ABSTRACT
Atherosclerotic renovascular disease (ARVD) represents the most common type of renal artery stenosis. In the last decade, a few large trials failed to demonstrate the superiority of standard medical therapy plus percutaneous transluminal renal angioplasty (PTRA) compared with medical therapy alone in lowering blood pressure levels or preventing adverse renal and cardiovascular outcomes in patients with ARVD. However, this issue remains controversial and an ongoing debate focusses on the benefits that selected patients could experience from renal revascularization procedures. In this regard, several pieces of observational data show that PTRA is associated with future cardiorenal benefits in patients presenting with high-risk ARVD phenotypes. Such evidence resulted in a progressive shift in relevant recommendations, with most recent not-graded suggestions supporting that revascularization should be offered in these high-risk subjects. Existing evidence clearly calls for a properly designed randomized controlled trial with selected patients presenting high-risk ARVD phenotypes, in order to confirm the superiority of PTRA versus non-invasive management in this patient group and objectively guide everyday clinical practice.
Keywords: atherosclerotic renovascular disease, high-risk patients, PTRA, RCTs, renal artery stenosis
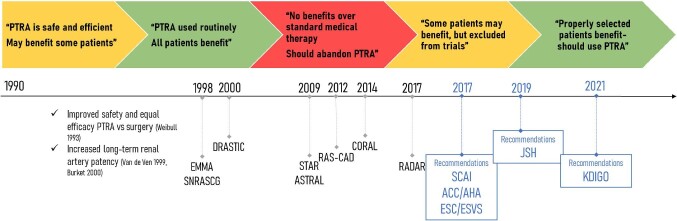
Atherosclerotic renovascular disease (ARVD) represents the most common type of renal artery stenosis (RAS) and is independently associated with various comorbidities including hypertension, chronic kidney disease (CKD), heart failure (HF), peripheral and coronary artery disease [1]. After some early years of enthusiasm towards the benefit of revascularization without supporting data from properly conducted randomized controlled trials (RCTs; Fig. 1), a few large studies attempted to properly test the superiority of standard medical therapy plus renal artery angioplasty compared with medical therapy alone in lowering blood pressure (BP) levels or preventing adverse renal and cardiovascular outcomes in patients with ARVD. Among the most important of these studies, the STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery (STAR) (140 patients with RAS ≥50%) [2] and the Angioplasty and STenting for Renal Artery Lesions (ASTRAL) (806 patients) trials [3] showed no significant differences in BP levels and kidney disease progression in patients treated with percutaneous transluminal renal angioplasty (PTRA) and standard medication compared with those with medication only. Lastly, in the multicentre Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study, with 947 patients and a median follow-up of 43 months, PTRA had no additional benefit on BP control, renal function and adverse cardiovascular or renal outcomes when compared with medical therapy alone [4].
FIGURE 1:
Historical timeline showing the different practice patterns and attitudes towards PTRA in the management of ARVD during the last 30 years and associations with published randomized trials and most recent recommendations.
The aforementioned trials met severe criticism due to numerous limitations in study design, methodology and execution. All of them had non-standardized inclusion criteria, resulting in the enrolment of large numbers of patients with mild/asymptomatic RAS, mild hypertension or advanced CKD with small kidneys, i.e. individuals with almost certain absence of benefit from RAS revascularization [5, 6]. In contrast, the aforementioned RCTs on PTRA have almost entirely excluded patients with a clinical presentation highly suggestive of functionally important RAS, such as those with flash pulmonary oedema, refractory hypertension or rapid loss of kidney function after the use of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. The presence of systematic biases in radiological assessment of RAS and poor laboratory proof of critical RAS is also highly possible, as there was great variability not only between, but also within study protocols in imaging techniques used for RAS diagnosis and evaluation, often resulting in an overestimation of the degree of stenosis. Additional methodological limitations include a large number of patients fulfilling the inclusion criteria who were not randomized based on the investigators’ judgement without specific justification, enrolment delays, protocol amendments during the trial, high crossover rates between the arms and low event rates for major outcomes [5–9].
Despite the severe criticism, the publication of the aforementioned negative clinical trials gave rise to widespread doubt of the utility of PTRA and led to significant changes in everyday clinical practice worldwide. With time progressing, revascularization for ARVD was given a IIb class of recommendation and a level of evidence of ‘C’ in the last 2017 American College of Cardiology/American Heart Association (AHA/ACC) hypertension guidelines [10]. However, this issue remains controversial and an ongoing debate focusses on the benefits that selected patients could experience from renal revascularization procedures. Proper patient selection for RAS revascularization may be of major importance, due to significant differences in prognostic associations between the different ARVD clinical phenotypes and the obvious significant differences in the actual benefit of revascularization. To this end, there are numerous reports of individual cases and cohorts showing that patients with high-risk presentations of ARVD benefit the most from revascularization [11]. In a previous prospective cohort study in 467 patients with RAS >50%, patients presenting with flash pulmonary oedema had a markedly increased risk of mortality and cardiovascular events compared with those with low-risk phenotypes (i.e. without flash pulmonary oedema, refractory hypertension or rapid loss of kidney function) [11]. In the same study, PTRA was associated with a major decrease in the risk of death {hazard ratio [HR] 0.15 [95% confidence interval (CI) 0.02–0.9]} and cardiovascular events [HR 0.23 (95% CI 0.1–0.6)] in patients with high-risk presentation, but no apparent benefit in low-risk patients without these presentations [HR 0.8 (95% CI 0.7–1.2) and 1.0 (95% CI 0.8–1.2), respectively]. In addition, a prospective observational study in 611 patients with RAS >50% (of which 152 patients had coexistent HF) showed that there was no difference in mortality in the non-HF group between PTRA and standard medical therapy [HR 0.8 (95% CI 0.5–1.1)], while for those with HF, the corresponding HR was 0.6 (95% CI 0.3–0.9) [12].
In this regard, a recent report by Reinhard et al. [13] of a prospective cohort study investigating the effects of PTRA on ambulatory BP levels, estimated glomerular filtration rate (eGFR) and HF recurrence in a group of well-defined patients with severe ARVD sheds more light on the subject. This study has several strengths, including a proper methodology and recruitment of patients with well-documented severe ARVD (≥70% stenosis) and harsh clinical presentations (resistant hypertension, rapidly declining kidney function or recurrent HF/flash pulmonary oedema). The results clearly demonstrated that PTRA is associated with better ambulatory BP levels [24-h systolic BP change from baseline: −25.7 mmHg (95% CI −30.8 to −20.6)] and control [change in the number of antihypertensives: −0.9 (95% CI −1.3 to −0.5)], improved kidney function [eGFR change: +7.2 mL/min/1.73 m2 (95% CI 3.2–11.2)] at the 24-month evaluation and decreased hospital admissions due to HF/flash pulmonary oedema (of 17 patients with a history of hospitalizations for HF, 14 patients had no new episodes after PTRA). This study is added to several reports of individual cases and cohorts showing that patients with high-risk presentations of ARVD, like those above, benefit the most from revascularization [11]. These patients often have nearly occluded renal arteries or bilateral RAS or single RAS with a solitary kidney, and revascularization has immediate beneficial effects with substantial decreases in BP and significant improvement in kidney function [5].
In conclusion, several pieces of observational data show that PTRA is associated with future renal and cardiovascular benefits in patients presenting high-risk ARVD phenotypes. Such evidence resulted in a progressive shift in relevant recommendations, with the most recent not-graded suggestions supporting that revascularization should be offered in patients with these phenotypes (Table 1) [14, 15]. Lastly, and most importantly, existing evidence clearly calls for a properly designed RCT with selected patients with severe and haemodynamically significant ARVD and high-risk clinical presentations in order to confirm the superiority of PTRA versus non-invasive management in this patient group and objectively guide everyday clinical practice.
Table 1.
Current indications for PTRA in existing clinical recommendations
| Recommendations | Year | Recommendations |
|---|---|---|
| SCAI | 2017 |
Appropriate
Cardiac disturbance syndromes (flash pulmonary oedema or ACS with hypertension and moderate RAS with a resting translesional mean gradient of ≥10 mmHg and/or several RAS (AUC 9) CKD stage 4 and bilateral moderate RAS with a resting translesional mean gradient of ≥10 mmHg with kidney size >7 cm (pole-to-pole length) (AUC 8) CKD stage 4 and bilateral severe RAS or unilateral severe RAS with a solitary kidney (AUC 7) Resistant hypertension and bilateral or solitary severe RAS (AUC 7) May be appropriate Resistant hypertension and unilateral severe RAS (AUC 6) CKD stage 4 and unilateral moderate RAS with a resting translesional mean gradient of ≥10 mmHg without other explanation (AUC 6) Recurrent CHF with unilateral moderate RAS with a resting translesional mean gradient of ≥10 mmHg (AUC 5) CKD stage 2 with bilateral severe RAS (AUC 5) CKD stage 3 stable for 1 year with bilateral severe RAS (AUC 5) Resistant hypertension with severe unilateral RAS and anatomically challenging or high-risk lesion (AUC 4) |
| ACC/AHA | 2017 | In adults with RAS for whom medical management has failed (refractory hypertension, worsening renal function and/or intractable HF) and those with nonatherosclerotic disease, including fibromuscular dysplasia, it may be reasonable to refer the patient for consideration of revascularization (percutaneous renal artery angioplasty and/or stent placement) (Class: IIb/LOE: C) |
| ESC/ESVS | 2017 | Routine revascularization is not recommended in RAS secondary to atherosclerosis (Class: III/LOE: A) In cases of hypertension and/or signs of renal impairment related to FMD, balloon angioplasty without bailout stenting should be considered (Class: IIa/LOE: B) Balloon angioplasty with or without stenting may be considered in selected patients with RAS and unexplained recurrent CHF or sudden pulmonary oedema (Class: IIb/LOE: C) |
| JSH | 2019 | PTRA for renovascular hypertension could be considered in patients with hemodynamically significant stenosis of the renal artery and one of the following conditions: FMD, resistant hypertension, exacerbating/malignant hypertension, unexplained or repeated pulmonary oedema/HF or bilateral renal artery stenosis or renal artery stenosis in solitary kidney |
| KDIGO | 2021 |
Definite indications
Acute pulmonary oedema or acute decompensations of HF and high-grade RAS Progressive CKD in high-grade (>75%) RAS (bilateral or solitary kidney) AKI due to acute renal artery occlusion or high-grade RAS ACEi or ARB intolerance in high-grade RAS Kidney transplant with RAS (symptomatic or asymptomatic) Possible indications Chronic HF and high-grade RAS Coexistence of progressive CKD and uncontrolled hypertension Asymptomatic high-grade RAS (either bilateral or supplying solitary kidney) with viable renal parenchyma (to prevent atrophy) New (<3 months) dialysis patients with non-functioning but possibly viable kidneys |
AUC, appropriate use criteria: 4–6 (indicated under certain circumstances), 7–9 (usually indicated); SCAI, Society for Cardiovascular Angiography and Interventions; ACS, acute coronary syndrome; CHF, congestive heart failure; ACC/AHA; American College of Cardiology/American Heart Association; ESC/ESVS; European Society of Cardiology/European Society of Vascular Surgery; LOE, level of evidence; FMD, fibromuscular dysplasia; JSH, Japanese Society of Hypertension; ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; KDIGO, Kidney Disease: Improving Global Outcomes.
ACKNOWLEDGEMENTS
This work represents original research by the authors.
Contributor Information
Marieta P Theodorakopoulou, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Artemios G Karagiannidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Charles J Ferro, Department of Renal Medicine, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Pantelis A Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
FUNDING
None.
CONFLICT OF INTEREST STATEMENT
A.O. is Editor Emeritus of CKJ. The other authors disclose that they do not have any financial or other relationships,which might lead to a conflict of interest regarding this article. The results presented in this work have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this work.
REFERENCES
- 1. Prince M, Tafur JD, White CJ. When and how should we revascularize patients with atherosclerotic renal artery stenosis? JACC Cardiovasc Interv 2019; 12: 505–517 [DOI] [PubMed] [Google Scholar]
- 2. Bax L, Woittiez A-JJ, Kouwenberg HJet al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150: 840–848 [DOI] [PubMed] [Google Scholar]
- 3. ASTRAL Investigators, Wheatley K, Ives Net al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361: 1953–1962 [DOI] [PubMed] [Google Scholar]
- 4. Cooper CJ, Murphy TP, Cutlip DEet al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van der Niepen P, Rossignol P, Lengelé J-Pet al. Renal artery stenosis in patients with resistant hypertension: stent it or not? Curr Hypertens Rep 2017; 19: 5. [DOI] [PubMed] [Google Scholar]
- 6. Sarafidis PA, Stavridis KC, Loutradis CNet al. To intervene or not? A man with multidrug-resistant hypertension, endovascular abdominal aneurysm repair, bilateral renal artery stenosis and end-stage renal disease salvaged with renal artery stenting. Blood Press 2016; 25: 123–128 [DOI] [PubMed] [Google Scholar]
- 7. Murphy TP, Cooper CJ, Cutlip DEet al. Roll-in experience from the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study. J Vasc Interv Radiol 2014; 25: 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarzwälder U, Zeller T. Critical review of indications for renal artery stenting: do randomized trials give the answer? Catheter Cardiovasc Interv 2009; 7251–256 [DOI] [PubMed] [Google Scholar]
- 9. White CJ. Kiss my ASTRAL: one seriously flawed study of renal stenting after another. Catheter Cardiovasc Interv 2010; 75: 305–307 [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Carey RM, Aronow WSet al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71: e13–e115 [DOI] [PubMed] [Google Scholar]
- 11. Ritchie J, Green D, Chrysochou Cet al. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 2014; 63: 186–197 [DOI] [PubMed] [Google Scholar]
- 12. Green D, Ritchie JP, Chrysochou Cet al. Revascularisation of renal artery stenosis as a therapy for heart failure: an observational cohort study. Lancet 2015; 385(Suppl 1): S11. [DOI] [PubMed] [Google Scholar]
- 13. Reinhard M, Schousboe K, Andersen UBet al. Renal artery stenting in consecutive high-risk patients with atherosclerotic renovascular disease: a prospective 2-center cohort study. J Am Heart Assoc 2022; 11: e024421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansen KL, Garimella PS, Hicks CWet al. Central and peripheral arterial diseases in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021; 100: 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hicks CW, Clark TWI, Cooper CJet al. Atherosclerotic renovascular disease: a KDIGO (Kidney Disease: Improving Global Outcomes) Controversies Conference. Am J Kidney Dis 2022; 79: 289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this work.