Abstract
Complement component 5 (C5), an important molecule in the complement cascade, blockade by antibodies shows clinical efficacy in treating complement-mediated disorders. However, insufficient blockading induced by single-nucleotide polymorphisms in the C5 protein or frequent development of “breakthrough” intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab have been reported. Herein, we developed a lipid nanoparticle (LNP)-formulated siRNA targeting C5 that was efficiently delivered to the liver and silenced C5 expression. We identified a potent C5-siRNA with an in vitro IC50 of 420 pM and in vivo ED50 of 0.017 mg/kg following a single administration. Single or repeated administrations of the LNP-formulated C5-siRNA allowed robust and durable suppression of liver C5 expression in mice. Complement C5 silencing ameliorated C5b-dependent anti-acetylcholine receptor antibody-induced myasthenia gravis and C5a-dependent collagen-induced arthritis symptoms. Similarly, in nonhuman primates, a single administration of C5-siRNA/LNP-induced dose-dependent plasma C5 suppression and concomitantly inhibited serum complement activity; complement activity recovered to the pre-treatment levels at 65 days post administration, thus indicating that the complement activity can be controlled for a specific period. Our findings provide the foundation for further developing C5-siRNA delivered via LNPs as a potential therapeutic for complement-mediated diseases.
Keywords: MT: Delivery strategies, small interfering RNA, complement component 5, lipid nanoparticle, myasthenia gravis, collagen-induced arthritis
Graphical abstract
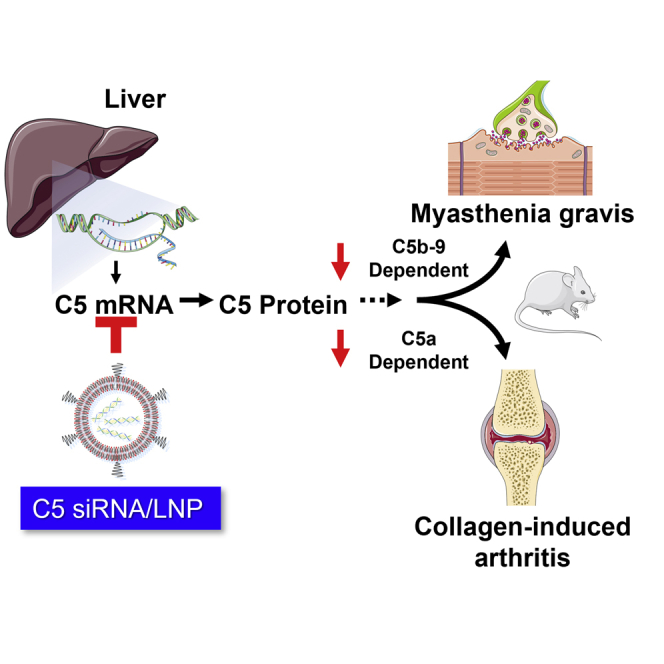
We identify a potent C5-siRNA and develop a lipid nanoparticle (LNP) that was efficiently delivered to the liver. LNP-formulated C5-siRNA showed robust and durable suppression of liver C5 expression not only in mice but also in nonhuman primates. Complement C5 silencing also ameliorated C5b- and C5a-dependent animal disease models.
Introduction
Complement component 5 (C5) is a critical molecule that controls the center of the complement pathway in innate immunity.1,2 The initiation of the terminal pathway via the assembly of C5 convertases is achieved through the activation of any of the three canonical activation routes: the classical pathway, lectin pathway, and alternative pathway.3 C5 is composed of alpha and beta polypeptide chains with a disulfide bridge and is cleaved by C5 convertases to produce C5a and C5b. C5a is an anaphylatoxin that induces chemotactic activity. C5a can also activate neutrophils that release inflammatory cytokines such as IL-8.4 Alternatively, C5b forms a complex with complement C6 and other complement components to produce C5b-9, a membrane attack complex (MAC) that eventually disrupts the cell membrane of target cells. Several reports indicate that C5a and C5b-9 are involved and play a critical role in several autoimmune/inflammatory disorders such as paroxysmal nocturnal hemoglobinuria (PNH),5,6 anti-neutrophil cytoplasmic antibody-associated vasculitis,7,8 atypical hemolytic uremic syndrome (aHUS),9 systemic lupus erythematosus,10,11 rheumatoid arthritis (RA),12 ischemia/reperfusion injury,13 myasthenia gravis (MG),14,15 neuromyelitis optica spectrum disorder (NMOSD),16 and Guillain-Barré syndrome.17 In addition, C5a and C5b-9 are also implicated in antibody-mediated rejection.18 Therefore, blockade of C5 cleavage has emerged as an attractive therapeutic approach that has led to the approval of eculizumab as a first-in-class C5 inhibitor for the treatment of complement-mediated disorders such as PNH,19,20 aHUS,21 MG,22,23 and NMOSD.24 In addition to clinical benefits, long-term safety and tolerability were also observed in those phase 2 or 3 studies and its open-label extension study; however, there are several limitations to anti-C5 antibody therapy. First, genetic polymorphisms can occur within the antibody-binding region of C5.25,26 Of the 345 Japanese patients with PNH who received eculizumab, 11 had a poor response. All 11 patients had a single missense C5 heterozygous mutation, c.2654G→A, which predicts the polymorphism p.Arg885His. The prevalence of this mutation among the patients with PNH (3.2%) was similar to that among healthy Japanese persons (3.5%).26
Second, anti-C5 antibodies require large dosages and frequent intravenous (i.v.) administration because of the high plasma concentration of C5.19,27 Furthermore, patients with PNH on eculizumab frequently develop “breakthrough” intravascular hemolysis owing to incomplete inhibition for strong complement activation during bacterial or viral infections.28 Finally, because of the limited treatment options for C5 blockade therapy, approved indication has been mostly limited for orphan diseases.1 Therefore, other therapeutic approaches that circumvent these concerns are likely to be preferred by patients and clinicians.
RNA interference (RNAi) is a naturally occurring cellular system that degrades mRNA and consequently suppresses protein synthesis.29 Synthetic small interfering RNAs (siRNAs) have emerged as potent yet specific therapeutics that can target any endogenous mRNA within cells.30 Since siRNA targets mRNA, upstream of protein production, siRNA targeting C5 can potentially bypass the above-mentioned issues of anti-C5 monoclonal antibodies. However, the major challenge in developing such therapeutics is the delivery of negatively charged large molecules into target cells.31 Complement C5 is predominantly produced within hepatocytes and secreted to circulation.32 Lipid nanoparticles (LNPs) represent one of the most clinically advanced delivery materials33,34 that are currently used for three approved siRNA- and mRNA-based medicines. We previously reported the development of unique ionizable lipids and LNPs that can efficiently deliver RNA molecules to hepatocytes.35,36 i.v. administration of LNP-formulated siRNA that targets liver-derived factor VII or proprotein convertase subtilisin kexin 9 (PCSK9) resulted in robust gene silencing with improved tolerability derived from the rapid clearance of biodegradable lipid.37
Evidence indicates that complement deposition and complement fragment are implicated in various autoimmune diseases, such as MG and RA. MG is caused by autoantibodies directed at proteins of the neuromuscular junction, primarily the acetylcholine receptor (AChR), resulting in neuromuscular transmission failure.38 Several studies suggest that complement activation is the primary effector mechanism in patients with MG and MG animal models.39,40 The administration of antibodies against C5 or C5-specific inhibitors has been shown to reduce the severity of MG in animal models.41,42 Collagen-induced arthritis (CIA) is an animal model of autoimmune polyarthritis that resembles human RA.43 As in RA, both humoral and cellular immune systems contribute to disease pathogenesis.44 Previous studies evaluated the role of the complement system in CIA and showed that the administration of anti-C5 antibodies diminished arthritis development and that C5-deficient mice were resistant to CIA.45,46
In this study, we describe the development of LNP-formulated C5-siRNA and their ability to suppress the synthesis of liver-derived C5. Our approach targeting C5 mRNA clearly ameliorated the severity in pre-clinical models of MG and RA.
Results
In vitro and in vivo screening identified candidate C5-siRNA
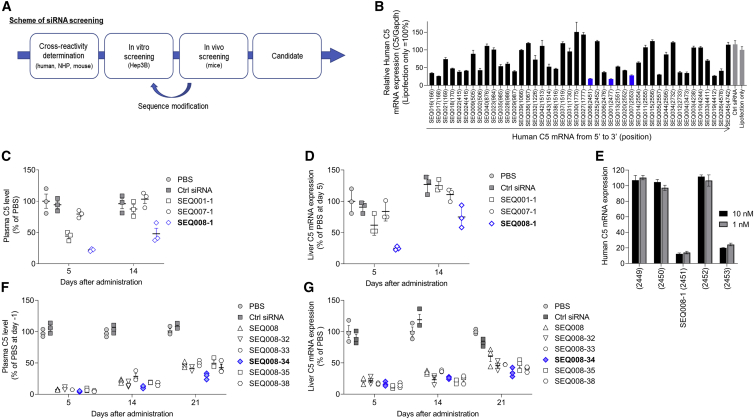
We designed siRNA sequences that are cross-reactive to mice, nonhuman primates, and human C5 mRNA (Figure 1A). All siRNAs were chemically modified with 2′-OMe nucleotides to suppress undesired immune responses as described previously.47 First, siRNAs were tested in vitro in Hep3B cells for C5 silencing by transfection (Figure 1B). The top 3 sequences (SEQ001-1, SEQ007-1, and SEQ008-1) based on silencing performance were selected and then subjected to in vivo investigation to confirm the durability of their effects on C5. Three siRNAs targeting C5 gene expression and control siRNA targeting luciferase gene expression (Ctrl siRNA) were formulated in LNPs as described previously.37 i.v. injection of LNP-formulated siRNAs into mice revealed that SEQ008-1 had the best effect on lowering both plasma C5 and liver C5 mRNA (Figures 1C and 1D). Notably, siRNA derivatives of SEQ008 targeting ±1 and ±2 positions of C5 mRNA showed no or at least 10-fold lower activity compared with that of SEQ008-1, implicating high siRNA specificity (Figure 1E). To further increase the treatment durability, we chemically and structurally modified SEQ008 and generated a panel of derivatives. The transfection of modified siRNAs into Hep3B cells allowed the identification of siRNAs that showed an equal to or greater C5 mRNA silencing activity than that of SEQ008 (Figure S1). Finally, six sequences in LNPs were tested in mice using a single i.v. administration at 0.3 mg/kg siRNA dose. We identified SEQ008-34 as the most durable candidate C5-siRNA that significantly lowered both plasma C5 and liver C5 mRNA (Figures 1F and 1G).
Figure 1.
Screening and identification of candidate C5-siRNA
(A) Schematic representation of siRNA screening. Sequences were designed to have cross-reactivity with mouse, nonhuman primates, and human C5 mRNA. All siRNAs were chemically modified with 2′-OMe nucleotides to suppress unwanted immune responses. (B) Human C5 mRNA levels in Hep3B cells transfected with a panel of siRNAs (3 nM) overnight were quantified using qRT-PCR and normalized to the housekeeping gene GAPDH. The top 3 sequences are described in the blue bar. (C and D) In vivo screening. The selected three C5-siRNAs and Ctrl-siRNA were formulated with lipid nanoparticles (LNPs). Mice (n = 3/group) received a single i.v. administration of 0.1 mg/kg PBS, Ctrl-siRNA/LNP, or C5-siRNA/LNP. Plasma C5 protein and liver C5 mRNA levels (mouse C5/mouse GAPDH) were quantified using ELISA and qRT-PCR on days 5 and 14 post administration. (E) Sequence walk. The mRNA position of top preforming SEQ008 was 2451 on C5 mRNA. Four siRNAs targeting ±1 and ±2 positions were prepared. C5 mRNA levels in Hep3B cells treated with siRNAs (1 and 10 nM) were quantified using qRT-PCR. (F and G) In vivo screening to identify SEQ008-34 as a candidate C5-siRNA. SEQ008 was chemically or structurally modified to produce derivatives. After in vitro screening, the six selected siRNAs were formulated with LNPs and then subject to in vivo screening using single i.v. administration of 0.3 mg/kg. Plasma C5 protein and liver C5 mRNA levels were quantified on days 5, 14, and 21 post administration. Data are presented as mean ± SEM.
Characterization of C5 suppression in mice
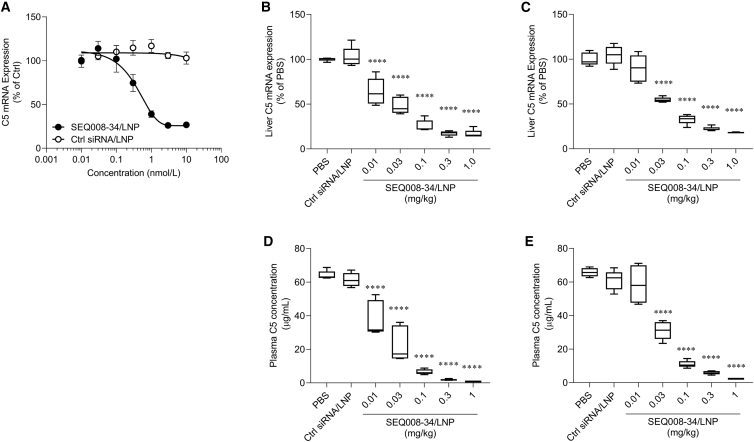
The effect of candidate compounds was characterized in Huh7 cells and in BALB/c mice. LNP-formulated SEQ008-34 exhibited a half-maximal inhibitory concentration of 420 pM in Huh7 cells (Figure 2A). Single i.v. administration of LNP-formulated SEQ008-34 at doses ranging between 0.01 and 1 mg/kg resulted in a clear dose-dependent reduction in C5 mRNA in the liver (Figures 2B and 2C) and circulating C5 levels (Figures 2D and 2E) on days 7 and 14 post administration. The half-maximal effective concentration of plasma C5 suppression (ED50) was estimated to be 0.017 mg/kg on day 7 and 0.028 mg/kg on day 14. The potent suppression of plasma C5 was achieved on days 7 (>95%) and 14 (>90%) using a dose of 0.3 mg/kg compared with that observed in the PBS-treated group (Figures 2D and 2E).
Figure 2.
Robust C5 suppressive activity of LNP-formulated C5-siRNA (SEQ008-34)
(A) Half-maximal inhibitory concentration measurement. Huh7 cells were treated with LNP-formulated C5-siRNA or Ctrl siRNA at concentrations of 0.01–10 nM. After overnight incubation, human C5 mRNA levels normalized to human GAPDH were quantified using qPCR. (B–D) C5 suppressive activity of C5-siRNA. Mice (n = 5/group) received a single i.v. administration of either PBS, 1 mg/kg of Ctrl-siRNA/LNP, or 0.01–1 mg/kg of C5-siRNA/LNP on day 0. Blood and the liver were collected on days 7 (B and D) and 14 (C and E) to evaluate plasma C5 concentration and liver C5 mRNA levels (mouse C5/mouse GAPDH). Data are presented as mean ± SEM. ∗∗∗∗p < 0.0001 versus PBS. The differences in complement C5 mRNA expression levels or plasma complement C5 protein levels between vehicle-treated and SEQ008-34/LNP-treated groups were calculated using Dunnett’s multiple comparison test.
SEQ008-34 single and repeated dosing induced robust suppression of hemolytic activity
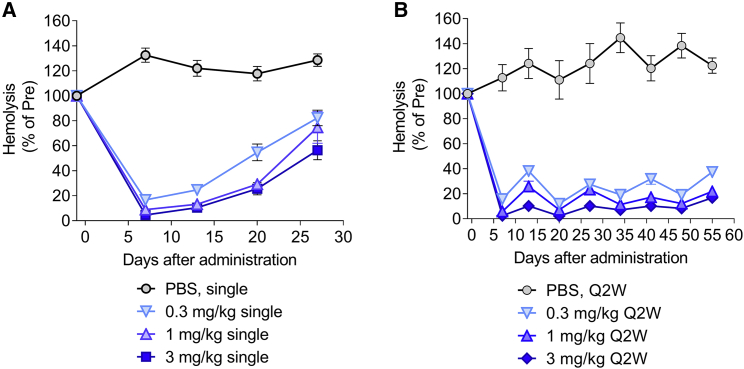
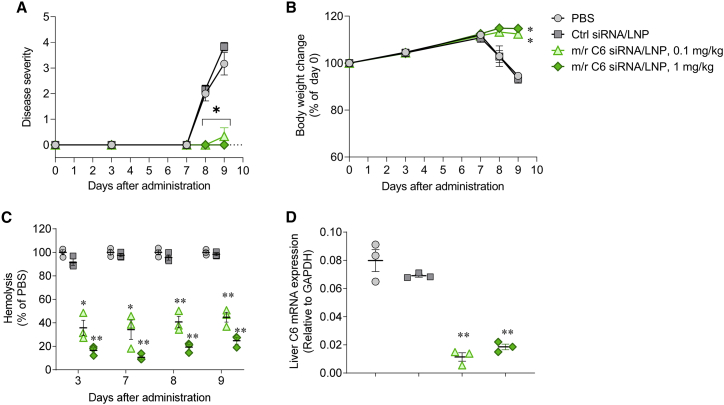
Complement activity triggered by the MAC was quantified by hemolysis based on a sensitized sheep red blood cell (RBC) lysis assay. A decrease in the levels of circulating C5 is expected to reduce MAC formation, leading to a decrease in hemolytic activity. To further characterize the candidate compound, SEQ008-34 was evaluated with single and repeated dosing schedules. As expected, single i.v. administration of LNP-formulated SEQ008-34 siRNA at 0.3, 1, and 3 mg/kg led to a dose-dependent reduction in hemolytic activity (Figure 3A). Importantly, repeated i.v. administration every 2 weeks resulted in a sustained reduction in hemolytic activity over a test period of 8 weeks (Figure 3B).
Figure 3.
Single and repeated administration of LNP-formulated C5-siRNA (SEQ008-34) lowers complement hemolytic activity in mice
(A and B) Mice (n = 4/group) received a single or repeated i.v. administration of either PBS or 0.3, 1, and 3 mg/kg of C5-siRNA/LNP. Blood samples were collected prior to and post administration (twice a week) and analyzed for complement hemolytic activity using a sheep RBC hemolysis assay. Hemolysis values were normalized to a “maximal hemolysis” control (lysis by water). Data are presented as mean ± SEM; q2w, every 2 week dosing.
Treatment with mouse/rat C5-siRNA/LNP suppresses disease symptoms and loss of neuromuscular junctions in experimental MG model in rats
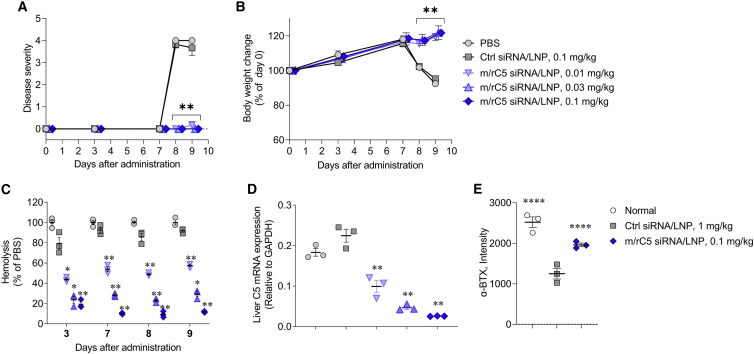
Next, we tested LNP-formulated mouse/rat surrogate C5-siRNA/LNP (m/r C5-siRNA/LNP) in experimental animal models. Based on previous reports, pre-clinical MG rat models were generated through the i.v. injection of antibodies against AChR.41,42 Since SEQ008-34 does not react with rat C5 mRNA, we prepared a surrogate siRNA (m/r C5-siRNA) that cross-reacts with mice and rats. We used the surrogate siRNA in the following rodent pre-clinical model. In brief, normal rats received i.v. injection of PBS, Ctrl-siRNA/LNP, or m/r C5-siRNA/LNP on day 0. After 7 days, MG symptoms were induced via i.v. injection of 1 mg/kg of anti-AChR mAb (mAb35). Rats treated with PBS or 1 mg/kg of Ctrl-siRNA/LNP began to display clinical symptoms, such as reduced fore limb grip strength, hindlimb weakness, and/or partial paralysis on days 8 and 9 (Figure 4A), accompanied by a reduction in body weight (Figure 4B). In contrast, all animals treated with 0.01, 0.03, and 0.1 mg/kg of m/r C5-siRNA/LNP showed reduced or no clinical manifestations of MG on days 8 and 9 (Figure 4A), with no reduction in body weight (Figure 4B). Hemolytic activity on day 9 was significantly suppressed in a dose-dependent manner in the m/r C5-siRNA/LNP treatment group compared with that observed in the PBS or Ctrl-siRNA/LNP treatment groups (Figure 4C). On day 9, m/r C5-siRNA/LNP significantly suppressed the liver complement C5 mRNA expression in a dose-dependent manner compared with that in PBS-treated animals. (Figure 4D).
Figure 4.
Treatment with mouse/rat C5-siRNA/LNP suppresses disease symptoms and loss of neuromuscular junctions in experimental MG model in rats
Disease severity (A), body weight change (B), hemolysis (C), and complement C5 expression levels in the liver (D) of MG rats (n = 3/group) treated with PBS, Ctrl-siRNA/LNP, or 0.01, 0.03, or 0.1 mg/kg of mouse/rat (m/r) C5-siRNA/LNP are shown. (A) Rats received i.v. administration of PBS, 0.1 mg/kg Ctrl-siRNA/LNP, or 0.01–0.1 mg/kg of m/r C5-siRNA/LNP on day 0. On day 7, anti-AChR mAb (mAb35, 1 mg/kg) was i.v. administered to rats to induce the symptoms of MG. Disease severity was monitored on days 8 and 9. (B) Body weights of rats treated with PBS, Ctrl-siRNA/LNP, or rat C5-siRNA/LNP were expressed as a ratio of the initial body weight (day 0). (C and D) Blood samples were collected from the jugular vein on days 3, 7, 8, and 9 and livers were collected on day 9 to evaluate the complement hemolytic activity in the serum and liver complement C5 mRNA expression levels (rat C5/rat GAPDH). (E) Soleus muscle sections were stained with Alexa Fluor 488-labeled α-BTX, and fluorescence signal was quantified for at least 50 or more neuromuscular junctions in each treated rat. Data are presented as the mean ± SEM. n = 3 in each group; ∗∗p < 0.01 versus Ctrl-siRNA/LNP.
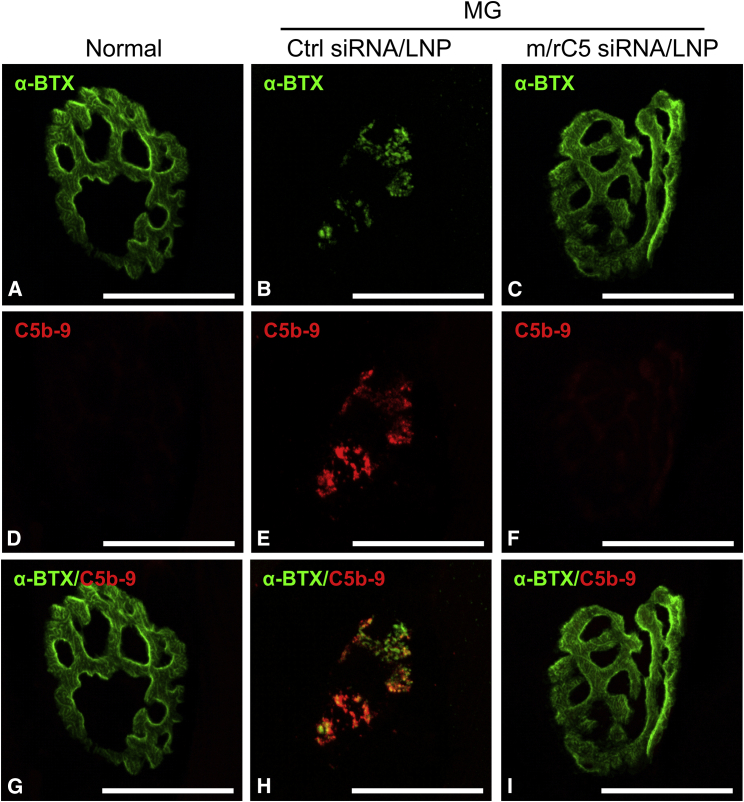
The fluorescence intensity of α-bungarotoxin (α-BTX), a marker of neuromuscular junction, in both normal and m/r C5-siRNA/LNP-treated rats was significantly greater than that of Ctrl-siRNA/LNP-treated rats, indicating protection from injury (Figure 4E). Signals of C5b-9 were clearly observed at the neuromuscular junction in rats treated with Ctrl-siRNA/LNP, and the intensity of C5b-9 was decreased upon treatment with m/r C5-siRNA/LNP, demonstrating that m/r C5-siRNA/LNP treatment could suppress the progression of complement cascade followed by C5 (Figures 5A–5I). These data indicate that m/r C5-siRNA/LNP suppressed MG symptoms with a marked reduction in serum hemolytic activity and prevention of neuromuscular junctions via the inhibition of complement C5 expression in the liver.
Figure 5.
Treatment with mouse/rat C5-siRNA/LNP suppresses the deposition of C5b-9 at the neuromuscular junction in experimental MG model in rats
Tissue sections from normal rats (A, D, and G), rats treated with Ctrl-siRNA/LNP (B, E, and H), or 0.1 mg/kg of mouse/rat (m/r) C5-siRNA/LNP (C, F, and I) were stained with α-bungarotoxin (α-BTX) (A–C) and C5b-9 (D–F) at the neuromuscular junctions in soleus muscles as shown. The merged composite of red and green images shows the localization of C5b-9 deposition at the neuromuscular junction. Soleus muscle sections were stained with Alexa Fluor 488-labeled α-BTX or anti-C5b-9 antibody, followed by Cy3-labeled secondary antibody to detect the C5b-9 signals. Maximum intensity projection (10 μm thick). Scale bars, 10 μm.
Treatment with mouse/rat C6-siRNA/LNP suppresses MG symptoms in rats
To evaluate the involvement of MAC in the onset of MG, we attempted to downregulate complement C6 in the liver, an essential component of the C5b-9 complex. Surrogate siRNA targeting mouse and rat C6 (i.e., m/r C6-siRNA) was prepared and formulated into LNP. Rats treated with PBS or 1 mg/kg of Ctrl-siRNA/LNP exhibited severe symptoms and began to lose weight consistently after MG induction (Figures 6A and 6B). In contrast, rats treated with 0.1 or 1 mg/kg of m/r C6-siRNA/LNP showed the complete suppression of clinical manifestations of MG without losing body weight (Figures 6A and 6B). Hemolytic activity on day 9 following administration was suppressed in a dose-dependent manner in the m/r C6-siRNA/LNP-treated group, but not in the PBS or Ctrl-siRNA/LNP-treated groups (Figure 6C). On day 9 following m/r C6-siRNA/LNP administration, the mRNA expression of complement C6 remained significantly suppressed (Figure 6D). These data indicate the importance of complement C6 in the onset of MG, suggesting that C5b-9 MAC, but not anaphylatoxin C5a, mainly contributes to MG pathogenesis.
Figure 6.
Mouse/rat C6-siRNA/LNP treatment suppresses MG symptoms in rats
Disease severity (A), body weight change (B), hemolysis (C), and complement C6 expression levels in the liver (D) of MG rats (n = 3/group) treated with PBS, Ctrl-siRNA/LNP, or 0.1 and 1 mg/kg mouse/rat (m/r) C6-siRNA/LNP are shown. (A) Rats received i.v. administration of PBS, 0.1 mg/kg of Ctrl-siRNA/LNP, or 0.01 or 0.1 mg/kg m/r C6-siRNA/LNP on day 0. On day 7, anti-AChR mAb (Mab35, 1 mg/kg) was i.v. administered to rats to induce the symptoms of MG. Disease severity was monitored on days 8 and 9 (B). Body weights of rats treated with PBS, Ctrl-siRNA/LNP, or rat C6-siRNA/LNP were expressed as a ratio of the initial body weight (day 0). (C and D) Blood samples were collected from the jugular vein on days 3, 7, 8, and 9 and livers were collected on day 9 to evaluate the complement hemolytic activity in serum and liver complement C6 mRNA expression levels (rat C6/rat GAPDH). Data are presented as the mean ± SEM. n = 4 in each group; ∗p < 0.05 and ∗∗p < 0.01 versus Ctrl-siRNA/LNP.
Treatment with m/r C5-siRNA/LNP, but not m/r C6-siRNA/LNP, suppressed arthritis symptoms in CIA mice
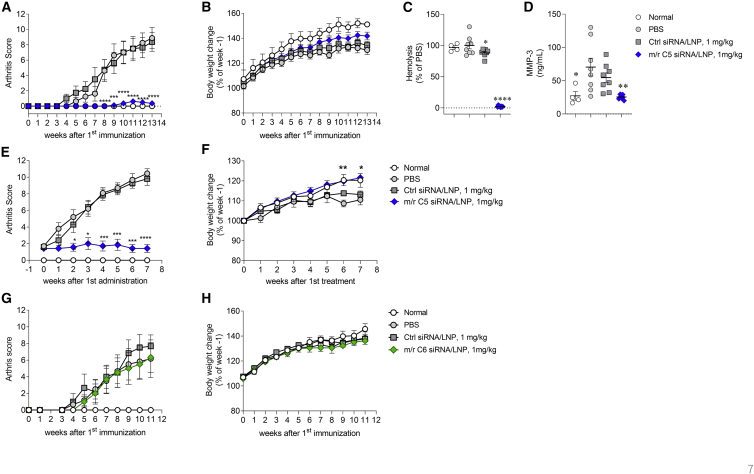
Based on previous reports,45,46 we tested the effect of surrogate m/r C5-siRNA/LNP on CIA animal models using both prophylactic and therapeutic protocols. In the prophylactic protocol, m/r C5-siRNA/LNP was administered once 4 days before the first immunization and then administered once a week. We observed a significant and almost complete inhibition of CIA progression, as indicated by a reduction in arthritis scores, with a slight recovery in weight loss, compared with those observed in the animals treated with PBS or Ctrl-siRNA/LNPs (Figures 7A and 7B). Serum hemolytic activity on week 13 also clearly decreased following treatment with m/r C5-siRNA/LNP, accompanied by a robust reduction in serum complement C5 protein compared with that of PBS-treated animals. The levels of plasma matrix metalloproteinase-3 (MMP-3) were also increased in PBS-treated animals and those levels were robustly suppressed by the treatment with m/r C5-siRNA/LNP (Figures 7C, 7D, and S2), along with the reduction of arthritis score. To evaluate the therapeutic efficacy of m/r C5-siRNA/LNP, the treatment was administration once per week after the onset of arthritis. The m/r C5-siRNA/LNP treatment significantly reduced the arthritis score, with a trend toward recovery in weight loss, compared with those observed following treatments with PBS or Ctrl-siRNA/LNP (Figures 7E and 7F). To assess the involvement of MAC in the onset of CIA, m/r C6-siRNA/LNP was used to deplete complement C6 in CIA mice. Notably, m/r C6-siRNA/LNP treatment had no impact on the development of CIA and body weight (Figures 7G and 7H). Importantly, plasma complement C6 protein levels were completely suppressed by the m/r C6-siRNA/LNP treatment (Figure S3). This clearly indicates that anaphylatoxin C5a, but not C5b-9, may play a critical role in the induction and development of CIA.
Figure 7.
Mouse/rat C5-siRNA/LNP treatment, but not of m/r C6-siRNA/LNP, suppresses the development of arthritis in CIA mice
(A–C) Prophylactic treatment with m/r C5-siRNA/LNP. Arthritis score (A), body weight change (B), and hemolysis (C) in CIA mice treated with PBS (n = 8), Ctrl-siRNA/LNP (n = 8), or m/r C5-siRNA/LNP (n = 8) and in normal mice (n = 4) are shown. Mice received i.v. administration of PBS, 1 mg/kg Ctrl-siRNA/LNP, or 0.1 mg/kg m/r C5-siRNA/LNP once per week from the day of the first immunization (day 0). Arthritis score was monitored from second immunization (day 21) onward, once or twice per week. (b) Body weights of mice treated with PBS, Ctrl-siRNA/LNP, or m/r C5-siRNA/LNP were expressed as a ratio of the initial body weight (day −4). (C and D) Blood samples were collected to evaluate the complement hemolytic (C) activity and MMP-3 level (D). Data are presented as the mean ± SEM. n = 4–8/group; ∗p < 0.05 and ∗∗∗∗p < 0.0001 versus PBS. (E and F) Therapeutic treatment with m/r C5-siRNA/LNP. Arthritis score (E) and body weight change (F) in CIA mice treated with PBS (n = 10), Ctrl-siRNA/LNP (n = 10), or m/r C5-siRNA/LNP (n = 7) and in normal mice (n = 4) are shown. (E) Mice with arthritis scores of 1–3 were randomly enrolled into groups and received i.v. administration of PBS, 1 mg/kg Ctrl-siRNA/LNP, or 1 mg/kg m/r C5-siRNA/LNP once per week from the day of enrollment. Arthritis score was sequentially monitored from enrollment once or twice per week. (F) Body weights of mice treated with PBS, Ctrl-siRNA/LNP, or m/r C5-siRNA/LNP were expressed as a ratio of the initial body weight (week −1). Data are presented as the mean ± SEM. n = 4–10/group; ∗∗p < 0.01 and ∗∗∗∗p < 0.0001 versus PBS. (G and H) Prophylactic treatment with m/r C6-siRNA/LNP. Arthritis score (G) and body weight change (H) in CIA mice treated with PBS (n = 6), Ctrl-siRNA/LNP (n = 6), or m/r C6-siRNA/LNP (n = 6) and in normal mice (n = 4) are shown. (F) Mice received i.v. administration of PBS, 1 mg/kg Ctrl-siRNA/LNP, or 1 mg/kg m/r C6-siRNA/LNP once per week from the day of the first immunization (day 0).
Single dose of C5-siRNA/LNP induced a potent, dose-dependent plasma C5 reduction in nonhuman primates
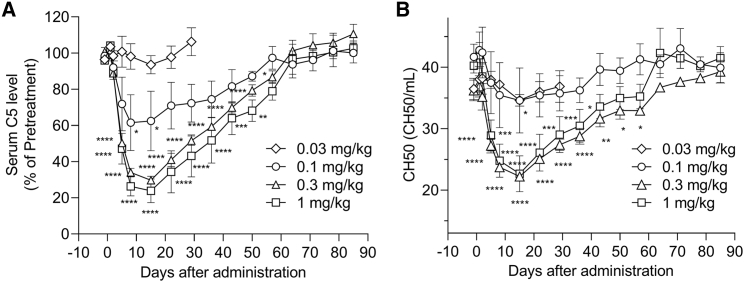
Finally, we tested our candidate compound in nonhuman primates. Cynomolgus monkeys (n = 4/group) received a single i.v. dose of 0.03, 0.1, 0.3, or 1 mg/kg of LNP-formulated SEQ008-34. In this experiment, we evaluated the total hemolytic complement activity using the 50% hemolytic unit of complement (CH50) levels, which are widely used as a tool for routine clinical diagnosis. Circulating C5 and CH50 levels were normalized to those in the average pre-dose samples for each animal. With a single injection, we observed a clear dose-dependent reduction in circulating C5 levels (Figure 8A). In the 1 mg/kg dose group, a maximum of 70%–89% C5 reduction was observed on days 8–15 post administration and the levels returned to the baseline 65 days post injection. Accordingly, CH50 levels showed similar reduction curve as that of circulating C5 (Figure 8B). These data clearly demonstrate that a single administration of LNP-formulated SEQ008-34 induces a potent decrease in blood C5 levels in nonhuman primates.
Figure 8.
Dose-dependent reduction in C5 protein levels and CH50 in nonhuman primates treated with a single dose of SEQ008-34/LNP
Cynomolgus monkeys (n = 3/group) received a single treatment with 0.03, 0.1, 0.3, or 1 mg/kg LNP-formulated C5-siRNA (SEQ008-34) on day 0 at an injection volume of 10 mL/kg by 30 min intravenous infusion with a syringe pump. (A) Serum C5 levels were quantified using ELISA and normalized to the average of three repeats. Values are expressed as the post-dose/pre-dose ratio for each animal. (B) Serum CH50 levels were quantified using a biochemistry automatic analyzer using diagnostics. Data are presented as the mean ± SEM (n = 3 in each group).
Discussion
In this study, we identified a potent and durable C5-siRNA, SEQ008-34, which has cross-reactivity with mice, nonhuman primates, and human C5 mRNA. Our LNPs allowed the delivery of C5-siRNA to hepatocytes, the main producers of circulating C5. We showed that a single administration of SEQ008-34/LNP in mice and nonhuman primates resulted in a significant suppression of serum C5 and hemolytic activity. In pre-clinical models, surrogate C5-siRNA/LNP showed robust efficacies in MG and arthritis models.
The depletion of circulating C5 leads to inactivated innate immunity, which is associated with increased infection susceptibility.48 Thus, vaccination for meningococcal disease is highly recommended before treatment with eculizumab49 because the clearance of Neisseria meningitidis is highly dependent on the terminal complement pathway.50 Therefore, treatment that can suppress C5 and allow the recovery to baseline sooner is favorable. Kusner et al. suggested the conjugation of C5-siRNA with the liver-targeting ligand N-acetylgalactosamine (GalNAc).51 In general, GalNAc-conjugated siRNA shows highly durable silencing activity, with single-dose siRNA treatments targeting PCSK952 or TTR53 eliciting long-term gene suppression between 6 and 12 months in humans. Accordingly, a single dose of 5–25 mg/kg GalNAc-conjugated C5-siRNA showed robust serum C5 knockdown in cynomolgus monkeys and its effects were sustained with approximately 75%–95% serum C5 suppression over 71 days post administration.51 In contrast, a single administration of 0.3–1 mg/kg SEQ008-34/LNP led to approximately 70%–80% serum C5 suppression in cynomolgus monkeys, and the serum C5 levels returned to baseline 65 days post administration (Figure 8A). Brown et al. reported that GalNAc-conjugated siRNA accumulates in the endosomal compartments of hepatocytes with a slow release into the cytoplasm, which results in their prolonged activity; conversely, LNPs facilitate endosomal escape in hepatocytes and the rapid release of siRNA into the cytoplasm.54 The observed difference in the duration of C5 suppression is likely mainly derived from the siRNA delivery method to the hepatocytes.54
Zilucoplan is a small peptide inhibitor of complement C5,55 and pharmacodynamic results were confirmed in a phase 2 study of patients with generalized MG.56 Interestingly, zilucoplan exhibited equipotent binding and inhibition of hemolysis induced by C5 variants, including the mutation associated with poor response to eculizumab,55 The efficacy of zilucoplan in patients with anti-AChR+ generalized MG was assessed in a phase 2 trial. Treatment with zilucoplan resulted in rapid, clinically meaningful, and statistically significant improvements compared with placebo in quantitative MG score at 12 weeks.56 Zilucoplan can be self-administered; however, zilucoplan needs to be administered subcutaneously once a day.
Collectively, our results illustrate the potential advantage of LNP-formulated siRNA for C5 suppression because the impaired complement function status can be adequately controlled for the required treatment period.
To test the efficacy of C5 inhibition using siRNA/LNP, we employed rat MG and mouse CIA models, wherein the involvement of the complement pathway is essential for etiopathogenesis.51,57,58 In the rat MG model, C5-siRNA/LNP completely suppressed the MG symptoms (Figures 4A and 4B), indicating that the C5 and the complement pathway are closely related to the onset of MG.51 Furthermore, we used C6-siRNA/LNP to validate the involvement of C5b-9 and MAC in MG pathogenesis. C6-siRNA/LNP markedly prevented the onset of MG (Figures 6A and 6B), demonstrating that MAC formation is essential for MG pathogenesis. In contrast, C5-siRNA/LNP ameliorated arthritis symptoms in the mouse CIA model, whereas C6-siRNA/LNP showed no effects (Figures 7A and 7F). Ji et al. showed that C5a plays a more important role in arthritis development than C5b-9 (i.e., MAC) by using complement C5 or C6 gene-disrupted mice.57 Consistent with this finding, our data clearly highlighted the importance of C5a in CIA pathology. Our pre-clinical data in two disease models suggest that the therapeutic approach using C5-siRNA/LNP could be effective for targeting the complement pathway, C5a, and C5b-9 (i.e., MAC).
In summary, we developed a potent siRNA therapeutic targeting C5 mRNA. Repeated administration of the identified SEQ008-34/LNP showed robust efficacy in two complement-mediated disease models. Our findings provide a foundation for the further development of C5-siRNA delivered via LNP as potential therapeutics for complement C5-mediated diseases.
Material and methods
Animals
Female or male BALB/c mice as well as male DBA/1 mice and female Lewis rats were purchased from Charles River Laboratories (Japan). Male cynomolgus monkeys were purchased from HAMRI (Japan). Animal care and experimental procedures were performed in an animal facility accredited by the Health Science Center for Accreditation of Laboratory Animal Care and Use of the Japan Health Sciences Foundation. The mice and rats were housed under controlled temperature (23°C ± 3°C) and humidity (55% ± 5%) conditions, with a 12 h light-dark cycle and were provided access to water and standard pelleted food ad libitum. Animal care and experimental procedures were performed in the animal facility accredited by the Health Science Center for Accreditation of Laboratory Animal Care and Use of the Japan Health Sciences Foundation. All protocols were approved by the Institutional Animal Care and Use Committee and carried out in accordance with the Animal Experimentation Regulations of KAN Research Institute or Eisai to laboratory animal welfare guidelines.
siRNA design and lipid nanoparticles formulation
For in vitro screening, siRNAs were designed to exhibit cross-reactivity with human, mouse, and nonhuman primate C5 mRNA. The siRNA sequences were chemically modified with 2′-OMe nucleotides to minimize potential immune responses.47 The designed siRNAs were synthesized using the standard phosphoramidite method at GeneDesign (Osaka, Japan). In general, the synthesized sense or antisense strands were purified using either a silica-based column for in vitro study or reverse-phase HPLC for in vivo study. The oligonucleotides purified using reverse-phase HPLC have a purity of more than 90%. Identification of molecular weight was performed with MALDI-TOF-MS. The identified sense and antisense strands were annealed to form siRNA, which were stored at −20°C prior to use. Surrogate siRNA targeting C5 (m/rC5-siRNA) and C6 (m/rC6-siRNA) were designed to exhibit cross-reactivity with mouse and rat C5 and C6 mRNA, respectively (Table S1). As Ctrl-siRNA, we used luciferase-targeting siRNA as described previously.59,60 Each siRNA was formulated into LNP and characterized as described previously.37 In general, siRNA was dissolved in 10 mM citric acid (pH 4.0). A proprietary ionizable lipid, cholesterol (Nippon Fine Chemical, Japan), 1,2-distearoyl-sn-glycero-3-phosphocholine (Nippon Fine Chemical), and mPEG2000-DMG (NOF no. SUNBRIGHT GM-020) were dissolved in ethanol. The siRNA solutions and lipid solutions were mixed at a flow ratio of 3:1 using a pump. The solutions were dialyzed with PBS using a 100 kDa dialysis tube (Spectrum Labs no. G235071) and filtered through a 0.22 μm membrane filter to produce LNP-formulated siRNA.
In vitro siRNA experiment
Each siRNA in combination with the transfection reagent Lipofectamine RNAiMAX (Invitrogen no. 13778150) was diluted in Opti-MEM medium (Gibco no. 31985062) to prepare the siRNA/RNAiMAX mixed solution. The siRNA/RNAiMAX mixtures were dispensed into 96-well plates and cells (Hep3B [ATCC no. HB-8064] or Huh7 [NIBIOHN JCRB Cell Bank no. JCRB0403]) were seeded in each well. Following overnight incubation, cells were harvested, and C5 mRNA expression levels were quantified using qRT-PCR and TaqMan primers. The C5 mRNA expression level from cells treated with either diluent or only the transfection reagent was considered the baseline level, and a C5 mRNA residual rate (relative value) was calculated for each siRNA treatment.
RNA preparation and qRT-PCR
For in vitro experiments, total RNA templates were prepared from cultured cells using a CellAmp Direct RNA Prep Kit for RT-PCR (no. 3732, TaKaRa, Japan) and Proteinase K (no. 9034, TaKaRa). For in vivo experiments, total RNA was isolated from harvested organs using the RNeasy Mini Kit (QIAGEN no. 74106). Next, cDNA was synthesized from total RNA using the PrimeScript RT Master Mix (no. RR036A, TaKaRa). Finally, RT-PCR was performed using cDNA and TaqMan probes (Applied Biosystems, Förster City, CA), with TaqMan Fast Advanced Master Mix (no. 4444963, Applied Biosystems) in a QuantStudio 3 Real-time PCR System (Applied Biosystems) or TaqMan Gene Expression Master Mix (Applied Biosystems, no. 4369016) in a 7500 Fast Real-Time PCR System (Applied Biosystems). The expression levels of human C5 (no. Hs00156197_m1), human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (no. Hs02758891_g1), mouse C5 (no. Mm00439275_m1), mouse C6 (no. Mm00489521_m1), mouse GAPDH (no. Mm99999915_g1), rat C5 (Rn01436156_m1), rat C6 (no. Rn00566466_m1), and rat GAPDH (no. Rn01775763_g1) were measured. In Huh7, the relative standard curves for complement C5 and GAPDH were generated using five-point serial dilutions of cDNA samples from nontreated Huh-7 cells. A five-point relative standard curve was used for the relative quantitation of complement C5 mRNA levels in each cDNA sample. The relative gene expression of complement C5 compared with that of GAPDH was calculated. In other experiments, GAPDH was used as an internal standard to normalize mRNA expression levels. PCR products were quantified using the comparative cycle threshold (Ct) method (2−ΔΔCt). Data are presented as the relative expression of the target gene compared with that of GAPDH.
C5 protein level measurement
Blood was collected from the abdominal vein of each mouse under light anesthesia with isoflurane. The blood was then transferred to an EDTA-coated tube and the sample was centrifuged at 3,000 rpm at 4°C for 15 min. The supernatant was then collected and stored at −80°C until use. The mouse C5 protein was quantified using ELISA as follows: the mouse anti-C5 antibody BB5.1 (no. HM1073-FS, Hycult Biotech) was diluted in PBS to a concentration of 2 μg/mL and immobilized in a 96-well assay plate (no. 442404, Nunc). After incubation at 4°C overnight, blocking solution (PBS containing 1% BSA [no. DY995, R&D systems]) was added and the plate was incubated at room temperature for 1 h. The plate was washed with washing buffer (PBS containing 0.02% Tween 20) and the diluted plasma sample was added to the plate. Recombinant mouse C5 protein (no. CO5-M52H4, ACROBiosystems) was used as the standard. The plate was incubated at room temperature for 1 h, and then washed with washing buffer. Diluted goat anti-human C5 antisera (no. A306, Quidel Corporation) solution was added and the plate was incubated at room temperature for 1 h. The plate was washed with washing buffer, diluted HRP-labeled bovine anti-goat IgG (H + L) (no. 805-035-180, Jackson ImmunoResearch) was added, and the plate was incubated at room temperature for 1 h. The plate was washed with washing buffer and 3,3′,5,5′-tetramethylbenzidine working solution (no. 5120-0053, Seracare Life Sciences) was added to each well to develop color. Stop solution (1 M/L of sulfuric acid [no. 198-09595, Wako]) was added as a quenching solution, and absorbance was measured using the Multiskan GO microplate reader with SkanIt Software version 3.2.1.4 (Thermo Fisher Scientific, Waltham, MA) at 450 and 650 nm. Calibration curves were constructed by plotting nominal concentrations of C5 and the mean absorbance values with sigmoidal 4-parameter regression using GraphPad Prism 9.0.2. (GraphPad Software, La Jolla, CA).
The monkey C5 protein was quantified using a commercially available C5 human ELISA kit (no. KA2114, Abnova Corporation) and measurement was conducted by the LSI Medience Corporation (Tokyo, Japan).
Complement activity measurement
Serum isolation was conducted according to the method as described previously.61 In brief, blood was collected from the abdominal vein of each mouse or from the jugular vein from each rat under light anesthesia with isoflurane. The collected blood was transferred to Bloodsepar (no. 31203, Immuno-Biological Laboratories) tubes, and the tubes were then subjected to tumbling and mixing. The samples were maintained at room temperature for 30 min until clot retraction was completely established, and the serum was collected after centrifugation (3,000 rpm, 4°C, 15 min). The collected serum was stored at −80°C until use. Rat and mouse serum complement activities were quantified using a serum complement titer CH50 kit (no. 400017, DENKA) in accordance with the manufacturer’s instructions. Serum samples were diluted with the same diluting medium. For mouse serum samples, sheep erythrocytes, mouse serum samples, and zymosan (final concentration of 10 μg/mL, no. 263-01491, Wako) were mixed, and the mixture was incubated at 37°C for 16 h. For rat serum samples, sheep erythrocytes and mouse serum samples were mixed, and the mixture was incubated at 37°C for 1 h. After incubation, the assay plate was centrifuged at 2,000 rpm at room temperature for 10 min. The absorbance of the supernatant was then measured at 415 nm using a Multiskan GO microplate reader (Thermo Fisher Scientific). The post-administration to pre-administration relative rates of hemolytic activity in mice, rats, and monkeys were calculated using the following formula: R1 (%) = 100 × [(Abs Post administration – Abs sRBC background)]/[(Abs Pre administration – Abs sRBC background)]. Monkey CH50 levels in the serum samples were measured using a biochemistry automatic analyzer (7180 Clinical Analyzer; Hitachi High-Tech, Tokyo, Japan) and an Auto CH50-L “Seiken” (DENKA).
C5 suppression in mice
Mice were divided into groups based on their average weights. Mice were i.v. administered either PBS or siRNA/LNP at various siRNA doses and sacrificed at the indicated time points to collect blood and liver samples. By using the frozen mouse liver, C5 mRNA expression levels were quantified using qRT-PCR and TaqMan primers. The liver C5 mRNA residual rate on each day of measurement for the PBS administration group was defined as 100%, and liver C5 mRNA residual rates (relative values) were calculated for each siRNA administration group. The serum C5 concentration levels and hemolytic activity were also measured.
C5 suppression in nonhuman primate
Male cynomolgus monkeys (3–4 years old, naive, n = 3/group) were intravenously administered with 0.03, 0.1, 0.3, and 1 mg/kg of LNP-formulated SEQ008-34, with an injection volume of 10 mL/kg by 30 min of intravenous infusion with a syringe pump. Serum was obtained on days −1, 0, 3, 6, 9, 16, 23, 30, 37, 44, 51, 58, 65, 72, 79, and 86. The serum C5 concentration levels and hemolytic activity were measured.
MG model in rats
Rats were divided into groups based on their average weights. To test the prophylactic efficacy of C5-siRNA in MG, 0.01, 0.1, and 1 mg/kg doses of mouse/rat (m/r) surrogate C5-siRNA/LNP (n = 3/group), 1 mg/kg of Ctrl-siRNA/LNP (n = 3/group), or PBS (n = 3/group) were i.v. administered to female Lewis rats at day 0. After 7 days, rats were inoculated intravenously with 1 mg/kg of antibodies against the AChR (clone Mab35 no. BE0123, BioXCell). One and 2 days after the anti-AChR mAb administration, the disease severity of MG was assessed according to the following scale: 0, can grip and lift lid of a cage; 1, can grip but cannot lift the lid of a cage; 2, unable to grip cage lid; 3, unable to grip and has forelimb paralysis, and 4, moribund, severe paralytic disease as described previously.41 To test the prophylactic efficacy of C6-siRNA in MG, 0.1 and 1 mg/kg of m/r surrogate C6-siRNA/LNP (n = 3/group), Ctrl-siRNA/LNP (n = 3/group), and PBS (n = 3/group) were i.v. administered to female Lewis rats at day 0. After 7 days, rats were inoculated intravenously with 1 mg/kg of antibodies against the AChR. One and 2 days after the anti-AChR mAb administration, the disease severity of rats was assessed as described above.
Immunohistochemistry
Upon conclusion of the animal study, rats were deeply anesthetized with isoflurane and perfused with PBS and 2% paraformaldehyde in PBS. After perfusion, soleus muscles were collected from each rat, post-fixed with 2% paraformaldehyde in PBS at 4°C for 24 h, treated with 20% sucrose in PBS, and embedded in Tissue-Tek O.C.T. (Sakura Finetek Japan, Tokyo, Japan). Tissue blocks were sliced into 12-μm-thick sections. Cryosections were blocked in Block Ace (DS Pharma Biomedical, Osaka, Japan) at room temperature for 1 h and incubated with Alexa Fluor 488-conjugated α-BTX (no. B-13422, Life Technologies) and mouse anti-rat C5b-9 monoclonal antibody (no. HM3033, Hycult) at room temperature for 2 h. The sections were washed three times with 0.1% Triton X-100 in PBS and then incubated at room temperature for 1 h with Cy3-conjugated donkey anti-mouse IgG (no. 715-166-151, Jackson ImmunoResearch Laboratories). The sections were washed three times with 0.1% Triton X-100 in PBS, mounted in mounting medium (no. P10144, ProLong Gold, Thermo Fisher Scientific), and analyzed using confocal laser microscopy (Nikon A1, Nikon, Tokyo, Japan). Neuromuscular junctions were determined by fluorescently labeled α-BTX and were defined as areas of interest. At least 50 or more of neuromuscular junctions were included for observation in each rat. Fluorescence intensities were determined using the NIS-Elements software (Nikon). The mean values were obtained for each animal (n = 3/group).
CIA mouse model
In the prophylactic experiment, mice were divided into groups based on their average weights. Bovine collagen type II (CII) (no. K42, Collagen Research Center) was dissolved in 0.05 M acetic acid at 3 mg/mL and emulsified in an equal volume of complete Freund’s adjuvant (no. 263810, Difco Laboratories). Mice were immunized intracutaneously with 100 μL of the emulsion at the base of the tail. At day 21 post immunization, mice were boosted with the same amount of bovine CII, emulsified in incomplete Freund’s adjuvant (no. 263910, Difco Laboratories). The progression of arthritis was monitored continuously after the first immunization by assessing arthritis score (the sum of scores on a 0–4 scale for all 4 limbs, based on the swelling of the limbs, for a total possible score of 16 for each mouse), using methods modified from those of Seeuws et al.62 and Huang et al.63 A disease severity score of 0 indicated a normal joint, without inflammation or redness; 1 = redness and swelling in one digit; 2 = redness and swelling in two digits, or redness and mild swelling in the ankle and wrist joints; 3 = redness and swelling in >3 digits, or moderate swelling in the ankle and wrist joints, and the foot; and 4 = severe swelling in the ankle and wrist joints, and the foot. The arthritis score for each mouse was defined as the sum of the scores for all four paws. For the prophylactic treatment experiment using C5-siRNA, PBS (n = 8/group), 1 mg/kg of Ctrl-siRNA/LNP (n = 8/group), or 1 mg/kg of m/r C5-siRNA/LNP (n = 8/group) were i.v. administered. In the prophylactic treatment experiment using C6-siRNA, PBS (n = 6/group), 1 mg/kg of Ctrl-siRNA/LNP (n = 6/group), or 1 mg/kg of m/r C6-siRNA/LNP (n = 6/group) were i.v. administered. For both prophylactic experiments, compounds were administered once 4 days before the first immunization, and then administered once a week. In the therapeutic treatment experiment, mice were equally distributed across groups based on their clinical score after an arthritis score of 1–3 was attained. PBS (n = 10/group), 1 mg/kg of Ctrl-siRNA/LNP (n = 10/group), or 1 mg/kg of m/r C5-siRNA/LNP (n = 7/group) were i.v. administered to mice, once per week from the day of enrollment.
Measurement of plasma parameters
Plasma levels of mouse MMP-3 were assessed using ELISA kits (no. MMP-300, R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed in GraphPad Prism 9.0.2 (GraphPad Software, La Jolla, CA). Data are expressed as the mean ± SEM. For statistical analysis, Dunnett’s multiple comparison test was performed for the data represented in Figure 2. At other instances, the nonparametric Kruskal-Wallis test was performed, followed by the Mann-Whitney U test.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Acknowledgments
Funding: This research was supported by AMED under grant no. JP17pc0101019. The authors would like to thank LSI Medience Corporation for measuring the C5 concentration in monkey serum. The authors would also like to thank Tetsu Kawano and Hiroshi Ishihara for helpful discussions; and Hikaru Maeda-Yamamoto, Masayo Kondo, Shinya Tanahashi, Tetsuya Fukuta, Yusuke Niwa, Eiji Kawada, Shunsuke Sanada, Natsuko Yao, Kei Miyazaki, Tomoyo Nishida, and Toru Sakairi for conducting the experiments and their support. In addition, we thank all members of KAN Research Institute and Eisai, Co., Ltd., for helpful discussions and advice.
Author contributions
Conceptualization, Y.S. and S.M.; methodology and investigation, Y.S., S.M., C.M., N.T., T.N., K.T., Y.T., Y.I., and A.T.; project administration, Y.K., M.S., and T.L.; supervision, Y.K., M.S., and T.L.; resources, M.S. and T.L.; writing – original draft, Y.K. and Y.S.; writing – review & editing, Y.K., Y.S., and T.I.
Declaration of interests
All authors were employees of the KAN Research Institute, Inc., and Eisai Co., Ltd., during the execution of this research project. KAN Research Institute, Inc., is a subsidiary of Eisai Co., Ltd. There are no conflicts of interest to declare.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2023.01.005.
Supplemental information
References
- 1.Morgan B.P., Harris C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 2015;14:857–877. doi: 10.1038/nrd4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mastellos D.C., Ricklin D., Lambris J.D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 2019;18:707–729. doi: 10.1038/s41573-019-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan C., Gao H. New insights for C5a and C5a receptors in sepsis. Front. Immunol. 2012;3:368. doi: 10.3389/fimmu.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peffault de Latour R., Fremeaux-Bacchi V., Porcher R., Xhaard A., Rosain J., Castaneda D.C., Vieira-Martins P., Roncelin S., Rodriguez-Otero P., Plessier A., et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125:775–783. doi: 10.1182/blood-2014-03-560540. [DOI] [PubMed] [Google Scholar]
- 6.Wakamiya N., Ohtani K., Hidaka Y., Inoue N. [New complement therapeutics in complement-related diseases] Brain Nerve. 2019;71:555–564. doi: 10.11477/mf.1416201316. [DOI] [PubMed] [Google Scholar]
- 7.Halbwachs L., Lesavre P. Endothelium-neutrophil interactions in ANCA-associated diseases. J. Am. Soc. Nephrol. 2012;23:1449–1461. doi: 10.1681/ASN.2012020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuta S., Jayne D.R.W. Antineutrophil cytoplasm antibody-associated vasculitis: recent developments. Kidney Int. 2013;84:244–249. doi: 10.1038/ki.2013.24. [DOI] [PubMed] [Google Scholar]
- 9.Noris M., Remuzzi G. Terminal complement effectors in atypical hemolytic uremic syndrome: C5a, C5b-9, or a bit of both? Kidney Int. 2019;96:13–15. doi: 10.1016/j.kint.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma Y., Nagai T., Yoshio T., Hirohata S. Differential activation mechanisms of serum C5a in lupus nephritis and neuropsychiatric systemic lupus erythematosus. Mod. Rheumatol. 2017;27:292–297. doi: 10.1080/14397595.2016.1193965. [DOI] [PubMed] [Google Scholar]
- 11.Wenderfer S.E., Ke B., Hollmann T.J., Wetsel R.A., Lan H.Y., Braun M.C. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J. Am. Soc. Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
- 12.Andersson C., Wenander C.S., Usher P.A., Hebsgaard J.B., Sondergaard B.C., Rønø B., Mackay C., Friedrichsen B., Chang C., Tang R., Hornum L. Rapid-onset clinical and mechanistic effects of anti-C5aR treatment in the mouse collagen-induced arthritis model. Clin. Exp. Immunol. 2014;177:219–233. doi: 10.1111/cei.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arumugam T.V., Shiels I.A., Woodruff T.M., Granger D.N., Taylor S.M. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Albazli K., Kaminski H.J., Howard J.F., Jr. Complement inhibitor therapy for myasthenia gravis. Front. Immunol. 2020;11:917. doi: 10.3389/fimmu.2020.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao L., Li H., Guo S. Eculizumab treatment for myasthenia gravis subgroups: 2021 update. J. Neuroimmunol. 2022;362:577767. doi: 10.1016/j.jneuroim.2021.577767. [DOI] [PubMed] [Google Scholar]
- 16.Mader S., Kümpfel T., Meinl E. Novel insights into pathophysiology and therapeutic possibilities reveal further differences between AQP4-IgG- and MOG-IgG-associated diseases. Curr. Opin. Neurol. 2020;33:362–371. doi: 10.1097/WCO.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 17.Halstead S.K., Zitman F.M.P., Humphreys P.D., Greenshields K., Verschuuren J.J., Jacobs B.C., Rother R.P., Plomp J.J., Willison H.J. Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain. 2008;131:1197–1208. doi: 10.1093/brain/awm316. [DOI] [PubMed] [Google Scholar]
- 18.Ishigooka H., Katsumata H., Saiga K., Tokita D., Motoi S., Matsui C., Suzuki Y., Tomimatsu A., Nakatani T., Kuboi Y., et al. Novel complement C5 small-interfering RNA lipid nanoparticle prolongs graft survival in a hypersensitized rat kidney transplant model. Transplantation. 2022;106:2338–2347. doi: 10.1097/TP.0000000000004207. [DOI] [PubMed] [Google Scholar]
- 19.Hillmen P., Young N.S., Schubert J., Brodsky R.A., Socié G., Muus P., Röth A., Szer J., Elebute M.O., Nakamura R., et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky R.A., Young N.S., Antonioli E., Risitano A.M., Schrezenmeier H., Schubert J., Gaya A., Coyle L., de Castro C., Fu C.L., et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 21.Legendre C.M., Licht C., Muus P., Greenbaum L.A., Babu S., Bedrosian C., Bingham C., Cohen D.J., Delmas Y., Douglas K., et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N. Engl. J. Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 22.Howard J.F., Utsugisawa K., Benatar M., Murai H., Barohn R.J., Illa I., Jacob S., Vissing J., Burns T.M., Kissel J.T., et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–986. doi: 10.1016/S1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]
- 23.Nowak R.J., Muppidi S., Beydoun S.R., O'Brien F.L., Yountz M., Howard J.F., Jr. Concomitant immunosuppressive therapy use in eculizumab-treated adults with generalized myasthenia gravis during the REGAIN open-label extension study. Front. Neurol. 2020;11:556104. doi: 10.3389/fneur.2020.556104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittock S.J., Berthele A., Fujihara K., Kim H.J., Levy M., Palace J., Nakashima I., Terzi M., Totolyan N., Viswanathan S., et al. Eculizumab in aquaporin-4–positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381:614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 25.Razzak M. Anaemia: mutations in C5 explain eculizumab resistance. Nat. Rev. Nephrol. 2014;10:182. doi: 10.1038/nrneph.2014.30. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura J.I., Yamamoto M., Hayashi S., Ohyashiki K., Ando K., Brodsky A.L., Noji H., Kitamura K., Eto T., Takahashi T., et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 2014;370:632–639. doi: 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]
- 27.Holers V.M. Complement and its receptors: new insights into human disease. Annu. Rev. Immunol. 2014;32:433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 28.Harder M.J., Kuhn N., Schrezenmeier H., Höchsmann B., von Zabern I., Weinstock C., Simmet T., Ricklin D., Lambris J.D., Skerra A., et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood. 2017;129:970–980. doi: 10.1182/blood-2016-08-732800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 30.Setten R.L., Rossi J.J., Han S.-p. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 31.Roberts T.C., Langer R., Wood M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020;19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin X., Gao B. The complement system in liver diseases. Cell. Mol. Immunol. 2006;3:333–340. [PubMed] [Google Scholar]
- 33.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020;154-155:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki Y., Hyodo K., Tanaka Y., Ishihara H. siRNA-lipid nanoparticles with long-term storage stability facilitate potent gene-silencing in vivo. J. Control. Release. 2015;220:44–50. doi: 10.1016/j.jconrel.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y., Ishihara H. Structure, activity and uptake mechanism of siRNA-lipid nanoparticles with an asymmetric ionizable lipid. Int. J. Pharm. 2016;510:350–358. doi: 10.1016/j.ijpharm.2016.06.124. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y., Hyodo K., Suzuki T., Tanaka Y., Kikuchi H., Ishihara H. Biodegradable lipid nanoparticles induce a prolonged RNA interference-mediated protein knockdown and show rapid hepatic clearance in mice and nonhuman primates. Int. J. Pharm. 2017;519:34–43. doi: 10.1016/j.ijpharm.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Mané-Damas M., Molenaar P.C., Ulrichts P., Marcuse F., De Baets M.H., Martinez-Martinez P., Losen M. Novel treatment strategies for acetylcholine receptor antibody-positive myasthenia gravis and related disorders. Autoimmun. Rev. 2022;21:103104. doi: 10.1016/j.autrev.2022.103104. [DOI] [PubMed] [Google Scholar]
- 39.Kusner L.L., Losen M., Vincent A., Lindstrom J., Tzartos S., Lazaridis K., Martinez-Martinez P. Guidelines for pre-clinical assessment of the acetylcholine receptor--specific passive transfer myasthenia gravis model-Recommendations for methods and experimental designs. Exp. Neurol. 2015;270:3–10. doi: 10.1016/j.expneurol.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano S., Engel A.G. Myasthenia gravis: quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 1993;43:1167–1172. doi: 10.1212/wnl.43.6.1167. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y., Gong B., Lin F., Rother R.P., Medof M.E., Kaminski H.J. Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J. Immunol. 2007;179:8562–8567. doi: 10.4049/jimmunol.179.12.8562. [DOI] [PubMed] [Google Scholar]
- 42.Soltys J., Kusner L.L., Young A., Richmonds C., Hatala D., Gong B., Shanmugavel V., Kaminski H.J. Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann. Neurol. 2009;65:67–75. doi: 10.1002/ana.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courtenay J.S., Dallman M.J., Dayan A.D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 44.Seki N., Sudo Y., Yoshioka T., Sugihara S., Fujitsu T., Sakuma S., Ogawa T., Hamaoka T., Senoh H., Fujiwara H. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J. Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- 45.Wang Y., Rollins S.A., Madri J.A., Matis L.A. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl. Acad. Sci. USA. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Kristan J., Hao L., Lenkoski C.S., Shen Y., Matis L.A. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J. Immunol. 2000;164:4340–4347. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 47.Judge A.D., Bola G., Lee A.C.H., MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Skattum L., van Deuren M., van der Poll T., Truedsson L. Complement deficiency states and associated infections. Mol. Immunol. 2011;48:1643–1655. doi: 10.1016/j.molimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 49.McNamara L.A., Topaz N., Wang X., Hariri S., Fox L., MacNeil J.R. High risk for invasive meningococcal disease among patients receiving eculizumab (soliris) despite receipt of meningococcal vaccine. MMWR Morb. Mortal. Wkly. Rep. 2017;66:734–737. doi: 10.15585/mmwr.mm6627e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westra D., Wetzels J.F.M., Volokhina E.B., van den Heuvel L.P., van de Kar N.C.A.J. A new era in the diagnosis and treatment of atypical haemolytic uraemic syndrome. Neth. J. Med. 2012;70:121–129. [PubMed] [Google Scholar]
- 51.Kusner L.L., Yucius K., Sengupta M., Sprague A.G., Desai D., Nguyen T., Charisse K., Kuchimanchi S., Kallanthottathil R., Fitzgerald K., et al. Investigational RNAi therapeutic targeting C5 is efficacious in pre-clinical models of myasthenia gravis. Mol. Ther. Methods Clin. Dev. 2019;13:484–492. doi: 10.1016/j.omtm.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzgerald K., White S., Borodovsky A., Bettencourt B.R., Strahs A., Clausen V., Wijngaard P., Horton J.D., Taubel J., Brooks A., et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 2017;376:41–51. doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habtemariam B.A., Karsten V., Attarwala H., Goel V., Melch M., Clausen V.A., Garg P., Vaishnaw A.K., Sweetser M.T., Robbie G.J., Vest J. Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-Small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin. Pharmacol. Ther. 2021;109:372–382. doi: 10.1002/cpt.1974. [DOI] [PubMed] [Google Scholar]
- 54.Brown C.R., Gupta S., Qin J., Racie T., He G., Lentini S., Malone R., Yu M., Matsuda S., Shulga-Morskaya S., et al. Investigating the pharmacodynamic durability of GalNAc–siRNA conjugates. Nucleic Acids Res. 2020;48:11827–11844. doi: 10.1093/nar/gkaa670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard J.F., Vissing J., Gilhus N.E., Leite M.I., Utsugisawa K., Duda P.W., Farzaneh-Far R., Murai H., Wiendl H. Zilucoplan: an investigational complement C5 inhibitor for the treatment of acetylcholine receptor autoantibody–positive generalized myasthenia gravis. Expert Opin. Investig. Drugs. 2021;30:483–493. doi: 10.1080/13543784.2021.1897567. [DOI] [PubMed] [Google Scholar]
- 56.Howard J.F., Jr., Nowak R.J., Wolfe G.I., Freimer M.L., Vu T.H., Hinton J.L., Benatar M., Duda P.W., MacDougall J.E., Farzaneh-Far R., et al. Clinical effects of the self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized myasthenia gravis: results of a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol. 2020;77:582–592. doi: 10.1001/jamaneurol.2019.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji H., Ohmura K., Mahmood U., Lee D.M., Hofhuis F.M.A., Boackle S.A., Takahashi K., Holers V.M., Walport M., Gerard C., et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 58.Morgan B.P., Chamberlain-Banoub J., Neal J.W., Song W., Mizuno M., Harris C.L. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin. Exp. Immunol. 2006;146:294–302. doi: 10.1111/j.1365-2249.2006.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Speicher T., Siegenthaler B., Bogorad R.L., Ruppert R., Petzold T., Padrissa-Altes S., Bachofner M., Anderson D.G., Koteliansky V., Fässler R., Werner S. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat. Commun. 2014;5:3862. doi: 10.1038/ncomms4862. [DOI] [PubMed] [Google Scholar]
- 60.Kawase W., Kurotaki D., Suzuki Y., Ishihara H., Ban T., Sato G.R., Ichikawa J., Yanai H., Taniguchi T., Tsukahara K., Tamura T. Irf5 siRNA-loaded biodegradable lipid nanoparticles ameliorate concanavalin A-induced liver injury. Mol. Ther. Nucleic Acids. 2021;25:708–715. doi: 10.1016/j.omtn.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lachmann P.J. Preparing serum for functional complement assays. J. Immunol. Methods. 2010;352:195–197. doi: 10.1016/j.jim.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Seeuws S., Jacques P., Van Praet J., Drennan M., Coudenys J., Decruy T., Deschepper E., Lepescheux L., Pujuguet P., Oste L., et al. A multiparameter approach to monitor disease activity in collagen-induced arthritis. Arthritis Res. Ther. 2010;12:R160. doi: 10.1186/ar3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang G., Xu Z., Huang Y., Duan X., Gong W., Zhang Y., Fan J., He F. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J. Clin. Immunol. 2013;33:550–557. doi: 10.1007/s10875-012-9839-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.