ABSTRACT
Background
This study aimed to characterize the temporal trends of chronic kidney disease (CKD) burden in China during 1990–2019, evaluate their age, period and cohort effects, and predict the disease burden for the next 10 years.
Methods
Data were obtained from the Global Burden of Disease (GBD) 2019 study. Join-point regression model was used to estimate the average annual percentage change (AAPC) of CKD prevalence and mortality, and the age-period-cohort analysis was used to estimate the age, period and cohort effects. We extended the autoregressive integrated moving average (ARIMA) model to predict the disease burden of CKD in 2020–2029.
Results
In 2019, there were 150.5 million cases of (10.6%) and 196 726 deaths from (13.8 per 100 000 general population) CKD in China. Between 1990 and 2019, the prevalence and mortality rate of CKD increased significantly from 6.7% to 10.6%, and from 8.3/100 000 to 13.8/100 000. The AAPC was estimated as 1.6% and 1.8%, respectively. Females had a higher CKD prevalence of CKD but a lower mortality rate. Setting the mean level of age, period and cohort as reference groups, the risk of developing CKD increased with age [RRage(15–19) = 0.18 to RRage(85–89) = 2.45]. The cohort risk was significantly higher in the early birth cohort [RRcohort(1905–1909) = 1.56]. In contrast, the increase in age-specific CKD mortality rate after 60–64 years was exponential [RRage(60–64) = 1.24]. The cohort-based mortality risk remained high prior to the 1945–1949 birth cohorts (RRcohort ranging from 1.69 to 1.89) and then declined in the 2000–2004 birth cohort [RRcohort(2000–2004) = 0.22]. The CKD prevalence and mortality are projected to rise to 11.7% and 17.1 per 100 000, respectively, by 2029.
Conclusions
To reduce the disease burden of CKD, a comprehensive strategy that includes risk factors prevention at the primary care level, CKD screening among the elderly and high-risk population, and access to high-quality medical services is required.
Keywords: age-period-cohort analysis, ARIMA model, chronic kidney disease, disease burden, join-point regression
INTRODUCTION
Chronic kidney disease (CKD) is a growing public health concern worldwide, with a 40% increase in prevalence and incidence over the last 30 years [1, 2]. According to the KDIGO 2012 guidelines, CKD is defined as abnormalities in kidney structure or function, present for 3 months, with health implications [3, 4]. The diagnostic thresholds for CKD are an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 and an albumin–creatinine ratio (ACR) of 30 mg/g or more [3, 4]. Substantial evidence proves that CKD can increase the risk of cardiovascular disease (CVD) mortality and act as a multiplier in patients with hypertension and diabetes [5–7]. In 2017, there were 697.5 million cases of CKD globally, with a prevalence of 9.1% [8]. If not detected and treated in time, CKD can progress to end-stage renal disease (ESRD) requiring dialysis. It was estimated that 2.6 million patients worldwide received renal replacement therapy in 2010 [9]. Moreover, CKD caused 1.2 million deaths in 2017, with an additional 1.4 million CVD deaths attributed to impaired kidney function [8], making CKD the 12th leading cause of death [10]. China had the most CKD patients (132 million in 2017), accounting for nearly one-fifth of the global total. According to a nationwide survey in China in 2009–2010, the estimated prevalence of CKD was 10.8% [11].
Age was the major contributor to mortality and ESRD risks in CKD patients [12, 13]. Advances in medical technology over time also influenced the quality and accessibility of CKD healthcare. Meanwhile, early-life exposure to famine has been demonstrated to play a significant role in the development of kidney dysfunction in adulthood [14, 15]. However, these time-related factors frequently interacted with each other, making it difficult to quantify the independent contributions of individual factors in epidemiological analysis. An age-period-cohort (APC) model can control the interaction between the three factors, allowing the trend of prevalence and mortality to be more clearly reflected [16]. There was no known comprehensive study to explore the longitudinal trends of CKD from age, period and cohort dimensions. In this study, we used the most recent Global Burden of Disease (GBD) data to characterize the temporal trends of CKD prevalence and mortality in China from 1990 to 2019, evaluate their age, period and cohort effects, and predict the CKD burden for the next 10 years.
MATERIALS AND METHODS
Data sources
The CKD disease burden metrics in China from 1990 to 2019 were obtained from the GBD 2019 public database (https://ghdx.healthdata.org/) [2]. The GBD 2019 provides the incidence, prevalence, mortality, years lived with disability, years of life lost, disability-adjusted life-years (DALYs) and related 95% uncertainty intervals (UI) due to 369 diseases and injuries, for both sexes, for 87 risk factors, and 204 countries and territories [17, 18]. It contains a total of 86 249 data sources from censuses, household surveys, civil registration and vital statistics, disease registries, health service use, air pollution monitors, satellite imaging, disease notifications and other sources [17]. Every dataset is standardized separately, with the crucial step being the disease coding according to the International Classification of Diseases (ICD). Methods for processing, standardization and modeling of CKD prevalence and mortality had been introduced by the GBD Chronic Kidney Disease Collaboration [8]. Briefly, the GBD definition of CKD used one measurement of eGFR and ACR. The CKD data in China are mainly from China disease surveillance points and death registration, China chronic disease and risk factor surveillance, China health and nutrition survey, Hong Kong/Macau vital registration, and relevant published literature (https://ghdx.healthdata.org/gbd-2019/data-input-sources). Several studies of various diseases have been conducted using Chinese GBD data, validating the data reliability and population representativeness [19–21]. DisMod-MR 2.1 model (Bayesian mixed-effects meta-regression tool) was applied to produce CKD prevalence estimates by age, sex and year. Vital registration, verbal autopsy data and surveillance system data for 1990–2019 were used to model the mortality rate due to CKD. Data were standardized and mapped according to the GBD causes of death ICD mapping method, which assigns each death to a single underlying cause. The ICD-9 codes mapped to CKD include 250.4, 403–404.9, 581–583.9, 585–585.9, 589–589.9 and 753–753.3. The ICD-10 codes mapped to CKD include D63.1, E10.2, E11.2, E12.2, E13.2, E14.2, I12-I13.9, N02-N08.8, N15.0, N18-N18.9 and Q61-Q62.8. A standard CODEm model with location level was used to model deaths due to CKD. Furthermore, the burden of CKD for each of five causes: type 1 diabetes, type 2 diabetes, glomerulonephritis, hypertension and unspecified causes. A more detailed description was linked in Supplementary data, Text S1. Human subjects were not directly involved, hence ethical approval was not required.
Join-point regression model
Join-point regression model is firstly proposed by Kim in 2000 [22]. Based on the temporal characteristics of the disease distribution, this model builds a piecewise regression and performs trend fitting and optimization on the data points in each segment. It allows for an in-depth examination of disease variance characteristics specific to different intervals on a global time scale. The equation of the log-linear model is: 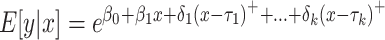 , where y is disease prevalence or mortality rate, x is year,
, where y is disease prevalence or mortality rate, x is year, 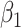 is regression coefficient, k is the number of join-points, the
is regression coefficient, k is the number of join-points, the 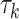 are the unknown join-points and
are the unknown join-points and 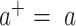 for
for 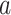 > 0 and 0 otherwise. Model results can be summarized using the metrics of annual percentage change (APC) and average annual % change (AAPC). The APC is calculated as
> 0 and 0 otherwise. Model results can be summarized using the metrics of annual percentage change (APC) and average annual % change (AAPC). The APC is calculated as 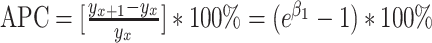 , evaluating the trend of independent intervals of piecewise functions. The AAPC is calculated as
, evaluating the trend of independent intervals of piecewise functions. The AAPC is calculated as 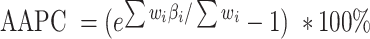 , assessing the average trend over the entire study interval.
, assessing the average trend over the entire study interval.
Age-period-cohort model
The age-period-cohort model examines the effect of age, period and cohort on health outcomes. Age effect refers to the risk of outcomes at different ages. Period effect refers to the effect of temporal changes on outcomes across all age groups. Cohort effect refers to the changes in outcomes among participants with the same birth cohorts. The log-linear regression model is expressed as: 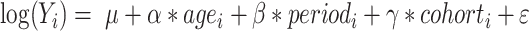 , where
, where 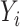 is the CKD prevalence or mortality rate,
is the CKD prevalence or mortality rate, 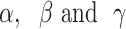 are the coefficients of age, period and cohort, respectively, μ is the intercept and
are the coefficients of age, period and cohort, respectively, μ is the intercept and 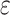 is the residual of model. The intrinsic estimator (IE) method integrated into age-period-cohort model was used to get the net effects for three dimensions [23].
is the residual of model. The intrinsic estimator (IE) method integrated into age-period-cohort model was used to get the net effects for three dimensions [23].
Autoregressive integrated moving average model
The autoregressive integrated moving average (ARIMA) model consists of the autoregressive (AR) model and moving average (MA) model. The underlying assumption is that data series are time-dependent random variables, whose autocorrelation can be characterized by the ARIMA model, and future values can be predicted based on past values. The equation is expressed as 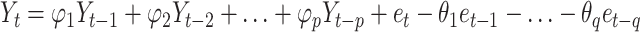 , where
, where 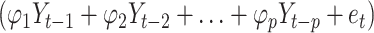 is the AR model part,
is the AR model part, 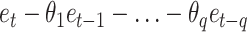 is the MA model part,
is the MA model part, 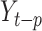 is the observed value at the period of (
is the observed value at the period of (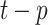 ),
), 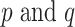 represent the model order of AR and MA, and
represent the model order of AR and MA, and 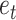 is the random error at the period of t [24]. The time series in the ARIMA model should be a stationary and stochastic sequence with zero mean. The time series in the ARIMA model should be a stationary random series with zero mean.
is the random error at the period of t [24]. The time series in the ARIMA model should be a stationary and stochastic sequence with zero mean. The time series in the ARIMA model should be a stationary random series with zero mean.
Statistical analysis
In GBD 2019, the CKD prevalence is determined as the percentage of CKD patients in the total population, and the CKD mortality rate refers to CKD deaths per 100 000 total population. Furthermore, the age-standardized prevalence and mortality rate were calculated by using the world population as the reference population. To run the age-period-cohort model, we divided the data series into consecutive 5-year intervals from 1994 to 2019. Data from 1990 to 1993 data were not analyzed because they did not span a 5-year interval. Ages were also divided into 5-year age groups ranging from 15–19 to 85–89 years old. Subjects under the age of 15 years old and those above 89 years old were excluded from the age-period-cohort analysis. In addition, 20 cohorts were summarized, covering subjects born from 1905–1909 to 2000–2004. The mean level of age, period and cohort was selected as the reference groups [25, 26]. The relative risk (RR) values for each age, period and cohort represent the independent risks in comparison with the reference group [Exp(α – αmean), Exp(β – βmean), Exp(γ – γmean)]. During the ARIMA modeling process, the difference method was initially used to stabilize the time-series data. The auto.arima() function was used to select the best-optimized model based on the Akaike information criterion (AIC) [27]. The distribution of the residuals was checked for normality using Q-Q plots, autocorrelation function (ACF) plots and partial autocorrelation function (PACF) plots. We then used the Ljung-Box test to examine whether the residual series were white noise. Join-point analysis was run in the joinpoint regression 4.9 software (Statistical Research and Applications Branch, National Cancer Institute, USA). Age-period-cohort model was created in Stata 14.0 software (StataCorp LP, TX, USA). ARIMA analysis and plot drawing were carried out in R 4.1 software (R Development Core Team) using the package ‘forecast’, ‘tseries’ and ‘ggplot2’. Statistical significance was considered at a two-tailed P < .05.
RESULTS
Description of CKD prevalence and mortality in China
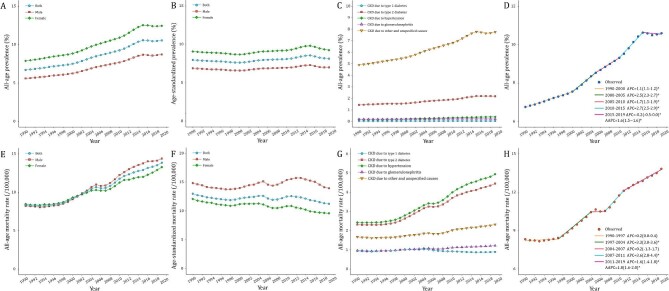
In 2019, there were 150.5 million (95% UI 138.6–162.3 million) cases of CKD in China, and CKD resulted in 196 726 deaths (95% UI 168 241–224 684). The trends of the CKD prevalence and mortality rate in China from 1990 to 2019 are presented in Fig. 1 and Supplementary data, Table S1. The CKD prevalence continued to rise from 6.7% in 1990 to 10.6% in 2019, with females consistently outnumbering males. The age-standardized prevalence increased by 2.1%. Stratified by CKD causes, we found a significant increase in the prevalence of CKD due to type 2 diabetes (from 1.4% to 2.2%) and unspecified causes (from 4.9% to 7.8%), while the prevalence of CKD due to type 1 diabetes, hypertension and glomerulonephritis remained stable. The CKD mortality rate increased from 8.3 per 100 000 population in 1990 to 13.8 per 100 000 population in 2019, despite the 13.5% decrease in the age-standardized rate. Males had a higher mortality rate as compared with females. Similarly, CKD caused by hypertension and type 2 diabetes had the highest mortality rates, increasing significantly from 2.4/2.3 per 100 000 to 4.9/4.5 per 100 000, respectively.
Figure 1:
Trends of CKD prevalence and mortality rate in China during 1990–2019. (A) CKD prevalence; (B) age-standardized CKD prevalence; (C) CKD prevalence for different causes; (D) join-point model of CKD prevalence; (E) CKD mortality rate; (F) age-standardized CKD mortality rate; (G) CKD mortality rate for different causes; and (H) join-point model of CKD mortality rate.
Join-point analysis of CKD prevalence and mortality in China
The segmental trends of prevalence and mortality rate of CKD were divided using join-point analysis (Fig. 1D and H). The CKD prevalence had significantly increased since 1990, with two major increases during 2000–2005 and 2010–2015 (APC2000-2005 = 2.5% and APC2010-2015 = 2.7%). The AAPC for prevalence in the entire period was 1.6% (95% CI 1.5%–1.6%). In contrast, the mortality rate remained steady from 1990 to 1997 before beginning to increase significantly, with an overall AAPC of 1.8% (95% CI 1.6%–2.0%). The highest APCs of mortality were found in 1997–2004 (APC1997-2004 = 3.3%) and 2007–2011 (APC2007-2011 = 3.6%).
Age, period and cohort effects on CKD prevalence and mortality
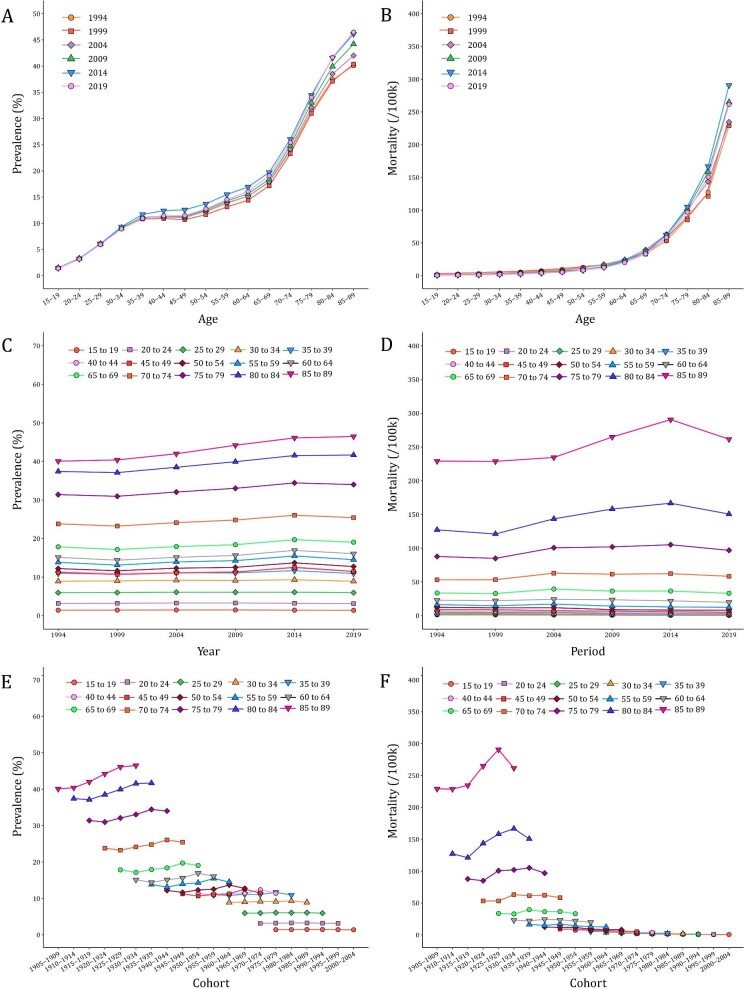
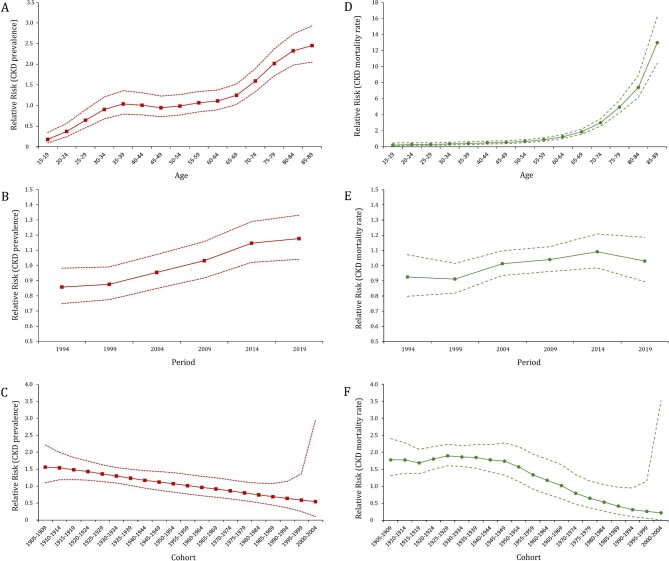
Figures 2 and 3 illustrated the age-period-cohort effect of CKD prevalence and mortality rate. The CKD prevalence increased from the ages of 15–19 years, peaking at the ages of 85–89 years. After controlling the effects of period and cohort, the risk of CKD in the 85–89 years group was 13.6 times the risks in the 15–19 years [exp((αage(85–89) – αmean) – (αage(15–19) – αmean)) = exp(αage(85–89) – αage(15–19)) = 2.45/0.18]. The period-based trends of CKD prevalence were relatively steady in the younger groups and trended upward over the period in the 80–89 age groups. The RR value increased from 0.86 (95% CI 0.75–0.98) in 1994 to 1.18 (95% CI 1.04–1.33) in 2019. The birth cohort of each age group showed that the CKD prevalence in the early period was lower than that in the later period. The cohort risk was significantly higher in the early birth cohort (RRcohort(1905–1909) = 1.56, 95% CI 1.10–2.21) and decreased in the recent cohorts (RRcohort(2000–2004) = 0.54, 95% CI 0.10–2.97). In contrast, the increase in age-specific CKD mortality rate was exponentially distributed. The increase of RR values was initially slow in the groups aged under 59 years old and then became exponential [RRage(60–64) = 1.24, 95% CI 1.02–1.52]. The period-based mortality rate had a turning point in 2014. The risk peaked in 2014 [RRperiod(2014) = 1.09, 95% CI 1.00–1.21] and then decreased afterwards [RRperiod(2019) = 1.03, 95% CI 0.89–1.19]. Similarly, the early birth cohort has a greater effect on CKD mortality. The RR maintained the level of 1.69–1.89 before the 1945–1949 birth cohort and then displayed a downward trend from 1.57 in the 1950–1954 birth cohort to 0.22 in the 1995–2000 birth cohort (Supplementary data, Table S2).
Figure 2:
Trends of age-specific, period-based and cohort-based variation of CKD prevalence and mortality rate in China. (A, B) Age-specific CKD prevalence and mortality rate; (C, D) period-based CKD prevalence and mortality rate; (E, F) cohort-based CKD prevalence and mortality rate.
Figure 3:
Age, period and cohort effects on CKD prevalence and mortality rate in China during 1990–2019. (A–C) The red dot line represents the 95% CI of age, period and cohort effects for CKD prevalence; (D–F) the green dot line represents the 95% CI of age, period and cohort effects for CKD mortality rate.
Risk factors for CKD prevalence and mortality
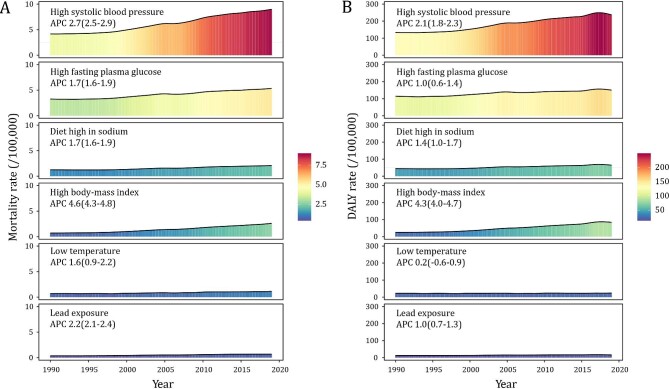
High systolic blood pressure (SBP), high fasting plasma glucose, high body-mass index (BMI), a diet high in sodium, low ambient temperature and lead exposure were the risk factors for CKD quantified in GBD contributing to 65.0%, 38.6%, 18.8%, 15.1%, 8.2% and 5.0%, respectively, of the CKD deaths in 2019. Between 1990 and 2019, high SBP contributed to the largest proportion of CKD mortality, rising from 50.0% to 65.0%. Besides, CKD mortality attributable to high BMI should not be ignored, as it had the fastest rise (APC = 4.6%, Fig. 4A). Further analyses of the DALY rate of CKD across risk factors revealed similar patterns (Fig. 4B).
Figure 4:
Trends of CKD mortality rate and DALY rate attributable to the major risk factors in China during 1990–2019.
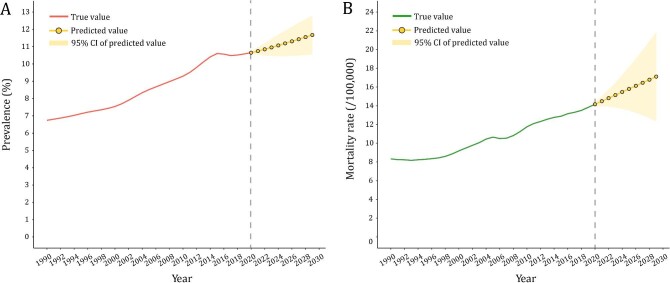
Prediction of CKD prevalence and mortality in the next decade
The ARIMA model was used to quantitatively depict the trends of CKD prevalence and mortality over the following 10 years. The optimized model was chosen to be (1,1,1) for CKD prevalence with an AIC value of –93.7 after being filtered by the auto.arima() function. The residuals were normally distributed according to Q-Q plots, ACF and PACF plots (Supplementary data, Figs S1–S3). The Ljung-Box test confirmed that the residuals of the model were white noise (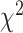 = 1.132, P = .287). By repeating the aforementioned steps, the ARIMA model (0,2,0) for CKD mortality rate (AIC = –35.3) was developed. It also was proven robust by the Ljung-Box test (
= 1.132, P = .287). By repeating the aforementioned steps, the ARIMA model (0,2,0) for CKD mortality rate (AIC = –35.3) was developed. It also was proven robust by the Ljung-Box test (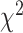 = 0.449, P = .503). According to calibration plots, the true values were in agreement with the predicted values (Supplementary data, Fig. S4). The CKD prevalence was expected to increase from 10.7% in 2020 to 11.7% in 2029. The predicted mortality rate also kept growing over the next decade, increasing from 14.2 per 100 000 in 2020 to 17.1 per 100 000 in 2029 (Fig. 5). Further predictions of age-standardized CKD burden were delineated in Supplementary data, Fig. S5. It was estimated that the future trends of age-standardized prevalence and mortality rate maintained the level of 8.0% and 11.1/100 000 in 2029 (compared with 8.1% and 11.2/100 000 in 2019).
= 0.449, P = .503). According to calibration plots, the true values were in agreement with the predicted values (Supplementary data, Fig. S4). The CKD prevalence was expected to increase from 10.7% in 2020 to 11.7% in 2029. The predicted mortality rate also kept growing over the next decade, increasing from 14.2 per 100 000 in 2020 to 17.1 per 100 000 in 2029 (Fig. 5). Further predictions of age-standardized CKD burden were delineated in Supplementary data, Fig. S5. It was estimated that the future trends of age-standardized prevalence and mortality rate maintained the level of 8.0% and 11.1/100 000 in 2029 (compared with 8.1% and 11.2/100 000 in 2019).
Figure 5:
Predicted trends of CKD prevalence and mortality rate in China over the next 10 years (2020–29). Red and green lines represent the true trend of CKD prevalence and mortality rate during 1990–2019; yellow dot lines and shaded regions represent the predicted trend and its 95% CI.
DISCUSSION
In the present study, we analyzed the temporal trends in CKD burden in China from 1990 to 2019. The prevalence and mortality rate of CKD have both increased significantly over the past 30 years. In 2019, the number of CKD patients reached 150.5 million in China, accounting for 10.6% of the total population. Furthermore, CKD resulted in 196 726 deaths (13.8 per 100 000 total population) and ranked as the 16th leading cause of death in China [20]. Compared with the global burden of CKD, we found that the CKD prevalence in China was significantly higher than the global estimate and was comparable to countries with a high social-demographic index [8]. Compared with a an increase beyond 50% in the crude prevalence between 1990 and 2019, the age-standardized CKD prevalence increased by only 2.1%. It indicates the growing crude prevalence was mainly due to population aging, longer life expectancy, changes in lifestyle and risk factors [20, 28, 29]. According to China's latest census, there were 1.35 billion people aged over 65 years in 2020, a 2.4-fold increase from 570 million in 1990 [30]. The life expectancy of Chinese people also rose from 68.6 in 1990 to 77.3 in 2019 [31]. Meanwhile, the age-standardized CKD mortality rate decreased by 13.5% in the past three decades. This indicates a shift in CKD mortality occurring at an older age and reduced severity of non-fatal CKD. With the advance in renal replacement technologies, more ESRD patients had survival benefits from dialysis. In China, the estimated number of dialysis patients reached 578 000 [32]. However, many patients in need of dialysis do not receive it, especially in regions with limited healthcare resources. It was estimated that the number of premature deaths due to inaccessibility to dialysis is three times the number of patients who receive dialysis treatment [33]. We found CKD had a higher prevalence in females and a higher mortality rate in males, consistent with previous studies [34]. The longer life expectancy on the natural decline of kidney function with age may partly contribute to the greater prevalence of CKD in females [35]. In contrast, the side effects of testosterone, combined with unhealthy lifestyles, might cause rapid progression of ESRD and mortality in males [35].
Previous epidemiological evidence has demonstrated that age was an independent and crucial risk factor for CKD, and the death due to impaired kidney function varied across different age groups [12, 13]. According to the age-period-cohort analysis, the CKD prevalence and mortality rate increase with age. After the age group of 60–64 years, the risk trends of age effect were approximately exponential. Older people were more vulnerable to hypertension, diabetes and CVD, putting them at higher risk of developing CKD [36]. Additionally, complications of CKD are more common and severe in older patients, which confers a negative effect on prognosis [37]. Period effects refer to medical technology, diagnostic methodologies, and economic and cultural changes in a specific period that influence the disease burden of CKD. The period effect increased modestly in CKD prevalence as per the current study. This could be attributed to the popularization of medical examinations in recent years in China, and more CKD cases were screened at the early stage. However, there was a risk of overdiagnosis of CKD by using the same level of eGFR regardless of patient age. It may classify many elderly people with a normal physiological age-related GFR decline as having the disease [38]. It should be recommended that the definition of CKD be modified to include age-specific thresholds for eGFR [39]. The cohort effect highlighted the socio-economic, behavioral and environmental exposures of earlier life and risks in different birth cohorts. In our study, the cohort effect of CKD prevalence trended downward: the early birth cohort had a higher risk of developing CKD, whereas the recent ones had a lower risk. Aside from the age factor, this declining effect could be attributed to receiving a good education and high health awareness in the younger generations. In terms of CKD mortality, the RR was stable before the 1945–1949 birth cohort. This was mainly because of the unstable socioeconomic development, and poor nutrition intake and healthcare before 1949. It has been reported that early malnutrition might be critical for the development of human kidney structure and function [14, 15]. The total number of glomeruli is directly related to birth weight [40]. Nutrition restriction in uterus may lead to low birth weight, resulting in fewer glomeruli and an increased risk of CKD in adulthood. With the rapid development of China's economy and medical advancements, the mortality risk had significantly decreased [41].
The CKD due to hypertension and type 2 diabetes had the highest mortality rate, which almost doubled in the past 30 years. Impaired fasting plasma glucose, high SBP, high BMI and a diet high in sodium were identified as CKD risk factors in GBD analysis. These factors have also been linked to an increased risk of CVD and mortality [42, 43]. Cardiovascular function and metabolic status are closely interrelated to kidney function [44, 45]. In a Canadian cohort, CVD disease was responsible for 27.5% of deaths in individuals with normal kidney function versus 58.0% in those with kidney failure [46]. Individuals with CKD should be identified as one of the high-risk groups for CVD [7]. However, only a few guidelines on CVD prevention have paid specific attention to CKD as a notable risk factor [47]. Screening for CKD in high-risk groups is cost-effective to reduce CKD progression to ESRD and mortality [48]. Long-term salt reduction in CKD patients has been proven to lower blood pressure, ESRD progression and cardiovascular events [49]. Furthermore, extreme ambient temperature and occupational exposure also have an impact on kidney function [50–52]. People who were exposed to lead, cadmium and mercury had a higher risk of CKD mortality [53]. As a result, reducing the exposure to nephrotoxic metals in China cannot be ignored.
Monitoring disease epidemics and predicting trends is an important link for disease prevention and control. According to the ARIMA model, CKD prevalence and mortality are expected to rise to 11.7% and 17.1 per 100 000 within the year 2029. Globally in 2017, the number of people with CKD has exceeded that of diabetes, osteoarthritis, chronic obstructive pulmonary disease, asthma or depression [54]. However, public health authorities and the general public place less emphasis on kidney health than they do on hypertension, diabetes and CVD. Only 8.7% of CKD patients in China were aware of their diagnosis and 4.9% were receiving treatment [55]. The huge disparity between high CKD prevalence and low awareness and treatment may partly explain the continued rise in mortality in recent years. Therefore, a comprehensive strategy that includes risk factors prevention at the primary care level, CKD screening among the elderly and high-risk people, and access to high-quality medical services, is necessary to reduce the CKD burden and achieve better health outcomes for CKD patients.
This study has some limitations to declare. First, while the GBD 2019 adjusted data sources to account for the heterogeneity of different studies and standardized data, these revisions inevitably increased the uncertainty of analytical data. Second, most of the data sources were cross-sectional studies that only measured eGFR and ACR once, which could result in the overestimation of CKD prevalence. Third, our study lacked a geographic description of the CKD burden due to the unavailability of provincial data. Last, the age-period-cohort analysis revealed the results at the population level, and it will be affected by ecological fallacy.
CONCLUSIONS
This study estimated the temporal trends of CKD prevalence and mortality in China from 1990 to 2019. We discovered that the prevalence and mortality of CKD have increased significantly over the past 30 years, and this upward trend is expected to continue until 2029. According to age-period-cohort analysis, the CKD mortality rate increased with age, and the risk trends of age effect were approximately exponential after the age group of 60–64 years. The independent period effects increased modestly on CKD prevalence and mortality, but cohort effects gradually decreased. The aged, as well as patients with hypertension and diabetes, are the high-risk groups for CKD death. Therefore, effective strategies and increased awareness are essential to improve the current situation of CKD in China, thereby improving the quality of life in CKD patients and preventing avoidable deaths.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate the work of the Global Burden of Disease study 2019 collaborators.
Contributor Information
Yang Li, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
Yichun Ning, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
Bo Shen, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
Yiqin Shi, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
Nana Song, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
Yi Fang, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
Xiaoqiang Ding, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China; Shanghai Medical Center of Kidney, Shanghai, China; Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, China.
FUNDING
This analytic study was sponsored by the National Natural Science Foundation of China (82103911), Natural Science Foundation of Shanghai (21ZR1412400), Shanghai Key Laboratory of Kidney and Blood Purification (20DZ2271600) and Yangtze River Delta scientific and technological Innovation Community project (21002411500).
DATA AVAILABILITY STATEMENT
The Global Burden of Disease study 2019 is an open-access resource; data are available at http://ghdx.healthdata.org/gbd-results-tool.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
REFERENCES
- 1. Webster AC, Nagler EV, Morton RLet al. Chronic kidney disease. Lancet North Am Ed 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 2. Institute for Health Metrics and Evaluation . 2019 Global Burden of Disease (GBD). http://ghdx.healthdata.org/gbd-results-tool (16 March 2022, date last accessed).
- 3. Levin A, Stevens P, Bilous RWet al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 4. Stevens PE, Levin A.. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 5. Couser WG, Remuzzi G, Mendis Set al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258–70. 10.1038/ki.2011.368 [DOI] [PubMed] [Google Scholar]
- 6. Provenzano M, Coppolino G, Faga Tet al. Epidemiology of cardiovascular risk in chronic kidney disease patients: the real silent killer. Rev Cardiovasc Med 2019;20:209–20. [DOI] [PubMed] [Google Scholar]
- 7. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet North Am Ed 2013;382:339–52. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 8. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed 2020;395:709–33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liyanage T, Ninomiya T, Jha Vet al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet North Am Ed 2015;385:1975–82. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 10. GBD 2017 DALYs and HALE Collaborators . Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed 2018;392:1859–922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang L, Wang F, Wang Let al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet North Am Ed 2012;379:815–22. 10.1016/S0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]
- 12. De Nicola L, Minutolo R, Chiodini Pet al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int 2012;82:482–8. 10.1038/ki.2012.174 [DOI] [PubMed] [Google Scholar]
- 13. Rapp JL, Lieberman-Cribbin W, Tuminello Set al. Male sex, severe obesity, older age, and chronic kidney disease are associated with COVID-19 severity and mortality in New York city. Chest 2021;159:112–5. 10.1016/j.chest.2020.08.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lv S, Shen Z, Zhang Het al. Association between exposure to the Chinese famine during early life and the risk of chronic kidney disease in adulthood. Environ Res 2020;184:109312. 10.1016/j.envres.2020.109312 [DOI] [PubMed] [Google Scholar]
- 15. Abate KH, Abdulahi M, Abdulhay Fet al. Consequences of exposure to prenatal famine on estimated glomerular filtration rate and risk of chronic kidney disease among survivors of the great Ethiopian famine (1983-85): a historical cohort study. Nutr J 2021;20:19. 10.1186/s12937-021-00675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fienberg SE, Mason WM. Identification and estimation of age-period-cohort models in the analysis of discrete archival data. Sociol Methodol 1979;10:1–67. [Google Scholar]
- 17. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet North Am Ed 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. GBD 2019 viewpoint collaborators. Five insights from the Global Burden of Disease Study 2019. Lancet 2020;396:1135–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu S, Li Y, Zeng Xet al. Burden of cardiovascular diseases in China, 1990-2016: findings from the 2016 Global Burden of Disease Study. JAMA Cardiol 2019;4:342–52. 10.1001/jamacardio.2019.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou M, Wang H, Zeng Xet al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L, Peng W, Zhao Zet al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA 2021;326:2498–506. 10.1001/jama.2021.22208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HJ, Fay MP, Feuer EJet al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 23. Luo L. Assessing validity and application scope of the intrinsic estimator approach to the age-period-cohort problem. Demography 2013;50:1945–67. 10.1007/s13524-013-0243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen HV, Naeem MA, Wichitaksorn Net al. A smart system for short-term price prediction using time series models. Comput Electr Eng 2019;76:339–52. [Google Scholar]
- 25. Liu X, Yu Y, Wang Met al. The mortality of lung cancer attributable to smoking among adults in China and the United States during 1990-2017. Cancer Commun 2020;40:611–9. 10.1002/cac2.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Y, Cui Y, Hu Qet al. Long-term changes of HIV/AIDS incidence rate in China and the U.S. population from 1994 to 2019: a join-point and age-period-cohort analysis. Front Public Health 2021;9:652868. 10.3389/fpubh.2021.652868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao X, Li C, Ding Get al. The burden of Alzheimer's disease mortality in the United States, 1999-2018. J Alzheimers Dis 2021;82:803–13. 10.3233/JAD-210225 [DOI] [PubMed] [Google Scholar]
- 28. Hao L, Xu X, Dupre MEet al. Adequate access to healthcare and added life expectancy among older adults in China. BMC Geriatrics 2020;20:129. 10.1186/s12877-020-01524-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yin P, Brauer M, Cohen AJet al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990-2017: an analysis for the Global Burden of Disease Study 2017. Lancet Planet Health 2020;4:e386–98. 10.1016/S2542-5196(20)30161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Bureau of Statistics of China . The seventh population census of China 2020. http://www.stats.gov.cn/tjsj/pcsj/rkpc/7rp/zk/indexch.htm (1 June 2022, date last accessed). [Google Scholar]
- 31. National Health Commission of China . The Chinese Health Statistical Yearbook 2021. http://www.nhc.gov.cn/mohwsbwstjxxzx/tjzxtjsj/tjsj_list.shtml (1 June 2022, date last accessed).
- 32. Yang C, Gao B, Zhao Xet al. Executive summary for china kidney disease network (CK-NET) 2016 annual data report. Kidney Int 2020;98:1419–23. 10.1016/j.kint.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 33. Thurlow JS, Joshi M, Yan Get al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol 2021;52:98–107. 10.1159/000514550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bikbov B, Perico N, Remuzzi G.. Disparities in chronic kidney disease prevalence among males and females in 195 countries: analysis of the Global Burden of Sisease 2016 study. Nephron 2018;139:313–8. 10.1159/000489897 [DOI] [PubMed] [Google Scholar]
- 35. Carrero JJ, Hecking M, Chesnaye NCet al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 36. Corsonello A, Fabbietti P, Formiga Fet al. Chronic kidney disease in the context of multimorbidity patterns: the role of physical performance: the screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr 2020;20:350. 10.1186/s12877-020-01696-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roderick PJ, Atkins RJ, Smeeth Let al. CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis 2009;53:950–60. 10.1053/j.ajkd.2008.12.036 [DOI] [PubMed] [Google Scholar]
- 38. Liu P, Quinn RR, Lam NNet al. Accounting for age in the definition of chronic kidney disease. JAMA Intern Med 2021;181:1359–66. 10.1001/jamainternmed.2021.4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delanaye P, Jager KJ, Bökenkamp Aet al. CKD: a call for an age-adapted definition. J Am Soc Nephrol 2019;30:1785–805. 10.1681/ASN.2019030238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hughson M, Farris AB III, Douglas-Denton Ret al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 2003;63:2113–22. 10.1046/j.1523-1755.2003.00018.x [DOI] [PubMed] [Google Scholar]
- 41. Li H, Liu K, Gu Jet al. The development and impact of primary health care in China from 1949 to 2015: a focused review. Int J Health Plann Manage 2017;32:339–50. 10.1002/hpm.2435 [DOI] [PubMed] [Google Scholar]
- 42. Resnick HE, Howard BV.. Diabetes and cardiovascular disease. Annu Rev Med 2002;53:245–67. 10.1146/annurev.med.53.082901.103904 [DOI] [PubMed] [Google Scholar]
- 43. Stevens SL, Wood S, Koshiaris Cet al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016;354:i4098. 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu M, Li XC, Lu Let al. Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci 2014;18:2918–26. [PubMed] [Google Scholar]
- 45. Li Y, Zhu B, Xie Yet al. Effect modification of hyperuricemia, cardiovascular risk, and age on chronic kidney disease in China: a cross-sectional study based on the China Health and Nutrition Survey Cohort. Front Cardiovasc Med 2022;9:853917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turin TC, Tonelli M, Manns BJet al. Chronic kidney disease and life expectancy. Nephrol Dial Transplant 2012;27:3182–6. 10.1093/ndt/gfs052 [DOI] [PubMed] [Google Scholar]
- 47. Visseren FLJ, Mach F, Smulders YMet al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 48. Komenda P, Ferguson TW, Macdonald Ket al. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 2014;63:789–97. 10.1053/j.ajkd.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 49. McMahon EJ, Campbell KL, Bauer JDet al. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2021;6:Cd010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim E, Kim H, Kim YCet al. Association between extreme temperature and kidney disease in South Korea, 2003-2013: stratified by sex and age groups. Sci Total Environ 2018;642:800–8. 10.1016/j.scitotenv.2018.06.055 [DOI] [PubMed] [Google Scholar]
- 51. Chapman CL, Hess HW, Lucas RAIet al. Occupational heat exposure and the risk of chronic kidney disease of nontraditional origin in the United States. Am J Physiol Regul Integr Comp Physiol 2021;321:R141–51. 10.1152/ajpregu.00103.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scammell MK. Trust, conflict, and engagement in occupational health: North American epidemiologists conduct occupational study in communities affected by chronic kidney disease of unknown origin (CKDu). Curr Environ Health Rep 2019;6:247–55. 10.1007/s40572-019-00244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orr SE, Bridges CC.. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci 2017;18:1039. 10.3390/ijms18051039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. James SL, Abate D, Abate KHet al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang S, Chen R, Liu Qet al. Prevalence, awareness and treatment of chronic kidney disease among middle-aged and elderly: the China Health and Retirement Longitudinal Study. Nephrology 2015;20:474–84. 10.1111/nep.12449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Global Burden of Disease study 2019 is an open-access resource; data are available at http://ghdx.healthdata.org/gbd-results-tool.