Understanding energy balance requires robust estimates of energy intake. Bioenergetics provides a key tool to investigate the effects of disturbance in marine mammals. We review the mechanistic components that affect energy intake and the processes from ingested energy to net utilizable energy. We summarize existing datasets and highlight key research gaps to advance this field to ensure effective conservation and management.
Keywords: marine mammals, energy intake, Bioenergetics
Abstract
Bioenergetics is the study of how animals achieve energetic balance. Energetic balance results from the energetic expenditure of an individual and the energy they extract from their environment. Ingested energy depends on several extrinsic (e.g prey species, nutritional value and composition, prey density and availability) and intrinsic factors (e.g. foraging effort, success at catching prey, digestive processes and associated energy losses, and digestive capacity). While the focus in bioenergetic modelling is often on the energetic costs an animal incurs, the robust estimation of an individual’s energy intake is equally critical for producing meaningful predictions. Here, we review the components and processes that affect energy intake from ingested gross energy to biologically useful net energy (NE). The current state of knowledge of each parameter is reviewed, shedding light on research gaps to advance this field. The review highlighted that the foraging behaviour of many marine mammals is relatively well studied via biologging tags, with estimates of success rate typically assumed for most species. However, actual prey capture success rates are often only assumed, although we note studies that provide approaches for its estimation using current techniques. A comprehensive collation of the nutritional content of marine mammal prey species revealed a robust foundation from which prey quality (comprising prey species, size and energy density) can be assessed, though data remain unavailable for many prey species. Empirical information on various energy losses following ingestion of prey was unbalanced among marine mammal species, with considerably more literature available for pinnipeds. An increased understanding and accurate estimate of each of the components that comprise a species NE intake are an integral part of bioenergetics. Such models provide a key tool to investigate the effects of disturbance on marine mammals at an individual and population level and to support effective conservation and management.
Introduction
Achieving energetic balance is a key to survival and reproduction in animals (Costa and Williams, 1999; Parsons, 2005; Stubbs and Tolkamp, 2006). Energetic balance results from an animal’s energetic costs (e.g. the effort expended on movement, maintenance of body processes, growth and reproduction), and the energy they can extract from their environment (Schneider, 2004). Bioenergetics, the study of how animals achieve such balance, integrates biotic and abiotic influences, including intrinsic and extrinsic factors (see Pirotta, 2022, this Special Issue), and can be used in conservation to understand the effects of stressors on an individual and the resulting dynamics on populations (Costa, 2012; Chimienti et al., 2020; Gallagher et al., 2021; Keen et al., 2021). Although several recent studies have advanced our understanding of the processes that influence energy use in animals, relatively little is known of the trade-offs that control energy intake. Mammalian species exhibit a wide range of life-history strategies, from long-lived species with long inter-birth intervals to species reaching sexual maturity early and with high reproductive output. Quantifying energy balance is critical to understand a species’ biology, the effect of a changing ecosystem on a species and informing effective conservation measures.
The past 40 years have seen significant advances in bioenergetic models to achieve a variety of research and conservation objectives for marine mammals. A key concern is that anthropogenic disturbance can cause behavioural, physiological and health changes that can affect an individual’s vital rates, such as survival and reproduction (Nabe-Nielsen et al., 2018; Pirotta et al., 2018a). The probability and effects of disturbance are ultimately mediated by the state of the individual (e.g. life history stage, exposure history) and the environment (e.g. resource availability) (Pirotta et al., 2018a; Keen et al., 2021). Globally, climate change is altering ecosystems, and assessing the effects of this and other anthropogenic stressors (and their complex interactions) remains a critical knowledge gap (National Academies of Sciences Engineering and Medicine, 2017; Hazen et al., 2019; Malhi et al., 2020; Gallagher et al. 2021b; Pirotta et al., 2022).
Energy intake is an important component of bioenergetic models (Pirotta, 2022, this Special Issue). While accounting models generally use a summary of energetic costs and efficiencies to estimate food intake requirements, dynamic models predict the mutual relationship between energy expenditure and energy intake. In this review, we compiled existing data on all aspects of energy intake relevant to bioenergetics models, ranging from prey acquisition and ingestion of food (including maximum rate of food intake, nutritional value of prey, prey density and food processing rates) to net energy (NE) (including losses associated with faecal energy [FE], urinary energy (UE) and heat increment of feeding [HIF]). This mechanistic approach to energy intake is population specific as it encompasses information about prey type, distribution and density and therefore individual foraging strategies and behaviours (McHuron et al., 2018; Pirotta et al., 2018b; Guilpin et al., 2019; Pirotta et al., 2019). However, it provides a useful framework to review each of these key parameters separately and identify data gaps to guide future efforts.
Review Scope and Structure
This review examines the parameters and constraints used in determining the amount of energy consumed and retained by an individual. This review is novel in bioenergetics as it follows a mechanistic approach to energy intake, highlighting parameters relevant for bioenergetic models (Figure 1). We summarize the current state of knowledge regarding how acquired energy flows from ingested energy (IE) to NE. NE is subsequently used for maintenance, which includes energy used for activity, basal metabolism and/or thermoregulation and production energy, which includes growth, reproduction and storage. Energy use and allocation is addressed in other reviews of this Special Issue (growth: Adamczak et al., reproduction: McHuron et al.; metabolic rates: Noren). This review focuses on cetaceans and pinnipeds. Thus, literature on polar bears (Ursus maritimus), sirenians and sea otters (Enhydra lutris) has not been as thoroughly covered.
Figure 1.
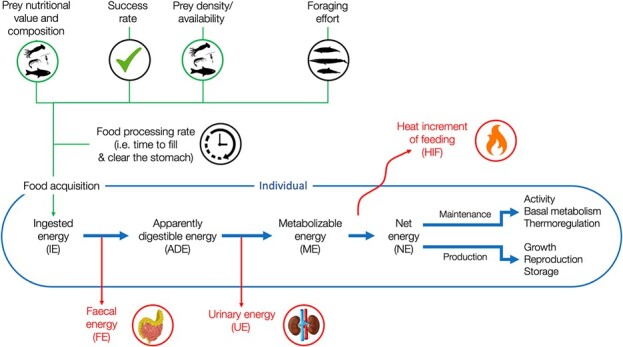
Schematic representation of the energy flow through a marine mammal (adapted from Cost a, 2009 and Lavigne et al. 1982). Green lines represent parameters influencing food acquisition an d ingested energy (IE). The outer blue line represents the individual and blue arrows represent the e nergy flow from ingested energy (IE) to net energy (NE) and subsequent allocation. Red arrows ind icate energy losses. NB: this schematic representation is not intended to represent parameters intera ctions but to provide a conceptual framework to visualize parameters and the underlying equations of a bioenergetic mechanistic approach.
Prey Acquisition
Foraging effort and success rate
Meeting energetic requirements is dependent upon the ability of an animal to find, ingest and digest suitable prey. Generating estimates of IE begins with robust estimates of the amount of time an animal spends foraging; for marine mammals the time allocated to this activity is also constrained by having to return to the surface to breathe and/or to land to haul out (Mori, 1998; Thompson and Fedak, 2001; Rosen et al., 2007). Estimates of energy intake can come from functional response relationships (Mackinson et al., 2003), time-activity budgets (McClintock et al., 2013; Jeanniard-du-Dot et al., 2017) and estimates of prey capture attempts and their success rate (i.e. the percentage of attempts that result in the successful capture of prey) (Johnson et al., 2006; Wisniewska et al., 2016).
Biologging deployments provide information on the movement and dive behaviour of individuals and increasingly greater detail on foraging behaviour across a wide range of species (McIntyre, 2014). Such data, particularly from longer-term tags (e.g. McConnell et al., 1999 ; Schorr et al., 2017 ; Savoca et al., 2021), can also be used to estimate the number of typical foraging dives over defined time intervals and allow for the characterization of time-activity budgets for cetacean and pinniped species (Russell, 2015; Jeanniard-du-Dot et al., 2017) and how those budgets can be altered when animals are disturbed from baseline behaviour (Bejder et al., 2009; Isojunno et al., 2017). For example, Isojunno et al. (2016) observed that sperm whales (Physeter macrocephalus) spent more time in an ‘active non-foraging state’ following exposure to a low-frequency active sonar or killer whale (Orcinus orca) sound playback (thereby reducing time spent foraging over the tag deployment). As discussed below, these data have allowed for estimates of energy intake or balance in a variety of marine mammal species (e.g. Goldbogen et al., 2019; Booth, 2020; Czapanskiy et al., 2021; Kienle et al., 2022).
In pinniped species, data from non-acoustic animal-borne sensors (e.g. accelerometry and cameras) have been widely used to identify foraging events at different temporal resolutions (Viviant et al., 2010; Iwata et al., 2012; Gallon et al., 2013; Cole et al., 2021; Vance et al., 2021). Head movements, associated with either raptorial or suction feeding techniques, can successfully be detected using the rate of change in acceleration (or also called jerk) from sensors when adequately deployed close to the head of the individuals (Ydesen et al., 2014; Volpov et al., 2015; Cole et al., 2021). Experiments on captive animals or the concomitant use of camera tags on wild animals (Volpov et al., 2015) corroborated the use of rate of change in acceleration to identify prey-capture events.
In cetaceans, prey-capture attempts have typically been estimated using tags that collect acoustic and 3D accelerometry data (e.g. Johnson and Tyack, 2003; Lewis et al., 2018). For odontocetes, high-resolution acoustic biologging sensors record echolocation behaviour (e.g. the fast production of successive clicks qualified as terminal or foraging buzzes), which can be combined with associated movement characteristics (e.g. jerk) to estimate the number of prey capture attempts occurring in a dive. Foraging buzzes (with or without estimates of jerk) have been quantified in beaked whales (Family Ziphiidae) (Johnson et al., 2006; Stimpert et al., 2014; Siegal, 2020; Alcázar-Treviño et al., 2021; Visser et al., 2022), sperm whales (including Kogia spp.) (Fais et al., 2016; Tønnesen et al., 2020; Malinka et al., 2021), short-finned pilot whales (Globicephala macrorhynchus) (Aguilar Soto et al., 2008; Holt et al., 2021), narwhals (Monodon monoceros) (Ngô et al., 2021), smaller delphinids (Wisniewska et al., 2014; Arranz et al., 2016) and harbour porpoises (Phocoena phocoena) (Wisniewska et al., 2014; Wisniewska et al., 2018).
In mysticetes, biologging devices have been used to record a broader range of foraging strategies than observed for odontocetes, with studies of blue whales (Balaenoptera musculus) (Doniol-Valcroze et al., 2011; Goldbogen et al., 2011), fin whales (Balaenoptera physalis) (Panigada et al., 1999; Goldbogen et al., 2006), humpback whales (Megaptera novaeangliae) (Friedlaender et al., 2009, 2013; Owen et al., 2015; Burrows et al., 2016), Antarctic minke whales (Balaenoptera bonaerensis) (Friedlaender et al., 2014), Bryde’s whales (Balaenoptera brydei) (Alves et al., 2010), North Atlantic right whales (Eubalaena glacialis) (Baumgartner et al., 2003; Laidre et al., 2007; van der Hoop et al., 2019) and bowhead whales (Balaena mysticetus) (Simon et al., 2009; Heide-Jørgensen et al., 2013). Balaenopterids (rorquals) are bulk feeders that engulf large volumes of water and ensnared prey by expanding their ventral groves, following an abrupt acceleration phase (Croll et al., 2001; Goldbogen et al., 2006). This characteristic feeding technique called lunge feeding is associated with distinct kinematics (e.g. swim speed, overall dynamic body acceleration [ODBA], minimum specific acceleration [MSA] or rate of acceleration [jerk]), which are used to identify feeding events on dive profiles obtained from biologging devices (e.g. DTAG, TDR Wildlife computer, Acousonde, Crittercam, CATs tag, Little Leonardo). Body kinematics during foraging can inform on feeding attempts, indeed the ODBA, the rate of change in acceleration or differential of the three acceleration axes (jerk) or MSA are proxies of energy expenditure and obtained from three-dimensional accelerometry (Wilson et al., 2006). Although very useful, measures of ODBA can differ from small to larger marine mammals and therefore could underestimate energy expenditure in large animals (Martín López et al., 2022) but limitations are covered in another review on metabolic rates of this Special Issue (Noren). Nevertheless, sounds produced by odontocetes can then be used to identify potential feeding attempts. Body kinematics remains an accurate metric to identify feeding attempts (Goldbogen et al., 2017). Contrary to balaenopterids, balaenids engage in continuous ram filter-feeding, during which they skim through a layer of prey at low speed to reduce drag (Werth, 2004). As they do not have discrete feeding attempts, identifying foraging time within balaenid dive profiles is less straightforward and relies on the shapes of dives and fluking gaits (i.e. types) (Nowacek et al., 2001; Baumgartner et al., 2003; Laidre et al., 2007). One time-depth–recorder tag has been deployed on a benthic-feeding grey whale (Eschrichtius robustus) (Malcolm et al., 1996), from which dive types were classified (Malcolm and Duffus, 2000) and used to assess foraging activity in focal-follow observations (Feyrer and Duffus, 2015). In shallow intertidal habitats, benthic feeding has been confirmed from mud plumes and feeding pits (Calambokidis et al., 2018).
It is important to highlight that most tag deployments are short duration and thus provide only a snapshot of foraging behaviour, the representativeness of which is hard to assess. Furthermore, foraging metrics are likely to be site specific, depending on environmental, seasonal and biotic conditions. For example, blue whales tagged with DTAGs (Johnson and Tyack, 2003), VTDRs (Mk8; Wildlife Computers), National Geographic CritterCam (Marshall, 1998) and Bioacoustic Probe (B-probe; Greeneridge Sciences) in Southern California (Oleson et al., 2007), the St. Lawrence estuary (Doniol-Valcroze et al., 2011; Guilpin et al., 2019) and northern Chilean Patagonia (Caruso et al., 2021) exhibited different feeding rates. While they follow the diurnal vertical migration of their prey in each location, feeding depth and dive duration varied across locations (Oleson et al., 2007; Doniol-Valcroze et al., 2011; Guilpin et al., 2019; Caruso et al., 2021). This highlights the need to take prey density and availability into account to contextualize foraging behaviour.
Prey-capture success is likely to vary significantly across predator and prey species, depending on the foraging strategy of the predator, the morphology of the feeding apparatus, and prey density, predictability and behaviour (e.g. diurnal or nocturnal, shoaling or burring, escape strategies). Stomach temperature telemetry has been used in pinnipeds to quantify prey-capture success, as the stomach temperature recovers faster after water ingestion than after the ingestion of a prey item, although the method does not prevent false detection of prey captures (Kuhn and Costa, 2006). Additionally, distinct jerk movements and jaw movements have been identified in pinniped tag deployments, which may be used to estimate foraging success. Despite the large number of acoustic tags deployed, few studies have estimated prey-capture success rate in odontocetes. Wisniewska et al. (2016) estimated that harbour porpoises, fitted with DTAGs, had mean prey-capture success rates between 92–99% (n = 4). The prey-capture success rate has been difficult to estimate for bulk feeders, such as mysticetes. Because of the high costs associated with lunging for rorquals and the increased drag costs of skimming balaenids, it is likely that all feeding attempts would be at least partly successful (Goldbogen et al., 2012; Potvin et al., 2012). For example, the angle of approach and speed of lunging allow rorquals to minimize prey escape (Cade et al., 2020). Prey-capture success rate has been explicitly (Goldbogen et al., 2011; Guilpin et al., 2019; Guilpin et al., 2020) or implicitly (Wiedenmann et al., 2011; Pirotta et al., 2018b; Pirotta et al., 2019; Pirotta et al., 2021) assumed to be 100% in bioenergetic models for rorquals whales.
The importance of prey
Estimates of time spent foraging or the number of successful foraging attempts are most useful in bioenergetics when combined with resource availability and quality estimates. In the past, studies have expressed energy intake requirements in weight of prey or as a percentage of body mass (e.g. Perez et al., 1990; Kastelein et al., 1997; Rosen and Worthy, 2018). However, as the energetic quality of prey items varies significantly with prey type and size, as well as in time and space, a more nuanced exploration of these factors is required.
A number of reviews exist that explore the diet of different marine mammal species and highlight the significant overlap between cetacean and pinniped diet (Tollit et al., 1997; Santos et al., 2001; Skern-Mauritzen et al., 2011; Andreasen et al., 2017; West et al., 2017; Trites and Spitz, 2018; Wilson and Hammond, 2019). However, many reviews on cetacean diet rely on stomach content samples that come from bycaught or stranded individuals, which may therefore provide a biassed assessment. Reviews of diet reveal that the composition varies by age, sex, region, season and inter-annually. Species fall on a generalist-specialist continuum (Jiang and Morin, 2005), though intra-population variation may exist (such that a generalist population may actually be composed of individual specialists) (Bolnick et al., 2003; Araújo et al., 2008; Araújo et al., 2011). Understanding where marine mammal species or individual are positioned on this continuum, and thus their plasticity in target habitat or prey, is important to understanding their resilience to disturbance (Gill et al., 2001; Booth, 2020; Hanson et al., 2021).
Generally, marine mammal diet is high in lipids and proteins and low in carbohydrates. While energy density is a useful metric when considering energetic balance, different energy metabolism pathways may exist, meaning different priorities for various micronutrients (Derous et al., 2021). Marine mammal metabolism may have evolved in response to a glucose-poor diet (a key difference from terrestrial mammals) and different macronutrients may have essential roles in metabolism, foraging behaviour and dive physiology (Derous et al., 2021).
A database was compiled using existing literature on prey type, size, nutritional content and availability (see Supplementary Information 1 and 2). A total of 146 literature sources were included, mainly consisting of peer-reviewed journal publications and publicly available grey literature. In some instances, species-level information was not available and prey data were presented at the family level only.
Weight–length relationships were collated for 42 families and 78 species; the majority of these were fish species. Because mass is a cubic function of length in fish (Froese, 2006) and cephalopods (e.g. Dawe, 1988; Brunetti and Ivanovic, 1997) and the caloric value of a prey item is a product of mass, larger prey offer substantially greater energy gains (see Figure 1 of Booth, 2020). Energy density values were sourced for 114 families comprising 172 species, with many records from outside the marine mammal literature (Figure 2). Cephalopod species were consistently between 4 and 5 kJ g−1Ww, irrespective of the water depth they inhabit, with estimates available for a moderate number of meso- and bathypelagic prey. Energy density of fish species was much more variable than for most other taxa. Other pelagic invertebrates generally had the lowest energy density, but a wide range of energy density of benthic invertebrates was found. Most are also typically small in size, yielding low total energy per item (Born et al., 2003). Marine mammals are also prey for some species (e.g. Kryukova et al., 2012; Pistorius et al., 2012; van Neer et al., 2015) and energy densities range between 4.6 and 5.1 kJ g−1Ww and 23 and34 kJ g−1Ww for muscle and blubber, respectively (Kuhnlein et al., 2002). Energy density varies within prey species, depending on length, sex and season (Hislop et al., 1991; Pierce et al., 1991; Pedersen and Hislop, 2001) (Figure 3). Macronutrient content in different prey types was available for only 22% of energy density records, suggesting this is a knowledge gap in the marine mammal field. However, understanding fish and cephalopod macronutrient composition is a burgeoning field of human-fisheries science (e.g. Hicks et al., 2019). Both weight–length relationships and energy densities were available for 34 families and 26 species, which could be used to estimate the energy density of observed prey species and size (e.g. from dietary studies).
Figure 2.
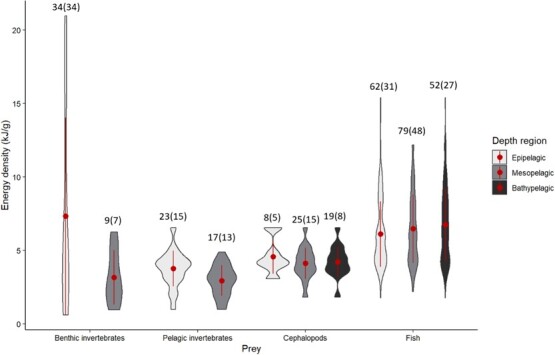
Violin plot for energy density of marine mamma l prey (wet weight) using information compiled in Supplementary Information 2. The red dot is the mean, lines are +/- 1 standard deviation. Numbers indicate the number of species (with families in p arentheses) for which energy density values were sourced.
Figure 3.
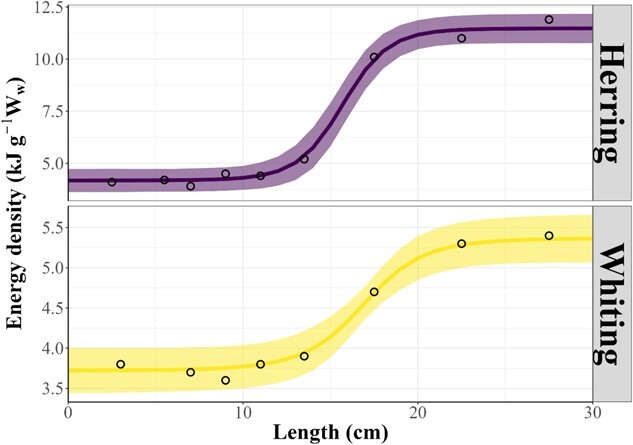
Predicted energy density (kJ g-1Ww) as a function of fish length using reported values from Pedersen and Hislop (2001) from Ju ly-September to fit a logistic model. Data in open circles, predicted mean in solid line, and confiden ce intervals displayed as ribbons per size class for herring and whiting.
Prey density and availability
An organism’s fitness is determined by the relationship it has with its environment (i.e. resources, risks etc.) (Matthiopoulos et al., 2020). Consequently, the population size and individual fitness of a predator is linked to prey availability (MacArthur and Pianka, 1966; Trites and Donnelly, 2003; Øigård et al., 2013; Benoit-Bird, 2017; Benoit-Bird et al., 2020), prey quality (Österblom et al., 2008) and catchability (Brown et al., 1999; Balme et al., 2007). Of course, animals can partly compensate for reduced food availability depending on their ability to, for example, move to different foraging areas, alter their diet, reduce their metabolic costs or use stored energy. However, prey density is a key parameter for affecting marine mammal intake. We collated prey density information from 32 peer-reviewed marine mammal publications and a further 15 papers from outside the marine mammal literature (e.g. fishery data; Sala, 2018) (summarized in Supplementary Information 2).
Increasingly, prey density estimates are incorporated into marine mammal studies to understand spatio-temporal distribution patterns, predator–prey interactions, foraging ecology and costs of disturbance (Friedlaender et al., 2016; Stäbler et al., 2019; Guilpin et al., 2020; Pirotta et al., 2021). However, estimating prey availability to predators is difficult as it requires estimation of the spatio-temporal overlap between predator and prey species. Several approaches can be taken; some studies use proxies (Booth et al., 2013), average prey densities (Goldbogen et al., 2011), regional stock assessment estimates (Astarloa et al., 2021) or combine telemetry with fish survey data (Nowacek et al., 2011; Smout et al., 2014). Depending on the research question, large-scale stock trends may or may not be representative of food availability to a predator. Large-scale averages could help understanding broader spatial patterns, especially if prey distribution is persistent. However, the productivity of many fish species has undergone changes due to climate variability, commercial harvest, habitat degradation and/or alterations in competition (Brander, 2007; Baudron et al., 2020). Therefore, prey density estimates on a smaller spatio-temporal scale are required to increase understanding of foraging ecology and to quantify the consequences of anthropogenic disturbance. These could come from species distribution models (Smout et al., 2014; Pendleton et al., 2020), real-time monitoring (Friedlaender et al., 2006) or inter-prey spacing (Southall et al., 2019).
The varying diets of generalist marine mammals reflect changes in the availability of multiple prey species. Functional responses are crucial to understand trophic interactions and provide information on predation pressure, prey preference and population dynamics (Smout and Lindstrøm, 2007; Ransijn et al., 2021). To gain insight into diet adaptability, a Multi-Species Functional Response (MSFR) must be modelled. The MSFR describes how the consumption rate of a predator varies in relation to the availability of several prey species. Furthermore, it allows exploration of the consequences of future changes in prey-driven bottom-up processes, or the impact of top-down control on the rest of the ecosystem and the fisheries that depend on it.
Food processing rates
While foraging, marine mammals make decisions affected by the rate at which they can acquire prey, which depends on the distribution and accessibility of prey and prey handling time (other factors such as predation, body condition, etc., may also factor into decision making). Additionally, the amount of food that an individual can consume is ultimately limited by digestive constraints, that is, the rate at which an animal can physically digest or process food (Williams et al., 2001; Rosen and Trites, 2004; Williams and Yeates, 2004). Food processing rates vary depending on the size and anatomy of the gastrointestinal tract, prey proximate composition (i.e. percent protein, lipid, and water) and prevalence of non-digestible structures (Trumble et al., 2003).
Few estimates of maximum food intake exist for marine mammals, but estimates are available from observations of animals in managed care (Goldblatt, 1993; Kastelein et al., 2019) or where a generalized relationship is assumed (sensu Taylor et al., 2007). Studies on juvenile Steller sea lions (Eumetopias jubatus) and Northern fur seals (Callorhinus ursinus) indicated that animals generally reached their digestive limit once food intake reached 14–32% of their body mass (Rosen and Trites, 2004; Rosen et al., 2012). This work highlighted that animals could alter their food intake in response to short-term changes in prey quality or availability, but that food intake levels could exceed their short-term physiological digestive capacities, impacting animal health (Kastelein et al., 2019). The wider taxonomic literature indicates that satiation levels can be impacted by numerous factors, including water intake, body weight and temperature (Reese and Hogenson, 1962; McFarland and L'Angellier, 1966; Arnason et al., 2009).
Rates of food ingestion are partly constrained by the rate at which animals can process it through the digestive system. This has been studied in several pinniped species. In harbour seals (Phoca vitulina), stomachs started to empty 1 h following feeding and some prey remained in the stomach after 5 h (Markussen, 1993). Kastelein et al. (2019) observed that porpoises had a large extensible forestomach (up to six times the relaxed size), are capable of ingesting > 90% of their daily energetic requirements (i.e. ~ 12–20 MJ) in 1 h and can feed again shortly afterwards. Most studies have measured processing time by measuring the time it takes for ingested chemical markers to appear in the faeces (Table 1). In general, passage rate is relatively uniformly rapid and among most studied pinniped species.
Table 1.
Summary of empirically measured processing times (in hours) from marine mammal studies with associated prey type and markers used.
| Species | Marker | Prey | Processing time (hours) | Reference |
|---|---|---|---|---|
| Pacific walrus | N:A. | unspecified fish | 5–9 | Fisher et al. (1992); Kastelein et al. (2003a) |
| Australian sea lion | T.O. | N.A. | 6.5 (± 4.3) | Bodley et al. (1999) |
| New Zealand fur seal | T.O. | N.A. | 4 (± 3) | Bodley et al. (1999) |
| Harbour seal | C | N.A. | 6–14 | Havinga (1933) |
| Harbour seal | C.R.D/B.S. | Fish | 2.5–6.3 | Markussen (1993) |
| Hawaiian monk seal | C.O. | Herring | 14.0 (±4.8) | Goodman-Bacon (2018) |
| Bottlenose dolphin | C.R.D. | Herring and mackerel | 3.9 (± 0.8) | Kastelein et al. (2003b) |
| False killer whale | C.R.D. | Herring and mackerel | 3.9 (± 0.5) | Kastelein et al. (2000a) |
| Dusky dolphin | C.R.D. | Hake, squid, octopus, cuttlefish, misc. teleosts | 2.5 (1.7–4.2) | Kastelein et al. (2000b) |
| Harbour porpoise | C.R.D. | Herring and sprat | 2.4–3.3 | Kastelein et al. (1997) |
| Beluga whale | C.R.D. | Herring, smelt, mackerel | 4.5 | Kastelein et al. (1994) |
| Amazon river dolphin | C.R.D. | Trout, carp, tench | 4.2 | Kastelein et al. (1999) |
| Manatee | N.A. | Water hyacinth | 146 | Lomolino and Ewel (1984) |
SD or range is reported when available. N.A. data non available, T.O.—Titanium oxide, C—charcoal, C.R.D.—Carmine red dye, B.S.—Barium sulphate, C.O.—Chromic oxide
Little is known about the rate at which cetaceans process food. Most cetacean species have a forestomach, except beaked whales (Ziphiidae), Franciscana dolphin (Pontoporia blainvillei) and Baiji (Lipotes vexillifer) (Tarpley et al., 1987; Mead, 2009). Processing time for ingested food was estimated to ~ 14–15 h in common dolphins (Delphinus delphis) (Tomilin and Heptner, 1967), 2.5 h in harbour porpoises (Kastelein et al., 1997) and 3.6–4.5 h in bottlenose dolphins (Tursiops truncatus), dusky dolphins (Sagmatias obscurus), false killer whales (Pseudorca crassidens) and beluga whales (Delphinapterus leucas) (Kastelein et al, 1994, 2000ab, 2003b) (Table 1).
For baleen whales and balaenopterids in particular, the first volumetric estimate of forestomach capacity comes from Víkingsson (1997) for fin whales caught off Iceland. The forestomach volumetric capacity was estimated by either filling up the forestomach with water and subsequently measuring the volume, or estimating volume from natural gas expansion (Víkingsson, 1997). The author estimated a digestion time of ~15 h between the forestomach and the rectum and a clearance rate of the forestomach of ~3 h by establishing the relationship between length L (in m) and size of the forestomach S (in kg) (S = 0.47 L2.36) (Víkingsson, 1997). Wiedenmann et al. (2011) estimated a forestomach’s clearance rate of 4 h for blue whales using the equation of Víkingsson (1997). They then defined the rate at which the forestomach is filled to depend on forestomach capacity, swarm density and engulfment volume of a lunge. This food processing rate was used in bioenergetic models for blue whales (Wiedenmann et al., 2011; Pirotta et al., 2018b, 2019). In balaenid species, the rates of filling and clearing the forestomach might be different for rorquals, for which the forestomach accounts for a larger part of the total stomach volume (e.g. Tarpley et al., 1987; Víkingsson, 1997), but they have not been measured or modelled to date.
From IE to NE
Not all chemical energy ingested as food (gross energy intake or IE) is available to the animal to fuel its biological functions. The difference between IE and the resulting NE is due to several losses along the digestive process (Figure 4). Once ingested, the energy remaining after digestion and loss of FE is called apparently digested energy (ADE). Metabolizable energy (ME) is the remaining energy after subtraction of the UE from the ADE, that is, the energy lost as urea and other compounds in the urine. According to traditional bioenergetic schemes (e.g. Kleiber, 1975; Lavigne et al., 1982), the energy lost via the HIF resulting from digestive processes is subtracted from ME, leaving NE. The NE can be divided into energy available for growth, reproduction and storage, also termed production energy, and maintenance energy, which is the energy used to fuel other metabolic processes. The current state of knowledge of each parameter is reviewed below. While the emphasis of this review was on cetaceans, the literature on pinnipeds was reviewed where data for cetaceans were limited.
Figure 4.
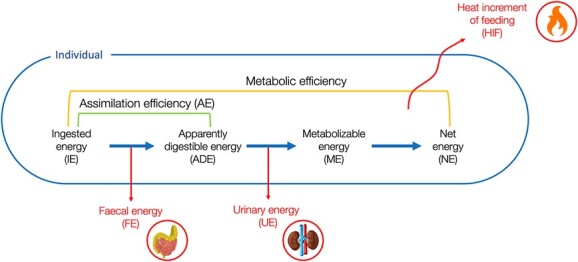
Detailed pathway from ingested energy (IE) to net energy (NE) and representation of associated losses and efficiencies.
Assimilation efficiency and FE
The efficiency with which an individual processes food can be differentiated between assimilation efficiency (AE) and metabolic efficiency (Figure 4).
To calculate AE, both the IE and energy lost through faecal material must be known. These two values are used to calculate the ADE:
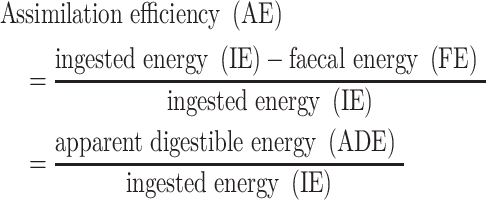 |
AE is also referred to as digestive efficiency (Rosen and Trites, 2000) or apparent digestibility (Costa and Williams, 1999). However, some studies use these terms when they are actually reporting dry matter digestibility (Lawson et al., 1997a; Rosen and Trites, 2000) and dry matter disappearance (Nordøy et al., 1993), which are measures of the ratio of lost dry organic matter, rather than energy per se. Dry matter digestibility is usually lower than AE (Fadely et al., 1994); while similar, the two measures are not equivalent (Rosen and Trites, 2000). In some literature, including some studies on cetaceans (Fortune et al., 2013) and other taxa (i.e. reptiles, McConnachie and Alexander, 2004), the term AE is used to account for both faecal and UE losses, but this is more correctly termed metabolic efficiency. Metabolic efficiency is a measure of how efficient an individual is at processing food and covers both urinary and FE losses, resulting in ME (Lavigne et al., 1982; Rosen and Worthy, 2018).
As AE’s quantification relies on the analysis of faecal material, it has been mostly measured in pinnipeds for logistical reasons (Table 2). In general, AE for pinnipeds is high, particularly for fish prey species compared with invertebrates. AE is affected by the biochemical composition of the prey (Schneider and Flatt, 1975; Lawson et al., 1997a; Rosen and Trites, 2000) but does not seem to be affected by meal size or frequency of feeding (Keiver et al., 1984; Ronald et al., 1984; Lawson et al., 1997a; Lawson et al., 1997b; Rosen et al., 2000). In experiments using fish species differing in energy density and lipid/protein content, AE was higher for prey species with a higher energy and lipid content than those with lower energy, higher protein content (Lawson et al., 1997a, 1997b; Rosen et al., 2000; Rosen and Trites, 2000; Diaz Gomez et al., 2016). A study with northern fur seals demonstrated that AE was negatively related to protein content, possibly because proteins decrease lipid digestibility (Diaz Gomez et al., 2020).
Table 2.
Summary of values measured or used for marine mammal species for both AE (%) representing the fraction of IE retained after FE losses, and FE loss (expressed as a % of IE) is the amount of energy lost in the faecal matter.
| Species | Parameter | Value | Unit | Prey | Reference |
|---|---|---|---|---|---|
| Bowhead whale | AE | 90 | % | Calanoids | Laidre et al. (2007) ** |
| Baleen whales | AE | 80 | % | N.A. | Lockyer (1981) ** |
| Minke whale | AE | 92.1 83.4 |
% | Herring Krill |
Nordøy et al. (1993) * |
| Minke whale | AE | 87–93 | % | Krill | Mårtensson et al. (1994a) * |
| Crabeater seal | 84.0 | % | Krill | Mårtensson et al. (1994a) * | |
| Grey seal | AE | 92.6 | % | Herring | Ronald et al. (1984) * |
| Grey seal | AE | 92.8 | % | Mixed diet | Prime and Hammond (1987) * |
| Harbour seal | AE | 92.0 88.5 |
Herring pollock |
Trumble et al. (2003) * | |
| Harp seal | AE | 72.2 92.5–95 |
% | Shrimp herring |
Keiver et al. (1984) * |
| Harp seal | AE | 91.0 95.0 84.3 |
% | Capelin Herring Atlantic cod |
Lawson et al. (1997b) * |
| Harp seal | AE | 93–94 81–83 |
% | Capelin Invertebrates |
Mårtensson et al. (1994b) * |
| Ringed seal | AE | 97.0 | % | Herring | Parsons (1977) * |
| Ringed seal | AE | 92.0–95.0 72.2 |
% | Herring Shrimp |
Keiver et al. (1984) * |
| Ringed seal | AE | 83.2 86.6 88.3 93.8 82.1 |
% | Red fish Capelin Cod Herring Herring/shrimp |
Lawson et al. (1997a) * |
| Walrus | AE | 92.7 | % | Herring clam |
Fisher et al. (1992) * |
| Steller sea lion | AE | 95.4 93.9 90.4 93.4 |
% | Herring Pollock Squid Salmon |
Rosen and Trites (2000) * |
| Northern fur seal | AE | 96.0 96.9 96.3 95.9–96.7 |
% | Capelin Herring Pollock Mixed prey diets |
Diaz Gomez et al. (2016) * |
| Northern fur seal | AE | 90.0 | % | Fish | Fadely et al. (1990) * |
| Californian sea lion | AE | 88.0–91.0 83.0–91.0 |
% | Herring Pollock |
Fadely et al. (1994) † |
| West Indian manatee | AE | 80.0–88.8 | Water hyacinths, lettuce | Lomolino and Ewel (1984) * | |
| North Atlantic right whale | FE | 6 | % of IE | Copepods | Fortune et al. (2013) ** |
| Minke whale | FE | ~8 | % of IE | na | Folkow et al. (2000) ** |
| Grey whale | FE | 20 | % of IE | na | Greenwald (2005) ** |
| Sea otter | FE | 18 | % of IE | na | Costa (1982) * |
Symbols associated with references indicate the methodology used for the values listed: * from experiment, ** assumed or † − original reference not available. N.A. data not available
Differences in AE between fish species with different proximate composition are relatively minor compared to differences between fish and invertebrate prey. For example, several studies on captive harp seals (Phoca groenlandica) found that AE was higher when seals were fed fish (92.5–97.0%) compared with small crustaceans like krill (Family Euphausiidae) (81–83%; Mårtensson et al., 1994b) or shrimp (Family Caridea) (72.2%; Keiver et al., 1984). A study with crabeater seals (Lobodon carcinophagus) reported a similarly low AE (84.0%) for krill (Mårtensson et al., 1994a). No such difference in AE was reported with walruses (Odobenus rosmarus) that were fed herring (Clupea harengus) versus clams (Mercenaria mercenaria) (92.7%), even though the lipid content of herring was 23.5% higher than that of the clams (Fisher et al., 1992). AE was significantly higher in female (94.4%) than male (91%) walruses but was not correlated with age (Fisher et al., 1992).
The AE of baleen whales was first estimated at 80% (Lockyer, 1981), based on the assumption that this upper limit could not be exceeded because of the indigestible exoskeleton of chitinous prey, while also accounting for fish being in the diet of many baleen whales species. This estimate has been widely used in the literature (Kenney et al., 1986; Sigurjónsson and Víkingsson, 1997; Baumgartner et al., 2003; Laidre et al., 2007; Goldbogen et al., 2011; Wiedenmann et al., 2011; Braithwaite et al., 2015; Pirotta et al., 2018b; Guilpin et al., 2019; Pirotta et al., 2019; Guilpin et al., 2020; Pirotta et al., 2021). Digestive tracts and microbiomes of baleen whales have been studied for nearly 40 years (Herwig et al., 1984; Herwig and Staley, 1986; Tarpley et al., 1987; Mårtensson et al., 1994a; Olsen et al., 1994a; Olsen et al., 1994b; Haug et al., 1995; Mathiesen et al., 1995; Miller et al., 2020). More recently, it has been shown that baleen whales have specialized gut microbiome, such as chitinolytic bacteria, that allow them to digest chitin (e.g. the exoskeleton of euphausiids) and extract the nutrients therein (Olsen et al., 2000; Sanders et al., 2015), suggesting that 80% is an underestimate. AE may thus be closer to the 93% estimated for krill-eating minke whales (Mårtensson et al., 1994a) (estimated using dietary manganese as an inert marker).
UE loss
UE is the chemical energy lost as urea and other metabolic end products in the urine. UE is represented as a percentage of the ADE and is proportional to the nitrogen content of prey items (Keiver et al., 1984; Worthy, 1990; Rosen and Worthy, 2018). That is, it is proportional to the nitrogen absorbed in the gut and not the nitrogen ingested (i.e. discounting the fraction lost through the faeces). UE was first assumed to be ~ 8% of the digestible energy (DE) based on a review of values from terrestrial mammals (Lavigne et al. (1982). Literature on UE for marine mammals is limited to pinnipeds, for which few measurements exist from feeding experiments of captive individuals, that is, for harp seal, grey seal (Halichoerus grypus) and ringed seal (Phoca hispida) (Table 3). Feeding experiments vary in prey type (which differ in biochemical composition) and meal size. In one of the most thorough studies, Keiver et al. (1984) analysed urine samples for energy and nitrogen content, urea, creatinine and uric acid. They found that UE was strongly dependent on the apparent digestible nitrogen intake, allowing predictions of UE given measures of AE and/or prey proximate composition (e.g. Diaz Gomez et al., 2016)).
Table 3.
UE losses measured or used for marine mammal species, expressed as either a percentage of the IE or a percentage of the ADE, which accounts for FE losses.
| Species | UE loss | Unit | Reference |
|---|---|---|---|
| Sea otter | 10 | % of IE | Costa (1982) * |
| North Atlantic right whale | 8 | % of IE | Fortune et al. (2013) ** |
| Grey seal | 7.9 | % of ADE | Ronald et al. (1984) * |
| Ringed seals | 8.6 | % of ADE | Parsons (1977) † |
| Pinnipeds | 8 | % of ADE | Lavigne et al. (1982) ** |
| Grey whale | 10 | % of ADE | Greenwald (2005) ** |
| Harp seal | 6.5–9.5 | % of ADE | Keiver et al. (1984) * |
| Minke whale | 8 | % of ADE | Folkow et al. (2000) ** |
Symbols associated with references indicate the methodology used for the values listed: * from experiment, ** assumed or † − original reference not available
For cetaceans, information on the proportion of energy lost through urine is limited and no estimates of UE exist. In bioenergetic studies, UE has either been overlooked, taken from measurements and estimates from pinnipeds or terrestrial taxa (Lavigne et al., 1982), or estimated based on the nitrogen content of prey (Fortune et al., 2013) (Table 3).
Heat increment of feeding
The HIF, also referred to as specific dynamic action (SDA), is a postprandial obligatory metabolic mechanism. It represents the increase in metabolic rate resulting from the physical and biochemical processes of digestion (preabsorptive, absorptive and post-absorptive) (Brody, 1945). The physiological processes underpinning the HIF are numerous, complex and non-exhaustively described in McCue (2006). The HIF can account for a substantial portion of IE and should ideally be included as a separate parameter in bioenergetic models. Nevertheless, this is not always possible, as the costs of HIF are incorporated in metabolic rates estimated from doubly labelled water. Given the scarcity of values for this parameter for marine mammals, and cetaceans in particular, most studies have not explicitly taken these costs into account (Wiedenmann et al., 2011; Pirotta et al., 2018b; Pirotta et al., 2019; Guilpin et al., 2020). Indeed, digestion costs are oftentimes assumed to be included in estimates of field metabolic rate (Blix and Folkow, 1995; Nordøy et al., 1995; McHuron et al., 2020). The HIF has been explicitly accounted for in a small number of bioenergetic studies of large whales, based on estimates from the pinniped literature, for example, grey whale or North Atlantic right whale (Greenwald, 2005; Fortune et al., 2013).
The HIF depends on the size and composition of the meal (Hoch, 1971), and the age and nutritional state of the animal (Brody, 1945). The chemical composition of the meal affects total HIF, given that the digestion of carbohydrates, proteins or lipids increases metabolism differently in amplitude and duration (Blaxter, 1989). The cost of processing carbohydrates has been estimated to be 6% of the IE, 13% of IE for processing fat and up to 30% of IE when processing protein (Bartholomew, 1977). The duration of an increase in metabolism linked to the HIF has been empirically estimated to 5 h for carbohydrates, 9 h for lipid and 12 h for protein (Hoch, 1971; Worthy, 1990). Consistent with other vertebrates, both the total increase in metabolism and the duration of the effect in marine mammals have been shown to depend on diet composition and meal size (Rosen and Trites, 1997; Costa and Williams, 1999; Costa, 2009). Unfortunately, the HIF cannot be calculated directly from diet composition, as the mixed composition of food items results in a lower than predicted HIF (Hoch, 1971).
Although accurate estimates are needed for bioenergetic models, measurements of the amplitude and duration of HIF are only possible for captive animals, for which fasting, meal size and composition can be controlled and monitored. The HIF is empirically measured by quantifying the increase in metabolism (measured as rate of oxygen consumption) over several hours following a meal of known size and composition. The HIF has been measured in few species: sea otter, harp seal, harbour seal, ringed seals, northern elephant seals (Mirounga angustirostris), Steller sea lions and northern fur seals (Table 4).
Table 4.
Summary of measured HIF values (% IE and ± SD, when available) and associated characteristics in marine mammal species, specifically pinnipeds and mustelids.
| Species | HIF (% IE) | Duration of HIF | Prey | Reference |
|---|---|---|---|---|
| Sea otter | 13.2 ± 1.4SD | 4-5 h | Squid | Costa and Kooyman (1984) * |
| 10 | 4-5 h | Clam | ||
| Steller sea lion | 9.9 ± 0.9 (small meal) 12.4 ± 0.9 (large meal) |
6-8 h (small meal) 8-10 h (large meal) |
Herring + other unspecified fish species | Rosen and Trites (1997) * |
| Harp seal | 17 | 5-6 h | Herring | Gallivan and Ronald (1981) * |
| Harbour seal | 14.9 | Max 12 h | Herring | Markussen et al. (1994) * |
| 4.7 5.7 |
10 h | Herring Pollock |
Ashwell-Erickson (1981) * | |
| Ringed seals | 27–35% increase in metabolism over RMR | 12-13 h and peak after 4-6 h | N.A. | Parsons (1977) * |
| Northern fur seals | 4.3 ± 1.0 6.5 ± 3.8 12.4 ± 2.0 7.1 ± 2.3 7.9 ± 3.0 6.0 ± 1.5 6.9 ± 2.0 5.2 ± 1.1 |
N.A. | Pacific herring walleye pollock capelin herring + pollock herring + capelin herring + magister armhook squid pollock + capelin herring + pollock + capelin |
Diaz Gomez et al. (2016) * |
| South American fur seals | 61% increase in metabolism over RMR | N.A. | white croaker + striped weakfish + Brazilian menhaden | Dassis et al. (2014) * |
| Northern elephant seal | 9.1–11.4 11.5–13.0 |
N.A. | herring capelin |
Barbour (1993) * |
Symbols associated with references indicate the methodology used to estimate the values listed: * from experiment. N.A. data non available
Although generally considered a waste product in most bioenergetic models, there are cases where the HIF can be repurposed (Rosen et al., 2007). For endothermic animals, the heat produced during digestion could be used to offset costs associated with thermoregulation (Costa and Kooyman, 1984), in a process termed thermal substitution. This hypothesis is difficult to verify and quantify as its effect would likely depend upon multiple factors, including the temperature of the environment and the nutritional state and body condition of the individual. Thermal substitution with HIF has been demonstrated in sea otters (Costa and Kooyman, 1984). However, it should be noted that this species is very different from other marine mammals, such that they rely on fur for thermoregulation while inhabiting cold environments. In contrast, thermal substitution with HIF did not occur in the much larger Steller sea lion (Rosen and Trites, 2003), another species which inhabits cold environments.
Discussion/Conclusions
The focus in bioenergetic modelling is often on the energetic costs an animal incurs, but the robust estimation of an individual’s energy intake is equally critical for producing meaningful predictions. We have reviewed the components and processes that affect energy intake from ingested gross energy to biologically useful units of NE. Processes that determine energy intake can be conceptually separated into sets (Figure 1) with some parameters contributing to the estimation of IE and other parameters associated with digestive processes.
The study of marine mammal foraging effort has tremendously benefitted from the ever-advancing field of biologging. Biologging devices that allow the measurement of foraging effort at different temporal and spatial scales exist or are being developed (Williams and Ponganis, 2021). Furthermore, advances in tag technology and analytical methods make it possible to monitor energy intake and health metrics like body condition regularly and across a large number of individuals (Arce et al., 2019; Hooker et al., 2019; Aoki et al., 2021; Siegal et al., 2022). Limitations would then be more associated with the costs of such studies and the logistical challenges of deploying biologgers. Regarding prey, our review showed that valuable data on prey distribution, behaviour, biomass, density, energy content and composition exist in the literature. This is an area that is being advanced with novel prey monitoring techniques, for example, autonomous underwater vehicles with autonomous echosounder systems or environmental DNA (eDNA) (Southall et al., 2019; Benoit-Bird et al., 2020; Urmy and Benoit-Bird, 2021; Visser et al., 2021). Prey-capture success rates remain relatively uncertain, but estimates for pinnipeds, odontocetes and mysticetes are increasingly available from biologgers (Kuhn and Costa, 2006; Wisniewska et al., 2016; Cade et al., 2020).
Once energy is ingested, it goes through the digestive process and associated energy losses, resulting in NE available to the individual for maintenance and production. The energy losses associated with faecal matter, urine production and the HIF are difficult to measure in free-ranging marine mammals, especially cetaceans. Most of the parameters used in cetaceans bioenergetic studies (Greenwald, 2005; Wiedenmann et al., 2011; Fortune et al., 2013; Villegas-Amtmann et al. 2015; Pirotta et al., 2018a) are either modelled or scaled from terrestrial mammal species or empirically measured from pinnipeds and mustelids. While adapting pinniped estimates to cetaceans could provide a first step, some parameters cannot be applied across all taxonomic groups. For instance, estimating UE loss based on known estimates from pinnipeds, adjusted based on the biochemical composition of cetacean prey, can provide an interim solution (Fortune et al., 2013). In contrast, applying an estimate of HIF from mustelids to cetaceans might not be appropriate considering their highly different physiology. The accurate estimation of these parameters represents the largest knowledge gap when quantifying energy intake, for cetaceans in particular.
With the ultimate goal of improving bioenergetic modelling, this review highlights the current empirical information on important parameters, which can be utilized in the latest modelling approaches (Pirotta, 2022, this Special Issue) to collectively drive this research topic ahead and improve conservation efforts for impacted species and populations. Sensitivity analyses of available models, which now span the reproductive strategies of most marine mammals, would be very useful to help identify uncertain and impactful parameters and guide research effort.
Animals achieving energetic balance are a key to their reproduction and survival (Costa and Williams, 1999; Parsons, 2005). As climate change is affecting terrestrial and marine ecosystems, understanding how the energetic landscape is being impacted (e.g. via changes in prey composition, size distribution or energetic content), how different species are responding and robustly projecting how this will propagate in the future (e.g. Gallagher et al. 2021b) remains critical to inform conservation and management.
Funding
This work was primarily funded under an award from Office of Naval Research: N000142012392, and with support from the Marine Mammal Commission project: “A priority setting exercise to identify key unanswered questions in marine mammal bioenergetics”. Funding from the Joint Nature Conservation Committee supported fish energy analyses - award C18-0241-1285.
Data Availability
All data are incorporated into the article and its online supplementary material.
Supplementary Material
Acknowledgements
We are grateful to Shawn Noren, Stephanie Adamczak and Anna Stevens for feedback and contributions that improved this manuscript.
Contributor Information
Cormac G Booth, SMRU Consulting, Scottish Oceans Institute, University of St Andrews, East Sands, St Andrews, KY16 8LB, UK.
Marie Guilpin, Department of Coastal Systems, NIOZ Royal Netherlands Institute for Sea Research, 1790 AB, Den Burg, Texel, the Netherlands; Department of Freshwater and Marine Ecology, IBED, University of Amsterdam,1090 GE, Amsterdam, the Netherlands.
Aimee-Kate Darias-O’Hara, SMRU Consulting, Scottish Oceans Institute, University of St Andrews, East Sands, St Andrews, KY16 8LB, UK.
Janneke M Ransijn, Sea Mammal Research Unit, Scottish Oceans Institute, East Sands, University of St. Andrews, St. Andrews, KY16 8LB, UK.
Megan Ryder, SMRU Consulting, Scottish Oceans Institute, University of St Andrews, East Sands, St Andrews, KY16 8LB, UK.
Dave Rosen, Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver, BC V6T 1Z4, Canada.
Enrico Pirotta, Centre for Research into Ecological and Environmental Modelling, The Observatory, Buchanan Gardens, University of St. Andrews, St. Andrews, KY16 9LZ, UK.
Sophie Smout, Sea Mammal Research Unit, Scottish Oceans Institute, East Sands, University of St. Andrews, St. Andrews, KY16 8LB, UK.
Elizabeth A McHuron, Cooperative Institute for Climate, Ocean, and Ecosystem Studies, University of Washington, 3737 Brooklyn Ave NE, Seattle, WA, 98105, USA.
Jacob Nabe-Nielsen, Marine Mammal Research, Department of Ecoscience, Aarhus University, Aarhus, DK-4000 Roskilde, Denmark.
Daniel P Costa, Ecology and Evolutionary Biology Department, University of California Santa Cruz, 130 McAlister Way, Santa Cruz, CA, 95064, USA.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Aguilar Soto N, Johnson MP, Madsen PT, Díaz F, Domínguez I, Brito A, Tyack P (2008) Cheetahs of the deep sea: deep foraging sprints in short-finned pilot whales off Tenerife (Canary Islands). J Anim Ecol 77: 936–947. [DOI] [PubMed] [Google Scholar]
- Alcázar-Treviño J, Johnson M, Arranz P, Warren VE, Pérez-González CJ, Marques T, Madsen PT, Aguilar de Soto N (2021) Deep-diving beaked whales dive together but forage apart. Proc R Soc B 288: 20201905. 10.1098/rspb.2020.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F, Dinis A, CASCao I, Freitas L (2010) Bryde's whale (Balaenoptera brydei) stable associations and dive profiles: New insights into foraging behavior. Mar Mamm Sci 26: 202–212. 10.1111/j.1748-7692.2009.00333.x. [DOI] [Google Scholar]
- Andreasen H, Ross SD, Siebert U, Andersen NG, Ronnenberg K, Gilles A (2017) Diet composition and food consumption rate of harbor porpoises (Phocoena phocoena) in the western Baltic Sea. Mar Mamm Sci 33: 1053–1079. [Google Scholar]
- Aoki K, Isojunno S, Bellot C, Iwata T, Kershaw J, Akiyama Y, Martin Lopez LM, Ramp C, Biuw M, Swift Ret al. (2021) Aerial photogrammetry and tag-derived tissue density reveal patterns of lipid-store body condition of humpback whales on their feeding grounds. Proc Biol Sci 288: 20202307. 10.1098/rspb.2020.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14: 948–958. [DOI] [PubMed] [Google Scholar]
- Araújo MS, Guimarães PR, Svanbäck R, Pinheiro A, Guuimarães P, Dos Reis SF, Bolnick DI (2008) Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89: 1981–1993. [DOI] [PubMed] [Google Scholar]
- Arce F, Bestley S, Hindell MA, McMahon CR, Wotherspoon S (2019) A quantitative, hierarchical approach for detecting drift dives and tracking buoyancy changes in southern elephant seals. Sci Rep 9: 1–13. 10.1038/s41598-019-44970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnason T, Bjoernsson B, Steinarsson A (2009) Allometric growth and condition factor of Atlantic cod (Gadus morhua) fed to satiation: effects of temperature and body weight. J Appl Ichthyol 25: 401–406. 10.1111/j.1439-0426.2009.01259.x. [DOI] [Google Scholar]
- Arranz P, DeRuiter S, Stimpert A, Neves S, Friedlaender A, Goldbogen J, Visser F, Calambokidis J, Southall B, Tyack P (2016) Discrimination of fast click-series produced by tagged Risso's dolphins (Grampus griseus) for echolocation or communication. J Exp Biol 219: 2898–2907. 10.1242/jeb.144295. [DOI] [PubMed] [Google Scholar]
- Ashwell-Erickson SM (1981) The energy cost of free existence for Bering Sea harbor and spotted seals. PhD Thesis. University of Alaska Fairbanks. [Google Scholar]
- Astarloa A, Louzao M, Andrade J, Babey L, Berrow S, Boisseau O, Brereton T, Dorémus G, Evans PG, Hodgins NK (2021) The role of climate, oceanography, and prey in driving decadal Spatio-temporal patterns of a highly Mobile top predator. Front Mar Sci 8: 665474. 10.3389/fmars.2021.665474. [DOI] [Google Scholar]
- Balme G, Hunter L, Slotow R (2007) Feeding habitat selection by hunting leopards Panthera pardus in a woodland savanna: prey catchability versus abundance. Anim Behav 74: 589–598. 10.1016/j.anbehav.2006.12.014. [DOI] [Google Scholar]
- Barbour AS (1993) Heat increment of feeding in juvenile northern elephant seals. University of California, Santa Cruz [Google Scholar]
- Bartholomew GA (1977) Animal physiology: principles and adaptations. Macmillan, New York. [Google Scholar]
- Baudron AR, Brunel T, Blanchet MA, Hidalgo M, Chust G, Brown EJ, Kleisner KM, Millar C, MacKenzie BR, Nikolioudakis Net al. (2020) Changing fish distributions challenge the effective management of European fisheries. Ecography 43: 494–505. 10.1111/ecog.04864. [DOI] [Google Scholar]
- Baumgartner MF, Cole TV, Campbell RG, Teegarden GJ, Durbin EG (2003) Associations between North Atlantic right whales and their prey, Calanus finmarchicus, over diel and tidal time scales. Mar Ecol Prog Ser 264: 155–166. 10.3354/meps264155. [DOI] [Google Scholar]
- Bejder L, Samuels A, Whitehead H, Finn H, Allen S (2009) Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar Ecol Prog Ser 395: 177–185. 10.3354/meps07979. [DOI] [Google Scholar]
- Benoit-Bird KJ (2017) Linking deep-water prey fields with odontocete population structure and behavior. In 2017 Marine Mammal & Biology Program Review, Book of Abstracts 20–24 March 2017. Office of Naval Research, Arlington, VA. [Google Scholar]
- Benoit-Bird KJ, Southall BL, Moline MA, Claridge DE, Dunn CA, Dolan KA, Moretti DJ (2020) Critical threshold identified in the functional relationship between beaked whales and their prey. Mar Ecol Prog Ser 654: 1–16. 10.3354/meps13521. [DOI] [Google Scholar]
- Blaxter K (1989) Energy matabolism in animals and man. Cambridge University Press, Cambridge [Google Scholar]
- Blix A, Folkow L (1995) Daily energy expenditure in free living minke whales. Acta Physiol Scand 153: 61–66. 10.1111/j.1748-1716.1995.tb09834.x. [DOI] [PubMed] [Google Scholar]
- Bodley B, Mercer the late JR, Bryden MM (1999) Rate of passage of digesta through the alimentary tract of the New Zealand fur seal (Arctocephalus forsteri) and the Australian sea lion (Neophoca cinerea) (Carnivora : Otariidae) Aust J Zool 47: 193–198. [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161: 1–28. 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Booth C, Embling C, Gordon J, Calderan SV, Hammond P (2013) Habitat preferences and distribution of the harbour porpoise Phocoena phocoena west of Scotland. Mar Ecol Prog Ser 478: 273–285. 10.3354/meps10239. [DOI] [Google Scholar]
- Booth CG (2020) Food for thought: harbor porpoise foraging behavior and diet inform vulnerability to disturbance. Mar Mam Sci 36: 195–208. 10.1111/mms.12632. [DOI] [Google Scholar]
- Born E, Rysgaard S, Ehlmé G, Sejr M, Acquarone M, Levermann N (2003) Underwater observations of foraging free-living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biol 26: 348–357. 10.1007/s00300-003-0486-z. [DOI] [Google Scholar]
- Braithwaite JE, Meeuwig JJ, Hipsey MR (2015) Optimal migration energetics of humpback whales and the implications of disturbance. Conserv Physiol 3: cov001. 10.1093/conphys/cov001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander KM (2007) Global fish production and climate change. Proc Natl Acad Sci 104: 19709–19714. 10.1073/pnas.0702059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S (1945) Bioenergetics and Growth. Hafner Publishing Company Incorporated, New York, New York, USA. [Google Scholar]
- Brown JS, Laundré JW, Gurung M (1999) The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80: 385–399. 10.2307/1383287. [DOI] [Google Scholar]
- Brunetti N, Ivanovic M (1997) Description of Illex argentinus beaks and rostral length relationships with size and weight of squids. Revista de Investigación y Desarrollo Pesquero. 11. Mar del Plata: Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP).
- Burrows J, Johnston D, Straley J, Chenoweth E, Ware C, Curtice C, DeRuiter S, Friedlaender A (2016) Prey density and depth affect the fine-scale foraging behavior of humpback whales Megaptera novaeangliae in Sitka Sound, Alaska, USA. Mar Ecol Prog Ser 561: 245–260. 10.3354/meps11906. [DOI] [Google Scholar]
- Cade DE, Carey N, Domenici P, Potvin J, Goldbogen JA (2020) Predator-informed looming stimulus experiments reveal how large filter feeding whales capture highly maneuverable forage fish. Proc Natl Acad Sci 117: 472–478. 10.1073/pnas.1911099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calambokidis J, Flynn K, Dobson E, Huggins JL, Perez A (2018) Return of the Giants of the Salish Sea: Increased occurrence of humpback and gray whales in inland waters. Proceedings of Salish Sea Ecosystem Conference (2018: Seattle, Wash.).
- Caruso F, Hickmott L, Warren JD, Segre P, Chiang G, Bahamonde P, Español-Jiménez S, Li S, Bocconcelli A (2021) Diel differences in blue whale (Balaenoptera musculus) dive behavior increase nighttime risk of ship strikes in northern Chilean Patagonia. Integr Zool 16: 594–611. 10.1111/1749-4877.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimienti M, Desforges J-P, Beumer LT, Nabe-Nielsen J, Beest FM, Schmidt NM (2020) Energetics as common currency for integrating high resolution activity patterns into dynamic energy budget-individual based models. Ecol Model 434: 109250. 10.1016/j.ecolmodel.2020.109250. [DOI] [Google Scholar]
- Cole MR, Zeligs JA, Skrovan S, McDonald BI (2021) Head-mounted accelerometry accurately detects prey capture in California sea lions. Anim Biotelemetry 9: 44. 10.1186/s40317-021-00267-7. [DOI] [Google Scholar]
- Costa DP (1982) Energy, nitrogen, and electrolyte flux and sea water drinking in the sea otter Enhydra lutris. Physiol Zool 55: 35–44. 10.1086/physzool.55.1.30158441. [DOI] [Google Scholar]
- Costa, D. P. 2009. Energetics. Encyclopedia of Marine Mammals. (Second Edition). Elsevier, London, UK, pages 383–391 10.1016/B978-0-12-373553-9.00091-2. [DOI] [Google Scholar]
- Costa, D. P. 2012. A bioenergetics approach to developing a population consequences of acoustic disturbance model. The Effects of Noise on Aquatic Life. Part of the Advances in Experimental Medicine and Biology book series (AEMB, vol 730). Springer, pages 423–426. 10.1007/978-1-4419-7311-5_96. [DOI] [PubMed] [Google Scholar]
- Costa DP, Kooyman GL (1984) Contribution of specific dynamic action to heat balance and thermoregulation in the sea otter Enhydra lutris. Physiol Zool 57: 199–203. 10.1086/physzool.57.2.30163705. [DOI] [Google Scholar]
- Costa DP, Williams TM (1999) Marine mammal energetics. In Biology of Marine Mammals. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Croll DA, Acevedo-Gutiérrez A, Tershy BR, Urbán-Ramírez J (2001) The diving behavior of blue and fin whales: is dive duration shorter than expected based on oxygen stores? Comp Biochem Physiol A Mol Integr Physiol 129: 797–809. 10.1016/S1095-6433(01)00348-8. [DOI] [PubMed] [Google Scholar]
- Czapanskiy MF, Savoca MS, Gough WT, Segre PS, Wisniewska DM, Cade DE, Goldbogen JA (2021) Modelling short-term energetic costs of sonar disturbance to cetaceans using high-resolution foraging data. J Appl Ecol 58: 1643–1657. 10.1111/1365-2664.13903. [DOI] [Google Scholar]
- Dassis M, Rodriguez DH, Ieno E, Denuncio PE, Loureiro J, Davis R (2014) Resting metabolic rate and heat increment of feeding in juvenile South American fur seals (Arctocephalus australis). Comp Biochem Physiol A Mol Integr Physiol 168: 63–68. 10.1016/j.cbpa.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Dawe EG (1988) Length–weight relationships for short-finned squid in Newfoundland and the effect of diet on condition and growth. Trans Am Fish Soc 117: 591–599. . [DOI] [Google Scholar]
- Derous D, Sahu J, Douglas A, Lusseau D, Wenzel M (2021) Comparative genomics of cetartiodactyla: energy metabolism underpins the transition to an aquatic lifestyle. Conserv Physiol 9: coaa136. 10.1093/conphys/coaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Gomez M, Rosen DA, Forster IP, Trites AW (2020) Prey composition impacts lipid and protein digestibility in northern fur seals (Callorhinus ursinus). Can J Zool 98: 681–689. 10.1139/cjz-2020-0007. [DOI] [Google Scholar]
- Diaz Gomez M, Rosen DA, Trites AW (2016) Net energy gained by northern fur seals (Callorhinus ursinus) is impacted more by diet quality than by diet diversity. Can J Zool 94: 123–135. 10.1139/cjz-2015-0143. [DOI] [Google Scholar]
- Doniol-Valcroze T, Lesage V, Giard J, Michaud R (2011) Optimal foraging theory predicts diving and feeding strategies of the largest marine predator. Behav Ecol 22: 880–888. 10.1093/beheco/arr038. [DOI] [Google Scholar]
- Fadely BS, Worthy GAJ, Costa DP (1990) Assimilation efficiency of northern fur seals determined using dietary manganese. J Wildl Manag 54: 246–251. 10.2307/3809037. [DOI] [Google Scholar]
- Fadely B, Zeligs J, Costa D (1994) Assimilation efficiencies and maintenance requirements of California sea lions (Zalophus californianus) fed walleye pollock (Theragra chalcogramma) and herring (Clupea harengus). Final Report to the National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service 7600: 98115–90070. [Google Scholar]
- Fais A, Johnson M, Wilson M, Soto NA, Madsen P (2016) Sperm whale predator-prey interactions involve chasing and buzzing, but no acoustic stunning. Sci Rep 6: 1–13. 10.1038/srep28562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyrer LJ, Duffus DA (2015) Threshold foraging by gray whales in response to fine scale variations in mysid density. Mar Mamm Sci 31: 560–578. 10.1111/mms.12178. [DOI] [Google Scholar]
- Fisher K, Stewart R, Kastelein R, Campbell L (1992) Apparent digestive efficiency in walruses (Odobenus rosmarus) fed herring (Clupea harengus) and clams (Spisula sp.). Can J Zool 70: 30–36. 10.1139/z92-005. [DOI] [Google Scholar]
- Folkow LP, Haug T, Nilssen KT, Nordøy ES (2000) Estimated food consumption of minke whales Balaenoptera acutorostrata in Northeast Atlantic waters in 1992-1995. NAMMCO Sci Publ 2: 65–80. 10.7557/3.2972. [DOI] [Google Scholar]
- Fortune SM, Trites AW, Mayo CA, Rosen DA, Hamilton PK (2013) Energetic requirements of North Atlantic right whales and the implications for species recovery. Mar Ecol Prog Ser 478: 253–272. 10.3354/meps10000. [DOI] [Google Scholar]
- Friedlaender A, Goldbogen J, Nowacek D, Read A, Johnston D, Gales N (2014) Feeding rates and under-ice foraging strategies of the smallest lunge filter feeder, the Antarctic minke whale (Balaenoptera bonaerensis). J Exp Biol 217: 2851–2854. 10.1242/jeb.106682. [DOI] [PubMed] [Google Scholar]
- Friedlaender A, Tyson R, Stimpert A, Read A, Nowacek D (2013) Extreme diel variation in the feeding behavior of humpback whales along the western Antarctic peninsula during autumn. Mar Ecol Prog Ser 494: 281–289. 10.3354/meps10541. [DOI] [Google Scholar]
- Friedlaender AS, Halpin PN, Qian SS, Lawson GL, Wiebe PH, Thiele D, Read AJ (2006) Whale distribution in relation to prey abundance and oceanographic processes in shelf waters of the Western Antarctic Peninsula. Mar Ecol Prog Ser 317: 297–310. 10.3354/meps317297. [DOI] [Google Scholar]
- Friedlaender AS, Hazen E, Goldbogen J, Stimpert A, Calambokidis J, Southall B (2016) Prey-mediated behavioral responses of feeding blue whales in controlled sound exposure experiments. Ecol Appl 26: 1075–1085. 10.1002/15-0783. [DOI] [PubMed] [Google Scholar]
- Friedlaender AS, Hazen EL, Nowacek DP, Halpin PN, Ware C, Weinrich MT, Hurst TP, Wiley DN (2009) Diel changes in humpback whale Megaptera novaeangliae feeding behavior in response to sand lance Ammodytes spp. Mar Ecol Prog Ser 395: 91–100. 10.3354/meps08003. [DOI] [Google Scholar]
- Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22: 241–253. 10.1111/j.1439-0426.2006.00805.x. [DOI] [Google Scholar]
- Gallagher C, Grimm V, Kyhn L, Nabe-Nielsen J (2021) Movement and seasonal energetics mediate vulnerability to disturbance in marine mammal populations. Am Nat 197: 296–311. 10.1086/712798. [DOI] [PubMed] [Google Scholar]
- Gallagher CA, Chimienti M, Grimm V, Nabe-Nielsen J (2022) Energy-mediated responses to changing prey size and distribution in marine top predator movements and population dynamics. J Anim Ecol 91: 241–254. 10.1111/1365-2656.13627. [DOI] [PubMed] [Google Scholar]
- Gallivan G, Ronald K (1981) Apparent specific dynamic action in the harp seal (Phoca groenlandica). Comp Biochem Physiol A Physiol 69: 579–581. 10.1016/0300-9629(81)93024-3. [DOI] [Google Scholar]
- Gallon S, Bailleul F, Charrassin J-B, Guinet C, Bost C-A, Handrich Y, Hindell M (2013) Identifying foraging events in deep diving southern elephant seals, Mirounga leonina, using acceleration data loggers. Deep-Sea Res II Top Stud Oceanogr 88-89: 14–22. 10.1016/j.dsr2.2012.09.002. [DOI] [Google Scholar]
- Gill JA, Norris K, Sutherland WJ (2001) Why behavioural responses may not reflect the population consequences of human disturbance. Biol Conserv 97: 265–268. 10.1016/S0006-3207(00)00002-1. [DOI] [Google Scholar]
- Goldblatt A (1993) Behavioural needs of captive marine mammals. Aquat Mamm 19: 149–149. [Google Scholar]
- Goldbogen JA, Cade DE, Calambokidis J, Friedlaender AS, Potvin J, Segre PS, Werth AJ (2017) How baleen whales feed: the biomechanics of engulfment and filtration. Ann Rev Mar Sci 9: 367–386. 10.1146/annurev-marine-122414-033905. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Cade DE, Wisniewska DM, Potvin J, Segre PS, Savoca MS, Hazen EL, Czapanskiy M, Kahane-Rapport SR, DeRuiter SLet al. (2019) Why whales are big but not bigger: physiological drivers and ecological limits in the age of ocean giants. Science 366: 1367–1372. 10.1126/science.aax9044. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Calambokidis J, Croll DA, McKenna MF, Oleson E, Potvin J, Pyenson ND, Schorr G, Shadwick RE, Tershy BR (2012) Scaling of lunge-feeding performance in rorqual whales: mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct Ecol 26: 216–226. 10.1111/j.1365-2435.2011.01905.x. [DOI] [Google Scholar]
- Goldbogen JA, Calambokidis J, Oleson E, Potvin J, Pyenson ND, Schorr G, Shadwick RE (2011) Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density. J Exp Biol 214: 131–146. 10.1242/jeb.048157. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Calambokidis J, Shadwick RE, Oleson EM, McDonald MA, Hildebrand JA (2006) Kinematics of foraging dives and lunge-feeding in fin whales. J Exp Biol 209: 1231–1244. 10.1242/jeb.02135. [DOI] [PubMed] [Google Scholar]
- Goodman-Bacon A (2018) Difference-in-Differences With Variation in Treatment Timing. National Bureau of Economic Research, Cambridge, MA, pp. 161–170. [Google Scholar]
- Greenwald NLE (2005) A Theoretical Approach To Assessing Annual Energy Balance In Gray Whales (eschrichtius Robustus). Electronic Theses and Dissertations, 2004--2019. 329. https://stars.library.ucf.edu/etd/329.
- Guilpin M, Lesage V, McQuinn I, Brosset P, Doniol-Valcroze T, Jeanniard-du-Dot T, Winkler G (2020) Repeated vessel interactions and climate-or fishery-driven changes in prey density limit energy acquisition by foraging blue whales. Front Mar Sci 7: 626. 10.3389/fmars.2020.00626. [DOI] [Google Scholar]
- Guilpin M, Lesage V, McQuinn I, Goldbogen JA, Potvin J, Jeanniard-du-Dot T, Doniol-Valcroze T, Michaud R, Moisan M, Winkler G (2019) Foraging energetics and prey density requirements of western North Atlantic blue whales in the estuary and gulf of St. Lawrence, Canada. Mar Ecol Prog Ser 625: 205–223. 10.3354/meps13043. [DOI] [Google Scholar]
- Hanson MB, Emmons CK, Ford MJ, Everett M, Parsons K, Park LK, Hempelmann J, Van Doornik DM, Schorr GS, Jacobsen JK (2021) Endangered predators and endangered prey: seasonal diet of southern resident killer whales. PLoS ONE 16: e0247031. 10.1371/journal.pone.0247031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug T, Gjøsæter H, Lindstrøm U, Nilssen KT (1995) Diet and food availability for north-east Atlantic minke whales (Balaenoptera acutorostrata), during the summer of 1992. ICES Journal of Marine Science 52: 77–86. 10.1016/1054-3139(95)80017-4. [DOI] [Google Scholar]
- Havinga B (1933) Der seehund (Phoca vitulina L.) in den Hollandischen gewassern. Tijdschr Ned Dierkd Ver 3: 79–111. [Google Scholar]
- Hazen EL, Abrahms B, Brodie S, Carroll G, Jacox MG, Savoca MS, Scales KL, Sydeman WJ, Bograd SJ (2019) Marine top predators as climate and ecosystem sentinels. Front Ecol Environ 17: 565–574. 10.1002/fee.2125. [DOI] [Google Scholar]
- Heide-Jørgensen MP, Laidre KL, Nielsen NH, Hansen RG, Røstad. A (2013) Winter and spring diving behavior of bowhead whales relative to prey. Animal Biotelemetry 1: 15–14. 10.1186/2050-3385-1-15. [DOI] [Google Scholar]
- Herwig R, Staley J, Nerini M, Braham H (1984) Baleen whales: preliminary evidence for forestomach microbial fermentation. Appl Environ Microbiol 47: 421–423. 10.1128/aem.47.2.421-423.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig RP, Staley JT (1986) Anaerobic bacteria from the digestive tract of North Atlantic fin whales (Balaenoptera physalus). FEMS Microbiol Ecol 38: 361–371. 10.1111/j.1574-6968.1986.tb01749.x. [DOI] [Google Scholar]
- Hicks CC, Cohen PJ, Graham NA, Nash KL, Allison EH, D’Lima C, Mills DJ, Roscher M, Thilsted SH, Thorne-Lyman ALet al. (2019) Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574: 95–98. 10.1038/s41586-019-1592-6. [DOI] [PubMed] [Google Scholar]
- Hislop JRG, Harris MP, Smith JGM (1991) Variation in the calorific value and total energy content of the lesser sandeel (Ammodytes marinus) and other fish preyed on by seabirds. J Zool 224: 501–517. 10.1111/j.1469-7998.1991.tb06039.x. [DOI] [Google Scholar]
- Hoch FL (1971) Energy transformation in mammals: regulatory mechanisms. University of Chicago Press. [Google Scholar]
- Holt MM, Tennessen JB, Hanson MB, Emmons CK, Giles DA, Hogan JT, Ford MJ (2021) Vessels and their sounds reduce prey capture effort by endangered killer whales (Orcinus orca). Mar Environ Res 170: 105429. 10.1016/j.marenvres.2021.105429. [DOI] [PubMed] [Google Scholar]
- Hooker SK, De Soto NA, Baird RW, Carroll EL, Claridge D, Feyrer L, Miller PJO, Onoufriou A, Schorr G, Siegal Eet al. (2019) Future directions in research on beaked whales. Front Mar Sci 5: 105429. [Google Scholar]
- Hoop JM, Nousek-McGregor AE, Nowacek DP, Parks SE, Tyack P, Madsen PT (2019) Foraging rates of ram-filtering North Atlantic right whales. Funct Ecol 33: 1290–1306. 10.1111/1365-2435.13357. [DOI] [Google Scholar]
- Isojunno S, Curé C, Kvadsheim PH, Lam FPA, Tyack PL, Wensveen PJ, Miller PJOM (2016) Sperm whales reduce foraging effort during exposure to 1–2 kHz sonar and killer whale sounds. Ecol Appl 26: 77–93. 10.1890/15-0040. [DOI] [PubMed] [Google Scholar]
- Isojunno S, Sadykova D, DeRuiter S, Curé C, Visser F, Thomas L, Miller P, Harris C (2017) Individual, ecological, and anthropogenic influences on activity budgets of long-finned pilot whales. Ecosphere 8: e02044. [Google Scholar]
- Iwata T, Sakamoto KQ, Takahashi A, Edwards EW, Staniland IJ, Trathan PN, Naito Y (2012) Using a mandible accelerometer to study fine-scale foraging behavior of free-ranging Antarctic fur seals. Mar Mamm Sci 28: 345–357. 10.1111/j.1748-7692.2011.00482.x. [DOI] [Google Scholar]
- Jeanniard-du-Dot T, Trites AW, Arnould JP, Speakman JR, Guinet C (2017) Activity-specific metabolic rates for diving, transiting, and resting at sea can be estimated from time–activity budgets in free-ranging marine mammals. Ecol Evol 7: 2969–2976. 10.1002/ece3.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Morin PJ (2005) Predator diet breadth influences the relative importance of bottom-up and top-down control of prey biomass and diversity. Am Nat 165: 350–363. 10.1086/428300. [DOI] [PubMed] [Google Scholar]
- Johnson M, Madsen P, Zimmer W, De Soto NA, Tyack P (2006) Foraging Blainville's beaked whales (Mesoplodon densirostris) produce distinct click types matched to different phases of echolocation. J Exp Biol 209: 5038–5050. 10.1242/jeb.02596. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Tyack PL (2003) A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Ocean Eng 28: 3–12. 10.1109/JOE.2002.808212. [DOI] [Google Scholar]
- Kastelein RA, Ford J, Berghout E, Wiepkema PR, van Boxsel M (1994) Food consumption, growth and reproduction of Belugas (Delphinapterus leucas) in human care. Aquat Mamm 20: 81–97. [Google Scholar]
- Kastelein RA, Hardeman J, Boer H (1997) Food consumption and body weight of harbour porpoises (Phocoena phocoena). In A. J. Read (Ed.), The biology of the harbour porpoise (pp. 217-233). De Spil Publishers.
- Kastelein RA, Helder-Hoek L, Booth C, Jennings N, Leopold M (2019) High levels of food intake in harbor porpoises (Phocoena phocoena): insight into recovery from disturbance. Aquat Mamm 45: 380–388. 10.1578/AM.45.4.2019.380. [DOI] [Google Scholar]
- Kastelein RA, Klasen WJC, Postma J, Boer H, Wiepkema PR (2003a) Food consumption, growth and food passage times in Pacific Walrus. Int Zoo Yearb 38: 192–203. [Google Scholar]
- Kastelein RA, Mosterd J, Schooneman NM, Wiepkema PR (2000a) Food consumption, growth, body dimensions, and respiration rates of captive false killer whales (Pseudorca crassidens). Aquat Mamm 26: 33–34. [Google Scholar]
- Kastelein RA, Neurohr B, Nieuwstraten SH, Wiepkema PR, Wiepkema PR (1999) Food consumption and body measurements of Amazon river dolphins (Inia geoffrensis). Aquat Mamm 25: 173–182. [Google Scholar]
- Kastelein RA, Staal C, Wiepkema PR (2003b) Food consumption, food passage time, and body measurements of captive Atlantic bottlenose dolphins (Tursiops truncatus). Aquat Mamm 29: 53–66. [Google Scholar]
- Kastelein RA, van der Elst CA, Tennant HK, Wiepkema PR (2000b) Food consumption and growth of a female dusky dolphin (Lagenorhynchus obscurus).. Zoo Biol 19: 131–142. . [DOI] [Google Scholar]
- Keen KA, Beltran RS, Pirotta E, Costa DP (2021) Emerging themes in population consequences of disturbance models. Proc Biol Sci 288: 20210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiver KM, Ronald K, Beamish FWH (1984) Metabolizable energy requirements for maintenance and faecal and urinary losses of juvenile harp seals (Phoca groenlandica). Can J Zool 62: 769–776. 10.1139/z84-110. [DOI] [Google Scholar]
- Kenney RD, Hyman MAM, Owen RE, Scott GP, Winn HE (1986) Estimation of prey densities required by western North Atlantic right whales. Mar Mamm Sci 2: 1–13. [Google Scholar]
- Kienle SS, Friedlaender AS, Crocker DE, Mehta RS, Costa DP (2022) Trade-offs between foraging reward and mortality risk drive sex-specific foraging strategies in sexually dimorphic northern elephant seals. R Soc Open Sci 9: 210522. 10.1098/rsos.210522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M (1975) Metabolic turnover rate: a physiological meaning of the metabolic rate per unit body weight. J Theor Biol 53: 199–204. 10.1016/0022-5193(75)90110-1. [DOI] [PubMed] [Google Scholar]
- Kryukova NV, Kruchenkova EP, Ivanov DI (2012) Killer whales (Orcinus orca) hunting for walruses (Odobenus rosmarus divergens) near Retkyn spit, Chukotka. Biol Bull 39: 768–778. 10.1134/S106235901209004X. [DOI] [Google Scholar]
- Kuhn CE, Costa DP (2006) Identifying and quantifying prey consumption using stomach temperature change in pinnipeds. J Exp Biol 209: 4524–4532. 10.1242/jeb.02530. [DOI] [PubMed] [Google Scholar]
- Kuhnlein H, Chan H, Leggee D, Barthet V (2002) Macronutrient, mineral and fatty acid composition of Canadian Arctic traditional food. J Food Compos Anal 15: 545–566. 10.1016/S0889-1575(02)91066-5. [DOI] [Google Scholar]
- Laidre KL, Heide MP, Nielsen TG (2007) Role of the bowhead whale as a predator in West Greenland. Mar Ecol Prog Ser 346: 285–297. 10.3354/meps06995. [DOI] [Google Scholar]
- Lavigne D, Barchard W, Innes S, Øritsland N (1982) Pinniped bioenergetics. Mammals in the seas 4: 191–235. [Google Scholar]
- Lawson JW, Hare JA, Noseworthy E, Friel JK (1997a) Assimilation efficiency of captive ringed seals (Phoca hispida) fed different diets. Polar Biol 18: 107–111. 10.1007/s003000050164. [DOI] [Google Scholar]
- Lawson JW, Miller EH, Noseworthy E (1997b) Variation in assimilation efficiency and digestive efficiency of captive harp seals (Phoca groenlandica) on different diets. Can J Zool 75: 1285–1291. 10.1139/z97-152. [DOI] [Google Scholar]
- Lewis LA, Calambokidis J, Stimpert AK, Fahlbusch J, Friedlaender AS, McKenna MF, Mesnick SL, Oleson EM, Southall BL, Szesciorka ARet al. (2018) Context-dependent variability in blue whale acoustic behaviour. R Soc Open Sci 5: 180241. 10.1098/rsos.180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer C (1981) Growth and energy budgets of large baleen whales from the southern hemisphere. Food and Agriculture Organization 3: 379–489. [Google Scholar]
- Lomolino MW, Ewel KC (1984) Digestive Efficiencies of the West Indian Manatee (Trichechus manatus). Florida Sci 47: 176–179. [Google Scholar]
- MacArthur, R. H., and Pianka E. R.. 1966. On optimal use of a patchy environment. Am Nat 100: 603–609. 10.1086/282454. [DOI] [Google Scholar]
- Mackinson S, Blanchard JL, Pinnegar JK, Scott R (2003) Consequences of alternative functional response formulations in models exploring whale-fishery interactions. Mar Mamm Sci 19: 661–681. 10.1111/j.1748-7692.2003.tb01123.x. [DOI] [Google Scholar]
- Malcolm C, Duffus D (2000) Comparison of subjective and statistical methods of dive classification using data from a time-depth recorder attached to a gray whale (Eschrichtius robustus). J Cetacean Res Manag 2: 177–182. [Google Scholar]
- Malcolm CD, Duffus DA, Wischniowski SG (1996) Small scale behaviour of large scale subjects: diving behaviour of a gray whale (Eschrichtius robustus). Western Geography 6: 35–44. [Google Scholar]
- Malhi Y, Franklin J, Seddon N, Solan M, Turner MG, Field CB, Knowlton N (2020) Climate change and ecosystems: threats, opportunities and solutions. R. Soc. 375: 20190104. 10.1098/rstb.2019.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinka CE, Tønnesen P, Dunn CA, Claridge DE, Gridley T, Elwen SH, Teglberg Madsen P (2021) Echolocation click parameters and biosonar behaviour of the dwarf sperm whale (Kogia sima). J Exp Biol 224. 10.1242/jeb.240689. [DOI] [PubMed] [Google Scholar]
- Markussen NH (1993) Transit time of digesta in captive harbour seals (Phoca vitulina). Can J Zool 71: 1071–1073. 10.1139/z93-144. [DOI] [Google Scholar]
- Markussen NH, Ryg M, Øritsland NA (1994) The effect of feeding on the metabolic rate in harbour seals (Phoca vitulina). J Comp Physiol B 164: 89–93. 10.1007/BF003016. [DOI] [PubMed] [Google Scholar]
- Marshall GJ (1998) Crittercam: an animal-borne imaging and data logging system. Mar Technol Soc J 32: 11. [Google Scholar]
- Mårtensson PE, Nordøy E, Blix A (1994b) Digestibility of crustaceans and capelin in harp seals (Phoca groenlandica). Mar Mamm Sci 10: 325–331. 10.1111/j.1748-7692.1994.tb00486.x. [DOI] [Google Scholar]
- Mårtensson P-E, Nordøy E, Blix A (1994a) Digestibility of krill (Euphausia superba and Thysanoessa sp.) in minke whales (Balaenoptera acutorostrata) and crabeater seals (Lobodon carcinophagus). Br J Nutr 72: 713–716. 10.1079/BJN19940073. [DOI] [PubMed] [Google Scholar]
- Martín López LM, Aguilar de Soto N, Madsen PT, Johnson M (2022) Overall dynamic body acceleration measures activity differently on large versus small aquatic animals. Methods Ecol Evol 13: 447–458. 10.1111/2041-210X.13751. [DOI] [Google Scholar]
- Mathiesen, S., Aagnes T., Sørmo W., Nordøy E., Blix A., and Olsen M.. 1995. Digestive physiology of minke whales. Developments in Marine Biology 4: 351–359. 10.1016/S0163-6995(06)80036-3. [DOI] [Google Scholar]
- Matthiopoulos J, Fieberg JR, Aarts G (2020) Species-Habitat Associations: Spatial data, predictive models, and ecological insights. https://conservancy.umn.edu/handle/11299/217469. [Google Scholar]
- McClintock BT, Russell DJF, Matthiopoulos J, King R (2013) Combining individual animal movement and ancillary biotelemetry data to investigate population-level activity budgets. Ecology 94: 838–849. 10.1890/12-0954.1. [DOI] [Google Scholar]
- McConnachie S, Alexander G (2004) The effect of temperature on digestive and assimilation efficiency, gut passage time and appetite in an ambush foraging lizard, Cordylus melanotus melanotus. J Comp Physiol B 174: 99–105. 10.1007/s00360-003-0393-1. [DOI] [PubMed] [Google Scholar]
- McConnell BJ, Fedak MA, Lovell P, Hammond PS (1999) Movements and foraging areas of grey seals in the North Sea. J Appl Ecol 36: 573–590. 10.1046/j.1365-2664.1999.00429.x. [DOI] [Google Scholar]
- McCue M (2006) Specific dynamic action: a century of investigation. Comp Biochem Physiol A Mol Integr Physiol 144: 381–394. 10.1016/j.cbpa.2006.03.011. [DOI] [PubMed] [Google Scholar]
- McFarland D, L'Angellier A (1966) Disinhibition of drinking during satiation of feeding behaviour in the Barbary dove. Anim Behav 14: 463–467. 10.1016/S0003-3472(66)80046-5. [DOI] [PubMed] [Google Scholar]
- McHuron EA, Luxa K, Pelland NA, Holsman K, Ream R, Zeppelin T, Sterling JT (2020) Practical application of a bioenergetic model to inform management of a declining fur seal population and their commercially important prey. Front Mar Sci 7: 1027. 10.3389/fmars.2020.597973. [DOI] [Google Scholar]
- McHuron EA, Schwarz LK, Costa DP, Mangel M (2018) A state-dependent model for assessing the population consequences of disturbance on income-breeding mammals. Ecol Model 385: 133–144. 10.1016/j.ecolmodel.2018.07.016. [DOI] [Google Scholar]
- McIntyre T (2014) Trends in tagging of marine mammals: a review of marine mammal biologging studies. Afr J Mar Sci 36: 409–422. 10.2989/1814232X.2014.976655. [DOI] [Google Scholar]
- Mead, J. G. 2009. Gastrointestinal tract. Encyclopedia of marine mammals. Pages 472–477Elsevier, London, UK. 10.1016/B978-0-12-373553-9.00113-9. [DOI] [Google Scholar]
- Miller CA, Holm HC, Horstmann L, George JC, Fredricks HF, Van Mooy BA, Apprill A (2020) Coordinated transformation of the gut microbiome and lipidome of bowhead whales provides novel insights into digestion. ISME J 14: 688–701. 10.1038/s41396-019-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y (1998) Optimal choice of foraging depth in divers. J Zool 245: 279–283. 10.1111/j.1469-7998.1998.tb00102.x. [DOI] [Google Scholar]
- Nabe-Nielsen J, Beest F, Grimm V, Sibly R, Teilmann J, Thompson PM (2018) Predicting the impacts of anthropogenic disturbances on marine populations. Conserv Lett 11: e12563. 10.1111/conl.12563. [DOI] [Google Scholar]
- National Academies of Sciences Engineering and Medicine (2017) Approaches to Understanding the Cumulative Effects of Stressors on Marine Mammals. The National Academies Press, Washington, DC. [Google Scholar]
- Neer A, Jensen LF, Siebert U (2015) Grey seal (Halichoerus grypus) predation on harbour seals (Phoca vitulina) on the island of Helgoland, Germany. J Sea Res 97: 1–4. 10.1016/j.seares.2014.11.006. [DOI] [Google Scholar]
- Ngô MC, Selvan R, Tervo O, Heide-Jørgensen MP, Ditlevsen S (2021) Detection of foraging behavior from accelerometer data using U-net type convolutional networks. Eco Inform 62: 101275. 10.1016/j.ecoinf.2021.101275. [DOI] [Google Scholar]
- Nordøy, E., Folkow L., Mårtensson P., and Blix A.. 1995. Food requirements of Northeast Atlantic minke whales. Proceedings of the International on the biology of Marine Mammals in the North East Atlantic. Whales, Seals, Fish and Man. Blix AS, Walløe L., and Ulltang Ø. (eds.). Dev. Mar. Bio 4:307–317, 10.1016/S0163-6995(06)80032-6. [DOI] [Google Scholar]
- Nordøy ES, Sørmo W, Blix AS (1993) In vitro digestibility of different prey species of minke whales (Balaenoptera acutorostrata). Br J Nutr 70: 485–489. 10.1079/BJN19930142. [DOI] [PubMed] [Google Scholar]
- Nowacek DP, Friedlaender AS, Halpin PN, Hazen EL, Johnston DW, Read AJ, Espinasse B, Zhou M, Zhu YW (2011) Super-aggregations of krill and humpback whales in Wilhelmina Bay, Antarctic peninsula. PLoS ONE 6: e19173. 10.1371/journal.pone.0019173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek DP, Johnson MP, Tyack PL, Shorter KA, McLellan WA, D. AP (2001) Buoyant balaenids: the ups and downs of buoyancy in right whales. Proc Biol Sci 268: 1811–1816. 10.1098/rspb.2001.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øigård TA, Lindstrøm U, Haug T, Nilssen KT, Smout S (2013) Functional relationship between harp seal body condition and available prey in the Barents Sea. Mar Ecol Prog Ser 484: 287–301. 10.3354/meps10272. [DOI] [Google Scholar]
- Oleson EM, Calambokidis J, Burgess WC, McDonald MA, LeDuc CA, Hildebrand JA (2007) Behavioral context of call production by eastern North Pacific blue whales. Mar Ecol Prog Ser 330: 269–284. 10.3354/meps330269. [DOI] [Google Scholar]
- Olsen M, Blix A, Utsi T, Sørmo W, Mathiesen S (2000) Chitinolytic bacteria in the minke whale forestomach. Can J Microbiol 46: 85–94. 10.1139/cjm-46-1-85. [DOI] [PubMed] [Google Scholar]
- Olsen M, Nordøy E, Blix A, Mathiesen S (1994a) Functional anatomy of the gastrointestinal system of Northeastern Atlantic minke whales (Balaenoptera acutorostrata). J Zool 234: 55–74. 10.1111/j.1469-7998.1994.tb06056.x. [DOI] [Google Scholar]
- Olsen MA, Aagnes TH, Mathiesen SD (1994b) Digestion of herring by indigenous bacteria in the minke whale forestomach. Appl Environ Microbiol 60: 4445–4455. 10.1128/aem.60.12.4445-4455.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österblom H, Olsson O, Blenckner T, Furness RW (2008) Junk-food in marine ecosystems. Oikos 117: 967–977. 10.1111/j.0030-1299.2008.16501.x. [DOI] [Google Scholar]
- Owen K, Warren JD, Noad MJ, Donnelly D, Goldizen AW, Dunlop RA (2015) Effect of prey type on the fine-scale feeding behaviour of migrating east Australian humpback whales. Mar Ecol Prog Ser 541: 231–244. 10.3354/meps11551. [DOI] [Google Scholar]
- Panigada S, Zanardelli M, Canese S, Jahoda M (1999) How deep can baleen whales dive? Mar Ecol Prog Ser 187: 309–311. 10.3354/meps187309. [DOI] [Google Scholar]
- Parsons JL (1977) Metabolic studies in ringed seals (Phoca hispida). MSc Thesis, University of Guelph, Ontario. [Google Scholar]
- Parsons PA (2005) Environments and evolution: interactions between stress, resource inadequacy and energetic efficiency. Biol Rev 80: 589–610. 10.1017/S1464793105006822. [DOI] [PubMed] [Google Scholar]
- Pedersen J, Hislop J (2001) Seasonal variations in the energy density of fishes in the North Sea. J Fish Biol 59: 380–389. 10.1111/j.1095-8649.2001.tb00137.x. [DOI] [Google Scholar]
- Pendleton DE, Holmes EE, Redfern J, Zhang J (2020) Using modelled prey to predict the distribution of a highly mobile marine mammal. Divers Distrib 26: 1612–1626. 10.1111/ddi.13149. [DOI] [Google Scholar]
- Perez MA, McAlister WB, Mooney EE (1990) Estimated feeding rate relationship for marine mammals based on captive animal data.NOAA Technical Memorandum NMFS F/ NWC-184. US Dept of Commerce.
- Pierce GJ, Miller A, Thompson PM, Hislop J (1991) Prey remains in grey seal (Halichoerus grypus) faeces from the Moray Firth, north-east Scotland. J Zool 224: 337–341. 10.1111/j.1469-7998.1991.tb04813.x. [DOI] [Google Scholar]
- Pirotta E (2022) A review of bioenergetic modelling for marine mammal populations. Conserv Physiol 10: 1–16. 10.1093/conphys/coac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Booth CG, Cade DE, Calambokidis J, Costa DP, Fahlbusch JA, Friedlaender AS, Goldbogen JA, Harwood J, Hazen ELet al. (2021) Context-dependent variability in the predicted daily energetic costs of disturbance for blue whales. Conser Physiol 9. 10.1093/conphys/coaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Booth CG, Costa DP, Fleishman E, Kraus SD, Lusseau D, Moretti D, New LF, Schick RS, Schwarz LKet al. (2018a) Understanding the population consequences of disturbance. Ecol Evol 8: 9934–9946. 10.1002/ece3.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Mangel M, Costa DP, Goldbogen J, Harwood J, Hin V, Irvine LM, Mate BR, McHuron EA, Palacios DMet al. (2019) Anthropogenic disturbance in a changing environment: modelling lifetime reproductive success to predict the consequences of multiple stressors on a migratory population. Oikos 128: 1340–1357. 10.1111/oik.06146. [DOI] [Google Scholar]
- Pirotta E, Mangel M, Costa DP, Mate B, Goldbogen JA, Palacios DM, Hückstädt LA, McHuron EA, Schwarz L, New L (2018b) A dynamic state model of migratory behavior and physiology to assess the consequences of environmental variation and anthropogenic disturbance on marine vertebrates. Am Nat 191: E40–E000, E56. 10.1086/695135. [DOI] [PubMed] [Google Scholar]
- Pirotta E, Thomas L, Costa DP, Hall AJ, Harris CM, Harwood J, Kraus SD, Miller PJ, Moore M, Photopoulou Tet al. (2022) Understanding the combined effects of multiple stressors: a new perspective on a longstanding challenge. Sci Total Environ 821: 153322. 10.1016/j.scitotenv.2022.153322. [DOI] [PubMed] [Google Scholar]
- Pistorius PA, Meyer MA, Reisinger RR, Kirkman SP (2012) Killer whale predation on subantarctic fur seals at Prince Edward Island, southern Indian Ocean. Polar Biology 35: 1767–1772. 10.1007/s00300-012-1216-1. [DOI] [Google Scholar]
- Potvin J, Goldbogen JA, Shadwick RE (2012) Metabolic expenditures of lunge feeding Rorquals across scale: implications for the evolution of filter feeding and the limits to maximum body size. PLoS ONE 7. 10.1371/journal.pone.0044854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime JH, Hammond PS (1987) Quantitative assessment of gray seal diet from fecal analysis. In AC Huntley, DP Costa, GAJ Worthy, MA Castellini, eds, Approaches to marine mammal energetics. Special Publication Number 1. The Society for Marine Mammalogy, pp. 165–181. [Google Scholar]
- Ransijn JM, Hammond PS, Leopold MF, Sveegaard S, Smout SC (2021) Integrating disparate datasets to model the functional response of a marine predator: a case study of harbour porpoises in the southern North Sea. Ecol Evol 11: 17458–17470. 10.1002/ece3.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T, Hogenson MJ (1962) Food satiation in the pigeon. J Exp Anal Behav 5: 239–245. 10.1901/jeab.1962.5-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald K, Keiver KM, Beamish FWH, Frank R (1984) Energy requirements for maintenance and faecal and urinary losses of the grey seal (Halichoerus grypus). Can J Zool 62: 1101–1105. 10.1139/z84-160. [DOI] [Google Scholar]
- Rosen D, Young B, Trites A (2012) Rates of maximum food intake in young northern fur seals (Callorhinus ursinus) and the seasonal effects of food intake on body growth. Can J Zool 90: 61–69. 10.1139/z11-112. [DOI] [Google Scholar]
- Rosen DA, Trites A (2000) Digestive efficiency and dry-matter digestibility in Steller Sea lions fed herring, pollock, squid, and salmon. Can J Zool 78: 234–239. 10.1139/z99-201. [DOI] [Google Scholar]
- Rosen DA, Trites AW (2003) No evidence for bioenergetic interaction between digestion and thermoregulation in Steller sea lions Eumetopias jubatus. Physiol Biochem Zool 76: 899–906. 10.1086/378140. [DOI] [PubMed] [Google Scholar]
- Rosen DA, Williams L, Trites AW (2000) Effect of ration size and meal frequency on assimilation and digestive efficiency in yearling Stellar Sea lions, Eumetopias jubatus. Aquatic Mammals 26: 76–82. [Google Scholar]
- Rosen DA, Winship AJ, Hoopes LA (2007) Thermal and digestive constraints to foraging behaviour in marine mammals. Philos Trans R Soc Biol Sci 362: 2151–2168. 10.1098/rstb.2007.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DA, Worthy GA (2018) Nutrition and energetics. In CRC Handbook of marine Mammal Medicine. CRC Press, Boca Raton, Florida, USA, pp. 695–738. [Google Scholar]
- Rosen DAS, Trites AW (1997) Heat increment of feeding in Steller sea lions, Eumetopias jubatus. Comp Biochem Physiol A Physiol 118: 877–881. 10.1016/S0300-9629(97)00039-X. [DOI] [PubMed] [Google Scholar]
- Rosen DAS, Trites AW (2004) Satiation and compensation for short-term changes in food quality and availability in young Steller sea lions (Eumetopias jubatus). Can J Zool 82: 1061–1069. 10.1139/z04-082. [DOI] [Google Scholar]
- Russell DJF (2015) Activity classification using state space modelling. In Sea Mammal Research Unit. University of St Andrews, Report to Scottish Government, no. MR 5.2, St Andrews, Scotland. [Google Scholar]
- Sala A (2018) Influence of tow duration on catch performance of trawl survey in the Mediterranean Sea. PLoS ONE 13: e0191662. 10.1371/journal.pone.0191662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JG, Beichman AC, Roman J, Scott JJ, Emerson D, McCarthy JJ, Girguis PR (2015) Baleen whales host a unique gut microbiome with similarities to both carnivores and herbivores. Nat Commun 6: 1–8. 10.1038/ncomms9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Clarke M, Pierce GJ (2001) Assessing the importance of cephalopods in the diets of marine mammals and other top predators: problems and solutions. Fish Res 52: 121–139. 10.1016/S0165-7836(01)00236-3. [DOI] [Google Scholar]
- Savoca MS, Czapanskiy MF, Kahane-Rapport SR, Gough WT, Fahlbusch JA, Bierlich K, Segre PS, Di Clemente J, Penry GS, Wiley DN (2021) Baleen whale prey consumption based on high-resolution foraging measurements. Nature 599: 85–90. 10.1038/s41586-021-03991-5. [DOI] [PubMed] [Google Scholar]
- Schneider BH, Flatt WP (1975) The evaluation of feeds through digestibility experiments. University of Georgia Press, Athens, GA, USA. [Google Scholar]
- Schneider JE (2004) Energy balance and reproduction. Physiol Behav 81: 289–317. 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Schorr, G. S., Falcone E. A., and Rone B.. 2017. Movements and diving behavior of beaked whales in Monterey Bay, CA: A comparative study site in the California Current Ecosystem. 2017 Marine Mammal & Biology Program Review, Book of Abstracts 20-24 March 2017.
- Siegal E (2020) The foraging behaviour and body condition of northern bottlenose whales (Hyperoodon ampullatus). PhD Thesis. University of St Andrews. [Google Scholar]
- Siegal E, Hooker SK, Isojunno S, Miller PJO (2022) Beaked whales and state-dependent decision-making: how does body condition affect the trade-off between foraging and predator avoidance? Proc R Soc B 289. 10.1098/rspb.2021.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurjónsson J, Víkingsson GA (1997) Seasonal abundance of and estimated food consumption by cetaceans in Icelandic and adjacent waters. J Northwest Atl Fish Sci 22: 271–287. 10.2960/J.v22.a20. [DOI] [Google Scholar]
- Simon M, Johnson M, Tyack P, Madsen PT (2009) Behaviour and kinematics of continuous ram filtration in bowhead whales (Balaena mysticetus). Proc R Soc Biol Sci 276: 3819–3828. 10.1098/rspb.2009.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skern-Mauritzen M, Johannesen E, Bjorge A, Oien N (2011) Baleen whale distributions and prey associations in the Barents Sea. Mar Ecol Prog Ser 426: 289–301. 10.3354/meps09027. [DOI] [Google Scholar]
- Smout S, Lindstrøm U (2007) Multispecies functional response of the minke whale Balaenoptera acutorostrata based on small-scale foraging studies. Mar Ecol Prog Ser 341: 277–291. 10.3354/meps341277. [DOI] [Google Scholar]
- Smout S, Rindorf A, Hammond PS, Harwood J, Matthiopoulos J (2014) Modelling prey consumption and switching by UK grey seals. ICES J Mar Sci 71: 81–89. 10.1093/icesjms/fst109. [DOI] [Google Scholar]
- Southall BL, Benoit-Bird KJ, Moline MA, Moretti D (2019) Quantifying deep-sea predator-prey dynamics: implications of biological heterogeneity for beaked whale conservation. J Appl Ecol 56: 1040–1049. 10.1111/1365-2664.13334. [DOI] [Google Scholar]
- Stäbler M, Kempf A, Smout S, Temming A (2019) Sensitivity of multispecies maximum sustainable yields to trends in the top (marine mammals) and bottom (primary production) compartments of the southern North Sea food-web. PLoS ONE 14: e0210882. 10.1371/journal.pone.0210882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpert A, DeRuiter SL, Southall B, Moretti D, Falcone E, Goldbogen J, Friedlaender A, Schorr G, Calambokidis J (2014) Acoustic and foraging behavior of a Baird's beaked whale, Berardius bairdii, exposed to simulated sonar. Sci Rep 4: 7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs R, Tolkamp B (2006) Control of energy balance in relation to energy intake and energy expenditure in animals and man: an ecological perspective. Br J Nutr 95: 657–676. 10.1079/BJN20041361. [DOI] [PubMed] [Google Scholar]
- Tarpley RJ, Sis RF, Albert TF, Dalton LM, George JC (1987) Observations on the anatomy of the stomach and duodenum of the bowhead whale, Balaena mysticetus. American journal of anatomy 180: 295–322. 10.1002/aja.1001800310. [DOI] [PubMed] [Google Scholar]
- Taylor C, Koyuk H, Coyle J, Waggoner R, Newman K (2007) An Agent-Based Model of Predator-Prey Relationships Between Transient Killer Whales and Other Marine Mammals. Final report to the Marine Mammal Commission, Bethesda, Maryland, USA. [Google Scholar]
- Thompson D, Fedak M (2001) How long should a dive last? A simple model of foraging decisions by breath-hold divers in a patchy environment. Anim Behav 61: 287–296. 10.1006/anbe.2000.1539. [DOI] [Google Scholar]
- Tollit D, Steward M, Thompson P, Pierce GJ, Santos M, Hughes S (1997) Species and size differences in the digestion of otoliths and beaks: implications for estimates of pinniped diet composition. Can J Fish Aquat Sci 54: 105–119. 10.1139/f96-264. [DOI] [Google Scholar]
- Tomilin A, Heptner V (1967) Cetacea. In vol. 9 Mammals of the USSR and adjacent countries. Transl. from Russian by Israel Program for Sei. Transl, Jérusalem. [Google Scholar]
- Tønnesen P, Oliveira C, Johnson M, Madsen PT (2020) The long-range echo scene of the sperm whale biosonar. Biol Lett 16: 20200134. 10.1098/rsbl.2020.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trites A, Donnelly C (2003) The decline of Steller sea lions Eumetopias jubatus in Alaska: a review of the nutritional stress hypothesis. Mamm Rev 33: 3–28. 10.1046/j.1365-2907.2003.00009.x. [DOI] [Google Scholar]
- Trites, A. W., and Spitz J.. 2018. Diet. Encyclopedia of marine mammals. Pages 255–259. Elsevier, London, UK, 10.1016/B978-0-12-804327-1.00105-9. [DOI] [Google Scholar]
- Trumble S, Barboza P, Castellini M (2003) Digestive constraints on an aquatic carnivore: effects of feeding frequency and prey composition on harbor seals. J Comp Physiol B 173: 501–509. 10.1007/s00360-003-0358-4. [DOI] [PubMed] [Google Scholar]
- Urmy SS, Benoit-Bird KJ (2021) Fear dynamically structures the ocean’s pelagic zone. Curr Biol 31: 5086–5092.e3. 10.1016/j.cub.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Vance H, Hooker S, Mikkelsen L, Neer A, Teilmann J, Siebert U, Johnson M (2021) Drivers and constraints on offshore foraging in harbour seals. Sci Rep 11: 1–14. 10.1038/s41598-021-85376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Víkingsson GA (1997) Feeding of fin whales (Balaenoptera physalus) off Iceland-diurnal and seasonal variation and possible rates. J Northwest Atl Fish Sci 22: 77–89. 10.2960/J.v22.a7. [DOI] [Google Scholar]
- Villegas-Amtmann S, Schwarz LK, Sumich JL, Costa DP (2015) A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere 6: 1–19. [Google Scholar]
- Visser F, Merten V, Bayer T, Oudejans M, Jonge D, Puebla O, Reusch TB, Fuss J, Hoving H (2021) Deep-sea predator niche segregation revealed by combined cetacean biologging and eDNA analysis of cephalopod prey. Sci Adv 7: eabf5908. 10.1126/sciadv.abf5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser F, Oudejans MG, Keller OA, Madsen PT, Johnson M (2022) Sowerby's beaked whale biosonar and movement strategy indicate deep-sea foraging niche differentiation in mesoplodont whales. J Exp Biol 225: jeb243728. 10.1242/jeb.243728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviant M, Trites AW, Rosen DA, Monestiez P, Guinet C (2010) Prey capture attempts can be detected in Steller Sea lions and other marine predators using accelerometers. Polar Biol 33: 713–719. 10.1007/s00300-009-0750-y. [DOI] [Google Scholar]
- Volpov BL, Hoskins AJ, Battaile BC, Viviant M, Wheatley KE, Marshall G, Abernathy K, Arnould JP (2015) Identification of prey captures in Australian fur seals (Arctocephalus pusillus doriferus) using head-mounted accelerometers: field validation with animal-borne video cameras. PLoS ONE 10: e0128789. 10.1371/journal.pone.0128789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth AJ (2004) Models of hydrodynamic flow in the bowhead whale filter feeding apparatus. J Exp Biol 207: 3569–3580. 10.1242/jeb.01202. [DOI] [PubMed] [Google Scholar]
- West KL, Walker WA, Baird RW, Mead JG, Collins PW (2017) Diet of Cuvier’s beaked whales Ziphius cavirostris from the North Pacific and a comparison with their diet world-wide. Mar Ecol Prog Ser 574: 227–242. 10.3354/meps12214. [DOI] [Google Scholar]
- Wiedenmann J, Cresswell KA, Goldbogen J, Potvin J, Mangel M (2011) Exploring the effects of reductions in krill biomass in the Southern Ocean on blue whales using a state-dependent foraging model. Ecol Model 222: 3366–3379. 10.1016/j.ecolmodel.2011.07.013. [DOI] [Google Scholar]
- Williams CL, Ponganis PJ (2021) Diving physiology of marine mammals and birds: the development of biologging techniques. Philos Trans R Soc B 376: 20200211. 10.1098/rstb.2020.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Haun J, Davis R, Fuiman L, Kohin S (2001) A killer appetite: metabolic consequences of carnivory in marine mammals. Comp Biochem Physiol A Mol Integr Physiol 129: 785–796. 10.1016/S1095-6433(01)00347-6. [DOI] [PubMed] [Google Scholar]
- Williams, T. M., and Yeates L.. 2004. The energetics of foraging in large mammals: a comparison of marine and terrestrial predators. International Congress Series 1275: 351–358. 10.1016/j.ics.2004.08.069. [DOI] [Google Scholar]
- Wilson L, Hammond P (2019) The diet of harbour and grey seals around Britain: examining the role of prey as a potential cause of harbour seal declines. Aquat Conserv 29: 71–85. 10.1002/aqc.3131. [DOI] [Google Scholar]
- Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ (2006) Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J Anim Ecol 75: 1081–1090. 10.1111/j.1365-2656.2006.01127.x. [DOI] [PubMed] [Google Scholar]
- Wisniewska DM, Johnson M, Nachtigall PE, Madsen PT (2014) Buzzing during biosonar-based interception of prey in the delphinids Tursiops truncatus and Pseudorca crassidens. J Exp Biol 217: 4279–4282. 10.1242/jeb.113415. [DOI] [PubMed] [Google Scholar]
- Wisniewska DM, Johnson M, Teilmann J, Rojano-Doñate L, Shearer J, Sveegaard S, Miller LA, Siebert U, Madsen PT (2016) Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr Biol 26: 1441–1446. 10.1016/j.cub.2016.03.069. [DOI] [PubMed] [Google Scholar]
- Wisniewska DM, Johnson M, Teilmann J, Siebert U, Galatius A, Dietz R, Madsen PT (2018) High rates of vessel noise disrupt foraging in wild harbour porpoises (Phocoena phocoena). Proceedings of the Royal Society B: Biological Sciences 285: 20172314. 10.1098/rspb.2017.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthy G (1990) Nutritional energetics of marine mammals. Handbook of Marine Mammal Medicine: Health Disease and Rehabilitation 504–508. [Google Scholar]
- Ydesen KS, Wisniewska DM, Hansen JD, Beedholm K, Johnson M, Madsen PT (2014) What a jerk: prey engulfment revealed by high-rate, super-cranial accelerometry on a harbour seal (Phoca vitulina). J Exp Biol 217: 2239–2243. 10.1242/jeb.100016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.


