Abstract
Background
Though honey has long been used as medicine, there is a scarcity of knowledge on how it interacts with the body.
Scope and approach
While different types of honey have different chemical and medicinal properties according to their origin, this narrative review seeks to analyse the current knowledge on the chemical composition and therapeutic use of honey. With numerous chemical components, honey has a range of health benefits in multiple disciplines of medicine, and provides an interesting prospect in chemical analysis with regards to identification of its origin.
Key findings and conclusions
There is a great potential for the use of honey in medicine, primarily due to its antioxidant and antimicrobial properties. Recent studies on the phenolic and enzymatic components of honey have made honey's therapeutic method of action in relation to the above properties clearer, still more research needs to be conducted and more innovations need to be tested, for the full potential of honey to be understood.
Keywords: Honey, Traditional medicine, Modern medicine, Medical grade honey, Antioxidant
Graphical abstract
Highlights
-
•
Honey, propolis, pollen, and royal jelly, are studied for their role in medicine.
-
•
Different honeys have different chemical and medicinal properties.
-
•
Honey has a wide range of health benefits and has great potential in modern medicine.
-
•
Honey components give benefits, such as antioxidant and antimicrobial properties.
Honey; Traditional medicine; Modern medicine; Medical grade honey; Antioxidant.
1. Introduction
Apitherapy is the use of beehive products for their medicinal and pharmacological properties. It is many times termed an alternative medicine, a last resort to try when everything else fails, yet as more research is done on the subject, more information about its true therapeutic value is being confirmed (Weis et al., 2022).
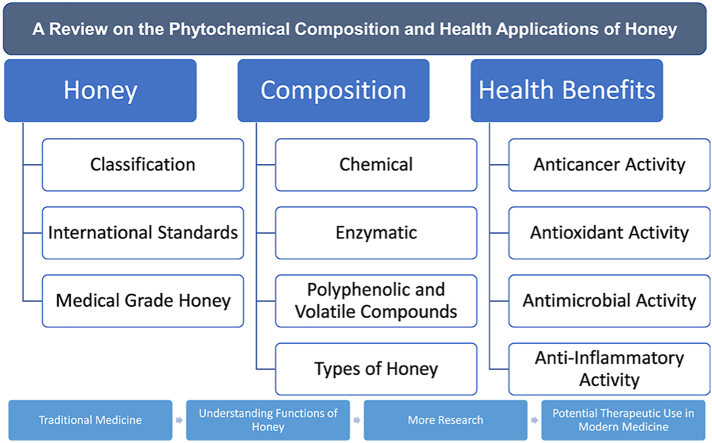
This narrative review analyses the classification and standards regulating honey, its chemical, phenolic and enzymatic composition, and the various health benefits stemming from the use of honey clinically and in daily life.
While many times it is easy to try to extrapolate the findings of one study to all the types of honey that exist, one would be misguided to do so. The geographical, botanical and even seasonal differences between different honeys leads to different chemical compositions, leading to different therapeutic activities. One therefore needs to research honey from different regions in order to obtain its global properties. In doing so one would also be highlighting and investigating the specific properties and specific types of honey, such as Manuka and Tualang honey (Cebrero et al., 2020).
2. International standards and regulations regarding honey
Legislation on what qualifies a substance to be called “honey” emanates from two international standards published by two of the largest international regulatory bodies, that is, the Codex Alimentarius (CA) and the European Council Directive Relating to Honey which are the main legislative standards applicable to most countries around the world. The CA was adopted by the United Nations (UN) via the Food and Agriculture Organization of the UN and the World Health Organisation (WHO) in 1981, revised in 1987 and 2001 and further amended in 2019. The European Directive was adopted in 2001 via the Council of the European Union and amended in 2014 by the European Parliament and Council (Bogdanov et al., 1999). Application of the CA is generally not imposed upon the UN member states, and thus many nations have their own legislation in place, instead of or parallel to the CA. Similarly, some European Union (EU) countries chose to adopt their own forms of regulation (Thrasyvoulou et al., 2018).
2.1. Classification of honey
Both standards classify honey as a sweet substance produced by the honeybee, Apis mellifera. They both underline the difference between blossom honey or nectar honey and honeydew honey, while the EU legislation also highlights differences between the method of production of different honeys. Furthermore, EU legislation also requires honey to be labelled by country of origin Codex Alimentarius Standard For Honey (2019); Council Directive (2001)/110/EC Relating to Honey (2001); Directive 2014/63/EU of The European Parliament and of the Council Relating to Honey, 2014.
2.2. Composition criteria of honey
The two documents have similar but slightly different sets of criteria with regards to the maximum and minimum amounts of the substances making up honey, as explained in Table 1 below (Bogdanov et al., 1999; Codex Alimentarius Standard For Honey, 2019; Council Directive 2001/110/EC Relating to Honey, 2001).
Table 1.
Composition criteria of honey according to the codex alimentarius and EU regulations.
| Criteria | Honey Type | CA | EU |
|---|---|---|---|
| Moisture content | General | ≤20% | ≤20% |
| Heather, Clover | ≤23% | ≤23% | |
| Sucrose Content | False acacia (Robinia pseudoacacia), leatherwood (Eucryphia lucida, Eucryphia milliganii), alfalfa (Medicago sativa), Banksia (Banksia menziesii), Menzies French honeysuckle (Hedysarum), Citrus spp., red gum (Eucalyptus camadulensis) | ≤10% | ≤10% |
| Lavender | ≤15% | ≤15% | |
| Others | ≤5% | ≤5% | |
| Sum of Glucose and Fructose content | Honeydew honey or blends of honeydew and blossom honey | ≥45 g/100 g | ≥45 g/100 g |
| Others (blossom honey) | ≥60 g/100 g | ≥60 g/100 g | |
| Water Insoluble Solids content | General | ≤0.1 g/100 g | ≤0.1 g/100 g |
| Pressed Honey | ≤0.5 g/100 g | ≤0.5 g/100 g | |
| Hydroxymethylfurfural content | General | N/A | ≤40 mg/kg |
| Tropical Honeys or Tropical Blends | N/A | ≤80 mg/kg | |
| Acidity | General | N/A | ≤50 meq/kg |
| Baker's Honey | N/A | ≤80 meq/kg | |
| Diastase Activity (Schade Scale) | General | N/A | ≤8 |
| Honeys with low natural enzyme content and an HMF ≤15 mg/kg | N/A | ≤3 |
2.3. Medical grade honey
Medical Grade Honey (MGH) is a type of honey specifically processed to be safe for use in a clinical scenario. This is usually considered sterile and so does not contain any microorganisms. For MGH to be useful, it should be free of toxic material and contaminants and comply with the legal and physicochemical criteria outlined in 2.1 above. Following strict production and storage guidelines organic honey is usually irradiated using gamma radiation to eliminate any pathogenic microorganisms, (Hermanns et al., 2020; Watts and Frehner, 2017).
Honey used to make MGH should not be of commercial origin, but rather organic, extracted from reputable sources. Commercially-available honeys have a lesser degree of antibacterial activity, probably due to thermal treatments, prolonged storage conditions and any possible adulteration which the honey might be subjected to (Bucekova et al., 2020; Hermanns et al., 2020).
It should be noted that in sterilising honey to make MGH, some of its antibacterial properties are lost, with honey samples acquired from beekeepers in Slovakia showing a higher degree of antibacterial activity than MGH (Bucekova et al., 2020). While sterilisation might reduce the functional antimicrobial activity of honey, irradiation is still important in order to eliminate any traces of pathogens which may be present in the honey due to the non-sterile collection and storage processes (Olaitan et al., 2007).
MGH may be used in a variety of ways, including in ointments, gels, or impregnated into wound dressings. They are also used in cases of infection (de Groot et al., 2021; Nolan et al., 2020), wound care (Holubová et al., 2021; Smaropoulos and Cremers, 2020), and burns (Krishnakumar et al., 2020).
3. The composition of honey
3.1. Chemical composition of honey
Honey is a very complex mixture of various nutrients and components, which vary in percentage concentrations depending on numerous factors. Most honeys only share circa 80% of their physical and chemical composition. Changes in composition could result from geographical and environmental conditions, the floral source that the bee consumes, the type of bee that produces the honey, and the extraction method used. Such variations would lead to different colours, viscosity, taste, and properties of the honey (Ranneh et al., 2021).
Honey is made up of mainly carbohydrates and water, with other substances such as proteins, amino acids, enzymes, polyphenols and other minerals present in much lower quantities. Carbohydrates as a whole represent around 80% of the honey's composition, the bulk of which (75%) is made up of the monosaccharides glucose and fructose. Fructose is usually more abundant than glucose with the exception of a small fraction of honeys such as those coming from Brassica napus and Taraxacum officinale (Miguel et al., 2017).
There can also be traces of vitamins such as Riboflavin, Pantothenic acid, Niacin, Thiamin, Pyridoxine, and Ascorbic acid as well as minerals such as Potassium, Sulphur, Chlorine, Calcium, Phosphorus, Magnesium, Sodium, Iron, Copper, and Manganese in honey. These can be both from natural sources or from environmental pollutants (Ball, 2007; Bogdanov et al., 2007).
Nectar is the raw material from which the honeybee produces honey, and thus the composition of the nectar from which the honey is produced will greatly affect the composition of the final product. Nectar itself varies greatly in its sugar content, and many bees would prefer nectars with higher sugar content depending on the amount of water availability (Waller, 1972). When nectar is scarce, bees collect the needed nutrients from honeydew produced by smaller insects such as aphids. This type of honey would have a characteristic presence of melezitose. When greater sugar content is present in the collected nectar or honeydew, the resulting honey would have a greater concentration of carbohydrates (Formosa, 2017).
Amino acids can also be detected in concentrations amounting to around 0.5%. They can be found either as free amino acids or as part of proteins. Amino acids such as proline, arginine, glutamic acid, cysteine and aspartic acid can all be detected in honey (Miguel et al., 2017).
Honey adulteration may play a significant role in changing the general composition of honey, especially with regards to its sugar content and physical properties. Generally, adulterated honey contains significantly lower fructose and glucose content and a slight decrease in the glass transition temperature of adulterated honey (Dranca et al., 2022).
Finally, minerals can be detected in varying amounts within honey, as can be seen in Table 2 (Ashagrie Tafere, 2021).
Table 2.
Minerals found in Honey.
| Minerals | Average amount in 100 g honey (mg) |
|---|---|
| Calcium | 4–30 |
| Chlorine | 2–20 |
| Copper | 0.01–0.1 |
| Iron | 1–3.4 |
| Magnesium | 0.7–13 |
| Phosphorous | 2–60 |
| Potassium | 10–470 |
| Sodium | 0.6–40 |
| Zinc | 0.2–0.5 |
3.2. Enzymes found in honey
Bees utilise the enzymes trypsin, chymotrypsin, elastase, and exopeptidase leucine aminopeptidases, which are found in their midgut, to digest dietary proteins and so be able to produce the proteins and enzymes found in honey (Burgess et al., 1996; Chua et al., 2015).
The vast majority (90%) of proteins found in honey are those belonging to the Major Royal Jelly Protein (MRJP) family, of which there are nine (MRJP1-9). Thus it is quite difficult to detect less abundant low molecular weight proteins found in honey (Rossano et al., 2012). The exact function of all these proteins is not known, but it is thought that they exhibit antioxidant activity (Chua et al., 2015). MRJP1 and MRJP2 have also been shown to have a hypocholesterolemic effect (Chiu et al., 2017), while MRJP3 has been found to have an immunomodulatory effect (Okamoto et al., 2003).
Enzymes related to carbohydrate metabolism, such as diastase, invertase, glucosidase, glucose oxidase, and catalase have been documented as components of honey (Babacan et al., 2002; Pontoh and Low, 2002).
While it is unclear how the enzymes found in honey are made, it is believed that these may either originate from the nectar used by the bee, by microorganisms in the honey, or from the bee itself. Diastase concentration may also be used as an indicator of honey quality, with higher quality honey usually containing more diastase (Ranneh et al., 2021).
In addition to the above-mentioned enzymes, a number of proteolytic enzymes, mostly serine proteases, are also present in honey. These usually originate from the nectar, pollen or from glandular secretions of the bees themselves. Proteolytic enzymes identified in honey mostly correspond to different forms of trypsin and chymotrypsin when compared to their molecular weight and biochemical function (Rossano et al., 2012). Apart from having a digestive function, some of the proteases detected in honey are suspected to have a developmental, defensive or immune function in bees (Paget et al., 2022; Zou et al., 2006). The proteolytic action of these enzymes may explain why honey has much lower amounts of MRJPs when compared with other bee-derived products (Rossano et al., 2012).
3.3. Polyphenols and volatile compounds in honey
Phenolic compounds differ greatly from one honey to another, and therefore can be used to determine the origin of honey via high performance liquid chromatography array detection (Miguel et al., 2017). In flowering plants, these compounds may serve as chemical attractants for pollinators such as bees, while in humans these have an impact on the taste and colour of honey (Formosa, 2017).
Polyphenols in honey are mainly flavonoids and phenolic acids and its derivatives, which are thought to give honey its antioxidant and antibacterial properties (Bogdanov et al., 2008). Numerous studies have shown the presence of various phenolic compounds known to have antibacterial properties, as outlined in Table 3.
Table 3.
A table Linking phenolic and flavonoid compounds found in honey with their antibacterial method of action.
| Compound | Molecular Formula | Structure | Mechanism | Reference |
|---|---|---|---|---|
| Gallic Acid | C7H6O5 | 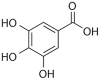 |
Cell membrane disruption and increased pore formation | (Borges et al., 2013; Ranneh et al., 2018) |
| Ferulic Acid | C10H10O4 | 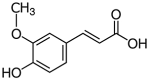 |
Cell membrane disruption and increased cytoplasmic leakage | (Borges et al., 2013; Lima et al., 2022) |
| Caffeic Acid | C9H8O4 | 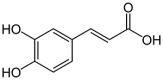 |
Damage to cell membrane integrity and oxidative stress | (Khan et al., 2021; Ranneh et al., 2018) |
| Chlorogenic acid | C16H18O9 | 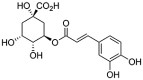 |
Increased cell membrane permeability and cytoplasmic and nucleotide leakage | (Cheung et al., 2019; Górniak et al., 2018) |
| p-Coumaric acid | C9H8O3 | 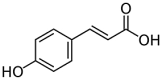 |
Disruption of cell membrane and bacterial DNA binding | (Borges et al., 2013; Ranneh et al., 2018) |
| Syringic acid | C9H10O5 | 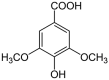 |
Dysfunction of cell membrane and inhibition of cellular enzymes | (Ranneh et al., 2018; Srinivasulu et al., 2018) |
| Vanillic acid | C8H8O4 | 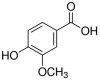 |
Disruption of cell membrane and inhibition of biofilm formation | (Cheung et al., 2019; Qian et al., 2020) |
| Apigenin | C15H10O5 | 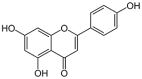 |
Increase in superoxide production and DNA fragmentation | (Kim et al., 2020; Ranneh et al., 2018) |
| Catechin | C15H14O6 | 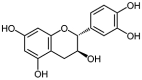 |
Production of hydrogen peroxide | (Wu and Brown, 2021; Yayinie et al., 2022) |
| Luteolin | C15H10O6 | 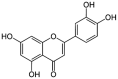 |
Disruption of cell wall and cell membrane, protein expression, and nucleic acid synthesis | (Guo et al., 2020; Tanleque-Alberto et al., 2020) |
| Pinocembrin | C15H12O4 | 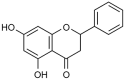 |
Increase in cell membrane permeability and disruption of protein and DNA metabolism | (Tanleque-Alberto et al., 2020; Wu et al., 2022) |
| Galangin | C15H10O5 | 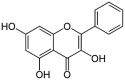 |
Inhibition of murein hydrolase gene expression | (Nešović et al., 2020; Ouyang et al., 2018) |
| Myricetin | C15H10O8 | 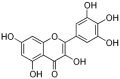 |
Inhibition of DnaB helicase | (Cheung et al., 2019; Griep et al., 2007) |
Phenolic acids and flavonoids can be further subclassified by their structural arrangement and their degree of oxidation. Flavonoids are more abundant than phenolic acids. A study of the digestion and absorption of these compounds would be of great help in understanding the physiology behind the beneficial effect of honey on human health. Still, this is not yet clearly understood. The studies currently available tend to focus more on the flavonoid component of honey. Flavonoids are known to be hydrolysed in the intestine and transported into the epithelium via sodium-dependent glucose transporter 1, where they may have an inhibitory action on Na-dependent facilitated diffusion of monosaccharides into the cells from the intestinal lumen (Cianciosi et al., 2018).
Compounds such as acids, alcohols, aldehydes, ketones, terpenes, norisoprenoids, benzene compounds and their derivatives, furan and pyran derivatives and other hydrocarbons all contribute as volatile compounds within honey. Similar to polyphenols, the fraction of these compounds which come from floral origins may be used to determine the botanical and geographical origin of the honey. Furthermore, the variation of these volatile compounds contribute to the aromas of different honeys (Manyi-Loh et al., 2011).
3.4. Different types of honey
In this section some different types of honey according to their visual appearance as well as their floral origins will be listed and discussed. These should not be taken as fully conclusive, as honey is affected by other factors such as weather and environmental conditions. Mixtures of honeys of various floral origins may also not follow Table 4 (Collins, 2010; National Honey Board, n.d.).
Table 4.
A table comparing various types of honey with their country of origin, colours and flavours.
| Name | Country of Origin | Colour and Flavour |
|---|---|---|
| Dark Honeys | ||
| Avocado | USA, especially California; Mexico; Australia | Dark amber colour; rich flavour of caramelised molasses |
| Buckwheat | USA; Canada; China; Russia | Very dark brown colour; strong molasses and malt flavour |
| Chestnut | Southern Europe | Dark amber colour; sharp, bitter flavour |
| Eucalyptus | USA, especially California; Australia; New Zealand; Italy | Dark amber colour; caramel flavour |
| Hawthorn | North America; New Zealand; Western and Northern Europe | Dark amber colour; nutty flavour |
| Heather | Europe, especially UK, Ireland and Scnadinavia | Dark amber or reddish brown colour; bitter-sweet taste |
| Honeydew | Europe, especially Germany, Greece and Turkey; New Zealand | Dark amber colour; strong rich flavour |
| Manuka | New Zealand | Dark colour; herbaceous taste |
| Rewarewa | New Zealand | Dark amber colour; rich malty flavour |
| Tulip Tree | Eastern USA | Very dark amber colour; strong flavour |
| Medium Honeys | ||
| Blackberry | UK; Canada | Light chestnut colour; coarse flavour |
| Coconut Palm | USA; West Indies | Amber colour; strong flavour |
| Dandelion | Worldwide | Golden yellow colour; strong flowery taste |
| Lavender | Europe, especially France and Spain | Golden colour; flowery taste |
| Lime Tree | Europe; Canada; USA | Amber or light yellowish green colour; strong flavour with vanilla taste |
| Mixed Meadow Flowers | Worldwide | Golden yellow; full flavour |
| Orange Blossom | USA; New Zealand; Asia | Medium amber colour; fruity taste |
| Rosemary | Mediterranean Europe | White to reddish gold colour; medium flavour |
| Thyme | Mediterranean Europe; North America; New Zealand | Bright amber colour; intense aromatic flavour |
| Tupelo | Southeastern USA | Light amber to medium yellow colour; sweet flavour |
| Light Honeys | ||
| Acacia | USA; Europe | Pale golden yellow colour; sweet, delicate flavour |
| Alfalfa | USA, especially California; Canada | Light amber or pale white colour; mild minty flavour |
| Apple | Worldwide | Light amber colour; good flavour with apple hints |
| Blueberry/Cranberry | USA; Canada; Europe | Light amber colour; full fruity flavour |
| Borage | UK; Canada; New Zealand | Pale yellow or white; light flavour |
| Clover | USA; Canada; Egypt; Europe; Australia; New Zealand | Pale amber, yellow or white colour; flowery flavour |
| Cotton | Egypt; Southern USA | Pale amber colour; light flavour |
| False Acacia | Europe; North America | Light colour; sweet flavour |
| Fireweed | Worldwide | Pale amber to white colour; subtle tea flavour |
| Fuchsia | Europe; New Zealand; North and South America | Light colour; mild flavour |
| Goldenrod | Europe; North America | Light-medium gold colour; slightly strong spicy flavour |
| Holly | Southern USA; Western and Southern Europe | Pale colour; fine flavour |
| Ivy | Europe; Asia; North America | Grey-white or yellow colour; bitter flavour |
| Knapweed | Ireland | Light amber colour; mild tangy flavour |
| Leatherwood | Tasmania | Light golden yellow colour; strong spicy taste |
| Maple | Canada; USA; UK | Pale yellow sometimes greenish colour; mild taste |
| Melilot | Worldwide | Pale greenish-yellow colour; slight cinnamon flavour |
| Mesquite | Mexico and USA | Light colour; smoky molasses flavour |
| Oil-seed rape | USA; Europe; Asia | White to light amber colour; mild taste |
| Rata | New Zealand | Water white colour, medium-bodied flavour |
| Sainfoin | North America; Europe | Lemon yellow colour; aromatic flavour |
| Sunflower | North America; Europe; Russia; China | Yellow colour; light flavour |
4. The health benefits of honey
4.1. The anticancer activity of honey
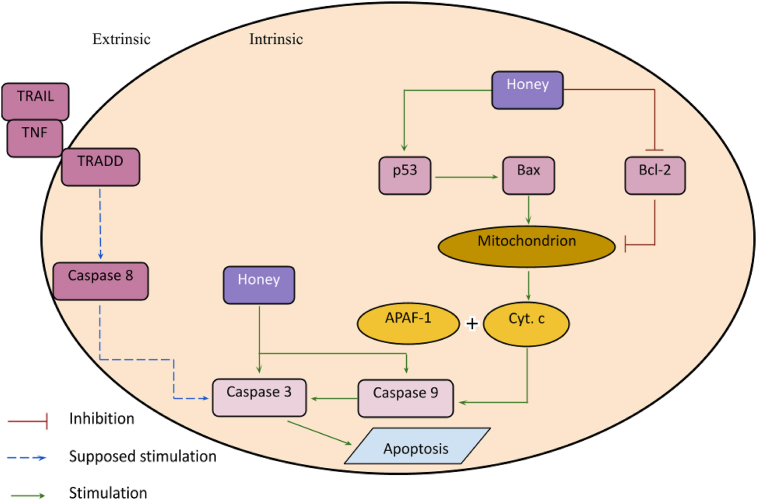
Cancer treatment generally involves drugs which induce apoptosis in the cancerous cells. Honey has a similar effect in depolarising the mitochondrial membrane and stimulating expression of caspase 3 and 9 in cancer cells, thus inducing apoptosis (Fauzi et al., 2011).
Honey also stimulates p53 expression, while down-regulating B cell lymphoma 2 protein (Bcl-2), which are proapoptotic and antiapoptotic proteins respectively. p53 thus depolarises the mitochondrial membrane via Bcl-2-associated X protein while Bcl-2 stops maintaining the resting potential of the mitochondrial membrane. The membrane depolarisation induces activation of cytochrome C, which in the presence of apoptotic protease activating factor 1 stimulates caspase 9 expression and so upregulates the apoptotic pathway. This thus induces apoptosis of cancerous cells. This process is explained in Figure 1 below (Ahmed and Othman, 2013).
Figure 1.
The effect of honey on the apoptotic pathway.
Furthermore, honey has been shown to decrease nuclear protein Ki-67 presence when administered orally with Aloe vera. This protein is present in the cell proliferation phases (G1, S, G2 and mitosis) but absent during the resting phase (G0). The decrease in Ki-67 was noted during all phases of cell replication, thus decreasing the rate by which cancerous cells divide, and so decreasing tumour growth (Tomasin and Gomes-Marcondes, 2011).
Another theory is that honey prevents cancer due to its antimutagenic activity against physical and chemical mutagens. Honey suppresses the error-prone repair pathway in bacterial cells and thus decrease mutations in these bacteria when dividing (Saxena et al., 2012).
Proteins in honey have been shown to induce the release of tumour necrosis factor α (TNFα) by macrophages (Majtán et al., 2006; Simúth et al., 2004). This increase in TNFα induces the release of reactive oxygen species (ROS) and induces the immunologic response to destroy the cancerous cells (Chan-Zapata and Segura-Campos, 2021; MacEwan, 2002).
4.2. The antioxidant activity of honey
The oxidation of molecules, which is crucial for the healthy operation of the cell, is prevented by antioxidants. Oxidation would successively harm tissues, organs and hence, the physiological functioning of the organism. Antioxidants are maintained in balance by a sophisticated system within the body. Food containing antioxidants has been demonstrated to help control this system and so promote health. While not yet confirmed through clinical trials, in vivo studies have shown that honey alleviates oxidative stress in various organ systems (Meo et al., 2017).
Honey contains a number of molecules, such as flavonoids, glucose oxidase, catalase, phenolic acids, ascorbic acid, and carotenoids which have been shown to have antioxidant activity both in vitro and in vivo. Many of these compounds exhibit a combined synergistic effect, and hence, honey is regarded as a natural antioxidant (Bogdanov et al., 2008).
While the handling and processing of honey may impact the antioxidant activity it exhibits, the most important factor affecting its antioxidant capacity is the geographical and botanical origin of the honey itself. This is correlated with the total phenolic content of honey, with honeys containing higher amounts of phenolic acids exhibiting higher antioxidant activity. As darker honeys tend to contain more phenolic acids, colour may be associated with antioxidant activity (Eteraf-Oskouei and Najafi, 2013).
While classical examples of antioxidants such as Vitamins C and E become pro-oxidants themselves when in large doses, honey has been shown not to exhibit this behaviour when given in larger amounts. This is thought to be due to the presence of more than one compound having antioxidant activity within honey, which helps to reconvert any compounds which become pro-oxidants into their active, antioxidant forms (Erejuwa et al., 2012).
The antioxidant activity of honey has been correlated with the prevention of several disorders such as cardiovascular diseases (Rakha et al., 2008), diabetes (Erejuwa et al., 2010) and cancer (Hassan et al., 2012).
4.3. The antimicrobial activity of honey
Honey has a number of properties which make it an ideal antimicrobial agent. The high sugar content, low pH, hydrogen peroxide, polyphenol compounds, and antimicrobial peptides all contribute in fighting against various types of pathogenic organisms, and further down the line, tissue repair (Almasaudi, 2021).
Throughout history, honey has been used as an antibacterial agent. Recent research has shown that honey does actually have antibacterial effects on aerobic, anaerobic, Gram-positive and Gram-negative bacteria, while it may also contain spores introduced during the production process (Olaitan et al., 2007).
A potential use of honey in modern medicine is in the treatment of patients presenting with Methicillin-resistant Staphylococcus aureus (MRSA) (Ayefoumi Adinortey et al., 2022). Numerous studies have shown positive results in the susceptibility of MRSA to honey. This provides a potential for a route of alternative treatment for antibiotic resistant bacteria, as well as reducing the number of antibiotics used during treatment, which may themselves be a cause to further antibiotic resistances (Chambers, 2006; Maeda et al., 2008; Natarajan et al., 2001).
Honey has also been found to have antifungal properties. In vitro studies have concluded that honey has an effect on Candida (de Groot et al., 2021) and Rhodotorula (Ahmed et al., 2013), which are opportunistic pathogenic yeasts. While no information on the clinical use of honey to treat fungal infections could be found at the time of writing, researchers are hopeful that the antifungal property of honey may be used in the near future for the treatment of antifungal resistant strains (Irish et al., 2006; Moussa et al., 2012).
Further to the antibacterial and antifungal properties exhibited by honey, it has been found to show a degree of antiviral activity as well. The antiviral activity of honey has been best shown in the treatment of skin lesions caused by the herpes simplex virus. In this regard, honey has shown faster treatment time than that taken by the normally prescribed antiviral acyclovir (Al-Waili, 2004; Rocha et al., 2022).
Despite antimicrobial resistance being constantly on the rise worldwide, there has never been a case of microbial resistance to honey reported, leading to honey frequently being used as a last resort. This is possibly due to honey having a number of different components which may exhibit antimicrobial properties (Almasaudi, 2021; Mandal and Mandal, 2011).
4.4. The anti-inflammatory activity of honey
Honey contains various compounds which have anti-inflammatory potential. The most notable anti-inflammatory effect exhibited by a component of honey is that of flavonoids, which mediates various cytokines such as TNF-α, interleukin (IL)-2, IL-10, IL-12p70, nitric oxide and interferon‐gamma (Silva et al., 2021, Silva et al., 2020).
Honey has been linked to its anti-inflammatory properties through in vitro studies (Candiracci et al., 2012), in animal studies (Leong et al., 2012) and also in clinical trials (Al-Waili and Boni, 2003; Subrahmanyam, 1998). With further research and clinical trials on this effect of honey, new drugs may be produced from this natural product. They may have all the necessary anti-inflammatory properties needed while also reducing or eliminating altogether the side effects associated with mainstream drugs used to treat chronic inflammation such as corticosteroids and non-steroidal anti-inflammatory drugs (Eteraf-Oskouei and Najafi, 2013).
Honey is attributed with the inhibition of the formation of ROS and the suppression of cyclooxygenase-2 enzymes, both of which are related to the pro-inflammatory pathway. This may be due to the mediation of the above-mentioned cytokines or due to gene suppression by transcription factors triggered by honey. Through this activity, honey thus exhibits an inhibitory effect on chronic inflammation (Ahmed and Othman, 2013; Leong et al., 2012; Ranneh et al., 2021).
5. Conclusion and discussion
Since the beginning of civilisation many claims were aimed at honey and its therapeutic use. Even today, a lot of research is being done to prove or disprove claims made in traditional medicine about the action of honey and hive products. While in vitro and in vivo studies provide valuable insight into the potential therapeutic benefits of honey, it would be misguided to draw the conclusion that these effects hold true in the human body. More research and most importantly more clinical trials are needed to evaluate the use of honey and other products of the beehive in medicine. There is still no clear consensus on the practice of apitherapy between regions of the world, and as such one should highlight the need to standardise and expand the present knowledge on apitherapy through collaborative research between chemists, medics and professionals within the industry.
Other issues which still need to be addressed are the standardisation of MGH components, the types of honey used to make MGH, and the process used to sterilise honey of any potentially pathogenic components while sparing its beneficial properties.
Furthermore, adulteration leads to drastic changes in honey content, which in turn may adversely affect body organ systems, especially the renal system (Fakhlaei et al., 2020).
As can be seen throughout this article, a lot of potential exists surrounding apitherapy, especially with regards to the treatment of multidrug resistant infections with honey. Although there have been significant advances in recent years in understanding the function and physiology of apitherapy, there is still a need to explore further and build upon this knowledge, as evidenced by a large number of articles that were referred to which highlighted the need for further research on the subject. In addition to this, contrary to other products such as pollen and bee venom, honey allergies are extremely uncommon. While these occur very rarely, care should be taken to make sure that there is no adverse reaction when using honey medically, as symptoms may range from simple to very severe (Altameemi et al., 2022).
It should be noted that effectively, the intake of honey has little to no side effects, and it is only in rare cases that humans have exhibited signs and symptoms of honey allergies (Altameemi et al., 2022).
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
References
- Ahmed S., Othman N.H. Honey as a potential natural anticancer agent: a review of its mechanisms. Evid. base Compl. Alternative Med. 2013;2013:829070. doi: 10.1155/2013/829070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M., Djebli N., Aissat S., Khiati B., Meslem A., Bacha S. In vitro activity of natural honey alone and in combination with curcuma starch against Rhodotorula mucilaginosa in correlation with bioactive compounds and diastase activity. Asian Pac. J. Trop. Biomed. 2013;3(10):816–821. doi: 10.1016/S2221-1691(13)60161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waili N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2004;10(8):MT94–MT98. [PubMed] [Google Scholar]
- Al-Waili N.S., Boni N.S. Natural honey lowers plasma prostaglandin concentrations in normal individuals. J. Med. Food. 2003;6(2):129–133. doi: 10.1089/109662003322233530. [DOI] [PubMed] [Google Scholar]
- Almasaudi S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021;28(4):2188–2196. doi: 10.1016/j.sjbs.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altameemi H., Sarheed N.M., Zaker K.A., Zaidan S. Honey allergy, first documentation in Iraq – a case report. Macedonian J. Med. Sci. 2022;10(C):243–245. Open Access. [Google Scholar]
- Ashagrie Tafere D. Chemical composition and uses of honey: a review. J. Food Sci. Nutr. Res. 2021;4(3) [Google Scholar]
- Ayefoumi Adinortey C., Wilson M., Kojo Kwofie S. In: The Global Antimicrobial Resistance Epidemic - Innovative Approaches and Cutting-Edge Solutions. Tellez-Isaias G., editor. IntechOpen; 2022. Honey as a natural product worthy of Re-consideration in treating MRSA wound infections. [Google Scholar]
- Babacan S., Pivarnik L.F., Rand A.G. Honey amylase activity and food starch degradation. J. Food Sci. 2002;67(5):1625–1630. [Google Scholar]
- Ball D.W. The chemical composition of honey. J. Chem. Educ. 2007;84(10):1643. [Google Scholar]
- Bogdanov S., Lüllmann C., Martin P., von der Ohe W., Russmann H., Vorwohl G., Oddo L.P., Sabatini A.-G., Marcazzan G.L., Piro R., Flamini C., Morlot M., Lhéritier J., Borneck R., Marioleas P., Tsigouri A., Kerkvliet J., Ortiz A., Ivanov T., Vit P. Honey quality and international regulatory standards: review by the International Honey Commission. Bee World. 1999;80(2):61–69. [Google Scholar]
- Bogdanov S., Haldimann M., Luginbühl W., Gallmann P. Minerals in honey: environmental, geographical and botanical aspects. J. Apicult. Res. 2007;46(4):269–275. [Google Scholar]
- Bogdanov S., Jurendic T., Sieber R., Gallmann P. Honey for nutrition and health: a review. J. Am. Coll. Nutr. 2008;27(6):677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Bucekova M., Bugarova V., Godocikova J., Majtan J. Demanding new honey qualitative standard based on antibacterial activity. Foods. 2020;9(9) doi: 10.3390/foods9091263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess E.P.J., Malone L.A., Christeller J.T. Effects of two proteinase inhibitors on the digestive enzymes and survival of honey bees (Apis mellifera) J. Insect Physiol. 1996;42(9):823–828. [Google Scholar]
- Candiracci M., Piatti E., Dominguez-Barragán M., García-Antrás D., Morgado B., Ruano D., Gutiérrez J.F., Parrado J., Castaño A. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J. Agric. Food Chem. 2012;60(50):12304–12311. doi: 10.1021/jf302468h. [DOI] [PubMed] [Google Scholar]
- Cebrero G., Sanhueza O., Pezoa M., Báez M.E., Martínez J., Báez M., Fuentes E. Relationship among the minor constituents, antibacterial activity and geographical origin of honey: a multifactor perspective. Food Chem. 2020;315:126296. doi: 10.1016/j.foodchem.2020.126296. [DOI] [PubMed] [Google Scholar]
- Chambers J. Topical manuka honey for MRSA-contaminated skin ulcers. Palliat. Med. 2006;20(5):557. doi: 10.1191/0269216306pm1160xx. [DOI] [PubMed] [Google Scholar]
- Chan-Zapata I., Segura-Campos M.R. Honey and its protein components: effects in the cancer immunology. J. Food Biochem. 2021;45(5):e13613. doi: 10.1111/jfbc.13613. [DOI] [PubMed] [Google Scholar]
- Cheung Y., Meenu M., Yu X., Xu B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019;22(1):290–308. [Google Scholar]
- Chiu H.-F., Chen B.-K., Lu Y.-Y., Han Y.-C., Shen Y.-C., Venkatakrishnan K., Golovinskaia O., Wang C.-K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharmaceut. Biol. 2017;55(1):497–502. doi: 10.1080/13880209.2016.1253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua L.S., Lee J.Y., Chan G.F. Characterization of the proteins in honey. Anal. Lett. 2015;48(4):697–709. [Google Scholar]
- Cianciosi D., Forbes-Hernández T.Y., Afrin S., Gasparrini M., Reboredo-Rodriguez P., Manna P.P., Zhang J., Bravo Lamas L., Martínez Flórez S., Agudo Toyos P., Quiles J.L., Giampieri F., Battino M. Phenolic compounds in honey and their associated health benefits: a review. Molecules. 2018;23(9) doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codex Alimentarius Standard For Honey . 2019. CXS 12-1981 CXS 12-1981.https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf [Google Scholar]
- Collins . Harpercollins Reference Hardbacks; 2010. Collins Beekeepers Bible (L. Gray, A. Kirstie, & W. Ione, Eds.; 1st ed.) [Google Scholar]
- Council Directive 2001/110/EC Relating to Honey, 2001/110/EC. 2001. [Google Scholar]
- de Groot T., Janssen T., Faro D., Cremers N.A.J., Chowdhary A., Meis J.F. Antifungal activity of a medical-grade honey formulation against Candida auris. J. Fungi. 2021;7(1) doi: 10.3390/jof7010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directive 2014/63/EU of the European Parliament and of the Council Relating to Honey, 2014/63/EU. 2014. [Google Scholar]
- Dranca F., Ropciuc S., Pauliuc D., Oroian M. Honey adulteration detection based on composition and differential scanning calorimetry (DSC) parameters. LWT (Lebensm.-Wiss. & Technol.) 2022;168(113910) [Google Scholar]
- Erejuwa O.O., Sulaiman S.A., Wahab M.S., Sirajudeen K.N.S., Salleh M.S.M., Gurtu S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann. Endocrinol. 2010;71(4):291–296. doi: 10.1016/j.ando.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Erejuwa Omotayo O., Sulaiman S.A., Ab Wahab M.S. Honey: a novel antioxidant. Molecules. 2012;17(4):4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eteraf-Oskouei T., Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran. J. Basic Med. Sci. 2013;16(6):731–742. [PMC free article] [PubMed] [Google Scholar]
- Fakhlaei R., Selamat J., Khatib A., Razis A.F.A., Sukor R., Ahmad S., Babadi A.A. The toxic impact of honey adulteration: a review. Foods. 2020;9(11) doi: 10.3390/foods9111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauzi A.N., Norazmi M.N., Yaacob N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011;49(4):871–878. doi: 10.1016/j.fct.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Formosa J.P. University of Malta; 2017. Chemical Profiling of Honey Produced in the Maltese Islands [Master Thesis] [Google Scholar]
- Górniak I., Bartoszewski R., Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Reviews. Proc. Phytochem. Soc. Eur. 2018;18(1):1–32. [Google Scholar]
- Griep M.A., Blood S., Larson M.A., Koepsell S.A., Hinrichs S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorg. Med. Chem. 2007;15(22):7203–7208. doi: 10.1016/j.bmc.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Guo Y., Liu Y., Zhang Z., Chen M., Zhang D., Tian C., Liu M., Jiang G. The antibacterial activity and mechanism of action of Luteolin against Trueperella pyogenes. Infect. Drug Resist. 2020;13:1697–1711. doi: 10.2147/IDR.S253363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.I., Mabrouk G.M., Shehata H.H., Aboelhussein M.M. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012;11(4):354–363. doi: 10.1177/1534735410387422. [DOI] [PubMed] [Google Scholar]
- Hermanns R., Mateescu C., Thrasyvoulou A., Tananaki C., Wagener F.A.D.T.G., Cremers N.A.J. Defining the standards for medical grade honey. J. Apicult. Res. 2020;59(2):125–135. [Google Scholar]
- Holubová A., Chlupáčová L., Cetlová L., Cremers N.A.J., Pokorná A. Medical-grade honey as an alternative treatment for antibiotics in non-healing wounds-A prospective case series. Antibiotics (Basel, Switzerland) 2021;10(8) doi: 10.3390/antibiotics10080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish J., Carter D.A., Shokohi T., Blair S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006;44(3):289–291. doi: 10.1080/13693780500417037. [DOI] [PubMed] [Google Scholar]
- Khan F., Bamunuarachchi N.I., Tabassum N., Kim Y.-M. Caffeic acid and its derivatives: antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021;69(10):2979–3004. doi: 10.1021/acs.jafc.0c07579. [DOI] [PubMed] [Google Scholar]
- Kim S., Woo E.-R., Lee D.G. Apigenin promotes antibacterial activity via regulation of nitric oxide and superoxide anion production. J. Basic Microbiol. 2020;60(10):862–872. doi: 10.1002/jobm.202000432. [DOI] [PubMed] [Google Scholar]
- Krishnakumar G.S., Mahendiran B., Gopalakrishnan S., Muthusamy S., Malarkodi Elangovan S. Honey based treatment strategies for infected wounds and burns: a systematic review of recent pre-clinical research. Wound Med. 2020;30(100188) [Google Scholar]
- Leong A.G., Herst P.M., Harper J.L. Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun. 2012;18(3):459–466. doi: 10.1177/1753425911422263. [DOI] [PubMed] [Google Scholar]
- Lima Â.C.O., Dias E.R., Reis I.M.A., Carneiro K.O., Pinheiro A.M., Nascimento A.S., Silva S.M.P.C., Carvalho C.A.L., Mendonça A.V.R., Vieira I.J.C., Braz Filho R., Branco A. Ferulic acid as major antioxidant phenolic compound of the Tetragonisca angustula honey collected in Vera Cruz - Itaparica Island, Bahia, Brazil. Brazil. J. Biol. Revista Brasleira de Biologia. 2022;84:e253599. doi: 10.1590/1519-6984.253599. [DOI] [PubMed] [Google Scholar]
- MacEwan D.J. TNF ligands and receptors–a matter of life and death. Br. J. Pharmacol. 2002;135(4):855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Loughrey A., Earle J.A.P., Millar B.C., Rao J.R., Kearns A., McConville O., Goldsmith C.E., Rooney P.J., Dooley J.S.G., Lowery C.J., Snelling W.J., McMahon A., McDowell D., Moore J.E. Antibacterial activity of honey against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Compl. Ther. Clin. Pract. 2008;14(2):77–82. doi: 10.1016/j.ctcp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Majtán J., Kovácová E., Bíliková K., Simúth J. The immunostimulatory effect of the recombinant apalbumin 1-major honeybee royal jelly protein-on TNFalpha release. Int. Immunopharm. 2006;6(2):269–278. doi: 10.1016/j.intimp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Mandal M.D., Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011;1(2):154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyi-Loh C.E., Ndip R.N., Clarke A.M. Volatile compounds in honey: a review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011;12(12):9514–9532. doi: 10.3390/ijms12129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., Al-Asiri S.A., Mahesar A.L., Ansari M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017;24(5):975–978. doi: 10.1016/j.sjbs.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel M.G., Antunes M.D., Faleiro M.L. Honey as a complementary medicine. Integr. Med. Insights. 2017;12 doi: 10.1177/1178633717702869. 1178633717702869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa A., Noureddine D., Saad A., Abdelmelek M., Abdelkader B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012;2(7):554–557. doi: 10.1016/S2221-1691(12)60096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S., Williamson D., Grey J., Harding K.G., Cooper R.A. Healing of an MRSA-colonized, hydroxyurea-induced leg ulcer with honey. J. Dermatol. Treat. 2001;12(1):33–36. doi: 10.1080/095466301750163563. [DOI] [PubMed] [Google Scholar]
- National Honey Board. (n.d.). Honey floral source guide. Retrieved April 21, 2022, from http://www.hcbees.org/HONEY_floral_guide.pdf.
- Nešović M., Gašić U., Tosti T., Horvacki N., Šikoparija B., Nedić N., Blagojević S., Ignjatović L., Tešić Ž. Polyphenol profile of buckwheat honey, nectar and pollen. R. Soc. Open Sci. 2020;7(12):201576. doi: 10.1098/rsos.201576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan V.C., Harrison J., Wright J.E.E., Cox J.A.G. Clinical significance of manuka and medical-grade honey for antibiotic-resistant infections: a systematic review. Antibiotics (Basel, Switzerland) 2020;9(11) doi: 10.3390/antibiotics9110766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto I., Taniguchi Y., Kunikata T., Kohno K., Iwaki K., Ikeda M., Kurimoto M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003;73(16):2029–2045. doi: 10.1016/s0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- Olaitan P.B., Adeleke O.E., Ola I.O. Honey: a reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007;7(3):159–165. doi: 10.5555/afhs.2007.7.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J., Sun F., Feng W., Xie Y., Ren L., Chen Y. Antimicrobial activity of galangin and its effects on murein hydrolases of vancomycin-intermediate Staphylococcus aureus (VISA) strain Mu50. Chemotherapy. 2018;63(1):20–28. doi: 10.1159/000481658. [DOI] [PubMed] [Google Scholar]
- Paget B.W., Kleffmann T., Whiteman K.E., Thomas M.F., McMahon C.D. Quantitative comparison of manuka and clover honey proteomes with royal jelly. BioRxiv. 2022 doi: 10.1371/journal.pone.0272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoh J., Low N.H. Purification and characterization of β-glucosidase from honey bees (Apis mellifera) Insect Biochem. Mol. Biol. 2002;32(6):679–690. doi: 10.1016/s0965-1748(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Qian W., Yang M., Wang T., Sun Z., Liu M., Zhang J., Zeng Q., Cai C., Li Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Protect. 2020;83(4):576–583. doi: 10.4315/JFP-19-469. [DOI] [PubMed] [Google Scholar]
- Rakha M.K., Nabil Z.I., Hussein A.A. Cardioactive and vasoactive effects of natural wild honey against cardiac malperformance induced by hyperadrenergic activity. J. Med. Food. 2008;11(1):91–98. doi: 10.1089/jmf.2006.172. [DOI] [PubMed] [Google Scholar]
- Ranneh Y., Ali F., Zarei M., Akim A.M., Hamid H.A., Khazaai H. Malaysian stingless bee and Tualang honeys: a comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT (Lebensm.-Wiss. & Technol.) 2018;89:1–9. [Google Scholar]
- Ranneh Y., Akim A.M., Hamid H.A., Khazaai H., Fadel A., Zakaria Z.A., Albujja M., Bakar M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Therap. 2021;21(1):30. doi: 10.1186/s12906-020-03170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M.P., Amorim J.M., Lima W.G., Brito J.C.M., da Cruz Nizer W.S. Effect of honey and propolis, compared to acyclovir, against Herpes Simplex Virus (HSV)-induced lesions: a systematic review and meta-analysis. J. Ethnopharmacol. 2022;287:114939. doi: 10.1016/j.jep.2021.114939. [DOI] [PubMed] [Google Scholar]
- Rossano R., Larocca M., Polito T., Perna A.M., Padula M.C., Martelli G., Riccio P. What are the proteolytic enzymes of honey and what they do tell us? A fingerprint analysis by 2-D zymography of unifloral honeys. PLoS One. 2012;7(11):e49164. doi: 10.1371/journal.pone.0049164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Gautam S., Maru G., Kawle D., Sharma A. Suppression of error prone pathway is responsible for antimutagenic activity of honey. Food Chem. Toxicol. 2012;50(3–4):625–633. doi: 10.1016/j.fct.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Silva B., Biluca F.C., Mohr E.T.B., Caon T., Gonzaga L.V., Fett R., Dalmarco E.M., Costa A.C.O. Effect of Mimosa scabrella Bentham honeydew honey on inflammatory mediators. J. Funct.Foods. 2020;72:104034. [Google Scholar]
- Silva B., Biluca F.C., Gonzaga L.V., Fett R., Dalmarco E.M., Caon T., Costa A.C.O. In vitro anti-inflammatory properties of honey flavonoids: a review. Food Res. Int. 2021;141:110086. doi: 10.1016/j.foodres.2020.110086. [DOI] [PubMed] [Google Scholar]
- Simúth J., Bíliková K., Kovácová E., Kuzmová Z., Schroder W. Immunochemical approach to detection of adulteration in honey: physiologically active royal jelly protein stimulating TNF-alpha release is a regular component of honey. J. Agric. Food Chem. 2004;52(8):2154–2158. doi: 10.1021/jf034777y. [DOI] [PubMed] [Google Scholar]
- Smaropoulos E., Cremers N.A.J. Treating severe wounds in pediatrics with medical grade honey: a case series. Clin. Case Rep. 2020;8(3):469–476. doi: 10.1002/ccr3.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh Kumar C. Syringic acid (SA) ‒ A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24(2):157–161. doi: 10.1016/s0305-4179(97)00113-7. [DOI] [PubMed] [Google Scholar]
- Tanleque-Alberto F., Juan-Borrás M., Escriche I. Antioxidant characteristics of honey from Mozambique based on specific flavonoids and phenolic acid compounds. J. Food Compos. Anal. 2020;86(103377) [Google Scholar]
- Thrasyvoulou A., Tananaki C., Goras G., Karazafiris E., Dimou M., Liolios V., Kanelis D., Gounari S. Legislation of honey criteria and standards. J. Apicult. Res. 2018;57(1):88–96. [Google Scholar]
- Tomasin R., Gomes-Marcondes M.C.C. Oral administration of Aloe vera and honey reduces Walker tumour growth by decreasing cell proliferation and increasing apoptosis in tumour tissue. Phytother Res. 2011;25(4):619–623. doi: 10.1002/ptr.3293. [DOI] [PubMed] [Google Scholar]
- Waller G.D. Evaluating responses of honey bees to sugar solutions using an artificial-flower feeder. Ann. Entomol. Soc. Am. 1972;65(4):857–862. [Google Scholar]
- Watts R., Frehner E. Evidence summary: wound management: medical-grade honey. Wound Pract. Res.: J. Austr. Wound Manag. Assoc. 2017;25(2):117–120. [Google Scholar]
- Weis W.A., Ripari N., Conte F.L., Honorio M., da S., Sartori A.A., Matucci R.H., Sforcin J.M. An overview about apitherapy and its clinical applications. Phytomedicine. 2022;2(2):100239. [Google Scholar]
- Wu M., Brown A.C. Applications of catechins in the treatment of bacterial infections. Pathogens. 2021;10(5) doi: 10.3390/pathogens10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen J., Wei W., Miao Y., Liang C., Wu J., Huang X., Yin L., Geng Y., Chen D., Ouyang P. A study of the antibacterial mechanism of pinocembrin against multidrug-resistant Aeromonas hydrophila. Int. Microbiol. 2022;25(3):605–613. doi: 10.1007/s10123-022-00245-w. [DOI] [PubMed] [Google Scholar]
- Yayinie M., Atlabachew M., Tesfaye A., Hilluf W., Reta C., Alemneh T. Polyphenols, flavonoids, and antioxidant content of honey coupled with chemometric method: geographical origin classification from Amhara region, Ethiopia. Int. J. Food Prop. 2022;25(1):76–92. [Google Scholar]
- Zou Z., Lopez D.L., Kanost M.R., Evans J.D., Jiang H. Comparative analysis of serine protease-related genes in the honey bee genome: possible involvement in embryonic development and innate immunity. Insect Mol. Biol. 2006;15(5):603–614. doi: 10.1111/j.1365-2583.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.