Abstract
Severe cases of acute hepatitis have been reported all around the world since 5 April 2022. Common viral hepatitis agents (HAV, HBV, HCV, HDV, and HEV) were ruled out by laboratory investigations, impelling the term “acute non-A-E hepatitis”. Common manifestations consist of abdominal pain, jaundice, and vomiting. A highly elevated level of liver enzymes was a remarkable laboratory finding among the patients. Currently, there is no clear etiology and thus treatment for the condition. Adenovirus serotype 41 (ad-41) was detected in most of the patients even though there is no elucidated link between Adenovirus and acute hepatitis. Other viral agents such as SARS-CoV-2 tested positive in a few cases. Treatment strategies depend on the severity, complications, and sequela of acute hepatitis and can vary widely from supportive therapy to liver transplantation. As of 8 July 2022, 1010 probable cases were reported from 35 countries. More than half were from the European region and were mostly children under the age of 6 years. Among different hypotheses about the etiology of severe acute non-A-E hepatitis, adenovirus-41 is of great importance but further assessments are needed to prove any definite link between ad-41 and severe acute hepatitis.
Keywords: Acute hepatitis, Children, Diagnosis, Epidemiology, Etiology, Pediatrics, Treatment, Unknown hepatitis
Highlights
-
•
Common viral hepatitis agents were ruled out by laboratory investigations, impelling the term “acute non hep A-E hepatitis”.
-
•
Severe cases of acute hepatitis of unknown etiology in children have been reported all around the world since 5 April 2022.
-
•
Common manifestations consist of abdominal pain, jaundice, vomiting, pale stool, and diarrhea.
-
•
So far, the underlying cause of the Unknown Hepatitis is not determined.
-
•
Adenovirus infection may be a probable cause.
1. Introduction
On April 5th, 2022, 10 cases of severe acute hepatitis of unknown cause between the age of 11 months to 5 years were reported by the United Kingdom (UK) to the World Health Organization's International Health Regulations (IHR) notification system. Further investigations excluded Viral hepatitis types A, B, C, D, and E, as well as other known causes of acute hepatitis [1,2].
Until 8 April 2022, the number of cases has risen by 74, mainly in previously healthy children and the common presentations were jaundice, vomiting, and pale stool. Six cases have undergone liver transplantation and no deaths were reported at that time [1,3].
The lack of a link between this condition commonly referred to as "acute non-A-E hepatitis," and the currently recognized viral hepatitis agents (HAV, HBV, HCV, HDV, and HEV), made the researchers eager to further investigate the etiology, pathophysiology, and outcome of this emerging illness [4] [1,3,5].
As of 8 July 2022, 1010 cases in five World Health Organization (WHO) regions have been reported, 46 (5%) children have required transplants, and 22 (2%) deaths have been reported to WHO which is an appalling feature of the condition [1,3,[6], [7], [8]].
In this review, we aim to summarize the currently available information about acute hepatitis of unknown etiology.
2. Methods
Search Strategy: We searched PubMed/Medline and Embase for all case reports or case series of ReA following COVID-19 published up to 12th January 2023. The following terms were used for the search strategy: [“unknown hepatitis” OR “hepatitis of unknown origin” OR “hepatitis of unknown etiology” OR “non-A-E hepatitis” OR “novel hepatitis”].
2.1. Working case definition for acute Non-A-E hepatitis
Currently the probable, epidemiologically linked, pending, and discarded cases of acute non-A-E hepatitis are defined by WHO. Due to the lack of precise etiology, the definition of a confirmed case has not been provided.
A probable case is defined as an individual who presents with acute hepatitis (non-hepatitis viruses A-E) with Alanine transaminase (ALT) or aspartate transaminase (AST) over 500 U/L with the age of 16 years or younger, since 1 October 2021.
An epi-linked case is an individual who presents with acute hepatitis of any age who was in close contact with a probable case since 1 October 2021.
If the serological tests for hepatitis A to E are waited for but other criteria are met, the patient is classified as a pending case. The discarded case is a formerly classified case that did not meet the case definition criteria with further investigations [9].
2.2. Common symptoms and associated lab data
The majority of symptoms described in the weeks leading up to hospital admissions were abdominal pain, vomiting, and diarrhea [3]. Together with jaundice, very high levels of liver enzymes (ALT and AST) were discovered [3,4]. Severely high levels of serum aminotransferases, which exceed 500 IU/L, are a remarkable feature. As Julia M. Baker et al. have figured out, severely high levels of serum aminotransferases, which exceed 500 IU/L were reported in nine affected children in Alabama with the ALT level ranging from 603 to 4696 IU/L, whereas the AST levels ranged from 447 to 4000 IU/L [10].
The WHO and the European Centre for Disease Prevention and Control (ECDC) both stated that fever was absent in the majority of patients [1,3]. The same pattern was followed in cases from Scotland, where no fever was noted in the weeks leading up to admission to the hospital [4]. The report on Alabama cases, on the other hand, revealed that fever was present in 5/9 (55.6 percent) of the cases [10]. Upper respiratory tract symptoms were noted in one-third of the children in Alabama cases before admission [10]. More than two-thirds of the cases in both Alabama and Scotland experienced vomiting and diarrhea [4,10].
Seven patients in Alabama presented with hepatomegaly; one had encephalopathy upon admission, with seven recovering without liver transplantation and two recovering after transplantation demonstrating the severity of acute non-A-E hepatitis [10].
2.3. Geographic distribution of reported cases
From 1 October 2021 to July 8th,2022, 1010 probable cases from 35 countries in five WHO Regions were reported. Approximately, half of the probable cases (484 cases) were from the WHO European Region, including 272 cases (27% of global cases) from the United Kingdom. The region of the Americas reported the second-highest number of probable cases (n = 435, including 334 cases from the United States of America). Western Pacific Region (n = 70), the South-East Asia Region (n = 19), and Eastern Mediterranean Region (n = 2) come next [8].
2.4. Demographic data
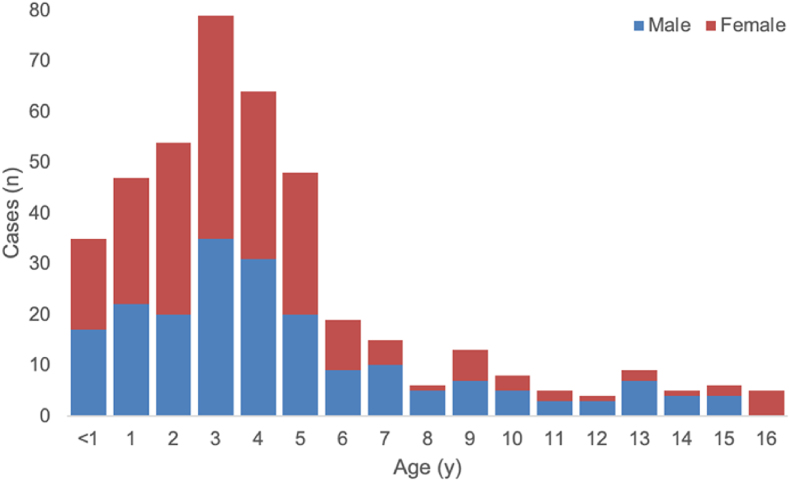
As of June 22nd, among 422 cases with available information on age and gender, 48% of cases are male (n = 202), and most of the cases (78%, n = 327) are under 6 years of age [6].(Fig. 1)
Fig. 1.
Distribution of probable cases of severe acute hepatitis of unknown aetiology in children by country, as of 8 July 2022 (n = 1010), 5 PM CEST [8].
3. The hypothesis of probable etiologies
The exact underlying cause of acute non-A-E hepatitis is still unknown. All of the hypothesized etiologies in the literature are mentioned as the following.(Fig. 2)
Fig. 2.
Gender and age distribution of 422 probable cases of severe acute hepatitis with the unknown etiology with available data (n = 422) [6].
3.1. Adenovirus infection
The role of adenovirus infection in cases of acute non-A-E hepatitis in children is now the most probable theory [1,3,5]. PCR detected adenovirus in 52% of cases (193/368) with available results [8]. The working hypothesis by the UK Health Security Agency is that a normal adenovirus infection in children can be worsened by a cofactor that causes the infection to become severe or immunopathologic [5]. During the COVID-19 pandemic, due to the lockdown restrictions, children did not encounter common infections, therefore they did not become immune to the common pathogens that they normally would be infected with. Thereafter, when the restrictions were lifted, exposure to these viruses without prior immunity resulted in severe manifestations such as acute liver failure (ALF) [11]. Former or concurrent infection with other viruses, such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or exposure to toxins or other environmental chemicals are also probable cofactors [1,5].
Another possible explanation is a novel variant of adenovirus which may cause acute non-A-E, with or without the mentioned cofactors [1,5]. The discovery that more than three-quarters of the reported cases were positive for adenoviruses supports the theory that adenoviruses have a role in acute non-A-E hepatitis [1,3,5]. Scientists and clinicians should study whether the virus's genome has changed in a way that allows for hepatotropism, which can cause severe liver inflammation.
The reporting of acute non-A-E hepatitis during the COVID-19 pandemic is a vital point to be considered. This pandemic coincided with increased global virus molecular testing capabilities; consequently, the discovery of previously unknown instances of hepatitis linked to Adenovirus–41 exacerbated by delayed exposure to this prevalent infection could be a reasonable hypothesis that requires additional proof [12].
3.2. COVID-19
A new variant of SARS-CoV-2 was considered to be a probable cause of non-A-E hepatitis, however, given the vast numbers of cases that tested negative for SARS-CoV-2, this hypothesis is not shown to be highly considered as in the European region, polymerase chain reaction (PCR) testing detected SARS-CoV-2 in 15% of cases (47/307) with available results. 10% of cases (8/83) with available results tested positive for COVID-19 in the United States [6] Moreover, acute hepatitis is not a prevalent sequela of COVID-19 in children despite reports of its incidence [13].
3.3. COVID-19 vaccines
Given that the majority of acute hepatitis of unknown etiology occurred in children who had not received the COVID-19 vaccine, a link between acute non-A-E hepatitis cases and COVID-19 vaccination seems unreasonable [3]. Furthermore, of the 99 cases reported by ECDC with available data on COVID-19 vaccination, 85 (85.9%) were unvaccinated [14].
3.4. Superantigen-mediated immune cell activation and immunopathology (immunogenicity)
The persistence of SARS-CoV-2 in the gastrointestinal tract can result in repeated viral protein releases across the intestinal epithelium, causing immunological activation [15]. A superantigen pattern of the SARS-CoV-2 spike protein that resembles Staphylococcal enterotoxin B6 may cause broad and non-specific T-cell activation, resulting in recurrent immunological activation. Superantigen-mediated immune-cell activation may be the probable cause of multisystem inflammatory syndrome [16,17]. Children with multisystem inflammatory syndrome have been diagnosed with acute hepatitis, but no other concurrent viral infections have been identified [18].
Adenovirus infection can make mice more susceptible to Staphylococcal enterotoxin-B-mediated toxic shock, which results in liver failure and death [19]. This result was explained by adenovirus-induced type-1 immunological skewing, which led to increased IFN-γ production and IFN-γ mediated hepatocyte death after Staphylococcal enterotoxin B administration [19]. One hypothesis is that the recent cases of severe acute hepatitis in children could be due to adenovirus infection with intestinal tropism in children who had been previously infected with SARS-CoV-2 and were carrying viral reservoirs. Given the recent scenario, testing for SARS-CoV-2 persistence in stool, T-cell receptor skewing, and IFN-upregulation are recommended since these observations potentially reveal a SARS-CoV-2 superantigen mechanism in a host sensitized to adenovirus type 41F.Immunomodulatory therapy should be evaluated in children with severe acute hepatitis if evidence of superantigen-mediated immune activation is detected [20].
3.5. Other viral agents
Other viral agents scarcely detected in UK patients include the Epstein-Barr virus, enterovirus, cytomegalovirus, respiratory syncytial virus, and human herpes viruses 6 and 7 [14].
3.6. Toxins/environmental agents
The possibility of a toxin originating from food or the environment is not excluded. The United Kingdom Health Security Agency (UKHSA) is undertaking epidemiological studies in which the children's environment, demographics, and food intake are assessed retrospectively. Some food has likely been contaminated with something, but there's no proof of that so far [21].
The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) has recently published research mentioning foodborne toxins as a probable underlying cause of the disease [22]. Given that food manufacturing can be a centralized process with distribution from a single producer to several locations around the world, this hypothesis should be tested. Due to the probable correlation of severe hepatic damage of the exposed individuals, aflatoxins appear as the leading candidate in this case scenario [22,23].
4. Treatment
Due to the emerging nature and lack of sufficient information on the cause of this disease, there is still no specific treatment strategy. However, symptomatic and supportive treatments are at the top of the current treatment strategies.
Adequate rest, avoidance of excessive protein consumption, and prevention of complications are the main supportive treatments [24].
The noteworthy points to which doctors must pay attention during the treatment period are the patient's consciousness, blood electrolytes, liver function, and coagulation function, as well as water, electrolyte, and acid-base balance.
As the next step, to prevent severe complications such as hepatic encephalopathy and hepatorenal syndrome, symptoms such as hypovolemia, hypoproteinemia, gastrointestinal bleeding, infection, and hypoglycemia must be taken seriously [25].
Another treatment strategy is against adenovirus infection. Adenoviruses have been detected in almost half of the known cases of this disease through PCR tests. For these cases, supportive treatment is still at the top of the order, although cidofovir has shown promise [25,26].
Acute liver failure (ALF) may result in coagulation abnormalities due to many reasons, including decreased coagulation factor synthesis, decreased hepatic thrombopoietin, and secondary bacterial infection [27]. In clinical practice, coagulation function can be evaluated using prothrombin activity (PTA) and INR. Newer techniques including thrombo-elastography (TEG) are also suggested [28]. There are no blood transfusion recommendations for kids with ALF at this moment. Plasma transfusion in large quantities can change the INR trend of ALF during therapy. Therefore, it is not advised to use fresh frozen plasma or platelets to treat coagulation problems on a preventative basis [29]. In case of vitamin K deficiency, it should be prescribed; the dosage of cryoprecipitate can be changed following the TEG value to keep fibrinogen within the normal range [25].
In the case of hepatic encephalopathy, treatment may be limited to general methods, serum ammonia-lowering therapy, treatment for intracranial hypertension, and artificial liver support. To prevent unnecessary interference, having a supine position with the head of the bed raised by 20 to 30 °C is recommended. Grading of hepatic encephalopathy needs to be assessed periodically. Endotracheal intubation is advised for children with grades III and IV hepatic encephalopathy to protect the airway and manage ventilation to maintain efficient ventilation and sufficient oxygenation [25,30].
To increase intestinal peristalsis and decrease the absorption of intestinally generated ammonia and toxins, lactulose and lactitol can be administrated orally. Ammonia-lowering medications like arginine can be taken if necessary, depending on the patient's internal environment, including electrolytes and acid-base imbalances [25,31,32].
Three main therapeutic medications to lower intracranial pressure consist of hypertonic saline, mannitol, and furosemide. Maintaining serum sodium at 145–150 mmol/L is advised when using hypertonic saline [25,31]. The drug mannitol is frequently used to reduce sudden increases in intracranial pressure. In case of renal failure, hypovolemia, serum osmolality greater than 320 mOsm/L [33], or prophylactic use, it is not recommended.
An important treatment for ALFis artificial liver support. Plasma exchange (PE), molecular adsorption recycling, and continuous blood purification are artificial liver support systems that are appropriate for children. The pathophysiological traits of the patient's illness, the idea behind the artificial liver model, and the intended effects of the therapy should all be taken into consideration while deciding when to start artificial liver therapy [34].
4.1. Liver transplant
About 6–10% of patients, particularly those with poor prognoses, require a liver transplant (LT) [35]. Children with ALF who don't improve or even progress gradually after receiving active, comprehensive medical care require urgent LT [36,37]. It’s not generally accepted whether there is any indication of LT in ALF children. Nadalin S et al. established the pursuing standards: INR >2, or INR >1.5 and linked with HE, bilirubin >18 mg/dL, increased propensity to hypoglycemia, and decreased liver size evaluated by sonography are all signs of grade III HE[36,38].
4.2. Prevention
Since the etiology and specific treatments of severe acute hepatitis in children are still unknown, it is crucial to prevent the disease from spreading. Currently, routine preventative measurements against respiratory viruses and adenovirus, such as hand cleanliness and respiratory hygiene, are advised. Clinicians are recommended to identify and report any possible cases of children presenting with hepatitis symptoms and indications that may call for serum transaminase testing [24,25].
4.3. Conclusion
The global outbreak of severe acute non-A-E hepatitis is a matter of great concern. Further investigation is needed to point out any probable epidemiologic links. So far, the underlying cause of the disease is not determined. Adenovirus infection may be a probable cause, however, there is no proven link between adenovirus infection and acute hepatitis in children.
Author contribution
KN, ANG and SFA contributed equally to this paper. KN and ANG contributed to the investigation, supervision, validation, reviewing, and editing and revised it critically under supervision of SFA. All the authors approved the final version of the manuscript.
Transparency declaration
There are no conflicts of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Control EcfDPa . 2022. Update: hepatitis of unknown origin in children.https://www.ecdc.europa.eu/en/news-events/update-hepatitis-unknown-origin-children [Available from: [Google Scholar]
- 2.World Health Organization . 2022. Acute hepatitis of unknown aetiology – the United Kingdom of great britain and northern Ireland.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON368 [Available from: [Google Scholar]
- 3.World Health Organization . 2022. Multi-Country – acute, severe hepatitis of unknown origin in children.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 [Available from: [Google Scholar]
- 4.Marsh K., Tayler R., Pollock L., Roy K., Lakha F., Ho A., et al. vol. 27. 2022. (Investigation into cases of hepatitis of unknown aetiology among Eurosurveillance). 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agency Uhs . 2022. Acute hepatitis: technical briefing.https://www.gov.uk/government/publications/acute-hepatitis-technical-briefing [Available from: [Google Scholar]
- 6.World Health Organization . 2022. Severe acute hepatitis of unknown aetiology in children - multi-country.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON394 [Available from: [Google Scholar]
- 7.Alexander E.C., Deep A. Characterization of a hepatitis outbreak in children, 2021 to 2022. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.37091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Severe acute hepatitis of unknown aetiology in children - multi-country. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400 [Available from:
- 9.World Health Organization . 2022. Suggested minimum variables for reporting cases of severe acute hepatitis of unknown aetiology in children: line list.https://www.who.int/publications/i/item/WHO-UnkHep-Surveillance-Line_list-2022.1 17 June 2022. [Available from: [Google Scholar]
- 10.Baker J.M., Buchfellner M., Britt W., Sanchez V., Potter J.L., Ingram L.A., et al. Acute hepatitis and adenovirus infection among children—Alabama. Morbidity Mortality Weekly Rep. 2022;71(18):638. doi: 10.15585/mmwr.mm7118e1. October 2021–February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Q.S., Wood T., Jelley L., Jennings T., Jefferies S., Daniells K., et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12(1):1–7. doi: 10.1038/s41467-021-21157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallam M., Mahafzah A., Şahin G.Ö. 2022. Clusters of hepatitis of unknown origin and etiology (acute non HepA–E hepatitis) among children in 2021/2022: a review of the current findings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisca G., Mallamaci M., Tardini G., Martino L., Chianucci B., Ricci M., et al. SARS-CoV-2 infection may present as acute hepatitis in children. The Pediatr Infect Dis J. 2021;40(5):e214–e215. doi: 10.1097/INF.0000000000003098. [DOI] [PubMed] [Google Scholar]
- 14.Control EcfDPa . 2022. Surveillance summary.https://www.ecdc.europa.eu/en/hepatitis/joint-weekly-hepatitis-unknown-origin-children-surveillance-bulletin [Available from: [Google Scholar]
- 15.Yonker L.M., Gilboa T., Ogata A.F., Senussi Y., Lazarovits R., Boribong B.P., et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131(14) doi: 10.1172/JCI149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodin P. SARS-CoV-2 infections in children: understanding diverse outcomes. Immunity. 2022;55(2):201–209. doi: 10.1016/j.immuni.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porritt R.A., Paschold L., Rivas M.N., Cheng M.H., Yonker L.M., Chandnani H., et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J Clin Invest. 2021;131(10) doi: 10.1172/JCI146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantor A., Miller J., Zachariah P., DaSilva B., Margolis K., Martinez M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report. Hepatology. 2020;72(5):1522–1527. doi: 10.1002/hep.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarovinsky T.O., Mohning M.P., Bradford M.A., Monick M.M., Hunninghake G.W. Increased sensitivity to staphylococcal enterotoxin B following adenoviral infection. Infect Immun. 2005;73(6):3375–3384. doi: 10.1128/IAI.73.6.3375-3384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodin P., Arditi M. The Lancet Gastroenterology & Hepatology; 2022. Severe acute hepatitis in children: investigate SARS-CoV-2 superantigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siva N. Hunt begins for the cause of acute hepatitis cases. The Lancet. 2022;399(10337):1765. doi: 10.1016/S0140-6736(22)00818-2. [DOI] [PubMed] [Google Scholar]
- 22.de Valdoleiros Src F., Beeching N., di Caro A., Petrosillo N., Ergonul O., Petersen . ESCMID; 2022. Hepatitis in children – could it be aflatoxins? [Google Scholar]
- 23.Sallam M., Mahafzah A., Şahin G.Ö., On Behalf Of Escmid Study Group For Viral H.-E. Hepatitis of unknown origin and etiology (acute non HepA-E hepatitis) among children in 2021/2022: review of the current findings. Healthcare (Basel) 2022;10(6):973. doi: 10.3390/healthcare10060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . 2022. Disease outbreak news; multicountryacute, severe hepatitis of unknown origin in children.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 [Available from: [Google Scholar]
- 25.Chen Y.H., Lou J.G., Yang Z.H., Chen Q.J., Hua C.Z., Ye S., et al. Diagnosis, treatment, and prevention of severe acute hepatitis of unknown etiology in children. World J Pediatr. 2022:1–7. doi: 10.1007/s12519-022-00581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florescu D.F., Schaenman J.M. Adenovirus in solid organ transplant recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9) doi: 10.1111/ctr.13527. [DOI] [PubMed] [Google Scholar]
- 27.Bulut Y., Sapru A., Roach G.D. Hemostatic balance in pediatric acute liver failure: epidemiology of bleeding and thrombosis, physiology, and current strategies. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.618119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hum J., Amador D., Shatzel J.J., Naugler W.E., Ahn J., Zaman A., et al. Thromboelastography better reflects hemostatic abnormalities in cirrhotics compared with the international normalized ratio. J Clin Gastroenterol. 2020;54(8):741–746. doi: 10.1097/MCG.0000000000001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Leary J.G., Greenberg C.S., Patton H.M., Caldwell S.H. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34–43.e1. doi: 10.1053/j.gastro.2019.03.070. [DOI] [PubMed] [Google Scholar]
- 30.Lutfi R., Abulebda K., Nitu M.E., Molleston J.P., Bozic M.A., Subbarao G. Intensive care management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2017;64(5):660–670. doi: 10.1097/MPG.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 31.Lutfi R., Abulebda K., Nitu M.E., Molleston J.P., Bozic M.A., Subbarao G. Intensive care management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2017;64(5):660–670. doi: 10.1097/MPG.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 32.Nath A., Haktanirlar G., Varga Á., Molnár M.A., Albert K., Galambos I., et al. Biological activities of lactose-derived prebiotics and symbiotic with probiotics on gastrointestinal system. Medicina. 2018;54(2):18. doi: 10.3390/medicina54020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schizodimos T., Soulountsi V., Iasonidou C., Kapravelos N. An overview of management of intracranial hypertension in the intensive care unit. J Anesth. 2020;34(5):741–757. doi: 10.1007/s00540-020-02795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consensus on diagnosis and treatment of invasive fungal infection in patients with severe liver disease. Zhonghua Gan Zang Bing Za Zhi. 2022;30(2):159–168. doi: 10.3760/cma.j.cn501113-20220130-00053. [DOI] [PubMed] [Google Scholar]
- 35.Khan Y.H., Mallhi T.H., Alanazi A.S., Butt M.H., Khan A., Salman M. Outbreak of acute hepatitis of unknown etiology in children: the critical role of healthcare professionals in neutralizing misleading narratives during the COVID-19 pandemic. J Med Virol. 2022 doi: 10.1002/jmv.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Kleine R.H., Lexmond W.S., Buescher G., Sturm E., Kelly D., Lohse A.W., et al. Severe acute hepatitis and acute liver failure of unknown origin in children: a questionnaire-based study within 34 paediatric liver centres in 22 European countries and Israel, April 2022. Eurosurveillance. 2022;27(19) doi: 10.2807/1560-7917.ES.2022.27.19.2200369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Squires J.E., Alonso E.M., Ibrahim S.H., Kasper V., Kehar M., Martinez M., et al. North American society for pediatric gastroenterology, hepatology, and nutrition position paper on the diagnosis and management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2022;74(1):138–158. doi: 10.1097/MPG.0000000000003268. [DOI] [PubMed] [Google Scholar]
- 38.Nadalin S., Heuer M., Wallot M., Auth M., Schaffer R., Sotiropoulos G.C., et al. Paediatric acute liver failure and transplantation: the University of Essen experience. Transpl Int. 2007;20(6):519–527. doi: 10.1111/j.1432-2277.2007.00474.x. [DOI] [PubMed] [Google Scholar]