ABSTRACT
Peritoneal dialysis (PD) for acute kidney injury (AKI) has been available for nearly 80 years and has been through periods of use and disuse largely determined by availability of other modalities of kidney replacement therapy and the relative enthusiasm of clinicians. In the past 10 years there has been a resurgence in the use of acute PD globally, facilitated by promotion of PD for AKI in lower resource countries by nephrology organizations effected through the Saving Young Lives program and collaborations with the World Health Organisation, the development of guidelines standardizing prescribing practices and finally the COVID-19 pandemic.
This review highlights the history of PD for AKI and looks at misconceptions about efficacy as well as the available evidence demonstrating that acute PD is a safe and lifesaving therapy with comparable outcomes to other modalities of treatment.
Keywords: acute kidney injury, COVID, dialysis, PD, peritoneal dialysis
HISTORY OF PD FOR AKI
Peritoneal dialysis (PD) has been used to treat acute kidney injury (AKI) since 1946 when Frank, Seligman and Fine successfully treated the first patient until recovery of function. Their series described the early difficulties encountered with access, fluid constituents and infections, as well as the solutions they developed. Their initial treatments used a steel, rubber and glass catheter and sump drain to instil Tyrodes solution supplemented with 1%–1.5% glucose and 1% gelatin [1, 2]. Initially the fluid caused hyperchloraemia and sodium chloride was substituted with sodium bicarbonate or sodium lactate. Concerns that glucose concentrations greater than 1.5% would cause significant irritation of the peritoneum along with the continuation of intravenous fluid replacement in the face of oliguria meant that many patients suffered from and often succumbed to pulmonary oedema. Peritonitis was common and thought due to the open fluid system and bedside mixing of fluids. Developments in peritoneal access further improved outcomes with better flow rates and fewer mechanical complications. Rigid plastic catheters introduced over a sharp stylet were used until very recently, however flexible catheters inserted at the bedside are now considered preferable [3].
PD has been the dominant modality for treating AKI in children, however in adults the proportion of patients has significantly fallen since the advent of pump-driven continuous kidney replacement therapy (CKRT) [4–7]. A study from Canada showed that in the 2 year periods beginning in 1994 and 1999 the reduced number of patients on PD was associated with an increase in CKRT use (9%–26%) [5]. A multicentre study of AKI management in intensive care units (ICU) globally showed that PD was used in only 3.2% patients. It must be noted that all countries except one were in the high-income category and this has a significant bearing on PD utilization [6]. A survey of 560 nephrologists, predominantly from Europe, found that PD for AKI was only available in 24% of centres. CKRT was the predominant modality used by intensivists whereas nephrologists were more likely to use intermittent therapies and PD [7]. This highlights the disconnect between those who feel that PD is suitable for treating AKI and those who actually practice it. A survey of nephrologists from three international conferences reported that 50.8% and 36.4% of respondents felt that PD was suitable for treating AKI in the wards and ICU, respectively. In contrast only 15.7% and 22% actually used it in these settings [8]. PD was used in 35% of ICU patients in Asia compared with only 13% and 8.2% in Europe and North America [8]. Most acute PD is practiced in low/low-middle income countries, as in many countries CKRT is prohibitively expensive whereas PD carries a lower cost and requires minimal infrastructure thus can be performed in remote areas without the need for electricity or significant volumes of water. As it is a cardiovascularly stable modality it is used preferentially over intermittent therapies in critically ill patients requiring vasopressor support.
Although there was a fall in use of PD in high income countries (HIC) in lower resource settings there has been growth in part due to the Saving Young Lives (SYL) initiative, the publication of the International Society for Peritoneal Dialysis (ISPD) guidelines for PD in AKI in 2014 and 2020 as well as the International Society of Nephrology (ISN) framework for dialysis recommending PD as the preferential modality in low-resource environments [3, 9–20].
The coronavirus disease 2019 (COVID-19) pandemic has challenged the perception that PD is not suitable for critically ill patients, and many nephrologists in HICs turned to acute PD in preference to CKRT. Reasons included the exceptional clotting of the extracorporeal circuit, reduced nursing staff availibility and haemodialysis machine/consumable demand outstripping supply. Case series from the UK and USA have shown good results in these settings [21–29].
The ICU at King’s College London treated 108 patients with AKI secondary to COVID-19 with 34 treated with acute PD. All patients on PD were mechanically ventilated and 61% required vasopressor support. Despite this, survival was 70.3% with 85% of patients recovering renal function after a median of 12 days [22]. Four separate units in New York who had rapidly implemented acute PD programs during the pandemic published their experiences separately and later combined the data highlighting the success of each, along with the barriers that needed to be overcome [26–28, 30, 31]. Ninety-four patients were included, with 87% requiring mechanical ventilation, with a mean modified Acute Physiology And Chronic Health Evaluation (APACHE II) score of 13. PD was the initial modality in 56 patients, with 32 transferring from intermittent haemodialysis (IHD) and the remainder from CKRT. All PD catheters were placed by either surgeons (85%) or interventional radiologists (16%). Mean PD volumes were 1.5 L, with 86% of prescribed volume being achieved with a mean ultrafiltration of 0.7 L (interquartile range 0.3–1.7). Twenty patients were either switched to or supplemented with IHD/CKRT. The reason for switch was split evenly between mechanical complications, persistent hyperkalaemia or fluid overload. After a median follow-up of 30 days 46% of patients had died, 22% had recovered kidney function, 28% were still in hospital on dialysis and 4% had been discharged on dialysis. There was concern that prone positioning would not be feasible in patients on PD, however in this analysis, 47% required prone positioning during their stay. Being prone did not affect potassium or bicarbonate clearance; although adjustments needed to be made to fluid volumes due to increased leaks, it did not require a switch of modality. Analysis of 11 patients in one centre confirmed that patients ventilated in the prone position while on PD showed no worsening of gas exchange. Certain techniques such as a more lateral exit-site were beneficial [27].
This resurgence in the use of PD for AKI in high-resource countries has sparked new interest in the use of PD for AKI in all settings.
BENEFITS OF PD
There are a number of demonstrable as well as theoretical benefits of PD over other modalities (Table 1).
Table 1:
Advantages and disadvantages of PD compared with other modalities.
| Advantages | Disadvantages |
|---|---|
| Cost effective | Few nephrologists trained to insert PD catheters |
| Low infrastructure requirement | PD fluids difficult to source in LMICs |
| Easier training of staff | Mechanical complications may delay therapy |
| More rapid recovery of renal function | Ultrafiltration is unreliable |
| No anticoagulation necessary | Peritonitis more likely |
| No vascular access required | High glucose exposureb |
| No disequilibrium syndromea | Reduced enteral toleranceb |
| Less bacteraemia | Raised intra-abdominal pressure |
| Less blood lossb | |
| No myocardial stunningb | |
| No exposure to foreign extracorporeal circuit |
Compared with IHD only.
bUnproven.
Cost effectiveness is extremely important for LMICs where acute dialysis is often poorly resourced and, in many cases, funded out of pocket. Kilonzo and Cagliari both showed that the cost of acute PD in Africa was significantly cheaper than haemodialysis and fell well within the World Health Organisation’s criteria for Choosing Interventions that are Cost-Effective (CHOICE) treatments which are deemed to be highly cost effective per life saved [32–34]. Many LMICs offer haemodialysis for AKI, but only in major centres which may be several days’ travel from patients in the rural districts and thus many patients with AKI do not survive. PD does not require machines, water, electricity or highly trained staff, and can easily be delivered in these communities. For these reasons training in PD for AKI is chosen preferentially by the SYL program [9, 12, 15–19, 35, 36].
Recovery of kidney function occurred on average 3 days earlier in two large randomized trials in critically ill patients [37, 38]. The reason for this is not clear but it is possible that relative cardiovascular stability of PD preserves renal perfusion better than the intermittent hypotension of IHD or CKRT. Another theory is that PD does not expose the patient to an extracorporeal circuit and the resulting pro-inflammatory state. It has been clearly demonstrated that in chronic dialysis preservation of residual kidney function has a significant impact on survival and one wonders whether this might be similar for patients with AKI [39–42].
The cardiovascular stability mentioned above is an advantage for critically ill patients with hypotension or shock where CKRT is not available. It has been shown in chronic patients that myocardial stunning does not occur during peritoneal dialysis, whereas this is commonplace in those on IHD [43–45]. A small study of 11 patients was suggestive of stunning in CKRT patients and needs to be confirmed with larger numbers, however considering that patients with AKI in HICs often have cardiovascular comorbidities it is enticing to think that PD may prove to be superior in these settings [46].
PD does not require anticoagulation and is the preferred modality in those with high risk for or ongoing postoperative bleeding, especially those following brain injury or surgery. It also limits blood loss which commonly occurs through clotting in extracorporeal circuits.
PD is not associated with dialysis disequilibrium syndrome and may be more appropriate for patients with raised intracranial pressure.
Vascular access is not required for PD and carries a significant advantage in small children where peritoneal catheter insertion is often easier than dialysis catheter insertion.
Specific to the COVID-19 pandemic, acute PD reduced the time renal nurses were required to be at the bedside, thus reducing personal protective equipment usage as well as reducing spread of infection back into the chronic dialysis unit. Remote patient monitoring allowed optimal care with fewer face to face visits [25]. Concerns about infectivity of PD fluid to staff and other patients are likely unfounded but universal precautions should continue to be used [47].
BARRIERS TO PD
Whilst there are a number of advantages of PD over other modalities, a number of disadvantages and barriers to higher PD uptake exist.
Clinician perceptions are a significant factor, Gaião et al.’s survey showed that 62% of clinicians did not believe PD was suitable for treating AKI in ICU [8]. This is not surprising as, until recently, there was little evidence to support the use of PD and even less to guide prescription thereof. Evidence of equivalent outcomes has accumulated over the past two decades and along with guidelines on prescription there has been an increase in acceptance of PD, especially in low resource countries.
Timely PD access has been a further stumbling block as it is not universal practice for nephrologists to place PD catheters at the bedside and taking a critically ill patient to a surgical theatre is often not feasible. Training sufficient nephrologists has been challenging especially when PD numbers are small, however programs such as the SYL initiative have used pork-belly models to train over 360 doctors and nurses to place PD catheters at the bedside [12] (Fig. 1). The ISPD guidelines recommend flexible PD catheters, but in many countries, PD is still performed using the rigid nylon catheters introduced over a sharp introducer stylet. Although these catheters are prone to infection and generally have poor flow characteristics, they are simple to insert and do save lives. Assistance of surgeons and interventional radiologists with bedside placement has also improved uptake as was seen in the COVID-19 pandemic [22, 23].
Obtaining PD fluid supplies in low-resource countries may be extremely troublesome. Most PD fluids are produced in HICs and shipped by sea and then by land, often crossing multiple borders to landlocked countries. The high costs of both shipping fees as well as legal and illicit border charges result in the cost-effectiveness being reduced. There is often unreliable delivery of fluids to remote areas especially when there are small numbers of patients treated. Although the ISPD guidelines recommend commercially produced solutions, in the event of fluid not being available, clinicians are trained to mix their own solutions using the most sterile technique possible. The simplest is the combination of modified Ringers lactate and 50% dextrose. There have been three case series from SYL sites showing this to be safe, with equivalent peritonitis rates to patients using commercial solutions [16, 17, 48].
There have been concerns that an increased intraperitoneal volume will impact on diaphragmatic movement in critically ill patients and impair gas exchange. This has not been shown to be the case. Studies from Brazil and the USA showed daily improvement in gas exchange rather than worsening. These are observational data and need to be confirmed with comparative studies between PD and extracorporeal therapies [27, 49].
Mechanical complications are seen in between 5% and 13% of cases [37, 50, 51]. This cannot be predicted and in a patient with life-threatening hyperkalaemia or pulmonary oedema the risk of delays due to a mechanical complication make it safer to place vascular access and perform extracorporeal therapy rather than PD in the first instance.
Adequate ultrafiltration is essential in critically ill patients to ensure a neutral fluid balance despite high volumes of fluids used for antibiotics, feeding, vasopressors, etc. Although PD can reliably achieve ultrafiltration rates of 1000–2500 mL/24 h, this may not be sufficient in a profoundly fluid-overloaded patient and here haemofiltration/dialysis may be more effective [38, 50, 52, 53]. Many intensive care specialists are reluctant to use PD as they are unable to reliably dial in an ultrafiltration volume and must adjust glucose concentrations to achieve fluid removal. It must be noted that extracorporeal therapies rely on a stable intravascular volume in order to achieve ultrafiltration without inducing hypotension and clotting. This may not be feasible if there is significant third space loss and low intravascular volumes. PD does not rely on this to such an extent and may occasionally be more effective than extracorporeal methods at removing fluid whilst maintaining perfusion.
The COVID-19 experience highlighted the difficulties in rapidly setting up a functional acute PD team [31]. There needs to be preparation and procurement of supplies, training of nursing and medical staff, and communication between clinicians (nephrologists, surgeons, intensivists and radiologists) prior to implementation. Collaboration with other acute PD units for advice and support will enhance the program.
Figure 1:
SYL training course using pork-belly model.
ACCESS FOR PD
When deciding on the optimal access options consider the flow rate (much higher than chronic PD), ease of insertion and training, cost and complication risk. The ISPD guidelines recommend a flexible catheter as optimal, but acknowledge that rigid and other makeshift catheters will indeed save lives. In many low resource countries where flexible catheters are not available for cost or logistical reasons, clinicians have no option but to use alternatives such as intercostal drains, nasogastric tubes, haemodialysis catheters and in children, central venous catheters [9, 15, 16, 35, 54]. Flexible catheters have the advantage of larger lumens and side holes and are less prone to blockage. In children they are associated with better patency and lower incidence of malfunction, infection and organ damage compared with rigid catheters [55, 56]. Catheters can be placed at the bedside using a modified Seldinger technique, thus allowing more rapid initiation of PD, and have the advantage that they can be tunnelled under the skin to allow easier nursing and less risk of leak.
ADEQUACY AND SURVIVAL OUTCOMES OF PD FOR AKI
A common cause of hesitancy amongst clinicians to use PD is based on the misconception that PD is unable to achieve the same clearance seen with extra-corporeal therapies. Those who practice acute PD are often perplexed by the excellent outcomes despite what at first glance appear to be suboptimal clearances, which begs the question what is the adequate clearance required for acute PD?
There are many case series and randomized trials of PD in AKI which demonstrate rapid correction of hyperkalaemia, acidosis and fluid overload usually returning to normal levels within 24–48 h [37, 38, 50, 57]. As these still remain the indications for starting acute dialysis it is clear that acute PD is adequate for saving lives by rapidly correcting these parameters.
There is much debate in critical care nephrology about the impact that dialysis/filtration has on modulating the inflammatory milleu and although it makes theoretical sense that removing cytokines and other larger molecules might improve outcomes, this has never conclusively been shown to impact on survival or recovery of renal function [58, 59]. As a result, the lower middle-molecule clearance seen with PD compared with CKRT may not be relevant when comparing modalities.
Prior to standardization of PD dosing proposed in the ISPD guidelines there were marked variations in dialysis prescriptions with fluid volumes ranging from 13 to 70 L per day [33, 37, 50, 52, 57, 60–62].
The intensive care nephrology literature for extracorporeal therapies, especially CKRT, focus on clearance of small molecules (Kt/V urea) or effluent volumes. Small molecule clearance is easy to measure and has outcome evidence in chronic dialysis patients [63]. AKI is a completely different disease, especially in the ICU where it is a component of multiple organ dysfunction and there is little evidence that small solute clearance targets have an impact on survival. Effluent rates of CKRT are a surrogate measure of clearance of small and larger molecules and are currently the target in most ICUs. These effluent rates are based on randomized studies which have suggested a lower threshold of 22 mL/kg/h, above which there is no survival benefit even in sepsis [64–66]. One study compared CKRT (at 22 mL/kg/h) with alternate day dialysis with a Kt/V urea of 1.2 per session and found no difference in survival [64]. If this is converted to a weekly Kt/V urea (a clearance target used in studies on acute PD) the target would be a weekly Kt/V urea of ∼2.2. Acute PD has been shown to achieve these clearances easily with weekly Kt/Vs in excess of 4.1 being achieved in some cases using high volume automated PD (APD) [37, 50, 52, 53, 57]. It must be noted though that rapid cycling in PD will often achieve good urea clearances, but larger molecule clearances which are dependent on dwell time and convection may be suboptimal as rapid cycling increases the time spent draining fluid in and out and reduces overall dwell time. For this reason, it may be more appropriate to target larger molecule clearance such as creatinine or others in future studies [62]. A randomized trial of acute PD versus daily IHD in critically ill patients showed no difference in survival when a weekly Kt/V of 3.5 was achieved [37]. The same group then randomized patients to higher targets and achieved weekly Kt/Vs of 3 and 4.13, respectively. There was no difference in survival between these groups suggesting a threshold effect with dosing as is seen in CKRT [64–66]. It was based on this data that the ISPD guidelines in 2015 recommended a target weekly Kt/V of 3.5 as the optimal, however it was the feeling of many of the authors that a lower dose may be suitable after extrapolation from the CKRT literature. For this reason, a minimum standard, weekly Kt/V of 2.1 was suggested [10]. Parapiboon et al. performed a randomized trial using these two targets in 75 critically ill patients, 88% of whom were on mechanical ventilation with an average APACHE 2 score of 26.3. They achieved weekly Kt/Vs of 2.26 and 3.3 in the conservative and intensive groups, respectively. There was no difference in survival between the groups. It must be noted that ultrafiltration was higher in the intensive group [53].
Based on the above findings and expert opinion, the most recent ISPD guidelines have set a recommended weekly Kt/V target of 2.2 [3].
Al-Hwiesh et al. adopted a different approach to delivery of acute PD—rather than focussing on Kt/V and its inherent shortfalls, they report on using tidal APD and a fixed volume of 25 L of fluid per 24 h. This was compared with CKRT with an achieved effluent volume of 23 mL/kg/h. Tidal APD may be beneficial in that there is always a residual volume of fluid in the abdomen and this may improve larger molecule clearance [38, 67].
Certainly, acute PD is appropriate for use in critically ill patients with good outcomes, this is demonstrated in the studies in Table 2.
Table 2:
Randomized trials of acute PD performed in ICU.
| Study | Ponce 2013 [69] | Gabriel 2008 [37] | Al-Hwiesh 2018 [38] | Ponce 2011 [52] | Parapiboon 2017 [53] |
|---|---|---|---|---|---|
| High volume PD vs daily EHD | High volume PD vs daily HD | Tidal APD vs CVVHDF | Higher vs lower intensity | Intensive vs minimal standard | |
| Number of patients | 63 vs 201 | 60 vs 60 | 63 vs 62 | 31 vs 30 | 41 vs 39 |
| Dosage | Weekly Kt/V 3.6 vs 4.1 | Weekly Kt/V 3.6 vs 4.7 | 25 L 70% tidal vs CVVHDF effluent 23 mL/kg/h | Weekly Kt/V 4.13 vs 3.0 | Weekly Kt/V 3.3 vs 2.26 |
| Ventilated | 83.6% vs 86.6% | 68% vs 75% | 62% vs 69% | 68% vs 72% | 87% vs 89% |
| APACHE II score | 27.5 vs 26.7 | 26.9 vs 24.1 | 22.1 vs 21.3 | 26.4 vs 24.8 | 26.9 vs 25.7 |
| Ultrafiltration (L) | 0.6 vs 1.4 | 2.1 vs 2.4 | 0.95 vs 1.39 | 2.4 vs 2.1 | 1.5 vs 0.5 (day 1), 2.1 vs 0.9 (day 2) |
| Mortality | 63.9% vs 63.4% (P = .94) | 58% vs 53% (P = .48) | 30.2 vs 53.2 (P = .002) | 55 vs 53% (P = .42) | 79% vs 63% (P = .13) |
| Limitations | Single centre, significantly different baseline characteristics | Single centre, patients not surviving 24 h excluded, underpowered for mortality | Single centre, adequate effluent rates on CKRT, however creatinine levels remained higher than expected for the dose achieved | Single centre | Single centre, small body surface area of patients |
EHD, extended haemodialysis; CVVHDF, continuous veno-veno haemodiafiltration.
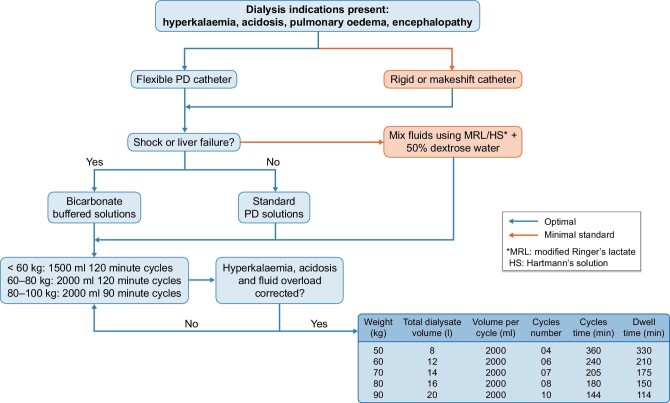
Figure 2 shows the suggested initial dosing schedule as recommended in the ISPD guidelines [3].
Figure 2:
ISPD guidelines for PD in AKI recommended dosing schedule (reproduced with permission) [3].
With all the shortfalls of dosing and clearance targets mentioned above, it is important that clinically relevant outcome measures are assessed when comparing therapies. Chionh et al. performed a systematic review and meta-analysis of all observational studies and found no difference in survival between PD and extracorporeal therapy [68]. Six randomized controlled trials have compared acute PD with extracorporeal therapy, four of which have significant flaws in methodology; the final two are single-centre studies but of a slightly higher quality [37, 38, 60, 61, 69, 70].
A randomized trial from Vietnam comparing acute PD using rigid catheters, acetate-based solutions and rapid cycling with CKRT in patients with sepsis and malaria. It was stopped early, after 35 patients were recruited in each arm, due to a higher mortality in the PD group. Not only was the study underpowered, the mortality in the CKRT arm was unusually low for this group of patients compared with other international studies of AKI in sepsis and suggested a type 1 error [6, 60].
George et al. in India compared PD with CKRT but the trial was stopped early due to poor recruitment and thus was underpowered. It showed mortality was lower in the PD group, however the CKRT effluent dose was suboptimal [61].
Arogundade et al. randomized patients with acute and chronic kidney disease to PD or IHD. There were only four patients in each arm with AKI and therefore could not be analysed [70].
Ponce et al. from Brazil compared acute PD with extended daily dialysis and found no difference in mortality; however, the randomization resulted in significant differences in baseline characteristics [69].
Gabriel et al. further randomized 60 patients to either high volume acute PD or daily haemodialysis. There was no significant difference in mortality although it was not powered to detect this. There was however recovery of renal function 3 days earlier in the PD group [37].
Al-Hwiesh et al. compared tidal APD using biocompatible solutions with CKRT. The effluent dose achieved in the CKRT group was appropriate. The patients on APD had a better survival than those on CKRT (69.8% versus 46.8% P < .01) and recovery of renal function was again 3 days earlier [38].
A Cochrane review of PD versus extracorporeal therapies concluded that based on moderate level of evidence there appeared to be little or no difference in mortality or recovery of renal function between the two groups [71]. The ISPD guidelines have given a 1B recommendation in adults and 1C in paediatrics that PD is suitable for treating AKI in all settings [3, 10, 13].
FUTURE DIRECTIONS FOR PD IN AKI
As with CKD patients, PD appears to be equivalent to other modalities for treating AKI in all settings. Although the study from Al-Hwiesh does suggest improved outcomes compared with CKRT, unless there is a multicentre randomized trial confirming this, there will remain reluctance for its use in adults in high resource countries. Certainly, the evidence is sufficient to justify its preferential use in low resource settings and paediatrics where there are significant benefits over other therapies, which is why it is recommended by the SYL program and ISN.
Suggested areas for research which may bring PD into the mainstream if results are confirmed include:
Assessing the comparative stimulation of the inflammatory cascade between PD (using the bodies biocompatible membrane) and extracorporeal circuits.
Tidal APD and the effect on outcomes including larger molecule clearance.
The use of biocompatible, bicarbonate containing solutions as well as newer solutions being trialled for liver failure and hepatorenal syndrome.
The comparative incidence of myocardial and gut hypoperfusion in extracorporeal therapies and PD.
CONCLUSION
The recent COVID-19 pandemic has led to a renewed interest in PD in high-resource countries. There is sufficient evidence of comparable outcomes between PD and extracorporeal therapies even in critically ill ICU patients. The benefits of cost effectiveness, ease of training, and reduced need for electricity and water make it the optimal form of therapy for low-resource environments. Standardization of practice through the ISPD guidelines will hopefully improve outcomes for patients across the globe.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
B.C. has received lecture fees and honoraria from Baxter Healthcare and Adcock Ingram Critical Care. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Fine J, Frank H, Seligman A. Peritoneal dialysis. Lancet North Am Ed 1947;249:577. 10.1016/S0140-6736(47)91705-4 [DOI] [PubMed] [Google Scholar]
- 2. Fine J, Frank HA, Seligman AM. The treatment of acute renal failure by peritoneal irrigation. Ann Surg 1946;124:857–78. 10.1097/00000658-194611000-00004 [DOI] [PubMed] [Google Scholar]
- 3. Cullis B, Al-Hwiesh A, Kilonzo Ket al. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (adults). Perit Dial Int 2021;41:15–31. 10.1177/0896860820970834 [DOI] [PubMed] [Google Scholar]
- 4. Guzzo I, de Galasso L, Mir Set al. Acute dialysis in children: results of a European survey. J Nephrol 2019;32:445–51. 10.1007/s40620-019-00606-1 [DOI] [PubMed] [Google Scholar]
- 5. Hyman A, Mendelssohn DC. Current Canadian approaches to dialysis for acute renal failure in the ICU. Am J Nephrol 2002;22:29–34. 10.1159/000046671 [DOI] [PubMed] [Google Scholar]
- 6. Uchino S, Kellum JA, Bellomo Ret al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294:813–8. 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 7. Ricci Z, Ronco C, D'Amico Get al. Practice patterns in the management of acute renal failure in the critically ill patient: an international survey. Nephrol Dial Transplant 2006;21:690–6. 10.1093/ndt/gfi296 [DOI] [PubMed] [Google Scholar]
- 8. Gaião S, Finkelstein FO, de Cal Met al. Acute kidney injury: are we biased against peritoneal dialysis? Perit Dialysis Int 2012;32:351–5. 10.3747/pdi.2010.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdou N, Antwi S, Koffi LAet al. Peritoneal dialysis to treat patients with acute kidney injury-the saving young lives experience in West Africa: proceedings of the Saving Young Lives session at the First International Conference of Dialysis in West Africa, Dakar, Senegal, December 2015. Perit Dial Int 2017;37:155–8. 10.3747/pdi.2016.00178 [DOI] [PubMed] [Google Scholar]
- 10. Cullis B, Abdelraheem M, Abrahams Get al. Peritoneal dialysis for acute kidney injury. Perit Dial Int 2014;34:494–517. 10.3747/pdi.2013.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cullis B, Feehally J. Locally prepared solutions for treating AKI in low-resource environments. Perit Dial Int 2018;38:240–1. 10.3747/pdi.2018.00029 [DOI] [PubMed] [Google Scholar]
- 12. Cullis B, Lalya F, Smoyer WE. Saving more young lives in Africa. Perit Dial Int 2020;40:438–40. 10.1177/0896860820931662 [DOI] [PubMed] [Google Scholar]
- 13. Nourse P, Cullis B, Finkelstein Fet al. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (paediatrics). Perit Dial Int 2021;41:139–57. 10.1177/0896860820982120 [DOI] [PubMed] [Google Scholar]
- 14. Sola L, Levin NW, Johnson DWet al. Development of a framework for minimum and optimal safety and quality standards for hemodialysis and peritoneal dialysis. Kidney Int Suppl (2011)2020;10:e55–62. 10.1016/j.kisu.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finkelstein FO, Smoyer WE, Carter Met al. Peritoneal dialysis, acute kidney injury, and the Saving Young Lives program. Perit Dial Int 2014;34:478–80. 10.3747/pdi.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nkoy AB, Ndiyo YM, Matoka TTet al. A promising pediatric peritoneal dialysis experience in a resource-limited setting with the support of saving young lives program. Perit Dial Int 2020;40:504–8. 10.1177/0896860819887286 [DOI] [PubMed] [Google Scholar]
- 17. Palmer D, Lawton WJ, Barrier C Jret al. Peritoneal dialysis for AKI in Cameroon: commercial vs locally-made solutions. Perit Dial Int 2018;38:246–50. 10.3747/pdi.2017.00190 [DOI] [PubMed] [Google Scholar]
- 18. Smoyer WE, Finkelstein FO, McCulloch Met al. Saving Young Lives: provision of acute dialysis in low-resource settings. Lancet North Am Ed 2015;386:2056. 10.1016/S0140-6736(15)00971-X [DOI] [PubMed] [Google Scholar]
- 19. Wilkie M. The role of peritoneal dialysis in Saving Young Lives from acute kidney injury. Perit Dial Int 2014;34:476–7. 10.3747/pdi.2014.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tonelli M, Nkunu V, Varghese Cet al. Framework for establishing integrated kidney care programs in low- and middle-income countries. Kid Int Suppl (2011)2020;10:e19–23. 10.1016/j.kisu.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Hwiesh AK, Mohammed AM, Elnokeety Met al. Successfully treating three patients with acute kidney injury secondary to COVID-19 by peritoneal dialysis: case report and literature review. Perit Dial Int 2020;40:496–8. 10.1177/0896860820953050 [DOI] [PubMed] [Google Scholar]
- 22. Bowes E, Joslin J, Braide-Azikiwe DCBet al. Acute peritoneal dialysis with percutaneous catheter insertion for COVID-19-Associated acute kidney injury in intensive care: experience from a UK tertiary center. Kidney Int Rep 2021;6:265–71. 10.1016/j.ekir.2020.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caplin NJ, Zhdanova O, Tandon Met al. Acute peritoneal dialysis during the COVID-19 pandemic at Bellevue Hospital in New York City. Kidney360 2020;1:1345–52. 10.34067/KID.0005192020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher R, Clarke J, Al-Arfi Ket al. Provision of acute renal replacement therapy, using three separate modalities, in critically ill patients during the COVID-19 pandemic. An after action review from a UK tertiary critical care centre. J Crit Care 2021;62: 190–6. 10.1016/j.jcrc.2020.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naljayan M, Yazdi F, Struthers Set al. COVID-19 in New Orleans: a nephrology clinical and education perspective and lessons learned. Kidney Med 2021;3:99–104. 10.1016/j.xkme.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shankaranarayanan D, Neupane SP, Varma Eet al. Peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City. Kidney Int Rep 2020;5:1532–4. 10.1016/j.ekir.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soomro QH, Mukherjee V, Amerling Ret al. Case series of acute peritoneal dialysis in the prone position for acute kidney injury during the Covid-19 pandemic: prone to complications? Perit Dial Int 2021;41:328–32. 10.1177/0896860820983670 [DOI] [PubMed] [Google Scholar]
- 28. Sourial MY, Sourial MH, Dalsan Ret al. Urgent peritoneal dialysis in patients with COVID-19 and acute kidney injury: a single-center experience in a time of crisis in the United States. Am J Kidney Dis 2020;76:401–6. 10.1053/j.ajkd.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parapiboon W, Ponce D, Cullis B. Acute peritoneal dialysis in COVID-19. Perit Dial Int 2020;40:359–62. 10.1177/0896860820931235 [DOI] [PubMed] [Google Scholar]
- 30. El Shamy O, Patel N, Abdelbaset MHet al. Acute start peritoneal dialysis during the COVID-19 pandemic: outcomes and experiences. J Am Soc Nephrol 2020;31:1680–2. 10.1681/ASN.2020050599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen W, Caplin N, El Shamy Oet al. Use of peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City: a multicenter observational study. Kidney Int 2021;100:2–5. 10.1016/j.kint.2021.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kilonzo KG, Akrabi HF, Yeates KE. Cost-effectiveness of acute peritoneal dialysis: considerations from Africa. Clin Nephrol 2020;93:72–5. 10.5414/CNP92S112 [DOI] [PubMed] [Google Scholar]
- 33. Kilonzo KG, Ghosh S, Temu SAet al. Outcome of acute peritoneal dialysis in northern Tanzania. Perit Dial Int 2012;32:261–6. 10.3747/pdi.2012.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Callegari J, Antwi S, Wystrychowski Get al. Peritoneal dialysis as a mode of treatment for acute kidney injury in sub-Saharan Africa. Blood Purif 2013;36:226–30. 10.1159/000356627 [DOI] [PubMed] [Google Scholar]
- 35. McCulloch M, Luyckx VA, Cullis Bet al. Challenges of access to kidney care for children in low-resource settings. Nat Rev Nephrol 2021;17:33–45. 10.1038/s41581-020-00338-7 [DOI] [PubMed] [Google Scholar]
- 36. Smoyer WE, Finkelstein FO, McCulloch MIet al. “Saving Young Lives” with acute kidney injury: the challenge of acute dialysis in low-resource settings. Kidney Int 2016;89:254–6. 10.1016/j.kint.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 37. Gabriel DP, Caramori JT, Martim LCet al. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int 2008;73:S87–93. 10.1038/sj.ki.5002608 [DOI] [PubMed] [Google Scholar]
- 38. Al-Hwiesh A, Abdul-Rahman I, Finkelstein Fet al. Acute kidney injury in critically ill patients: a prospective randomized study of tidal peritoneal dialysis versus continuous renal replacement therapy. Ther Apher Dial 2018;22:371–9. 10.1111/1744-9987.12660 [DOI] [PubMed] [Google Scholar]
- 39. Obi Y, Rhee CM, Mathew ATet al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 2016;27:3758–68. 10.1681/ASN.2015101142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. You AS, Kalantar-Zadeh K, Obi Yet al. Residual urine output and mortality in a prospective hemodialysis cohort. Kidney Int Rep 2020;5:643–53. 10.1016/j.ekir.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bargman JM. The CANUSA study and the importance of residual kidney function in dialysis patients. Kidney Int 2010;77:931; author reply931–2. 10.1038/ki.2010.44 [DOI] [PubMed] [Google Scholar]
- 42. Paniagua R, Amato D, Vonesh Eet al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002;13:1307–20. 10.1681/ASN.V1351307 [DOI] [PubMed] [Google Scholar]
- 43. Burton JO, Jefferies HJ, Selby NMet al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Ame Soc Nephrol 2009;4:914–20. 10.2215/CJN.03900808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McIntyre CW. Hemodynamic effects of peritoneal dialysis. Perit Dial Int 2011;31Suppl:73–6. 10.3747/pdi.2010.00069 [DOI] [PubMed] [Google Scholar]
- 45. Selby NM, McIntyre CW. Peritoneal dialysis is not associated with myocardial stunning. Perit Dial Int 2011;31:27–33. 10.3747/pdi.2010.00007 [DOI] [PubMed] [Google Scholar]
- 46. Slessarev M, Salerno F, Ball IMet al. Continuous renal replacement therapy is associated with acute cardiac stunning in critically ill patients. Hemodial Int 2019;23:325–32 [DOI] [PubMed] [Google Scholar]
- 47. Candellier A, Scohy A, Gillet Net al. Absence of SARS-CoV-2 in the effluent of peritoneal dialysis patients. Perit Dial Int 2020;40:499–503. 10.1177/0896860820953061 [DOI] [PubMed] [Google Scholar]
- 48. McCulloch MI, Nourse P, Argent AC. Use of locally prepared peritoneal dialysis (PD) fluid for acute PD in children and infants in Africa. Perit Dial Int 2020;40:441–5. 10.1177/0896860820920132 [DOI] [PubMed] [Google Scholar]
- 49. Almeida CP, Balbi AL, Ponce D. Effect of peritoneal dialysis vs. haemodialysis on respiratory mechanics in acute kidney injury patients. Clin Exp Nephrol 2018;22:1420–6. 10.1007/s10157-018-1598-7 [DOI] [PubMed] [Google Scholar]
- 50. Gabriel DP, Nascimento GV, Caramori JTet al. High volume peritoneal dialysis for acute renal failure. Perit Dial Int 2007;27:277–82. 10.1177/089686080702700312 [DOI] [PubMed] [Google Scholar]
- 51. Htay H, Johnson DW, Craig JCet al. Urgent-start peritoneal dialysis versus haemodialysis for people with chronic kidney disease. Cochrane Database Syst Rev 2021;1:Cd012899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ponce D, Brito GA, Abrão JGet al. Different prescribed doses of high-volume peritoneal dialysis and outcome of patients with acute kidney injury. Adv Perit Dial 2011;27:118–24 [PubMed] [Google Scholar]
- 53. Parapiboon W, Jamratpan T. Intensive versus minimal standard dosage for peritoneal dialysis in acute kidney injury: a randomized pilot study. Perit Dial Int 2017;37:523–8. 10.3747/pdi.2016.00260 [DOI] [PubMed] [Google Scholar]
- 54. Esezobor CI, Ladapo TA, Lesi FE. Peritoneal dialysis for children with acute kidney injury in Lagos, Nigeria: experience with adaptations. Perit Dial Int 2014;34:534–8. 10.3747/pdi.2013.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wong SN, Geary DF. Comparison of temporary and permanent catheters for acute peritoneal dialysis. Arch Dis Child 1988;63:827–31. 10.1136/adc.63.7.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chadha V, Warady BA, Blowey DLet al. Tenckhoff catheters prove superior to cook catheters in pediatric acute peritoneal dialysis. Am J Kidney Dis 2000;35:1111–6. 10.1016/S0272-6386(00)70048-5 [DOI] [PubMed] [Google Scholar]
- 57. Chitalia VC, Almeida AF, Rai Het al. Is peritoneal dialysis adequate for hypercatabolic acute renal failure in developing countries? Kidney Int 2002;61:747–57. 10.1046/j.1523-1755.2002.00177.x [DOI] [PubMed] [Google Scholar]
- 58. Cole L, Bellomo R, Hart Get al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med 2002;30:100–6. 10.1097/00003246-200201000-00016 [DOI] [PubMed] [Google Scholar]
- 59. Payen D, Mateo J, Cavaillon JMet al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med 2009;7:803–10. 10.1097/CCM.0b013e3181962316 [DOI] [PubMed] [Google Scholar]
- 60. Phu NH, Hien TT, Mai NTet al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med 2002;347:895–902. 10.1056/NEJMoa020074 [DOI] [PubMed] [Google Scholar]
- 61. George J, Varma S, Kumar Set al. Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: a pilot study. Perit Dial Int 2011;31:422–9. 10.3747/pdi.2009.00231 [DOI] [PubMed] [Google Scholar]
- 62. Cullis B, Ponce D, Finkelstein F. What is the adequate dose for peritoneal dialysis in acute kidney injury: lower the bar or shift the goalposts? Perit Dial Int 2017;37:491–3. 10.3747/pdi.2017.00087 [DOI] [PubMed] [Google Scholar]
- 63. Eknoyan G, Beck GJ, Cheung AKet al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002;347:2010–9. 10.1056/NEJMoa021583 [DOI] [PubMed] [Google Scholar]
- 64. Palevsky PM, Zhang JH, O'Connor TZet al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ronco C, Bellomo R, Homel Pet al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet North Am Ed 2000;356:26–30. 10.1016/S0140-6736(00)02430-2 [DOI] [PubMed] [Google Scholar]
- 66. Bellomo R, Cass A, Cole Let al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009;361:1627–38 [DOI] [PubMed] [Google Scholar]
- 67. Öberg CM, Rippe B. Optimizing automated peritoneal dialysis using an extended 3-Pore model. Kidney Int Rep 2017;2:943–51. 10.1016/j.ekir.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chionh CY, Soni SS, Finkelstein FOet al. Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol 2013;8:1649–60. 10.2215/CJN.01540213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ponce D, Berbel MN, Abrão JMet al. A randomized clinical trial of high volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. Int Urol Nephrol 2013;45:869–78. 10.1007/s11255-012-0301-2 [DOI] [PubMed] [Google Scholar]
- 70. Arogundade FA, Ishola DA Jr, Sanusi AAet al. An analysis of the effectiveness and benefits of peritoneal dialysis and haemodialysis using Nigerian made PD fluids. Afr J Med Med Sci 2005;34:227–33 [PubMed] [Google Scholar]
- 71. Liu L, Zhang L, Liu GJ, Fu P. Peritoneal dialysis for acute kidney injury. Cochrane Database Syst Rev 2017;12:Cd011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.