Abstract
Background:
Isolated complete atrioventricular block is a rare disease often associated with maternal autoantibodies. This study aimed to present the mid-term data of patients at our clinic diagnosed with isolated complete atrioventricular block.
Methods:
We evaluated 108 patients diagnosed with isolated complete atrioventricular block. Demographic data of the patients, electrocardiography, echocardiography, 24-hour Holter monitoring data, and follow-up and complications of the patients who underwent pacemaker implantation were evaluated retrospectively.
Results:
The mean age of the patients at diagnosis was 5.51 ± 5.05 years. At the time of diagnosis, 74.8% of the patients had no symptoms associated with complete atrioventricular block. The most common symptom was fatigue. Pacemaker implantation was needed in 88 (81.4%) patients during follow-up. Significant bradycardia was the most common pacemaker implantation indication. The mean battery life was 5.41 ± 2.65 years. The battery replacement-free period of 68 patients who underwent pacemaker implantation and continued their follow-up was 4.18 ± 2.89 (0.1-10) years. Pacemaker-related complications developed in 8 patients during follow-up. Left ventricular dysfunction developed (dyssynchrony induced) in 3 patients at follow-up, and all were paced from the right ventricular anterior wall. Those patients underwent cardiac resynchronization therapy and their left ventricular dysfunction improved.
Conclusion:
Isolated complete atrioventricular block is a rare disease requiring careful clinical follow-up. Patients are often asymptomatic and significant bradycardia is the most common indication for pacemaker implantation. Left ventricular dysfunction is an important cause of morbidity, especially in patients with right ventricular anterior wall pacing. Physicians should be aware of left ventricular dysfunction during follow-up. Cardiac resynchronization therapy should be considered as a treatment option for left ventricular dysfunction.
Keywords: Isolated complete atrioventricular block, pacemaker, congenital complete atrioventricular block, cardiac resynchronization therapy
Highlights
Complete atrioventricular block is an uncommon disease, and most patients are asymptomatic when they are diagnosed.
The most frequent reason for pacemaker implantation is significant bradycardia.
Left ventricular dysfunction is a major cause of morbidity in patients with pacemakers, especially those who have right ventricular anterior pacing.
In patients who develop left ventricular dysfunction, cardiac resynchronization therapy implantation can be utilized as a therapeutic option.
Introduction
Isolated congenital complete atrioventricular block (CAVB) is an uncommon disease linked to SSA/Ro and SSB/La autoantibodies that come from the mother.1 Some patients are diagnosed prenatally or in the first month as having a congenital heart condition. In the latter case, the diagnosis is considered to be childhood CAVB. Complete atrioventricular block affects 1/15 000-20 000 persons with a structurally normal heart.2 In 90%-99% of infants identified before the age of 6 months, the cause is maternal antibodies that enter the fetal circulation and trigger inflammation, causing irreversible fibrosis in the conduction system and down-regulation of L-type calcium channels.3,4 Most patients with isolated CAVB are diagnosed later in life and the condition is not related to maternal autoantibodies.1 Complete atrioventricular block can occur with congenital heart diseases, such as left atrial isomerism, atrial septal defect, and congenitally corrected transposition (ccTGA) or acquired due to acute rheumatic fever, myocarditis, and Lyme carditis.2
Left ventricular (LV) dysfunction-dilated cardiomyopathy (DCMP) may develop in the follow-up of patients with CAVB and is the main cause of morbidity and mortality. Different studies have identified age at early diagnosis, maternal antibody positivity, pacemaker (PM) localization, male gender, and pre-pacing LV dysfunction as risk factors for post-pacing LV dysfunction.1,5-9 Studies in the literature on isolated CAVB have mostly been performed with maternal antibody status, PM monitoring, and timing. Less data are available on isolated CAVB follow-ups at all ages. Our country's and other underdeveloped countries' pediatric experience is relatively limited. Thus, this study aimed to present the early and mid-term outcomes of patients who were diagnosed with isolated CAVB and examined in our hospital.
Methods
The data of patients diagnosed with CAVB who applied to our clinic between October 2011 and 2021 were evaluated retrospectively. The study received approval from the Local Ethics Committee (2022.01-06). Patients' age at admission, weight, follow-up periods, symptoms at presentation, maternal autoantibody positivity, time of birth, history of hydrops, and age at CAVB diagnosis were obtained from medical records using the internet database system Filemaker®. The Muse® electrocardiograph recording device was used to examine the patients' admission electrocardiographic data (General Electric HC, Menomonee Falls, Wis, USA). The 12-lead electrocardiograms (ECGs) of CAVB patients were analyzed for atrial and ventricular rates, QRS, QT intervals, and QT (QTc) intervals corrected for heart rate using Bazett's algorithm. Moreover, 24-hour Holter ECG monitoring, maximum, minimum, mean heart rates, pause duration, complex ventricular ectopia, and ventricular escape findings were assessed. Patients with AV block due to congenital heart disease or postoperative AV block were excluded from the study. Left ventricular dilatation, dysfunction, and mitral valve regurgitation were all detected by echocardiography. The effort response of patients who underwent an exercise test was also assessed. The indication for PM implantation was assessed according to current guidelines during the follow-up period.10-12 If the patient's weight is less than 15 kg, the epicardial approach is used for PM implantation. The transvenous route is utilized if the patient's weight is greater than 20 kg. Either of these 2 ways is employed as a PM implantation strategy if the patient's weight is between 15 and 20 kg. At our clinic, epicardial PM ventricular leads are inserted into the apical LV and transvenous PM leads are implanted in the right ventricular (RV) mid-septum. The PM controls are conducted after PM implantation on the first day, third day, and first week, then at 1-month, 3-month, and 6-month intervals. Electrocardiogram, chest x-ray, and lead and battery control are performed by experienced electrophysiologists (Y.E., H.C.K.) at each follow-up. Moreover, every 1-2 years, echocardiography and Holter monitoring examinations are conducted. The following information was collected: PM implantation indication, initial age at time of PM implantation, epicardial-transvenous PM implantation, PM implantation location, and PM mode. The onset and duration of LV dysfunction, PM-related complications, the need for battery replacement, and average battery life were all examined during the follow-up. During follow-up, total pace durations and LV end-diastolic dimension (LVEDd) z scores were assessed.
Statistical Analysis
The data were analyzed using the Statistical Package for Social Sciences statistical software program. Percentages were given for the categorical data, and mean and standard derivations were given for the numerical data. Battery survival and complication-free survival were assessed by using Kaplan–Meier analysis.
Results
Clinical Characteristics
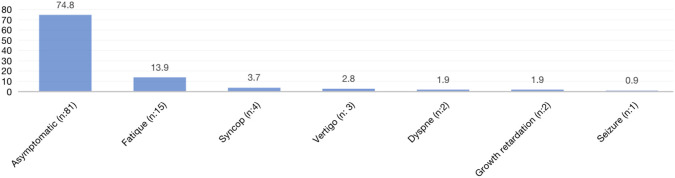
The study included 108 patients with an isolated CAVB diagnosis who did not have any CAVB-related congenital heart disease. Of those, 53.7% were male. At the time of presentation, the mean age was 7.1 ± 5.57 (0-18) years. The mean weight at admission was 25.34 ± 6.1 (1.72-80) kg. The mean age at diagnosis of CAVB was 5.51 ± 5.05 (0-17) years, and some patients had diagnosis and follow-up periods at different centers before arriving at our clinic. At the time of admission, 74.8% of the patients presented no signs and symptoms associated with AV block. The complaints of the other patients included fatigue (13.9%), syncope (3.7%), dizziness (2.8%), dyspnea (1.9%), growth retardation (1.9%), and seizure (0.9%). Complete atrioventricular block was detected prenatally and in the first month in a total of 27 (25%) cases, 14 (13%) of whom were in the prenatal period. Nineteen of the 22 patients evaluated for maternal antibody levels were positive and 3 were negative. Six of the patients had a history of preterm birth (5.6%). One of the patients had a history of hydrops and had been born prematurely. While 4 patients initially had milder cases (first- and second-degree AV block), CAVB occurred during the follow-up. While 1 patient had 2 : 1 AVB at admission, CAVB occurred during follow-up, leading to speculation that this patient had previously suffered from viral myocarditis. There was no clear etiology in the other patients. The onset of CAVB in many patients is unknown due to the lack of regular healthy child follow-ups and standard electrocardiographic scans in our country; however, it is hypothesized that these individuals may have late-diagnosed congenital AV block.
Electrocardiogram, Holter, and Exercise Stress Test Data
Table 1 shows the initial ECG data and the maximum, minimum, and mean 24-hour Holter heart rates of 75 patients who had comprehensive Holter and ECG data retrieved. On the baseline ECG, the mean ventricular rate was 52.63 ± 11 (min-max: 30-98)/bpm. The mean heart rate was 50.43 ± 7.89 (35-72)/bpm during the 24-hour Holter monitorization. A pause of more than 3 seconds was detected in the Holter data in 13% (14/108) of the patients. Ventricular escape beats were found in 9.6% (10/108) of the patients. Seven (7/108, 6.5%) patients had a history of severe symptomatic bradycardia/syncope that necessitated the use of a temporary PM. A temporary PM was not required for the other 101 patients.
Table 1.
Patients' 12-Lead Electrocardiography and Holter ECG Data in First Admission
| Mean ± SD | Minimum-Maximum | |
|---|---|---|
| Atrium rate (bpm/min) | 105.01 ± 29.56 | 56-192 |
| Ventricular rate (bpm/min) | 52.63 ± 11 | 30-98 |
| QRS interval (ms) | 73.34 ± 15.53 | 40-130 |
| QT interval (ms) | 446.23 ± 43.29 | 346-560 |
| QTc interval (ms) | 419.31 ± 36.16 | 350-539 |
| Maximum heart rate in Holter (bpm/min) | 85.75 ± 19.48 | 40-147 |
| Minimum heart rate in Holter (bpm/min) | 38.98 ± 8.17 | 26-62 |
| Mean heart rate in Holter (bpm) | 50.43 ± 7.89 | 35-72 |
| Pause > 3 seconds | 13% | |
| Ventricular escape beat | 9.6% |
ECG, electrocardiogram; SD, standard deviation.
In 33 patients over the age of 6, an exercise test was available. Apart from 2 patients, an increase in heart rate was detected with exercise but at a suboptimal level. Patients who underwent exercise testing had basal and maximal heart rates of 57.18 ± 10.61 and 120.51 ± 31.92, respectively. The patients' average time to achieve their projected maximal heart rate was 57.12 ± 15.16%. Two of the patients were chronotropically incompetent.
Echocardiography Findings
At the initial assessment, the echocardiography results were normal in 42 (39%) of the patients. At the time of diagnosis, 14 (13%) of the patients had LV dilatation. Before admission, 2 of these patients had PM implantation and LV dysfunction. While 48 (44.4%) of the patients had mild mitral regurgitation at the initial visit, 1 patient had significant mitral valve regurgitation and 2 patients had moderate mitral valve regurgitation. In 3 cases, the ascending aorta was mildly dilated. Moreover, 14 patients had congenital heart disease that did not affect their hemodynamics (small patent ductus arteriosus, small secundum atrial/ventricular septal defect, and bicuspid aortic valve). Mild aortic regurgitation was seen in 2 patients, although it was not clinically significant.
Follow-Up Data
Every patient was asked about PM syndrome during each follow-up appointment, and lead tension and fracture controls were also carried out, along with ECGs and chest x-rays. Furthermore, PM life, heart rate, mode, lead impedance, sensing, and threshold value controls were carried out during PM control, and specific changes were done by experienced electrophysiologists based on the patient's age and PM characteristics. All patients underwent annual ECG and Holter monitoring checks.
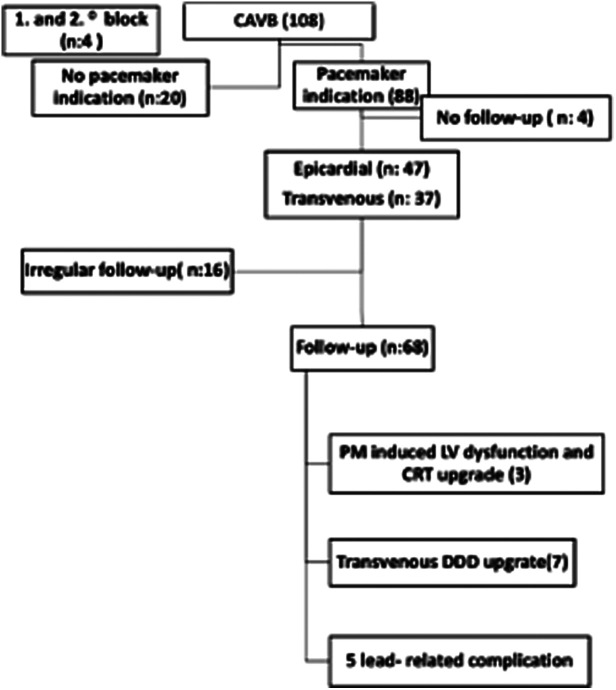
The follow-ups of the patients after diagnosis are summarized in Figure 1. None of patients in this study died. Of the 85 patients who were followed up, 68 had PMs implanted and 17 had no PM. The mean age at the last control of 68 patients who had pacemaker implanted and followed up was 11.78 ± 6.1 years, 41 of them were boys and 27 of them were girls. The data of patients who could not be followed regularly were not included in the follow-up data. The mean follow-up time for these 85 patients was 3.63 ± 3.32 (0.1-11.75) years.
Figure 1.
Summary of patients’ follow-up data after diagnosis.
Of the 20 (18.5%) patients without indication for PM implantation, 17 were followed-up, with a mean age at the last visit of 10.5 (1.2-18.8) years, a mean age at diagnosis of 4.67 (0-15.35) years, and a follow-up period of 2.69 (0.15-9.75) years.
Pacemaker-Related Data
According to the guidelines, 88 (81.4%) of the CAVB patients received a PM; the indication rates are shown in Figure 2. Some individuals experienced multiple signs and symptoms. Significant bradycardia was the most common reason for PM implantation.
Figure 2.
Indication rate for pacemaker implantation.
Epicardial pacing was performed on 47 patients. Transvenous pacing was performed on 37 patients. In 84 individuals who had PMs implanted, the average period from diagnosis to implantation was 1.43 ± 2.88 (0-13) years. Because 4 of the patients did not continue their follow-up in our clinic despite the need for PM implantation, it is unknown if implantation was done.
During battery replacement intervals, 3 patients had their cardiac resynchronization therapy (CRT) upgraded, 7 had their VVI(R) mode upgraded to the DDD(R) mode, and 3 had their transvenous pace upgraded from epicardial pace.
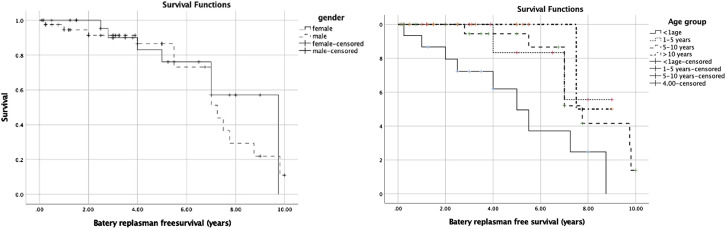
Twenty-four patients needed a battery replacement (once in 21 cases, twice in 2 cases, and 3 times in 1 case). The average battery life is 5.41 ± 2.65 (0.25-9.8) years. The battery belonging to the patient whose battery was changed due to infection was replaced after 3 months. The average replacement-free time was 4.18 ± 2.89 (0.1-10) years in 68 individuals who had PMs implanted and followed up. The replacement-free period was 98.5% at 1 year, 85% at 5 years, 74.5% at 7 years, and 11.3% at 10 years. Battery replacement-free survival Kaplan–Maier analysis by gender and age groups is given in Figure 3.
Figure 3.
Battery replacement free survival Kaplan–Maier analysis by gender and age groups.
Complications
Five patients suffered lead-related complications and 3 had LV dysfunction. Patients with PM implantation in the neonatal period accounted for 5 of the 8 patients who developed complications. Seven of the 8 patients had epicardial PM implantation. The follow-up time for patients that were free of complications was 4.86 ± 4.12 (0.1-19) years.
Left Ventricle Dysfunction
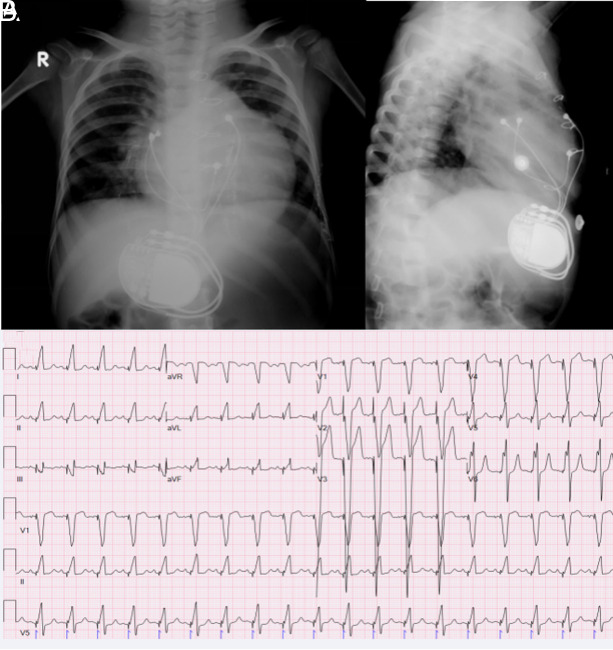
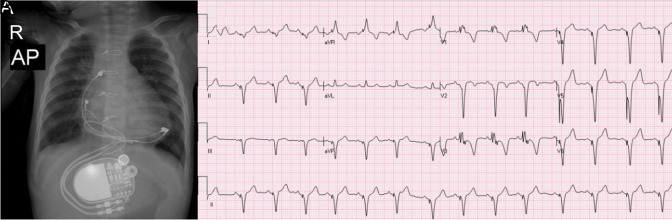
Due to an LV ejection fraction (LVEF) of <35% (mean LVEF 27% with Simpson) throughout the follow-up, CRT was implanted in 3 (2.7%) patients, 2 from different centers and 1 from our clinic. Due to a borderline reduction in LV systolic function, 1 patient is being followed up. Furthermore, 1 patient displayed mild LV hypokinesia, which is likely to be the result of previous myocarditis. The LV size and functioning of the other patients were within normal ranges. The LV functions of all 3 patients who had CRT implantation improved. The diagnosis and PM implantation were done during the neonatal period, and RV anterior pacing was done in 2 of the 3 CRT patients. The third patient was 8.25 years of age at the time of diagnosis and PM implantation. As seen in Figure 4, cardiomegaly is indicated by anterior-posterior and lateral chest x-rays and the ECG of the patient who was paced from the anterior RV wall. Figure 5 shows an ECG sample and chest x-ray of another patient who underwent LV apical pacing. The maternal antibody status was positive in 1 of the 3 cases, but the maternal antibody status of the other 2 was unknown.
Figure 4.
Cardiomegaly and pacemaker lead on the right ventricle anterior localization anterior/posterior x-ray (A) and laterally x-ray (B), (C): ECG with LBBB and inferior axis QRS in a patient with dilated cardiomyopathy who was paced from the anterior RV anterior wall. ECG, electrocardiogram; RV, right ventricular; LBBB, left bundle branch block.
Figure 5.
Chest x-ray of a patient who underwent LV apical pacing (A) and ECG with RBBB and superior QRS axis (B). ECG, electrocardiogram; LV, left ventricular; RBBB, Right bundle branch block.
Discussion
Our study is a single-center retrospective evaluation of the largest number of CAVB patients in our country and it was performed with a relatively large number of patients from a single center in the literature. There is a multicenter study in the literature that included 141 individuals with partial AV blocks (30%) and CAVB (70%) (congenital: 18, childhood: 82), as well as 15 centers from France. Complete atrioventricular block patients up to the age of 15 were enrolled in that study for a total of 29 years, and 84.4% of the patients were asymptomatic. In our study, 74.8% of the patients were asymptomatic, which was lower than the study, although incomplete blocks were not included, unlike in the study.13 The mean age at diagnosis was 3.6 ± 4.2 years, and the mean age of PM implantation was 32 ± 69.8 months in this study. In our study, the mean age at diagnosis was 5.51 ± 5.05 years, and the mean age of PM implantation was 6.54 ± 5.09 years, which was higher in the study. This might be due to the absence of a neonatal intensive care unit and perinatal follow-up and therapy in our hospital. After diagnosis, the PM implantation time was found to be 2.6 years in this study, which was longer than in our study, although it was considered to be due to the inclusion of incomplete blocks.13 The mean period from diagnosis to PM implantation was 1.43 ± 2.88 years in our study. The most prevalent reason for PM implantation was prophylactic (62.5%), and bradycardia was the most common reason for PM implantation in our study, with a rate of 72%. While Weinreb et al8 examined 114 patients with a CAVB diagnosis during a study period of 40 years, the age of diagnosis was 0.0 (0-2) years in that study, which is much younger than in our study. The mean age of PM implantation was 1.9 (0.1-8) years, which was significantly younger than the age of PM implantation in our research. The patients' prenatal diagnosis rate was 32%, and maternal antibody positivity was 43%; in comparison, in our study, the fetal diagnostic rate was 12.9%, and maternal antibody evaluation rates were very low. One reason for this difference may be the inadequacies in the diagnosis and follow-up system of patients in developing countries, as well as the fact that our clinic is located in a heart hospital where routine neonatal follow-up and treatment are not performed.
Left ventricle dilatation/dysfunction is an important cause of morbidity in patients with CAVB block. Complete AVB-related LV dilatation has been reported to occur at a rate of 7%-14% in some studies.8,9 While some studies have shown a 75% mortality rate in patients with DCMP, others have reported varied rates in various patient groups.7 The mortality rate for DCMP was found to be 30% and the morbidity rate for DCMP was 30% in a study analyzing the long-term results of newborn patients with PMs. Mortality was observed to be higher before 1995.7 Many studies have shown that RV anterior pacing is the most important risk factor for developing DCMP.11 Right ventricle free wall pacing was revealed to be the most important independent risk factor for patients with LV dysfunction in a study of 34 patients (odds ratio 52.5; 95% CI: 3.9-700; P = .003). The RV apical pacing and the LV apical pacing were similar for LV dysfunction.1 In the same study, there was no difference in the single or dual chamber pacing modes.1 The incidence of LV dysfunction was found to be 6% in a study of 63 patients with a mean follow-up of 9.9 years, and the most important risk variables were identified to be RV pacing and longer QRS duration. However, RV apical pacing is still an acceptable initial therapy.6 Numerous studies have demonstrated that LV dysfunction is influenced by variables other than RV pacing. In a study including 99 patients who underwent RV pacing, the LV shortening fraction (SF) was assessed.14 That study showed that RV pacing had no effect on LV systolic functions and that LV dysfunction was related to complex congenital heart disease. No correlation was observed with QRS duration.14 In another trial, patients were assigned to 2 groups based on maternal antibody positivity, but no difference was identified between the 2 groups. Pre-PM dysfunction has been found to be the most essential predisposition for LV dysfunction, although early diagnosis and male gender are related to a poor prognosis.5 Left ventricle diameters, LVEF, SF, and strain were monitored prospectively in another trial of 20 patients; LV functions and diameters were preserved with LV pace and synchronization was determined to be satisfactory.15 It was revealed that the presence of maternal antibodies had no effect.15 In comparison to other studies, the frequency of LV dysfunction in our study was much lower (2.7%). This might be due to the fact that our clinic uses LV apical pacing as a PM placement approach. Furthermore, several studies have shown that low fetal diagnosis rates and advanced age after PM implantation are risk factors.3,7,16 In our study, due to DCMP, CRT upgrades were performed on 3 patients, 2 of whom were from other clinics. There was a history of RV anterior pacing in all 3 patients. As reported in the literature,1 LV dysfunction also improved in the follow-up of our patients who had CRT implantation. In our patients, RV anterior pacing appears to play a major role in LV dysfunction, as reported in several other studies.1,11 Since the LV apical pacing strategy was adopted at our clinic, it was not possible to compare our findings with those of other studies that used an RV pacing strategy. In our study, the maternal antibody status of 2 of the 3 patients who developed LV dysfunction was unknown, and the status of 1 of the patients was positive. Again, it was not possible to compare our findings for the maternal antibody status data in our research was restricted. Although some researchers13 have reported that 30% of individuals had wide QRS, our patients had a QRS rate of 1.85%.
Two of the 3 patients that required CRT implantation in our study underwent PM implantation during the neonatal period. Udink Ten Cate et al16 published the findings of a trial comprised of 149 patients with congenital AVB, 121 patients with pacemakers, and 9 patients who acquired CMP. Prenatal diagnosis and a low heart rate were revealed to be major risk factors for DCMP.16 In our study, LV dysfunction was not detected in individuals who did not undergo RV pacing, even though the pacing was implanted in 12 of our patients during the neonatal period.
Lead problems occurred in 7.3% of our patients, which was shown to be fairly low in comparison to other studies.17,18 However, because our center is relatively new, the mean follow-up duration was less than in previous studies.17,18
The average follow-up duration for 48 patients under the age of 1 who had epicardial pacing implantation was 8.5 years, and the percentage of patients who did not require battery replacement was 97.8%, 76.2%, and 46.3% in the first, fifth, and tenth years, respectively.19 In our study, the mean battery replacement-free period of 68 patients who had PMs implanted and were followed-up on was 4.18 ± 2.89 years. The battery replacement-free rate was 98.5% at 1 year, 85% at 5 years, 74.5% at 7 years, and 11.3% at 10 years in our study. The battery replacement-free rates in our analysis were similar in the first year but higher in the fifth year. In the tenth year, there was a significant decline. During this time, a large number of patients required battery replacements.
In a study that included 102 patients, 11% of neonates and 12% of children did not require PM implantation until the age of 20.20 Our study did not analyze age groupings, although PM implantation was not done in 20 (18.5%) patients with a mean age of 10.5 years, and 17 of them are being followed-up. The average age of 17 of those patients at their latest visit was 10.5 (1.2-18.8) years, the mean age at diagnosis was 4.67 (0-15.35) years, and the mean follow-up duration was 2.69 (0.15-9.75) years.
In 84 patients with PM implantation, the average period from diagnosis to implantation was 1.43 ± 2.88 years. The mean PM implantation time was 0.7 years in another trial with 127 patients, which was shorter than ours.2 In a trial with a mean follow-up of 122 months, PM implantation was done in 111 (74%) of the 149 patients. The mean follow-up period for the 38 patients who did not get a PM was 124 months.16 Although the PM rates in that study are similar to those seen in our study, the follow-up durations are longer. However, the patients in that trial were younger at the initial diagnosis, and the time between diagnosis and PM implantation was longer. The patients' ages at the time of the last visit were not disclosed.21
Study Limitations
Our study has some limitations. It used a retrospective design, some of our patients were excluded from follow-up, and the follow-up period was short. However, because of the clinic’s status as a pediatric cardiology-heart surgery reference hospital, it can treat patients with more complicated situations, which has an impact on the data's homogeneity. Other limitations of our study include the fact that the number of patients diagnosed in the prenatal and natal period is somewhat lower than in other studies, and the maternal antibody levels are unknown, as our institution is only a pediatric cardiology-heart surgery reference hospital.
Conclusion
Complete AVB is an uncommon disease category that requires extensive clinical follow-up. Most patients are asymptomatic when they are diagnosed. Significant bradycardia was the most frequent reason for PM implantation. Moreover, LV dysfunction is a major cause of morbidity in patients with PMs, especially those who have RV anterior pacing. In patients who develop LV dysfunction, CRT implantation can be utilized as a therapeutic option.
Footnotes
Ethics Committee Approval: University of Health Sciences İstanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Education and Research Hospital Non-Interventional Researches Ethics Committee approval was obtained (2022.01-06).
Informed Consent: The data were obtained retrospectively from electronic file records.
Peer-review: Externally peer-reviewed.
Author Contributions: Study conception and design: Y.E., A.S., A.G., S.H., I.S.O.; data collection: A.S., H.K., S.B.G.; analysis and interpretation of results: A.S., H.C.K., H.K., draft manuscript preparation: Y.E., A.S., H.C.K., H.K., S.B.G., I.S.O., Supervisor; Y.E., A.G., S.H., I.S.O. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments: We would like to thank Enver Karakaruk, employee of Medtronic company, because of his support in intraoperative and postoperative pacemaker controls..
Declaration of Interests: All of the authors had no conflict of interest.
Funding: This research received no specific grant from any funding agency, commercial or not for profit sectors.
References
- 1. Song MK, Kim NY, Bae EJ.et al. Long-term follow-up of epicardial pacing and left ventricular dysfunction in children with congenital heart block. Ann Thorac Surg. 2020;109(6):1913 1920. ( 10.1016/j.athoracsur.2019.09.063) [DOI] [PubMed] [Google Scholar]
- 2. Friedman RA, Fenrich AL, Kertesz NJ. Congenital complete atrioventricular block. Pacing and Clinical Electrophysiology. 2001;24(11):1681 1688. ( 10.1046/j.1460-9592.2001.01681.x) [DOI] [PubMed] [Google Scholar]
- 3. Garcia S, Nascimento JH, Bonfa E.et al. Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J Clin Invest. 1994;93(2):718-724. ( 10.1172/JCI117025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao GQ, Hu K, Boutjdir M. Direct inhibition of expressed cardiac l and t- type calcium channels by igg from mothers whose children have congenital heart block. Circulation. 2001;103(11):1599 1604. ( 10.1161/01.cir.103.11.1599) [DOI] [PubMed] [Google Scholar]
- 5. Eliasson H, Sonesson SE, Salomonsson S. et al. Outcome in young patients with isolated complete atrioventricular block and permanent pacemaker treatment: a nationwide study of 127 patients. Heart Rhythm. 2015;12(11):2278 2284. ( 10.1016/j.hrthm.2015.06.028) [DOI] [PubMed] [Google Scholar]
- 6. Kim JJ, Friedman RA, Eidem BW.et al. Ventricular function and long-term pacing in children with congenital complete atrioventricular block. J Cardiovasc Electrophysiol. 2007;18(4):373- 377. ( 10.1111/j.1540-8167.2006.00741.x) [DOI] [PubMed] [Google Scholar]
- 7. Kurosaki K, Miyazaki A, Watanabe K, Echigo S. Long-term outcome of isolated congenital complete atrioventricular block pacing since neonatal period experience at a single Japanese institution. Circ J. 2008;72(1):81 87. ( 10.1253/circj.72.81) [DOI] [PubMed] [Google Scholar]
- 8. Weinreb SJ, Okunowo O, Griffis H, Vetter V. Incidence of morbidity and mortality in a cohort of congenital complete heart block patients followed over 40 years. Heart Rhythm. 2022;5271(22):00200 00204. ( 10.1016/j.hrthm.2022.02.019) [DOI] [PubMed] [Google Scholar]
- 9. Chandler SF, Fynn-Thompson F, Mah DY. Role of cardiac pacing in congenital complete heart block. Expert Rev Cardiovasc Ther. 2017;15(11):853 861. ( 10.1080/14779072.2017.1376655) [DOI] [PubMed] [Google Scholar]
- 10. Writing Committee Members, Shah MJ, Silka MJ. et al. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Heart Rhythm. 2021;18(11):1888 1924. ( 10.1016/j.hrthm.2021.07.038) [DOI] [PubMed] [Google Scholar]
- 11. Tracy CM, Epstein AE, Darbar D.et. al. ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2012;9:1737 1753. [DOI] [PubMed] [Google Scholar]
- 12. European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA), Brignole M. et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. 2013;15(8):1070 1118. ( 10.1093/europace/eut206) [DOI] [PubMed] [Google Scholar]
- 13. Baruteau AE, Fouchard S, Behaghel A.et al. Characteristics and long-term outcome of nonimmune isolated atrioventricular block diagnosed in utero or early childhood: a multicentre study. Eur Heart J. 2012;33(5):622 629. ( 10.1093/eurheartj/ehr347) [DOI] [PubMed] [Google Scholar]
- 14. Shalganov TN, Paprika D, Vatasescu R.et al. Mid-term echocardiographic follow up of left ventricular function with permanent right ventricular pacing in pediatric patients with and without structural heart disease. Cardiovasc Ultrasound. 2007;5:13. ( 10.1186/1476-7120-5-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silvetti MS, Muzi G, Unolt M.et al. Left ventricular (LV) pacing in newborns and infants: echo assessment of LV systolic function and synchrony at 5-year follow-up. Pacing Clin Electrophysiol. 2020;43(6):535 541. ( 10.1111/pace.13908) [DOI] [PubMed] [Google Scholar]
- 16. Udink ten Cate FEA, Breur JMPJ, Cohen MI.et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block:early and long-term risk in children. J Am Coll Cardiol. 2001;37(4):1129 1134. ( 10.1016/s0735-1097(00)01209-2) [DOI] [PubMed] [Google Scholar]
- 17. Manolis AA, Manolis TA, Melita H, Manolis AS. Congenital heart block: pace earlier (Childhood) than later (Adulthood). Trends Cardiovasc Med. 2020;30(5):275 286. ( 10.1016/j.tcm.2019.06.006) [DOI] [PubMed] [Google Scholar]
- 18. Van De Bruaene A, Willems R, Troost E,et al. Long-term follow-up of children with heart block born from mothers with systemic lupus erythematosus: a retrospective study from the database pediatric and congenital heart disease in university hospitals leuven. Pace. 2016;39:935 943. [DOI] [PubMed] [Google Scholar]
- 19. Kwak JG, Cho S, Kim WH. Surgical outcomes of permanent epicardial pacing in neonates and young infants less than 1 year of age. Heart Lung Circ. 2019;28(7):1127 1133. ( 10.1016/j.hlc.2018.06.1039) [DOI] [PubMed] [Google Scholar]
- 20. Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution experience of 30 years. J Am Coll Cardiol. 2002;39(1):130 137. ( 10.1016/s0735-1097(01)01697-7) [DOI] [PubMed] [Google Scholar]
- 21. Breur JM, Udink Ten Cate FEA, Kapusta L.et al. Pacemaker therapy in isolated congenital complete atrioventricular block. Pace. 2002;25(12):1685- 1691. ( 10.1046/j.1460-9592.2002.01685.x) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a