ABSTRACT
The effect of donor obesity on kidney transplantation success has long been an overlooked clinical research area. Even though there is no strict guideline in most countries prohibiting donation from obese individuals, most candidates with a body mass index >35–40 kg/m2 are rejected due to concerns regarding long-term renal functional deterioration in the donor. The effects of excessive fat mass on renal function and allograft survival have been analysed by several longitudinal and follow-up studies. These studies have documented the deleterious effect on long-term graft outcomes of excessive body mass in living kidney donors and de novo obesity or pre-existing obesity worsening after transplantation on kidney outcomes. However, there is a paucity of clinical trials aimed at countering overweight and obesity in living and deceased kidney donors and in transplant patients. In this review we will briefly discuss the mechanism whereby fat excess induces adverse kidney outcomes and describe the effects on graft function and survival in living obese donors.
Keywords: donor obesity, fatty kidney, graft survival, inflammation, kidney transplantation
INTRODUCTION
Renal transplantation is the gold standard treatment for kidney failure and >80 000 kidney transplants were performed worldwide in 2020 [1]. Multiple factors influence the success of this treatment, including age, comorbidities, aetiology of kidney failure, pre-dialysis vintage, human leucocyte antigen compatibility, living or deceased donor, delayed graft function, proteinuria and estimated glomerular filtration rate (eGFR) after transplantation [2, 3]. The effect of the donor’s body mass index (BMI) is a potential additional risk factor for kidney graft loss. Obesity, defined as a BMI >30 kg/m2, produces structural changes in the kidney, including effacement of podocyte foot processes, focal segmental glomerulosclerosis (FSGS) and glomerular hypertrophy, which are collectively referred as obesity-related glomerulopathy, a condition that may progress to kidney failure over time [4]. Although the exact underlying pathophysiology is still incompletely defined, one hypothesis holds that glomerular hypertension and hyperfiltration and glomerular hypertrophy develop in response to an inability to match the metabolic demand of a high BMI [5]. Despite similar serum creatinine, eGFR and iothalamate clearance in obese living donors show a higher glomerular planar surface area and tubular dilatation [6]. However, whether these alterations may engender kidney dysfunction and eventually kidney failure is still an unsettled clinical research issue. Herein we will briefly review the evolution of kidney grafts from obese donors as well as the effect of weight gain after transplantation on the risk of graft failure. In this review we will briefly review the mechanisms whereby overweight and obesity may have an adverse effect on kidney outcomes in transplant patients and then illustrate the effect of the donor's BMI on kidney function and discuss BMI thresholds for kidney donation and the effect of weight gain post-transplantation.
METHODS
We performed an extensive literature search through three databases—Embase (Elsevier), the Cochrane Central Register of Controlled Trials (Wiley) and PubMed/MEDLINE Web of Science—in November 2021 by using the following terms and their combinations: ‘kidney transplantation’, ‘renal transplantation’, ‘transplantation’, ‘end stage renal disease’, ‘chronic kidney disease’, ‘donor obesity’, ‘obesity’, ‘overweight’, ‘donor criteria’, ‘body mass index’ and ‘long term outcome’. Each study was individually assessed by the authors. Reference lists of each study were evaluated manually in order not to miss any relevant studies. After preliminary elimination of the studies with evaluation of the titles and abstracts, the full text of the studies were evaluated by the authors independently. Inclusion criteria for this review study were as follows and studies not fitting these criteria are excluded from the review: study design was a randomized clinical trial, prospective or retrospective cohort study, case–control study or cross-sectional study; the aim of study was to determine the effects of donor obesity or weight gain after transplantation on kidney transplantation outcomes; and the study was published in a peer-reviewed journal in English through May 2022. We additionally included pre-clinical and clinical studies investigating potential pathophysiological mechanisms underlying clinical outcomes associated with donor obesity or weight gain after the transplantation procedure.
Pathophysiology
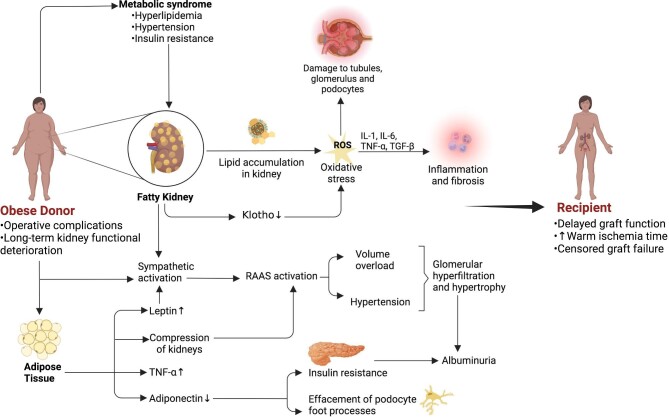
Several hypothetical mechanisms have been postulated to explain the effect of BMI on kidney function after transplantation from an obese donor. These mechanisms are depicted in Fig. 1.
Figure 1:
The summary of hypothetical mechanisms behind the effect of donor obesity on the transplanted kidney and its consequences on the donor and recipient.
Abdominal obesity most often precedes other components of metabolic syndrome, including hyperlipidaemia, hypertension and insulin resistance, and may have a causal role in their development [7–9]. Other potential mechanisms include adipose tissue–mediated neuro-hormonal factors, inflammation and fibrosis, oxidative stress, renal haemodynamic alterations and hypoxia-inducible factor signalling.
Abdominal obesity and higher BMI have been linked to fatty kidney, a recently identified area of research implicating adipose tissue accumulation in renal sinus and perirenal tissues [10]. Fatty kidney predisposes individuals to the development of chronic kidney disease (CKD) via various pathophysiological mechanisms, most of which are applicable to kidney transplantation from obese or overweight donors.
Inflammation and oxidative stress
Accumulation of cholesterol and lipoproteins in the glomerulus and in renal tubules leads to the formation of reactive oxygen species (ROS) that in turn trigger pro-inflammatory (i.e. interleukin-1, interleukin-6, tumour necrosis factor α) and pro-fibrotic cytokines [i.e. transforming growth factor (TGF)-β] [11, 12]. Such alterations result in the deposition of inflammatory cells—monocytes, macrophages and neutrophils—that are crucial for the progression towards irreversible injury and CKD. These alterations eventually disrupt cellular energy metabolism, the glomerular filtration barrier and tubular secretion/re-absorption systems. Additionally, accumulation of cholesterol and fatty acids in mitochondria in renal tubular cells alters the lipolysis:lipogenesis ratio in favour of lipogenesis and affects energy metabolism [13, 14].
Renal haemodynamics
Via renin–angiotensin–aldosterone system (RAAS) activation and hyperinsulinemia triggered by peripheral insulin resistance, obesity and weight gain increase tubular sodium reabsorption, thereby generating hypertension and glomerular hypertrophy/hyperfiltration [15, 16]. Furthermore, obesity activates the sympathetic system. Endothelin-1 levels are elevated in obese individuals and physical compression of the kidneys and volume overload may contribute to the adverse health effects of obesity [17].
Adipose tissue
High leptin and low adiponectin levels are hallmarks of overweight and obesity. These alterations lead to insulin resistance, impaired glucose and fatty acid metabolism, increased fatty acid oxidation and the formation of ROS and secretion of pro-inflammatory cytokines [18–20]. A decrease in adiponectin levels also results in podocyte foot process effacement, as demonstrated in studies in adiponectin knockout mice, a genetically engineered model characterized by albuminuria [21, 22]. In contrast, high leptin results in nitric oxide synthesis inhibition and sympathetic nervous system activation [17]. As a result of these derangements in adiponectin and leptin levels, oxidative stress is promoted and pro-inflammatory pathways are upregulated. Such processes lead to acute glomerular and tubular injury and fibrosis and kidney function loss. As a consequence of insulin resistance, oxidative stress through nicotinamide adenine dinucleotide phosphate oxidase activation, growth factor releases via the protein kinase B–mammalian target of rapamycin pathway and renal tissue fibrosis caused by TGF-β and collagen IV secretion eventually develop in experimental obesity models [23].
Other factors
Renal adipose tissue accumulation has been linked to a decrease in renal tissue Klotho expression that further shifts the balance towards pro-oxidant over antioxidant mechanisms [24]. Accumulation of fatty acids, especially palmitic acid, has been linked to unfolded protein response, which may disrupt new protein synthesis and induce apoptosis [25, 26]. Lastly, obesity-associated inflammatory changes have been associated with glomerular hyperfiltration with insulin resistance and hyperinsulinemia [27].
Effect of donor BMI on long-term transplanted kidney function
Available information on the effect of donor BMI on kidney function derives mainly from studies based on UK [28] and US [29–31] kidney transplant registries. Arshad et al [28]. investigated the effect of high donor BMI on kidney graft outcomes in a cohort of 17 590 consecutive deceased donors in the UK Transplant Registry between January 2003 and January 2015. The prevalence of delayed graft function increased in parallel with donor BMI and grafts from donors with moderate–severe obesity (BMI >35 kg/m2) had a 38% increased risk of delayed graft function as compared with grafts from donors with a BMI of 18.5–25.0 kg/m2. However, donor BMI was unrelated to serum creatinine 1 year after transplantation or graft survival. Similarly, in overweight patients, increasing donor BMI associated with an increase in warm ischaemia time and functional warm ischaemia, but these variables were unrelated to delayed graft function or graft loss [28]. In this and other studies, higher donor BMI associated with type 2 diabetes and hypertension. Furthermore, in the same database, high BMI donors were preferentially utilized in elderly recipients with high baseline BMI in the same registry. However, analyses adjusting for these confounders did not modify the associations seen in unadjusted analyses.
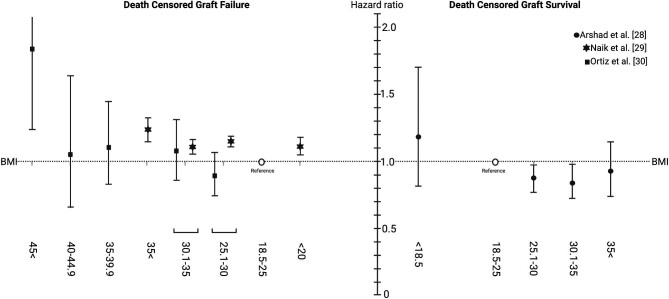
In an equally large study based on the US transplant registry, including 18 734 deceased donor and 84 377 living donor kidney transplants, there was a graded increase in delayed graft function risk in overweight [living donors hazard ratio (HR) 1.06, deceased donors HR 1.04), mildly obese (living donors HR 1.16, deceased donors HR 1.10) and very obese (HR 1.22 in both living and deceased donors) compared with normal BMI donors (P < .05). The effect of BMI on graft outcomes was apparent in the early phases post-transplant and persisted over time. In contrast with the UK study, donor obesity status in the US registry study remained an independent risk factor for graft loss in analyses adjusting for donor and recipient size mismatch and other donor, recipient and transplant potential confounders. In the third study, the largest registry based analysis so far, including 90 158 non-cardiac death deceased donors and 6932 cardiac death deceased donors, an excess risk for delayed graft function was registered but no excess risk by donor BMI on graft survival except for kidneys from donors with extremely severe obesity (BMI >45 kg/m2) was apparent [30]. In the fourth study, based on a cohort of deceased donor recipients in the USA between 2000 and 2014 with 115 124 kidney transplant recipients in total, donor–recipient weight mismatch (donor < recipient) was associated with a higher risk of death-censored graft loss and with gender mismatch [31]. A similar pattern of worse clinical outcome with BMI mismatch was reported in two additional studies [32, 33]. Overall, even if differences among studies exist, donor BMI seems to have an effect on delayed graft function and long-term graft survival. Fig. 2 provides a summary of the three studies [28–30], showing the association of donor BMI with death-censored allograft failure and survival. Data extracted from these three studies can be found in Supplementary Table 1.
Figure 2:
A cumulative graphic of three studies [28–30] illustrating the adjusted relationship between deceased donor BMI and death-censored graft failure/survival by multivariate analysis.
The optimal graft function curve is thought to be achieved with a donor–recipient pair of normal BMI. As donor BMI increases, the graft function curve shifts to the right, as graft function is delayed and peak eGFR that can be achieved is less. In addition, the concept of censored graft function appears earlier in the recipient. The changes are also seen in donor–recipient pairs who are both obese.
It is also crucial to remember that in an obese donor with normal eGFR and no proteinuria, FSGS is less likely. In obese patients with minimal comorbidities and healthy allografts, careful inclusion into the donor pool should be considered.
There are significant discrepancies between living and deceased donors that considerably limit their comparability since donation from a deceased donor is associated with an increase in ischaemia time, a technically complicated surgery and a higher risk of complications.
Donor and recipient BMI mismatch is another crucial topic that needs to be addressed and adjusted for when evaluating the effect of BMI on clinical outcome. The hypothetical framework behind this issue is glomerular hypertension and hypertrophy in response to the demands of a recipient with a high BMI.
BMI thresholds for kidney donation
The main kidney transplantation guidelines are conflicting about cut-off values for living donor BMI. While the 2015 European Renal Best Practice document recommends a BMI >35 kg/m2 as a contraindication for living kidney donors, while the British Transplantation Society and Kidney Disease: Improving Global Outcomes guidelines recommend careful and individual assessment of donor candidates with a BMI >30 kg/m2 without specifying any absolute cut-off value [34–36]. The major concerns for donors with a high BMI are the potential long-term kidney function deterioration and higher risk of intraoperative and early postoperative complications in the donor. In a single-centre retrospective study in 553 subjects undergoing laparoscopic living kidney donation [37], the duration of surgery was longer and the rates of minor, mostly wound-related, complications were higher in donors with a BMI >35 kg/m2, but there was no difference in the duration of hospital stay and major complications in donors with a BMI >35 kg/m2 or <35 kg/m2. However, at least three studies showed that obesity is among the strongest predictors of perioperative complications in living kidney donors [38–40]. Advancements in surgical techniques and preferential utilization of laparoscopic or robotic surgery minimize surgical complications. Analyses in clinical series applying these techniques are needed to update our knowledge of the surgical risk of obesity in living donor kidney transplantation.
In a study with a >20-year follow-up involving 3752 living donors, among which 656 were obese, obese donors experienced a higher decline in eGFR and a higher incidence of diabetes mellitus and hypertension without any excess risk of end-stage renal disease (ESRD) development [39]. These findings contrast with another study in 10 000 living donors where the incident risk for ESRD in obese donors (93.9/10 000) was more than double that of non-obese donors (39.7/10 000) [41]. In this study, the cut-off value for the risk for post-transplantation ESRD risk was 27 kg/m2, i.e. a cut-off in the overweight range rather than in the obesity range. In studies with a short follow-up, which are inherently limited by the fact that they are based on surrogates, no difference in eGFR or proteinuria emerged in obese subjects compared with normal weight subjects [42–44]. Lifestyle modifications, pharmacological interventions and bariatric surgery may lead to significant weight loss and may encourage donors to participate in kidney donation who would otherwise have been discouraged [45]. Furthermore, the poor performance of eGFR based on serum creatinine in obese subjects cannot be overemphasized [46].
Effect of weight gain in the post-transplantation period
Weight gain after transplantation is an issue of obvious relevance that requires prompt recognition and intervention. It differs from donor obesity in terms of allograft injury, as transplanted patients have solitary kidneys and are exposed to more hyperfiltration. A study conducted in 96 kidney transplantation recipients showed that younger age, higher carbohydrate consumption, higher trunk fat percentage and the mental health quality of life dimension pre-transplantation were all predictors of weight gain during the first year after transplantation [47]. These early predictors may be useful to design strategies aimed at mitigating the obesity risk in transplant patients.
A special type of obesity called sarcopenic obesity (or obese sarcopenia) can be seen in renal transplant patients. This can start at the CKD stage and persist through being wait-listed and the post-transplant period [48]. Both sarcopenia and obesity in transplant patients are associated with poor transplant outcomes and this special type of obesity should be adequately addressed to prevent graft loss. Physical exercise along with a diet balanced in calories and proteins that favours muscle mass while preventing obesity should be suggested in the post-transplant period.
Of note, sleep apnoea—a pervasive complication in ESRD patients—improves early on after transplantation but re-emerges in later stages and worsens over time and longitudinal changes of sleep apnoea metrics are directly related to simultaneous changes in BMI [49, 50].
An increase in BMI >5% in the first year post-transplantation was associated with an increased graft loss risk [HR 2.82 (95% confidence interval 1.11–7.44), P = .015] in a study of 292 transplant recipients [51]. An accelerated decline in eGFR and higher rates of new-onset diabetes mellitus was reported in patients with weight gain in a cohort study of 433 transplant patients [52]. Similar results have been registered in other studies [53–55]. The efficacy of therapeutic interventions, including lifestyle modifications and bariatric surgery, for optimizing renal transplant outcomes is a promising but still scarcely investigated clinical research area [56–58].
PERSPECTIVES
Studies targeting excessive fat mass in living kidney donors are a priority to test whether fat mass excess reduction may improve the outcomes of transplanted kidneys. Also, intervention studies to mitigate body weight gain after transplantation are fundamental to understand whether preventing de novo obesity or aggravation of pre-existing obesity translates into clinical benefits in the transplant population. Dietary and lifestyle modifications, bariatric surgery and, limited to patients with diabetes insulin therapy, glucagon-like peptide 1 (GLP-1) analogues, dipeptidyl peptidase-4 (DPP-IV) inhibitors, RAAS blockade and sodium–glucose cotransporter 2 (SGLT-2) inhibitors represent promising opportunities for clinical research [59, 60] (Fig. 3). Several clinical trials have examined the role of various types of exercise in renal transplant recipients (clinicaltrials.gov: NCT04123951, NCT04745169, NCT04954690, NCT04489043, NCT04044963, NCT05003193). A meta-analysis including 17 randomized controlled trials showed that this intervention is safe and effective for weight reduction both before and after kidney transplantation [61]. There are two ongoing clinical trials evaluating the effect of SGLT-2 inhibitors on renal transplantation in terms of cardio-renal health and glycaemic control (NCT04906213, NCT04965935). The role of DPP-IV inhibitors in kidney transplant recipients has been sparsely investigated in methodologically weak studies [62–64]. A short-duration follow-up period, study designs including crossover techniques, small numbers of patients and analysis of patients with new-onset diabetes mellitus after transplantation are the major limitations of these studies. Nonetheless, initial results indicate that GLP-1 analogues are safe options for glycaemic control in renal transplant patients with only limited beneficial effects on weight control. GLP-1 analogues are still untested in transplant recipients and there is an ongoing placebo-controlled double-blind trial investigating the effect of semaglutide on the eligibility of obese ESRD patients for kidney transplantation (NCT04741074).
Figure 3:
Potential therapeutic interventions to improve graft function during the post-transplantation period.
Another significant consideration in kidney transplant recipients is long-term dietary interventions, among which the Mediterranean and the Dietary Approaches to Stop Hypertension (DASH) diets are most commonly investigated. The Mediterranean diet includes consumption of greater amounts of fish, fruit, vegetables, legumes, nuts and olive oil, together with a low intake of dairy and meat products, while the DASH diet includes consumption of vegetables, fruits, legumes and low-fat dairy products [65, 66]. Adherence to the Mediterranean diet in renal transplant recipients has been linked to better nutritional status and lower risk for metabolic syndrome in long-term follow-up, along with better preservation of allograft function [67–71]. Similarly, the DASH diet is also linked to better long-term renal allograft function preservation [72, 73]. Thus adherence to such a diet is associated with better allograft function, but future large-scale randomized studies are required in order to make a strong recommendation.
Robotic-assisted kidney transplantation is an alternative renal transplantation procedure. The latest studies indicate that such procedures may be performed with no difference in terms of complications or graft function compared with non-obese kidney donors when performed in experienced centres [74–76].
This large research portfolio will hopefully produce in the medium term the knowledge needed to inform clinical policies aimed at optimizing body weight and related risk factors and to mitigate the renal and cardiovascular sequelae of overweight and obesity in transplant patients.
Supplementary Material
Contributor Information
Mehmet Kanbay, Department of Medicine, Division of Nephrology, Koc University School of Medicine, Istanbul, Turkey.
Sidar Copur, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Duygu Ucku, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Carmine Zoccali, Renal Research Institute, New York, NY, USA; Department of Medicine, Division of Nephrology, Associazione Ipertensione, Nefrologia e Trapianto Renale, Nefrologia, Ospedali Riuniti, Reggio Calabria, Italy.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
M.K. and C.Z. are members of the CKJ editorial board.
REFERENCES
- 1. Statista . Estimated number of worldwide kidney transplants in 2020, by region. https://www.statista.com/statistics/398657/kidney-transplants-by-world-region/(7 October 2022, date last accessed). [Google Scholar]
- 2. Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int 2014;27:19–27. [DOI] [PubMed] [Google Scholar]
- 3. Richie RE, Niblack GD, Johnson HKet al. Factors influencing the outcome of kidney transplants. Ann Surg 1983;197:672–7. 10.1097/00000658-198306000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Agati VD, Chagnac A, de Vries APet al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016;12:453–71. 10.1038/nrneph.2016.75 [DOI] [PubMed] [Google Scholar]
- 5. Brenner BM, Milford EL. Nephron underdosing: a programmed cause of chronic renal allograft failure. Am J Kidney Dis 1993;21:66–72. 10.1016/0272-6386(93)70097-I [DOI] [PubMed] [Google Scholar]
- 6. Rea DJ, Heimbach JK, Grande JPet al. Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int 2006;70:1636–41. 10.1038/sj.ki.5001799 [DOI] [PubMed] [Google Scholar]
- 7. Chang Y, Ryu S, Suh BSet al. Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int J Obes (Lond) 2012;36:1187–94. 10.1038/ijo.2011.247 [DOI] [PubMed] [Google Scholar]
- 8. Cameron AJ, Boyko EJ, Sicree RAet al. Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring) 2008;16:2707–16. 10.1038/oby.2008.412 [DOI] [PubMed] [Google Scholar]
- 9. Kotsis V, Stabouli S, Papakatsika Set al. Mechanisms of obesity-induced hypertension. Hypertens Res 2010;33:386–93. 10.1038/hr.2010.9 [DOI] [PubMed] [Google Scholar]
- 10. Kanbay M, Copur S, Demiray Aet al. Fatty kidney: a possible future for chronic kidney disease research. Eur J Clin Invest 2022;52:e13748. [DOI] [PubMed] [Google Scholar]
- 11. de Vries AP, Ruggenenti P, Ruan XZet al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol 2014;2:417–26. 10.1016/S2213-8587(14)70065-8 [DOI] [PubMed] [Google Scholar]
- 12. Yang P, Xiao Y, Luo Xet al. Inflammatory stress promotes the development of obesity-related chronic kidney disease via CD36 in mice. J Lipid Res 2017;58:1417–27. 10.1194/jlr.M076216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandel LJ. Metabolic substrates, cellular energy production, and the regulation of proximal tubular transport. Annu Rev Physiol 1985;47:85–101. 10.1146/annurev.ph.47.030185.000505 [DOI] [PubMed] [Google Scholar]
- 14. Wang XX, Jiang T, Shen Yet al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 2010;59:2916–27. 10.2337/db10-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Lerman LO. Obesity and renovascular disease. Am J Physiol Renal Physiol 2015;309:F273–9. 10.1152/ajprenal.00547.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Câmara NO, Iseki K, Kramer Het al. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol 2017;13:181–90. 10.1038/nrneph.2016.191 [DOI] [PubMed] [Google Scholar]
- 17. Silva GB Jr, Bentes AC, Daher EFet al. Obesity and kidney disease. J Bras Nefrol 2017;39:65–9. 10.5935/0101-2800.20170011 [DOI] [PubMed] [Google Scholar]
- 18. Hall JE, do Carmo JM, da Silva AAet al. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol 2019;15:367–85. 10.1038/s41581-019-0145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 1998;31:409–14. 10.1161/01.HYP.31.1.409 [DOI] [PubMed] [Google Scholar]
- 20. Yamagishi SI, Edelstein D, Du XLet al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 2001;276:25096–100. 10.1074/jbc.M007383200 [DOI] [PubMed] [Google Scholar]
- 21. Sharma K, Ramachandrarao S, Qiu Get al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008;118:1645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunley TE, Ma LJ, Kon V. Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens 2010;19:227–34. 10.1097/MNH.0b013e3283374c09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García-Carro C, Vergara A, Bermejo Set al. A nephrologist perspective on obesity: from kidney injury to clinical management. Front Med 2021;8:655871. 10.3389/fmed.2021.655871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sastre C, Rubio-Navarro A, Buendía Iet al. Hyperlipidemia-associated renal damage decreases Klotho expression in kidneys from ApoE knockout mice. PLoS One 2013;8:e83713. 10.1371/journal.pone.0083713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanda T, Matsuoka S, Yamazaki Met al. Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol 2018;24:2661–72. 10.3748/wjg.v24.i25.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rives C, Fougerat A, Ellero-Simatos Set al. Oxidative stress in NAFLD: role of nutrients and food contaminants. Biomolecules 2020;10:1702. 10.3390/biom10121702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mima A, Yasuzawa T, King GLet al. Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio 2018;8:664–70. 10.1002/2211-5463.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arshad A, Hodson J, Chappelow Iet al. The impact of donor body mass index on outcomes after deceased kidney transplantation – a national population-cohort study. Transpl Int 2018;31:1099–109. 10.1111/tri.13263 [DOI] [PubMed] [Google Scholar]
- 29. Naik AS, Zhong Y, Parasuraman Ret al. The temporal and long-term impact of donor body mass index on recipient outcomes after kidney transplantation – a retrospective study. Transpl Int 2020;33:59–67. 10.1111/tri.13505 [DOI] [PubMed] [Google Scholar]
- 30. Ortiz J, Gregg A, Wen Xet al. Impact of donor obesity and donation after cardiac death on outcomes after kidney transplantation. Clin Transplant 2012;26:E284–92. 10.1111/j.1399-0012.2012.01649.x [DOI] [PubMed] [Google Scholar]
- 31. Miller AJ, Kiberd BA, Alwayn IPet al. Donor-recipient weight and sex mismatch and the risk of graft loss in renal transplantation. Clin J Am Soc Nephrol 2017;12:669–76. 10.2215/CJN.07660716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. el-Agroudy AE, Hassan NA, Bakr MAet al. Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am J Nephrol 2003;23:294–9. 10.1159/000072819 [DOI] [PubMed] [Google Scholar]
- 33. Kasiske BL, Snyder JJ, Gilbertson D. Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol 2002;13:2152–9. 10.1097/01.ASN.0000024564.22119.3D [DOI] [PubMed] [Google Scholar]
- 34. Lentine KL, Kasiske BL, Levey ASet al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 2017;101:1783–92. 10.1097/TP.0000000000001770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abramowicz D, Cochat P, Claas FHet al. European Renal Best Practice guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant 2015;30:1790–7. 10.1093/ndt/gfu216 [DOI] [PubMed] [Google Scholar]
- 36. Andrews PA, Burnapp L. British Transplantation Society /Renal Association UK guidelines for living donor kidney transplantation 2018: summary of updated guidance. Transplantation 2018;102:e307. 10.1097/TP.0000000000002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heimbach JK, Taler SJ, Prieto Met al. Obesity in living kidney donors: clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant 2005;5:1057–64. 10.1111/j.1600-6143.2005.00791.x [DOI] [PubMed] [Google Scholar]
- 38. Friedman AL, Cheung K, Roman SAet al. Early clinical and economic outcomes of patients undergoing living donor nephrectomy in the United States. Arch Surg 2010;145:356–62; discussion 362. 10.1001/archsurg.2010.17 [DOI] [PubMed] [Google Scholar]
- 39. Serrano OK, Sengupta B, Bangdiwala Aet al. Implications of excess weight on kidney donation: long-term consequences of donor nephrectomy in obese donors. Surgery 2018;164:1071–6. 10.1016/j.surg.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 40. Patel S, Cassuto J, Orloff Met al. Minimizing morbidity of organ donation: analysis of factors for perioperative complications after living-donor nephrectomy in the United States. Transplantation 2008;85:561–5. 10.1097/TP.0b013e3181643ce8 [DOI] [PubMed] [Google Scholar]
- 41. Locke JE, Reed RD, Massie Aet al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int 2017;91:699–703. 10.1016/j.kint.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thukral S, Mazumdar A, Ray DS. Long-term consequences of complex living renal donation: is it safe? Transplant Proc 2018;50:3185–91. 10.1016/j.transproceed.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 43. Tavakol MM, Vincenti FG, Assadi Het al. Long-term renal function and cardiovascular disease risk in obese kidney donors. Clin J Am Soc Nephrol 2009;4:1230–8. 10.2215/CJN.01350209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerkeni W, Rebai MH, Bouzouita Aet al. The effect of body mass index at the time of donation on postoperative and remote consequences of nephrectomy in 189 living-related kidney donors. Arab J Urol 2015;13:221–4. 10.1016/j.aju.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nguyen MJP, Carpenter D, Tadros Jet al. Bariatric surgery prior to living donor nephrectomy: a solution to expand the living donor kidney pool – a retrospective study. Transpl Int 2019;32:702–9. 10.1111/tri.13408 [DOI] [PubMed] [Google Scholar]
- 46. Aggarwal N, Porter AC, Tang IYet al. Creatinine-based estimations of kidney function are unreliable in obese kidney donors. J Transplant 2012;2012:872894. 10.1155/2012/872894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cashion AK, Hathaway DK, Stanfill Aet al. Pre-transplant predictors of one yr weight gain after kidney transplantation. Clin Transplant 2014;28:1271–8. 10.1111/ctr.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tantisattamo E, Kalantar-Zadeh K, Halleck Fet al. Novel approaches to sarcopenic obesity and weight management before and after kidney transplantation. Curr Opin Nephrol Hypertens 2021;30:14–26. 10.1097/MNH.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 49. Moradzadeh M, Mirmohammadkhani M, Tamadon MRet al. Prevalence of sleep apnea and its associated factors in chronic kidney disease patients. Tanaffos 2021;20:116–25. [PMC free article] [PubMed] [Google Scholar]
- 50. Mallamaci F, Tripepi R, D'Arrigo Get al. Long-term changes in sleep disordered breathing in renal transplant patients: relevance of the BMI. J Clin Med 2020;9:1739. 10.3390/jcm9061739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ducloux D, Kazory A, Simula-Faivre Det al. One-year post-transplant weight gain is a risk factor for graft loss. Am J Transplant 2005;5:2922–8. 10.1111/j.1600-6143.2005.01104.x [DOI] [PubMed] [Google Scholar]
- 52. Nöhre M, Schieffer E, Hanke Aet al. Obesity after kidney transplantation—results of a KTx360°Substudy. Front Psychiatry 2020;11:399. 10.3389/fpsyt.2020.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang SH, McDonald SP. Post-kidney transplant weight change as marker of poor survival outcomes. Transplantation 2008;85:1443–8. 10.1097/TP.0b013e31816f1cd3 [DOI] [PubMed] [Google Scholar]
- 54. Hoogeveen EK, Aalten J, Rothman KJet al. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation 2011;91:869–74. 10.1097/TP.0b013e3182100f3a [DOI] [PubMed] [Google Scholar]
- 55. Kim IK, Choi SH, Son Set al. Early weight gain after transplantation can cause adverse effect on transplant kidney function. Transplant Proc 2016;48:893–6. 10.1016/j.transproceed.2015.10.064 [DOI] [PubMed] [Google Scholar]
- 56. Cohen JB, Lim MA, Tewksbury CMet al. Bariatric surgery before and after kidney transplantation: long-term weight loss and allograft outcomes. Surg Obes Relat Dis 2019;15:935–41. 10.1016/j.soard.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Modanlou KA, Muthyala U, Xiao Het al. Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States Renal Data System and literature review. Transplantation 2009;87:1167–73. 10.1097/TP.0b013e31819e3f14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klaassen G, Zelle DM, Navis GJet al. Lifestyle intervention to improve quality of life and prevent weight gain after renal transplantation: design of the Active Care after Transplantation (ACT) randomized controlled trial. BMC Nephrol 2017;18:296. 10.1186/s12882-017-0709-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martin-Moreno PL, Shin HS, Chandraker A. Obesity and post-transplant diabetes mellitus in kidney transplantation. J Clin Med 2021;10:2497. 10.3390/jcm10112497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Halden TAS, Kvitne KE, Midtvedt Ket al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 2019;42:1067–74. 10.2337/dc19-0093 [DOI] [PubMed] [Google Scholar]
- 61. Conley MM, McFarlane CM, Johnson DWet al. Interventions for weight loss in people with chronic kidney disease who are overweight or obese. Cochrane Database Syst Rev 2021;3:CD013119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haidinger M, Werzowa J, Hecking Met al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation—a randomized, double-blind, placebo-controlled trial. Am J Transplant 2014;14:115–23. 10.1111/ajt.12518 [DOI] [PubMed] [Google Scholar]
- 63. Strøm Halden TA, Åsberg A, Vik Ket al. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant 2014;29:926–33. 10.1093/ndt/gft536 [DOI] [PubMed] [Google Scholar]
- 64. Soliman AR, Fathy A, Khashab Set al. Sitagliptin might be a favorable antiobesity drug for new onset diabetes after a renal transplant. Exp Clin Transplant 2013;11:494–8. 10.6002/ect.2013.0018 [DOI] [PubMed] [Google Scholar]
- 65. Sofi F, Abbate R, Gensini GFet al. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96. 10.3945/ajcn.2010.29673 [DOI] [PubMed] [Google Scholar]
- 66. Salehi-Abargouei A, Maghsoudi Z, Shirani Fet al. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition 2013;29:611–8. 10.1016/j.nut.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 67. Vučković M, Radić J, Gelemanović Aet al. Associations between depression, nutritional status and Mediterranean diet in Dalmatian kidney transplant recipients. Nutrients 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vučković M, Radić J, Gelemanović Aet al. Mediterranean diet adherence and nutritional status in Dalmatian kidney transplant recipients–are they related? Nutrients 2021;13:3246.w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nafar M, Noori N, Jalali-Farahani Set al. Mediterranean diets are associated with a lower incidence of metabolic syndrome one year following renal transplantation. Kidney Int 2009;76:1199–206. 10.1038/ki.2009.343 [DOI] [PubMed] [Google Scholar]
- 70. Barbagallo CM, Cefalù AB, Gallo Set al. Effects of Mediterranean diet on lipid levels and cardiovascular risk in renal transplant recipients. Nephron 1999;82:199–204. 10.1159/000045403 [DOI] [PubMed] [Google Scholar]
- 71. Gomes-Neto AW, Osté MCJ, Sotomayor CGet al. Mediterranean style diet and kidney function loss in kidney transplant recipients. Clin J Am Soc Nephrol 2020;15:238–46. 10.2215/CJN.06710619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tantisattamo E, Kalantar-Zadeh K, Molnar MZ. Nutritional and dietary interventions to prolong renal allograft survival after kidney transplantation. Curr Opin Nephrol Hypertens 2022;31:6–17. 10.1097/MNH.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 73. Osté MCJ, Gomes-Neto AW, Corpeleijn Eet al. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am J Transplant 2018;18:2523–33. 10.1111/ajt.14707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matthew AN, Hampton LJ, Autorino Ret al. Evolution of robotic-assisted kidney transplant: successes and barriers to overcome. Curr Opin Urol 2021;31:29–36. 10.1097/MOU.0000000000000834 [DOI] [PubMed] [Google Scholar]
- 75. Tzvetanov IG, Spaggiari M, Tulla KAet al. Robotic kidney transplantation in the obese patient: 10-year experience from a single center. Am J Transplant 2020;20:430–40. 10.1111/ajt.15626 [DOI] [PubMed] [Google Scholar]
- 76. Prudhomme T, Beauval JB, Lesourd Met al. Robotic-assisted kidney transplantation in obese recipients compared to non-obese recipients: the European experience. World J Urol 2021;39:1287–98. 10.1007/s00345-020-03309-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.