar probe Surveyor 3 after its recovery and return to Earth by the Apollo 12 mission (111).
Launching Spores into Space
What natural process(es) could result in ejection of microorganisms from the gravity well of a planet such as Earth, with its escape velocity of 11.2 km/s? The options are rather limited to either volcanic eruptions or large meteorite impacts. Thermodynamic considerations limit the ejection velocities from volcanic explosions to about 1 km/s, much lower than the escape velocity of either Mars (5.1 km/s) or the Moon (2.4 km/s), yet at least two dozen meteorites have been found on Earth which definitely originated on Mars or the Moon (55). It now seems almost certain that the only natural process capable of ejecting surface material from a planet or moon into space is the impact of a large object such as a meteorite, asteroid, or comet. Thus, two of the central questions of microbial interplanetary transfer theory are: What are the physical conditions prevailing in the vicinity of an impact sufficiently energetic to accelerate ejecta material to planetary escape velocities? and what are the chances of spores or other microorganisms surviving these launch conditions? Because impacts are highly energetic processes, the major factors predicted to influence bacterial survival during an impact-generated launch are extremes of heat, pressure, and acceleration resulting from transfer of energy during the shock of collision (109).
Heat.
Until recently it was thought that the shock pressure from an impact sufficiently large to launch crustal fragments from a planetary surface into space would melt or even partially vaporize the ejecta, effectively rendering them sterile. However, many of the meteorites originating on Mars or the Moon show evidence of little or no shock-induced internal heating; in the case of Martian meteorite ALH84001, recent measurements of the alignment of magnetic minerals within the meteorite indicate that, except for its thin (0.1 mm) surface fusion crust, it has not been heated above 40°C since its formation at least 4 billion years ago, including its ejection from Mars, transfer through space, entry through Earth's atmosphere over Antarctica, time of residence on the Earth's surface, and recovery and storage at the Johnson Spaceflight Center in Houston. (B. P. Weiss, J. L. Kirschvink, F. J. Baudenbacher, N. T. Peters, F. A. MacDonald, J. P. Wikswo, and H. Vali, Abstr. 1999 Fall Meet. Am. Geophys. Union, abstr. B42F-06). Clearly, spores and even vegetative bacterial cells would be little affected by such a small degree of heating. Moreover, spores would most likely be present inside ejecta fragments in a dry or semidry environment, and dry heat is known to be a less effective sporocidal treatment than moist heat; at any given temperature, spores survive approximately 1,000-fold longer in dry heat than in moist heat (Fig. 1). Indeed, bacteria embedded deep within rock may not even be subjected to appreciable thermal stress at all, as the transit time of ejecta through the atmosphere would be on the order of seconds.
How do such lightly shocked meteorites arise? Melosh (105) first proposed that around the margin of an impact site is located a spall zone (spalls are fragments or chips from a piece of stone) where the reflected shock wave of the impact would create a very large pressure gradient near the surface crust. Because the pressure above the crustal surface is effectively zero, most of the energy of the reflected shock wave would be translated directly into the acceleration of surface rocks upwards, rather than causing their compression and resultant heating. Current estimates predict that an impact of the magnitude of the 180-km diameter Chicxulub impact (which reputedly contributed to the Cretaceous-Tertiary mass extinctions 65 million years ago) could eject many billions of meter-sized terrestrial surface rock fragments from Earth's gravity well with relatively little shock damage. An extensive discussion of the physics and mathematics of spall theory and its relation to the interplanetary transfer of microbial life can be found in reference 64.
The above considerations incite a fundamental microbiological question: Do bacteria live inside rocks? Extensive recent literature indicates that a wide variety of microbial life (called endolithic microbes), including several Bacillus spp., does indeed reside within rock, extending from the surface well into the deep subsurface (8, 16, 49, 129a). These new finds from deep subsurface environments have forced us to reexamine some of our long-cherished taxonomic classifications. For example, the terrestrial subsurface depth record is currently held by the first strictly anaerobic Bacillus spp. described, designated Bacillus infernus, which was isolated from a borehole at a depth of >2.5 km, living in a thermic (60°C), saline (2 M NaCl), anoxic environment (16). Although a strict anaerobe, phylogenetic analysis by 16S rRNA sequence comparison places B. infernus solidly within the species Bacillus, most closely related to B. methanolicus, B. lentus, B. firmus, and B. circulans (16). In addition, numerous deep-subsurface Bacillus spp. have been isolated from deep paleosol (ancient buried soils) and lacustrine (lake) sediments at the Department of Energy's Hanford, Wash., site, including isolates most closely related by 16S rRNA analysis to Bacillus thuringiensis, B. cohnii, B. polymyxa, and B. cereus (8).
Pressure.
Material near the free surface adjacent to an impact or explosion is ejected at high speed as one or more spall plates. Spallation is produced by the interference of the direct compressional wave and a reflected tensile wave. In common experience, spalls are fast-moving chips of rock ejected from near the site of a hammer blow or bullet impact on brittle rock. By necessity, the pressure is zero at the surface itself but increases rapidly with increasing depth below the surface. Thus, only the top few meters of surface crust would be ejected in an unshocked or lightly shocked state. Current estimates of the shock pressures that rock could withstand before collapse of its internal pore spaces and heating to temperatures of above 100°C set the probable upper limit on shock pressure to approximately 1 GPa (or approximately 10,000 atm). What levels of shock pressure can bacteria, and especially spores, withstand? The current laboratory model of spore survival to ultrahigh hydrostatic pressure (see above) is largely insufficient to answer this question, as it relates mainly to isotropic conditions, in which pressure is applied slowly and held for periods far exceeding the time of an impact event. In addition, little information on the survival of bacterial spores to shock-induced pressure is available. In one of the few studies exploring this question, a recovery experiment with an explosive set-up, B. subtilis spores were subjected to a simulated impact calculated to generate a peak shock pressure of 32 GPa (nearly 320,000 atm!), and an appreciable fraction of the population (approximately 10−4) survived (71; G. Horneck, D. Stöffler, U. Eschweiler, and U. Hornemann, unpublished data).
Acceleration.
Spallation theory predicts that ejecta arising from an impact will be accelerated to escape velocity within a very short rise time (on the order of 0.5 ms) (64); the ejecta are therefore exposed not only to extreme accelerations but also to extremes in the rate of change of acceleration (or “jerk”). While macroscopic organisms are readily crushed by accelerations of only a few times Earth gravity of 9.8 m/s2, it is well known that bacteria and spores can tolerate accelerations in laboratory centrifuges exceeding 10,000 to 15,000 × g for virtually indefinite periods of time. What are the upper limits of acceleration and jerk which spores can tolerate? When B. subtilis spores were subjected to ultracentrifugation at 436,000 × g, it was observed that spores were inactivated with first-order kinetics and a D value of approximately 65 h (R. M. E. Mastrapa, H. Glanzberg, J. N. Head, H. J. Melosh, and W. L. Nicholson, Abstr. 30th Conf. Lunar Planetary Soc., abstr. 2045, 2000). In the ultracentrifuge used in this experiment, the rise time to achieve 436,000 × g, was on the order of 2 min, slower by a factor of 2.4 × 105 than would be predicted to occur in an impact-launched rock with a rise time of 0.5 ms.
In order to explore bacterial survival to high accelerations with short rise times, researchers have recently turned to ballistics experiments. Mileikowsky et al. (C. Mileikowsky, E. Larsson, and B. Eiderfors, paper presented at the 25th General Assembly of the European Geophysical Society, April 1999) designed a projectile containing 10 sample compartments. Spores or vegetative cells of B. subtilis or cells of the radioresistant bacterium Deinococcus radiodurans were embedded within different materials, including simulated rock, and loaded inside each compartment. The projectile was then fired from a cannon at an acceleration test facility, and the viability of the samples was determined. While a large fraction of all cell types survived firing up to the maximal acceleration of 33,800 × g with a rise time on the order of 1 ms, B. subtilis spores demonstrated 100% survival under these conditions.
The upper limit of bacterial survival to extreme acceleration and jerk has recently been extended considerably by impact experiments in which projectiles carrying films of air-dried B. subtilis spores or D. radiodurans cells were fired into a chilled plasticene clay target, resulting in rapid deceleration over very short distances (Mastrapa et al., abstr.). Spores and cells were shown to survive well at the maximum deceleration of 4.5 × 106 m/s2 (or 460,000 × g), with a rise time of 0.5 ms, giving a jerk value of 1.5 × 1011 m/s3. Comparing these estimates with the results from computer simulations of impacts, in this experiment spores and cells experienced jerks and accelerations ranging from 2.5 to 25 times larger than the values computed to be necessary for ejection from Mars. The authors therefore concluded that high acceleration and jerk are probably not important factors constraining ejection of rocks containing living microorganisms from planetary surfaces (Mastrapa et al., abstr.).
Interplanetary Travel of Spores
Once rocks have been ejected from the surface of their home planet, the microbial passengers now face an entirely new set of problems affecting their survival, namely, exposure to the space environment. With space technology, a new tool is available for testing experimentally whether microorganisms are capable of surviving a hypothetical journey from one planet to another. In order to study the survival of resistant microbial forms in the upper atmosphere or in free space, microbial samples have been exposed in situ by use of balloons, rockets, and spacecraft and their responses investigated after recovery (reviewed in references 68 and 71). For this purpose, several facilities were developed, such as the exposure device on Gemini, MEED on Apollo, ES029 on Spacelab 1, ERA on EURECA, UV-RAD on Spacelab D2, and Biopan on Foton. These investigations were supported by studies in the laboratory in which certain parameters of space (high and ultrahigh vacuum, extreme temperature, UV radiation of different wavelengths, and ionizing radiation) were simulated. The microbial responses (physiological, genetic, and biochemical changes) to selected factors applied separately and in combination were determined. Because of their high resistance to environmental extremes, spores of B. subtilis have been used as model systems for many of these studies. However, since Bacillus ssp. are not found at the deepest branch of the phylogenetic tree, they are certainly not considered representative of a hypothetical microbial “first colonizer” on the early Earth.
Effects of space vacuum.
Because of its extreme dehydrating effect, space vacuum has been considered to be one of the factors that may prevent interplanetary transfer of life (141). However, space experiments have shown that up to 70% of bacterial and fungal spores survive short-term (e.g., 10 days) exposure to space vacuum, even without any protection (66). The chances of survival in space are increased if the spores are embedded in chemical protectants such as sugars or salt crystals or if they are exposed in thick layers. For example, 30% of B. subtilis spores survived nearly 6 years of exposure to space vacuum when embedded in salt crystals, whereas 80% survived in the presence of glucose (72). It has been suggested that sugars and polyalcohols stabilize the structure of the cellular macromolecules, especially during vacuum-induced dehydration, leading to increased rates of survival. In addition, endolithic microorganisms in nature are probably distributed in microcracks and pore spaces within rock as consortial biofilms embedded in a complex extracellular polysaccharide matrix (27, 100), which would also be expected to provide a degree of protection from extreme vacuum.
To determine the protective effects of different meteorite materials, “artificial meteorites” were constructed by embedding B. subtilis spores in clay, meteorite dust, or simulated Martian soil as described by Horneck et al. (74). Their survival has been determined after exposure to the space environment. Crystalline salt provided sufficient protection for osmophilic microbes to survive at least 2 weeks in space (97). For example, a species of the cyanobacterium Synechococcus that inhabits gypsum-halite crystals was capable of nitrogen and carbon fixation, and about 5% of a species of the extreme halophile Haloarcula survived after exposure to the space environment for 2 weeks in connection with a Foton space flight (97).
The mechanisms of cellular damage due to vacuum exposure are based on the extreme desiccation imposed. If not protected by internal or external substances, cells in a vacuum experience dramatic changes in such important biomolecules as lipids, carbohydrates, proteins, and nucleic acids. Upon desiccation, the lipid membranes of cells undergo dramatic phase changes from planar bilayers to cylindrical bilayers (28). The carbohydrates, proteins, and nucleic acids undergo so-called Maillard reactions, i.e., amino-carbonyl reactions, to give products that become cross-linked, eventually leading to irreversible polymerization of the biomolecules (28). Concomitant with these structural changes are functional changes, including altered selective membrane permeability, inhibited or altered enzyme activity, decreased energy production, alteration of genetic information, etc. Vacuum-induced damage to DNA is especially dramatic, because it may lead to death or mutation. A 10-fold-increased mutation rate over the spontaneous rate has been observed in spores of B. subtilis after exposure to space vacuum (73). This mutagenic effect of vacuum is probably based on a unique molecular signature of tandem double base changes at restricted sites in the DNA (127). In addition, DNA strand breaks have been observed to be induced by exposure to space vacuum (35, 37). Such damage would accumulate during long-term exposure to space vacuum, because DNA repair is not active during anhydrobiosis. As discussed above, spore survival ultimately depends on the efficiency of DNA repair after rehydration and germination. In accordance with this notion, it was shown that the high resistance to vacuum observed in repair-proficient strains of B. subtilis was lost in rec mutant strains that were deficient in DNA repair (127).
The high resistance of bacterial endospores to desiccation is mainly due to a dehydrated core enclosed in a thick protective envelope, the cortex and the spore coat layers (Fig. 6), and the saturation of spore DNA with SASP whose binding greatly alters the chemical and enzymatic reactivity of the DNA (190). However, the strategies by which B. subtilis spores protect their integrity, including that of the DNA, against vacuum damage are not yet fully understood. The accumulation of nonreducing sugars, such as trehalose and sucrose, which are used by other anhydrobiotes (31), is not observed in spores. However, the addition of glucose to the spores substantially increased the survival rate of spores in vacuum (72, 74). These molecules help to prevent damage to the DNA, membranes, and proteins by replacing the water molecules during the desiccation process and thereby preserving the three-dimensional structure of the biomolecules (31). Similar but not yet identified protective molecules may also exist in bacterial spores that may stabilize the DNA double helix by taking the place of the hydrate water which eventually leaves the spore during long-term exposure to vacuum. This may be a time-consuming procedure at room temperatures and even more at increasingly low temperatures. However, in experiments simulating the conditions of interstellar space, B. subtilis spores in vacuum survived even exposures to temperatures as low as 10 K (220).
Effects of extraterrestrial solar UV radiation.
Solar UV radiation has been found to be the most deleterious factor of space, as tested with dried preparations of viruses and of bacterial and fungal spores (66). The reason for this is the highly energetic UV-C and vacuum UV radiation that is directly absorbed by the DNA, as demonstrated by action spectroscopy in space (Fig. 8) (74). The full spectrum of extraterrestrial UV radiation kills unprotected spores of B. subtilis within seconds (71). These harmful UV ranges do not reach the surface of the Earth because they are effectively absorbed by the Earth's atmosphere (Fig. 5 and 7). The photobiological and photochemical effects of extraterrestrial UV radiation are based on the production of specific photoproducts in the DNA that are highly mutagenic and lethal (reviewed in reference 18). The most damaging photochemical lesions are thymine-containing dimers, cyclobutadipyrimidines, the (6-4) pyrimidine-pyrimidone adducts, and their Dewar valence isomer (reviewed in reference 19).
FIG. 8.
Action spectrum of extraterrestrial solar UV inactivation of B. subtilis spores from three space experiments on board the missions Spacelab (SL) 1 (triangles), D2 (squares), and EURECA (circles). Using an optical filtering system, dry layers of spores were exposed to defined wavelengths and intensities of extraterrestrial solar UV radiation during periods when the samples were pointing towards the sun. The spores were either kept at atmospheric pressure in argon (solid symbols) or exposed to the vacuum of space during the whole mission (open symbols). (Data are modified from reference 74.)
If spores of B. subtilis are simultaneously exposed to solar UV radiation and space vacuum, they respond with an increased sensitivity to a broad spectrum of solar UV (>170 nm) as well as to selected wavelengths (Fig. 8). Upon dehydration (e.g., in space vacuum), DNA undergoes substantial conformational changes. This conversion in the physical structure leads to altered DNA photochemistry, resulting in a 10-fold increase in the UV sensitivity of B. subtilis spores in space vacuum compared to spores irradiated at atmospheric pressure (69). Several attempts were made to isolate and identify the photoproducts generated within the DNA of B. subtilis spores exposed to UV radiation in vacuum. Two thymine decomposition products, the cis-syn and trans-syn cyclobutane thymine dimers, as well as DNA-protein cross-linking were tentatively characterized in addition to SP (73, 89).
Effects of cosmic ionizing radiation.
Among the ionizing components of radiation in space, the heavy primaries, the so-called HZE particles, are the most biologically effective species (reviewed in references 32, 67, and 83). Because of their low flux (they contribute to approximately 1% of the flux of particulate radiation in space), methods have been developed to localize precisely the trajectory of an HZE particle relative to the biological object and to correlate the physical data of the particle relative to the observed biological effects along its path. In the Biostack method, visual track detectors are sandwiched between layers of biological objects in a resting state, e.g., Bacillus subtilis spores (17) (Fig. 9). This method allows us (i) to localize each HZE particle's trajectory in relation to the biological specimens, (ii) to investigate the responses of each biological individual hit separately with regard to its radiation effects, (iii) to measure the impact parameter b (i.e., the distance between the particle track and the sensitive target); (iv) to determine the physical parameters charge (Z), energy (E), and linear energy transfer (LET); and finally (v) to correlate the biological effect with each HZE particle's parameters. These studies provide important basic information in the attempt to assess the radiation risks for humans in space.
FIG. 9.
Biostack experiment, a method to study the biological effects of single heavy ions of cosmic radiation. (A) The complete Biostack experiment package (about 10 × 10 × 10 cm in size) accommodated in a hermetically sealed aluminum container was stowed inside the spacecraft during several space missions (Apollo and Spacelab) and exposed to cosmic radiation. (B) The Biostack consists of a sandwich of monolayers of biological objects in a resting state (in this example, B. subtilis spores) mounted on a particle track detector. After irradiation and processing of the track detector, the biological effect on individual spores was investigated and correlated to impact parameter, i.e., the distance from the particle's trajectory (C) (17). CN, cellulose nitrate; PC, polycarbonate; PVA, polyvinyl alcohol.
Bacterial spores having a cytoplasmic core with a geometrical cross section of 0.2 to 0.3 μm2 (Fig. 6) are suitable test systems in HZE particle research, especially for probing the biological effectiveness in dependence of the impact parameter for each particle's trajectory when applying the Biostack concept (Fig. 9). The small size of spores required the development of special techniques: (i) dry monolayers of spores of B. subtilis were mounted directly on the track detector: (ii) the track detector with the spores was etched under microscopic control on the spore-free side only; and (iii) spores located around the track of an HZE particle (at a radius of ≤5 μm) were removed by micromanipulation and deposited in individual incubation chambers (17) (Fig. 10). The accuracy in determining the impact parameter was ≥0.2 μm—depending on the dip angle of the trajectory—which is well below the size of a single spore. Figure 10 shows the frequency of inactivated spores as a function of the impact parameter b. Spores within the range b ≤0.2 μm were inactivated by 73%. The frequency of inactivated spores dropped abruptly at b >0.2 μm. However, 15 to 30% of spores located within 0.2 < b < 3.8 μm were still inactivated. A statistical analysis showed that all data at b ≤3.8 μm are significantly different from the control value (at b >10 μm) (95% confidence) (41a). Hence, spores were inactivated well beyond 1 μm, which distance would roughly correspond to the dimensions of a spore. At the distance of 1 μm, the mean dose of δ-rays (δ-rays are secondary electrons produced by interaction of the primary ions with matter) ranges between 0.1 and 1 Gy, depending on the particle, and declines rapidly with increasing b (40). This value of 0.1 to 1 Gy is several orders of magnitude below the D37 (dose reducing survival by e−1) of electrons, which amounts to 800 Gy. Therefore, the radial long-ranging effect around the trajectory of an HZE particle (up to b = 3.8 μm) cannot be explained merely by the δ-ray dose. These results were largely confirmed by experiments at heavy ion accelerators (67). Combining the results from the experiments in space with those obtained at accelerators, one can draw the following general conclusions. (i) The inactivation probability for spores, centrally hit, is always substantially less than 1. (ii) The effective range of inactivation extends far beyond the range of impact parameter where inactivation of spores by δ-rays can be expected. This far-reaching effect is less pronounced for ions of low energies (1.4 MeV/μm), a phenomenon which might reflect the “thin-down effect” at the end of the ion's path (80). (iii) The dependence of inactivated spores from impact parameter points to a superposition of two different inactivation mechanisms: a short-range component reaching up to about 1 μm may be traced back to the δ-ray dose, and a long-range one that extends at least to somewhere between 4 and 5 μm off the particle's trajectory, for which additional mechanisms are conjectured, such as shock waves, UV radiation, or thermophysical events (41).
FIG. 10.
Frequency of inactivated spores of B. subtilis as a function of the impact parameter. During several space missions, spores were exposed to the HZE particles of cosmic radiation in a Biostack experiment, and the viability of each spore in the vicinity of the trajectory of an HZE particle was analyzed separately under the microscope after micromanipulation and incubation in small incubation chambers. Data are based on the analysis of 121 HZE particle tracks, 919 spores from the target area, and 580 control spores. (Data modified from reference 67.)
As shown above, B. subtilis spores can survive even a central hit of an HZE particle of cosmic radiation (reviewed in reference 66). Such HZE particles of cosmic radiation are conjectured to set the ultimate limit on the survival of spores in space because they penetrate even thick shielding. However, since the flux of HZE particles is relatively low, a spore may exist in space for several hundred thousand years before being hit by an HZE particle (e.g., iron of LET > 100 keV/μm). This time span complies with estimates of the time required for boulder-sized rocks to travel from one planet of our solar system to another, e.g., from Mars to Earth, by random motion (107). However, only a few months have been estimated to be sufficient for an interplanetary transfer of microscopic particles (112).
Concerning shielding against radiation in space, a few micrometers of meteorite material are sufficient to give efficient protection against UV if the material is without cracks, and a shielding of less than 0.5 g/cm2 is required against diffuse X-rays and about 30 g/cm2 against solar particles. (Shielding is determined by the thickness of the material [in centimeters] times its density [in grams per cubic centimeter].) However, because the particles of cosmic radiation interact with the shielding material by creating secondary radiation, the dose rates go through a maximum with increasing shielding thickness. The dose rate has been calculated behind different shielding thicknesses, based on (i) inactivation cross sections for B. subtilis spores from experimental data obtained at heavy ion accelerators (9), (ii) an estimated density for the meteorite of 1.8 g/cm3 (taken from data for a Martian regolith), and (iii) the National Aeronautics and Space Administration HZETRN transport code using distribution of cosmic ray particles, their interaction with matter, and the track structure model for cell inactivation, which has been developed to calculate the radiation risks for humans in space missions (32). The dose rate reaches its maximum behind a shielding layer of about 10 cm; behind about 130 cm, the value is the same as obtained without any shielding, and only for higher shielding thickness is the dose rate reduced significantly (Fig. 11) (109). The calculations also show that even after 25 million years in space, a substantial fraction of a spore population (10−6) would survive the exposure to cosmic radiation if shielded by 2 to 3 m of meteorite material. The same surviving fraction would be reached after about 500,000 years without any shielding or a shielding of less than 1 m. However, one has to bear in mind that radiation is only one of the harmful parameters in space and that for the success of a viable transfer, the microbes have ultimately to cope with the complex matrix of all environmental factors governing the space milieu (reviewed in reference 109).
FIG. 11.
Shielding of bacterial spores against galactic cosmic radiation by meteorite material. The dashed line gives the annual dose profile (in Grays per year), which increases for the outer 10 cm due to secondary radiation and then decreases. The solid line gives the exposure time for a survival rate of 10−6 (survival of 100 spores from an initial population of 108 spores). (Data from reference 109.)
Combined effects of space.
With the LDEF mission, for the first time B. subtilis spores were exposed to the full environment of space for an extended period of time (nearly 6 years), and their survival was determined after retrieval. The samples were separated from space by a perforated aluminum dome only, which allowed access of space vacuum, solar UV radiation, and most of the components of cosmic radiation (72). Figure 12 shows that even in the unprotected samples, thousands of spores survived the space journey (from an initial sample size of 108 spores). All spores were exposed in multilayers and predried in the presence of glucose. The spore samples had turned from white to yellow during the mission, a phenomenon which is probably due to photochemical processes. It was suggested that all spores in the upper layers were completely inactivated by the high flux of solar UV radiation. With time, they formed a protective crust which considerably attenuated the solar UV radiation for the spores located beneath them. Therefore, the survivors probably originated from the innermost part of the samples (72).
FIG. 12.
Survival of B. subtilis spores on the LDEF experiment after nearly 6 years in space. The spores in multilayers were either exposed under a perforated dome to the full environment of space or shielded either by a quartz window 2 mm thick, which allowed access of solar UV radiation (λ > 170 nm), or an aluminum cover of 2 mm. (Graph drawn from data in reference 72.)
Time scales of interplanetary or interstellar transport of life.
To travel from one planet of our solar system to another, e.g., from Mars to Earth, by random motion, a mean time of several hundred thousands to millions of years has been estimated for boulder-sized rocks (107), but only 2 months for microscopic particles (112). Therefore, can the LDEF experiment be considered a reasonable simulation of an interplanetary transfer event? Because LDEF was in a near-equatorial low-altitude Earth orbit, during each orbit the satellite travelled about 40,650 km every 90 min. Thus, over the nearly 6-year duration of the LDEF experiment, the satellite travelled over 109 km, the approximate equivalent of the distance from Earth to Saturn if it had been flying on a straight course rather than in an elliptical orbit. The likelihood of spore survival over the time spans involved in interplanetary transfer is therefore quite high (109). For an interstellar transport, the following picture has been drawn. The clouds of gaseous and particulate matter between the stars move in a random fashion at speeds of about 10 km/s. If a bacterial spore is captured in such a cloud, it will be swept along with the gas. Given the distance between neighboring stars of 0.1 to 1 parsec (1 parsec = 3.26 light years), this corresponds to a passage time of 105 to 106 years (220). Thus, for interstellar infection, microorganisms would have to survive for at least 105 to 106 years in such a cloud, and as in interplanetary transfer models, this probability is quite low for naked spores but rises dramatically with minimal shielding material (109). Results from exposing microorganisms to space for up to several years are available (66, 72). More data on long-term effects are required. Exposure platforms, such as the Expose on the International Space Station (ISS), are especially suited for this kind of research. Such studies will eventually allow extrapolation to the time spans required for interplanetary or interstellar transport of life.
Deposition of Spores from Space to a Planet
Assuming that spores trapped inside ejecta survive launch and transit through space, they must be captured by the gravity of a recipient planet and survive entry. Our current understanding of what happens to a rock fragment upon reentry is generally good, thanks to years of study of natural meteors and the reentry of spacecraft (24, 108, 147). It turns out that planets with atmospheres are almost ideal recipients of life-bearing rock fragments. Although small (0.001 to 1 kg) fragments may burn up entirely in Earth's atmosphere, larger fragments are slowed dramatically upon reentry to a terminal velocity of a few hundred meters per second. Because the entire reentry process, from first encounter with the atmosphere to impact, takes only about 1 min or so, the interior of the meteorite (except for a few millimeters of ablation crust at the surface) is not heated significantly above its in-space temperature. This accounts for the well-documented phenomenon that recently landed meteorites are cold to the touch. Aerodynamic drag forces often disaggregate the rock in the lower atmosphere, and the resulting fragments strike Earth's surface in the characteristic pattern of a strewn field (147). Upon impact, these fragments are further shattered and mixed with the surface material of the destination planet. It is therefore possible for endolithic microbes not only to survive reentry embedded in a meteorite, but actually to be effectively inoculated into the recipient planet's crust, thereby encountering an environment potentially conductive to growth.
Outlook for the Future
For future research on bacterial spores and other microorganisms in space, the European Space Agency is developing the Expose facility that is to be attached to the external pallet of the truss structure of the ISS for 1.5 years during the ISS early utilization period. Expose will support long-term in situ studies of microbes in artificial meteorites as well as of microbial communities from special ecological niches, such as endolithic (within rocks) and endoevaporitic (within salt crystals, e.g., evaporitic gypsum-halite crusts that form along the marine intertidal or evaporitic NaCl crystal from salt evaporation ponds) ecosystems (77). These experiments on the responses of organisms to the space environment (ROSE) include the study of photobiological processes in simulated radiation climates of planets (e.g., early Earth, early and present Mars, and the role of the ozone layer in protecting the biosphere from harmful UV-B radiation), as well as studies of the probabilities and limitations for life to be distributed beyond its planet of origin. Results from the Expose experiments will provide us with a better understanding of the processes regulating the interactions of life with its environment.
ACKNOWLEDGMENTS
Work in the Nicholson lab has been supported by grants from the National Institutes of Health (GM47461), the American Cancer Society (JFRA-410), the Arizona Agricultural Experimental Station (USDA-Hatch), and the University of Arizona/NASA Space Grant Undergraduate Research Internship Program. Work in the Setlow lab has been supported by grants from the Army Research Office and the NIH (GM19698).
REFERENCES
- 1.Alderton G, Snell N. Chemical states of bacterial spores: dry-heat resistance. Appl Microbiol. 1969;17:745–749. doi: 10.1128/am.17.5.745-749.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrhenius S. Die Verbreitung des Lebens im Weltenraum. Umschau. 1903;7:481–485. [Google Scholar]
- 3.Arroyo G, Sanz P D, Prestamo G. Response to high-pressure, low-temperature treatment in vegetables: determination of survival rates of microbial populations using flow cytometry and detection of peroxidase activity using confocal microscopy. J Appl Microbiol. 1999;86:544–556. doi: 10.1046/j.1365-2672.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 4.Atrih A, Zollner P, Allmaier G, Foster S F. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babitzke P. Regulation of tryptophan biosynthesis: trp-ing the TRAP, or how Bacillus subtilis reinvented the wheel. Mol Microbiol. 1997;26:1–9. doi: 10.1046/j.1365-2958.1997.5541915.x. [DOI] [PubMed] [Google Scholar]
- 6.Bagyan I, Casillas-Martinez L, Setlow P. The katX gene which codes for the catalase in spores of Bacillus subtilis is a forespore specific gene controlled by ςF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol. 1998;180:2057–2062. doi: 10.1128/jb.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balassa G, Milhaud P, Raulet E, Silva M T, Sousa J C F. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J Gen Microbiol. 1979;110:365–379. doi: 10.1099/00221287-110-2-365. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill D L, Reeves R H, Drake G R, Reeves J Y, Crocker F H, King M B, Boone D R. Phylogenetic characterization of bacteria in the subsurface microbial culture collection. FEMS Microbiol Rev. 1997;20:201–216. doi: 10.1111/j.1574-6976.1997.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 9.Baltschukat K, Horneck G. Responses to accelerated heavy ions of spores of Bacillus subtilis of different repair capacity. Radiat Environ Biophys. 1991;30:87–103. doi: 10.1007/BF01219343. [DOI] [PubMed] [Google Scholar]
- 10.Beaman T C, Gerhardt P. Heat resistance of bacterial spores correlated with protoplast dehydration, mineralization, and thermal adaptation. Appl Environ Microbiol. 1986;52:1242–1246. doi: 10.1128/aem.52.6.1242-1246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belliveau B H, Beaman T C, Pankratz S, Gerhardt P. Heat killing of bacterial spores analyzed by differential scanning calorimetry. J Bacteriol. 1992;174:4463–4474. doi: 10.1128/jb.174.13.4463-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender G R, Marquis R E. Spore heat resistance and specific mineralization. Appl Environ Microbiol. 1985;50:1414–1421. doi: 10.1128/aem.50.6.1414-1421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieger-Dose A, Dose K, Meffert R, Mehler M, Risi S. Extreme dryness and DNA-protein crosslinks. Adv Space Res. 1992;12:265–270. doi: 10.1016/0273-1177(92)90181-v. [DOI] [PubMed] [Google Scholar]
- 14.Björn L O, Teramura A H. Simulation of daylight UVR. In: Young A R, Björn L O, Moan J, Nultsch W, editors. Environmental UV photobiology. New York, N.Y: Plenum Press; 1993. pp. 38–72. [Google Scholar]
- 15.Bloomfield S F, Arthur M. Mechanisms of inactivation and resistance of spores to chemical biocides. J Appl Bacteriol. 1994;76:91S–104S. doi: 10.1111/j.1365-2672.1994.tb04361.x. [DOI] [PubMed] [Google Scholar]
- 16.Boone D R, Liu Y, Zhao Z-J, Balkwill D L, Drake G R, Stevens T O, Aldrich H C. Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int J Syst Bacteriol. 1995;45:441–448. doi: 10.1099/00207713-45-3-441. [DOI] [PubMed] [Google Scholar]
- 17.Bücker H, Horneck G. Studies on the effects of cosmic HZE-particles in different biological systems in the biostack experiments I and II, flown on board of Apollo 16 and 17. In: Nygaard O F, Adler H I, Sinclair W K, editors. Radiation research. New York, N.Y: Academic Press; 1975. pp. 1138–1151. [Google Scholar]
- 18.Cadet J, Voituriez L, Grand A, Hruska F E, Vigny P, Kan L S. Photosensitized reactions of nucleic acids. Biochimie. 1985;67:277. doi: 10.1016/s0300-9084(85)80070-5. [DOI] [PubMed] [Google Scholar]
- 19.Cadet J, Weinfeld M. Detecting DNA damage. Anal Chem. 1993;65:675A–682A. doi: 10.1021/ac00063a001. [DOI] [PubMed] [Google Scholar]
- 20.Cano R J, Borucki M K. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science. 1995;268:1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 21.Casillas-Martinez L, Setlow P. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J Bacteriol. 1997;179:7420–7425. doi: 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N-Y, Zhang J-J, Paulus H. Chromosomal location of the Bacillus subtilis aspartokinase II gene and nucleotide sequence of the adjacent genes homologous to uvrC and trx of Escherichia coli. J Gen Microbiol. 1989;135:2931–2940. doi: 10.1099/00221287-135-11-2931. [DOI] [PubMed] [Google Scholar]
- 23.Cheo D L, Bayles K W, Yasbin R E. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J Bacteriol. 1991;173:1696–1703. doi: 10.1128/jb.173.5.1696-1703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chyba C F, Thomas P J, Zahnle K J. The 1908 Tunguska explosion: atmospheric disruption of a stony asteroid. Nature. 1993;361:40–44. [Google Scholar]
- 25.Cohn F. Untersuchungen über Bakterien. IV. Beiträge zur Biologie der Bacillen. Beiträge zur Biologie der Pflanzen. 1876;2:249–276. [Google Scholar]
- 26.Condon S, Bayante M, Sala F J. Influence of the sporulation temperature upon the heat resistance of Bacillus subtilis. J Appl Bacteriol. 1992;73:251–256. doi: 10.1111/j.1365-2672.1992.tb02985.x. [DOI] [PubMed] [Google Scholar]
- 27.Costerton J W, Cheng K J, Geesey G G, Ladd T I, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 28.Cox C S. Roles of water molecules in bacteria and viruses. Origins Life Evol Biosph. 1993;23:29–36. doi: 10.1007/BF01581988. [DOI] [PubMed] [Google Scholar]
- 29.Crawford Y J, Murano E A, Olson D G, Shenoy K. Use of high hydrostatic pressure and irradiation to eliminate Clostridium sporogenes spores in chicken breast. J Food Prot. 1996;59:711–715. doi: 10.4315/0362-028X-59.7.711. [DOI] [PubMed] [Google Scholar]
- 30.Crick F H C, Orgel L E. Directed panspermia. Icarus. 1973;19:341–346. [Google Scholar]
- 31.Crowe L M, Crowe J H. Anhydrobiosis: a strategy for survival. Adv Space Res. 1992;12:(4)239–(4)247. doi: 10.1016/0273-1177(92)90178-z. [DOI] [PubMed] [Google Scholar]
- 32.Cucinotta F A, Wilson J W, Katz R, Atwell W, Badhuser G D, Shavers M R. Track structure and radiation transport model for space radiobiology studies. Adv Space Res. 1995;18:183–194. [Google Scholar]
- 33.Daniel R A, Errington J. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipcolinic acid synthetase required for sporulation in Bacillus subtilis. J Mol Biol. 1993;232:468–483. doi: 10.1006/jmbi.1993.1403. [DOI] [PubMed] [Google Scholar]
- 34.Donnellan J E, Jr, Setlow R B. Thymine photoproducts but not thymine dimers are found in ultraviolet irradiated bacterial spores. Science. 1965;149:308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- 35.Dose K, Bieger-Dose A, Dillmann R, Gill M, Kerz O, Klein A, Meinert H, Nawroth T, Risi S, Stridde C. ERA-experiment “space biochemistry.”. Adv Space Res. 1995;16:(8)119–(8)129. [PubMed] [Google Scholar]
- 36.Dose K, Bieger-Dose A, Kerz O, Gill M. DNA-strand breaks limit survival in extreme dryness. Origins Life Evol Biosph. 1991;21:177–188. doi: 10.1007/BF01809446. [DOI] [PubMed] [Google Scholar]
- 37.Dose K, Bieger-Dose A, Labusch M, Gill M. Survival in extreme dryness and DNA-single-strand breaks. Adv Space Res. 1992;12:221–229. doi: 10.1016/0273-1177(92)90176-x. [DOI] [PubMed] [Google Scholar]
- 38.Driks A. Bacillus subtilis spore coat. Microbiol Mol Biol Rev. 1999;63:1–20. doi: 10.1128/mmbr.63.1.1-20.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driks A, Setlow P. Morphogenesis and properties of the bacterial spore. In: Brun Y, Shimkets L, editors. Prokaryotic development. Washington, D.C.: American Society for Microbiology; 1999. pp. 191–218. [Google Scholar]
- 40.Facius R, Bücker H, Hildebrand D, Horneck G, Höltz G, Reitz G, Schäfer M, Toth B. Radiobiological results from the Bacillus subtilis Biostack experiments within the Apollo and ASTP spaceflights. Life Sci Space Res. 1978;16:151–156. doi: 10.1016/b978-0-08-022022-2.50028-9. [DOI] [PubMed] [Google Scholar]
- 41.Facius R, Bücker H, Reitz G, Schäfer M. Radial dependence of biological response of spores of Bacillus subtilis around tracks of heavy ions. 1978. pp. 977–986. . Proceedings of the 6th Symposium on Microdosimetry, EUR 6064 DE-EN-FR. Harwood Academic Press, London, U.K. [Google Scholar]
- 41a.Facius R, Reitz G, Schäfer M. Inactivation of individual Bacillus subtilis spores in dependence on their distance to single heavy ions. Adv Space Res. 1994;14:(10)1027–(10)1038. doi: 10.1016/0273-1177(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 42.Fairhead H, Setlow B, Waites W M, Setlow P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from being killed by freeze-drying. Appl Environ Microbiol. 1994;60:2647–2649. doi: 10.1128/aem.60.7.2647-2649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fajardo-Cavazos P, Nicholson W L. Molecular dissection of mutations in the Bacillus subtilis spore photoproduct lyase gene which affect repair of spore DNA damage caused by UV radiation. J Bacteriol. 1995;177:4402–4409. doi: 10.1128/jb.177.15.4402-4409.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fajardo-Cavazos P, Nicholson W L. The TRAP-like SplA protein is a trans-acting negative regulator of spore photoproduct lyase synthesis during Bacillus subtilis sporulation. J Bacteriol. 2000;182:555–560. doi: 10.1128/jb.182.2.555-560.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fajardo-Cavazos P, Salazar C, Nicholson W L. Molecular cloning and characterization of the Bacillus subtilis spore photoproduct lyase (spl) gene, which is involved in repair of UV-induced DNA damage during spore germination. J Bacteriol. 1993;175:1735–1744. doi: 10.1128/jb.175.6.1735-1744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster S J, Johnstone K. The trigger mechanism of bacterial spore germination. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C.: American Society for Microbiology; 1989. pp. 89–108. [Google Scholar]
- 47.Fox K, Eder B D. Comparison of survivor curves of Bacillus subtilis spores subjected to wet and dry heat. J Food Sci. 1969;34:518–521. [Google Scholar]
- 48.Frederick J E. Ultraviolet sunlight reaching the Earth's surface: a review of recent research. Photochem Photobiol. 1993;57:175–178. [Google Scholar]
- 49.Fredrickson J K, Onstott T C. Microbes deep inside the earth. Sci Am. 1996;275:68–73. doi: 10.1038/scientificamerican1096-68. [DOI] [PubMed] [Google Scholar]
- 50.Friedberg E C. Relationships between DNA repair and transcription. Annu Rev Biochem. 1996;65:15–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- 51.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 52.Gerhardt P, Marquis R E. Spore thermoresistance mechanisms. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C.: American Society for Microbiology; 1989. pp. 43–63. [Google Scholar]
- 53.Gerhardt P, Scherrer R, Black S H. Molecular sieving by dormant spore structures. In: Halvorson H O, Hanson R, Campbell L L, editors. Spores V. Washington, D.C.: American Society for Microbiology; 1972. pp. 68–74. [Google Scholar]
- 54.Gest H, Mandelstam J. Longevity of microorganisms in natural environments. Microbiol Sci. 1987;4:69–71. [PubMed] [Google Scholar]
- 55.Gladman B J, Burns J A, Duncan M, Lee P, Levinson H F. The exchange of impact ejecta between the terrestrial planets. Science. 1996;271:1387–1392. [Google Scholar]
- 55a.Gogarten J P, Taiz L. Evolution of proton pumping ATPases: rooting the tree of life. Photosynthesis Res. 1992;33:137–146. doi: 10.1007/BF00039176. [DOI] [PubMed] [Google Scholar]
- 56.Gould G W. Mechanisms of resistance and dormancy. In: Gould G W, Hurst A, editors. The bacterial spore. Vol. 2. London, U.K: Academic Press; 1983. pp. 397–444. [Google Scholar]
- 56a.Grahn D, editor. HZE particle effects in manned spaceflight. Radiobiological Advisory Board, Committee on Space Medicine, National Academy of Sciences. Washington, D.C.: National Academy Press; 1973. [Google Scholar]
- 57.Grossman A D. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 58.Hadden C T. Postirradiation recovery dependent on the uvr-1 locus in Bacillus subtilis. J Bacteriol. 1976;128:317–324. doi: 10.1128/jb.128.1.317-324.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanlin J H, Lombardi S J, Slepecky R A. Heat and UV light resistance of vegetative cells and spores of Bacillus subtilis Rec− mutants. J Bacteriol. 1985;163:774–777. doi: 10.1128/jb.163.2.774-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanson R S, Curry M V, Garner J V, Halvorson H O. Mutants of Bacillus cereus strain T that produce thermoresistant spores lacking dipicolinic acid and have low levels of calcium. Can J Microbiol. 1972;18:1139–1143. doi: 10.1139/m72-175. [DOI] [PubMed] [Google Scholar]
- 61.Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Sussex, England: John Wiley and Sons; 1990. [Google Scholar]
- 62.Hayes C S, Illades-Aguiar B, Casillas-Martinez L, Setlow P. In vitro and in vivo oxidation of methionine residues in small, acid-soluble spore proteins from Bacillus species. J Bacteriol. 1998;180:2694–2700. doi: 10.1128/jb.180.10.2694-2700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayes C S, Setlow P. Analysis of deamidation of small acid-soluble spore proteins from Bacillus subtilis in vitro and in vivo. J Bacteriol. 1997;179:6020–6027. doi: 10.1128/jb.179.19.6020-6027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Head J N. Fragmentation and ejection of the Martian clan meteorites. Ph.D. thesis. Tucson: University of Arizona; 1999. [Google Scholar]
- 64a.Hecker M, Schumann W, Volker U. Heat shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 65.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 66.Horneck G. Responses of Bacillus subtilis spores to the space environment: results from experiments in space. Origins Life Evol Biosph. 1993;23:37–52. doi: 10.1007/BF01581989. [DOI] [PubMed] [Google Scholar]
- 67.Horneck G. The Biostack concept and its application in space and at accelerators: studies on Bacillus subtilis spores. NATO ASI Ser Ser A Life Sci. 1993;243A:99–115. [Google Scholar]
- 68.Horneck G. Exobiology, the study of the origin, evolution and distribution of life within the context of cosmic evolution: a review. Planet Space Sci. 1995;43:189–217. doi: 10.1016/0032-0633(94)00190-3. [DOI] [PubMed] [Google Scholar]
- 69.Horneck G. Exobiological experiments in Earth orbit. Adv Space Res. 1998;22:317–326. [Google Scholar]
- 70.Horneck G. European activities in exobiology in Earth orbit: results and perspectives. Adv Space Res. 1999;23:381–386. [Google Scholar]
- 71.Horneck G, Brack A. Study of the origin, evolution and distribution of life with emphasis on exobiology experiments in Earth orbit. In: Bonting S L, editor. Advances in space biology and medicine. Vol. 2. Greenwich, Conn: JAI Press; 1992. pp. 229–262. [DOI] [PubMed] [Google Scholar]
- 72.Horneck G, Bücker H, Reitz G. Long-term survival of bacterial spores in space. Adv Space Res. 1994;14:41–45. doi: 10.1016/0273-1177(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 73.Horneck G, Bücker H, Reitz G, Requardt H, Dose K, Martens K D, Menningmann H D, Weber P. Microorganisms in the space environment. Science. 1984;225:226–228. doi: 10.1126/science.225.4658.226. [DOI] [PubMed] [Google Scholar]
- 74.Horneck G, Eschweiler U, Reitz G, Wehner J, Willimek R, Strauch K. Biological responses to space: results of the experiment “exobiological unit” of ERA on EURECA I. Adv Space Res. 1995;16:105–111. doi: 10.1016/0273-1177(95)00279-n. [DOI] [PubMed] [Google Scholar]
- 75.Horneck G, Rettberg P, Rabbow E, Strauch W, Seckmeyer G, Facius R, Reitz G, Strauch K, Schott J U. Biological dosimetry of solar radiation for different simulated ozone column thicknesses. J Photochem Photobiol B Biol. 1996;32:189–196. doi: 10.1016/1011-1344(95)07219-5. [DOI] [PubMed] [Google Scholar]
- 76.Reference deleted.
- 77.Horneck G, Wynn-Williams D D, Mancinelli R, Cadet J, Munakata N, Rontó G, Edwards H G M, Hock B, Wänke H, Reitz G, Dachev T, Häder D P, Brillouet C. Biological experiments on the EXPOSE facility of the International Space Station. In: Wilson A, editor. Proceedings of the 2nd European Symposium on Utilisation of the International Space Station. Noordwijk, The Netherlands: ESA, ESTEC; 1999. pp. 459–468. [Google Scholar]
- 78.Irie R, Okamoto T, Fujita Y. Estimation of photoproduct formation in germinating spores of Bacillus subtilis. Photochem Photobiol. 1982;35:783–787. doi: 10.1111/j.1751-1097.1982.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 79.Jannasch H W, Wirsen C O, Molyneaux S J, Langworthy T A. Comparative physiological studies on hyperthermophilic archaea isolated from deep-sea hot vents with emphasis on Pyrococcus strain GB-D. Appl Environ Microbiol. 1992;58:3472–3481. doi: 10.1128/aem.58.11.3472-3481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katz R. Biological effects of heavy ions from the standpoint of target theory. Adv Space Res. 1986;6:(11)191–(11)198. doi: 10.1016/0273-1177(86)90292-9. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy M J, Reader S L, Swierczynski L M. Preservation records of micro-organisms: evidence of the tenacity of life. Microbiology. 1994;140:2513–2519. doi: 10.1099/00221287-140-10-2513. [DOI] [PubMed] [Google Scholar]
- 82.Kerr R A. Martian meteorites are arriving. Science. 1987;237:721–723. doi: 10.1126/science.237.4816.721. [DOI] [PubMed] [Google Scholar]
- 83.Kiefer J, Kost M, Schenk-Meuser K. Radiation biology. In: Moore D, Bie P, Oser H, editors. Biological and medical research in space. Berlin, Germany: Springer; 1996. pp. 300–367. [Google Scholar]
- 84.Koch R. Untersuchungen über Bakterien. V. Die Aetiologie der Milzbrand Krankheit, begrandet auf Entwicklurgs geschichte des Bacillus anthracis. Beiträge zur Biologie der Pflanzen. 1876;2:277–308. [Google Scholar]
- 85.Koike J, Oshima T, Koike K A, Taguchi H, Tanaka R, Nishimura K, Miyaji M. Survival rates of some terrestrial microorganisms under simulated space conditions. Adv Space Res. 1992;12:(4)271–(4)274. doi: 10.1016/0273-1177(92)90182-w. [DOI] [PubMed] [Google Scholar]
- 86.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 87.Lin J-J, Sancar A. Reconstitution of nucleotide excision nuclease with UvrA and UvrB proteins from Escherichia coli and UvrC protein from Bacillus subtilis. J Biol Chem. 1990;265:21337–21341. [PubMed] [Google Scholar]
- 88.Lin J-J, Sancar A. (A)BC excinuclease: the Escherichia coli nucleotide excision repair enzyme. Mol Microbiol. 1992;6:2219–2224. doi: 10.1111/j.1365-2958.1992.tb01398.x. [DOI] [PubMed] [Google Scholar]
- 89.Lindberg C, Horneck G. Action spectra for survival and spore photoproduct formation of Bacillus subtilis irradiated with short-wavelength (200–300 nm) UV at atmosphere pressure and in vacuo. J Photochem Photobiol B Biol. 1991;11:69–80. doi: 10.1016/1011-1344(91)80269-n. [DOI] [PubMed] [Google Scholar]
- 90.Lindsay J A, Murrell W G. A comparison of UV-induced DNA photoproducts from isolated and non-isolated bacterial forespores. Biochem Biophys Res Commun. 1983;113:618–625. doi: 10.1016/0006-291x(83)91771-0. [DOI] [PubMed] [Google Scholar]
- 91.Lorenz P R, Hemenway C L, Hotchin J. The biological effectiveness of solar electromagnetic radiation in space. Life Sci Space Res. 1968;6:100. [PubMed] [Google Scholar]
- 92.Lorenz P R, Orlob G B, Hemenway C L. Survival of microorganisms in space. Space Life Sci. 1969;1:491–500. doi: 10.1007/BF00924239. [DOI] [PubMed] [Google Scholar]
- 93.Loshon C A, Genest P C, Setlow B, Setlow P. Formaldehyde kills spores of Bacillus subtilis by DNA damage, and small, acid-soluble spore proteins of the α/β-type protect spores against this DNA damage. J Appl Microbiol. 1999;87:8–14. doi: 10.1046/j.1365-2672.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 94.Ludwig H, Bieler C, Hallbauer K, Scigalla W. Inactivation of microorganisms by hydrostatic pressure. Colloq INSERM. 1992;224:25–32. [Google Scholar]
- 95.Madronich S. The atmosphere and UV-B radiation at ground level. In: Young A R, et al., editors. Environmental UV photobiology. New York, N.Y: Plenum; 1993. pp. 1–37. [Google Scholar]
- 96.Madronich S, DeGruijl F R. Stratospheric ozone depletion between 1979 and 1992: implications for biologically active ultraviolet-B radiation and non-melanoma skin cancer incidence. Photochem Photobiol. 1994;59:541–546. doi: 10.1111/j.1751-1097.1994.tb02980.x. [DOI] [PubMed] [Google Scholar]
- 97.Mancinelli R L, White M R, Rothschild L J. Biopan survival I: exposure of the osmophiles Synechococcus sp. (Nageli) and Haloarcula sp. to the space environment. Adv Space Res. 1998;22:327–334. [Google Scholar]
- 98.Marquis R E, Shin S Y. Mineralization and responses of bacterial spores to heat and oxidative agents. FEMS Microbiol Rev. 1994;14:375–380. doi: 10.1111/j.1574-6976.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 99.Marquis R E, Sim J, Shin S Y. Molecular mechanisms of resistance to heat and oxidative damage. J Appl Bacteriol. 1994;76:40S–48S. doi: 10.1111/j.1365-2672.1994.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 100.Marshall K C. Colonization, adhesion, and biofilms. In: Hurst C J, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: ASM Press; 1997. pp. 358–365. [Google Scholar]
- 101.Reference deleted.
- 102.McDonnell G, Russell A D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKay D S, Gibson E K, Jr, Thomas-Keprta K L, Vali H, Romanek C S, Clemett S J, Chillier X D F, Maechling C R, Zare R N. Search for past life on Mars: possible relic biogenic activity in Martian meteorite ALH84001. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 104.McKenzie R L, Blumthaler M, Booth R, Diaz S B, Frederick J E, Ito T, Madronich S, Seckmeyer G. Surface ultraviolet radiation. In: Albritton D L, Watson R T, Aucamp P J, editors. United Nations Environment Programme-World Meteorological Organisation scientific assessment of ozone depletion: 1994. World Meteorological Organisation global ozone research and monitoring project report no. 37. Geneva, Switzerland: United Nations Environment Programme; 1995. pp. 9.1–9.22. [Google Scholar]
- 105.Melosh H J. Impact ejection, spallation, and the origin of meteorites. Icarus. 1984;59:234–260. [Google Scholar]
- 106.Melosh H J. Ejection of rock fragments from planetary bodies. Geology. 1985;13:144–148. [Google Scholar]
- 107.Melosh H J. The rocky road to panspermia. Nature. 1988;332:687–688. doi: 10.1038/332687a0. [DOI] [PubMed] [Google Scholar]
- 108.Melosh H J. Impact cratering: a geologic process. New York, N.Y: Oxford University Press; 1989. [Google Scholar]
- 109.Mileikowsky C, Cucinotta F A, Wilson J W, Gladman B, Horneck G, Lindegren L, Melosh H J, Rickman H, Valtonen M, Zheng J Q. Natural transfer of viable microbes in space, part 1: from Mars to Earth and Earth to Mars. Icarus. 2000;145:391–427. doi: 10.1006/icar.1999.6317. [DOI] [PubMed] [Google Scholar]
- 110.Reference deleted.
- 111.Mitchell F J, Ellis W L. Analysis of Surveyor 3 material and photographs returned by Apollo 12. Washington, D.C.: U.S. Government Scientific and Technical Information Office; 1972. Surveyor 3: bacterium isolated from lunar-retrieved television camera; pp. 239–251. [Google Scholar]
- 112.Moreno M A. Microorganism transport from Earth to Mars. Nature. 1988;336:209. [Google Scholar]
- 112a.Movahedi, S., and W. Waites. 2000. A two-dimensional protein gel electrophoresis study of the heat stress response of Bacillus subtilis during sporulation. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 113.Munakata N. Genetic analysis of a mutant of Bacillus subtilis producing ultraviolet-sensitive spores. Mol Gen Genet. 1969;104:258–263. doi: 10.1007/BF02539290. [DOI] [PubMed] [Google Scholar]
- 114.Munakata N. Ultraviolet sensitivity of Bacillus subtilis spores upon germination and outgrowth. J Bacteriol. 1974;120:59–65. doi: 10.1128/jb.120.1.59-65.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munakata N. Mapping of the genes controlling excision repair of pyrimidine photoproducts in Bacillus subtilis. Molec Gen Genet. 1977;156:49–54. [Google Scholar]
- 116.Munakata N. Killing and mutagenic action of sunlight upon Bacillus subtilis spores: a dosimetric system. Mutat Res. 1981;82:263–268. doi: 10.1016/0027-5107(81)90155-x. [DOI] [PubMed] [Google Scholar]
- 117.Munakata N. Genotoxic action of sunlight upon Bacillus subtilis spores: monitoring studies at Tokyo, Japan. Jpn J Radiat Res. 1989;30:338–351. doi: 10.1269/jrr.30.338. [DOI] [PubMed] [Google Scholar]
- 118.Munakata N. Biologically effective dose of solar ultraviolet radiation estimated by spore dosimetry since 1980. Photochem Photobiol. 1993;58:386–392. doi: 10.1111/j.1751-1097.1993.tb09579.x. [DOI] [PubMed] [Google Scholar]
- 119.Munakata N. Continual increase in biologically effective dose of solar UV radiation determined by spore dosimetry from 1980 to 1993 in Tokyo. J Photochem Photobiol B Biol. 1995;31:63–68. doi: 10.1111/j.1751-1097.1993.tb09579.x. [DOI] [PubMed] [Google Scholar]
- 120.Munakata N. Comparative measurements of solar UV radiation with spore dosimetry at three European and two Japanese sites. J Photochem Photobiol B Biol. 1999;53:7–11. doi: 10.1016/s1011-1344(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 121.Munakata N, Cornain S, Mulyadi K, Ichihashi M. Biologically effective doses of ambient solar-UV radiation in Indonesia and Japan. Med J Indonesia. 2000;9:123–128. [Google Scholar]
- 122.Munakata N, Ikeda Y. A mutant of Bacillus subtilis producing ultraviolet-sensitive spores. Biochem Biophys Res Commun. 1968;33:469–475. doi: 10.1016/0006-291x(68)90597-4. [DOI] [PubMed] [Google Scholar]
- 123.Munakata N, Morohoshi F, Hieda K, Suzuki K, Furusawa Y, Shimura H, Ito T. Experimental correspondence between spore dosimetry and spectral photometry of solar ultraviolet radiation. Photochem Photobiol. 1996;63:74–78. doi: 10.1111/j.1751-1097.1996.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 124.Munakata N, Rupert C S. Genetically controlled removal of “spore photoproduct” from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J Bacteriol. 1972;111:192–198. doi: 10.1128/jb.111.1.192-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Munakata N, Rupert C S. Dark repair of DNA containing “spore photoproduct” in Bacillus subtilis. Mol Gen Genet. 1974;130:239–250. doi: 10.1007/BF00268802. [DOI] [PubMed] [Google Scholar]
- 126.Munakata N, Rupert C S. Effects of DNA-polymerase-defective and recombination-deficient mutations on the ultraviolet sensitivity of Bacillus subtilis spores. Mutat Res. 1975;27:157–169. doi: 10.1016/0027-5107(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 127.Munakata N, Saito M, Takahashi N, Hieda K, Morihoshi F. Induction of unique tandem-base change mutations in bacterial spores exposed to extreme dryness. Mutat Res. 1997;390:189–195. doi: 10.1016/s0165-1218(97)00020-7. [DOI] [PubMed] [Google Scholar]
- 128.Murrell W G. The biochemistry of the bacterial endospore. Adv Microb Physiol. 1967;1:133–251. [Google Scholar]
- 129.Mussa D M, Ramaswamy H S, Smith J P. High-pressure destruction kinetics of Listeria monocytogenes on pork. J Food Prot. 1999;62:40–45. doi: 10.4315/0362-028x-62.1.40. [DOI] [PubMed] [Google Scholar]
- 129a.Nagy B, Nagy L A, Rigaly M J, Jones W D, Krinsley D H, Sinclair N A. Rock varnish in the Sonoran desert: microbiologically mediated accumulation of manganiferous sediments. Sedimentology. 1991;38:1153–1171. [Google Scholar]
- 130.Nicewonger R, Begley T P. Synthesis of the spore photoproduct. Tetrahedron Lett. 1997;38:935–936. [Google Scholar]
- 131.Nicholson W L. Photoreactivation in the genus Bacillus. Curr Microbiol. 1995;31:361–364. doi: 10.1007/BF00294700. [DOI] [PubMed] [Google Scholar]
- 132.Nicholson W L, Chooback L, Fajardo-Cavazos P. Analysis of spore photoproduct lyase operon (splAB) function using targeted deletion-insertion mutations spanning the Bacillus subtilis operons ptsHI and splAB. Mol Gen Genet. 1997;255:587–594. doi: 10.1007/s004380050532. [DOI] [PubMed] [Google Scholar]
- 133.Nicholson W L, Fajardo-Cavazos P. DNA repair and the ultraviolet radiation resistance of bacterial spores: from the laboratory to the environment. Recent Res Dev Microbiol. 1997;1:125–140. [Google Scholar]
- 134.Nicholson W L, Law J F. Method for purification of bacterial endospores from soils: UV resistance of natural Sonoran desert soil populations of Bacillus spp. with reference to B. subtilis strain 168. J Microbiol Methods. 1999;35:13–21. doi: 10.1016/s0167-7012(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 135.Nicholson W L, Setlow B, Setlow P. Ultraviolet irradiation of DNA complexed with α/β-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc Natl Acad Sci USA. 1991;88:8288–8292. doi: 10.1073/pnas.88.19.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicholson W L, Setlow P. Dramatic increase in the negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J Bacteriol. 1990;172:7–14. doi: 10.1128/jb.172.1.7-14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Sussex, England: John Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 138.Nicholson W L, Sun D, Setlow B, Setlow P. Promoter specificity of sigma G-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J Bacteriol. 1989;171:2708–2718. doi: 10.1128/jb.171.5.2708-2718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nishi K, Kato R, Tomita M. Activation of Bacillus spp. spores by hydrostatic pressure. J Jpn Soc Food Sci Technol. 1994;41:542–549. [Google Scholar]
- 140.Northrop J, Slepecky R A. Sporulation mutations induced by heat in Bacillus subtilis. Science. 1967;155:838–839. doi: 10.1126/science.155.3764.838. [DOI] [PubMed] [Google Scholar]
- 141.Nussinov M D, Lysenko S V. Cosmic vacuum prevents radiopanspermia. Origins Life Evol Biosph. 1983;13:153–164. [Google Scholar]
- 142.O'Keefe J D, Ahrens T J. Oblique impact: a process for obtaining meteorite samples from other planets. Science. 1986;234:346–349. doi: 10.1126/science.234.4774.346. [DOI] [PubMed] [Google Scholar]
- 143.Pagan R, Esplugas S, Gongora-Nieto M M, Barbosa-Canovas G V, Swanson B G. Inactivation of Bacillus subtilis spores using high intensity pulsed electric fields in combination with other food conservation technologies. Food Sci Technol Int. 1998;4:33–44. [Google Scholar]
- 144.Reference deleted.
- 145.Palop A, Rutherford G C, Marquis R E. Hydroperoxide inactivation of enzymes within spores of Bacillus megaterium ATCC19213. FEMS Microbiol Lett. 1996;142:283–287. [PubMed] [Google Scholar]
- 146.Palop A, Rutherford G C, Marquis R E. Inactivation of enzymes within spores of Bacillus megaterium ATCC 19213 by hydroperoxides. Can J Microbiol. 1998;44:465–470. [PubMed] [Google Scholar]
- 147.Passey Q R, Melosh H J. The effects of atmospheric breakup on crater field formation. Icarus. 1980;42:211–233. [Google Scholar]
- 148.Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson W L. Temporal regulation and forespore-specific expression of the spore photoproduct lyase gene by sigma G RNA polymerase during Bacillus subtilis sporulation. J Bacteriol. 1994;176:3983–3991. doi: 10.1128/jb.176.13.3983-3991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson W L. Spore photoproduct lyase operon (splAB) regulation during Bacillus subtilis sporulation: modulation of splB-lacZ fusion expression by P1 promoter mutations and by an in-frame deletion of splA. Curr Microbiol. 1997;34:133–137. doi: 10.1007/s002849900157. [DOI] [PubMed] [Google Scholar]
- 150.Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 151.Popham D L, Gilmore M E, Setlow P. Analysis of the role of low-molecular weight penicillin-binding proteins in spore peptidoglycan synthesis and spore properties in Bacillus subtilis. J Bacteriol. 1999;181:126–132. doi: 10.1128/jb.181.1.126-132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Popham D, Helin J, Costello C E, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Popham D L, Sengupta S, Setlow P. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA binding proteins. Appl Environ Microbiol. 1995;61:3633–3638. doi: 10.1128/aem.61.10.3633-3638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Priest F G. Systematics and ecology of Bacillus. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 3–16. [Google Scholar]
- 156.Priest F G, Girgorova R. Methods for studying the ecology of endospore-forming bacteria. In: Grigorova R, Norris J R, editors. Methods in microbiology. Vol. 22. London, U.K: Academic Press; 1991. pp. 565–591. [Google Scholar]
- 157.Puskeppeleit M, Quintern L E, El Naggar S, Schott J U, Eschweiler U, Horneck G, Bücker H. Long-term dosimetry of solar UV radiation in Antarctica with spores of Bacillus subtilis. Appl Environ Microbiol. 1992;58:2355–2359. doi: 10.1128/aem.58.8.2355-2359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Quintern L E, Horneck G, Eschweiler U, Bücker H. A biofilm used as ultraviolet dosimeter. Photochem Photobiol. 1992;55:389–395. [Google Scholar]
- 159.Quintern L E, Puskeppeleit M, Rainer P, Weber S, El Naggar S, Eschweiler U, Horneck G. Continuous dosimetry of the biologically harmful UV-radiation in Antarctica with the biofilm technique. J Photochem Photobiol B Biol. 1994;22:59–66. doi: 10.1016/1011-1344(93)06954-2. [DOI] [PubMed] [Google Scholar]
- 160.Raffalli J, Rosec J-P, Carlez A, Dumay E, Richard N, Cheftel J-C. High pressure stress and inactivation of Listeria innocua in inoculated dairy cream. Sci Aliment. 1994;14:349–358. [Google Scholar]
- 161.Raso J, Barbosa-Canovas G, Swanson B G. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J Appl Microbiol. 1998;85:17–24. doi: 10.1046/j.1365-2672.1998.00460.x. [DOI] [PubMed] [Google Scholar]
- 162.Rebeil R, Sun Y, Chooback L, Pedraza-Reyes M, Kinsland C, Begley T P, Nicholson W L. Spore photoproduct lyase from Bacillus subtilis spores is a novel iron-sulfur DNA repair enzyme which shares features with proteins such as class III anaerobic ribonucleotide reductases and pyruvate-formate lyases. J Bacteriol. 1998;180:4879–4885. doi: 10.1128/jb.180.18.4879-4885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reichard P. The evolution of ribonucleotide reduction. Trends Biochem Sci. 1997;22:81–85. doi: 10.1016/s0968-0004(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 164.Richter H. Zur Darwinschen Lehre. Schmidts Jahrb Ges Med. 1865;126:243–249. [Google Scholar]
- 165.Riesenman P J, Nicholson W L. Role of the spore coat layers in Bacillus subtilis resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/aem.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reference deleted.
- 167.Roberts T A, Hitchins A D. Resistance of spores. In: Gould G W, Hurst A, editors. The Bacterial Spore. New York, N.Y: Academic Press; 1969. pp. 611–670. [Google Scholar]
- 168.Russell A D. The destruction of bacterial spores. London, U.K: Academic Press; 1982. [Google Scholar]
- 169.Russell A D. Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev. 1990;3:99–119. doi: 10.1128/cmr.3.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reference deleted.
- 171.Schaeffer P, Millet J, Aubert J-P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schäfer M, Bücker H, Facius R, Hildebrand D. High precision localization method for HZE particles. Radiat Effects. 1977;34:129–130. [Google Scholar]
- 173.Schidlowski M. A 3.800 million-year isotopic record of life from carbon in sedimentary rocks. Nature. 1988;333:313–318. [Google Scholar]
- 174.Sedlak M, Vinter V, Adames J, Vohradsky J, Voburka Z, Chaloupka J. Heat shock applied early in sporulation affects heat resistance of Bacillus megaterium spores. J Bacteriol. 1993;175:8049–8052. doi: 10.1128/jb.175.24.8049-8052.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Setlow B, Setlow C A, Setlow P. Killing bacterial spores by organic hydroperoxides. J Ind Microbiol. 1997;18:384–388. [Google Scholar]
- 176.Setlow B, Setlow P. Thymine-containing dimers as well as spore photoproducts are found in ultraviolet-irradiated Bacillus subtilis spores that lack small, acid-soluble proteins. Proc Natl Acad Sci USA. 1987;84:421–423. doi: 10.1073/pnas.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Setlow B, Setlow P. Absence of transient elevated UV resistance during germination of Bacillus subtilis spores lacking small, acid-soluble spore proteins α and β. J Bacteriol. 1988;170:2858–2859. doi: 10.1128/jb.170.6.2858-2859.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Setlow B, Setlow P. Measurement of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci USA. 1988;77:2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Setlow B, Setlow P. Decreased UV light resistance of spores of Bacillus subtilis strains deficient in pyrimidine dimer repair and small, acid-soluble spore proteins. Appl Environ Microbiol. 1988;54:1275–1276. doi: 10.1128/aem.54.5.1275-1276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Setlow B, Setlow P. Heat inactivation of Bacillus subtilis spores lacking small, acid-soluble spore proteins is accompanied by generation of abasic sites in spore DNA. J Bacteriol. 1994;176:2111–2113. doi: 10.1128/jb.176.7.2111-2113.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Setlow B, Setlow P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl Environ Microbiol. 1995;61:2787–2790. doi: 10.1128/aem.61.7.2787-2790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Setlow B, Setlow P. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol. 1996;178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Setlow B, Tautvydas K J, Setlow P. Small, acid-soluble spore proteins of the α/β-type do not protect the DNA in Bacillus subtilis spores against base alkylation. Appl Environ Microbiol. 1998;64:1958–1962. doi: 10.1128/aem.64.5.1958-1962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Setlow P. Small acid-soluble, spore proteins of Bacillus species: structure, synthesis, genetics, function and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- 185.Setlow P. Resistance of bacterial spores to ultraviolet light. Comments Mol Cell Biophys. 1988;5:253–264. [Google Scholar]
- 186.Setlow P. DNA in dormant spores of Bacillus species is in an A-like conformation. Molec Microbiol. 1992;6:563–567. doi: 10.1111/j.1365-2958.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 187.Setlow P. I will survive: protecting and repairing spore DNA. J Bacteriol. 1992;174:2737–2741. doi: 10.1128/jb.174.9.2737-2741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Setlow P. Spore structural proteins. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 801–809. [Google Scholar]
- 189.Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol. 1994;76:49S–60S. doi: 10.1111/j.1365-2672.1994.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 190.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 191.Setlow P. Bacterial spore resistance. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: American Society for Microbiology; 1999. pp. 217–230. [Google Scholar]
- 192.Setlow P, Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970;245:3637–3644. [PubMed] [Google Scholar]
- 193.Setlow P, Kornberg A. Biochemical studies on bacterial sporulation and germination. XXIII. Nucleotide metabolism during spore germination. J Biol Chem. 1970;245:3645–3652. [PubMed] [Google Scholar]
- 194.Setlow P, Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975;250:623–630. [PubMed] [Google Scholar]
- 195.Setlow R B, Carrier W L. Pyrimidine dimers in ultraviolet-irradiated DNA. J Mol Biol. 1966;17:237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- 196.Simpson R K, Gilmour A. The effect of high hydrostatic pressure on Listeria monocytogenes in phosphate-buffered saline and model food systems. J Appl Microbiol. 1997;83:181–188. doi: 10.1046/j.1365-2672.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 197.Reference deleted.
- 198.Simpson R K, Gilmour A. The resistance of Listeria monocytogenes to high hydrostatic pressure in foods. Food Microbiol (London) 1997;14:567–573. [Google Scholar]
- 199.Slepecky R A, Foster J W. Alteration in metal content of spores of Bacillus megaterium and the effects on some spore properties. J Bacteriol. 1959;78:117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Slepecky R A, Leadbetter E R. Ecology and relationships of endospore-forming bacteria: changing perspectives. In: Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. pp. 195–206. [Google Scholar]
- 201.Slieman T A, Nicholson W L. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl Environ Microbiol. 2000;66:199–205. doi: 10.1128/aem.66.1.199-205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Somerville H J, Delafield F P, Rittenberg S C. Urea-mercaptoethanol-soluble protein from spores of Bacillus thuringiensis and other species. J Bacteriol. 1970;101:551–560. doi: 10.1128/jb.101.2.551-560.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. [Google Scholar]
- 204.Stafford R S, Donnellan J E., Jr Photochemical evidence for conformation changes in DNA during germination of bacterial spores. Proc Natl Acad Sci USA. 1968;59:822–829. doi: 10.1073/pnas.59.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Stewart G S A B, Eaton M W, Johnstone K, Barrat M D, Ellar D J. An investigation of membrane fluidity changes during sporulation and germination of Bacillus megaterium K.M. measured by electron spin and nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1980;600:270–290. doi: 10.1016/0005-2736(80)90432-0. [DOI] [PubMed] [Google Scholar]
- 206.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 207.Stuy J H. Studies on the mechanism of radiation inactivation on micro-organisms. III. Inactivation of germinating spores of Bacillus cereus. Biochim Biophys Acta. 1956;22:241–246. doi: 10.1016/0006-3002(56)90146-9. [DOI] [PubMed] [Google Scholar]
- 208.Sun D, Stragier P, Setlow P. Identification of a new ς-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989;3:141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- 209.Taylor G R. Space microbiology. Annu Rev Microbiol. 1974;28:121–137. doi: 10.1146/annurev.mi.28.100174.001005. [DOI] [PubMed] [Google Scholar]
- 209a.Tennen R, Setlow B, Davis K L, Loshon C A, Setlow P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J Appl Microbiol. 2000;89:330–338. doi: 10.1046/j.1365-2672.2000.01114.x. [DOI] [PubMed] [Google Scholar]
- 210.Thomson W. Popular Lectures and Addresses. New York, N.Y: MacMillan and Co.; 1894. 1871 presidential address to the British Association; pp. 132–205. [Google Scholar]
- 211.Traving C, Schauer R, Roggentin P. Gene structure of the “large” sialidase isoenzyme from Clostridium perfringens A99 and its relationship with other clostridial nanH proteins. Glycoconj J. 1994;11:141–151. doi: 10.1007/BF00731154. [DOI] [PubMed] [Google Scholar]
- 211a.Tyndall J. Further researches on the department and vital persistence of putrefactive and infective organisms from a physical point of view. Phil Trans R Soc. 1877;167:149–206. [Google Scholar]
- 212.Tyrrell R M. Solar dosimetry with repair deficient bacterial spores: action spectra, photoproduct measurements and a comparison with other biological systems. Photochem Photobiol. 1978;27:571–579. doi: 10.1111/j.1751-1097.1978.tb07648.x. [DOI] [PubMed] [Google Scholar]
- 213.Tyrrell R M. Inducible responses to UV-A exposure. In: Urbach F, editor. Biological responses to ultraviolet-A radiation. 1992. pp. 59–64. Valdenmar Publishing, Overland Park, Kansas. [Google Scholar]
- 214.Urbach F, Gange R W, editors. The biological effects of UVA radiation. New York, N.Y: Praeger Publishers; 1986. [Google Scholar]
- 215.Varghese A J. 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun. 1970;38:484–490. doi: 10.1016/0006-291x(70)90739-4. [DOI] [PubMed] [Google Scholar]
- 216.Vickery A M, Melosh H J. The large crater origin of SNC meteorites. Science. 1987;237:738–743. doi: 10.1126/science.237.4816.738. [DOI] [PubMed] [Google Scholar]
- 217.Wang T-C V. A simple convenient biological dosimeter for monitoring solar UV-B radiation. Biochem Biophys Res Commun. 1991;177:48–53. doi: 10.1016/0006-291x(91)91946-a. [DOI] [PubMed] [Google Scholar]
- 218.Wang T-C V, Rupert C S. Transitory germinative excision repair in Bacillus subtilis. J Bacteriol. 1977;129:1313–1319. doi: 10.1128/jb.129.3.1313-1319.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang T-C V, Rupert C S. Evidence for the monomerization of spore photoproduct to two thymines by the light independent “spore repair” process in Bacillus subtilis. Photochem Photobiol. 1977;25:123–127. doi: 10.1111/j.1751-1097.1977.tb07432.x. [DOI] [PubMed] [Google Scholar]
- 220.Weber P, Greenberg J M. Can spores survive in interstellar space? Nature. 1985;316:403–407. [Google Scholar]
- 221.Reference deleted.
- 221a.Whight I P, Grady M M, Pillinger C T. Organic materials in a Martian meteorite. Nature. 1989;340:220–222. [Google Scholar]
- 222.Woese C R, Kandler O, Wheelis M L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wong K K, Kozaritch J W. S-Adenosylmethionine-dependent radical formation in anaerobic systems. In: Sigel H, Sigel A, editors. Metal ions in biological systems, vol. 30: metalloenzymes involving amino acid-residue and related radicals. New York, N.Y: Marcel Dekker Press; 1994. pp. 279–313. [Google Scholar]
- 224.Wuytack E Y, Boven S, Michiels C W. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl Environ Microbiol. 1998;64:3220–3224. doi: 10.1128/aem.64.9.3220-3224.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wuytack E Y, Soons J, Poschet F, Michiels C W. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl Environ Microbiol. 2000;66:257–261. doi: 10.1128/aem.66.1.257-261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xue Y. Resistance of Bacillus subtilis spores lacking either nucleotide excision repair or spore photoproduct lyase to ultraviolet (UV) radiation from artificial or natural sources. M.S. thesis. Fort Worth, Tex: University of North Texas Health Science Center; 1996. [Google Scholar]
- 227.Xue Y, Nicholson W L. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl Environ Microbiol. 1996;62:2221–2227. doi: 10.1128/aem.62.7.2221-2227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yasbin R E, Cheo D, Bol D. DNA repair systems. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 529–537. [Google Scholar]
- 229.Young A R, Björn L O, Moan J, Nultsch W, editors. Environmental UV photobiology. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 230.Zamenhof S. Effects of heating dry bacteria and spores on their phenotype and genotype. Proc Natl Acad Sci USA. 1960;46:101–105. doi: 10.1073/pnas.46.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zytkovic T H, Halvorson H O. Some characteristics of dipicolinic acid-less mutant spores of Bacillus cereus, Bacillus megaterium and Bacillus subtilis. In: Halvorson H O, Hanson R, Campbell L L, editors. Spores V. Washington, D.C.: American Society for Microbiology; 1972. pp. 49–52. [Google Scholar]