Abstract
There are few data regarding adult protracted bacterial bronchitis (PBB). This study aimed to delineate the clinical features of PBB and evaluate their potential diagnostic value in adults. We recruited 55 adult patients with PBB and selected randomly 220 patients with non-PBB as control. A diagnosis of PBB was considered if patients had a cough lasting ≥3 weeks, no abnormalities of chest computed tomography, positive bacterial culture in sputum and/or response well to oral moxifloxacin for 1–4 weeks. The clinical manifestations and laboratory investigations were compared between PBB patients and non-PBB patients. Of the 55 patients with PBB, approximately three-fifths (34, 61.8%) were females with a median age of 46.0 years, which were similar to that of patients with non-PBB. We observed a shorter cough duration in PBB than non-PBB (median 3.0 versus 24.0 months, p < 0.001). Compared to non-PBB patients, PBB patients had higher incidences of productive cough, yellow phlegm and a sensation of mucus in the throat (SMIT) (all p < 0.001). Sputum neutrophils and lymphocytes were markedly elevated in PBB patients than non-PBB patients (both p = 0.004). Bacterial pathogens were detected in eight (28.6%) of 28 cases with PBB. The multivariate analyses showed yellow phlegm, productive cough, SMIT, increased sputum lymphocytes (≥2.3%) and cough duration ≤8.5 months with moderate sensitivity (50.9–81.8%) and moderate-high specificity (60.5–94.4%) for determining PBB. In summary, adults with PBB are characterized by productive cough, yellow phlegm, SMIT and neutrophilic airway inflammation. These cough features and increased sputum lymphocytes may be useful to indicate PBB.
Keywords: Protracted bacterial bronchitis, Adult, Clinical characteristics, Predicted value
Protracted bacterial bronchitisAdultClinical characteristicsPredicted value.
1. Introduction
Protracted bacterial bronchitis (PBB) is a common cause of chronic cough in young children, which presents with wet cough, neutrophilic airway inflammation and bacterial infection of the conducting airways [1]. To date, there are few data involving the clinical characteristics of adult PBB. Martin and colleagues previously reported that the clinical features of idiopathic chronic productive cough in adults were similar to pediatric PBB [2]. Of note, a significant proportion of them had a history of asthma, gastroesophageal reflux disease or upper airway cough syndrome (UACS). Another study showed that lower respiratory tract bacterial infection accounted for approximately one-third of patients undergoing bronchoscopy due to idiopathic chronic cough [3], thereby suggesting that PBB might be misdiagnosed as unexplained cough.
Indeed, failure to characterize endobronchial infections could result in under-recognition of PBB as an important clinical entity in children [4]. Microbiological-based diagnosis of PBB is still a challenging area thus far. Whereas, clinical characteristics were used as potential predictors for the common causes of chronic cough, including cough variant asthma, nonasthmatic eosinophilic bronchitis (NAEB) and UACS [5]. Identifying characteristics of PBB may assist clinicians to differentiate it from other causes of cough. This study, thus, aimed to investigate the clinical and laboratory features of PBB, and evaluate the potential diagnostic value in the adult population.
2. Materials and methods
2.1. Study design and participants
In this study, we consecutively screened patients with persistent cough from our cough database between January 2013 and December 2020, in which all patients presented with cough as a sole or predominant symptom lasting more than 3 weeks at age ≥18 years without obvious abnormal chest image. Patients were excluded if they were pregnant women, or could not finish follow-up at the early stages of evaluating cough-related causes, or had a ratio of forced expiratory volume in the first second to forced vital capacity of less than 0.7, or had other conditions with potential infection (bronchiectasis, chronic bronchitis, chronic tonsillitis, etc.).
A standardized questionnaire was used in our cough database [5, 6]. We gathered information on demographics, smoking status, cough features, concomitant symptoms, medical history, laboratory investigations, diagnosis and response to therapy. The cough features included duration, timing, characteristics, sputum color and triggers. A modified diagnostic algorithm of cough was used to determine the causes of cough in this study, as previously described (Figure S1) [6]. Given clinical diagnostic criteria of pediatric PBB [7], a diagnosis of PBB was considered if patients met the following criteria: (1) cough lasting at least 3 weeks, (2) a normal or near normal chest high-resolution computed tomography, (3) positive bacterial culture in sputum and/or good response to oral moxifloxacin for 1–4 weeks, (4) absence of evidences of alternative specific cause of cough (e.g., asthma, reflux disease, upper airway cough syndrome). Herein, if a patient had eosinophilic airway inflammation or bronchial hyperresponsiveness confirmed by methacholine provocation testing, the diagnosis of PBB would be not taken into account. The remaining eligible subjects were grouped as non-PBB. We selected the non-PBB patients as control based on a 1:4 ratio of PBB to non-PBB randomly by SPSS software, with the purpose of reducing the potential bias from the differences of sample size. The study protocol was approved by the Ethics Review Committees of the First Affiliated Hospital of Guangzhou Medical University (IRB No. 201921). All patients provided written informed consents prior to the study.
2.2. Cough severity assessment
The impact and severity of cough were evaluated in all enrolled subjects with persistent cough. Cough-related quality of life was assessed using cough symptom score (CSS), which is a two-part questionnaire during the day and at night [8]. Cough severity was measured using cough visual analogue scale (VAS), with a range of 0–100 mm, where 0 indicates no cough and 100 indicates for the worst cough imaginable [9]. A responder was defined as a decline of cough VAS ≥15 mm or CSS ≥2 points after treatment [10, 11].
2.3. Differential cells in induced sputum
Induced sputum was obtained and processed as previously described [12]. Briefly, sputum was induced by inhalation of 3% hypertonic saline for 21 min via an ultrasonic nebulizer. If no sufficient amount of qualified sputum was collected, the inhalation procedure was continued for a further 7 min. The cell smear was stained with hematoxylin and eosin for differential cell count. A total of 400 non-squamous cells were counted to obtain the differential count.
2.4. Sputum microbiological culture
If patients had an indicator of suspected tracheobronchial infection (e.g., productive cough or yellow phlegm production), they were required to conduct the detection of microbial pathogens. Spontaneous sputum was obtained and sent to the hospital laboratory for routine processing and bacterial culture [13]. A cut-off value of bacterial load ≥105 copies/mL was considered to be positive [14].
2.5. Statistical analysis
All statistical analyses were done with SPSS statistical software version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were non-normally distributed and presented as medians and interquartile ranges (IQRs). The comparisons of skewed data were analyzed by non-parametric Wilcoxon rank-sum tests within two groups. Categorical variables were expressed as frequencies and proportions, and calculated by Chi-square tests or Fisher's exact tests. A logistic regression test was used to evaluate the variables for diagnosing PBB. Univariate logistic regression analysis was performed to screen significant variables. If the p-value for the variable was <0.1, it was added to the multivariate regression model. Then, multiple logistic regression analysis was conducted with the method of forward stepwise (likelihood ratio) to identify independent predictors for PBB after adjusting age and sex. P value < 0.05 was considered as statistically significant.
3. Results
3.1. Characteristics of the study population
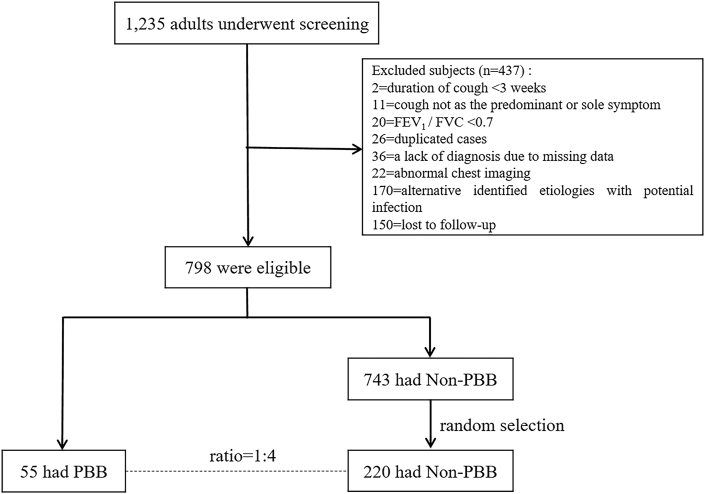
A total of 1,235 patients underwent screening and 437 (35.4%) were excluded in this study (Figure 1). Among the remaining 798 cases, 55 (7.0%) had a diagnosis of suspected PBB, and 220 (27.6%) with non-PBB were randomly selected as control subjects by a 1:4 ratio of PBB to non-PBB. There were 207 (94.1%) patients with single cause and 13 (5.9%) patients with dual causes in the non-PBB group. The four common causes included gastroesophageal reflux cough (51, 23.2%), asthmatic cough (35, 15.9%), NAEB (32, 14.5%), as well as refractory or unexplained chronic cough (49, 22.3%) (Figure S2).
Figure 1.
The flow diagram of patients screening. FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PBB, protracted bacterial bronchitis.
Of the 55 patients with PBB, approximately three-fifths (34, 61.8%) were females with a median (IQR) age of 46.0 (32.0–52.0) years, which were similar to non-PBB (both p > 0.05) (Table 1). However, we observed a shorter cough duration in patients with PBB than in patients with non-PBB (median [IQR]: 3.0 [1.4–8.5] versus 24.0 [9.0–90.0] months, p < 0.001). In addition, over half (31, 56.4%) of the patients with PBB had a cough lasting more than 8 weeks. Productive cough, yellow phlegm and a sensation of mucus in the throat (SMIT) more frequently occurred in patients with PBB than those without (all p < 0.001). Moreover, many more patients with PBB had upper respiratory tract infection (URTI) as an initiating factor (67.3% versus 42.7%, p = 0.001). No significant differences were found in cough timing, triggers, severity, concomitant symptoms (besides SMIT) between groups (all p > 0.05).
Table 1.
Demographics and clinical characteristics between the PBB and the non-PBB group.
| PBB (N = 55) | non-PBB (N = 220) | P-value | |
|---|---|---|---|
| Female sex | 34 (61.8) | 112 (50.9) | 0.147 |
| Age (ys) | 46.0 (32.0–52.0) | 39.5 (30.0–53.0) | 0.350 |
| Smoking status | |||
| Never smoker | 48 (87.3) | 192 (87.3) | 0.999 |
| Ex-smoker | 6 (10.9) | 17 (7.7) | 0.422 |
| Current smoker | 1 (1.8) | 11 (5.0) | 0.470 |
| Cough duration (mon) | 3.0 (1.4–8.5) | 24.0 (9.0–90.0) | <0.001 |
| Productive cough | 45 (81.8) | 69 (32.2) | <0.001 |
| Yellow phlegm | 28 (50.9) | 12 (5.6) | <0.001 |
| URTI as an initial factor | 37 (67.3) | 94 (42.7) | 0.001 |
| Seasonality | 7 (12.7) | 165 (22.2) | 0.120 |
| Occupational or environmental exposure | 14 (25.5) | 36 (16.4) | 0.118 |
| Timing of cough | |||
| Daily cough | 48 (87.3) | 194 (88.2) | 0.853 |
| Nocturnal cough | 22 (40.0) | 80 (36.4) | 0.618 |
| Triggers | |||
| Dust | 27 (49.1) | 96 (43.6) | 0.467 |
| Cold air | 24 (43.6) | 93 (42.3) | 0.855 |
| Cooking smell | 25 (45.5) | 102 (46.4) | 0.904 |
| Cigarette smoke | 20 (36.4) | 89 (40.5) | 0.579 |
| Lying down | 10 (18.2) | 36 (16.4) | 0.747 |
| Talking | 18 (32.7) | 85 (38.6) | 0.418 |
| Concomitant symptoms | |||
| Itchy throat | 33 (60.0) | 130 (59.1) | 0.902 |
| Itching below the throat | 14 (25.5) | 53 (24.1) | 0.833 |
| Pharyngeal foreign body sensation | 20 (36.4) | 82 (37.3) | 0.901 |
| Throat clearing | 16 (29.1) | 90 (40.9) | 0.107 |
| SMIT | 37 (67.3) | 87 (39.5) | <0.001 |
| Postnasal dripping | 16 (29.1) | 49 (22.3) | 0.287 |
| Runny nose | 15 (27.3) | 60 (27.3) | 0.999 |
| Acid regurgitation | 5 (9.1) | 38 (17.3) | 0.135 |
| Heartburn | 5 (9.1) | 20 (9.1) | 0.999 |
| Cough severity evaluation | |||
| Cough VAS (mm) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.236 |
| Daytime cough scores | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.283 |
| Night-time cough scores | 50.0 (30.0–70.0) | 60.0 (50.0–70.0) | 0.292 |
| Medical history | |||
| Rhinitis | 9 (16.4) | 52 (23.6) | 0.246 |
| Sinusitis | 6 (10.9) | 31 (14.1) | 0.536 |
Continuous variables were expressed as median (inter-quartile range, IQR). Categorical variables were presented as n (%). Among 220 patients with non-PBB, cough characteristics and sputum color were recorded in 214 patients, and cough seasonality in 212 patients. PBB: protracted bacterial bronchitis; URTI: upper respiratory tract infection; SMIT: a sensation of mucus in the throat; VAS: visual analogue scale.
3.2. Sputum cytology and bacterial culture
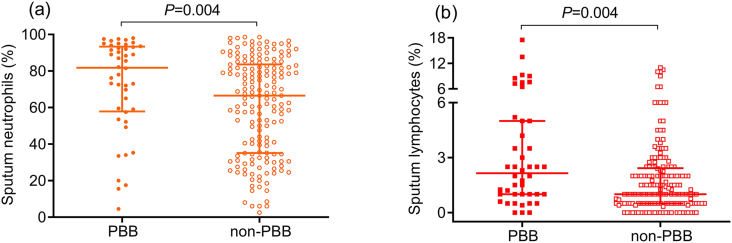
Sputum test for differential cells was performed in 44 subjects with PBB and 174 with non-PBB. Compared to patients with non-PBB, those with PBB had higher percentage of neutrophils (median [IQR]: 81.8% [57.9–93.4%] versus 66.5% [35.2–83.6%], p = 0.004) and lymphocytes in sputum (median [IQR]: 2.2% [1.0–5.0%] versus 1.0% [0.5–2.4%], p = 0.004) (Figures 2a and 2b).
Figure 2.
Sputum neutrophils (a) and lymphocytes (b) between the PBB (N = 44) and non-PBB (N = 174) group. PBB, protracted bacterial bronchitis.
Of those patients who developed PBB, 28 (50.9%) out of 55 patients performed the bacterial culture of sputum. And positive pathogenic bacteria were detected in eight (28.6%) patients. There were 7 cases with single pathogen and one case with dual pathogens, including Klebsiella pneumoniae (4, 14.3%), Haemophilus influenzae (2, 7.1%), Haemophilus parahaemolyticus (2, 7.1%) and Pseudomonas aeruginosa (1, 3.6%).
3.3. The predictive value of clinical parameters for PBB
As depicted in Table 2, the results of the multivariate analyses, adjusted for age and sex, showed that yellow phlegm (OR 6.602, 95% CI 2.147–20.300), productive cough (OR 3.891, 95% CI 1.410–10.743), SMIT (OR 3.122, 95% CI 1.197–8.142), elevated sputum lymphocytes level (OR 1.294, 95% CI 1.098–1.526) and cough duration (OR 0.985, 95% CI 0.972–0.998) remained independent predictors for PBB in adults. For distinguishing PBB from non-PBB, the optimal cutoff point for cough duration was 8.5 months while that for sputum lymphocytes was 2.3% (Figure S3). Of these predictors, yellow phlegm had the highest specificity of 94.4% and a moderate sensitivity of 50.9% (Table 3). In addition, the sensitivity of productive cough, SMIT, sputum lymphocytes ≥2.3% and cough duration ≤8.5 months were 51.2–81.8%, and their specificity were 60.5–75.6%.
Table 2.
Univariate and multiple logistic regression of predictors of PBB.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |
| Cough duration, months | 0.970 | 0.955–0.986 | <0.001 | 0.985 | 0.972–0.998 | 0.029 |
| Productive cough | 8.875 | 4.228–18.631 | <0.001 | 3.891 | 1.410–10.743 | 0.009 |
| Yellow phlegm | 17.457 | 7.950–38.331 | <0.001 | 6.602 | 2.147–20.300 | 0.001 |
| SMIT | 3.119 | 1.670–5.826 | <0.001 | 3.122 | 1.197–8.142 | 0.020 |
| Sputum Lym% | 1.226 | 1.088–1.383 | 0.001 | 1.294 | 1.098–1.526 | 0.002 |
| Previous history of URTI | 2.755 | 1.477–5.139 | 0.001 | |||
| Before-sleep cough | 1.774 | 0.912–3.450 | 0.091 | |||
| Sputum Neu% | 1.018 | 1.004–1.033 | 0.013 | |||
PBB: protracted bacterial bronchitis; SMIT: a sensation of mucus in the throat; Lym: lymphocyte; URTI: upper respiratory tract infection; Neu: neutrophil; CI: confidence interval.
Table 3.
Predictive value of clinical features for PBB.
| Variables | Sensitivity | Specificity |
|---|---|---|
| Cough duration ≤8.5 months | 75.9% | 75.6% |
| Productive cough | 81.8% | 67.8% |
| Yellow phlegm | 50.9% | 94.4% |
| SMIT | 67.3% | 60.5% |
| Sputum Lym ≥2.3% | 51.2% | 74.9% |
PBB: protracted bacterial bronchitis; SMIT: a sensation of mucus in the throat; Lym: lymphocyte.
4. Discussion
Using data from the cough database, we found that patients with PBB were characterized by productive cough, yellow sputum, SMIT and neutrophilic airway inflammation. Furthermore, these cough features and increased sputum lymphocytes may be useful in indicating adult PBB. These findings advance our understanding of PBB in adults and are conducive to improve diagnostic accuracy.
Our results showed that the majority of PBB patients were females with a middle-aged predominance. PBB and bronchiectasis might represent different parts of the same underlying process of endobronchial infection and inflammation [15]. Previous studies revealed that bronchiectasis mostly occurred in women, which was associated with heightened capsaicin cough sensitivity in females [16, 17]. Similarly, the maximum tolerable dose of inhaled capsaicin was lower in female patients with chronic cough [6, 18]. Thus, the preponderance of females in PBB may be explained by sex-related difference in the cough reflex sensitivity, just like bronchiectasis and chronic cough. In clinical practice, some female patients seeking medical help often complained of troublesome coughing secondary to childbirth. For general female individuals, there was a reduction in T-helper 1 pro-inflammatory immune molecules with a simultaneous increase in T-helper 2 immune molecules during pregnancy [19]. This immunomodulatory effects could directly contribute to an overall female-bias in susceptibility to infection compared to males [20]. In other words, decreased immunity to viral or bacterial infection may be another important reason for why more females had PBB.
The median duration of patients with PBB was 3.0 (IQR 1.4–8.5) months and over half of them had a cough lasting longer than 8 weeks in the present study, indicating adult PPB not only occurred in subacute cough but also in chronic cough. Although many patients had been treated with antibiotics before this visit, cephalosporins were used commonly in community hospitals in China. However, atypical pathogens, as one of possible causative organisms of PBB, could be not cleared by cephalosporins. In addition to the type of antibiotics, the duration of initial antibiotic treatment was considered as an important factor and significantly associated with recurrent PBB in children [21]. Despite we did not collect the information of initial antibiotic course in adults with PBB, inappropriate antibiotics use was very common in patients with chronic cough in China [22]. It was therefore reasonable to believe that many patients with PBB might not receive proper and efficient treatment and developed into chronic cough.
Chronic wet cough was the most leading complaint in pediatric PBB [1]. Likewise, our study demonstrated that productive cough mostly occurred in patients with PBB, and was one of independent factors for diagnosing PBB. The proliferation and hypertrophy of goblet cells were possible potential mechanisms for endobronchial mucus hypersecretion [23]. In addition, we also found that yellow phlegm appeared in approximately half of PBB patients with the highest specificity (94.4%) and a moderate sensitivity (50.9%) for determining PBB. A pooled analysis on acute exacerbations of chronic bronchitis showed that sputum color, particularly green and yellow, was a strong predictor of potential pathogenic bacteria [24]. Both PBB and chronic bronchitis are deemed to be chronic inflammatory disorders of the bronchus. Thus, we think that yellow phlegm is likely to be a marker of bacterial infection in patients with persistent cough. Of note, sputum color did not predict the need for antibiotic treatment in patients with acute exacerbation of chronic bronchitis [24]. Further study was needed.
In addition to productive cough, recurrent wheeze was often reported by parents in children with PBB, which might tend to misdiagnose as asthma [25]. However, adult patients with PBB hardly presented wheeze. Moreover, all patients had performed bronchial challenge and induced sputum test for differential cell count. If the patients had airway hyper-responsiveness or sputum eosinophilia, the diagnosis of PBB would not be considered in those patients. Therefore, there was little possibility that asthma patients were enrolled in the PBB group. In the clinical settings, what differentiated the two conditions were mainly the type of cough (often wet cough in PBB and dry and/or nocturnal in asthma), positive bronchial provocation test in asthma, and good response to antimicrobial agents in PBB [26].
Many patients with chronic cough had laryngeal hypersensitivity, such as laryngeal paresthesia [27]. In our study, SMIT was an independent predictor of adult PBB with moderate sensitivity (76.9%) and specificity (58.7%). It was also considered as one of clinical features of UACS [28], which was associated with excessive mucus from the nose adhering to the throat. Moreover, SMIT mostly occurred in laryngopharyngeal reflux disease as well [29]. However, reflux-related symptoms were less described in patients with PBB in the current study. Additionally, Ogawa and colleagues found that SMIT was an important clinical manifestation in patients with fungus-associated chronic cough [30]. In the subsequent studies, they reported that significant improvements of SMIT and cough were observed after antifungal treatment [31, 32], suggesting a link between fungal infection and SMIT. Although we did not perform the detection of pathogenic fungi in many patients, fungal atopy or infection could not explain the reasons for prolonged cough in PBB patients. The underlying mechanisms of SMIT are not yet well understood in patients with PBB. Adults with PBB had increased percentages of neutrophils in the lower airways, as described in pediatric PBB [1]. In addition to airway neutrophilia, we also observed a significant elevated level of sputum lymphocytes in adults with PBB than those with non-PBB. Similarly, bronchoalveolar lavage fluid (BALF) lymphocytosis was also reported in a cohort of subjects with unexplained chronic cough [33], and we speculated that these patients were likely to had potential infection. Majorities of chronic cough patients complained of a preceding viral infection and developed a persistent cough in our study population. Perhaps, the increased lymphocytes in sputum were induced by the viral infection initially. Moreover, there was another possibility that higher airway lymphocytes may be merely the consequence of a decrease in the proportion of other inflammatory cells.
With respect to pathogenic bacteria of adult PBB, our results suggested that slight differences existed in comparison with the findings of children PBB. The common pathogenic microorganisms of pediatric PBB included Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis, respectively [1]. Martin et al. also observed similar pathogens in idiopathic chronic productive cough [34]. In our study, Klebsiella pneumoniae, Haemophilus parahaemolyticus and Pseudomonas aeruginosa were identified in the cases with PBB, which were less described in pediatric PBB. It was worth noting that BALF sample was commonly used in children [1], The sensitivity of culture in sputum was lower than that in BALF. In addition, many factors, including medication history, detection methods and host's immune status, might affect the results of bacterial culture. Consequently, an unmet need for pathogenic organisms should be fulfilled in the further prospective studies.
Ideally, a lower airway specimen for microbiologic testing can be obtained before treatment in infectious disorders [35]. The original definition of children PBB required positive culture of respiratory pathogens from BALF [1]. Indeed, obtaining BALF via bronchoscopy for each patient with suspected PBB is impractical in most clinical settings [35]. Moreover, there is a situation of antibiotic abuse in primary and secondary medical institutions [22, 36], possibly leading to reduce the detection rate of bacterial pathogens. From a pragmatic perspective, researchers proposed that the criterion of recognizing respiratory bacterial pathogens growing in sputum or in BALF was replaced by absence of other causes of wet/productive cough during the diagnostic process of children PBB [35]. Furthermore, the definition of clinically based PBB have been gradually recognized and applied in clinical practice [37, 38, 39, 40], and recommended by the British Thoracic Society guidelines [41]. Therefore, the clinically based definition of PBB was also utilized in the current study. It is worthy note that other potential causes of persistent cough had been ruled out by comprehensive investigations or empiric treatment, and good responses to antibiotic treatment were observed in those patients with PBB. We believe that the definition of clinically based PBB is of great promise in clinical practice and researches. In the future, further study is required to enlarge the sample size to validate the definition and clinical features of clinically-based PBB among the adult population.
There are several limitations in our study. Firstly, this is a single center study with small sample size, which may not reflect the real situation in clinical practice. Secondly, although productive cough was common in adults with PBB, some patients could not cough up qualified sputum for bacterial culture, thereby lacking of etiological results. Therefore, the diagnosis of PBB may be not very accurate. Lastly, moxifloxacin was used to treatment suspected PBB patients in our study, which is effective to atypical pathogens such as mycoplasma and chlamydia. Thus, atypical pathogenic infection could not be ruled out in patients with PBB in the current study. Here, we think that the term “protracted infectious bronchitis” may be more suitable for these kind of patients.
5. Conclusions
Our study shows that adult patients with PBB were characterized by productive cough, yellow phlegm, SMIT and neutrophilic airway inflammation. In addition, these cough characteristics and elevated sputum lymphocytes can be utilized to indicate diagnosis of PBB and guide the treatment, especially in patients with suspected infection who failed to detect pathogens.
Declarations
Author contribution statement
Lianrong Huang: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kefang Lai: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chen Zhan and Li Long: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fang Yi, Jianmeng Zhou, Wenzhi Zhan, Hankun Lu, Ziyu Jiang, Yuehan Chen, Mei Jiang, Ruchong Chen, Jiaxing Xie and Wei Luo: Contributed reagents, materials, analysis tools or data.
Funding statement
Kefang Lai was supported by Incubative Project for Innovation Team of GMU [2017-159].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge Siqi Jiang, Baojuan Liu, Hongmei Yao, Jiayu Pan, Bonian Zhong, Jing Tian, Fagui Chen, Zhe Chen, Liqi Yang, Wen Peng, Hu Li, Xiaomei Chen for assistance with recruiting subjects. Special thanks to Yonglin Mai for assistance in manuscript editing.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Marchant J.M., Masters I.B., Taylor S.M., Cox N.C., Seymour G.J., Chang A.B. Evaluation and outcome of young children with chronic cough. Chest. 2006;129:1132–1141. doi: 10.1378/chest.129.5.1132. [DOI] [PubMed] [Google Scholar]
- 2.Martin M.J., Lee H., Clayton C., Pointon K., Soomro I., Shaw D.E., et al. Idiopathic chronic productive cough and response to open-label macrolide therapy: an observational study. Respirology. 2019;24:558–565. doi: 10.1111/resp.13483. [DOI] [PubMed] [Google Scholar]
- 3.Heching M., Rosengarten D., Shitenberg D., Shtraichman O., Abdel-Rahman N., Unterman A., et al. Bronchoscopy for chronic unexplained cough: use of biopsies and cultures increase diagnostic yield. J. Bronchology. Interv. Pulmonol. 2020;27:30–35. doi: 10.1097/LBR.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen L.M., de Groot R. Recurrent, protracted and persistent lower respiratory tract infection: a neglected clinical entity. J. Infect. 2015;71(Suppl 1):S106–111. doi: 10.1016/j.jinf.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Lai K., Zhan W., Li H., Yi F., Peng W., Zhou J., et al. The predicative clinical features associated with chronic cough that has a single underlying cause. J. Allergy Clin. Immunol. Pract. 2020;9:426–432. doi: 10.1016/j.jaip.2020.06.066. e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai K., Long L., Yi F., Tang J., Chen Z., Chen F., et al. Age and sex distribution of Chinese chronic cough patients and their relationship with capsaicin cough sensitivity. Allergy. Asthma. Immunol. Res. 2019;11:871–884. doi: 10.4168/aair.2019.11.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson P.G., Chang A.B., Glasgow N.J., Holmes P.W., Katelaris P., Kemp A.S., et al. CICADA: cough in children and adults: diagnosis and assessment. Australian cough guidelines summary statement. Med. J. Aust. 2010;192:265–271. doi: 10.5694/j.1326-5377.2010.tb03504.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsu J.Y., Stone R.A., Logan-Sinclair R.B., Worsdell M., Busst C.M., Chung K.F. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur. Respir. J. 1994;7:1246–1253. doi: 10.1183/09031936.94.07071246. [DOI] [PubMed] [Google Scholar]
- 9.Won H.K., Kang S.Y., Kang Y., An J., Lee J.H., Lee S.M., et al. Cough-related laryngeal sensations and triggers in adults with chronic cough: symptom profile and impact. Allergy. Asthma. Immunol. Res. 2019;11:622–631. doi: 10.4168/aair.2019.11.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumitsu K., Kanemitsu Y., Asano T., Takeda N., Ichikawa H., Yap J.M.G., et al. Tiotropium attenuates refractory cough and capsaicin cough reflex sensitivity in patients with asthma. J. Allergy Clin. Immunol. Pract. 2018;6:1613–1620. doi: 10.1016/j.jaip.2018.01.016. e1612. [DOI] [PubMed] [Google Scholar]
- 11.Kanemitsu Y., Matsumoto H., Oguma T., Nagasaki T., Ito I., Izuhara Y., et al. Independent factors contributing to daytime and nighttime asthmatic cough refractory to inhaled corticosteroids. J Investig. Allergol. Clin. Immunol. 2019;29:30–39. doi: 10.18176/jiaci.0281. [DOI] [PubMed] [Google Scholar]
- 12.Yi F., Chen R., Luo W., Xu D., Han L., Liu B., et al. Validity of fractional exhaled nitric oxide in diagnosis of corticosteroid-responsive cough. Chest. 2016;149:1042–1051. doi: 10.1016/j.chest.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 13.State health and Family Planning Commission of China . 2017. Performance Guideline for Bacterial Culture of Lower Respiratory Infections : WS/T 499-2017[S] [Google Scholar]
- 14.Gadsby N.J., Russell C.D., McHugh M.P., Mark H., Conway Morris A., Laurenson I.F., et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin. Infect. Dis. 2016;62:817–823. doi: 10.1093/cid/civ1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang A.B., Redding G.J., Everard M.L. Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr. Pulmonol. 2008;43:519–531. doi: 10.1002/ppul.20821. [DOI] [PubMed] [Google Scholar]
- 16.Guan W.J., Gao Y.H., Xu G., Lin Z.Y., Tang Y., Li H.M., et al. Capsaicin cough sensitivity and the association with clinical parameters in bronchiectasis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y.H., Guan W.J., Liu S.X., Wang L., Cui J.J., Chen R.C., et al. Aetiology of bronchiectasis in adults: a systematic literature review. Respirology. 2016;21:1376–1383. doi: 10.1111/resp.12832. [DOI] [PubMed] [Google Scholar]
- 18.Morice A.H., Jakes A.D., Faruqi S., Birring S.S., McGarvey L., Canning B., et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur. Respir. J. 2014;44:1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 19.Sherer M.L., Posillico C.K., Schwarz J.M. The psychoneuroimmunology of pregnancy. Front. Neuroendocrinol. 2018;51:25–35. doi: 10.1016/j.yfrne.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz J.M. Frank Beach Award Winner - the future of mental health research: examining the interactions of the immune, endocrine and nervous systems between mother and infant and how they affect mental health. Horm. Behav. 2019;114 doi: 10.1016/j.yhbeh.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross-Hodge E., Carroll W.D., Rainford N., Gamble C., Gilchrist F.J. Duration of initial antibiotic course is associated with recurrent relapse in protracted bacterial bronchitis. Arch. Dis. Child. 2020;105:1111–1113. doi: 10.1136/archdischild-2019-317917. [DOI] [PubMed] [Google Scholar]
- 22.Lai K., Li B., Wang F., Chen R., Liu X., Zhong N. Survey on the diagnosis and management of the patients with chronic cough. Chin. J. Asthma. 2011;5:8–10. [Google Scholar]
- 23.Choi W., Yang A.X., Waltenburg M.A., Choe S., Steiner M., Radwan A., et al. FOXA2 depletion leads to mucus hypersecretion in canine airways with respiratory diseases. Cell Microbiol. 2019;21 doi: 10.1111/cmi.12957. [DOI] [PubMed] [Google Scholar]
- 24.Miravitlles M., Kruesmann F., Haverstock D., Perroncel R., Choudhri S.H., Arvis P. Sputum colour and bacteria in chronic bronchitis exacerbations: a pooled analysis. Eur. Respir. J. 2012;39:1354–1360. doi: 10.1183/09031936.00042111. [DOI] [PubMed] [Google Scholar]
- 25.Wurzel D.F., Marchant J.M., Yerkovich S.T., Upham J.W., Mackay I.M., Masters I.B., et al. Prospective characterization of protracted bacterial bronchitis in children. Chest. 2014;145:1271–1278. doi: 10.1378/chest.13-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallucci M., Pedretti M., Giannetti A., di Palmo E., Bertelli L., Pession A., et al. When the cough does not improve: a review on protracted bacterial bronchitis in children. Front. Pediatr. 2020;8:433. doi: 10.3389/fped.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzone S.B., McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin. Pharmacol. Ther. 2021;109:619–636. doi: 10.1002/cpt.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese Thoracic Society (CTS) Asthma Cosortium Chinese guidelines for diagnosis and treatment of cough. Chin. J. Tuberc. Respir. Dis. 2015:323–354. [Google Scholar]
- 29.Massawe W.A., Nkya A., Abraham Z.S., Babu K.M., Moshi N., Kahinga A.A., et al. Laryngopharyngeal reflux disease, prevalence and clinical characteristics in ENT department of a tertiary hospital Tanzania, World. J. Otorhinolaryngol. Head. Neck. Surg. 2021;7:28–33. doi: 10.1016/j.wjorl.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa H., Fujimura M., Takeuchi Y., Makimura K. Chronic cough management: dealing with a sensation of mucus in the throat. Respirology. 2013;18:732–733. doi: 10.1111/resp.12064. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa H., Fujimura M., Takeuchi Y., Makimura K. Clinical experience with low-dose itraconazole in chronic idiopathic cough. Cough. 2013;9 doi: 10.1186/1745-9974-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa H., Fujimura M., Ohkura N., Makimura K. Effects of nebulized amphotericin B and budesonide inhalation for chronic cough-related laryngeal sensations. Auris Nasus Larynx. 2015;42:221–225. doi: 10.1016/j.anl.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Birring S.S., Brightling C.E., Symon F.A., Barlow S.G., Wardlaw A.J., Pavord I.D. Idiopathic chronic cough: association with organ specific autoimmune disease and bronchoalveolar lymphocytosis. Thorax. 2003;58:1066–1070. doi: 10.1136/thorax.58.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin M.J., Harrison T.W. Causes of chronic productive cough: an approach to management. Respir. Med. 2015;109:1105–1113. doi: 10.1016/j.rmed.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Chang A.B., Upham J.W., Masters I.B., Redding G.R., Gibson P.G., Marchant J.M., et al. Protracted bacterial bronchitis: the last decade and the road ahead. Pediatr. Pulmonol. 2016;51:225–242. doi: 10.1002/ppul.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain S.A., Garrod R., Douiri A., Masefield S., Powell P., Bücher C., et al. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193:401–408. doi: 10.1007/s00408-015-9701-2. [DOI] [PubMed] [Google Scholar]
- 37.Baines K.J., Upham J.W., Yerkovich S.T., Chang A.B., Marchant J.M., Carroll M., et al. Mediators of neutrophil function in children with protracted bacterial bronchitis. Chest. 2014;146:1013–1020. doi: 10.1378/chest.14-0131. [DOI] [PubMed] [Google Scholar]
- 38.Wurzel D.F., Mackay I.M., Marchant J.M., Wang C.Y., Yerkovich S.T., Upham J.W., et al. Adenovirus species C is associated with chronic suppurative lung diseases in children. Clin. Infect. Dis. 2014;59:34–40. doi: 10.1093/cid/ciu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Gast C.J., Cuthbertson L., Rogers G.B., Pope C., Marsh R.L., Redding G.J., et al. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann. Am. Thorac. Soc. 2014;11:1039–1048. doi: 10.1513/AnnalsATS.201312-456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang A.B., Van Asperen P.P., Glasgow N., Robertson C.F., Mellis C.M., Masters I.B., et al. Children with chronic cough: when is watchful waiting appropriate? development of likelihood ratios for assessing children with chronic cough. Chest. 2015;147:745–753. doi: 10.1378/chest.14-2155. [DOI] [PubMed] [Google Scholar]
- 41.Shields M.D., Bush A., Everard M.L., McKenzie S., Primhak R. BTS guidelines: recommendations for the assessment and management of cough in children. Thorax. 2008;63(Suppl 3):iii1–iii15. doi: 10.1136/thx.2007.077370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.