Abstract
Background
Anemia is a common complication of chronic kidney disease. The hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) is a new class of oral drugs for the treatment of renal anemia.
Summary
Clinical trials have consistently shown that HIF-PHIs can effectively increase hemoglobin in both the dialysis population and the nondialysis population. The effects of HIF-PHIs in treating renal anemia include promoting endogenous erythropoietin production and facilitating iron mobilization. Several studies suggest that the erythropoiesis effect of roxadustat is less affected by inflammation. Careful monitoring of thromboembolic events and tumor before and during HIF-PHI treatment is necessary.
Key Messages
HIF-PHIs are effective in correcting renal anemia. The long-term safety of HIF-PHIs needs to be further studied.
Keywords: Hypoxia-inducible factor, Chronic kidney disease, Anemia, Iron
Introduction
Anemia is a common complication of chronic kidney disease (CKD). CKD anemia is associated with reduced quality of life and increased mortality [1, 2, 3, 4]. The cause of anemia in CKD patients is multifactorial, including erythropoietin (EPO) deficiency, iron deficiency (absolute deficiency and functional deficiency), resistance to EPO signaling, bone marrow suppression, and shortened red cell lifespan [5, 6]. EPO-stimulating agents (ESAs) and iron are the main treatments for anemia in patients with CKD. However, approximately 10–20% of CKD patients are hyporesponsive to ESA therapy, which poses challenges to CKD anemia management in these patients [7, 8, 9]. Furthermore, ESA use has also raised some safety concerns, especially when targeting a “normal” Hb level [10, 11]. CKD patients usually have absolute iron deficiency and functional iron deficiency, resulting in reduced availability of iron for erythropoiesis. Dialysis patients frequently need intravenous iron therapy. Although a recent study shows proactive intravenous iron was effective and safe, long-term safety concerns still exist [12, 13, 14, 15].
The hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) is a new class of oral drugs for the treatment of renal anemia. Roxadustat (a HIF-PHI) was first approved for renal anemia in China in 2019. Japan then approved 5 HIF-PHIs including roxadustat, daprodustat, vadadustat, molidustat, and enarodustat for renal anemia. Recently, European Medicines Agency (EMA) also authorized roxadustat for the treatment of adult patients with symptomatic anemia associated with CKD in the European Union. This review provides a concise overview of the mechanism that hopefully will help us understand the effect of HIF-PHIs on erythropoiesis and other potential effects. This review will also address some of the potential safety concerns.
Mechanism of Action of HIF-PHIs in Treating Renal Anemia
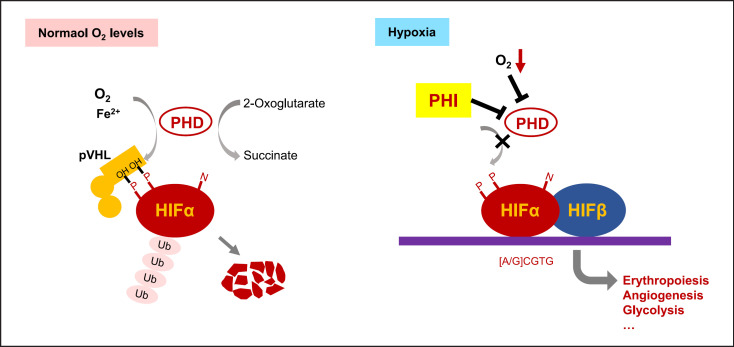
HIF is a key transcription factor that responds to hypoxia by activating the expression of a series of genes from a variety of cell types in the body. In normoxic conditions, HIFα is hydroxylated on proline residues by prolyl hydroxylase domain (PHD). Prolyl-hydroxylated HIFα is recognized and ubiquitylated by the von Hippel-Lindau (VHL) complex. Polyubiquitylated HIFα is then degraded by the proteasomal degradation system. HIF-PHIs stabilize HIFα by reversibly inhibiting PHD catalytic activity. HIFα translocates to the nucleus and forms a heterodimer with HIFβ. The heterodimer binds to hypoxia-response elements and induces the transcription of oxygen-regulated genes, including genes associated with erythropoiesis and iron metabolism (Fig. 1).
Fig. 1.
Prolyl hydroxylase domain (PHD) is a therapeutic target for CKD anemia. In normoxic conditions, HIFα is hydroxylated on proline residues by PHD. Prolyl-hydroxylated HIFα is recognized and ubiquitylated by the von Hippel-Lindau (VHL) complex. Polyubiquitylated HIFα is then degraded by the proteasomal degradation system. In hypoxic conditions or during HIF-PHI treatment, HIFα translocates to the nucleus and forms a heterodimer with HIFβ. The heterodimer binds to hypoxia-response elements and induces the transcription of oxygen-regulated genes, including genes associated with erythropoiesis, angiogenesis, glycolysis, etc.
In contrast to ESAs that activate the EPO receptor, HIF-PHIs may induce multiple target genes in multiple cell types in response to hypoxia, including EPO from the kidney or/and liver and genes associated with iron absorption and mobilization. Although hundreds of genes are induced by HIF activation, animal and human studies show that the genes associated with erythropoiesis are more sensitive to HIF activation. Furthermore, clinical data show that under the recommended dosing schedule, the increase of EPO after HIF-PHI use is intermittent, and the blood levels of EPO are lower than those treated with exogenous EPO [16, 17]. The pleiotropic effects of HIF-PHIs are associated with their effectiveness in correcting anemia but may also raise concerns about unwanted effects.
Efficacy of HIF-PHIs in Treating Renal Anemia
Nondialysis-Dependent CKD Patients
Clinical studies examining the efficacy of HIF-PHIs on hemoglobin in CKD patients consistently showed that HIF-PHIs are effective in correcting CKD anemia. The phase 3 study of roxadustat in Chinese nondialysis-dependent patients showed that the patients treated with roxadustat had a hemoglobin increase of 1.9 ± 1.2 g/dL after 8-week treatment, while the patients in the placebo group had a decrease of 0.4 ± 0.8 g/dL in hemoglobin levels from baseline (difference, 2.2 g/dL; 95% confidence interval, 1.9–2.6; p < 0.001) [18]. Consistently, roxadustat also showed superiority to placebo in increasing hemoglobin [19, 20]. Roxadustat was noninferior to darbepoetin alfa in increasing hemoglobin as shown by the DOLOMITES study (a part of the global phase 3 trial) [21]. The hemoglobin response was 89.5% (256/286) in the roxadustat group and 78% (213/273) in the darbepoetin alfa group [21].
The phase 3 studies of vadadustat also showed that vadadustat was noninferior to darbepoetin alfa in both ESA-untreated and ESA-treated nondialysis-dependent CKD patients [22, 23]. In patients who were under ESA treatment but whose hemoglobin levels were still less than 11.0 g/dL, vadadustat effectively improved hemoglobin levels to the target range (11.0–13.0 g/dL) [23].
The phase 3 studies of daprodustat also revealed noninferiority to ESA in efficacy outcomes [24, 25]. In ESA naive patients, daprodustat elevated hemoglobin levels to the target range at week 8 and maintained hemoglobin levels through week 52 [24]. In ESA-treated patients, switching to daprodustat maintained hemoglobin levels within the target range (11.0–13.0 g/dL) throughout the 52-week treatment [24]. Clinical trials on enarodustat, molidustat, and desidustat also demonstrated that these HIF-PHIs were efficacious for increasing hemoglobin in nondialysis-dependent CKD patients [26, 27, 28, 29].
Dialysis-Dependent Patients
The phase 3 study of roxadustat in China in patients undergoing maintenance dialysis under stable ESA showed that after switching to roxadustat, the increase in hemoglobin from baseline was 0.7 ± 1.1 g/dL compared with 0.5 ± 1.0 g/dL in the epoetin alfa group [30], showing a noninferiority to epoetin alfa (95% confidence interval, −0.02 to 0.5) [30]. The HIMALAYAS study (a global phase 3 trial) that recruited incident dialysis patients showed that roxadustat effectively corrected and maintained hemoglobin levels compared with epoetin alfa [31]. Vadadustat, in a global phase 3 study, also showed noninferior to darbepoetin alfa in correcting and maintaining hemoglobin concentrations in both incident dialysis patients and prevalent dialysis patients [32]. Daprodustat was also noninferior to ESA in increasing hemoglobin levels [33].
Studies with patients on peritoneal dialysis demonstrated that roxadustat, vadadustat, and daprodustat were effective in correcting or maintaining hemoglobin levels within the target range [34, 35, 36]. Clinical trials also showed that enarodustat and molidustat were efficacious for increasing hemoglobin in dialysis patients [28, 37].
Recently, some case reports showed that roxadustat was effective in treating anti-EPO antibody-associated pure red cell aplasia [38, 39, 40]. More studies are required to confirm the effectiveness of HIF-PHIs on pure red cell aplasia.
CKD Patients with Inflammation
Patients with CKD are frequently in an inflammatory state. Numerous studies showed that inflammation impairs erythropoiesis and is one of the most frequent causes of ESA resistance in CKD patients [41, 42, 43].
In the phase 3 trial in China with nondialysis-dependent patients, among 411 patients with elevated C-reactive protein (CRP) at baseline, roxadustat treatment showed an effective hemoglobin response, with an increase of hemoglobin of 1.75 g/dL after 28–52 weeks, compared with placebo of 0.62 g/dL (p < 0.001) [20]. In dialysis-dependent patients, as in the phase 3 study from China, roxadustat had a similar hemoglobin response in patients with high CRP compared with those with normal CRP, while in the epoetin alfa group, patients with high CRP had lower hemoglobin response although higher doses of epoetin alfa were used compared with patients with normal CRP [30]. In another phase 3 trial from Japan, in patients with high CRP, no increase of roxadustat dose was required to maintain target hemoglobin, while higher doses of darbepoetin alfa were required to maintain target hemoglobin levels [44]. These studies suggest that the erythropoiesis effect of roxadustat is less affected by inflammation. The mechanism is incompletely understood but may be associated with the suppression of hepcidin and more efficient iron utilization after the HIF-PHI use. Inflammation and impaired iron mobilization are important causes of ESA resistance. More studies are required to explore the role of HIF-PHIs in the management of ESA-resistant anemia.
Effects of HIF-PHIs on Iron Mobilization
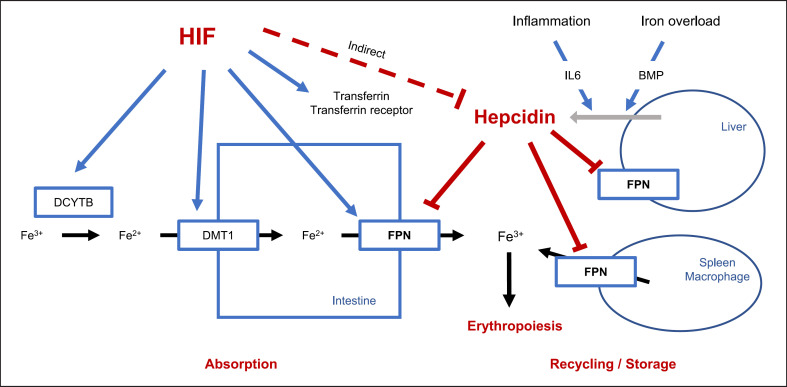
In the duodenum, Fe3+ is reduced to Fe2+ by duodenal cytochrome b reductase 1 (DCYTB), and then Fe2+ enters intestinal cells via divalent metal transporter 1 (DMT1). Intracellular Fe2+ exits cells through ferroportin (FPN) and is immediately oxidized by hephaestin to Fe3+, which binds to transferrin. Transferrin carries Fe3+ either to the bone marrow where it is used for erythropoiesis or to the liver and spleen where it is bound to ferritin for storage (Fig. 2).
Fig. 2.
Effect of HIF on iron absorption and mobilization. In the intestine, Fe3+ is reduced to Fe2+ by duodenal cytochrome b reductase 1 (DCYTB), and then Fe2+ enters intestinal cells via divalent metal transporter 1 (DMT1). Intracellular Fe2+ exits cells through ferroportin (FPN), and then Fe3+ is used for erythropoiesis. Dmt1, Dcytb, Fpn, transferrin, and transferrin receptor have been demonstrated to be target genes of HIF. Stabilizing HIF is associated with reduced levels of hepcidin. Hepcidin suppression increases FPN, which enhances iron absorption and utilization.
In CKD, patients may have absolute iron deficiency and functional iron deficiency. Absolute iron deficiency may be caused by increased blood loss such as chronic gastrointestinal bleeding, frequent venipuncture, and blood trapping in the dialysis apparatus [45]. Functional iron deficiency is characterized by impaired iron release from storage (also called reticuloendothelial cell iron blockade) due to increased hepcidin levels [45]. Decreased renal clearance, inflammation, and iron overload may contribute to the increased levels of hepcidin [8]. Studies demonstrated that in dialysis-dependent patients, oral iron is ineffective, suggesting impaired iron absorption in uremic conditions [46]. Therefore, intravenous iron is recommended in dialysis patients.
Dmt1, Dcytb, Fpn, transferrin, and transferrin receptor have been demonstrated to be target genes of HIF [47, 48, 49, 50, 51] (Fig. 2). Stabilizing HIF may promote iron absorption and utilization by inducing transcription of these genes. In addition, HIF-PHIs are associated with reduced levels of hepcidin and thus enhance iron absorption and utilization, particularly in inflammatory conditions [52, 53]. The mechanism by which HIF-PHIs inhibit hepcidin is incompletely understood. Studies showed that erythroid cells during erythropoiesis as stimulated by HIF-PHIs or ESA may release erythroferrone that inhibits hepcidin expression [54, 55]. Studies also suggested that reduced diferric transferrin as in iron deficiency or increased transferrin expression may inhibit hepcidin expression [56].
Some clinical studies demonstrated that HIF-PHIs depend less on intravenous iron in treating CKD anemia. Interestingly, a recent study showed that, in hemodialysis patients treated with roxadustat, oral iron was as effective as intravenous iron [57], in contrast to patients treated with ESA, where oral iron is ineffective [46]. The phase 3 trials of roxadustat in China in both dialysis-dependent and nondialysis-dependent patients allowed only oral iron but not intravenous iron, and roxadustat was still effective in raising or maintaining hemoglobin levels [30]. Based on the above results, the Asian Pacific Society of Nephrology HIF-PHI Recommendation Committee recommended oral iron first for patients who require iron supplementation and intravenous iron as an alternative for those who do not tolerate oral iron or when the physician considers intravenous iron is necessary [58].
The effect of HIF-PHIs on iron metabolism is also reflected in the iron parameters as demonstrated in the clinical trials. Our meta-analysis that included 12 randomized controlled trials (RCTs), involving 6 HIF-PHIs and 1,382 patients, showed that compared with placebo, HIF-PHIs decreased TSAT, ferritin, and hepcidin, increased TIBC, and did not change serum iron levels despite enhanced erythropoiesis [59]. These effects have been observed across all HIF-PHIs studies suggesting a class effect of HIF-PHIs on iron regulation [59]. Both roxadustat and epoetin alfa led to a decrease in TSAT [30], but their clinical implication is different. The reduction of TSAT after roxadustat may be associated with increased transferrin level, therefore may suggest an increase in iron transport capacity [30]. However, the reduced TSAT levels after ESA are driven primarily by reduced serum iron level, reflecting an imbalance between iron consumption and availability [30].
Other Potential Effects of HIF-PHIs
Effect of HIF-PHIs on blood lipids: phase 3 trials of roxadustat showed that roxadustat treatment was associated with lower LDL cholesterol levels [18, 19, 31, 60, 61]. Daprodustat has also been shown to decrease cholesterol levels in ASCEND-D study and ASCEND-ND study [25, 33]. However, no consistent trends were observed for cholesterol in vadadustat-treated patients and no changes in cholesterol have been observed after molidustat use in a phase 3 trial in Japan [62, 63]. The cause for this inconsistency of the effect of HIF-PHI on cholesterol is not clear. The clinical significance of the effect of certain HIF-PHIs on blood lipids remains explored.
Effect of HIF-PHI on blood pressure and CKD progression: pre-clinical studies and early clinical studies showed that HIF-PHIs corrected anemia without increasing blood pressure [57, 64]. A recent phase 3 study (ASCEND-TD) showed a similar effect of daprodustat on blood pressure compared with ESA [65]. There are no published clinical studies that specifically examined the effect of HIF-PHI on CKD progression. In the phase 3 trial (ASCEND-ND) of daprodustat examining its efficacy and safety, daprodustat failed to show superiority to darbepoetin in CKD progression [25].
Potential Safety Concerns
Tumorigenesis
The mechanism of action of HIF-PHIs may raise theoretical concerns about tumorigenesis. It is well documented that some genes that are induced by HIF activation are related to tumor formation and progression, including vascular endothelial growth factor (VEGF), genes associated with glycolysis, etc. [66, 67]. VHL is an E3 ligase that causes HIF degradation in response to oxygen. The mutation of the VHL gene, which results in HIF activation, is associated with the VHL disease that manifests as a neoplastic disorder including clear cell renal cell carcinoma, retinal and central nervous system hemangioblastoma, phaeochromocytoma, and pancreatic neuroendocrine tumor [68]. HIF-2α inhibitors are being developed to treat renal clear cell carcinoma in VHL disease [69]. Studies find that most people with VHL disease inherit a germline mutation of the VHL gene from the affected parent and a wild-type allele from the unaffected parent [70]. Further studies show that the tumor develops only when the wild-type VHL allele is also inactivated by somatic mutation or epigenetic inactivation (two-hit hypothesis of tumorigenesis). Interestingly, a homozygous 598C>T (R200W) VHL germline mutation has been identified to cause congenital Chuvash polycythemia that does not usually develop tumors [71, 72]. The difference in tumor risk among these VHL gene mutations may be associated with the extent HIF is activated. The R200W substitution only partially impairs the interaction of VHL with HIF1α, leading to partial HIF activation [71], while in VHL disease, loss of VHL leads to greater HIF activation, and also probably the activation of other tumor-associated genes such as p53 [70, 73]. EPO-associated polycythemia with or without paraganglioma has also been reported in carriers of heterozygous germline mutations in the PHD2 genes [74, 75, 76, 77]. Loss of heterozygosity or mutations that occur in the catalytic domain of the PHD2 gene are suggested to be associated with paraganglioma development [76, 77]. Similarly, a missense mutation in the HIF2A gene that impairs hydroxylation of the HIF-2α protein has been reported to cause familial erythrocytosis [78]. Interestingly, in patients with polycythemia and paraganglioma, somatic HIF2A gain-of-function mutations have been reported in tumor tissues [79]. These studies may suggest that the risk of tumor development may depend on the extent that HIF is activated.
VEGF is an angiogenic factor involved in the growth and metastasis of some tumors. Several studies examined the effect of HIF-PHIs on serum VEGF levels. High doses of daprodustat (10 mg or 25 mg per day) were shown to only slightly increase serum VEGF in healthy people, while the doses of daprodustat to treat CKD anemia in RCTs are usually less than 5 mg [80]. Some RCTs showed that there is no significant difference in serum VEGF between the HIF-PHI group and the control group in both dialysis patients and nondialysis patients (placebo or ESA) [17, 26, 81, 82, 83, 84].
So far, most clinical trials did not show an increased risk of tumor after HIF-PHI treatment. However, in the ASCEND-ND study, cancer-related death and tumor progression and recurrence occurred in 72 of 1,937 patients in the daprodustat group, significantly higher compared with 49 of 1,935 patients in the darbepoetin alfa group (relative risk, 1.47; 95% confidence interval, 1.03–2.10; p, 0.04) [25]. Post hoc analyses that accounted for the dosing frequencies showed an attenuation of the imbalance for cancer events [25]. The study of molidustat also reported more treatment-emergent adverse events of neoplasms (benign, malignant, and unspecified, including cysts and polyps) in the molidustat group (molidustat, 15/153; darbepoetin, 4/76) [85]. Long-term follow-up and careful monitoring of tumor in patients treated with HIF-PHIs are warranted.
Retinopathy
VEGF promotes retinal neovascularization and is implicated in the pathogenesis of several retinal diseases, including diabetic retinopathy, macular edema, age-related macular degeneration, and retinal vein occlusion. An animal study showed that enarodustat did not increase mRNA expression of retinal Vegf in rats at 2 h or 4 h after the administration of at a dose of 30 mg/kg [86].
In two phase 3 and double-blind studies of daprodustat, patients with diabetic retinopathy or macular edema or age-related macular degeneration at baseline were included in both groups and the incidence of ocular adverse events of special interest was similar throughout the 52-week treatment in both groups [24, 87]. In two phase 3 studies of roxadustat in Japan, ophthalmological tests by independent blinded central reviewers were conducted and revealed the proportion of patients with new or worsening retinal hemorrhages in the roxadustat group was slightly lower than that in the darbepoetin alfa group [44, 88]. In a phase 3 study of vadadustat in nondialysis-dependent CKD patients, 2 patients (1.3%) in the vadadustat group and 5 patients (3.3%) in the darbepoetin alfa group reported retinal hemorrhage during 52 weeks of treatment [23]. However, a phase 3 study of vadadustat in Japanese hemodialysis patients showed that retinal hemorrhage occurred more often in the vadadustat group (9.9%) than in the darbepoetin alfa group (6.2%) [89]. In the patients with retinal hemorrhage, the plasma VEGF levels at baseline were 44.6 (15.6–80.0) and 55.4 (33.2–329.0) in the vadadustat group and the darbepoetin alfa group, respectively; the plasma VEGF levels closest to the time of retinal hemorrhage were 47.4 (15.6–70.0) and 50.2 (29.5–92.7) in the vadadustat group and the darbepoetin alfa group, respectively [89]. Investigators of this study did not consider retinal hemorrhage is related to vadadustat [89].
The data on retinopathy as an adverse effect of HIF-PHIs are inconclusive. When a patient reports visual disturbance during HIF-PHI treatment, early ophthalmological assessment is recommended.
Hyperkalemia
The phase 3 studies of roxadustat in China reported a higher incidence rate of hyperkalemia among patients treated with roxadustat in both nondialysis patients (roxadustat, 16/101; placebo, 4/51) and dialysis patients (roxadustat, 15/204; epoetin alfa, 1/100) [18, 30]. However, analysis of serum potassium measured in the central laboratory did not find that patients in the roxadustat group were more likely to have hyperkalemia [18, 30]. Two global phase 3 studies in nondialysis patients also reported more hyperkalemia among patients treated with roxadustat. In the OLYMPUS study, 118 (8.5%) patients in the roxadustat group and 98 (6.9%) patients in the placebo group reported hyperkalemia [20]. In the ALPS study, hyperkalemia occurred in 39 (10%) patients assigned to roxadustat and 15 (7.4%) patients assigned to placebo [19]. Conversely, other two phase 3 studies of roxadustat, the DOLOMITES study and the HIMALAYAS study, reported higher incidence rates of hyperkalemia in the ESA group [21, 31]. Although a phase 2 study of vadadustat reported more hyperkalemia in the vadadustat group, recent published four long-term phase 3 studies of vadadustat did not report more hyperkalemia in the vadadustat group [22, 23, 81] [32, 89]. It is still not clear whether HIF-PHIs cause hyperkalemia. Considering that hyperkalemia can be life-threatening, serum potassium monitoring is recommended before and during HIF-PHI treatment [58].
Thrombotic Events and Cardiovascular Safety
Previous studies of ESA have found that targeting normal hemoglobin levels is associated with increased risk of cardiovascular events [90]. Cardiovascular safety is also a potential concern for HIF-PHIs.
The prespecified pooled analysis of the four global phase 3 studies of roxadustat (PYRENEES, SIERRAS, HIMALAYAS, and ROCKIES) in dialysis patients showed that the hazard ratio for the time to the first major adverse cardiovascular event (MACE) was 1.09 (95% confidence interval, 0.95–1.26), consistent with noninferiority to placebo according to the prespecified noninferiority margin of 1.3 [91]. The pooled analysis of the three global phase 3 studies (ANDES, ALPS, and OLYMPUS) in nondialysis-dependent CKD patients showed the hazard ratio for the time to the first MACE was 1.10 (95% confidence interval, 0.96–1.27), which met the prespecified noninferiority margin of 1.3 [92]. However, there are some inconsistent perspectives [93]. More cardiovascular safety end points of roxadustat are in Tables 1 and 2.
Table 1.
Cardiovascular safety end points in patients undergoing dialysis
| Cardiovascular safety end points | Hazard ratio (95% CI) |
|---|---|
| Roxadustat compared with ESAs (PYRENEES, SIERRAS, HIMALAYAS and ROCKIES) [91] | |
| MACE (all-cause mortality, myocardial infarction, and stroke) | 1.09 (0.95, 1.26) |
| MACE+ (MACE plus congestive heart failure or unstable angina requiring hospitalization) | 0.98 (0.86, 1.11) |
| All-cause mortality | 1.13 (0.95, 1.34) |
| Daprodustat compared with ESAs [33] | |
| MACE (death from any cause, nonfatal myocardial infarction, or nonfatal stroke) | 0.93 (0.81, 1.07) |
| MACE or thromboembolic event | 0.88 (0.78, 1.00) |
| MACE or hospitalization for heart failure | 0.97 (0.85, 1.11) |
| Death from any cause | 0.96 (0.82, 1.13) |
| Vadadustat compared with darbepoetin alfa [32] | |
| MACE (death from any cause, nonfatal myocardial infarction, or nonfatal stroke) | 0.96 (0.83, 1.11) |
| Expanded MACE (MACE plus hospitalization for either heart failure or a thromboembolic event, excluding vascular access failure) | 0.96 (0.84, 1.10) |
| Death from cardiovascular causes | 0.96 (0.77, 1.20) |
| Death from any cause | 0.95 (0.81, 1.12) |
| Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke | 0.95 (0.80, 1.14) |
Table 2.
Cardiovascular safety end points in nondialysis-dependent CKD patients
| Cardiovascular safety end points | Hazard ratio (95% CI) |
|---|---|
| Roxadustat compared with placebo (ANDES, ALPS, OLYMPUS) [92] | |
| MACE (all-cause mortality, myocardial infarction, and stroke) | 1.10 (0.96, 1.27) |
| MACE+ (MACE plus unstable angina and congestive heart failure requiring hospitalization) | 1.07 (0.94, 1.21) |
| All-cause mortality | 1.08 (0.93, 1.26) |
| Daprodustat compared with darbepoetin alfa [25] | |
| MACE (death from any cause, nonfatal myocardial infarction, or nonfatal stroke) | 1.03 (0.89, 1.19) |
| MACE or thromboembolic event | 1.06 (0.93, 1.22) |
| MACE or hospitalization for heart failure | 1.09 (0.95, 1.24) |
| Death from any cause | 1.03 (0.87, 1.20) |
| Vadadustat compared with darbepoetin alfa [22] | |
| MACE (death from any cause, nonfatal myocardial infarction, or nonfatal stroke) | 1.17 (1.01, 1.36) |
| Expanded MACE (MACE plus hospitalization for either heart failure or a thromboembolic event, excluding vascular access failure) | 1.11 (0.97, 1.27) |
| Death from cardiovascular causes | 1.01 (0.79, 1.29) |
| Death from any cause | 1.09 (0.93, 1.27) |
| Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke | 1.16 (0.95, 1.42) |
In the phase 3 study of daprodustat among dialysis patients (the ASCEND-D study), the first MACE occurred in 374 of 1,487 patients (25.2%) in the daprodustat group and in 394 of 1,477 patients (26.7%) in the ESA group (hazard ratio, 0.93; 95% confidence interval, 0.81–1.07) during a median follow-up of 2.5 years [33]. Among nondialysis-dependent patients (the ASCEND-ND study), a first MACE occurred in 378 of 1,937 patients (19.5%) in the daprodustat group and in 371 of 1,935 patients (19.2%) in the darbepoetin alfa group (hazard ratio, 1.03; 95% confidence interval, 0.89–1.19) during a median follow-up of 1.9 years [25]. These results met prespecified noninferiority margin of 1.25 [25, 33]. However, analysis of the on-treatment MACE, which occurred within 28 days after the last drug dose, showed a higher incidence of a first MACE in the daprodustat group (14.1%) than in the darbepoetin alfa group (10.5%) (hazard ratio, 1.40; 95% confidence interval, 1.17–1.68). Further analysis indicates that the different dosing frequencies between daprodustat and darbepoetin, at least in part, contributed to the higher incidence of the first MACE in daprodustat-treated patients [25]. More cardiovascular safety end points of daprodustat are in Tables 1 and 2.
In the phase 3 study of vadadustat among dialysis patients (the INNO2VATE study), a first MACE occurred in 355 of the 1,947 patients (18.2%) in the vadadustat group and in 377 of the 1,955 patients (19.3%) in the darbepoetin alfa group (hazard ratio, 0.96; 95% confidence interval, 0.83–1.11) [32]. Vadadustat was noninferior to darbepoetin alfa with respect to cardiovascular safety in dialysis patients [32]. However, vadadustat did not meet the prespecified noninferiority criterion for cardiovascular safety in patients with nondialysis-dependent CKD as compared with darbepoetin alfa (the PRO2TECT studies) [22]. In the study among nondialysis-dependent patients, a first MACE occurred in 382 of 1,739 patients (22.0%) in the vadadustat group and in 344 of 1,732 patients (19.9%) in the darbepoetin alfa group (hazard ratio, 1.17; 95% confidence interval, 1.01–1.36) [22]. Noninferiority margin was an upper boundary of the 95% confidence interval not exceeding 1.25 or 1.3 for the hazard ratio of MACE in this study [22]. Prespecified subgroup analyses showed that in the non-US regions, the risks of MACE and expanded MACE were considerably higher among patients in the vadadustat group than among those in the darbepoetin alfa group [22]. Whether this difference between regions is causally related to the difference in hemoglobin targets or other factors is unclear [22]. More cardiovascular safety end points of vadadustat are in Tables 1 and 2.
Based on the experience of ESA and the available clinical data about HIF-PHIs, careful monitoring of thromboembolic events before and during HIF-PHI treatment is necessary. Accordingly, a “warning” about the risk of thromboembolism is posted on the product information of all HIF-PHIs in Japan. In Europe, EMA has also marked roxadustat as being subject to “additional monitoring”.
Conclusion
Studies have consistently shown that HIF-PHIs can effectively increase hemoglobin in both the dialysis population and the nondialysis population. The effects of HIF-PHIs in treating renal anemia are multifaceted, including promoting endogenous EPO production and facilitating iron mobilization. In addition, the long-term safety of HIF-PHIs, the effectiveness of HIF-PHIs in ESA-resistant patients, and the potential pleiotropic effects of HIF-PHIs, and their clinical significance need to be further studied.
Conflict of Interest Statement
Chuan-Ming Hao received honoraria from FibroGen for speaking engagements and advisory board participation related to physician education. The other authors do not disclose any conflicts of interest. Volker H. Haase is a scientific advisor and has received honoraria from Akebia Therapeutics. Volker H. Haase is supported by the Krick-Brooks Chair in Nephrology.
Funding Sources
This research received no particular grant from any funding agency in the public, private, or not-for-profit sectors.
Author Contributions
All the authors have contributed to this review. Jing Li drafted the manuscript. Volker H. Haase revised the manuscript. Chuan-Ming Hao revised and approved the final manuscript.
Funding Statement
This research received no particular grant from any funding agency in the public, private, or not-for-profit sectors.
References
- 1.Li Y, Shi H, Wang WM, Peng A, Jiang GR, Zhang JY, et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease. Medicine. 2016;95((24)):e3872. doi: 10.1097/MD.0000000000003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St Peter WL, Guo H, Kabadi S, Gilbertson DT, Peng Y, Pendergraft T. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol. 2018;19((1)):67. doi: 10.1186/s12882-018-0861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4((1)):33–38. doi: 10.2215/CJN.00630208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshino J, Muenz D, Zee J, Sukul N, Speyer E, Guedes M, et al. Associations of hemoglobin levels with health-related quality of life, physical activity, and clinical outcomes in persons with stage 3-5 nondialysis CKD. J Ren Nutr. 2020;30((5)):404–414. doi: 10.1053/j.jrn.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Haase VH. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int. 2017;21((Suppl 1)):S110–24. doi: 10.1111/hdi.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31((3)):456–468. doi: 10.1681/ASN.2019020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossert J, Gassmann-Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transpl. 2007;22((3)):794–800. doi: 10.1093/ndt/gfl716. [DOI] [PubMed] [Google Scholar]
- 8.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55((4)):726–741. doi: 10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J, Jensen DE, Maroni BJ, Brunelli SM. Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68((5)):763–771. doi: 10.1053/j.ajkd.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355((20)):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361((21)):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 12.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19((8)):1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis. 1995;26((1)):41–46. doi: 10.1016/0272-6386(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Kusek JW, Pappas MK. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88((4)):905–914. doi: 10.1038/ki.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol. 2013;24((7)):1151–1158. doi: 10.1681/ASN.2012121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67((6)):912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Holdstock L, Meadowcroft AM, Maier R, Johnson BM, Jones D, Rastogi A, et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27((4)):1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381((11)):1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 19.Shutov E, Sulowicz W, Esposito C, Tataradze A, Andric B, Reusch M, et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, double-blind, placebo-controlled study (ALPS) Nephrol Dial Transplant. 2021;36((9)):1629–1639. doi: 10.1093/ndt/gfab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishbane S, El-Shahawy MA, Pecoits-Filho R, Van BP, Houser MT, Frison L, et al. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol. 2021;32((3)):737–755. doi: 10.1681/ASN.2020081150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barratt J, Andric B, Tataradze A, Schomig M, Reusch M, Valluri U, et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a Phase 3, randomized, open-label, active-controlled study (DOLOMITES) Nephrol Dial Transplant. 2021;36((9)):1616–1628. doi: 10.1093/ndt/gfab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chertow GM, Pergola PE, Farag YM, Agarwal R, Arnold S, Bako G, et al. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med. 2021;384((17)):1589–1600. doi: 10.1056/NEJMoa2035938. [DOI] [PubMed] [Google Scholar]
- 23.Nangaku M, Kondo K, Kokado Y, Ueta K, Kaneko G, Tandai T, et al. Phase 3 Randomized study comparing Vadadustat with Darbepoetin Alfa for anemia in Japanese patients with nondialysis-dependent CKD. J Am Soc Nephrol. 2021;32((7)):1779–1790. doi: 10.1681/ASN.2020091311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nangaku M, Hamano T, Akizawa T, Tsubakihara Y, Nagai R, Okuda N, et al. Daprodustat compared with epoetin beta pegol for anemia in Japanese patients not on dialysis: a 52-week randomized open-label phase 3 trial. Am J Nephrol. 2021;52:26–35. doi: 10.1159/000513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh AK, Carroll K, McMurray JJ, Solomon S, Jha V, Johansen KL, et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med. 2021;385((25)):2313–2324. doi: 10.1056/NEJMoa2113380. [DOI] [PubMed] [Google Scholar]
- 26.Akizawa T, Nangaku M, Yamaguchi T, Arai M, Koretomo R, Matsui A, et al. A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long-term trial. Am J Nephrol. 2019;49((2)):165–174. doi: 10.1159/000496929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akizawa T, Nangaku M, Yamaguchi T, Koretomo R, Maeda K, Yamada O, et al. Two long-term phase 3 studies of enarodustat (JTZ-951) in Japanese anemic patients with chronic kidney disease not on dialysis or on maintenance hemodialysis: SYMPHONY ND-Long and HD-Long studies. Ther Apher Dial. 2021;26((2)):345–356. doi: 10.1111/1744-9987.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14((1)):28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmar DV, Kansagra KA, Patel JC, Joshi SN, Sharma NS, Shelat AD, et al. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am J Nephrol. 2019;49((6)):470–478. doi: 10.1159/000500232. [DOI] [PubMed] [Google Scholar]
- 30.Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381((11)):1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 31.Provenzano R, Shutov E, Eremeeva L, Korneyeva S, Poole L, Saha G, et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant. 2021;36((9)):1717–1730. doi: 10.1093/ndt/gfab051. [DOI] [PubMed] [Google Scholar]
- 32.Eckardt KU, Agarwal R, Aswad A, Awad A, Block GA, Bacci MR, et al. Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N Engl J Med. 2021;384((17)):1601–1612. doi: 10.1056/NEJMoa2025956. [DOI] [PubMed] [Google Scholar]
- 33.Singh AK, Carroll K, Perkovic V, Solomon S, Jha V, Johansen KL, et al. Daprodustat for the treatment of anemia in patients undergoing dialysis. N Engl J Med. 2021;385((25)):2325–2335. doi: 10.1056/NEJMoa2113379. [DOI] [PubMed] [Google Scholar]
- 34.Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open-label study. Ther Apher Dial. 2020;24((2)):115–125. doi: 10.1111/1744-9987.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nangaku M, Kondo K, Takabe S, Ueta K, Kaneko G, Otsuka M, et al. Vadadustat for anemia in chronic kidney disease patients on peritoneal dialysis: a phase 3 open-label study in Japan. Ther Apher Dial. 2020;25((5)):642–653. doi: 10.1111/1744-9987.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanai H, Nangaku M, Nagai R, Okuda N, Kurata K, Nagakubo T, et al. Efficacy and safety of daprodustat in Japanese peritoneal dialysis patients. Ther Apher Dial. 2021;25((6)):979–987. doi: 10.1111/1744-9987.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akizawa T, Nangaku M, Yamaguchi T, Koretomo R, Maeda K, Miyazawa Y, et al. A phase 3 study of enarodustat (JTZ-951) in Japanese hemodialysis patients for treatment of anemia in chronic kidney disease: SYMPHONY HD study. Kidney Dis (Basel) 2021;7((6)):494–502. doi: 10.1159/000517053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Cai X, Ni J, Lin X. Resolution of epoetin-induced pure red cell aplasia, successful re-challenge with roxadustat. Int J Lab Hematol. 2020;42((6)):e291–3. doi: 10.1111/ijlh.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan K, Yin Y, Luo Z, Cheng J. Remarkable response to roxadustat in a case of anti-erythropoietin antibody-mediated pure red cell aplasia. Ann Hematol. 2021;100((2)):591–593. doi: 10.1007/s00277-020-04269-y. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, Liu S, Li Y, Zhao L, Song X, Chen T. Roxadustat in the treatment of a hemodialysis patient with anti-erythropoietin antibody-mediated pure red cell aplasia. Clin Kidney J. 2021;14((11)):2444–2445. doi: 10.1093/ckj/sfab134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradbury BD, Critchlow CW, Weir MR, Stewart R, Krishnan M, Hakim RH. Impact of elevated C-reactive protein levels on erythropoiesis- stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transpl. 2008;24((3)):919–925. doi: 10.1093/ndt/gfn543. [DOI] [PubMed] [Google Scholar]
- 42.Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A. Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29((1)):1–12. doi: 10.1159/000245041. [DOI] [PubMed] [Google Scholar]
- 43.de Francisco ALM, Stenvinkel P, Vaulont S. Inflammation and its impact on anaemia in chronic kidney disease: from haemoglobin variability to hyporesponsiveness. NDT Plus. 2009;2((Suppl 1)):i18–26. doi: 10.1093/ndtplus/sfn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31((7)):1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23((10)):1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macdougall IC, Tucker B, Thompson J, Tomson CR, Baker LR, Raine AE. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996;50((5)):1694–1699. doi: 10.1038/ki.1996.487. [DOI] [PubMed] [Google Scholar]
- 47.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest. 2009;119((5)):1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9((2)):152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, et al. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140((7)):2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272((32)):20055–62. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 51.Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999;274((34)):24142–6. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- 52.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115((17)):3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 53.Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, Rathanaswami P, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122((17)):3054–3061. doi: 10.1182/blood-2013-06-505792. [DOI] [PubMed] [Google Scholar]
- 54.Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of beta-thalassemia. Blood. 2015;126((17)):2031–2037. doi: 10.1182/blood-2015-07-658419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srole DN, Ganz T. Erythroferrone structure, function, and physiology: iron homeostasis and beyond. J Cell Physiol. 2021;236((7)):4888–4901. doi: 10.1002/jcp.30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganz T, Nemeth E. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol. 2016;36((2)):87–93. doi: 10.1016/j.semnephrol.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27((4)):1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yap DYH, McMahon LP, Hao CM, Hu N, Okada H, Suzuki Y, et al. Recommendations by the Asian Pacific Society of Nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrology. 2020;26((2)):105–118. doi: 10.1111/nep.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Xie QH, You L, Xu NX, Hao CM. Effects of hypoxia-inducible factor prolyl hydroxylase inhibitors on iron regulation in non-dialysis-dependent chronic kidney disease patients with anemia: a systematic review and meta-analysis. Pharmacol Res. 2021;163:105256. doi: 10.1016/j.phrs.2020.105256. [DOI] [PubMed] [Google Scholar]
- 60.Charytan C, Manllo-Karim R, Martin ER, Steer D, Bernardo M, Dua SL, et al. A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int Rep. 2021;6((7)):1829–1839. doi: 10.1016/j.ekir.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanghani NS, Haase VH. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26((4)):253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nangaku M, Farag YMK, DeGoma E, Luo W, Vargo D, Khawaja Z. Vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, for treatment of anemia of chronic kidney disease: two randomized phase 2 trials in Japanese patients. Nephrol Dial Transplant. 2020:gfaa060. doi: 10.1093/ndt/gfaa060. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto H, Nobori K, Matsuda Y, Hayashi Y, Hayasaki T, Akizawa T. Efficacy and safety of molidustat for anemia in ESA-naive nondialysis patients: a randomized, phase 3 trial. Am J Nephrol. 2021;52((10-11)):871–883. doi: 10.1159/000518071. [DOI] [PubMed] [Google Scholar]
- 64.Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014;9((11)):e111838. doi: 10.1371/journal.pone.0111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coyne DW, Singh AK, Lopes RD, Bailey CK, DiMino TL, Huang C, et al. Three times weekly dosing of daprodustat versus conventional epoetin for treatment of anemia in hemodialysis patients: ASCEND-TD: a phase 3 randomized, double-blind, noninferiority trial. Clin J Am Soc Nephrol. 2022;17((9)):1325–1336. doi: 10.2215/CJN.00550122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnarra JR, Zhou S, Merrill MJ, Wagner JR, Krumm A, Papavassiliou E, et al. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 1996;93((20)):10589–94. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93((20)):10595–9. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15((1)):55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 69.Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med. 2021;385((22)):2036–2046. doi: 10.1056/NEJMoa2103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. The Lancet. 2003;361((9374)):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 71.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32((4)):614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 72.Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103((10)):3924–3932. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 73.Semenza GL. VHL and p53: tumor suppressors team up to prevent cancer. Mol Cell. 2006;22((4)):437–439. doi: 10.1016/j.molcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Prehu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2008;40((2)):160–165. doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 75.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TRJ, Maxwell PH, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103((3)):654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359((25)):2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- 77.Ladroue C, Hoogewijs D, Gad S, Carcenac R, Storti F, Barrois M, et al. Distinct deregulation of the hypoxia inducible factor by PHD2 mutants identified in germline DNA of patients with polycythemia. Haematologica. 2012;97((1)):9–14. doi: 10.3324/haematol.2011.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358((2)):162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367((10)):922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hara K, Takahashi N, Wakamatsu A, Caltabiano S. Pharmacokinetics, pharmacodynamics and safety of single, oral doses of GSK1278863, a novel HIF-prolyl hydroxylase inhibitor, in healthy Japanese and Caucasian subjects. Drug Metab Pharmacokinet. 2015;30((6)):410–418. doi: 10.1016/j.dmpk.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90((5)):1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Martin ER, Smith MT, Maroni BJ, Zuraw QC, DeGoma EM. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol. 2017;45((5)):380–388. doi: 10.1159/000464476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akizawa T, Nangaku M, Yamaguchi T, Arai M, Koretomo R, Maeda K, et al. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo-controlled phase 2b trial followed by long-term trial. Nephron. 2019;143((2)):77–85. doi: 10.1159/000500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akizawa T, Tsubakihara Y, Nangaku M, Endo Y, Nakajima H, Kohno T, et al. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol. 2017;45((2)):127–135. doi: 10.1159/000454818. [DOI] [PubMed] [Google Scholar]
- 85.Akizawa T, Yamada T, Nobori K, Matsuda Y, Hayashi Y, Hayasaki T, et al. Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int Rep. 2021;6((10)):2604–2616. doi: 10.1016/j.ekir.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukui K, Shinozaki Y, Kobayashi H, Deai K, Yoshiuchi H, Matsui T, et al. JTZ-951 (enarodustat), a hypoxia-inducibe factor prolyl hydroxylase inhibitor, stabilizes HIF-alpha protein and induces erythropoiesis without effects on the function of vascular endothelial growth factor. Eur J Pharmacol. 2019;859:172532. doi: 10.1016/j.ejphar.2019.172532. [DOI] [PubMed] [Google Scholar]
- 87.Akizawa T, Nangaku M, Yonekawa T, Okuda N, Kawamatsu S, Onoue T, et al. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol. 2020;15((8)):1155–1165. doi: 10.2215/CJN.16011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akizawa T, Iwasaki M, Otsuka T, Yamaguchi Y, Reusch M. Phase 3 study of roxadustat to treat anemia in non-dialysis-dependant CKD. Kidney Int Rep. 2021;6((7)):1810–1828. doi: 10.1016/j.ekir.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nangaku M, Kondo K, Ueta K, Kokado Y, Kaneko G, Matsuda H, et al. Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemic patients on hemodialysis: a phase 3, multicenter, randomized, double-blind study. Nephrol Dial Transplant. 2021;36((9)):1731–1741. doi: 10.1093/ndt/gfab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369((9559)):381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 91.Barratt J, Sulowicz W, Schomig M, Esposito C, Reusch M, Young J, et al. Efficacy and cardiovascular safety of roxadustat in dialysis-dependent chronic kidney disease: pooled analysis of four phase 3 studies. Adv Ther. 2021;38((10)):5345–5360. doi: 10.1007/s12325-021-01903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Provenzano R, Szczech L, Leong R, Saikali KG, Zhong M, Lee TT, et al. Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non-dialysis-dependent CKD: pooled results of three randomized clinical trials. Clin J Am Soc Nephrol. 2021;16((8)):1190–1200. doi: 10.2215/CJN.16191020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winkelmayer WC, Walther CP. Cardiovascular safety of roxadustat in CKD anemia: a fig leaf named noninferiority. Clin J Am Soc Nephrol. 2021;16((8)):1155–1157. doi: 10.2215/CJN.08280621. [DOI] [PMC free article] [PubMed] [Google Scholar]