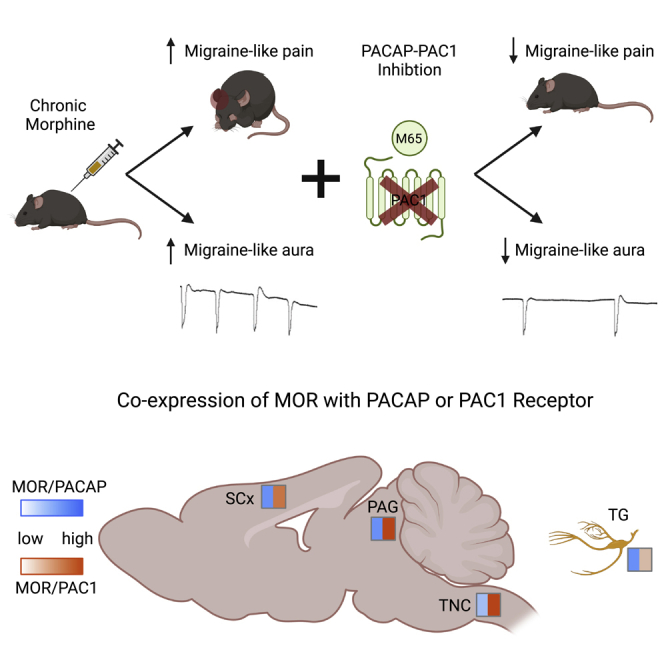
Summary
Opioids prescribed for pain and migraine can produce opioid-induced hyperalgesia (OIH) or medication overuse headache (MOH). We previously demonstrated that pituitary adenylate cyclase activating polypeptide (PACAP) is upregulated in OIH and chronic migraine models. Here we determined if PACAP acts as a bridge between opioids and pain chronification. We tested PACAP-PAC1 receptor inhibition in novel models of opioid-exacerbated trigeminovascular pain. The PAC1 antagonist, M65, reversed chronic allodynia in a model which combines morphine with the migraine trigger, nitroglycerin. Chronic opioids also exacerbated cortical spreading depression, a correlate of migraine aura; and M65 inhibited this augmentation. In situ hybridization showed MOR and PACAP co-expression in trigeminal ganglia, and near complete overlap between MOR and PAC1 in the trigeminal nucleus caudalis and periaqueductal gray. PACAPergic mechanisms appear to facilitate the transition to chronic headache following opioid use, and strategies targeting this system may be particularly beneficial for OIH and MOH.
Subject areas: Neuroscience, Sensory neuroscience, Cell biology
Graphical abstract
Highlights
-
•
Chronic morphine worsens allodynia and cortical excitability related to migraine
-
•
PACAP-PAC1 receptor inhibition mitigates these exacerbating effects of morphine
-
•
Mu opioid receptor is highly co-expressed with the PACAPergic system
-
•
PAC1 receptor is a promising target for opioid-induced medication overuse headache
Neuroscience; Sensory neuroscience; Cell biology
Introduction
Opioid analgesics in current use (e.g., hydrocodone, oxycodone, meperidine) act primarily at the mu opioid receptor (MOR) and are still commonly prescribed for migraine.1,2 Although opioids may provide acute relief, chronic use results in increased severity and progression of migraine from an episodic to a chronic state3,4; a phenomenon known as medication overuse headache (MOH).5 Furthermore, excessive use of prescription opioids by migraine patients is also a significant public health issue.6,7,8 Over 50% of patients in a recent study were prescribed opioids at some point for headache,2 and a large scale epidemiological study published in 2020 found that 36% of migraine patients continued to use or keep on hand opioids for headache management.1 In another study, opioids were administered in over half of all emergency room visits for migraine, and repeat visits to the emergency room were associated with opioid prescription.9 Even after successful initial opioid withdrawal, 20–50% of patients relapsed within the first year, and most within the first 6 months.10 The continued prescription of opioids for migraine puts a substantial number of patients at risk of developing MOH and potentially for prescription drug misuse and abuse.11,12 There is thus a desperate need to find effective therapies for opioid-induced MOH.
Pituitary adenylate cyclase-activating polypeptide (PACAP) has emerged as a therapeutic target for migraine.13,14,15,16 This neuropeptide is found in two forms as a 38 amino acid peptide (PACAP38) and a truncated version, PACAP27.17 PACAP38 is the predominant form making up 90% of the circulating peptide and is found in both the peripheral and central nervous system18 PACAP38 can bind to one of three Gs G-protein coupled receptors (GPCRs), VPAC1, VPAC2, or PAC1.19 However, its affinity for PAC1 is 100-fold higher relative to the other two receptors.20 In clinical studies, direct infusion of PACAP produces headache in healthy subjects and migraine in migraine patients.21,22 Further strengthening the relationship between PACAP and migraine, PACAP38 is increased in the ictal phase of migraine patients23; and the migraine therapeutic, sumatriptan, was correspondingly associated with reduced PACAP38 levels.24
Preclinical work also shows that PACAP produces migraine-like phenotypes as infusion in rodents causes allodynia25,26 light aversive behavior,27,28 meningeal dilation, and increased c-fos expression in the trigeminal nucleus caudalis (TNC).27 PACAP knockout mice also have reduced susceptibility to the effects of nitroglycerin (NTG), a known human migraine trigger commonly used to model migraine-associated effects in rodents.27 The PACAPergic system may play a distinct role in opioid-induced MOH. Our lab recently performed an unbiased large scale peptidomic study comparing mouse models of opioid induced hyperalgesia (OIH) and chronic migraine-associated pain.29 PACAP was one of the few neuropeptides with altered expression in both models. In confirmation experiments, we found that the PAC1 inhibitor, M65, blocked the development of cephalic allodynia in an NTG model of migraine-associated pain, as well as in a model of OIH using chronic escalating doses of morphine.29 PACAP may act as mechanistic bridge between opioid use and migraine chronification.
Although PACAP has been investigated as a target for migraine, to the best of our knowledge it has not been considered for opioid-induced MOH; and one of the aims of the current study was to explore this role further. To date, most preclinical studies either model chronic migraine or OIH. To better reflect clinical MOH, we first developed 2 models of opioid-exacerbated migraine phenotypes. We modified the commonly used NTG model of chronic migraine in which high doses of NTG result in the development of chronic allodynia.30,31,32,33,34 We found that much lower doses of NTG did not produce chronic hypersensitivity unless combined with chronic morphine. Furthermore, we investigated the effect of morphine on a mechanistically distinct model of migraine – cortical spreading depression (CSD), a physiological correlate of migraine aura.35 We found that repeated escalating doses of morphine also increased CSD events. We next tested the PAC1 inhibitor M65 within these models of opioid exacerbated migraine-associated pain and aura. M65 effectively reduced MOH-associated symptoms in both models. Finally, we investigated the cellular expression of the PACAPergic system relative to the mu opioid receptor (MOR) using in situ hybridization; and observed co-expression of MOR with PACAP or PAC1 in key headache processing regions. Together our results establish two new models of opioid induced MOH and demonstrate that inhibition of the PACAPergic system may be an effective therapeutic strategy for this disorder.
Results
Repeated opioid administration exacerbates cephalic allodynia induced by low dose NTG
MOR agonists are still prescribed for the treatment of headache disorders despite the potential to cause MOH.4,36,37 In this case opioids are overlaid on top of migraine pathophysiology, and our first aim was to establish migraine models that reflect this interaction. To this end we repeatedly treated animals with morphine (10 mg/kg, SC) or vehicle for 11 days and paired it with an intermittent low dose of the human migraine trigger NTG (0.01 mg/kg, IP) starting on day 3 (Figure 1A). We tested cephalic mechanical thresholds across the 11-day period; and responses were assessed before daily treatments (Basal Responses, Figure 1B), and 2 h following the NTG/Veh injection (Post-treatment Responses, Figure 1C). These doses of NTG or morphine alone did not produce chronic allodynia, but the combination of the two resulted in significant cephalic allodynia as observed on days 7 and 11 (Figure 1B). The low dose of NTG produced an acute allodynia 2 h post-injection (Figure 1C, open squares). This hypersensitivity was blocked by morphine on the first day of NTG treatment (Figure 1C, filled square, day 3), but the anti-allodynic effects of morphine were lost by day 7. We also separated this data by sex, and did not observe any significant differences between males and females (Figure S1). This data suggests that morphine tolerance is observed in this model, or that the combination of NTG and morphine facilitates signaling mechanisms that promote allodynia that is insensitive to opioids. Chronic opioid treatment exacerbates the effect of low dose NTG to produce chronic cephalic allodynia.
Figure 1.
Morphine combined with low dose NTG induces chronic cephalic allodynia
(A) Schematic of testing and injection schedule, M&F C57BL6/J mice were treated every day with vehicle or morphine (10 mg/kg, SC; Morph) for 11 days. On days 3,5,7,9, and 11, mice were co-administered vehicle or low-dose nitroglycerin (0.01 mg/kg, IP; NTG) represented by white circles. The green oval shows days in which cephalic allodynia was assessed.
(B) Periorbital mechanical thresholds were determined before drug treatment. Neither NTG nor morphine produced chronic allodynia alone. However, the combination of the two produced severe cephalic allodynia starting on day 7 which persisted to the end of treatment, p < 0.001 effect of treatment, time, and interaction, three-way RM ANOVA and Holm-Sidak post hoc analysis. ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 relative to Veh-Veh on the same day, n = 18/group.
(C) Periorbital mechanical thresholds were also assessed 2 h after vehicle, NTG, morphine administration to determine acute effects of treatment. On day 3, only the Veh-NTG group showed acute cephalic allodynia, but by day 7 the Morph-NTG groups also demonstrated severe allodynia. p< 0.001 effect of treatment, time, and interaction, three-way RM ANOVA and Holm-Sidak post hoc analysis. ++++p < 0.0001 Veh-NTG compared to Veh-Veh on same day. ∗∗∗∗p < 0.0001 Morph-NTG compared to Veh-Veh on same day n = 18/group. Data are represented as mean ± SEM.
Opioid induced hyperalgesia model results in increased cortical spreading depression
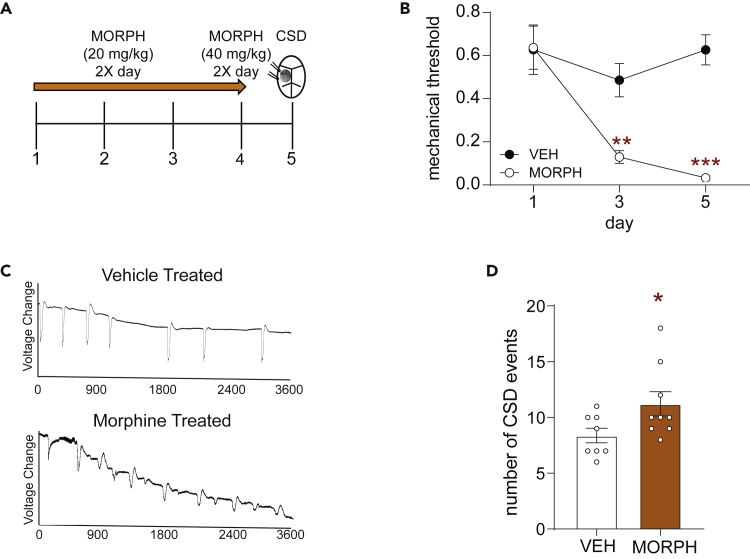
Approximately one-third of migraine patients experience migraine aura as part of their symptoms, and cortical spreading depression (CSD) is thought to be the electrophysiological correlate of aura.35 To examine if chronic MOR agonist treatment results in increased susceptibility to CSD we subjected mice to an established OIH model shown to cause cephalic allodynia.38,39,40,41,42 Mice were treated with morphine or vehicle twice daily for 4 days (Figure 2A, 20 mg/kg/injection on days 1–3 and 40 mg/kg/injection on day 4). As reported previously,38,41,42 this dosing regimen produced significant cephalic allodynia (Figure 2B). We tested mice in the CSD model30,38,43,44 on day 5, ∼16 h after the final morphine treatment. Mice were recorded for an hour during continual administration of 1M KCl directly onto the dura. The total number of CSD events was counted based on visual reflectance changes and local field potential (LFP) recordings (Figure 2C). Chronic opioid treatment significantly increased CSD events relative to vehicle treated controls (Figure 2D). This regimen of morphine did not alter amplitude of CSD (Figure S2). We also tested the dosing regimen of morphine used in the combined morphine-NTG model described in Figure 1. In this case, mice were treated with vehicle or morphine (10 mg/kg SC) daily for 11 days and CSD was tested on day 12. This dosing regimen of morphine does not cause cephalic allodynia (Figures 1B and 1C), and did not significantly exacerbate CSD (Figure S3). These data indicate that OIH not only increases cephalic hyperalgesia but also increases frequency of CSD events.
Figure 2.
Chronic opioid treatment results in increased cortical spreading depression events
(A) Schematic of testing and injection schedule. Female C57BL6/J mice were treated twice daily with vehicle or morphine (20 mg/kg/injection Days 1–3, 40 mg/kg/injection Day 4, SC). On day 5 mice underwent CSD testing.
(B) Periorbital mechanical thresholds were assessed before morning treatment of vehicle or morphine. Chronic morphine resulted in increased periorbital allodynia. p< 0.001 effect of treatment, time, and interaction, two-way ANOVA and Holm-Sidak Post hoc analysis. ∗∗p < 0.01, ∗∗∗p < 0.001. Morph compared to Veh on Day 1 n = 12/group.
(C) Representative line tracing of CSDs in vehicle (Top) or morphine (Bottom) treated mice over the 1 h recording session.
(D) Mice treated with morphine showed a significant increase in the average number of CSD events recorded over an hour. Unpaired t-test. ∗p < 0.05 n = 8–9/group. Data are represented as mean ± SEM.
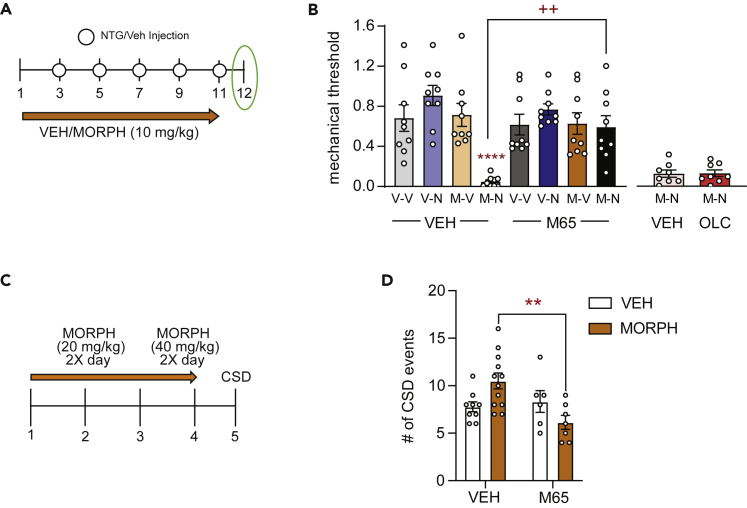
PAC1 antagonist inhibits allodynia induced by the mixed morphine-NTG model
We next determined if inhibition of the PACAPergic system could block cephalic allodynia in our opioid-facilitated migraine model. Mice were treated in the opioid facilitated migraine model as described in Figure 1 (Figure 3A). On day 12, 24 h, after their final drug treatment, mice were injected with vehicle or the PAC1 receptor antagonist, M65 (Figure 3A, 0.1 mg/kg IP)45 and tested for cephalic thresholds 30 min post-treatment. At this time point, mice treated with morphine and NTG continued to show significant allodynia (Figure 3B, morphine-NTG-vehicle). Acute treatment with M65 completely inhibited allodynia in this group (Figure 3B, morphine-NTG-M65). Of importance, we also determined the effect of olcegepant (1 mg/kg IP, 2h post-injection), a calcitonin gene related peptide (CGRP) receptor antagonist, in this model. Olcegepant did not significantly inhibit allodynia induced by chronic morphine plus NTG (Figure 3B, morphine-NTG-OLC). These data suggest that inhibition of the PACAPergic system specifically can ameliorate opioid-facilitated migraine.
Figure 3.
The PAC1 receptor inhibitor, M65, blocks opioid facilitation of NTG-induced allodynia and CSD
(A) Schematic of testing and injection schedule for model of opioid facilitated migraine-like pain. Male and female C57BL6/J mice were treated every day with vehicle or morphine (10 mg/kg, SC; Morph) for 11 days. Starting on day 3, mice were co-administered vehicle or low-dose nitroglycerin (0.01 mg/kg, IP; NTG) every other day for the remaining 9 days, represented by white circles. On day 12, 24 h follow the last treatment, mice were treated with either vehicle or M65 (0.1 mg/kg, IP) and tested 30 min later.
(B) Periorbital mechanical thresholds were determined following M65 or vehicle administration. Chronic Morph-NTG treated mice continued to show cephalic allodynia at this time point; an effect that was inhibited by M65. p< 0.001 interaction, three-way ANOVA and Holm-Sidak post hoc analysis. ∗∗∗∗p < 0.0001 Morph-NTG-Veh compared to Veh-Veh-Veh group; ++p < 0.01 Morph-NTG-M65 compared to Morph-NTG-Veh. n = 9/group. A separate group of mice were treated with morphine/NTG and then tested on day 12 with vehicle or olcegepant (1 mg/kg IP, 2h post-injection).
(C) Schematic of injection schedule for opioid facilitated CSD. Female C57BL6/J mice were treated twice daily with vehicle or morphine (20 mg/kg/injection days 1–3, 40 mg/kg/injection day 4, SC). On day 5 mice underwent CSD procedure. After the initial 400 s mice were treated with either vehicle or M65 (0.1 mg/kg IP) and recorded for an additional 3600 s.
(D) Mice treated with chronic morphine showed an increased number of CSD events, and this increase was inhibited by M65 treatment. p< 0.01 effect of M65, and interaction, two-way ANOVA and Holm-Sidak post hoc analysis. ∗∗p < 0.01 Morph-Veh compared to Morph-M65 n = 6–12/group. Data are represented as mean ± SEM.
PAC1 antagonist inhibits exacerbation of CSD by chronic morphine
We tested if PAC1 inhibition could affect CSD and/or opioid-exacerbation of CSD. Mice were treated in the 4 days OIH regimen described above (Figure 3C), and on day 5 were tested in the CSD paradigm. Mice were treated with vehicle or M65 (0.1 mg/kg IP) 400 s following the beginning of KCl stimulation and were recorded for an additional 3600 s. As was observed in Figure 2, chronic morphine resulted in a significant increase in CSD events in vehicle challenged mice (Figure 3D, left panel). Intriguingly, M65 did not reduce the number CSD events in chronic vehicle treated animals, but it significantly reduced the exacerbation of CSD by morphine (Figure 3D, right panel). These data suggest that a PACAPergic mechanism may mediate the facilitation of CSD by chronic opioid treatment.
MOR is co-expressed with PACAP and/or PAC1 in migraine processing regions
We investigated if there was evidence for direct interaction between MOR and PACAP-PAC1 receptor. We used fluorescent in situ hybridization to map transcripts for Oprm1 (MOR, pink), Adcyap1 (PACAP, blue), and Adcyap1R1 (PAC1, green); and examined the following migraine/pain processing regions: trigeminal ganglia (TG), trigeminal nucleus caudalis (TNC, layer 1–4), somatosensory cortex (SSC, layers 4–5), and lateral-ventrolateral periaqueductal gray (PAG)46,47 (Figure 4A). We also included nuclear DAPI staining (dark blue) for anatomical context. In the TG, approximately 42% of MOR+ cells also expressed PACAP, whereas only ∼23% expressed PAC1 (Figure 4B). In contrast, in the TNC almost all MOR+ cells also co-expressed PAC1, whereas only ∼22% expressed PACAP (Figure 4C). In the SSC, MOR was highly co-expressed with both PACAP and PAC1, ∼57% and ∼67%, respectively (Figure 4D). Similarly, in the PAG we observed ∼50% co-expression of MOR and PACAP, and almost 100% co-expression of MOR+ cells with PAC1 (Figure 4E). We also performed an initial study to determine if MOR and PAC1 transcripts were expressed in neurons. We found that MOR and PAC1 broadly co-localized with the neuronal marker, NeuN, in all four regions (Figure S4). These results demonstrate that there is very high cellular co-expression between MOR and components of the PACAPergic system.
Figure 4.
Co-expression of MOR with PACAP and PAC1 in key migraine and pain processing regions
In situ hybridization was performed on brain slices from adult M&F mice using probes for Oprm1 (MOR, pink), Adcyap1 (PACAP, light blue), and Adcyap1R1 (PAC1, green), as well as DAPI (dark blue).
(A) Representative images from trigeminal ganglia (TG), trigeminal nucleus caudalis (TNC), somatosensory cortex (SSC), and periaqueductal gray (PAG). Percentage of MOR+ cells that co-express PACAP or PAC1 in (B) trigeminal ganglia, (C) trigeminal nucleus caudalis, (D) somatosensory cortex, and (E) periaqueductal gray. Data are represented as mean ± SEM. Scale bar 100 μm.
Discussion
Opioids are still commonly prescribed for the treatment of headache disorders including migraine.1,2,36 Although opioids can provide limited relief from the acute migraine attack phase repeated use can result in transition to MOH.3,4 To better understand the drivers of opioid-induced MOH we developed two models which reflect opioid exacerbation of migraine-associated pain and aura. Chronic morphine treatment combined with a low dose of the human migraine trigger, NTG, resulted in chronic cephalic allodynia not observed with either treatment alone. We also demonstrated that a traditional OIH paradigm not only resulted in cephalic allodynia but also increased CSD events. We identified PAC1 receptor as a promising therapeutic target for opioid-induced MOH, as PAC1 inhibition blocked opioid facilitation in both of these models. Importantly, CGRP blockade was ineffective in reducing opioid-induced MOH. This finding suggests that opioid-induced MOH is distinctly regulated by the PACAPergic system; and not just by migraine mechanisms more generally. This study also demonstrates that MOR, PACAP, and PAC1 are co-expressed in numerous sites associated with head pain processing, further supporting the idea of direct interaction between these two systems.
Clinically, OIH is treated by opioid taper and cessation, which can be difficult to implement as patients are reluctant to stop using drugs which they believe are treating their pain, and because of opioid withdrawal.48 Even after successful initial opioid withdrawal, 20–50% of headache patients relapse within the first year, and most of those patients relapse within the first 6 months.10 A greater understanding of the pathophysiology underlying MOH would allow for discovery of more targeted treatments for this disorder. One of our aims was to produce preclinical models that reflected the mechanistic interactions between chronic opioid treatment and migraine pathology. NTG evokes migraine in migraine patients,31 and has been used as a human experimental model of migraine.49 Our laboratory has used chronic intermittent administration of higher dose NTG (10 mg/kg) to model chronic migraine-associated pain.50,51 In the current study we found that a much lower dose of NTG (0.01 mg/kg) induced acute cephalic allodynia but did not result in chronic hypersensitivity. Similarly, frequent and escalating doses of morphine results in OIH,40,52,53 however, the opioid regimen used in our mixed NTG-opioid model was insufficient to produce OIH. Only when low-dose NTG and morphine were combined did we observe the development of chronic cephalic allodynia. MOH patients show increased pain sensitivity in cephalic and extra-cephalic regions,54 and in epidemiological studies MOH is associated with increased pain severity and cutaneous allodynia.1,3 Therefore, the allodynia observed in this animal model of opioid-facilitated migraine pain reflects clinical observations and may be of translational significance.
CSD captures a mechanistically different aspect of migraine relative to the NTG model. CSD is an electrophysiological correlate of migraine aura and widely held to be the cause of aura symptoms.35 Susceptibility to CSD has been linked to increased cortical excitability. Genetic knock-in models of familial/monogenic forms of migraine show increased susceptibility to CSD.55,56 Furthermore, multiple migraine preventives with diverse sites of action inhibit CSD,57,58 and this model is used to screen novel preventives. We show that pretreatment of mice with an opioid paradigm shown to cause OIH,29,38,40,42,53 also increases the number of CSD events in response to KCl administration. Increased CSD events were also observed following chronic treatment with paracetamol,59 and chronic sumatriptan exposure resulted in a decrease in the stimulation threshold required to generate a CSD event.60 Together with our findings, these studies show that the mechanisms underlying CSD are sensitive to medication overuse, and a combined drug-CSD model can be used to model MOH.
In the NTG model, a less severe dosing regimen of morphine (10 mg/kg daily for 10 days) was sufficient to exacerbate NTG-induced chronic allodynia. In contrast, this dosing regimen did not increase CSD events. Although NTG and CSD are considered migraine triggers, they are mechanistically distinct. For example, NTG infusion in migraine patients does not evoke aura, even in migraine with aura patients.32 Our results suggest that mechanisms regulating migraine-like pain are more sensitive to opioid administration. Considering the high expression of MOR in pain processing regions, over-stimulation of these receptors would directly feed into mechanisms regulating allodynia and hyperalgesia. Outside of headache, many clinical studies also indicate that chronic opioids can result in increased hyperalgesia and allodynia, including in patients suffering from peripheral pain or opioid use disorders.48,61,62,63
In a previous study we had identified PACAP as a potential bridge between chronic opioid treatment and migraine chronicity.29 We followed up on these findings and demonstrated that PAC1 inhibition effectively blocked both the chronic allodynia induced by NTG-morphine treatment, as well as the facilitatory effects of morphine on CSD. The latter findings are particularly intriguing as M65 did not itself decrease CSD events (i.e. in vehicle treated mice), as has been demonstrated for migraine preventives.57,58 PAC1 inhibition only decreased the augmentation of CSD induced by chronic morphine. These results support the idea that PACAPergic mechanisms are specifically enhanced in response to chronic opioid treatment which can feed into migraine pathophysiology. A number of studies indicate that MOR, PACAP, and PAC1 are expressed at similar anatomical sites, including the PAG, trigeminal complex, and somatosensory cortex.13,64 Our in situ hybridization studies confirmed expression in these regions and show that at a cellular level MOR is abundantly co-expressed with PACAP and PAC1. Transcript expression does not always correspond with protein expression or functional interaction, and future studies will address these issues more specifically. Nevertheless, these findings are the first step to showing that chronic opioid action at MOR could directly impact PACAP and PAC1 expression and function.
Targeting PACAP and its receptor PAC1 is under investigation as a potential migraine therapy.13,16 A rodent specific PAC1 antibody was able to inhibit evoked nociceptive activity in rats,65 and we have shown that M65 can block the development of chronic migraine-associated pain induced by NTG.29 AMG-301, a human monoclonal antibody targeting the PAC1 receptor, recently completed a Phase II clinical trial for migraine; but did not show efficacy over placebo.66 Although disappointing, the antibody was well tolerated with minimal adverse effects, and there may still be a use for this strategy for the treatment of opioid-induced MOH or OIH. These results may also point to the need to target PACAP, which can also bind to VPAC1 and VPAC2. Anti-PACAP antibodies developed by Lundbeck and Lilly may address this question. Future clinical studies will also have to consider that PACAP levels increase in lactating women and is expressed in breast milk, therefore these strategies may be contraindicated for this population. In addition, peripheral PAC1 inhibition may be insufficient for efficacy in migraine. In our peptidomic study, PACAP levels were most altered in the PAG following chronic NTG or morphine treatment,29 supporting the role of central PACAPergic mechanisms in migraine and MOH.
The work presented in the current study suggests that PAC1 may be a particularly effective target for opioid-induced MOH. M65 effectively blocked chronic cephalic allodynia induced by morphine-NTG treatment, an effect not observed with olcegepant. Both PACAP and CGRP are implicated in migraine mechanisms, and both evoke migraine in migraine patients.67 How MOR regulates CGRP and its receptor is relatively underexplored. Immunohistochemical analysis has shown co-expression between MOR and CGRP in trigeminal ganglia in rats,68 but another study found very little expression of MOR in rat dura, and even rarer co-expression with CGRP.69 In higher order brain regions, high co-expression of MOR and CALCA transcripts were observed in the parabrachial nucleus.70 In addition, this co-expression may change following chronic opioid or pain conditions, and this will be explored in future studies. Headache patients are heterogeneous and spontaneous migraines may be endogenously generated in each individual by different combinations of pro-migraine peptides and signaling molecules. Our findings suggest that opioid-induced MOH may be particularly weighted toward PACAPergic mechanisms; and that PACAP-PAC1 is a therapeutic target for this disorder.
Limitations of the study
In this study we found that PACAP-PAC1 receptor facilitated behaviors associated with opioid-induced MOH. This work suggests that PACAP or PAC1 targeting therapies would be beneficial to patients suffering from this disorder. However, MOH patients usually have a long history of headache and have likely tried or continue to use or overuse many different therapies. Therefore, the mechanisms regulating their headaches are expected to be due to adaptations at many different levels in the central and peripheral nervous system. This heterogeneity is not captured in our preclinical study. Technically, this study was limited in that we only examined transcript expression of MOR, PACAP, and PAC1 which cannot fully reflect where the translated receptors and peptide are expressed in the cell. For example, proteins can be made in one part of the neuron but expressed or released in far-reaching projection sites. Unfortunately, antibodies targeting PACAP and PAC1 receptor are not always selective under standard immunohistochemistry protocols. Future studies will test new antibodies to overcome this limitation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Brain and trigeminal ganglia tissue from C57 mice used in the studies described in Animals section of methods | Mice described within paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| M65 | Bachem | CAS Number: 1872440-65-7 |
| Olcegepant | Tocris | Catalog Number: 4561 CAS Number:204697-65-4 |
| Nitroglycerin | American Regent | Pack NDC#:0517-4810-25 |
| Morphine | Henry Scheinn | Catalog Number: 1338023 |
| Potassium Chloride | Sigma-Aldrich | Catalog Number: P9541 CAS Number: 7447-40-7 |
| Critical commercial assays | ||
| RNAscope Fluorescent Multiplex Detection Reagents | ACDBio | Cat# 320851 |
| Experimental models: Organisms/strains | ||
| C57BL6/J mice | Jackson Laboratories | RRID: IMSR_JAX:000664 |
Resource availability
Lead contact
Further information and requests for specific reagents or antibodies can be directed to the lead contact Amynah Pradhan (amynah@wustl.edu).
Material availability
No unique plasmids, animal lines, or reagents were used in any of these studies. All materials are publicly available and distributers are listed in the manuscript where appropriate.
Experimental model and subject details
Animals
An equal number of adult male and female C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME. USA, RRID IMSR_JAX:000664) were used in this study; except for CSD experiments, where only females were used, as they are more sensitive to CSD induction.71 Mice were aged between 9 and 16 weeks. All mice were group housed with a 12hour–12hour light-dark cycle, in which lights were turned on at 07:00 and turned off at 19:00. Food and water were available ad libitum and mice weighed between 20 and 30g. On test days weights were recorded and the experimenters were blinded. All experimental procedures were approved by the University of Illinois at Chicago Office of Animal Care and Institutional Biosafety Committee, in accordance with Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International guidelines and the Animal Care policies of the University of Illinois at Chicago. All results are reported according to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All animals were monitored continuously throughout experiments and no adverse effects were observed. All injections were in a volume of 10 mL/kg.
Method details
Sensory sensitivity testing
At the beginning of each experiment a basal test for mechanical threshold was recorded, mice were counterbalanced into groups from this measurement. All sensory sensitivity tests were conducted in the same behavior room. The behavior room is separated from the vivarium and has low light (∼35–50 lux) and low noise conditions. All tests were performed during the light cycle between 08:00 and 17:00. The testing rack consisted of individual plexiglass boxes with a 4 oz paper cup in each box. Mice were habituated to the testing racks for 2 consecutive days before the initial test day. On test days mice were again habituated to the racks 20 minutes before the first test measurement. Mice were tested while in the paper cup. The periorbital region, caudal to the eyes and near the midline, was tested to assess cephalic mechanical thresholds. Manual von Frey hair filaments were used in an up-and-down method.72 The filaments had a bending force ranging from 0.008g to 2g. The first filament used was 0.4g. Following the first filament if there was no response a heavier filament (up) was used, alternatively a response would result in the use of a lighter filament (down). Response were defined as shaking of the head, repeated pawing, or cowering away from the filament following a bend. Following the first response the up-and-down method was continued for 4 additional filaments. Mice were tested with the PAC1 inhibitor, M65 (0.1 mg/kg IP); the CGRP receptor antagonist, olcegepant (1 mg/kg IP), or vehicle (0.9% saline) on day 12, 24h after their final morphine/NTG injection.
Model of opioid exacerbated migraine
Nitroglycerin (NTG) was purchased at a concentration of 5 mg/mL, in 30% alcohol, 30% propylene glycol and water (American Regent, NY, USA). NTG was diluted on each test day in 0.9% saline to make a working solution of 1 mg/mL. This solution was further diluted in saline for a final dose of 0.01 mg/kg (0.001 mg/mL). Morphine (10 mg/kg) or vehicle (saline) was administered once daily subcutaneously for 11 days. Beginning on day 3, 30 minutes after the morphine/vehicle injection, animals received NTG (0.01 mg/kg, IP) or vehicle (saline). Basal mechanical thresholds were assessed on days 1, 3, 7, and 11 prior to that days’ morphine/vehicle treatment. A post-treatment measurement was also taken 2 hours after the NTG/veh injection on days 3, 7, and 11.
Model of opioid exacerbated aura
Before CSD measurement, mice underwent an OIH protocol in a similar matter to previous studies in our lab and others.29,73,74 Only female mice were used in this model. Mice received morphine (Sigma Chemical, St. Louis, MO) 20 mg/kg SC twice daily, once in the morning around 09:00 and again at 17:00, for days 1–3. On day 4 the dose was escalated to 40 mg/kg SC/injection, which was also given twice that day. Vehicle(saline) was similarly administered to control mice. Mice were tested in the CSD model on day 5, 18–20h following their final morphine/vehicle injection. In a pilot experiment we also tested another morphine paradigm – 10 mg/kg SC daily for 11 days with CSD on day 12 – which did not significantly exacerbate CSD (Figure S3).
Cortical spreading depression model
The model of cortical spreading depression/depolarization (CSD) used in these studies is based on previously published work.30,38,75 Mice were randomly grouped into vehicle or M65 (0.1 mg/kg, IP). For the CSD procedure mice were anesthetized with isoflurane (induction 3–4%; maintenance 0.75 to 1.25%; in 67% N2/33% O2) and placed in a stereotaxic frame on a homoeothermic heating pad. Core temperature (37.0 ± 0.5°C), oxygen saturation (∼ 99%), heart rate, and respiratory rate (80–120 bpm) were continuously monitored (PhysioSuite; Kent Scientific Instruments, Torrington, CT, USA). Mice were repeatedly tested with tail and hind paw pinch to ensure proper anesthetic levels were maintained.
CSD was verified in two ways, optical intrinsic signal (OIS) and electrophysiological recordings, as has been shown in similar studies30,43,75 For OIS a thinned rectangular region of the skull was made about 2.5 × 3.3 mm2 (∼0.5 mm from sagittal, and ∼1.4 from coronal and lambdoid sutures) of the right parietal bone. A dental drill (Fine Science Tools, Inc., Foster City, CA, USA) was used to thin the skull. To increase transparency mineral oil was applied to the thinned region, which allowed for further visualization of the parenchyma and vasculature. For video recording a green LED (520 nm) light illuminated the skull throughout the experiment (1-UP; LED Supply, Randolph, VT, USA). Reflectance was collected with a lens (HR Plan Apo 0.5 × WD 136) through a 515LP emission filter on a Nikon SMZ 1500 stereomicroscope (Nikon Instruments, Melville, NY, USA). Images were acquired at 1–5 Hz using a high-sensitivity USB monochrome CCD (CCE-B013-U; Mightex, Pleasanton, CA, USA) with 4.65-micron square pixels and 1392 × 1040 pixel resolution.
Two burr holes were drilled lateral to the thinned window around the midpoint of the rectangle. The burr holes were drilled deeper than the thinned skull portion such that the dura was exposed, but not so deep that the dura was broken. Local field potentials (LFPs) were recorded using a pulled glass pipette filled with saline and attached to an electrode, which was further connected to an amplifier. The electrode was placed inside of the lateral burr hole such that it was inside of the cortical tissue. A separate ground wire was placed underneath the skin caudal to the skull, which was used to ground the LFPs. After set up, the LFP was recorded for an hour to allow for stabilization in the case that a CSD occurred during the placement of the electrode or the thinning surgery. After stabilization a second pulled glass pipette was filled with 1M KCl and placed into the rostral burr hole, ensuring there was no direct contact with the brain or surrounding skull. Once placed, an initial flow of KCl was started and an even flow was maintained so that a constant small pool of KCl filled the burr hole. Any excess liquid was removed with tissue paper. After initial KCl administration mice were recorded for 400 seconds. Following which mice were treated with M65 or vehicle and then recorded for a remaining 3,600 seconds, for a total recording time of 4,000 seconds. Animals were only included in the final analysis if at least 2 CSD events occurred within the first 400s of recording. No animals were excluded in this study. Following the recording, video and LFP were analyzed and used to count the number of CSD events that occurred within the hour recording. Following the procedure mice were euthanized by anesthetic overdose followed by decapitation.
RNAScope fluorescent in situ hybridization
RNAscope kit was purchased from Advanced Cell Diagnostics RNAScope Technology (ACD Bioscience). C57Bl6/J mice were anesthetized, brain and TG were collected and immediately frozen. Frozen tissue was cut on a cryostat at 14 μm, collected on slides, and processed per the manufacturer’s protocol. Every 4th section was quantified for each brain region. The probes used were targeted against the mouse genes for Oprm1, Adcyap1, and Adcyap1r1.
Quantification and statistical analysis
Statistical analysis
The sample size needed for each experiment was either based on similar previous experiments or calculated through power analysis where the minimal detectable difference in means = 0.3, expected standard deviation of residuals = 0.2, desired power = 0.8, alpha = 0.05. Each experiment was replicated with separate cohorts of animals to ensure reproducibility. All data were analyzed using GraphPad Prism (GraphPad, San Diego, CA). The level of significance (α) for all tests was set to p < 0.05. Unpaired, two-tailed, t-test was performed to determine effect of morphine on CSD; 2-way ANOVA to determine effect of M65 on morphine-CSD. Three-way ANOVA was performed for allodynia experiments. Post hoc analysis was conducted using Holm-Sidak analysis to correct for multiple comparisons. Post hoc analysis was only performed when F values achieved p < 0.05. All values in the text are reported as mean ± SEM.
Three-way repeated measure ANOVA was performed in Figure 1, following significant interaction within this test, post hoc analysis was performed using the Holm-Sidak test. Figure 1B ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 relative to the vehicle-vehicle group on the same test day. Figure 1C ++++p < 0.0001 vehicle-NTG group compared to vehicle-vehicle Group; ∗∗∗∗p < 0.0001, morphine-NTG group compared to vehicle-vehicle group same day.
Figure 2A a two-way ANOVA was performed followed by post hoc analysis using the Holm-Sidak test, ∗∗p < 0.01, ∗∗∗p < 0.001 morphine compared to vehicle on Day 1. An unpaired t-test was also used in Figure 2D ∗p < 0.05.
Figure 3 used a three-way ANOVA and post hoc analysis with the Holm-Sidak test; ∗∗∗∗p < 0.0001 morphine-nitroglycerin-vehicle Group compared to the vehicle-vehicle-vehicle group; ++p < 0.01 morpine-nitroglycerin-M65 group compared to the morphine-nitroglycerin-vehicle group. A two-way ANOVA and post hoc analysis through Holm-Sidak test, ∗∗p < 0.01 morphine-vehicle Group compared to the morphine-M65 group.
Acknowledgments
This work was funded by NIH grants: DA040688 (AAP) and NS109862 (AAP).
Author contributions
Z.B. and A.A.P. designed experiments; Z.B., E.M., K.S., A.T., and H.C., performed experiments; Z.B. and A.A.P. analyzed data; Z.B. and A.A.P. wrote the manuscript.
Declaration of interests
The authors declare no competing interest.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
We worked to ensure sex balance in the selection of non-human subjects.
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location.
One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research.
While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: February 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.105950.
Supplemental information
Data and code availability
-
•
No new code was used in these studies.
-
•
No large dataset was generated through these studies.
-
•
All other data are available upon request through the lead contact.
References
- 1.Lipton R.B., Buse D.C., Friedman B.W., Feder L., Adams A.M., Fanning K.M., Reed M.L., Schwedt T.J. Characterizing opioid use in a US population with migraine: results from the CaMEO study. Neurology. 2020;95:e457–e468. doi: 10.1212/wnl.0000000000009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minen M.T., Lindberg K., Wells R.E., Suzuki J., Grudzen C., Balcer L., Loder E. Survey of opioid and barbiturate prescriptions in patients attending a tertiary care headache center. Headache. 2015;55:1183–1191. doi: 10.1111/head.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwedt T.J., Alam A., Reed M.L., Fanning K.M., Munjal S., Buse D.C., Dodick D.W., Lipton R.B. Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study. J. Headache Pain. 2018;19:38. doi: 10.1186/s10194-018-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigal M.E., Lipton R.B. Excessive opioid use and the development of chronic migraine. Pain. 2009;142:179–182. doi: 10.1016/j.pain.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Author Anonymous Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 6.Colás R., Muñoz P., Temprano R., Gómez C., Pascual J. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338–1342. doi: 10.1212/01.wnl.0000120545.45443.93. [DOI] [PubMed] [Google Scholar]
- 7.Reid M.C., Engles-Horton L.L., Weber M.B., Kerns R.D., Rogers E.L., O'Connor P.G. Use of opioid medications for chronic noncancer pain syndromes in primary care. J. Gen. Intern. Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diener H.C., Holle D., Solbach K., Gaul C. Medication-overuse headache: risk factors, pathophysiology and management. Nat. Rev. Neurol. 2016;12:575–583. doi: 10.1038/nrneurol.2016.124. [DOI] [PubMed] [Google Scholar]
- 9.Friedman B.W., West J., Vinson D.R., Minen M.T., Restivo A., Gallagher E.J. Current management of migraine in US emergency departments: an analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301–309. doi: 10.1177/0333102414539055. [DOI] [PubMed] [Google Scholar]
- 10.Katsarava Z., Limmroth V., Finke M., Diener H.C., Fritsche G. Rates and predictors for relapse in medication overuse headache: a 1-year prospective study. Neurology. 2003;60:1682–1683. doi: 10.1212/01.wnl.0000063322.14078.90. [DOI] [PubMed] [Google Scholar]
- 11.Stovner L., Hagen K., Jensen R., Katsarava Z., Lipton R., Scher A., Steiner T., Zwart J.A. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 12.Bigal M.E., Serrano D., Reed M., Lipton R.B. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–566. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- 13.Vollesen A.L.H., Amin F.M., Ashina M. Targeted pituitary adenylate cyclase-activating peptide therapies for migraine. Neurotherapeutics. 2018;15:371–376. doi: 10.1007/s13311-017-0596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerman S., Goadsby P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci. Transl. Med. 2015;7:308ra157. doi: 10.1126/scitranslmed.aaa7557. [DOI] [PubMed] [Google Scholar]
- 15.Waschek J.A., Baca S.M., Akerman S. PACAP and migraine headache: immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J. Headache Pain. 2018;19:23. doi: 10.1186/s10194-018-0850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertels Z., Pradhan A.A.A. Emerging treatment targets for migraine and other headaches. Headache. 2019;59(Suppl 2):50–65. doi: 10.1111/head.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyata A., Jiang L., Dahl R.D., Kitada C., Kubo K., Fujino M., Minamino N., Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem. Biophys. Res. Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- 18.Arimura A., Somogyvári-Vigh A., Miyata A., Mizuno K., Coy D.H., Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 19.Harmar A.J., Arimura A., Gozes I., Journot L., Laburthe M., Pisegna J.R., Rawlings S.R., Robberecht P., Said S.I., Sreedharan S.P., et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- 20.Harmar A.J., Fahrenkrug J., Gozes I., Laburthe M., May V., Pisegna J.R., Vaudry D., Vaudry H., Waschek J.A., Said S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birk S., Sitarz J.T., Petersen K.A., Oturai P.S., Kruuse C., Fahrenkrug J., Olesen J. The effect of intravenous PACAP38 on cerebral hemodynamics in healthy volunteers. Regul. Pept. 2007;140:185–191. doi: 10.1016/j.regpep.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Schytz H.W., Birk S., Wienecke T., Kruuse C., Olesen J., Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 23.Tuka B., Helyes Z., Markovics A., Bagoly T., Szolcsányi J., Szabó N., Tóth E., Kincses Z.T., Vécsei L., Tajti J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia. 2013;33:1085–1095. doi: 10.1177/0333102413483931. [DOI] [PubMed] [Google Scholar]
- 24.Zagami A.S., Edvinsson L., Goadsby P.J. Pituitary adenylate cyclase activating polypeptide and migraine. Ann. Clin. Transl. Neurol. 2014;1:1036–1040. doi: 10.1002/acn3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo S., Ernstsen C., Hay-Schmidt A., Ashina M., Olesen J., Christensen S.L. PACAP signaling is not involved in GTN- and levcromakalim-induced hypersensitivity in mouse models of migraine. J. Headache Pain. 2022;23:155. doi: 10.1186/s10194-022-01523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernstsen C., Christensen S.L., Rasmussen R.H., Nielsen B.S., Jansen-Olesen I., Olesen J., Kristensen D.M. The PACAP pathway is independent of CGRP in mouse models of migraine: possible new drug target? Brain. 2022;145:2450–2460. doi: 10.1093/brain/awac040. [DOI] [PubMed] [Google Scholar]
- 27.Markovics A., Kormos V., Gaszner B., Lashgarara A., Szoke E., Sandor K., Szabadfi K., Tuka B., Tajti J., Szolcsanyi J., et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 2012;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Kuburas A., Mason B.N., Hing B., Wattiez A.S., Reis A.S., Sowers L.P., Moldovan Loomis C., Garcia-Martinez L.F., Russo A.F. PACAP induces light aversion in mice by an inheritable mechanism independent of CGRP. J. Neurosci. 2021;41:4697–4715. doi: 10.1523/jneurosci.2200-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anapindi K.D.B., Yang N., Romanova E.V., Rubakhin S.S., Tipton A., Dripps I., Sheets Z., Sweedler J.V., Pradhan A.A. PACAP and other neuropeptides link chronic migraine and opioid-induced hyperalgesia in mouse models. Mol. Cell. Proteomics. 2019;18:2447–2458. doi: 10.1074/mcp.RA119.001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pradhan A.A., Smith M.L., Zyuzin J., Charles A. delta-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br. J. Pharmacol. 2014;171:2375–2384. doi: 10.1111/bph.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iversen H.K., Olesen J., Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Pain. 1989;38:17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen I., Thomsen L.L., Daugaard D., Ulrich V., Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. discussion: 626. [DOI] [PubMed] [Google Scholar]
- 33.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Olesen J., Iversen H.K., Thomsen L.L. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Charles A.C., Baca S.M. Cortical spreading depression and migraine. Nat. Rev. Neurol. 2013;9:637–644. doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- 36.Buse D.C., Pearlman S.H., Reed M.L., Serrano D., Ng-Mak D.S., Lipton R.B. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. 2012;52:18–36. doi: 10.1111/j.1526-4610.2011.02050.x. [DOI] [PubMed] [Google Scholar]
- 37.Mifflin K.A., Kerr B.J. The transition from acute to chronic pain: understanding how different biological systems interact. Can. J. Anaesth. 2014;61:112–122. doi: 10.1007/s12630-013-0087-4. [DOI] [PubMed] [Google Scholar]
- 38.Dripps I.J., Bertels Z., Moye L.S., Tipton A.F., Siegersma K., Baca S.M., Kieffer B.L., Pradhan A.A. Forebrain delta opioid receptors regulate the response of delta agonist in models of migraine and opioid-induced hyperalgesia. Sci. Rep. 2020;10:17629. doi: 10.1038/s41598-020-74605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elhabazi K., Ayachi S., Ilien B., Simonin F. Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities. J. Vis. Exp. 2014:e51264. doi: 10.3791/51264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Yang C., Wang Z.J. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J. Neurosci. 2010;30:38–46. doi: 10.1523/jneurosci.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anapindi K.D.B., Yang N., Romanova E.V., Rubakhin S.S., Tipton A., Dripps I., Sheets Z., Sweedler J.V., Pradhan A.A. PACAP and other neuropeptide targets link chronic migraine and opioid-induced hyperalgesia in mouse models. Mol. Cell. Proteomics. 2019;18:2447–2458. doi: 10.1074/mcp.RA119.001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moye L.S., Tipton A.F., Dripps I., Sheets Z., Crombie A., Violin J.D., Pradhan A.A. Delta opioid receptor agonists are effective for multiple types of headache disorders. Neuropharmacology. 2019;148:77–86. doi: 10.1016/j.neuropharm.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertels Z., Singh H., Dripps I., Siegersma K., Tipton A.F., Witkowski W.D., Sheets Z., Shah P., Conway C., Mangutov E., et al. Neuronal complexity is attenuated in preclinical models of migraine and restored by HDAC6 inhibition. Elife. 2021;10:e63076. doi: 10.7554/eLife.63076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertels Z., Witkowski W.D., Asif S., Siegersma K., van Rijn R.M., Pradhan A.A. A non-convulsant delta-opioid receptor agonist, KNT-127, reduces cortical spreading depression and nitroglycerin-induced allodynia. Headache. 2021;61:170–178. doi: 10.1111/head.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida D., Tatsuno I., Tanaka T., Hirai A., Saito Y., Moro O., Tajima M. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP type 1 receptor. Ann. N. Y. Acad. Sci. 1998;865:253–258. doi: 10.1111/j.1749-6632.1998.tb11185.x. [DOI] [PubMed] [Google Scholar]
- 46.Goadsby P.J., Holland P.R., Martins-Oliveira M., Hoffmann J., Schankin C., Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol. Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burstein R., Noseda R., Borsook D. Migraine: multiple processes, complex pathophysiology. J. Neurosci. 2015;35:6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayhurst C.J., Durieux M.E. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology. 2016;124:483–488. doi: 10.1097/aln.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 49.Iversen H.K. Human migraine models. Cephalalgia. 2001;21:781–785. doi: 10.1111/j.1468-2982.2001.00250.x. [DOI] [PubMed] [Google Scholar]
- 50.Pradhan A.A., Smith M.L., McGuire B., Tarash I., Evans C.J., Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tipton A.F., Tarash I., McGuire B., Charles A., Pradhan A.A. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia. 2016;36:1048–1056. doi: 10.1177/0333102415623070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrini F., Trang T., Mattioli T.A.M., Laffray S., Del'Guidice T., Lorenzo L.E., Castonguay A., Doyon N., Zhang W., Godin A.G., et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat. Neurosci. 2013;16:183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu X., Huang F., Szymusiak M., Tian X., Liu Y., Wang Z.J. PLGA-curcumin attenuates opioid-induced hyperalgesia and inhibits spinal CaMKIIalpha. PLoS One. 2016;11:e0146393. doi: 10.1371/journal.pone.0146393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munksgaard S.B., Bendtsen L., Jensen R.H. Modulation of central sensitisation by detoxification in MOH: results of a 12-month detoxification study. Cephalalgia. 2013;33:444–453. doi: 10.1177/0333102412475235. [DOI] [PubMed] [Google Scholar]
- 55.Eikermann-Haerter K., Arbel-Ornath M., Yalcin N., Yu E.S., Kuchibhotla K.V., Yuzawa I., Hudry E., Willard C.R., Climov M., Keles F., et al. Abnormal synaptic Ca(2+) homeostasis and morphology in cortical neurons of familial hemiplegic migraine type 1 mutant mice. Ann. Neurol. 2015;78:193–210. doi: 10.1002/ana.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Maagdenberg A.M.J.M., Pietrobon D., Pizzorusso T., Kaja S., Broos L.A.M., Cesetti T., van de Ven R.C.G., Tottene A., van der Kaa J., Plomp J.J., et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 57.Bogdanov V.B., Multon S., Chauvel V., Bogdanova O.V., Prodanov D., Makarchuk M.Y., Schoenen J. Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol. Dis. 2011;41:430–435. doi: 10.1016/j.nbd.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Ayata C., Jin H., Kudo C., Dalkara T., Moskowitz M.A. Suppression of cortical spreading depression in migraine prophylaxis. Ann. Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 59.Potewiratnanond P., le Grand S.M., Srikiatkhachorn A., Supronsinchai W. Altered activity in the nucleus raphe magnus underlies cortical hyperexcitability and facilitates trigeminal nociception in a rat model of medication overuse headache. BMC Neurosci. 2019;20:54. doi: 10.1186/s12868-019-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green A.L., Gu P., De Felice M., Dodick D., Ossipov M.H., Porreca F. Increased susceptibility to cortical spreading depression in an animal model of medication-overuse headache. Cephalalgia. 2014;34:594–604. doi: 10.1177/0333102413515344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuelsen P.J., Nielsen C.S., Wilsgaard T., Stubhaug A., Svendsen K., Eggen A.E. Pain sensitivity and analgesic use among 10,486 adults: the Tromso study. BMC Pharmacol. Toxicol. 2017;18:45. doi: 10.1186/s40360-017-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Compton P., Canamar C.P., Hillhouse M., Ling W. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J. Pain. 2012;13:401–409. doi: 10.1016/j.jpain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Athanasos P., Smith C.S., White J.M., Somogyi A.A., Bochner F., Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120:267–275. doi: 10.1016/j.pain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Le Merrer J., Becker J.A.J., Befort K., Kieffer B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffmann J., Miller S., Martins-Oliveira M., Akerman S., Supronsinchai W., Sun H., Shi L., Wang J., Zhu D., Lehto S., et al. PAC1 receptor blockade reduces central nociceptive activity: new approach for primary headache? Pain. 2020;161:1670–1681. doi: 10.1097/j.pain.0000000000001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashina M., Doležil D., Bonner J.H., Zhou L., Klatt J., Picard H., Mikol D.D. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia. 2021;41:33–44. doi: 10.1177/0333102420970889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ashina M., Hansen J.M., Á Dunga B.O., Olesen J. Human models of migraine - short-term pain for long-term gain. Nat. Rev. Neurol. 2017;13:713–724. doi: 10.1038/nrneurol.2017.137. [DOI] [PubMed] [Google Scholar]
- 68.Li J.L., Ding Y.Q., Li Y.Q., Li J.S., Nomura S., Kaneko T., Mizuno N. Immunocytochemical localization of mu-opioid receptor in primary afferent neurons containing substance P or calcitonin gene-related peptide. A light and electron microscope study in the rat. Brain Res. 1998;794:347–352. doi: 10.1016/s0006-8993(98)00332-1. [DOI] [PubMed] [Google Scholar]
- 69.Rice F.L., Xie J.Y., Albrecht P.J., Acker E., Bourgeois J., Navratilova E., Dodick D.W., Porreca F. Anatomy and immunochemical characterization of the non-arterial peptidergic diffuse dural innervation of the rat and Rhesus monkey: implications for functional regulation and treatment in migraine. Cephalalgia. 2017;37:1350–1372. doi: 10.1177/0333102416677051. [DOI] [PubMed] [Google Scholar]
- 70.Huang D., Grady F.S., Peltekian L., Laing J.J., Geerling J.C. Efferent projections of CGRP/Calca-expressing parabrachial neurons in mice. J. Comp. Neurol. 2021;529:2911–2957. doi: 10.1002/cne.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brennan K.C., Romero Reyes M., López Valdés H.E., Arnold A.P., Charles A.C. Reduced threshold for cortical spreading depression in female mice. Ann. Neurol. 2007;61:603–606. doi: 10.1002/ana.21138. [DOI] [PubMed] [Google Scholar]
- 72.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 73.Liang D.Y., Liao G., Wang J., Usuka J., Guo Y., Peltz G., Clark J.D. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang P., Moye L.S., Southey B.R., Dripps I., Sweedler J.V., Pradhan A., Rodriguez-Zas S.L. Opioid-induced hyperalgesia is associated with dysregulation of circadian rhythm and adaptive immune pathways in the mouse trigeminal ganglia and nucleus accumbens. Mol. Neurobiol. 2019;56:7929–7949. doi: 10.1007/s12035-019-01650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bertels Z., Witkowski W.D., Asif S., Siegersma K., van Rijn R.M., Pradhan A.A. A non-convulsant delta-opioid receptor agonist, KNT-127, reduces cortical spreading depression and nitroglycerin-induced allodynia. Headache. 2021;61:170–178. doi: 10.1111/head.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
No new code was used in these studies.
-
•
No large dataset was generated through these studies.
-
•
All other data are available upon request through the lead contact.