Summary
Sepsis is a life-threatening condition caused by a dysregulated host response to infection. Despite continued efforts to understand the pathophysiology of sepsis, no effective therapies are currently available. While singular components of the aberrant immune response have been investigated, comprehensive studies linking different data layers are lacking. Using an integrated systems immunology approach, we evaluated neutrophil phenotypes and concomitant changes in cytokines and metabolites in patients with sepsis. Our findings identify differentially expressed mature and immature neutrophil subsets in patients with sepsis. These subsets correlate with various proteins, metabolites, and lipids, including pentraxin-3, angiopoietin-2, and lysophosphatidylcholines, in patients with sepsis. These results enabled the construction of a statistical model based on weighted multi-omics linear regression analysis for sepsis biomarker identification. These findings could help inform early patient stratification and treatment options, and facilitate further mechanistic studies targeting the trifecta of surface marker expression, cytokines, and metabolites.
Subject areas: Immunity, Components of the immune system, Cell biology, Systems biology
Graphical abstract

Highlights
-
•
Integrated systems immunology approach reveals unique neutrophil subsets in sepsis
-
•
Multi-omics modeling reveals biomarkers including neutrophil CD10, PTX3, and lysoPC
-
•
These findings could enable future mechanistic studies and patient stratification
Immunity; Components of the immune system; Cell biology; Systems biology
Introduction
Sepsis is defined as a dysregulated immune response to infection mounted by the host.1,2 Despite more than 100 clinical trials testing the hypothesis that modulating endogenous responses to sepsis will improve survival, no new treatments have been delivered in the last 40 years. Broad-spectrum antibiotics and fluid replenishment remain the standard of care.3 Many hypotheses have been proposed to explain the lack of new therapies, including poor translatability of animal models, inappropriate patient selection, and a limited understanding of the basic molecular and cellular underpinnings of sepsis.4 Nevertheless, over the course of the last decade, multiple studies have advanced our basic knowledge of this disease. Because of these studies, we now know that the disease course is complex and heterogeneous. It includes an initial hyper-inflammatory phase that varies over time and is mediated by cells of both the innate and adaptive immune systems, various inflammatory cytokines, proteases, metabolites, and lipids. Ultimately, the combined effects of both pro- and anti-inflammatory responses can result in irreversible tissue damage, endothelial dysfunction, organ failure, and death.5,6 Several studies have established the roles of apoptotic depletion of immune cells, monocytes, B cell populations, and T cell populations in the pathogenesis of sepsis.7,8,9 Within the immune compartment, it is recognized that neutrophils are crucial components of the innate immune response during sepsis,10 responsible for the release of important regulatory factors, phagocytosis of pathogens, and antimicrobial killing via expression of a range of antimicrobial peptides, proteases, and oxidants, and by formation of neutrophil extracellular traps (NETs).11 However, neutrophils can also exert detrimental effects: localized production of reactive oxygen species (ROS) and excessive NETs can contribute to tissue damage, vascular leak, and thrombosis.12,13 Neutrophil functions become dysregulated in sepsis, with altered migration due to dysfunctional interaction with the endothelium, altered actin cytoskeleton, impaired apoptosis, and altered G-protein-coupled receptor/toll-like receptor signaling.14,15,16 Furthermore, disease progression in sepsis has been shown to be associated with suppression of neutrophil genes encoding mediators of inflammation and immune modulation.17 Neutrophils can express pro- and anti-inflammatory cytokines including IFNγ, TNF, and IL-6 in response to host factors and pathogen-associated molecular patterns.18,19,20,21,22 These cytokines, and others such as IL-1beta, IL-12, and IL-17, comprise the so-called cytokine storm which develops in the early hyper-inflammatory state of the disease.23,24 IL-10-producing neutrophils have been reported in mice during sepsis.
Recent studies have started to investigate the potential crosstalk between cytokine and metabolites in infection/inflammation.25,26,27 The metabolic activity of host cells can be hijacked by bacteria and viruses to facilitate their replication, driving alteration of intracellular metabolites and dysregulation of metabolic enzymes that can directly and indirectly regulate immune responses.28,29,30 Indeed, dysregulated metabolites have been reported in sepsis,31 with a predominant catabolic state leading to the breakdown of carbohydrates, lipids, and proteins reported.32,33,34,35,36 Another hallmark of sepsis and central cause of devastating tissue damage, organ dysfunction, and vascular collapse is the breakdown of microvascular integrity and the resulting vascular leak.37,38,39,40 While there is some evidence that soluble factors (e.g., endotoxin, inflammatory cytokines, and growth factors such as VEGF and angiopoietin-2) are involved,37,38,39,40 extensive empirical data in animal models and human disease implicate a role for neutrophil-endothelial cell (EC) interactions in this process. Previous studies demonstrate that circulating neutrophils undergo “intravascular priming” coupled to microvascular sequestration, and this increased prevalence of primed neutrophils, as well as neutrophil clustering, correlates with leak and severity of disease.41,42,43 Moreover, the previous finding that septic patients who underwent leukapheresis to selectively deplete primed neutrophils from the circulation demonstrated reduced leak and organ dysfunction,44,45 provides further important scientific support for this mechanism.
While several hypotheses have been advanced to explain how aberrances in neutrophil-EC interactions might promote EC dysfunction and vascular leak, none have been formally tested in human sepsis. Most studies have focused on characterizing neutrophil responses, cytokines, or metabolites as singular data layers in sepsis, and this information taken by itself can be potentially limiting/misleading.46 Relating plasma levels of soluble factors and metabolites with cellular profiles of specific immune cell subsets in patients with sepsis could not only strengthen predictive findings but could also help elucidate the mechanisms of disease progression and enable stratification of patient populations. To this end, we used an integrated systems immunology approach, that included in-depth profiling at the cellular (high-dimensional flow cytometry), and molecular levels (plasma cytokines and metabolomics/lipidomics) to further characterize neutrophil signatures in patients with sepsis.
Results
Clinical cohort information and systems immunology approach
We utilized an integrated analysis approach to characterize the cellular, molecular, and metabolic immune signatures in sepsis. To this end, we performed flow cytometry on freshly isolated neutrophils,47,48 and cytokine profiling/metabolomics/lipidomics analyses on plasma samples from patients with sepsis (n = 24) at diagnosis and age/gender-matched healthy donors (n = 19) (Figure 1A). Clinical information for patient groups and parameters for identification of sepsis are summarized in (Table S1).
Figure 1.
Cell clustering and correlation analysis identifies multiple populations of neutrophils
(A) Clinical cohorts and experimental design used in this study.
(B) Circos plot showing relationships between features in the three data layers (neutrophil surface marker expression, cytokines, and metabolites/lipids); connections between neutrophil markers and metabolites/lipids (orange), connections between neutrophil markers and cytokines (purple), and connections between metabolites/lipids and cytokines (gray). Width of nodes on the circular layout corresponds to the sum of each feature’s degree distribution.
(C) UMAP plot of various neutrophil cell clusters obtained from pooled samples of patients with sepsis and healthy controls, as a single cohort. Each color represents a different cell cluster (identified by FlowSOM), and each dot represents a single cell.
(D) Hierarchical clustering and heatmap showing differential expression of individual surface markers across 17 neutrophil subclusters obtained from pooled samples of patients with sepsis and healthy controls., Scale: low median marker expression (blue) to high median marker expression (red).
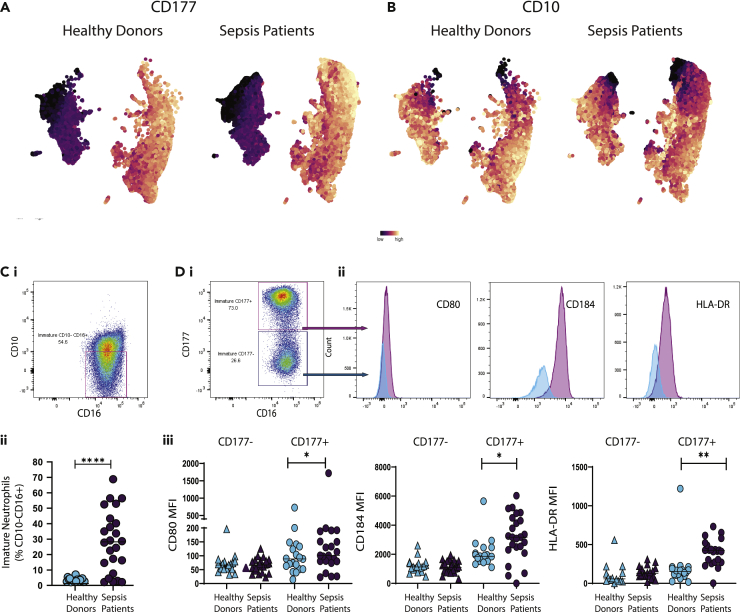
(E) UMAP plots of neutrophil clusters colored based on CD177 (left) and CD10 (right) expression in each cell. Scale: low marker expression (dark blue) to high marker expression (bright yellow).
To assess how different data layers relate to each other, we measured correlation between features in each of our three data layers. We employed a circos plot to visualize correlations between neutrophil surface markers, cytokines, and metabolites whose false discovery rate (FDR) was less than 0.05 (Figure 1B). A high level of correlation was observed between neutrophil cell surface markers, cytokines, metabolites, and lipids (Table S2). This finding reflects the association between neutrophils and soluble cytokine/metabolites in the pathophysiology of sepsis.
Identification of unique subsets of immature and mature neutrophils
We developed a customized analysis pipeline similar to CyTOF Workflow49 to analyze and visualize high-dimensional flow cytometry data on neutrophils. To elucidate neutrophil heterogeneity and identify different subsets of neutrophils, we employed a flow cytometry-specific version of the self-organizing map (SOM) algorithm, FlowSOM,50,51 to cluster cells based on expression of fourteen surface markers of interest. This analysis was first performed on both patients with sepsis and healthy volunteers as a single cohort, to identify different neutrophil cell subsets/clusters. FlowSOM along with ConsensusClusterPlus52 identified a total of 20 clusters, which were then combined to 17 based on similarity of marker expression patterns. Of these 17 neutrophil clusters, we saw two major distinct populations across patients with sepsis and healthy controls, using a uniform manifold approximation and projection (UMAP)53,54 (Figure 1C). We then calculated the median surface marker expression across all cells for each cluster and generated a heatmap with hierarchical clustering55,56 to characterize neutrophil subsets (Figure 1D). This allowed us to detect mature (CD10+) or immature (CD10−) neutrophil populations57 and other neutrophil subsets. For example, the most abundant clusters (2, 7, and 8) had a mature phenotype (CD10+) and differed in expression of CD80, CD177, CD184, and HLA-DR. Cluster 17 was characterized by a mature phenotype and high expression of CD274 and CD300f. Together with cluster 8, it may represent an immunosuppressive neutrophil population based on the known function of the markers expressed. We also identified subsets of CD10-/low immature neutrophils (clusters 9, 14, and 16) that differed in expression levels of CD11b, CD16, CD80, CD86, CD177, CD184, CD274, and HLA-DR.
As CD177 appears to be expressed approximately in half of the cluster that were identified (Figure 1D), we postulated that this neutrophil marker could be the driver of the large subgroups identified in the UMAP (Figure 1C). We were also interested in assessing the distribution of immature neutrophils across these two subgroups. We thus superimposed expression of CD10 and CD177 onto the UMAPs (Figure 1E), confirming that neutrophils can be split into two large subgroups characterized by presence or absence of CD177. Furthermore, there are also three distinct subsets of CD10-/low immature neutrophils, two of those in the CD177+ cluster and the remaining being CD177-. In summary, these analyses revealed multiple distinct subpopulations of neutrophils, some of which have not been observed previously.
Neutrophil subsets are differentially abundant in patients with sepsis compared to healthy donors
Next, we assessed the relative differences in neutrophil subsets between patients with sepsis and healthy controls, using surface marker expression across all neutrophils per sample and cluster abundance measurements. In an unsupervised approach, we calculated the median marker expression across all neutrophils to generate a principal component analysis plot58 (Figure 2A). Separation between the two cohorts suggested a distinction between patients with sepsis and healthy controls based on neutrophil markers alone. In a supervised approach, we further used linear regression to test which markers were differentially expressed between patients with sepsis and healthy controls and visualized this using a volcano plot.59 We found that 9 of the 14 markers showed statistically significant differences between the two groups: consistent with the literature, CD10, CD16,57,60 and CD86 are downregulated in sepsis, while HLA-DR, CD11b, CD80, CD184, CD63, and CD66b are upregulated in sepsis (Figure 2B). Hierarchical clustering of samples demonstrated that median marker expression clearly separated sepsis from control, which indicated that neutrophil surface markers are differentially regulated in the pathological state (Figures 2C, S1A, and S1B).
Figure 2.
Neutrophil populations display differential marker expression and abundance in patients with sepsis compared to healthy donors
(A) Principal component analysis (PCA) plot of median antigen expression in patients with sepsis (n = 24) and healthy controls (n = 22), showing cohort-specific sample clustering.
(B) Volcano plot showing effect size (see STAR Methods for details) and -log10 p value with the orange line representing the significance cutoff (FDR corrected p value <0.05).
(C) Heatmap of median marker expression across all cells per sample. The top dendrogram represents similarity between markers while the left dendrogram represents similarity between samples, both of which were determined by hierarchical clustering. Colored bar on the left represents group membership: teal for healthy controls, dark purple for patients with sepsis.
(D) Individual UMAP plots of neutrophil cell clusters in healthy controls (left) and patients with sepsis (right).
(E) Proportion (%) of each cluster in individual healthy controls (left) and patients with sepsis (right).
(F) Proportion (%) of each cluster in healthy controls (teal) versus patients with sepsis (dark purple). Differential abundance was determined by fitting data to a generalized linear model, and then testing coefficients with a log-ratio for significance (asterisks and FDR adjusted p value settings) (∗ = 0.05 > FDR>0.01, ∗∗ = 0.01 > FDR >0.001, ∗∗∗ = FDR <0.001).
While median marker expression suggested differential signals in the two cohorts, UMAPs indicated that specific subpopulations of neutrophils show variations in cluster abundances between healthy controls and patients with sepsis (Figures 2D, 2E, S2, and S3). We utilized a generalized linear model to test if clusters were differentially abundant between patients with sepsis and healthy controls. We found that clusters 3, 8, 9, 12, 14, and 15 were significantly more abundant in patients with sepsis, while clusters 1, 4, 5, 6, 7, and 10 were significantly less abundant in patients with sepsis (FDR adjusted p values ≤ 0.0556,61) (Figures 2E and 2F). Specifically, three distinct subsets of CD10-/low immature neutrophils clusters 9, 14, and 16 were more abundant in patients with sepsis compared to healthy controls (Figures 1D, 1E, and 2F). We also wanted to test if the increased presence of these clusters in patients with sepsis correlated with associated sequential organ failure assessment (SOFA) score (Table S1), a metric that has been utilized as a predictor of organ dysfunction and mortality in patients with sepsis.62 Spearman’s rank correlation analysis revealed that the percentage of immature neutrophil clusters 9 and 16, as shown in Figure 2F, positively correlated with SOFA score (Cluster 9: ρ = 0.54, p value = 0.008; Cluster 16: ρ = 0.60, p value = 0.002, Table S3). Additionally, while it did not reach statistical significance, abundance of cluster 14 also displayed trends of positive correlation with SOFA score (ρ = 0.18, p value = 0.422, Table S3).
Furthermore, we observed trends of negative correlation between CD10 expression and SOFA score in patients with sepsis (ρ = 0.18, p value = 0.412, Table S4). These findings suggested that both the abundance of immature neutrophil populations as well as CD10 expression were aligned with the SOFA metric in the cohort of patients with sepsis in this study, thus further confirming the critical role of immature neutrophils.
Distinct immature neutrophil subsets are differentially regulated in sepsis
Our results (Figures 1D and 1E) indicated the presence of three distinct populations of immature neutrophils (CD10-/low), largely differentiated by the presence or absence of CD177. Since immature neutrophils play a critical role in acute inflammation and the role of CD177 in neutrophil subsets remains unclear,63 we investigated whether these populations were differentially expressed in patients with sepsis and healthy controls. As expected, neutrophils from both healthy donors and patients with sepsis clustered accordingly to CD177 expression (Figures 3A and 3B). Within these two clusters, the populations of CD10− immature neutrophils were evident and clearly different in abundance in healthy controls versus patients with sepsis. This was confirmed by manual gating on CD10−CD16+ immature population of neutrophils (Figure 3Ci): while in healthy individuals the percentage of immature neutrophils was very low (1.837 ± 3.290%, mean ± SD), in septic patients it reached levels as high as 70% of all neutrophils (26.46% ± 20.079%, mean ± SD) (Figure 3Cii).
Figure 3.
Unique subpopulations of neutrophils are differentially expressed in patients with sepsis
(A and B) UMAP plot of neutrophil cell clusters in healthy controls and patients with sepsis colored by CD177 expression (A) and CD10 expression (B), scale: marker expression low (dark blue) to high (bright yellow).
(C) Detection of immature neutrophils via manual gating on CD10−CD16+ (i) and comparison of population percentage in healthy controls vs. patients with sepsis (ii).
(D) Separation of CD10−CD16+ immature neutrophils based on CD177 expression (i), comparison of CD80, CD184, and HLA-DR expression in the two separated populations (ii), and comparison of CD80, CD184, and HLA-DR median fluorescence intensity (MFI) between healthy controls and patients with sepsis in CD177- immature neutrophils and CD177+ immature neutrophils (iii). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t-test.
We confirmed the presence of the newly identified immature clusters 9 (CD177-) and combined 14 and 16 (CD177+) (Figures 1D and 1E) by manual gating, and assessed expression of their key differentiating markers CD80, CD184, and HLA-DR (Figures 3D and 3i-iii). Data showed that the two CD10−CD177+ immature neutrophils subsets, clusters 14 and 16, were significantly more abundant (t-test, p value < 0.05) in patients with sepsis (Figure 3Diii). On the other hand, the immature CD177- subset, cluster 9, did not display differential expression of CD80, CD184, or HLA-DR, between healthy subjects and septic patients.
Expression levels of surface markers on neutrophil subsets are correlated with cytokines and other soluble factors in patients with sepsis
Next, we evaluated the differential expression of cytokines and vascular-associated factors between patients with sepsis and healthy controls. Hierarchical clustering across cytokines and soluble factor levels (see STAR Methods for details) showed clear separation between the two cohorts (Figure 4A), confirming that their dysregulation accompanies sepsis pathology.64,65,66 An improved separation between healthy controls and septic patients was observed when simultaneously analyzing levels of surface markers measured by flow cytometry and levels of soluble factors measured in plasma (Figure 4B). We further visualized the levels of marker expression and cytokines between patients with sepsis and healthy donors using a volcano plot (Figure 4C).
Figure 4.
Cytokines are differentially expressed in patients with sepsis versus healthy controls and are correlated with specific neutrophil subsets
(A and B) Heatmap for significant cytokines from patients with sepsis (n = 24) and healthy controls (n = 20) (A) and heatmap for combined significant cytokines and flow cytometry markers for patients with sepsis (n = 21) and healthy controls (n = 17) (B). Dendrograms on the x and y axes show the unsupervised hierarchical clustering of features and subjects, respectively. Colored bar on the left represents group membership: teal for healthy controls, dark purple for patients with sepsis.
(C) Volcano plot for neutrophil surface markers (green) and cytokines (magenta). Processed data from these two data layers were concatenated and a comparison of all surface markers and cytokines isolated from the blood of patients with sepsis (n = 21) or healthy controls (n = 17) is depicted. Markers identified as significant were labeled on the plot (FDR t-test <0.05). Orange horizontal line represents cutoff for significance.
(D) Correlogram of significant features obtained from the combination of flow cytometry (green) and cytokine (magenta) data layers. Pairwise correlations between features are based on Spearman rank correlations.
We observed an overall upregulation of cytokines in the patients with sepsis (Figure 4C). However, there was notable heterogeneity across individual patients with sepsis. For example, although clearly distinguished from healthy donors, not all patients had an increase in TNF-α and IL-6 (Figure S4). Also, C-reactive protein, a known marker of sepsis,67 was not significantly differentially expressed between healthy subjects and septic patients (Figure S4). The highest upregulated factor by effect size (effect size = 1.64, adjusted p value = 5.69e-09; see STAR Methods for details) was instead pentraxin 3 (PTX3); other molecules involved in vascular function, such as endocan (ESM-1), E-Selectin (E-Sel), and Gas6 were also significantly upregulated (FDR p value ≤0.05) in septic patients (Figure 4C). Conversely, decreased levels of L-Selectin (L-Sel) and angiopoietin-1 (Ang-1) were seen in septic patients (Figures 4C and S4). We also observed upregulation of most surface markers, and a decrease in expression of CD10, CD16, and CD86 in the patients with sepsis (Figures 4B, 4C, 2C, S1A, and S1B).57,60
To identify relationships between various neutrophil subsets and cytokine secretion, we mapped associations both within and between the flow cytometry and cytokine data layers using Spearman’s rank correlation and visualized this using a correlogram.55 This analysis showed that CD10 expression in patients with sepsis was positively correlated with CD16 and L-Sel, and negatively correlated with PTX3, procalcitonin (PCT), Ang-2, ESM-1, ICAM-1, and others, indicating a possible role of immature neutrophils in the regulation of endothelial dysfunction and vascular leak (Figure 4D).
Expression levels of surface markers on neutrophil subsets are correlated with metabolites and lipids in patients with sepsis
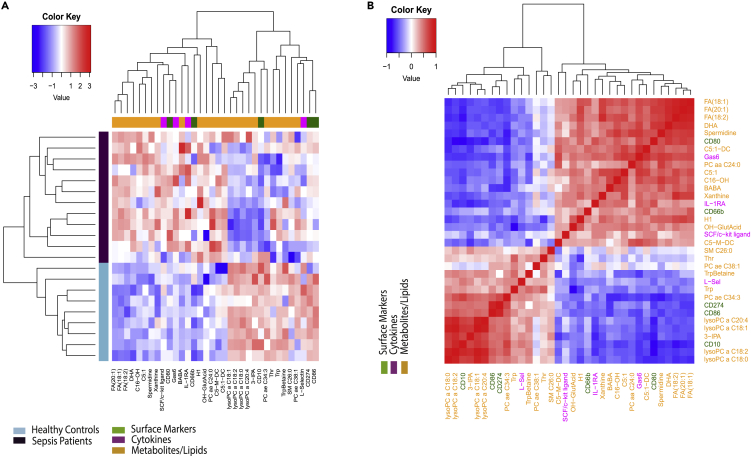
Metabolic alterations have been recognized to be part of the dysregulated response in sepsis.68 We therefore extended our analysis to include plasma metabolites and lipids. To this end, we carried out targeted metabolomics using triple quadrupole (QQQ) liquid chromatography/mass spectrometry (LC/MS) and subsequent analysis69 (see STAR Methods for details). We measured levels of 306 metabolites and lipids in plasma samples from a subset of 12 septic patients and 10 healthy controls for which we had previously generated flow cytometry data. As in the case of cytokines (Figure 4A), a heatmap generated by the significantly associated features (t-test) revealed a clear difference between patients with sepsis and healthy controls, with the two cohorts almost exclusively clustering with each other (Figure 5A). The concentration of several metabolites and lipids, such as lysophosphatidylcholines (lysoPCC16:0, C17:0, C18:0, C18:2), phosphatidylcholines (PC aa C34:4, PC ae C34:2, C34:3, C36:5, C40:1), tryptophan (Trp), and indole propionic acid (IPA), was decreased in patients with sepsis compared to healthy controls (Figure 5A). On the other hand, the concentration of some metabolites was significantly increased in septic patients compared to healthy controls, for example fatty acids (FA 18:1, 20:1, 18:2, 20:3), putrescine, spermidine, and L-kynurenine, among others (Figure 5A). To identify the relationship between various neutrophil subsets and metabolites, we integrated surface markers and metabolite layers, and visualized them using heatmaps with hierarchical clustering and volcano plots (Figures 5B and 5C). Strikingly, the heatmap showed perfect differential clustering of healthy controls from septic patients. In addition, a clear separation between two populations of septic patients was identified (Figure 5B). The change in metabolites/lipids was largely homogeneous in the septic group at the bottom part of the heatmap (Figure 5B, green arrow). The other septic subgroup, while clearly different from the healthy controls, was heterogeneous in terms of metabolite and lipid changes (Figure 5B, pink arrow).
Figure 5.
Metabolites and lipids are differentially expressed in patients with sepsis versus healthy controls and are correlated with specific neutrophil subsets
(A and B) Heatmap for significant metabolites for patients with sepsis (n = 14) and healthy controls (n = 11) (A) and heatmap for combined significant metabolites and neutrophil surface markers from patients with sepsis (n = 12) and healthy controls (n = 10) (B). Dendrograms on the x and y axes show unsupervised hierarchical clustering of features and subjects, respectively. Colored bar on the left represents group membership: teal for healthy controls, dark purple for patients with sepsis.
(C) Volcano plot for neutrophil surface markers (green) and metabolites (orange). Flow cytometry and metabolite data were concatenated for patients with sepsis (n = 12) and healthy controls (n = 10). Markers identified as significant are labeled on the plot (FDR t-test <0.05). Orange horizontal line represents cutoff for significance.
(D) Correlogram of significant features obtained from the combination of flow cytometry (green) and metabolite (orange) data layers. Pairwise correlations between features are based on Spearman rank correlations.
Next, we visualized the levels of marker expression and metabolites between patients with sepsis and healthy donors using a volcano plot (Figure 5C). While metabolites were differentially regulated between sepsis and healthy, we observed similar results of decreased expression of CD10, CD16, and CD86 in sepsis versus healthy (Figure 5C). We also evaluated correlations between surface markers and metabolites/lipids. Several significant (p value <0.01) positive and negative (Figure 5D) correlations were observed between all surface markers on immature and mature neutrophil subsets, and metabolite levels. For instance, significant downregulation of CD10 and CD16 and upregulation of CD80 in sepsis neutrophils was correlated with increased production of fatty acids and decreased production of lysoPC, PCaa/PCae species, and 3-IPA (Figures 5C and 5D).
Identifying unique associations between neutrophil surface markers, cytokines, and metabolites in patients with sepsis using weighted multi-omics linear regression
Finally, we developed a statistical model for sepsis by examining features in an intersection of subjects in all three data layers: we concatenated the data layers and used the intersection of subjects across all data layers to build a multi-omics statistical model with features selected using weighted LASSO regression70 and further analysis71,72 (see STAR Methods for details). This resulted in an accuracy measure of 0.967 ± 0.039 and an average predictive accuracy and area under the receiver operating curve measure of 0.969 ± 0.040. We then generated a heatmap to visualize the expression patterns of selected features from the 3 data layers sepsis versus healthy. Hierarchical clustering demonstrated perfect separation between sepsis and control groups (Figure 6A). Feature selection presented CD10 again as a critical neutrophil surface marker (Figure 6A). Other surface markers that were selected as having discriminatory power between sepsis and healthy subjects include CD66b, CD80, CD86, and CD274 (Figure 6A). We also observed additional metabolites such as Trp, Betaine, and OH-Glut Acid that did not have a significant fold change in previous linear analyses but were important in the non-linear classification of sepsis from healthy subjects (Figure 6A) Additionally, we employed Spearman rank correlation to identify pairwise associations between the selected discriminative features (Figure 6B). We observed similar associations between features from the three data layers as described previously (Figures 4C, 4D, 5C, and 5D), thus further validating our findings.
Figure 6.
Multivariate approach identifies features associated with sepsis
(A) Heatmap from multivariate multi-omics analysis showing expression patterns for all discriminative features. Processed data from all data layers (flow cytometry, metabolomics, and cytokines) measured from the blood of patients with sepsis (n = 12) or healthy controls (n = 9) were concatenated into a single dataset. Feature selection (employing LASSO regression, see STAR Methods for details) was used in nested cross validation framework to obtain the most important set of heterogeneous markers to distinguish between sepsis and healthy individuals.
(B) Correlogram showing pairwise Spearman rank correlations between discriminative features. Surface markers from flow cytometry analysis (green), cytokines (magenta), and metabolites (orange).
Of note, although each data layer contributed features to the classifier, there was a higher-than-expected increase (Fisher exact test, p = 0.012) in the observed proportion of features selected from the flow cytometry data layer (Figures 6A and S5). The flow cytometry data layer comprised only 4.1% of the overall features, but the multi-omics classifier selected features from the flow cytometry data layer was 14.3% and suggested that the flow cytometry data were over-represented in the multi-omics classifier (Figure S5). This implies that the flow cytometry panel utilized in this study presents potentially valuable biomarkers for patients with sepsis.
Discussion
Despite multiple efforts to characterize the host response and find a clear signature to support development of new therapeutic options, sepsis remains an unmet clinical need. Several groups have characterized immune responses based either on transcriptomics,7,73,74 surface protein expression,75 or metabolomics.36 Nevertheless, comprehensive approaches that profile the immune response more holistically in patients with sepsis are still lacking.7,76,77 Specifically, the functional impact of different neutrophil subsets in sepsis is not completely understood.57,75 Here, we focused on characterizing neutrophil-driven immune responses and associated pleiotropic effects, by developing an integrative platform including high-dimensional flow cytometry, multiplex bead array for cytokine measurement, metabolomics, and lipidomics. Firstly, we confirmed neutrophil heterogeneity (Figures 1C and 1D) and further identified unique subsets of neutrophils. Secondly, we showed how these different subsets are differentially regulated in sepsis compared to healthy controls (Figures 2 and 3). We also correlated for the first-time increased presence of immature neutrophils in patients with sepsis with endothelial dysfunction and metabolic alterations (Figures 4 and 5). Lastly, we built a multi-omics statistical model for sepsis by concatenating the three data layers in this study, to identify discriminatory markers across neutrophil surface markers, cytokines, and metabolites in patients with sepsis (Figure 6).
High levels of circulating, banded, immature neutrophils are an established, critical feature of the systemic inflammatory response in sepsis.78,79 Traditionally, it was thought that immature neutrophils are less competent, with a lower ability to combat infection80 and migrate to the site of infection.81,82,83 However, it has recently been shown that the banded immature neutrophil population has a higher phagocytic ability.84 This suggests that immature neutrophils may indeed be critical players in managing the infection, but possibly also in exacerbating the immune response.
Rather than relying on nuclear morphology, we defined immature neutrophils by evaluating the levels of the surface marker CD10.57 In this study, we identified two distinct subsets of immature neutrophils, CD10−CD177+ and CD10−CD177- (Figures 1D and 1E), that were differentially regulated in sepsis (Figures 2F and S1B). The function of CD177 remains poorly understood and distinct roles for CD177+ and CD177−neutrophils subsets have not yet been established.63 As CD177 is localized to the specific granules for rapid mobilization to the surface upon cell activation,85 we could speculate that this immature population of neutrophils might drive some of the critical neutrophil functions such as adhesion/transmigration,86 phagocytosis,86 ROS, and NETs production,87,88 based on the co-expression of other markers.
We also observed significant differential expression of CD184 (CXCR4) and HLA-DR in the CD10−CD177+ immature neutrophil subset in sepsis versus healthy individuals. Specifically, we identified a CD10−CD177+ immature neutrophil subset that resembles a CD10−CD16lowCD11blow population shown to express CD184 and have low phagocytic ability and ROS production.89 On the other hand, in other inflammatory settings mimicking sepsis, aged neutrophils expressing CD184 were shown to be particularly effective in migration to sites of inflammation and to exhibit a higher phagocytic activity as compared to subsequently recruited non-aged neutrophils.90 Though the impact of CD184 and aging on phagocytic activity is still unclear, our findings identified an increased expression of CD184 in the CD10− CD177+ population, in a subset of patients with sepsis (Figure 3D). Understanding the functional relevance of increased aging of immature neutrophils may provide interesting insights into predicting patient-specific disease outcomes in sepsis.
Results from our study also indicated an increased expression of HLA-DR in the CD10− CD177+ sepsis neutrophils (Figure 3D). While the expression and role of HLA-DR on neutrophils remains controversial,91,92 the notion that under appropriate inflammatory conditions neutrophils can acquire the ability to present antigen and stimulate (or inhibit) T cell responses has started to emerge.93 Cytokines such as GM-CSF, IFN-γ, IL-4, and TNF-α have been shown to induce antigen-presenting cell function in both mature and immature neutrophils.94,95 Induction of HLA-DR on neutrophils was also observed in patients with chronic inflammatory diseases and studies indicate that the ability of neutrophils to present antigen might be driven by a highly inflammatory environment.96,97,98 In fact, GM-CSF, IFN-γ, IL-4, and TNF-a are upregulated in a subset of the patients with sepsis in our cohort, together with increased expression of HLA-DR and CD80 (Figures 1 and 4). Therefore, our results support emerging evidence in the literature, and point toward a more important role for immature neutrophils in driving and maintaining antigen-specific T cell responses than had previously been appreciated.
Our data also identified a cluster of mature neutrophils (cluster 17, Figures 1 and 2) that were more abundant in a subset of patients with sepsis. This population resembled a recently characterized CD62Ldim/CD11bbright neutrophil population, shown to suppress human T cell proliferation.99 Additionally, we observed that this subset also expressed high levels of CD274 (PD-L1) and CD300f. Although blocking the PD-1-PD-L1 axis using an anti-PD-1 antibody improved survival in septic mice and patients with sepsis,100,101,102 the resulting excessive neutrophil engagement with T/B cells and inhibition of humoral immune responses were shown to be detrimental.103,104 Furthermore, recent studies in a model of septic peritonitis showed that absence of CD300f, or disruption of ceramide-CD300f interaction stimulated neutrophil recruitment to sites of infection and protected mice from septic death.105 This pointed toward a potential immunosuppressive and pathogenic role for cluster 17 identified in our studies.
To test if findings from our study aligned with existing metrics to define disease progression in patients with sepsis, we correlated the abundance of neutrophil subsets and associated marker expression with SOFA score in our sepsis patient cohort (Tables S3 and S4). Abundance of immature neutrophil clusters 9, 14, and 16 was positively correlated with SOFA score (Table S3) and expression of CD10 was negatively correlated with SOFA score (Table S4). Other markers identified from flow cytometry data analysis as well as by the multi-omics model, such as CD66b, CD63, CD274, and CD16, were significantly correlated (p < 0.05) with SOFA score to various degrees (Table S4). However, most of these correlations were rather weak and did not reach statistical significance (Table S4). Since the SOFA is not based on immune phenotype during disease progression, but only on degree of organ dysfunction over time,62,106 weak and/or non-significant (p > 0.05) correlation of SOFA score with singular, heterogeneous, and specific features such as neutrophil population dynamics or surface marker expression is not surprising. Nevertheless, trends of correlation between increased abundance of immature neutrophil subsets, decreased expression of CD10, and increased organ dysfunction as indicated by SOFA, are encouraging and further confirm the critical role of immature neutrophils in sepsis.
In conjunction with flow cytometry, we also observed differential plasma levels of several key cytokines and soluble factors in patients with sepsis. Interestingly, upregulation of classic pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 was heterogeneous, thus offering a potential explanation as to why all clinical trials targeting cytokine production in patients with sepsis have thus far failed to show efficacy. On the other hand, upregulation of PTX3, Ang-2, Endocan, Gas6, as well as inflammation marker PCT, was a hallmark of our cohort of septic patients (Figure S4). These factors have been previously reported to be increased in septic patients and to drive vascular leak and endothelial dysfunction.107,108 However, a relationship between these factors and specific immune cell subsets in the context of sepsis has not been discussed previously. Strikingly, the correlation of neutrophil surface marker expression and plasma levels of soluble factors observed in this study revealed that CD10 (a marker of neutrophil maturity) was inversely correlated with these soluble factors (Figure 4D). Thus, we can hypothesize that immature neutrophils might be the drivers of vascular inflammation/leak in patients with sepsis. Of note, immature neutrophils have been shown to store and release PTX3 during inflammation.109,110,111 Additionally, levels of PTX3 have been shown to predict and correlate with severity of disease and mortality in sepsis.112,113,114 Furthermore, though Ang-1/Ang-2 and Endocan have been associated with inflammation in sepsis,115,116,117 and can influence neutrophil functions such as migration and NETosis,118,119 their relationship to neutrophil subsets in sepsis has not been investigated. For the first time, our study demonstrated a significant correlation between immature neutrophils and soluble factors that drive vascular dysfunction.
Sepsis is also characterized by metabolic changes that impact normal metabolic homeostasis. Some of these changes have been suggested as prognostic factors/biomarkers for severity of the disease. For example, lactate, a metabolite of anaerobic metabolism, is used in clinical settings as a marker for sepsis diagnosis. However, the predictive value of this parameter alone remains controversial.120,121,122 In our cohort (Table S1), lactate levels varied from 0.1 to 10, falling outside of the lowest threshold recommended for diagnosis (>2.3 mmol. L-1),123,124 and highlighted the challenge of patient heterogeneity. Additional work has examined alterations in metabolite levels associated with sepsis and to date no single compound has shown sufficient sensitivity and specificity to be used as a routine biomarker for early diagnosis and prognosis.
In general, metabolites involved in glycolysis, TCA cycle, fatty acid oxidation, and amino acid pathways play important roles in sepsis and septic shock associated with different causal agents.125 Our results (Figure 5A) are in line with published work126,127,128 and the metabolic profile used in our study clearly separated healthy subjects from septic patients. One of the unique insights from the current study is a decreased concentration of IPA in septic patients compared to healthy donors. To our knowledge, this trend has not been reported previously. IPA is produced by Clostridium sporogenes129 in the gastrointestinal tract and has been shown to play a role in regulating endothelial function.130 Additionally, previous studies have demonstrated IPA’s anti-inflammatory properties,131,132 among other therapeutically beneficial effects,133,134 which is consistent with the decreased abundance in septic patients observed in this study. Further studies are necessary to better understand the role of IPA in sepsis. In this study, 4 out of 14 surface markers (CD10, CD16, CD80, and CD86), and 42 metabolites/lipids (out of 306) were significantly different between sepsis and control groups. CD10, whose expression discriminated between mature and immature neutrophils, was the top downregulated surface marker by effect size (see STAR Methods for details), inversely correlating with fatty acids and positively correlating with lysoPCs (Figure 5B). While several studies report dysfunctional metabolism in sepsis, findings from these studies are observational, not correlative. Our results reveal a strong correlation between expression of CD10 and aberrant levels of plasma metabolites in patients with sepsis, thereby indicating a previously unidentified association between immature neutrophils and impaired metabolism. Taken together, integrating data from flow cytometry, cytokine, and metabolite measurements, and performing univariate analysis, our findings reveal a unique link between CD10 expression level, increased vascular permeability, and dysfunctional metabolism in sepsis.
From a therapeutic perspective, many of the molecules identified in this study (i.e., PTX3, Ang2, Endocan, PCs, LPA, TNF-α, and IL6) have been tested separately as possible diagnostic, prognostic, or aids in sepsis patient stratification, but none have been successfully adopted. Using multivariate machine learning classifiers, we integrated neutrophil surface markers, pro/anti-inflammatory cytokines, vascular mediators, and metabolic profiles to identify a multi-omics model for sepsis (Figure 6). One key finding of our study is that features from each layer were selected by this model and that neutrophil surface markers were over-represented even though that data layer comprised the least number of possible features to select (Figures 6 and S5). This may suggest that a multivariate model that utilizes information from several data layers, including the surface marker panel presented in this paper, may prove valuable for diagnosing sepsis in the future.
Overall, we have developed a systems immunology approach to profile neutrophil signatures and their impact on various aspects of inflammation in patients with sepsis. Our approach reinforces the importance of data integration and provides valuable insights that will drive future mechanistic studies targeting the trifecta of surface marker expression, cytokines, and metabolites, in sepsis. Specifically, this study identified an association between CD10−immature neutrophils and markers of endothelial dysfunction indicated by increased PTX3 and dysregulated metabolism suggested by increased FA, in patients with sepsis.
Limitations of study
Distinguishing sepsis from other infections and organ failure conditions remains challenging in the clinic. Using an integrated systems immunology approach, our study was designed to characterize disease responses by correlating multiple data layers from individual patients with sepsis and healthy controls. While this approach has uncovered several associations between neutrophil surface markers, cytokines, and metabolites in patients wtih sepsis, it is predominantly observational and missing direct experimental evidence. Additionally, we conducted in-depth analysis and correlated neutrophil phenotypes with plasma levels of cytokines and other soluble factors, lipids, and metabolites, in a limited number of patients with sepsis. Although these findings shine light on strong disease-related correlations and present disease biomarkers for sepsis, additional experimental studies on differential expression of inflammatory cytokines, vascular-leak associated cytokines, and metabolites/lipids produced by mature and immature neutrophil subsets would be required to confirm the direct impact of neutrophil signature on vascular leak and metabolic alterations in sepsis.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-human CD11b (Mac-1), FITC (clone ICRF44) | BD Biosciences | Cat# 562793; RRID: AB_2737798 |
| Mouse anti-human CD66b, PerCP-Cy5.5 (clone G10F5) | BD Biosciences | Cat# 562254; RRID: AB_11154419 |
| Mouse anti-human CD63, Alexa Fluor 647 (clone H5C6) | BD Biosciences | Cat# 561983; RRID: AB_10897006 |
| Mouse anti-human CD45, Alexa Fluor 700 (clone HI30) | BD Biosciences | Cat# 560566; RRID: AB_1645452 |
| Mouse anti-human CD62L, APC-Cyanine 7 (clone DREG-56) | BioLegend | Cat# 304814; RRID: AB_493582 |
| Mouse anti-human CD184, (CXCR4) BV711 (clone 12G5) | BD Biosciences | Cat# 740799; RRID: AB_2740462 |
| Mouse anti-human CD11c, BV510 (clone B-Ly6) | BD Biosciences | Cat# 563026; RRID: AB_2737960 |
| Mouse anti-human CD10, BUV737 (clone HI10a) | BD Biosciences | Cat# 612826; RRID: AB_2870150 |
| Mouse anti-human CD16, PE (clone 3G8) | BD Biosciences | Cat# 555407; RRID: AB_395807 |
| Mouse anti-human CD300f, BUV395 (clone UP-D1) | BD Biosciences | Cat# 745678; RRID: AB_2743166 |
| Mouse anti-human CD80, BV786 (clone L307.4) | BD Biosciences | Cat# 564159; RRID: AB_2738631 |
| Mouse anti-human CD86, PE-CF594 (clone 2331) | BD Biosciences | Cat# 562390; RRID: AB_11154047 |
| Mouse anti-human CD177, BUV421 (clone MEM-166) | BD Biosciences | Cat# 564240; RRID: AB_2738694 |
| Mouse anti-human CD274, PE-Cy7 (clone MIH1) | BD Biosciences | Cat# 558017; RRID: AB_396986 |
| Mouse anti-human HLA-DR, BV650 (clone G46-6) | BD Biosciences | Cat# 564231; RRID: AB_2738685 |
| Biological samples | ||
| Sepsis patient whole blood | Massachusetts General Hospital (MGH) | This paper |
| Healthy donor whole blood | MRL volunteer blood donor program | This paper |
| Critical commercial assays | ||
| Human cytokine/chemokine magnetic bead kit (38-plex) | Millipore Sigma | Cat# HCYTMAG-60K-38X |
| Human cytokine/chemokine/soluble factors (custom-made, 36-plex) | R&D Systems | This paper |
| MxP Quant 500 | Biocrates | Cat# 21094.12 |
| Deposited data | ||
| Original code | This paper | [Harvard Dataverse]: [https://dataverse.harvard.edu/privateurl.xhtml?token=af411269-5320-4443-b5e2-44ae46c8f13e] |
| Software and algorithms | ||
| FlowJo | BD Biosciences | https://www.flowjo.com/solutions/flowjo |
| Customized flow cytometry data analysis pipeline | This paper | STAR Methods, this paper |
| FlowSOM | https://bioconductor.org/packages/release/bioc/html/FlowSOM.html | https://bioconductor.org/packages/release/bioc/html/FlowSOM.html |
| ConsensusClusterPlus | https://bioconductor.org/packages/release/bioc/html/ConsensusClusterPlus.html | https://bioconductor.org/packages/release/bioc/html/ConsensusClusterPlus.html |
| UMAP | https://cran.r-project.org/web/packages/umap/ | https://cran.r-project.org/web/packages/umap/ |
| xPONENT | Luminex | https://www.luminexcorp.com/xponent/#overview |
| Analyst software (1.6.3) | SCIEX | https://sciex.com/products/software/analyst-software |
| MetIDQ | Biocrates | https://biocrates.com/our-technology/ |
| LIMMA package | https://bioconductor.org/packages/release/bioc/html/limma.html | https://bioconductor.org/packages/release/bioc/html/limma.html |
| LASSO | https://cran.r-project.org/web/packages/glmnet/index.html | https://cran.r-project.org/web/packages/glmnet/index.html |
| Random Forest | https://cran.r-project.org/web/packages/randomForest/index.html | https://cran.r-project.org/web/packages/randomForest/index.html |
| gplots package, R v3.6.3 | https://CRANR-projectorg/package=gplots | https://CRANR-projectorg/package=gplots |
| Ggplot2 package, R v3.6.3 | https://cran.r-project.org/web/packages/ggplot2/ | https://cran.r-project.org/web/packages/ggplot2/ |
| ggpubr | https://CRANR-projectorg/package=ggpubr | https://CRANR-projectorg/package=ggpubr |
| Other | ||
| Percoll | GE Healthcare Life Sciences | Cat# 17-0891-02 |
| Human IL-2 | ThermoFisher Scientific | Cat# PHC0021 |
| RPMI-1640 | ThermoFisher Scientific | Cat# 11875093 |
| HBSS, no calcium, no magnesium, no phenol red | ThermoFisher Scientific | Cat# 14175095 |
| Fetal bovine serum | ThermoFisher Scientific | Cat# 10082-147 |
| Penicillin/Streptomycin | ThermoFisher Scientific | Cat# 15140-122 |
| LIVE/DEAD Fixable Yellow Dead Cell Stain Kit | ThermoFisher Scientific | Cat# L34967 |
| CellTrace CFSE Cell Proliferation Kit | ThermoFisher Scientific | Cat# C34554 |
| Trypan Blue Stain | ThermoFisher Scientific | Cat# 15250-061 |
| Phytohemagglutinin (PHA) | Sigma-Aldrich | Cat# L1668 |
| BSA | Sigma-Aldrich | Cat# A9418 |
| Fc block | BD Biosciences | Cat# 564220 |
| Stain buffer (FBS) | BD Biosciences | Cat# 554656 |
Resource availability
Lead contact
Further information and any related requests should be directed to and will be fulfilled by the lead contact, Roberta Martinelli (roberta.martinelli@merck.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human subjects and clinical protocol
Patient cohort comprised subjects with sepsis presenting to the Emergency Department at the Massachusetts General Hospital (MGH) between June 2018 and June 2020. This study was approved by the Institutional Review Boards Harvard and at Partners HealthCare (MA, USA), under protocols 2018P000224. Age- and sex-matched healthy control subjects were collected through MRL volunteer blood donor program. Patients were adjudicated as having sepsis based on Sepsis-3 criteria: Presence of organ dysfunction as a result of bacterial infection. As such, all subjects adjudicated as sepsis received 4 or more days of antibiotic therapy and had an elevated sequential organ failure assessment (SOFA) score upon enrollment. Sepsis severity was identified using the Acute Physiology and Chronic Health Evaluation (APACHE) II score and Sepsis-related Organ Failure Assessment (SOFA) score. Informed consent was obtained from all donors or their surrogates. Blood samples from patients and healthy controls were drawn with Na-Heparin Vacutainer tubes (BD Biosciences) and processed within 2 h of collection. Aliquots of blood from heparin tubes were stained for flow cytometry panels (see below) or centrifuged at 2,000g for 10 min and plasma stored at −80 °C. Patient data, including clinical course, relevant laboratory testing, sign of organ disfunction, and 90-day mortality, were collected. All studies were approved by the committee and informed consent was obtained from all subjects. Detailed information on sepsis clinical cohort can be found in Table S1. Detailed information on sample size can be found in Tables S5 and S6.
Method details
Reagents used
Percoll (Cat#17-0891-02) was purchased from GE Healthcare Life Sciences. HBSS (Cat#14175095), Human IL-2 (Cat#PHC0021), RPMI-1640 (Cat#11875093), FBS (Cat#10082-147), Penicillin/Streptomycin (Cat#15140-122), LIVE/DEAD Fixable Yellow Dead Cell Stain Kit (Cat#L34967), CellTrace CFSE Cell Proliferation Kit (Cat#C34554), and Trypan Blue Stain (Cat#15250-061) were all purchased from ThermoFisher Scientific. Phytohemagglutinin (PHA) (Cat#L1668) and BSA (Cat#A9418) were purchased from Sigma-Aldrich. This information is also included in the key resources table.
Neutrophil isolation
Neutrophils were isolated according to a single-step separation procedure with slight modifications.47 Blood (5 mL) was layered over a two-step Percoll gradient formed by 4 mL of 75% isotonic Percoll (75% Percoll, 10% PBS 10×, 15 mL of H2O, density (d) 1.103 g/mL, 300–310 mOsM) and 4 mL of 62% isotonic Percoll (62% Percoll, 10% PBS 10×, 28% H2O, d 1.078, 300–310 mOsM) in 15-mL conical test tubes. After centrifugation for 25 min (10 min at 200 × g and 15 min at 400 × g) at 20°C, granulocytes, located at the interface between the two Percoll solutions, were collected, diluted in Ca2+- and Mg2+-free HEPES-buffered saline solution containing BSA (HBSS-BSA, 145 mM NaCl, 5 mM KCl, 5 mM HEPES, 5 mM glucose, 0.2% BSA, pH 7.4), and centrifuged for 5 min at 200 × g. CD16 expression was used to distinguish neutrophils (CD45+CD16+) from eosinophils (CD45+CD16−).48 The procedure was conducted at room temperature and in the absence of divalent cations to prevent neutrophil aggregation and activation. All subsequent experiments were conducted in HBSS.
Flow cytometry staining and acquisition
Antibodies used for flow cytometry: Mouse anti-human CD11b (Mac-1), FITC (clone ICRF44), Cat#562793, BD Biosciences; Mouse anti-human CD66b, PerCP-Cy5.5 (clone G10F5),Cat#562254,BD Biosciences; Mouse anti-human CD63, Alexa Fluor 647 (clone H5C6),Cat#561983,BD Biosciences; Mouse anti-human CD45, Alexa Fluor 700 (clone HI30),Cat#560566,BD Biosciences; Mouse anti-human CD62L, APC-Cyanine 7 (clone DREG-56),Cat#304814,BioLegend; Mouse anti-human CD184, (CXCR4) BV711 (clone 12G5),Cat#740799,BD Biosciences; Mouse anti-human CD11c, BV510 (clone B-Ly6),Cat#563026,BD Biosciences; Mouse anti-human CD10, BUV737 (clone HI10a),Cat#612826,BD Biosciences; Mouse anti-human CD16, PE (clone 3G8),Cat#555407,BD Biosciences; Mouse anti-human CD300f, BUV395 (clone UP-D1),Cat#745678,BD Biosciences; Mouse anti-human CD80, BV786 (clone L307.4),Cat#564159,BD Biosciences; Mouse anti-human CD86, PE-CF594 (clone 2331),Cat#562390,BD Biosciences; Mouse anti-human CD177, BUV421 (clone MEM-166),Cat#564240,BD Biosciences; Mouse anti-human CD274, PE-Cy7 (clone MIH1),Cat#558017,BD Biosciences; Mouse anti-human HLA-DR, BV650 (clone G46-6),Cat#564231,BD Biosciences. Details of antibodies are also listed in the key resources table. Neutrophils were incubated with Fc block (BD Biosciences, Cat#564220) for 10 min prior to staining in Stain Buffer (FBS) (BD Biosciences, Cat#554656) for 30 min at 4°C. Samples were washed and resuspended in 200ul of staining buffer for acquisition by flow cytometry on a BD FACS Symphony and analyzed with FlowJo (BD Biosciences) analysis.
Input data
This study was comprised of three data layers, namely cytokines, neutrophil flow cytometry markers and metabolites. However, all data layers were not able to be collected from all subjects. Table S5 represents the total number of subjects for each data layer.
Flow cytometry data analysis pipeline
To analyze and visualize the neutrophil cell surface marker expression via high dimensional flow cytometry (HDFlow), we developed a customized analysis pipeline similar to CyTOF Workflow.49 This pipeline consists of a series of steps which are outlined below. The first step in the pipeline was to subset the total number of cells to a smaller, more manageable number (downsampling) and transform the single cell data. We downsampled each sample to 4000 cells. We used the hyperbolic arcsine transformation with a cofactor of 150 to transform the data from an exponential distribution into a normal distribution. The second step in the pipeline was to generate subject-level diagnostic plots, including multidimensional scaling (MDS) with euclidean distance,58 equivalent to principal component analysis (PCA). We produced this plot using the median transformed expression value for each antigen for each subject as input, and calculated similarity values between pairs of samples which defined the two-dimensional plot showing how similar samples are to each other. The third step in the pipeline was cell clustering. We clustered the cells using a cytometry-specific self-organizing map (SOM) algorithm51 called FlowSOM.50 The algorithm placed cells into a single node of a 10x10 node grid based on the similarity of that cell to other cells in the node. FlowSOM also performed clustering with ConsensusClusterPlus52 on top of the SOM to create a hierarchical clustering of the nodes. Tree cutting was then used to give a specific number of clusters for downstream analysis. First, we began with 20 clusters to identify broader subsets and balance between over- and under-clustering. Subsequently, based on similarity of marker expression patterns in each cluster, we merged six clusters into three, thus giving us the final 17 clusters. The fourth step in the pipeline was to generate a cell-level Uniform Manifold Approximation and Projection (UMAP) plot53,54 and then color cells based upon feature expression. UMAP plots contain all neutrophils in the dataset, colored according to either cluster membership from the FlowSOM step or according to transformed CD10 or CD177 transformed expression. Additional UMAP plots were generated based around the previous coordinates while separating cells from healthy donors and sepsis patients. For differential abundance we utilized a generalized linear mixed model (GLMM) with outcome variables as percentages, while for differential expression we utilized a linear mixed model (LMM) with the outcome variable as median marker expression. In each model, we used patient as a fixed effect and disease status as a random effect. Since the sepsis patients were age- and sex-matched to the healthy controls, we did not include age or sex as covariates in the model. To test coefficients of the model, a log-ratio test was used. To correct for multiple comparisons, we adjusted the nominal p values from the models using false discovery rate (FDR B&H method).
Cytokine analysis
Cytokines and chemokines in serum were measured using Magnetic Luminex Screening Assay using the Curiox DropArray System (Curiox Biosystems). The human cytokine/chemokine magnetic bead kit (38-plex) (Cat#HCYTOMAG-60K-38X, Millipore Sigma) and a custom-made panel of human cytokines/chemokines/soluble factors (36-plex) (R&D Systems) were used according to manufacturer’s instructions. Data were acquired using xPonent software and represented the median fluorescence intensity of the respective analyte. Sample concentration was calculated by the same software. Each value was measured in duplicates.
Serum endogenous metabolites and lipids analyses using Biocrates kits
Serum metabolites and lipids were analyzed using a commercial kit MxP Quant 500 (Cat#21094.12, Biocrates). Briefly, all reagents and consumables including analytical standards, internal standards and sample extraction plates were provided in the kit. Quality control (QC) samples containing expected levels of endogenous analytes were also shipped within the kit. 10 μL of each serum sample from the sepsis study was extracted in duplicates along the QCs to generate two extracts separating by polarities of the analytes using different eluents. One extract was analyzed for polar metabolites including amino acids and related, biogenic amines, fatty acids, bile acids, carbohydrates and related, hormones, vitamins, indoles and derivatives, nucleobases and related using liquid chromatography (LC) coupled with triple quadrupole mass spectrometry (QQQ MS). The other apolar extract was analyzed for lipids including acylcarnitines, phosphatidylcholines (PC, including lyso PC), sphingomyelins, cholesteryl esters using flow injection analysis and QQQ MS detection.
A Sciex 6500 QQQ MS equipped with an electrospray ionization source and a Waters Acquity UPLC chromatographic system coupled comprised the LC/MS system. The polar metabolites were analyzed using reversed phase chromatographic separation and scheduled multiple reaction monitoring (sMRM) detection at both positive and negative ionization modes. The lipid analysis was done using single ion monitoring mode on the same QQQ MS. All LC and QQQ MS conditions were provided by Biocrates Quant 500. LC/QQQ MS peak integration was performed using Analyst software (1.6.3). Quantification and quality control process for both LC/QQQ MS and flow injection/QQQMS analyses were conducted using Biocrates MetIDQ software Qaunt 500 workflow. Lipid species ceramides, diglycerides, and triglycerides in the apolar serum extract were analyzed using a high-resolution mass spectrometer Thermo Scientific Q Exactive HF equipped with HESI-II ion source. Samples were introduced using flow injection following conditions defined within Biocrates p400 kit. The mass resolution was set as 120,000 at m/z400. Data acquisition was performed as full MS scan at positive detection mode. The data process and quality control were completed using Biocrates MetIDQ p400 workflow.
Normalization and standardization for univariate and multivariate testing
To satisfy the assumptions of linear regression, each data layer was transformed and standardized as described below. The cytokine data was log2 transformed (log2(x+1)), the metabolites were transformed with variance stabilization transformation (normalize VSN function from LIMMA package69) and neutrophil flow cytometry marker expression data was transformed using the following functional form.
Here represents the mean and sigma the SD of a given feature. After transformation, inspection of both histograms and qqplots indicated that transformed data satisfies the assumption of linear regression. Each dataset was then standardized using the Z score to have data on the same scale.
Samples used for each analysis
For analyses performed that combined data layers, we used the intersection of subjects containing relevant data layers. Table S6 represents the various analyses performed and combinations used.
Univariate analysis
We defined the effect size for a given feature as the difference in mean transformed and normalized values computed over samples present in the two cohorts. Univariate hypothesis testing using the Student’s t test was performed to obtain the significant p value associated with the effect size for a given feature. P-values were corrected for multiple hypothesis testing using the False Discovery Rate method (FDR B&H method).
Multivariate machine learning methods
We built a predictive model for sepsis by examining features in all data layers (flow cytometry, cytokines, and metabolites/lipids) individually (single-omics analysis) to determine which layers had the most predictive signal. The predictive models were constructed in two steps. First, we performed feature selection (FS) using the least absolute shrinkage and selection operator (LASSO) regression,70 a well-established linear approach for feature selection and classification, followed by Random Forest (RF)71 to obtain the model on the selected features.
A stratified nested cross-validation (NCV) strategy72 was implemented to minimize overfitting of the predictive model. Data was stratified in such a manner that the proportion of subjects in each cohort was approximately maintained in each fold. We performed 1000 rounds of NCV loops. The outer cross-validation (CV) loop comprised of 5-folds while the inner loop consisted of 3-folds. The different numbers of k-folds in the inner and outer loops were chosen due to the limited number of samples. FS (LASSO regression along with its parameter estimation) and model building steps were performed on the inner loop. Only the features obtained from the FS step were used to build an RF model (using default setting of the ‘random forest’ package in R). The predictive scores of the classifier were obtained on the held out outer CV samples (never seen in the feature selection or the model building steps). We reported the average predictive accuracy and area under the receiver operating curve (AUROC) over 1000 runs of NCV. For multi-omics analysis, all data layers were concatenated. Except for feature selection, we employed all the steps described for the single-omics data on the collated heterogeneous data. Weighted LASSO regression70 was used to identify informative features in the multi-omics analysis. These weighting factors were used as a penalization factor in the regularization procedure of weighted LASSO. Lower weights (i.e., less penalty) increased the chances of the feature to be selected in the L1 regularized optimization step. For all features in a data layer, we computed the weights as the fraction of variation of a given single omics layer, quantified by the mean interquartile range (IQR) of all features divided by the total variation, computed as sum of individual mean IQR of each single omics layer).
Here represents the ith feature in the jth dataset, IQR is a function that computes the interquartile range for a given feature, Nj is the total number of features in the jth dataset and M is the total number of datasets in the study. An assumption for this analysis is that large mean IQR computed over of all features in given dataset is undesirable. The weights assigned to cytokine R&D panel, cytokine Millipore panel, flow cytometry panel and the metabolomics panel were 0.20, 0.22, 0.31 and 0.27 respectively. The final panel of features was selected based on the average value of beta, obtained in the FS step, over 1000 runs of NCV. The beta values corresponded to the coefficients obtained when one fits a LASSO model to the data. Average beta values in this analysis ranged from a minimum value of 0 to maximum value of 1.58. For the multi-omics analysis, we reported all features with an average beta greater than 0.01.
Packages used
Heatmaps and correlograms were generated using the function heatmaps2 in the gplots package55 in R v3.6.3, while volcano plots were generated using the ggplot2 package59 in R v3.6.3.56 The Student’s t test, FDR correction, and Fisher exact test were performed using base R, and the Wilcoxon test was done using the R package ggpubr.61
Quantification and statistical analysis
Detailed description of statistical methods is provided in STAR Methods under the following sections: Flow cytometry data analysis pipeline; Normalization and standardization for univariate and multivariate testing; Univariate analysis; Multivariate machine learning methods. Sample size (n) information for all analyses is provided in Tables S5 and S6. For normalization and standardization of univariate and multivariate testing, data was transformed and put through variance stabilization. Inspection of histogram and qqplots indicated that transformed data satisfied the assumptions of linear modeling. Random forest (RF) is a non-parametric method used in machine learning classification. RF assumes that the input data is continuous and the outcome variable in binary, e.g., sepsis and control. Significance for analyses was determined by an FDR-corrected p value (<0.05). For Figure 2, differential abundance was determined by fitting data to a generalized linear model, and then testing coefficients with a log-ratio for significance (asterisks and FDR adjusted p value settings: ∗ = 0.05 > FDR>0.01, ∗∗ = 0.01 > FDR >0.001, ∗∗∗ = FDR <0.001). For Figures 3, S1, and S4, statistical testing was performed using t-test and significance was defined as follows: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Acknowledgments
We are grateful to all the subjects for participating and donating blood for this study. Graphical abstract and Figure 1A were created with BioRender.com.
Author contributions
Conceptualization, R.M.; Methodology & Investigation, Y.K., T.P.W., J.E.N. Jr, J.R.K., B.S., R.N.Z., and R.M.; Methodology & Software, C.H.W.#, G.T., J.D.H., T.P.W., T.R.S., R.N.Z., and C.H.W.∗; Formal Analysis, U.P., Y.K., R.M., C.H.W.#, G.T., J.D.H., T.P.W., T.R.S., and R.N.Z.; Resources, R.M., Y.K., D.C., M.F., and C.B.; Writing – Original Draft, U.P., R.M., J.D.H., and C.H.W. #; Writing – Review & Editing, U.P., R.M., C.H.W. #, A.G.T., and D.A.G.; Project Administration, R.M. and C.H.W. #; Supervision, R.M.
# Cory H. White, ∗ Christopher H. Woelk.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: February 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.105948.
Contributor Information
Cory H. White, Email: cory.white@merck.com.
Roberta Martinelli, Email: roberta.martinelli@merck.com.
Supplemental information
Data and code availability
All original code generated as part of this study has been deposited at Harvard Dataverse: https://dataverse.harvard.edu/privateurl.xhtml?token=af411269-5320-4443-b5e2-44ae46c8f13e, and is publicly available as of the date of publication. A link to code has been included in the key resources table. Data reported in this paper will be shared by the lead contact upon request.
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bone R., Balk R., Cerra F., Dellinger R., Fein A., Knaus W., Schein R., Sibbald W. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101 doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 3.Marshall J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014;20:195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Cavaillon J.M., Singer M., Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020;12:e10128. doi: 10.15252/emmm.201810128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao C., Yu M., Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019;10:782. doi: 10.1038/s41419-019-2015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyes M., Filbin M.R., Bhattacharyya R.P., Billman K., Eisenhaure T., Hung D.T., Levy B.D., Baron R.M., Blainey P.C., Goldberg M.B., Hacohen N. An immune-cell signature of bacterial sepsis. Nat. Med. 2020;26:333–340. doi: 10.1038/s41591-020-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venet F., Chung C.S., Monneret G., Huang X., Horner B., Garber M., Ayala A. Regulatory T cell populations in sepsis and trauma. J. Leukoc. Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 9.Lorente-Sorolla C., Garcia-Gomez A., Català-Moll F., Toledano V., Ciudad L., Avendaño-Ortiz J., Maroun-Eid C., Martín-Quirós A., Martínez-Gallo M., Ruiz-Sanmartín A., et al. Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis. Genome Med. 2019;11:66. doi: 10.1186/s13073-019-0674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovach M.A., Standiford T.J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 11.Shen X.F., Cao K., Jiang J.P., Guan W.X., Du J.F. Neutrophil dysregulation during sepsis: an overview and update. J. Cell Mol. Med. 2017;21:1687–1697. doi: 10.1111/jcmm.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., Patel K.D., Chakrabarti S., McAvoy E., Sinclair G.D., et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 13.Phillipson M., Kubes P. The neutrophil in vascular inflammation. Nat. Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves-Filho J.C., Spiller F., Cunha F.Q. Neutrophil paralysis in sepsis. Shock. 2010;34(Suppl 1):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 15.Alves-Filho J.C., Freitas A., Souto F.O., Spiller F., Paula-Neto H., Silva J.S., Gazzinelli R.T., Teixeira M.M., Ferreira S.H., Cunha F.Q. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. USA. 2009;106:4018–4023. doi: 10.1073/pnas.0900196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danikas D.D., Karakantza M., Theodorou G.L., Sakellaropoulos G.C., Gogos C.A. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin. Exp. Immunol. 2008;154:87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang B.M.P., McLean A.S., Dawes I.W., Huang S.J., Lin R.C.Y. The use of gene-expression profiling to identify candidate genes in human sepsis. Am. J. Respir. Crit. Care Med. 2007;176:676–684. doi: 10.1164/rccm.200612-1819OC. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann M., Arruda-Silva F., Bianchetto-Aguilera F., Finotti G., Calzetti F., Scapini P., Lunardi C., Cassatella M.A., Tamassia N. IFNα enhances the production of IL-6 by human neutrophils activated via TLR8. Sci. Rep. 2016;6:19674. doi: 10.1038/srep19674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gideon H.P., Phuah J., Junecko B.A., Mattila J.T. Neutrophils express pro- and anti-inflammatory cytokines in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Mucosal Immunol. 2019;12:1370–1381. doi: 10.1038/s41385-019-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyadova I. Neutrophils in tuberculosis: heterogeneity shapes the way? Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/8619307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tecchio C., Cassatella M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016;28:119–128. doi: 10.1016/j.smim.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasten K.R., Muenzer J.T., Caldwell C.C. Neutrophils are significant producers of IL-10 during sepsis. Biochem. Biophys. Res. Commun. 2010;393:28–31. doi: 10.1016/j.bbrc.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte W., Bernhagen J., Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenfield K.G., Badovinac V.P., Griffith T.S., Knoop K.A. Sepsis, cytokine storms, and immunopathology: the divide between neonates and adults. ImmunoHorizons. 2021;5:512–522. doi: 10.4049/immunohorizons.2000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., Li K., Ran X., Long Q., Deng H., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chimenti M.S., Triggianese P., Conigliaro P., Candi E., Melino G., Perricone R. The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis. 2015;6:e1887. doi: 10.1038/cddis.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera-Van Oostdam A.S., Castañeda-Delgado J.E., Oropeza-Valdez J.J., Borrego J.C., Monárrez-Espino J., Zheng J., Mandal R., Zhang L., Soto-Guzmán E., Fernández-Ruiz J.C., et al. Immunometabolic signatures predict risk of progression to sepsis in COVID-19. PLoS One. 2021;16:e0256784. doi: 10.1371/journal.pone.0256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin C.M., Xu S., Munger J. Stealing the keys to the kitchen: viral manipulation of the host cell metabolic network. Trends Microbiol. 2015;23:789–798. doi: 10.1016/j.tim.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenreich W., Rudel T., Heesemann J., Goebel W. How viral and intracellular bacterial pathogens reprogram the metabolism of host cells to allow their intracellular replication. Front. Cell. Infect. Microbiol. 2019;9:42. doi: 10.3389/fcimb.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen P.J., Garcia G., Purkayastha A., Matulionis N., Schmid E.W., Momcilovic M., Sen C., Langerman J., Ramaiah A., Shackelford D.B., et al. SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition. Nat. Commun. 2021;12:1876. doi: 10.1038/s41467-021-22166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Englert J.A., Rogers A.J. Metabolism, metabolomics, and nutritional support of patients with sepsis. Clin. Chest Med. 2016;37:321–331. doi: 10.1016/j.ccm.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mickiewicz B., Thompson G., Blackwood J., Jenne C., Winston B., Vogel H., Joffe A. Development of metabolic and inflammatory mediator biomarker phenotyping for early diagnosis and triage of pediatric sepsis. Crit. Care. 2015;19 doi: 10.1186/s13054-015-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michie H.R. Metabolism of sepsis and multiple organ failure. World J. Surg. 1996;20:460–464. doi: 10.1007/s002689900072. [DOI] [PubMed] [Google Scholar]
- 34.Rogers A.J., McGeachie M., Baron R.M., Gazourian L., Haspel J.A., Nakahira K., Fredenburgh L.E., Hunninghake G.M., Raby B.A., Matthay M.A., et al. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS One. 2014;9:e87538. doi: 10.1371/journal.pone.0087538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mickiewicz B., Vogel H.J., Wong H.R., Winston B.W. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am. J. Respir. Crit. Care Med. 2013;187:967–976. doi: 10.1164/rccm.201209-1726OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langley R.J., Tsalik E.L., van Velkinburgh J.C., Glickman S.W., Rice B.J., Wang C., Chen B., Carin L., Suarez A., Mohney R.P., et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013;5:195ra95. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee W.L., Slutsky A.S. Sepsis and endothelial permeability. N. Engl. J. Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg N.M., Steinberg B.E., Slutsky A.S., Lee W.L. Broken barriers: a new take on sepsis pathogenesis. Sci. Transl. Med. 2011;3:88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 39.Aird W.C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 40.De Backer D., Orbegozo Cortes D., Donadello K., Vincent J. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5 doi: 10.4161/viru.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner J.G., Roth R.A. Neutrophil migration during endotoxemia. J. Leukoc. Biol. 1999;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- 42.Lewis S.M., Khan N., Beale R., Treacher D.F., Brown K.A. Depletion of blood neutrophils from patients with sepsis: treatment for the future? Int. Immunopharm. 2013;17:1226–1232. doi: 10.1016/j.intimp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Brown K.A., Brain S.D., Pearson J.D., Edgeworth J.D., Lewis S.M., Treacher D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet (London, England) 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 44.Groeneveld K.M., Koenderman L., Warren B.L., Jol S., Leenen L.P.H., Hietbrink F. Early decreased neutrophil responsiveness is related to late onset sepsis in multitrauma patients: an international cohort study. PLoS One. 2017;12:e0180145. doi: 10.1371/journal.pone.0180145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox E., Heffernan D., Cioffi W., Reichner J. Neutrophils from critically ill septic patients mediate profound loss of endothelial barrier integrity. Crit. Care. 2013;17 doi: 10.1186/cc13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuurman A.R., Reijnders T.D.Y., Kullberg R.F.J., Butler J.M., van der Poll T., Wiersinga W.J. Sepsis: deriving biological meaning and clinical applications from high-dimensional data. Intensive Care Med. Exp. 2021;9:27. doi: 10.1186/s40635-021-00383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dri P., Haas E., Cramer R., Menegazzi R., Gasparini C., Martinelli R., Scheurich P., Patriarca P. Role of the 75-kDa TNF receptor in TNF-induced activation of neutrophil respiratory burst. J. Immunol. 1999;162:460–466. [PubMed] [Google Scholar]
- 48.Pillay J., Tak T., Kamp V.M., Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell. Mol. Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowicka M., Krieg C., Crowell H., Weber L., Hartmann F., Guglietta S., Becher B., Levesque M., Robinson M. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Research. 2017;6:748. doi: 10.12688/f1000research.11622.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Gassen S., Callebaut B., Van Helden M.J., Lambrecht B.N., Demeester P., Dhaene T., Saeys Y. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A. 2015;87:636–645. doi: 10.1002/cyto.a.22625. [DOI] [PubMed] [Google Scholar]
- 51.Kohonen T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982;43:59–69. doi: 10.1007/BF00337288. [DOI] [Google Scholar]
- 52.Wilkerson M.D., Hayes D.N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26:1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McInnes L., Healy J., Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. arXiv. 2018 doi: 10.48550/arXiv.1802.03426. Preprint at. [DOI] [Google Scholar]
- 54.McInnes L., Healy J., Saul N., Großberger L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 2018;3:861. doi: 10.21105/joss.00861. [DOI] [Google Scholar]
- 55.Warnes G.R., Bolker B., Bonebakker L., Gentleman R., Huber W., Liaw A., Lumley T., Maechler M., Magnusson A., Moeller S., et al. 2020. Gplots: Various R Programming Tools for Plotting Data. R Package Version 3.1.1.https://CRAN.R-project.org/package=gplots [Google Scholar]
- 56.R Core Team . R Core Team; 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 57.Marini O., Costa S., Bevilacqua D., Calzetti F., Tamassia N., Spina C., De Sabata D., Tinazzi E., Lunardi C., Scupoli M.T., et al. Mature CD10 + and immature CD10 - neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129:1343–1356. doi: 10.1182/blood-2016-04-713206. [DOI] [PubMed] [Google Scholar]
- 58.Buja A., Swayne D.F., Littman M.L., Dean N., Hofmann H., Chen L. Data visualization with multidimensional scaling. J. Comput. Graph Stat. 2008;17:444–472. doi: 10.1198/106186008X318440. [DOI] [Google Scholar]
- 59.Wickham H. Springer-Verlag; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 60.Demaret J., Venet F., Friggeri A., Cazalis M.A., Plassais J., Jallades L., Malcus C., Poitevin-Later F., Textoris J., Lepape A., Monneret G. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J. Leukoc. Biol. 2015;98:1081–1090. doi: 10.1189/jlb.4A0415-168RR. [DOI] [PubMed] [Google Scholar]
- 61.Kassambara A. 2020. ggpubr: 'ggplot2' Based Publication Ready Plots.https://CRAN.R-project.org/package=ggpubr [Google Scholar]
- 62.Lambden S., Laterre P., Levy M., Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care. 2019;23 doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grieshaber-Bouyer R., Nigrovic P.A. Neutrophil heterogeneity as therapeutic opportunity in immune-mediated disease. Front. Immunol. 2019;10:346. doi: 10.3389/fimmu.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gouel-Chéron A., Allaouchiche B., Guignant C., Davin F., Floccard B., Monneret G., AzuRea Group Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One. 2012;7:e33095. doi: 10.1371/journal.pone.0033095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mera S., Tatulescu D., Cismaru C., Bondor C., Slavcovici A., Zanc V., Carstina D., Oltean M. Multiplex cytokine profiling in patients with sepsis. APMIS. 2011;119:155–163. doi: 10.1111/j.1600-0463.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 66.Chaudhry H., Zhou J., Zhong Y., Ali M., McGuire F., Nagarkatti P., Nagarkatti M. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669–684. [PMC free article] [PubMed] [Google Scholar]
- 67.Póvoa P., Almeida E., Moreira P., Fernandes A., Mealha R., Aragão A., Sabino H. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24:1052–1056. doi: 10.1007/s001340050715. [DOI] [PubMed] [Google Scholar]
- 68.Wasyluk W., Zwolak A. Metabolic alterations in sepsis. J. Clin. Med. 2021;10:2412. doi: 10.3390/jcm10112412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 71.Liaw A., Wiener M. R News; 2002. Classification and Regression by randomForest. [Google Scholar]
- 72.Krstajic D., Buturovic L.J., Leahy D.E., Thomas S. Cross-validation pitfalls when selecting and assessing regression and classification models. J. Cheminf. 2014;6:10. doi: 10.1186/1758-2946-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scicluna B.P., van Vught L.A., Zwinderman A.H., Wiewel M.A., Davenport E.E., Burnham K.L., Nürnberg P., Schultz M.J., Horn J., Cremer O.L., et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir. Med. 2017;5 doi: 10.1016/S2213-2600(17)30294-1. [DOI] [PubMed] [Google Scholar]
- 74.Sweeney T.E., Azad T.D., Donato M., Haynes W.A., Perumal T.M., Henao R., Bermejo-Martin J.F., Almansa R., Tamayo E., Howrylak J.A., et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit. Care Med. 2018;46:915–925. doi: 10.1097/CCM.0000000000003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meghraoui-Kheddar A., Chousterman B.G., Guillou N., Barone S.M., Granjeaud S., Vallet H., Corneau A., Guessous K., de Roquetaillade C., Boissonnas A., et al. Two new neutrophil subsets define a discriminating sepsis signature. Am. J. Respir. Crit. Care Med. 2022;205:46–59. doi: 10.1164/rccm.202104-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Itenov T.S., Murray D.D., Jensen J.U.S. Sepsis: personalized medicine utilizing 'omic' technologies-A paradigm shift? Healthcare. Healthcare. 2018;6:111. doi: 10.3390/healthcare6030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Resende C.B., Borges I., Gonçalves W.A., Carneiro R., Rezende B.M., Pinho V., Nobre V., Teixeira M.M. Neutrophil activity in sepsis: a systematic review. Braz. J. Med. Biol. Res. 2020;53:e7851. doi: 10.1590/1414-431X20207851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drifte G., Dunn-Siegrist I., Tissières P., Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 79.Manz M.G., Boettcher S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 80.Taneja R., Sharma A., Hallett M., Findlay G., Morris M. Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock. 2008;30 doi: 10.1097/SHK.0b013e318173ef9c. [DOI] [PubMed] [Google Scholar]
- 81.Olins A.L., Zwerger M., Herrmann H., Zentgraf H., Simon A.J., Monestier M., Olins D.E. The human granulocyte nucleus: unusual nuclear envelope and heterochromatin composition. Eur. J. Cell Biol. 2008;87:279–290. doi: 10.1016/j.ejcb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf K., Te Lindert M., Krause M., Alexander S., Te Riet J., Willis A.L., Hoffman R.M., Figdor C.G., Weiss S.J., Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park B., Dolen J., Snyder B. Defective chemotactic migration of polymorphonuclear leukocytes in Pelger-Huet anomaly. Proc. Soc. Exp. Biol. Med. 1977;155 doi: 10.3181/00379727-155-39743. [DOI] [PubMed] [Google Scholar]
- 84.Leliefeld P.H.C., Pillay J., Vrisekoop N., Heeres M., Tak T., Kox M., Rooijakkers S.H.M., Kuijpers T.W., Pickkers P., Leenen L.P.H., Koenderman L. Differential antibacterial control by neutrophil subsets. Blood Adv. 2018;2:1344–1355. doi: 10.1182/bloodadvances.2017015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu N., Mora-Jensen H., Theilgaard-Mönch K., Doornbos-van der Meer B., Huitema M.G., Stegeman C.A., Heeringa P., Kallenberg C.G.M., Westra J. Differential expression of granulopoiesis related genes in neutrophil subsets distinguished by membrane expression of CD177. PLoS One. 2014;9:e99671. doi: 10.1371/journal.pone.0099671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai M., Grieshaber-Bouyer R., Wang J., Schmider A.B., Wilson Z.S., Zeng L., Halyabar O., Godin M.D., Nguyen H.N., Levescot A., et al. CD177 modulates human neutrophil migration through activation-mediated integrin and chemoreceptor regulation. Blood. 2017;130:2092–2100. doi: 10.1182/blood-2017-03-768507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schreiber A., Luft F.C., Kettritz R. Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int. 2004;65:2172–2183. doi: 10.1111/j.1523-1755.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhou G., Yu L., Fang L., Yang W., Yu T., Miao Y., Chen M., Wu K., Chen F., Cong Y., Liu Z. CD177 + neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67:1052–1063. doi: 10.1136/gutjnl-2016-313535. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez-Rosales Y.A., Langereis J.D., Gorris M.A.J., van den Reek J.M.P.A., Fasse E., Netea M.G., de Vries I.J.M., Gomez-Muñoz L., van Cranenbroek B., Körber A., et al. Immunomodulatory aged neutrophils are augmented in blood and skin of psoriasis patients. J. Allergy Clin. Immunol. 2021;148:1030–1040. doi: 10.1016/j.jaci.2021.02.041. [DOI] [PubMed] [Google Scholar]
- 90.Uhl B., Vadlau Y., Zuchtriegel G., Nekolla K., Sharaf K., Gaertner F., Massberg S., Krombach F., Reichel C.A. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood. 2016;128:2327–2337. doi: 10.1182/blood-2016-05-718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davis R.E., Sharma S., Conceição J., Carneiro P., Novais F., Scott P., Sundar S., Bacellar O., Carvalho E.M., Wilson M.E. Phenotypic and functional characteristics of HLA-DR + neutrophils in Brazilians with cutaneous leishmaniasis. J. Leukoc. Biol. 2017;101:739–749. doi: 10.1189/jlb.4A0915-442RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chakravarti A., Rusu D., Flamand N., Borgeat P., Poubelle P.E. Reprogramming of a subpopulation of human blood neutrophils by prolonged exposure to cytokines. Lab. Invest. 2009;89:1084–1099. doi: 10.1038/labinvest.2009.74. [DOI] [PubMed] [Google Scholar]
- 93.Radsak M., Iking-Konert C., Stegmaier S., Andrassy K., Hänsch G.M. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–530. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iking-Konert C., Csekö C., Wagner C., Stegmaier S., Andrassy K., Hänsch G.M. Transdifferentiation of polymorphonuclear neutrophils: acquisition of CD83 and other functional characteristics of dendritic cells. J. Mol. Med. 2001;79:464–474. doi: 10.1007/s001090100237. [DOI] [PubMed] [Google Scholar]
- 95.Oehler L., Majdic O., Pickl W.F., Stöckl J., Riedl E., Drach J., Rappersberger K., Geissler K., Knapp W. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J. Exp. Med. 1998;187:1019–1028. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cross A., Bucknall R.C., Cassatella M.A., Edwards S.W., Moots R.J. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. 2003;48:2796–2806. doi: 10.1002/art.11253. [DOI] [PubMed] [Google Scholar]
- 97.Iking-Konert C., Ostendorf B., Sander O., Jost M., Wagner C., Joosten L., Schneider M., Hänsch G.M. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann. Rheum. Dis. 2005;64:1436–1442. doi: 10.1136/ard.2004.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davey M.S., Morgan M.P., Liuzzi A.R., Tyler C.J., Khan M.W.A., Szakmany T., Hall J.E., Moser B., Eberl M. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J. Immunol. 2014;193:3704–3716. doi: 10.4049/jimmunol.1401018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pillay J., Kamp V.M., van Hoffen E., Visser T., Tak T., Lammers J.W., Ulfman L.H., Leenen L.P., Pickkers P., Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J.F., Li J.B., Zhao Y.J., Yi W.J., Bian J.J., Wan X.J., Zhu K.M., Deng X.M. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: an animal study and a prospective case-control study. Anesthesiology. 2015;122:852–863. doi: 10.1097/ALN.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y., Zhou Y., Lou J., Li J., Bo L., Zhu K., Wan X., Deng X., Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit. Care. 2010;14 doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brahmamdam P., Inoue S., Unsinger J., Chang K.C., McDunn J.E., Hotchkiss R.S. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamenyeva O., Boularan C., Kabat J., Cheung G.Y.C., Cicala C., Yeh A.J., Chan J.L., Periasamy S., Otto M., Kehrl J.H. Neutrophil recruitment to lymph nodes limits local humoral response to Staphylococcus aureus. PLoS Pathog. 2015;11:e1004827. doi: 10.1371/journal.ppat.1004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hampton H.R., Bailey J., Tomura M., Brink R., Chtanova T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat. Commun. 2015;6:7139. doi: 10.1038/ncomms8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Izawa K., Maehara A., Isobe M., Yasuda Y., Urai M., Hoshino Y., Ueno K., Matsukawa T., Takahashi M., Kaitani A., et al. Disrupting ceramide-CD300f interaction prevents septic peritonitis by stimulating neutrophil recruitment. Sci. Rep. 2017;7:4298. doi: 10.1038/s41598-017-04647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rhee C., Zhang Z., Kadri S., Murphy D., Martin G., Overton E., Seymour C., Angus D., Dantes R., Epstein L., et al. Sepsis surveillance using adult sepsis events simplified eSOFA criteria versus sepsis-3 sequential organ failure assessment criteria. Crit. Care Med. 2019;47 doi: 10.1097/CCM.0000000000003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vincent J.L., Beumier M. Diagnostic and prognostic markers in sepsis. Expert Rev. Anti Infect. Ther. 2013;11:265–275. doi: 10.1586/eri.13.9. [DOI] [PubMed] [Google Scholar]
- 108.Parlato M., Cavaillon J.M. Host response biomarkers in the diagnosis of sepsis: a general overview. Methods Mol. Biol. 2015;1237:149–211. doi: 10.1007/978-1-4939-1776-1_15. [DOI] [PubMed] [Google Scholar]
- 109.Daigo K., Hamakubo T. Host-protective effect of circulating pentraxin 3 (PTX3) and complex formation with neutrophil extracellular traps. Front. Immunol. 2012;3:378. doi: 10.3389/fimmu.2012.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jaillon S., Peri G., Delneste Y., Frémaux I., Doni A., Moalli F., Garlanda C., Romani L., Gascan H., Bellocchio S., et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Imamura M., Kawasaki T., Savchenko A.S., Ohashi R., Jiang S., Miyamoto K., Ito Y., Iwanari H., Sagara M., Tanaka T., et al. Lipopolysaccharide induced expression of pentraxin 3 in human neutrophils and monocyte-derived macrophages. Cell. Immunol. 2007;248:86–94. doi: 10.1016/j.cellimm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Caironi P., Masson S., Mauri T., Bottazzi B., Leone R., Magnoli M., Barlera S., Mamprin F., Fedele A., Mantovani A., et al. Pentraxin 3 in patients with severe sepsis or shock: the ALBIOS trial. Eur. J. Clin. Invest. 2017;47:73–83. doi: 10.1111/eci.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee Y.T., Gong M., Chau A., Wong W.T., Bazoukis G., Wong S.H., Lampropoulos K., Xia Y., Li G., Wong M.C.S., et al. Pentraxin-3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta-analysis. J. Infect. 2018;76:1–10. doi: 10.1016/j.jinf.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Uusitalo-Seppälä R., Huttunen R., Aittoniemi J., Koskinen P., Leino A., Vahlberg T., Rintala E.M. Pentraxin 3 (PTX3) is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PLoS One. 2013;8:e53661. doi: 10.1371/journal.pone.0053661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ioakeimidou A., Pagalou E., Kontogiorgi M., Antoniadou E., Kaziani K., Psaroulis K., Giamarellos-Bourboulis E.J., Prekates A., Antonakos N., Lassale P., Gogos C., Hellenic Sepsis Study Group Increase of circulating endocan over sepsis follow-up is associated with progression into organ dysfunction. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1749–1756. doi: 10.1007/s10096-017-2988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saharinen P., Eklund L., Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017;16:635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 117.Fang Y., Li C., Shao R., Yu H., Zhang Q., Zhao L. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit. Care. 2015;19 doi: 10.1186/s13054-015-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burnett A., Gomez I., De Leon D.D., Ariaans M., Progias P., Kammerer R.A., Velasco G., Marron M., Hellewell P., Ridger V. Angiopoietin-1 enhances neutrophil chemotaxis in vitro and migration in vivo through interaction with CD18 and release of CCL4. Sci. Rep. 2017;7:2332. doi: 10.1038/s41598-017-02216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lavoie S.S., Dumas E., Vulesevic B., Neagoe P.E., White M., Sirois M.G. Synthesis of human neutrophil extracellular traps contributes to angiopoietin-mediated in vitro proinflammatory and proangiogenic activities. J. Immunol. 2018;200:3801–3813. doi: 10.4049/jimmunol.1701203. [DOI] [PubMed] [Google Scholar]
- 120.Kushimoto S., Akaishi S., Sato T., Nomura R., Fujita M., Kudo D., Kawazoe Y., Yoshida Y., Miyagawa N. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med. Surg. 2016;3:293–297. doi: 10.1002/ams2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moran J.L., Santamaria J. Reconsidering lactate as a sepsis risk biomarker. PLoS One. 2017;12:e0185320. doi: 10.1371/journal.pone.0185320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sauer C.M., Gómez J., Botella M.R., Ziehr D.R., Oldham W.M., Gavidia G., Rodríguez A., Elbers P., Girbes A., Bodi M., Celi L.A. Understanding critically ill sepsis patients with normal serum lactate levels: results from U.S. and European ICU cohorts. Sci. Rep. 2021;11:20076. doi: 10.1038/s41598-021-99581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wacharasint P., Nakada T., Boyd J., Russell J., Walley K. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38 doi: 10.1097/SHK.0b013e318254d41a. [DOI] [PubMed] [Google Scholar]
- 124.Nichol A., Egi M., Pettila V., Bellomo R., French C., Hart G., Davies A., Stachowski E., Reade M., Bailey M., Cooper D. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit. Care. 2010;14 doi: 10.1186/cc8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Wyngene L., Vandewalle J., Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol. Med. 2018;10:e8712. doi: 10.15252/emmm.201708712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jaurila H., Koivukangas V., Koskela M., Gäddnäs F., Myllymaa S., Kullaa A., Salo T., Ala-Kokko T.I. 1 H NMR based metabolomics in human sepsis and healthy serum. Metabolites. 2020;10:70. doi: 10.3390/metabo10020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kauppi A.M., Edin A., Ziegler I., Mölling P., Sjöstedt A., Gylfe Å., Strålin K., Johansson A. Metabolites in blood for prediction of bacteremic sepsis in the emergency room. PLoS One. 2016;11:e0147670. doi: 10.1371/journal.pone.0147670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J., Sun Y., Teng S., Li K. Prediction of sepsis mortality using metabolite biomarkers in the blood: a meta-analysis of death-related pathways and prospective validation. BMC Med. 2020;18:83. doi: 10.1186/s12916-020-01546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L.S., Davies S.S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tuomainen M., Lindström J., Lehtonen M., Auriola S., Pihlajamäki J., Peltonen M., Tuomilehto J., Uusitupa M., de Mello V.D., Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes. 2018;8:35. doi: 10.1038/s41387-018-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Mello V.D., Paananen J., Lindström J., Lankinen M.A., Shi L., Kuusisto J., Pihlajamäki J., Auriola S., Lehtonen M., Rolandsson O., et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017;7:46337. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bendheim P.E., Poeggeler B., Neria E., Ziv V., Pappolla M.A., Chain D.G. Development of indole-3-propionic acid (OXIGON) for Alzheimer's disease. J. Mol. Neurosci. 2002;19:213–217. doi: 10.1007/s12031-002-0036-0. [DOI] [PubMed] [Google Scholar]
- 134.Chyan Y.J., Poeggeler B., Omar R.A., Chain D.G., Frangione B., Ghiso J., Pappolla M.A. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem. 1999;274:21937–21942. doi: 10.1074/jbc.274.31.21937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All original code generated as part of this study has been deposited at Harvard Dataverse: https://dataverse.harvard.edu/privateurl.xhtml?token=af411269-5320-4443-b5e2-44ae46c8f13e, and is publicly available as of the date of publication. A link to code has been included in the key resources table. Data reported in this paper will be shared by the lead contact upon request.