Abstract
Introduction
Although asthma is a common disease, its diagnosis remains a challenge in clinical practice with both over- and underdiagnosis. Here, we performed a prospective observational study investigating the value of symptom intensity scales alone or combined with spirometry and exhaled nitric oxide fraction (FENO) to aid in asthma diagnosis.
Methods
Over a 38-month period we recruited 303 untreated patients complaining of symptoms suggestive of asthma (wheezing, dyspnoea, cough, sputum production and chest tightness). The whole cohort was split into a training cohort (n=166) for patients recruited during odd months and a validation cohort (n=137) for patients recruited during even months. Asthma was diagnosed either by a positive reversibility test (≥12% and ≥200 mL in forced expiratory volume in 1 s (FEV1)) and/or a positive bronchial challenge test (provocative concentration of methacholine causing a 20% fall in FEV1 ≤8 mg·mL−1). In order to assess the diagnostic performance of symptoms, spirometric indices and FENO, we performed receiver operating characteristic curve analysis and multivariable logistic regression to identify the independent factors associated with asthma in the training cohort. Then, the derived predictive models were applied to the validation cohort.
Results
63% of patients in the derivation cohort and 58% of patients in the validation cohort were diagnosed as being asthmatic. After logistic regression, wheezing was the only symptom to be significantly associated with asthma. Similarly, FEV1 (% pred), FEV1/forced vital capacity (%) and FENO were significantly associated with asthma. A predictive model combining these four parameters yielded an area under the curve of 0.76 (95% CI 0.66–0.84) in the training cohort and 0.73 (95% CI 0.65–0.82) when applied to the validation cohort.
Conclusion
Combining a wheezing intensity scale with spirometry and FENO may help in improving asthma diagnosis accuracy in clinical practice.
Short abstract
Misdiagnosis of asthma is common in clinical practice. Here, a predictive model was developed and validated, combining symptom intensity scales, spirometry and FENO, that offers a new simple and minimally invasive way to aid in diagnosing asthma. https://bit.ly/3hdpmvz
Introduction
Asthma is a common chronic respiratory disease defined by the conjunction of respiratory symptoms (breathlessness (dyspnoea), wheezing, cough and chest tightness) and the demonstration of excessive airway calibre fluctuation that varies over time [1]. This pathology is a major public health problem affecting approximately 334 million people worldwide [2]. In addition to its important prevalence, asthma is responsible for considerable direct economic costs (e.g. hospital admissions and cost of pharmaceutical medicines) and indirect economic costs (e.g. school and work days lost and income lost because of premature death) [3, 4]. Next to these public health implications of asthma, recent surveys in primary care have indicated that many patients living with asthma are underdiagnosed, while several studies point out an overdiagnosis in up to 30% of the patients who had received a diagnosis [5, 6]. Both under- and overdiagnosis produce adverse consequences for patient health-related quality of life (e.g. exposing the patient to adverse effects of the therapies prescribed and generating patient anxiety) and for healthcare systems (e.g. cost of inappropriate medicine prescription) [7].
The European Respiratory Society (ERS) has recently published guidelines for the diagnosis of asthma for both children [8] and adults [9]. These guidelines underline, for each patient complaining of recurrent asthma-like symptoms, the importance of spirometry combined with a bronchodilator reversibility test for diagnosing asthma in primary care. In the absence of significant bronchodilator reversibility in primary care, the guidelines recommend measuring exhaled nitric oxide fraction (FENO) level and highlight the value of bronchoprovocation testing in a secondary care setting if a diagnosis has not been established in primary care.
Although a common disease, the diagnosis of asthma may be all but trivial in clinical practice. In this respect, it is worth noting that in the recently published NOVELTY study only a minority of patients labelled as being asthmatic satisfied the bronchodilation criteria for asthma [10, 11]. The reason is that lung function tests are often not performed and consequently diagnosis is reduced to the clinical history and signs at the physical examination [12]. There has been much emphasis on the utility of discovering and using biomarkers to help in the diagnosis [13–15]. In contrast, the respective value of symptoms as reported directly by patients has been somewhat neglected [5, 11] and the recent ERS guidelines acknowledge the importance of new research focusing on detailed symptoms history [9]. Indeed, despite the increasing recognition of the need to include the patient's perspective in routine practice as captured by patient-reported outcome measures (PROMs) [16, 17], few asthma studies have explored the contribution of patient-reported asthma symptoms in the diagnosis [18, 19]. Therefore, in line with the growing importance given to the patient's perspective, we decided to explore the diagnostic performance of the intensity of each classical asthma symptom as reported directly by the patient and to investigate how their combination with spirometric indices and FENO might improve diagnostic accuracy.
Methods
Study design, setting and participants
We conducted a prospective observational study between November 2018 and December 2021 on adult patients (≥18 years) investigated in a real clinical practice setting at the asthma clinic of Liège University Hospital (secondary care centre) (Liège, Belgium). We recruited 303 untreated patients who sought medical attention and in whom asthma was suspected based on clinical history. We split our global cohort (n=303) into a training cohort that comprised patients recruited during odd months (n=166) and a validation cohort of patients recruited during even months (n=137). In accordance with the Global Initiative for Asthma (GINA) criteria, asthma diagnosis was based on the presence of typical symptoms (wheezing, dyspnoea, cough, sputum production and chest tightness) combined with ≥12% and ≥200 mL forced expiratory volume in 1 s (FEV1) reversibility after inhalation of 400 μg salbutamol and/or a provocative concentration of methacholine causing a 20% fall in FEV1 (PC20M) ≤8 mg·mL−1 when FEV1 ≥70% predicted. Patients attended the asthma clinic on 2 days at an interval of 1–2 weeks. On day 1, each patient underwent FENO measurement, spirometry with bronchodilation, sputum induction, gave a blood sample, and filled in asthma control and asthma quality of life questionnaires as well as symptom intensity scales. On day 2, subjects underwent methacholine challenge if baseline FEV1 was not <70% predicted.
This study was approved by the Liège University Hospital ethics committee. Signed informed consent was obtained from patients as soon as they entered the asthma clinic. They agreed to allow their clinical data and the health outcomes they reported in the routine setting to be used for research purpose.
Study parameters
Asthma symptoms intensity scales
The intensity levels of the five classic asthma symptoms (wheezing, dyspnoea, cough, sputum production and chest tightness) [20] were measured using Likert scales that extend over five levels (from 0 to 4), where level 0 means that the symptom is not present and level 4 expresses the greatest intensity of the symptom concerned.
Demographic and disease characteristics
Demographic characteristics were age, gender, atopy, smoking status and body mass index (BMI). Atopy was defined by a positive IgE test (>0.35 kU·L−1) to one or more common aeroallergens from our area (grass pollen, tree pollen, cat, dog, moulds and house dust mite). Smoking status was divided in three categories: never-smoker, ex-smoker (quit smoking at least 6 months previously) and current smoker.
Disease characteristics were lung function and systemic and airway inflammation. Lung function testing was performed by spirometry (PFT Spirostick; Geratherm, Geratal, Germany), according to the American Thoracic Society/ERS standard [21]. A post-bronchodilator (reversibility) test was done for each patient, irrespective of their baseline FEV1 and FEV1/forced vital capacity (FVC) ratio, as a standard procedure. Patients were administrated 400 μg inhaled salbutamol via a metered-dose inhaler (Ventolin), one puff at a time into the spacer, and spirometry was performed again 15 min later. Patients with baseline FEV1 ≥70% predicted underwent a methacholine challenge test, as previously described [22, 23]. Using tidal breathing, the subjects inhaled successive quadrupling methacholine concentrations from 0.06 to 16 mg·mL−1 for 30 s each through a Hudson jet nebuliser (Micro Mist; Hudson RCI/Teleflex, Research Triangle Park, NC, USA) [22]; FEV1 was measured 30 and 90 s after each concentration. The test was stopped if FEV1 fell at least 20% from its baseline value. The PC20M was calculated by linear interpolation from the last two points of the curve. Inflammatory parameters included FENO, sputum cell counts, blood cell counts and systemic markers. FENO was measured at a flow rate of 50 mL·s−1 (NIOX; Aerocrine, Solna, Sweden) before spirometry. Sputum induction and processing were performed as previously described [24]. Blood eosinophil counts and total serum IgE were determined by routine laboratory analysis at Liège University Hospital.
Statistical analysis
Quantitative variables were summarised as mean with standard deviation or median (interquartile range (IQR)), while count (percentage) was given for qualitative variables. Linear regression models were applied to analyse the comparison of the two cohorts (training and validation), as well as the comparison of asthmatic and nonasthmatic groups within both cohorts.
The objective of our study was to identify the parameters that could predict accurate asthma diagnosis. These analyses included symptom intensity scale scores (wheezing, dyspnoea, cough, sputum production and chest tightness), FEV1 (% pred), FEV1/FVC (%) and FENO. In order to assess the diagnostic performance of these different parameters, receiver operating characteristic (ROC) curves were drawn in the training cohort. Multivariable logistic regression analysis was then applied in the training cohort considering significant parameters found in the univariate logistic regression analysis to derive different predictive models. Odds ratios with corresponding 95% confidence intervals were provided to assess the strength of association between asthma diagnosis and parameters. Finally, the validity of the predictive models was evaluated by analysing the validation dataset. In order to achieve these objectives, the corresponding ROC curve was depicted, and sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) were also calculated. In order to compare the discriminant capacity of the different predictive models, the area under the curve (AUC) and corresponding 95% confidence intervals were calculated. All analyses were performed using R (www.r-project.org) at a significance level of p<0.05.
Results
Characteristics of the study population
Demographic, functional and inflammatory features of the training and validation cohorts are given in table 1. The training and validation cohorts were similar regarding demographics, functional and inflammatory features. The majority of the patients had preserved baseline spirometric values, and FENO and blood eosinophils counts within the normal range.
TABLE 1.
Patient demographic, functional and inflammatory characteristics in the training and validation cohorts
| Training cohort (n=166) | Validation cohort (n=137) | |
| Asthmatic subjects | 63 (105) | 58 (80) |
| Age (years) | 51±16 | 51±15 |
| Male | 43 (71) | 36 (50) |
| BMI (kg·m−2) | 27±4.8 | 26±4.9 |
| Smoking status | ||
| Nonsmoker | 52 (87) | 51 (70) |
| Ex-smoker | 25 (42) | 31 (42) |
| Current smoker | 23 (37) | 18 (25) |
| Atopy | 47 (78) | 42 (58) |
| FEV1 (% pred) | 92±17 | 94±17 |
| FEV1/FVC (%) | 79±8.3 | 79±7.9 |
| FENO (ppb) | 21 (14–34) | 19 (13–29) |
| Sputum eosinophils (%)# | 1 (0–3) | 1 (0.2–2.5) |
| Blood eosinophils (%) | 2.5 (1.3–4.2) | 2.1 (1.2–3.1) |
| Blood eosinophils (μL−1) | 170 (98–290) | 160 (81–250) |
| Total serum IgE (kU·L−1) | 60 (22–252) | 78 (27–158) |
Data are presented as % (n), mean±sd or median (interquartile range). BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FENO: exhaled nitric oxide fraction. #: n=95 in the training cohort and n=94 in the validation cohort.
Comparison between asthmatic and nonasthmatic subjects in the training cohort
105 out of 166 patients (63%) were found to be asthmatic in the training cohort. Asthmatic subjects displayed lower FEV1 (% pred) (p<0.0001) and FEV1/FVC (%) (p<0.0001) values and a higher FENO (p<0.05) value compared with nonasthmatic subjects, while there was no significant difference for blood eosinophils and serum IgE (table 2). With respect to symptoms, wheezing was the only symptom showing a difference between asthmatic and nonasthmatic subjects (p<0.001). Detailed analysis of asthmatic and nonasthmatic subjects in the validation cohort is provided in supplementary table S1.
TABLE 2.
Comparison between asthmatic and nonasthmatic subject demographic, functional and inflammatory characteristics in the training cohort (n=166)
| Asthmatic subjects (n=105) | Nonasthmatic subjects (n=61) | |
| Age (years) | 52±16 | 48±17 |
| Male | 40 (42) | 47 (29) |
| BMI (kg·m−2) | 27±4.3 | 27±5.5 |
| Smoking status | ||
| Nonsmoker | 48 (51) | 59 (36) |
| Ex-smoker | 28 (29) | 21 (13) |
| Current smoker | 24 (25) | 20 (12) |
| Atopy | 48 (50) | 44 (28) |
| FEV1 (% pred) | 86±18 | 98±16**** |
| FEV1 <80% pred | 27 (28) | 11 (7) |
| FEV1/FVC (%) | 76±9 | 82±6.7**** |
| FEV1/FVC <75% | 43 (46) | 16 (10) |
| FENO (ppb) | 22 (15–37) | 19 (13–27.5)* |
| FENO >25 ppb | 40 (42) | 36 (22) |
| Sputum eosinophils (%)# | 1 (0–5) | 0.8 (0.05–2.65) |
| Blood eosinophils (%) | 2.7 (1.45–4.5) | 2.1 (1.3–3.2) |
| Blood eosinophils (μL−1) | 190 (110–320) | 140 (98–260) |
| Blood eosinophils >300 μL−1 | 26 (27) | 18 (11) |
| Total serum IgE (kU·L−1) | 80 (27.5–272) | 42 (17–148) |
| Wheezing intensity score | 1.48±1.1 | 0.84±1.1*** |
| Dyspnoea intensity score | 2.09±1.1 | 1.87±1.1 |
| Cough intensity score | 1.63±1 | 1.52±1.1 |
| Airway secretion intensity score | 1.28±1 | 1.08±1.1 |
| Chest tightness intensity score | 1.57±1.2 | 1.52±1 |
Data are presented as % (n), mean±sd or median (interquartile range). BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FENO: exhaled nitric oxide fraction. #: n=57 asthmatic subjects and n=38 nonasthmatic subjects. *: p<0.05; ***: p<0.001; ****: p<0.0001.
Diagnostic power of symptoms, spirometric indices and FENO in the training cohort
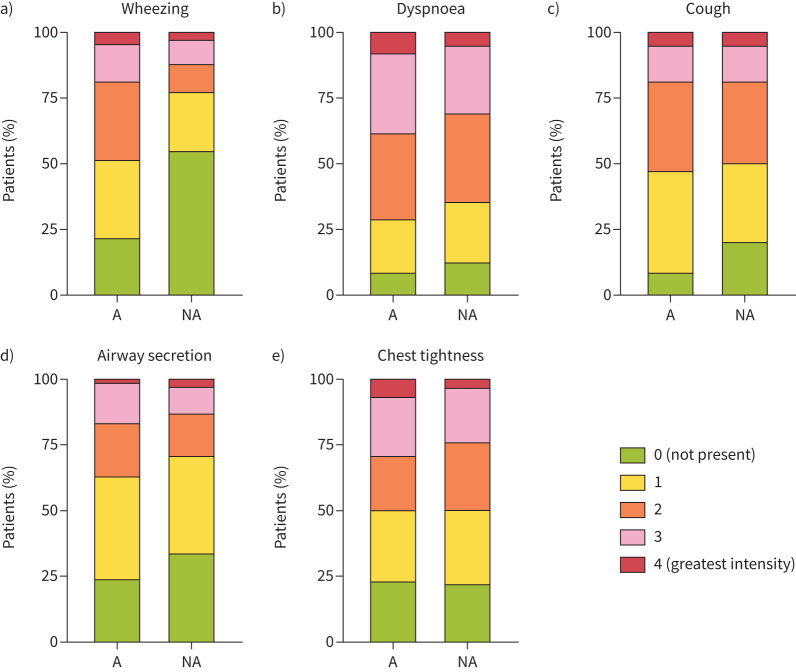
The performance of each symptom, spirometric indices and FENO in the training cohort was assessed by constructing ROC curves (table 3). Among symptoms, only wheezing provided a significant AUC (0.67 (95% CI 0.59–0.76); p<0.001). While wheezing was the most discriminant symptom, 22% of patients with an asthma diagnosis did not report any wheezing (figure 1 and supplementary figure S1). By comparison, only 9% of patients did not report dyspnoea, and the corresponding values for chest tightness, cough and airway secretion were 25%, 12% and 25%, respectively (figure 1 and supplementary figure S1). Both FEV1 (% pred) and FEV1/FVC (%) also provided a significant AUC (0.68 (95% CI 0.60–0.77); p<0.0001 and 0.69 (95% CI 0.61–0.77); p<0.0001, respectively), whereas FENO failed to provide a significant AUC (0.56 (95% CI 0.47–0.66); p=0.184).
TABLE 3.
Performance of each symptom intensity scale, spirometric indices and exhaled nitric oxide fraction (FENO) to diagnose asthma in the training cohort (n=166)
| Threshold | AUC (95% CI) | Sensitivity (%) | Specificity (%) | p-value AUC | |
| Wheezing intensity score | 0.5 | 0.67 (0.59–0.76) | 78 (69–86) | 54 (41–67) | 0.0002 |
| Dyspnoea intensity score | 2.5 | 0.56 (0.46–0.64) | 38 (29–48) | 69 (56–80) | 0.2485 |
| Cough intensity score | 0.5 | 0.53 (0.44–0.62) | 88 (80–93) | 20 (11–32) | 0.5410 |
| Airway secretion intensity score | 0.5 | 0.56 (0.47–0.65) | 75 (66–83) | 34 (23–48) | 0.1847 |
| Chest tightness intensity score | 2.5 | 0.51 (0.42–0.60) | 28 (19–37) | 77 (65–87) | 0.8762 |
| FEV1 (% pred) | 96 | 0.68 (0.60–0.77) | 71 (62–80) | 59 (46–71) | <0.0001 |
| FEV1/FVC (%) | 78 | 0.69 (0.61–0.77) | 54 (44–64) | 79 (66–88) | <0.0001 |
| FENO (ppb) | 33 | 0.56 (0.47–0.66) | 32 (23–43) | 83 (71–92) | 0.1839 |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. Bold indicates statistical significance.
FIGURE 1.
Asthma symptom intensity scales between asthmatic (A: n=105) and nonasthmatic (NA: n=61) subjects in the training cohort (n=166).
Building predictive models from the training cohort
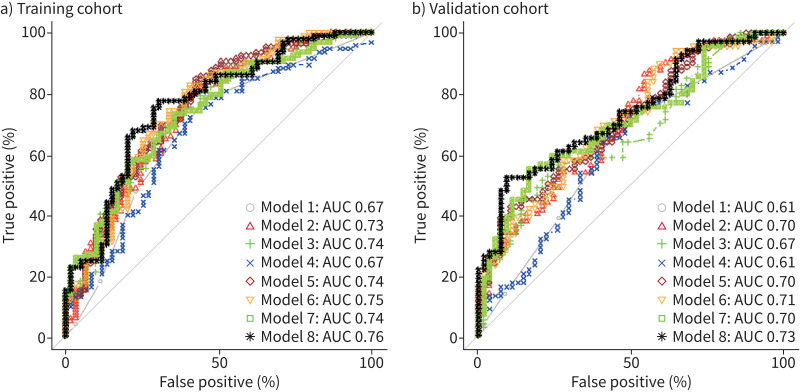
We performed univariate logistic regression for each parameter (table 4). Only wheezing, FEV1 (% pred), FEV1/FVC (%) and FENO were found to be significant. Then, we constructed eight different predictive models using a multivariable logistic regression based on the significant parameters of the univariate logistic regression (table 5). In each model, the probability of asthma diagnosis increased with wheezing intensity and FENO levels. Likewise, the probability of asthma increased when FEV1 (% pred) and FEV1/FVC (%) decreased. Only wheezing and FEV1 (% pred) were significant in any models where they were tested. The best performing model (Model 8) included wheezing, FEV1 (% pred), FEV1/FVC (%) and FENO, and provided an AUC of 0.76 (95% CI 0.66–0.84) with a sensitivity and specificity of 0.77 (95% CI 0.54–0.85) and 0.69 (95% CI 0.44–0.81), respectively (figure 2a). The NPV and PPV were 0.66 and 0.80, respectively (table 6).
TABLE 4.
Univariate logistic regression on the training cohort (n=166)
| OR (95% CI) | |
| Wheezing intensity score | 1.72 (1.26–2.40)*** |
| Dyspnoea intensity score | 1.20 (0.89–1.62) |
| Cough intensity score | 1.10 (0.81–1.49) |
| Airway secretion intensity score | 1.21 (0.89–1.66) |
| Chest tightness intensity score | 1.03 (0.79–1.35) |
| FEV1 (% pred) | 0.95 (0.93–0.97)**** |
| FEV1/FVC (%) | 0.90 (0.86–0.95)**** |
| FENO (ppb) | 1.02 (1.002–1.04)* |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FENO: exhaled nitric oxide fraction. *: p<0.05; ***: p<0.001; ****: p<0.0001.
TABLE 5.
Multivariate logistic regression on the training cohort (n=166)
| OR (95% CI) | ||
| Model 1 | Wheezing | 1.72 (1.26–2.40)** |
| Model 2 | Wheezing | 1.59 (1.16–2.22)** |
| FEV1 (%) | 0.96 (0.93–0.98)** | |
| Model 3 | Wheezing | 1.61 (1.17–2.27)** |
| FEV1/FVC (%) | 0.91 (0.86–0.96)** | |
| Model 4 | Wheezing | 1.62 (1.19–2.25)** |
| F ENO | 1.02 (0.99–1.04) | |
| Model 5 | Wheezing | 1.57 (1.14–2.19)** |
| FEV1 (% pred) | 0.97 (0.94–0.99)* | |
| FEV1/FVC (%) | 0.94 (0.88–0.99)* | |
| Model 6 | Wheezing | 1.53 (1.11–2.18)* |
| FEV1 (% pred) | 0.95 (0.93–0.98)*** | |
| F ENO | 1.02 (1.00–1.05)* | |
| Model 7 | Wheezing | 1.50 (1.08–2.11)* |
| FEV1/FVC (%) | 0.91 (0.86–0.96)*** | |
| F ENO | 1.01 (0.99–1.03) | |
| Model 8 | Wheezing | 1.48 (1.07–2.11)* |
| FEV1 (% pred) | 0.96 (0.94–0.99)* | |
| FEV1/FVC (%) | 0.94 (0.88–1.01) | |
| F ENO | 1.02 (0.99–1.04) |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FENO: exhaled nitric oxide fraction. *: p<0.05; **: p<0.01; ***: p<0.001.
FIGURE 2.
Receiver operating characteristic curves showing the performance of the predictive models (Models 1–8 in table 5) in the a) training and b) validation cohorts. AUC: area under the curve.
TABLE 6.
Diagnostic performance of the models derived from the training cohort and applied to the training cohort (n=166)
| AUC (95% CI) | Threshold | Sensitivity (95% CI) | Specificity (95% CI) | NPV | PPV | |
| Model 1 | 0.67 (0.59–0.76) | 0.20 | 0.78 (0.62–0.86) | 0.54 (0.35–0.67) | 0.59 | 0.74 |
| Model 2 | 0.73 (0.65–0.81) | 0.22 | 0.80 (0.58–0.89) | 0.59 (0.41–0.71) | 0.63 | 0.77 |
| Model 3 | 0.74 (0.65–0.82) | 0.33 | 0.75 (0.57–0.87) | 0.62 (0.44–0.74) | 0.59 | 0.77 |
| Model 4 | 0.67 (0.58–0.76) | 0.24 | 0.74 (0.53–0.85) | 0.58 (0.36–0.71) | 0.59 | 0.73 |
| Model 5 | 0.74 (0.67–0.83) | 0.12 | 0.85 (0.62–0.93) | 0.57 (0.41–0.69) | 0.69 | 0.77 |
| Model 6 | 0.75 (0.67–0.83) | 0.14 | 0.83 (0.64–0.91) | 0.59 (0.34–0.71) | 0.69 | 0.76 |
| Model 7 | 0.74 (0.65–0.82) | 0.34 | 0.73 (0.53–0.83) | 0.64 (0.42–0.76) | 0.60 | 0.76 |
| Model 8# | 0.76 (0.68–0.84) | 0.25 | 0.77 (0.54–0.85) | 0.69 (0.44–0.81) | 0.66 | 0.80 |
AUC: area under the curve; NPV: negative predictive value; PPV: positive predictive value. #: best performing model.
Application of the predictive models to the validation cohort
80 out of 137 patients (58%) in the validation cohort proved to be asthmatic. The application of the eight predictive models to the validation cohort is shown in table 7 and figure 2b. The best performing model (Model 8) was the one that included all the parameters with an AUC of 0.73 (95% CI 0.65–0.82) with a sensitivity and specificity of 0.52 (95% CI 0.21–0.64) and 0.91 (95% CI 0.63–0.96), respectively. The NPV and PPV were 0.60 and 0.88, respectively.
TABLE 7.
Diagnostic performance of the models derived from the training cohort and applied to the validation cohort (n=137)
| AUC (95% CI) | Threshold | Sensitivity (95% CI) | Specificity (95% CI) | NPV | PPV | |
| Model 1 | 0.61 (0.52–0.71) | 0.55 | 0.69 (0.50–0.80) | 0.54 (0.36–0.68) | 0.55 | 0.67 |
| Model 2 | 0.70 (0.61–0.77) | 0.46 | 0.86 (0.57–0.95) | 0.46 (0.28–0.58) | 0.70 | 0.69 |
| Model 3 | 0.67 (0.58–0.76) | 0.71 | 0.45 (0.22–0.59) | 0.86 (0.68–0.93) | 0.53 | 0.82 |
| Model 4 | 0.61 (0.51–0.71) | 0.57 | 0.64 (0.35–0.76) | 0.61 (0.37–0.72) | 0.57 | 0.68 |
| Model 5 | 0.70 (0.62–0.79) | 0.71 | 0.42 (0.22–0.54) | 0.89 (0.70–0.98) | 0.52 | 0.85 |
| Model 6 | 0.71 (0.61–0.80) | 0.37 | 0.88 (0.65–0.97) | 0.42 (0.24–0.54) | 0.74 | 0.66 |
| Model 7 | 0.70 (0.61–0.79) | 0.64 | 0.55 (0.33–0.68) | 0.83 (0.54–0.92) | 0.59 | 0.81 |
| Model 8# | 0.73 (0.65–0.82) | 0.70 | 0.52 (0.21–0.64) | 0.91 (0.63–0.96) | 0.60 | 0.88 |
AUC: area under the curve; NPV: negative predictive value; PPV: positive predictive value. #: best performing model.
Finally, including all the symptoms in the constructed models did not provide better diagnosis accuracy (supplementary tables S2 and S3).
Discussion
Here we provide a predictive model based on noninvasive measures that might improve the accuracy of asthma diagnosis in clinical practice. Our study shows that combining a wheezing intensity scale together with spirometric indices and FENO provides a fair model to predict asthma defined by excessive fluctuation of airway calibre that is demonstrated either by a positive reversibility test or by a positive bronchial challenge to methacholine.
Wheezing came out as the best symptom to predict asthma. Wheezing is the result of turbulent airflow passing through the airways as a consequence of a reduction in airway calibre. This obviously fits with asthma pathophysiology, which features episodes of airway calibre constriction [25]. Interestingly, as the best threshold of the wheezing AUC is in the lower part of the scale it would suggest that the symptom is already discriminant even if relatively mild. However, it should be noted that 22% of patients with a proven asthma diagnosis denied any wheezing. Our findings are in line with a study conducted by Sistek et al. [26], where the authors demonstrated that, among chronic respiratory symptoms, wheezing was the best single predictor of confirmed asthma. However, the authors considered the wheezing symptom in a dichotomous way (yes or no) and did not use a validation cohort to confirm their results. Contrarily, Shin et al. [27] developed a self-reported symptoms questionnaire to aid asthma diagnosis where they demonstrated that cough was the best symptom to discriminate in a trial conducted in secondary care. However, an important limit to their results was the size of their sample that only included 50 patients. Regarding the values of symptoms for diagnosing asthma, Schneider et al. [18] demonstrated that the diagnostic performance of each symptom was dependent of the healthcare sector. In this respect, they showed that dyspnoea and chest tightness were better to discriminate in primary care, while wheezing and expectorations were better in secondary care.
There has been much emphasis on the need for clinically objective parameters to help the clinician to make an asthma diagnosis [13–15]. Our data show that baseline spirometric indices provided a moderate accuracy (FEV1 (% pred): AUC 0.68; FEV1/FVC (%): AUC 0.69) to make a correct asthma diagnosis in patients complaining with chronic respiratory symptoms. This is in line with the value of low FEV1 (% pred) and low FEV1/FVC (%) as predictors of a significant bronchodilator response [22, 28]. However, spirometric indices performed better than measuring FENO (AUC 0.56) in that regard, which is in keeping with previous studies conducted in other cohorts [15, 29]. If FENO was found to be a component of the predictive model that proved to be the best when applied on the validation cohort, its contribution in the predictive models was, however, clearly less than that of spirometric indices. Adding FENO to the model that combined wheezing and spirometry resulted in a slight increase of the AUC from 0.74 to 0.76 and from 0.70 to 0.73 in the training and validation cohorts, respectively. By contrast, adding spirometric indices to the model that combined wheezing and FENO improved the AUC from 0.67 to 0.76 and from 0.61 to 0.73 in the training and validation cohorts, respectively. The modest contribution of FENO to asthma diagnosis does not discard its value as a predictive biomarker for good symptom response to inhaled corticosteroids irrespective of the asthma label [30].
Overall, our data indicate that combining a subjective parameter (wheezing intensity score) with clinical objective parameters (spirometric indices and FENO) provides a high PPV (0.88) for asthma diagnosis. However, if the objective is to rule out asthma, the model with the highest NPV should be chosen, i.e. Model 6 in our study. Regardless, our results support the idea recently recommended by Nawaz et al. [31] to combine subjective and objective parameters in order to improve the accuracy of asthma diagnosis. Using PROMs to help diagnosis has mainly been developed in detecting mental health conditions such as depression [32]. Nevertheless, developing PROMs in order to aid the diagnosis of chronic disease such as asthma is equally relevant [31]. In this regard, we believe that a systematic assessment by a symptom intensity scale contributes to improve asthma diagnosis by alerting clinicians about a symptom that the patient might not necessarily report spontaneously [33].
Given its high PPV, our model should now be tested in a primary care setting because it offers a simple and a quick way to make a first selection in patients with symptoms suggestive of asthma. Filling in a symptom questionnaire and performing a FENO measurement followed by spirometry will take only 10 min, which is less time consuming than the classic reversibility test, which requires 15–20 min and was, otherwise, found to be insensitive in capturing excess airway variability in patients with preserved baseline airway calibre [9, 11]. Furthermore, developing a digital health tool that integrates an algorithm based on our results might be a useful diagnostic/screening tool to disseminate in the primary care setting [34, 35]. Another algorithm for asthma diagnosis has recently been proposed by Drake et al. [36] including wheeze on auscultation, blood eosinophil count and peak flow variability. Compared with the algorithm proposed by Drake et al. [36], our algorithm would have the advantage of providing the probability of being asthmatic in only one visit. It would be of great interest to compare the diagnostic performance of these two algorithms in a new prospective study.
Strengths and limitations of the study
A strength of our study is that we combined symptoms together with FENO and spirometry, whereas many previous studies investigated an index test alone [13, 29]. Furthermore, we derived a predictive model in the training cohort that we applied in a validation cohort. The way we selected the training and validation cohorts with the odd months to set up the training cohort and the even months to build the validation cohort resulted in a validation cohort very similar to the training cohort. We believe this selection process may avoid bias linked to change in hospital organisation and patient attendance due to the coronavirus disease 2019 (COVID-19) pandemic, which strongly affected our region during early spring 2020 as well as during the fall of the same year. This mode of selection also allows us to escape the bias that may result from recruiting patients during different seasons, which might influence allergen exposure known to impact FENO values in sensitised patients. Our study has some limitations. First, it was performed in a secondary care centre and the type of patients recruited might not be representative of patients seen in primary care where most asthma diagnoses are performed. However, healthcare organisation in Belgium is such that mild asthmatic subjects typically seen in primary care may have access to secondary care and seek a diagnosis for their complaint in an ambulatory care setting of a university hospital without general practitioner gatekeeping. The demographic and functional characteristics of our patient cohorts are in fact close to what is seen in a primary care setting [37, 38] and in cohorts of adult incident asthma [39, 40]. Second, the symptom question only considered the intensity of the symptom but not the triggers. Third, the Likert scales extended over five levels, where patients had to choose one level (0, 1, 2, 3 or 4), whereas having a scale that extends over 10 levels might have refined the results [41]. Fourth, the criterion of significant reversibility after bronchodilation may be a subject of discussion and has not been extensively validated to differentiate asthmatic from nonasthmatic patients in clinical studies [42]. Fifth, some of the patients classified as nonasthmatic might have actually developed real asthma if followed over weeks or months, illustrating the concept of a fourth dimension in asthma diagnosis [35].
Conclusions
A wheezing intensity scale combined with spirometry and FENO enabled us to build a predictive model which offers a new simple and minimally invasive way to aid in diagnosing asthma, yielding a high PPV. Its value should now be externally validated in both another secondary care setting and, above all, in a primary care setting.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables 00451-2022.supplement (858.4KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: G. Louis, B. Pétré, F. Schleich and R. Louis contributed to the conception of the study. F. Schleich, F. Guissard, M. Henket, V. Paulus and R. Louis contributed to data acquisition. G. Louis, H.N. Zahrei and A-F. Donneau performed statistical analysis. G. Louis, B. Pétré, F. Schleich, R. Louis, M. Guillaume and D. Kirkove drafted and critically revised the work. All authors gave final approval of the manuscript.
Conflict of interest: Outside of this submitted work, R. Louis received unrestricted research grants from GSK, AstraZeneca and Chiesi, and lecture or advisory board fees from GSK, AstraZeneca, Novartis and Sanofi. Outside of this submitted work, F. Schleich received lecture or advisory board fees from Chiesi, AstraZeneca, GSK and Novartis. The rest of the authors declare that they have no relevant conflicts of interest.
Support statement: This study received funding from the European Union, FEDER APPS INTERREG (APPS (Approche Patient Partenaire de Soins) 032-3-06-013). The funders had no role in study design, data collection, and analysis and interpretation of the results. The study also received support from a federal grant of the Belgian Government (EOS 0013618F). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2020. Available from: http://ginasthma.org/
- 2.Enilari O, Sinha S. The global impact of asthma in adult populations. Ann Glob Health 2019; 85: 2. doi: 10.5334/aogh.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousquet J, Bousquet PJ, Godard P, et al. The public health implications of asthma. Bull World Health Organ 2005; 83; 548–554. [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss KB, Gergen PJ, Hodgson TA. An economic evaluation of asthma in the United States. N Engl J Med 1992; 326: 862–866. doi: 10.1056/NEJM199203263261304 [DOI] [PubMed] [Google Scholar]
- 5.Aaron SD, Boulet LP, Reddel HK, et al. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 2018; 198: 1012–1020. doi: 10.1164/rccm.201804-0682CI [DOI] [PubMed] [Google Scholar]
- 6.Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–279. doi: 10.1001/jama.2016.19627 [DOI] [PubMed] [Google Scholar]
- 7.MacNeil J, Loves RH, Aaron SD. Addressing the misdiagnosis of asthma in adults: where does it go wrong? Expert Rev Respir Med 2016; 10: 1187–1198. doi: 10.1080/17476348.2016.1242415 [DOI] [PubMed] [Google Scholar]
- 8.Gaillard EA, Kuehni CE, Turner S, et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur Respir J 2021; 58: 2004173. doi: 10.1183/13993003.04173-2020 [DOI] [PubMed] [Google Scholar]
- 9.Louis R, Satia I, Ojanguren I, et al. European Respiratory Society guidelines for the diagnosis of asthma in adults. Eur Respir J 2022; 60: 2101585. doi: 10.1183/13993003.01585-2021 [DOI] [PubMed] [Google Scholar]
- 10.Reddel HK, Vestbo J, Agustí A, et al. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur Respir J 2021; 58: 2003927. doi: 10.1183/13993003.03927-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis R, Louis G, Bonhomme O. NOVELTY: a landmark study in phenotyping and endotyping chronic obstructive airway diseases in real clinical practice. Eur Respir J 2021; 58: 2100627. doi: 10.1183/13993003.00627-2021 [DOI] [PubMed] [Google Scholar]
- 12.Heffler E, Crimi C, Mancuso S, et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med 2018; 142: 48–52. doi: 10.1016/j.rmed.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Hunter CJ, Brightling CE, Woltmann G, et al. A comparison of the validity of different diagnostic tests in adults with asthma. Chest 2002; 121: 1051–1057. doi: 10.1378/chest.121.4.1051 [DOI] [PubMed] [Google Scholar]
- 14.Yurdakul AS, Dursun B, Canbakan S, et al. The assessment of validity of different asthma diagnostic tools in adults. J Asthma 2005; 42: 843–846. doi: 10.1080/02770900500370981 [DOI] [PubMed] [Google Scholar]
- 15.Nekoee H, Graulich E, Schleich F, et al. Are type-2 biomarkers of any help in asthma diagnosis? ERJ Open Res 2020; 6: 00169-2022. doi: 10.1183/23120541.00169-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013; 346: f167. doi: 10.1136/bmj.f167 [DOI] [PubMed] [Google Scholar]
- 17.Nelson EC, Eftimovska E, Lind C, et al. Patient reported outcome measures in practice. BMJ 2015; 350: g7818. doi: 10.1136/bmj.g7818 [DOI] [PubMed] [Google Scholar]
- 18.Schneider A, Ay M, Faderl B, et al. Diagnostic accuracy of clinical symptoms in obstructive airway diseases varied within different health care sectors. J Clin Epidemiol 2012; 65: 846–854. doi: 10.1016/j.jclinepi.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 19.Tomita K, Sano H, Chiba Y, et al. A scoring algorithm for predicting the presence of adult asthma: a prospective derivation study. Prim Care Respir J 2013; 22: 51–58. doi: 10.4104/pcrj.2013.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gater A, Nelsen L, Fleming S, et al. Assessing asthma symptoms in adolescents and adults: qualitative research supporting development of the asthma daily symptom diary. Value Health 2016; 19: 440–450. doi: 10.1016/j.jval.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019; 200: E70–E88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis R, Bougard N, Guissard F, et al. Bronchodilation test with inhaled salbutamol versus bronchial methacholine challenge to make an asthma diagnosis: do they provide the same information? J Allergy Clin Immunol Pract 2020; 8: 618–625. doi: 10.1016/j.jaip.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 23.Louis G, Pétré B, Schleich F, et al. Predictors of asthma-related quality of life in a large cohort of asthmatics: a cross-sectional study in a secondary care center. Clin Transl Allergy 2021; 11: e12054. doi: 10.1002/clt2.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demarche SF, Schleich FN, Henket MA, et al. Effectiveness of inhaled corticosteroids in real life on clinical outcomes, sputum cells and systemic inflammation in asthmatics: a retrospective cohort study in a secondary care centre. BMJ Open 2017; 7: e018186. doi: 10.1136/bmjopen-2017-018186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim CS, Williams MH. Relationship of wheezing to the severity of obstruction in asthma. Arch Intern Med 1983; 143: 890–892. doi: 10.1001/archinte.1983.00350050044009 [DOI] [PubMed] [Google Scholar]
- 26.Sistek D, Wickens K, Amstrong R, et al. Predictive value of respiratory symptoms and bronchial hyperresponsiveness to diagnose asthma in New Zealand. Respir Med 2006; 100: 2107–2111. doi: 10.1016/j.rmed.2006.03.028 [DOI] [PubMed] [Google Scholar]
- 27.Shin B, Cole SL, Park SJ, et al. A new symptom-based questionnaire for predicting the presence of asthma. J Investig Allergol Clin Immunol 2010; 20: 27–34. [PubMed] [Google Scholar]
- 28.Tuomisto LE, Ilmarinen P, Lehtimäki L, et al. Clinical value of bronchodilator response for diagnosing asthma in steroid-naïve adults. ERJ Open Res 2021; 7: 00293-2021. doi: 10.1183/23120541.00293-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleich FN, Asandei R, Manise M, et al. Is FENO50 useful diagnostic tool in suspected asthma? Int J Clin Pract 2012; 66: 158–165. doi: 10.1111/j.1742-1241.2011.02840.x [DOI] [PubMed] [Google Scholar]
- 30.Price DB, Buhl R, Chan A, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med 2018; 6: 29–39. doi: 10.1016/S2213-2600(17)30424-1 [DOI] [PubMed] [Google Scholar]
- 31.Nawaz SF, Ravindran M, Kuruvilla ME. Asthma diagnosis using patient-reported outcome measures and objective diagnostic tests: now and into the future. Curr Opin Pulm Med 2022; 28: 251–257. doi: 10.1097/MCP.0000000000000871 [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh J, Dalkin S, Gooding K, et al. Functionality and feedback: a realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Health Serv Deliv Res 2017; 5: 1–280. doi: 10.3310/hsdr05020 [DOI] [PubMed] [Google Scholar]
- 33.Field J, Holmes MM, Newell D. PROMs data: can it be used to make decisions for individual patients? A narrative review. Patient Relat Outcome Meas 2019; 10: 233–241. doi: 10.2147/prom.s156291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez-Perea A, Dimov V, Popescu FD, et al. The applications of eHealth technologies in the management of asthma and allergic diseases. Clin Transl Allergy 2021; 11: e12061. doi: 10.1002/clt2.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Murray CS, Fowler SJ, et al. Asthma diagnosis: into the fourth dimension. Thorax 2021; 76: 624–631. doi: 10.1136/thoraxjnl-2020-216421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake S, Wang R, Healy L, et al. Diagnosing asthma with and without aerosol-generating procedures. J Allergy Clin Immunol Pract 2021; 9: 4243–4251. doi: 10.1016/j.jaip.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 37.de Sousa JC, Pina A, Cruz AM, et al. Asthma control, quality of life, and the role of patient enablement: a cross-sectional observational study. Prim Care Respir J 2013; 22: 181–187. doi: 10.4104/pcrj.2013.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw D, Green R, Berry M, et al. A cross-sectional study of patterns of airway dysfunction, symptoms and morbidity in primary care asthma. Prim Care Respir J 2012; 21: 283–287. doi: 10.4104/pcrj.2012.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rönmark E, Lindberg A, Watson L, et al. Outcome and severity of adult onset asthma-Report from the obstructive lung disease in northern Sweden studies (OLIN). Respir Med 2007; 101: 2370–2377. doi: 10.1016/j.rmed.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 40.Tuomisto LE, Ilmarinen P, Niemelä O, et al. A 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study. Respir Med 2016; 117: 223–229. doi: 10.1016/j.rmed.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 41.Krishnan JA, Lemanske RF, Canino GJ, et al. Asthma outcomes: symptoms. J Allergy Clin Immunol 2012; 129: S124–S135. doi: 10.1016/j.jaci.2011.12.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuomisto LE, Ilmarinen P, Lehtimäki L, et al. Immediate bronchodilator response in FEV1 as a diagnostic criterion for adult asthma. Eur Respir J 2019; 53: 1800904. doi: 10.1183/13993003.00904-2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables 00451-2022.supplement (858.4KB, pdf)