Abstract
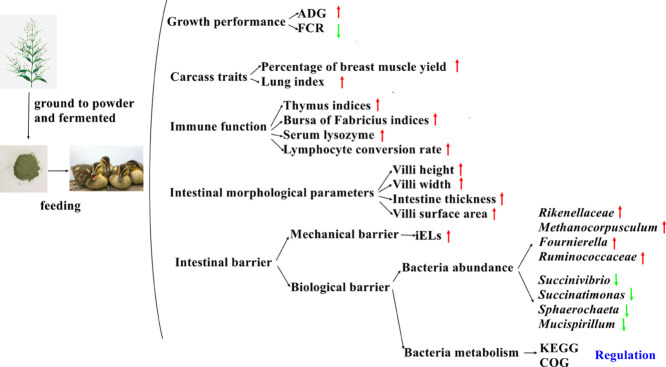
The study aimed to examine the effects of unfermented and fermented Andrographis paniculata on growth performance, carcass traits, immune function, and intestinal health in Muscovy ducks. A total of 450 (16-day-old) Muscovy ducks weighing 271.44 ± 8.25 g were randomly assigned to 5 dietary treatments (6 replicate pens of 15 ducks per treatment), consisting of one control treatment (basal diet without A. paniculata), one unfermented A. paniculata treatment (basal diet plus 30 g/kg unfermented A. paniculata) and 3 fermented A. paniculata treatments (basal diet plus 10, 30, and 50 g/kg). 30 g/kg unfermented A. paniculata increased the ADG, thymus index, peripheral blood lymphocyte conversion rate, villi height, intestinal thickness, villi surface area, intraepithelial lymphocytes rate, while decreased the FCR. 10 g/kg fermented A. paniculata markedly boosted ADG, bursa of fabricius index, thymus index, serum lysozyme, lymphocyte conversion rate, villi height, vilii width, intestinal thickness, villi surface area, while decreased the FCR. 30 g/kg fermented A. paniculata clearly improved ADG, bursa of fabricius index, thymus index, serum lysozyme, lymphocyte conversion rate, villi height, vilii width, intestinal thickness, villi surface area, intraepithelial lymphocytes, while decreased FCR. 50 g/kg fermented A. paniculata significantly increased villi height, vilii width, and villi surface area, while clearly reduced BW. Additionally, compared to 30 g/kg unfermented A. paniculata, 30 g/kg fermented A. paniculata obviously increased bursa of fabricius indices, lymphocyte conversion rate, vilii width, villi surface area. On top of that, supplementation with unfermented and fermented A. paniculata (30 g/kg each) decreased the relative abundance of harmful bacteria (Succinivibrio, Succinatimonas, Sphaerochaeta, and Mucispirillum) and increase the abundance of beneficial bacteria (Rikenellaceae, Methanocorpusculum, Fournierella, Ruminococcaceae) in the ceca of the ducks. However, fermented A. paniculata had considerable better effects than unfermented A. paniculate on all above measured indices. Overall, these results revealed that supplementation with unfermented and fermented A. paniculata across different treatments improved growth, immune status, intestinal morphology, and intestinal microbiota composition and structure in Muscovy ducks, making it a potential alternative to antibiotics in poultry production.
Key words: fermented A. Paniculata, growth performance, carcass traits, immune function, intestinal health
GRAPHICAL ABSTRACT
INTRODUCTION
Problems with drug resistance and residues in livestock products have resulted in the recent ban of antibiotic use in livestock and poultry breeding in several countries (Liu et al., 2020). Therefore, it is important to identify and evaluate alternatives to antibiotics, including “green additives”. Chinese herbal feed additives have been shown to improve feed quality and solve the problem of drug residues in livestock products (Lin et al., 2020). A. paniculata is a widely cultivated herb in East and Southeast Asia, belonging to Acanthaceae family (Jiang et al., 2021). It is commonly known as the “king of bitter” due to and has the ability to grow in most soil types and under shaded conditions. A. paniculata possess anti-inflammatory, antibacterial, antipyretic, and immunosuppressive properties (Julaton et al., 2022). Traditionally, it is used to treat several infectious diseases, including colds, fevers, laryngitis, malaria, dysentery, and diarrhea, in China, India, and other Southeast Asian countries. Additionally, it has been shown to significantly improve tibial length and body weight, prevent the occurrence of gastrointestinal diseases, improve antioxidant and immune functions, and promote growth in poultry (Arify et al., 2019; Aneesh et al., 2021). A. paniculata also possesses several bioactive compounds with complex structures often consisting of multiple asymmetric carbon atoms. Structural modifications using chemical reactions have demonstrated some associated disadvantages, such as low yield of bioactive compounds, poor reaction specificity, and the production of several by-products. However, fermentation of A. paniculata with probiotics has the potential to increase the production of active substances, promote the synthesis of specific active compounds, and facilitate the degradation of antinutritional factors and toxins in A. paniculata. Additionally, microorganisms used for fermentation can produce organic acids in the intestinal tract of animals, thereby inhibiting the growth of harmful bacteria and enhancing intestinal immunity. However, studies on the medicinal and growth promoting effects of fermented A. paniculata are limited.
Therefore, this study aimed to examine the effect of fermented A. paniculata on growth performance, immune function, intestinal morphology, and intestinal microbial composition in poultry. Microorganisms were firstly used to ferment Andrographis paniculata, and the Muscovy duck was used as the research poultry species. It is anticipated that our findings will provide scientific basis for the use of Chinese herbal medicinal technology in livestock and will contribute to the development of an effective alternative to antibiotics in poultry production.
MATERIALS AND METHODS
Preparation of Fermented A. Paniculata
To prepare the fermentation medium, 4% compound bacterial agent (mass ratio of Saccharomycetes to Lactobacillus was 1: 1), sugar, and other raw materials were mixed in water. The fermentation material and water were added into a vat containing A. paniculata powder, and evenly mixed. The moisture was controlled at 35 to 45%. The mixture was loaded into a breathing bag, sealed, and fermented at 30°C for 7 d. The number of viable bacteria after fermentation was ≥ 2.0 × 108 CFU/g.
Experimental Design
A total of 450 (16-day-old) Muscovy ducks weighing 271.44 ± 8.25 g (Ganzhou, Jiangxi, China) were randomly assigned to 5 dietary treatments (6 replicate per groups, 15 ducks per replicate), consisting of one control treatment (basal diets without A. paniculata/CG), one unfermented A. paniculata treatment(basal diet plus 30 g/kg unfermented A. paniculata/30 g/kg APG) and three fermented A. paniculata treatments.(basal diets plus 10, 30, and 50 g/kg of fermented A. paniculate/10 g/kg FAPG, 30 g/kg FAPG and 50 g/kg FAPG). Composition and nutrient levels of basal diets was shown in Table 1. Muscovy duck trial protocols used in this study and all experimental stages were conducted in accordance with the requirements of National Research Council's Guide for the Care and Use of Laboratory Animals.
Table 1.
Composition and nutrient levels of basal diets (air-dry basis).
| Items | Diet content |
|
|---|---|---|
| Ducklings (18–21 days) | Fattening stage ducks (22–70 days) | |
| Ingredients (%) | ||
| Corn | 61.80 | 62.00 |
| Soybean meal | 26.00 | 20.00 |
| Fish meal | 4.20 | 0 |
| Wheat bran | 4.00 | 14.00 |
| Met | 0.13 | 0.15 |
| Limestone | 1.22 | 1.50 |
| CaHPO4 | 0.95 | 1.07 |
| NaCl | 0.20 | 0.28 |
| Premix1 | 1.50 | 1.50 |
| Total | 100 | 100 |
| Nutrient levels2 | ||
| ME (MJ/Kg) | 11.69 | 11.29 |
| CP (%) | 20.00 | 15.50 |
| CF (%) | 2.80 | 3.12 |
| Ca (%) | 1.25 | 1.74 |
| P (%) | 0.70 | 0.65 |
| Met (%) | 0.45 | 0.40 |
| Met+Cys (%) | 0.80 | 0.68 |
| Lys (%) | 1.05 | 0.75 |
The premix provided the following per kg of diets: VA 8,000 IU, VD3 3,000 IU, VE 20 IU, VK3 2 mg, VB1 4 mg, VB2 3.6 mg, VB5 40 mg, VB6 4 mg, VB12 0.02 mg, biotin 0.15 mg, folic acid 1.0 mg, D-pantothenic acid 11 mg, nicotinic acid 10 mg, antioxidant 100 mg, Cu (as copper sulfate) 10 mg, Fe (as ferrous sulfate) 80 mg, Mn (as manganese sulfate) 80 mg, Zn (as zinc sulfate) 75 mg, I (as potassium iodide) 0.40 mg, Se (as sodium selenite) 0.30 mg.
ME was a calculated value, while the others were measured values.
Growth Performance and Carcass Traits Measurements
Body weight (BW) at 16-, 21-, 42-, and 70-day-old and daily feed consumption of the ducks were recorded to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR, ADFI/ADG). Carcass traits (dressed percentage, percentage of half-eviscerated yield with giblet, percentage of eviscerated yield, percentage of breast muscle yield, and percentage of leg muscle yield) were determined according to NY/T 823-2020. Additionally, visceral organ parameters (heart, liver, lung, kidney, gizzard, proventriculus, and pancreas index) and immune organ indices (spleen, bursa of Fabricius, and thymus index) were calculated using the following formula: Organs parameters (visceral organ parameters and immune organ indices) = the weight of organ (mg)/BW (g).
Immune Index
Serum samples (30) were used for the determination of 2 immune parameters, including serum lysozyme and lymphocyte conversion rate. Serum lysozyme and lymphocyte conversion rate were analyzed using standard kits (Ganzhou Beisite Biological Co. Ltd, Ganzhou, China) according to the manufacturer's instructions.
Histological Assay
Frozen sections were thawed, dried, and fixed with 4% paraformaldehyde for 15 min. They were differentiated using 75% alcohol, dyed for 3 to 5 min using hematoxylin, differentiated using hydrochloric acid alcohol, and dyed using ammonia solution. Subsequently, the stained sections were scanned using a panoramic scanner (3DHISTECH, Budapest, Hungary). Villi length, crypt depth, villi width, villi length/crypt depth ratio, and intestinal thickness were determined using Caseviewer Systems. Villi surface area was calculated as follows: Villi surface area = 2·π·r·h (where r is half of villi width; h is villi height).
Immunohistochemistry
All formalin-fixed, paraffin-embedded duodenal sections (5–6 µm) were placed on coated object-slides. Thereafter, the sections were deparaffinized in light and incubated with rabbit anti-human CD3 monoclonal antibody (1: 200; Proteintech, Chicago, Illinois), followed by incubation with secondary antibody (HRP-Goat anti-rabbit IgG [1:200; Solaribio, China]). Immunohistochemical staining was in accordance with the step of Wu et al. (2019). Positive cells ratio (Average Optical Density/AOD) were calculated as follows. AOD = Integrated option density (IOD)/the area of the target protein distribution region.
Microbial DNA Extraction, PCR, and 16s rRNA Sequencing
Total genome DNA from cecal samples was extracted using the CTAB/SDS method. DNA concentration and purity were monitored on 1% agarose gels. PCR amplification of the bacterial 16S rRNA gene V4 region was performed using the forward and reverse primers (515F/GTGCCAGCMGCCGCGGTAA and 806R/GGACTACHVGGGTWTCTAAT). Sequencing adapters were appended to the end of primers. PCR was performed to amplify the target genes, and the PCR products were purified using a Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, California) following the manufacturer's recommendations and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific, Waltham, Massachusetts) and Agilent Bioanalyzer 2100 system. The library was sequenced on an Illumina NovaSeq platform and 250 bp paired-end reads were generated.
Processing of Sequencing Data
High-quality reads were assembled using FLASH v1.2.7 software. Quality filtering on the raw tags was performed under specific filtering conditions to obtain the high-quality clean tags according to the QIIME V1.9.1 software quality-controlled process. UCHIME v4.2 software was used to identify and remove the chimeric sequences, and effective reads were generated. Further bioinformatic analyses were performed by Consure Biotechnology Co.Ltd (Beijing, China).
Statistical Analysis
All data were analyzed using SPSS software 17.0 software and were expressed as the mean ± standard deviation (SD). A multiple t test was performed to determine significant differences among the 5 groups. Means were considered statistically significant and highly significant at P < 0.05 and < 0.01, respectively.
RESULTS
Growth Performance
As shown in Table 2, the ADG of 22 to 42 d in 30 g/kg APG was increased (P < 0.05), but the FCR of 22 to 42 d (P < 0.01) and 16 to 70 d (P < 0.05) in 30 g/kg APG was decreased compared with CG. Additionally, the ADG of 16 to 70 d in 10 g/kg FAPG was increased (P < 0.01), but the FCR of 16 to 70 d in 10 g/kg FAPG was decreased (P < 0.01) compared with CG. Moreover, the ADG of 22 to 42 d (P < 0.05) and 16 to 70 d (P < 0.05) in 30 g/kg FAPG were increased, but the FCR of 22 to 42 d (P < 0.05) and 16 to 70 d (P < 0.05) in 30 g/kg FAPG were decreased compared with CG. Furthermore, 50 g/kg of fermented A. paniculata reduced the BW of 42 d (P < 0.05) and 70 d (P < 0.01) in 50 g/kg FAPG compared with CG.
Table 2.
Effects of fermented A. paniculata on growth performance in Muscovy ducks.
| Items | CG | 30 g/kg APG | 10 g/kg FAPG | 30 g/kg FAPG | 50 g/kg FAPG | P-value |
|---|---|---|---|---|---|---|
| Body weight (g) | ||||||
| 16 d | 267.33 ± 9.50 | 274.33 ± 8.50 | 270.00 ± 9.85 | 258.67 ± 7.51 | 277.33 ± 16.01 | 0.32 |
| 21 d | 386.69 ± 19.54 | 386.64 ± 18.39 | 388.57 ± 16.85 | 360.86 ± 15.36 | 375.19 ± 16.06 | 0.09 |
| 42 d | 1,089.25 ± 49.16ABa | 1,106.63 ± 45.89Aa | 1,091.25 ± 75.36ABa | 1,077.89 ± 41.98ABab | 1,035.54 ± 47.41 Bb | 0.05 |
| 70 d | 2,220.00 ± 82.46Aa | 2,211.25 ± 68.07ABa | 2,230.63 ± 90.85Aa | 2,205.00 ± 61.18ABa | 2,106.34 ± 94.04Bb | 0.03 |
| Average daily gain (g) | ||||||
| 16-21 d | 21.68 ± 4.36 | 24.60 ± 3.11 | 24.36 ± 3.72 | 22.34 ± 2.24 | 20.68 ± 4.25 | 0.63 |
| 22-42 d | 31.95 ± 0.54ABb | 36.32 ± 0.72Aa | 33.96 ± 3.03ABab | 35.78 ± 1.31ABa | 31.73 ± 1.58Bb | 0.02 |
| 42-70 d | 40.06 ± 3.25 | 39.22 ± 2.88 | 41.22 ± 3.99 | 40.24 ± 2.58 | 38.54 ± 4.17 | 0.59 |
| 16-70 d | 34.50 ± 1.21 Bc | 35.34 ± 0.11 ABbc | 37.24 ± 1.11Aa | 36.27 ± 0.70ABab | 34.80 ± 0.88Bc | 0.02 |
| Average daily feed intake (g) | ||||||
| 16-21 d | 43.22 ± 0.81 | 44.11 ± 1.27 | 43.04 ± 1.63 | 42.51 ± 1.01 | 43.47 ± 1.25 | 0.62 |
| 22-42 d | 89.11 ± 3.93 | 85.71 ± 2.16 | 88.87 ± 2.92 | 89.14 ± 1.50 | 87.99 ± 2.39 | 0.52 |
| 42-70 d | 160.95 ± 2.23 | 156.38 ± 2.07 | 161.15 ± 3.82 | 157.88 ± 2.44 | 163.35 ± 2.55 | 0.06 |
| 16-70 d | 120.68 ± 1.42 | 117.15 ± 1.93 | 120.65 ± 3.16 | 119.05 ± 1.80 | 121.50 ± 2.33 | 0.20 |
| Feed conversion ratio | ||||||
| 16-21 d | 2.05 ± 0.40 | 1.81 ± 0.22 | 1.80 ± 0.30 | 1.91 ± 0.20 | 2.17 ± 0.45 | 0.60 |
| 22-42 d | 2.79 ± 0.04Aa | 2.36 ± 0.05Bc | 2.63 ± 0.05ABab | 2.50 ± 0.10ABbc | 2.78 ± 0.14Aa | P < 0.01 |
| 42-70 d | 3.94 ± 0.22 | 3.75 ± 0.19 | 3.56 ± 0.16 | 3.74 ± 0.07 | 3.83 ± 0.21 | 0.31 |
| 16-70 d | 3.50 ± 0.12Aa | 3.31 ± 0.01ABb | 3.24 ± 0.10Bb | 3.28 ± 0.07ABb | 3.49 ± 0.09Aa | 0.01 |
In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05).
Different small letter superscripts: significant difference (P < 0.05).
Different capital letter superscripts: significant difference (P < 0.01). The same as below.
Carcass Traits
Compared with CG, dressed percentage in 10 g/kg FAPG was increased (P < 0.05); percentage of breast muscle yield in 30 g/kg APG was increased (P < 0.05); lung index in 30 g/kg APG, 30 g/kg FAPG and 50 g/kg FAPG were increased (P < 0.05), as shown in Table 3.
Table 3.
Effects of fermented A. paniculata on carcass traits in Muscovy ducks.
| Items | CG | 30 g/kg APG | 10 g/kg FAPG | 30 g/kg FAPG | 50 g/kg FAPG | P-value |
|---|---|---|---|---|---|---|
| Dressed percentage (%) | 83.89 ± 2.51bc | 83.69 ± 0.77c | 86.00 ± 0.85a | 85.67 ± 0.71ab | 84.48 ± 1.71abc | 0.04 |
| Percentage of half-eviscerated yield with giblet (%) | 75.96 ± 1.72 | 76.82 ± 1.19 | 78.03 ±1.47 | 76.86 ± 3.15 | 77.45 ± 0.74 | 0.40 |
| Percentage of eviscerated yield (%) | 66.39 ± 7.11 | 68.51 ± 3.49 | 71.22 ± 1.79 | 69.92 ± 3.15 | 70.14 ± 0.78 | 0.26 |
| Percentage of breast muscle yield (%) | 6.91 ± 1.13b | 8.69 ± 1.41a | 7.32 ± 0.99ab | 7.43 ± 0.72ab | 7.07 ± 1.45b | 0.10 |
| Percentage of leg muscle yield (%) | 17.80 ± 4.90 | 17.00 ± 5.09 | 16.01 ± 2.76 | 17.17 ± 1.26 | 15.90 ± 1.86 | 0.75 |
| Heart index (mg/g) | 4.79 ± 0.29 | 5.09 ± 0.38 | 4.55 ± 2.24 | 4.24 ± 2.12 | 5.03 ± 0.46 | 0.83 |
| Liver index (mg/g) | 21.09 ± 0.98 | 20.59 ± 0.74 | 22.40 ± 1.75 | 20.95 ± 2.30 | 21.91 ± 1.50 | 0.33 |
| Lung indexs (mg/g) | 7.18 ±0.32b | 9.07 ± 1.66a | 8.87 ± 1.89ab | 9.79 ± 1.38a | 9.65 ± 1.45a | 0.04 |
| Kidney index (mg/g) | 6.20 ±0.57 | 6.43 ± 1.11 | 6.87 ± 1.02 | 6.97 ± 0.95 | 7.57 ± 1.17 | 0.22 |
| Gizzard index (mg/g) | 24.61 ± 4.05 | 26.18 ± 4.42 | 22.87 ± 2.10 | 22.79 ± 3.44 | 25.06 ± 2.41 | 0.38 |
| Proventriculus index (mg/g) | 3.54 ± 0.56 | 3.65 ± 0.30 | 3.46 ± 0.42 | 3.52 ± 0.36 | 3.74 ± 0.58 | 0.85 |
| Pancreas index (mg/g) | 2.13 ± 0.36 | 2.26 ± 0.14 | 2.14 ± 0.89 | 1.76 ± 1.54 | 1.91 ± 0.98 | 0.88 |
In the same row, values with no letter or the same small letter superscripts mean no significant difference ( P > 0.05), while with different small letter superscripts mean significant difference (P < 0.05), and with different capital letter superscripts mean significant difference (P < 0.01).
Immune Function
In 30 g/kg APG, thymus index (P < 0.05) and lymphocyte conversion rate (P < 0.01) was increased compared with CG (Tables 4 and 5). However, in 10 g/kg FAPG and 30 g/kg FAPG, the bursa of Fabricius and thymus index, serum lysozyme level, and lymphocyte conversion rate were increased (P < 0.05) compared with CG. Additionally, in 30 g/kg FAPG, bursa of Fabricius index (P < 0.05) and lymphocyte conversion rate (P < 0.01) were increased compared with 30 g/kg APG.
Table 4.
Effects of fermented A. paniculata on immune organ indices in Muscovy ducks.
| Items | CG | 30 g/kg APG | 10 g/kg FAPG | 30 g/kg FAPG | 50 g/kg FAPG | P-value |
|---|---|---|---|---|---|---|
| Spleen index (mg/g) | 0.71 ± 0.05 | 0.80 ± 0.23 | 1.07 ± 0.47 | 0.87 ± 0.11 | 0.85 ± 0.16 | 0.50 |
| Bursa of Fabricius index (mg/g) | 0.76 ± 0.20Bc | 1.00 ± 0.20ABbc | 1.12 ± 0.29ABab | 1.45 ± 0.12 Aa | 0.93 ± 0.10 Bbc | 0.02 |
| Thymus index (mg/g) | 1.76 ± 0.14Bc | 2.82 ± 0.56 ABab | 2.76 ± 0.70ABab | 3.63 ± 0.57Aa | 2.31 ± 0.48ABbc | 0.02 |
In the same row, values with no letter or the same small letter superscripts mean no significant difference ( P > 0.05), while with different small letter superscripts mean significant difference (P < 0.05), and with different capital letter superscripts mean significant difference (P < 0.01).
Table 5.
Effects of fermented A. paniculata on immune-related indices in Muscovy ducks.
| Items | CG | 30 g/kg APG | 10 g/kg FAPG | 30 g/kg FAPG | 50 g/kg FAPG | P-value |
|---|---|---|---|---|---|---|
| Lysozyme (μg/L) | 51.15 ± 2.55b | 54.42 ± 1.33ab | 56.69 ± 1.79a | 55.11 ± 2.98a | 53.47 ± 0.31ab | 0.07 |
| Lymphocyte conversion rate (%) | 59.95 ± 1.38Cd | 67.45 ± 3.10Bb | 63.12 ± 2.33Cc | 74.59 ± 2.58Aa | 61.13 ± 1.38 Ccd | P < 0.01 |
In the same row, values with no letter or the same small letter superscripts mean no significant difference ( P > 0.05), while with different small letter superscripts mean significant difference (P < 0.05), and with different capital letter superscripts mean significant difference (P < 0.01).
Intestinal Parameters
In 30 g/kg APG, villi height, intestine thickness, intraepithelial lymphocytes (iELs) (P < 0.01), and villi surface area (P < 0.05) in the ducks were increased compared with CG (Table 6). However, in 10 g/kg FAPG, villi height, intestine thickness, villi surface area (P < 0.01), and villi width (P < 0.05) in the ducks were elevated compared with CG. Similarly, in 30 g/kg FAPG, villi height, villi width, intestine thickness, villi surface area, and iELs were increased (P < 0.01) in the ducks compared with CG. Additionally, in 50 g/kg FAPG, villi height, villi width, and villi surface area were increased (P < 0.01) compared with CG.
Table 6.
Effects of fermented A. paniculata on intestinal morphology in Muscovy ducks.
| Items | CG | 30 g/kg APG | 10 g/kg FAPG | 30 g/kg FAPG | 50 g/kg FAPG | P-value |
|---|---|---|---|---|---|---|
| Villi height (μm) | 995.82 ± 160.68B | 1,430.83 ± 139.16A | 1,263.66 ± 151.11A | 1,373.20 ± 198.41A | 1,157.08 ± 125.78A | P < 0.01 |
| Crypt depth (μm) | 213.80 ± 51.94 | 246.49 ± 38.92 | 239.09 ± 40.73 | 251.55 ± 60.52 | 198.75 ± 49.57 | 0.31 |
| Vilii width (μm) | 200.90 ± 63.91 Bb | 227.28 ± 40.84 Bab | 265.46 ± 32.56Ba | 406.27 ± 59.07Aa | 382.59 ± 47.04Aa | P < 0.01 |
| V/C ratio (μm) | 4.56 ± 1.13 | 5.79 ± 0.99 | 5.37 ± 0.85 | 5.68 ± 1.42 | 6.08 ± 1.38 | 0.30 |
| Intestine thickness (μm) | 254.74 ± 16.40C | 351.22 ± 65.10A | 329.85 ± 34.73A | 333.27 ± 19.25A | 298.32 ± 29.73AB | 0.35 |
| Villi surface area (mm2) | 0.65 ± 0.29Cc | 1.05 ± 0.27 BCb | 1.13 ± 0.11 Bb | 1.61 ± 0.33Aa | 1.40 ± 0.27 AaBb | P < 0.01 |
| Intraepithelial lymphocytes (%) | 3.34 ± 0.19B | 6.76 ± 1.80A | 4.36 ± 0.52B | 6.49 ± 1.06A | 5.23 ± 1.36AB | P < 0.01 |
In the same row, values with no letter or the same small letter superscripts mean no significant difference ( P > 0.05), while with different small letter superscripts mean significant difference (P < 0.05), and with different capital letter superscripts mean significant difference (P < 0.01).
16S rRNA Sequencing Data Statistics
To further verify the effect of fermented A. paniculata on the intestinal health of Muscovy ducks, we collected the cecal contents from the control treatments (basal diets without A. paniculata/CG), one unfermented A. paniculata treatment (30 g/kg APG) and one fermented A. paniculata treatment (30 g/kg FAPG) for 16S rRNA Sequencing. A total of 608,486 raw reads were generated from 9 samples, of which 563,624 clean reads were obtained after quality control. A minimum of 52,637 and an average of 62,625 clean reads were generated for each sample. Table 6 shows basic information for each sample. The results of raw data processing, including raw reads, clean reads, effective reads, avgLen, GC, Q20, and Q30 are shown in Table 7. The effective rates of each group were >80%, indicating that the data were reliable.
Table 7.
Summary on raw data processing.
| Items | CG | 30 g/kg APG | 30 g/kg FAPG | P-value |
|---|---|---|---|---|
| Raw Reads | 71,796.33 ± 6,946.93 | 64,127.33 ± 8916.69 | 66,905.00 ± 4,784.33 | 0.46 |
| Clean Reads | 66,592.33 ± 6,415.54 | 58,883.33 ± 7077.94 | 62,399.00 ± 4,470.57 | 0.36 |
| Effective Reads | 62,356.33 ± 6,123.90 | 54,716.67 ± 6210.68 | 58,169.33 ± 4,152.56 | 0.31 |
| AvgLen (bp) | 252 | 252 | 252 | |
| GC (%) | 53.22 ± 0.15 | 53.15 ± 0.89 | 52.73 ± 0.20 | 0.52 |
| Q20 (%) | 99.83 ± 0.01 | 99.83 ± 0.01 | 99.84 ± 0.01 | 0.37 |
| Q30 (%) | 99.09 ± 0.01 | 99.11 ± 0.03 | 99.13 ± 0.03 | 0.13 |
| Effective (%) | 86.84 ± 0.20 | 85.52 ± 2.15 | 86.95 ± 0.17 | 0.36 |
Note: Raw Reads: Counts of raw reads; Clean Reads: Counts of clean reads(post quality control and assembly); Effective Reads: Counts of effective reads after chimeric reads removal; AvgLen (bp): Average read length of each sample; GC(%): GC content, i.e. proportion of G and C in all bases; Q20(%): Percentage of bases with Q-score larger or equal to Q20; Q30(%): Percentage of bases with Q-score larger or equal to Q30; Effective(%): Percentage of effective reads in raw reads.
OTU/ASV Analysis
Operational taxonomic unit (OTU) refers to a cluster of sequences used to define a group (e.g., species, genus, strain, etc.) in phylogenetic or population genetic studies. These DNA sequences are clustered according to sequence similarity. Each OTU corresponds to a representative sequence.
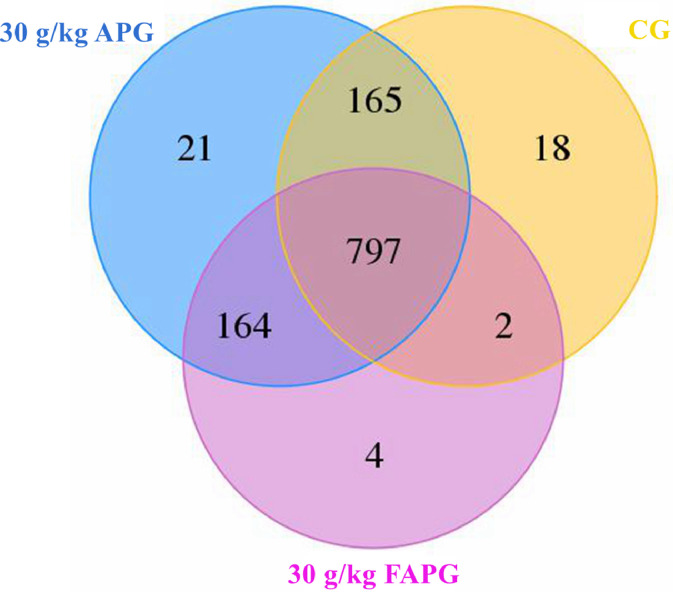
The results showed that adding unfermented and fermented A. paniculata to the diet significantly affected the number of unique OTUs in the intestines of Muscovy ducks. There were 797 common OTUs among CG, 30 g/kg APG and 30 g/kg FAPG, and 18 unique OTUs in CG, 21 unique OTUs in 30 g/kg APG and 4 unique OTUs in the 30 g/kg FAPG (Figure 1).
Figure 1.
Venn Diagram on operational taxonomic units (OTUs). Note: The number in each independent or overlapped area stands for number of unique or common features in each corresponding collection.
α-Diversity Index Statistics
The results of α-diversity analysis showed that there was a significant increase (P = 0.05) in the Shannon index in the 30 g/kg APG and 30 g/kg FAPG compared with CG (Table 8).
Table 8.
Summary of alpha diversity metrics.
| Items | CG | 30 g/kg APG | 30 g/kg FAPG | P-value |
|---|---|---|---|---|
| Feature | 897.00 ± 17.09 | 904.33 ± 28.75 | 885.67 ± 17.50 | 0.40 |
| ACE | 917.10 ± 12.28 | 931.16 ± 21.64 | 912.97 ± 17.12 | 0.46 |
| Chao1 | 934.71 ± 7.01 | 948.51 ± 25.33 | 922.42 ± 19.55 | 0.31 |
| Simpson | 0.96 ± 0.01 | 0.97 ± 0.01 | 0.96 ± 0.01 | 0.24 |
| Shannon | 6.45 ± 0.17b | 7.14 ± 0.42a | 7.01 ± 0.20a | 0.05 |
| PD-wholetree | 52.13 ± 0.64 | 53.52 ± 0.56 | 53.37 ± 0.83 | 0.09 |
| Coverage | 0.999 ± 0.003 | 0.999 ± 0.000 | 0.999 ± 0.001 | 0.60 |
β-Diversity Analysis
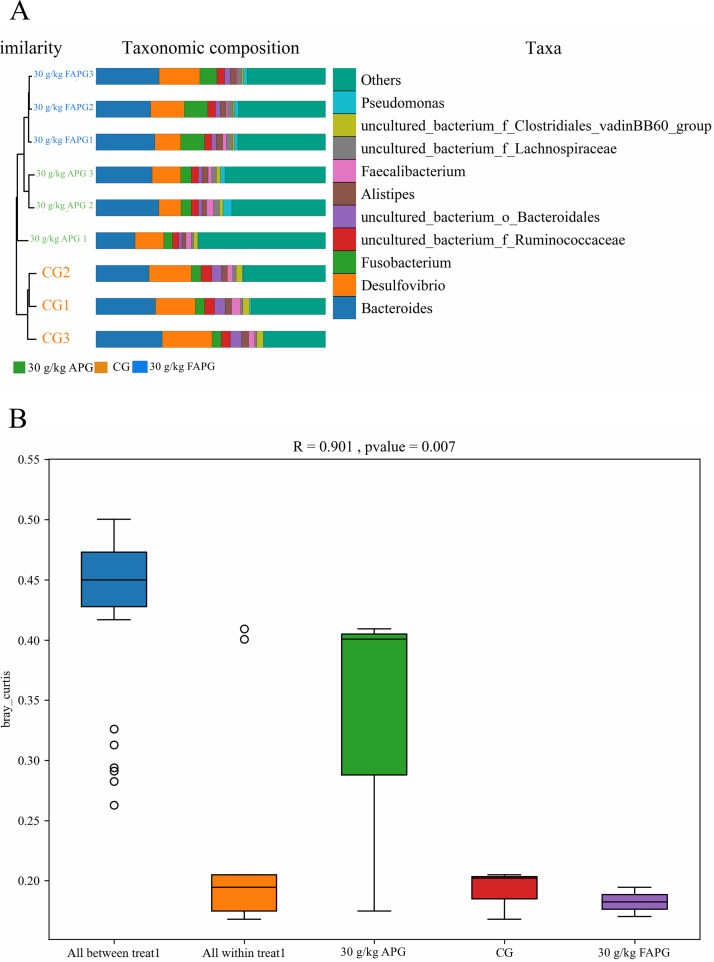
Sample clustering trees showing species between different samples and species compositions at the genus level are shown in a histogram (Figure 2A). Notably, between group differences were higher than within group differences (Figure 2A). Species compositions in genus level are shown using a histogram (Figure 2A). Bacteroides and Desulfovibrio were the dominant bacterial genera in each group.
Figure 2.
Intestinal microbiota β-diversity (A) Clustering tree and histogram. (B) PERMANOVA/Anosim analysis box plot.
Analysis of similarity (ANOSIM) indicated that between group differences were higher than within group differences (R = 0.901, P = 0.007), and confirmed the high reliability of the test (Figure 2B).
Differential Analysis Between Groups
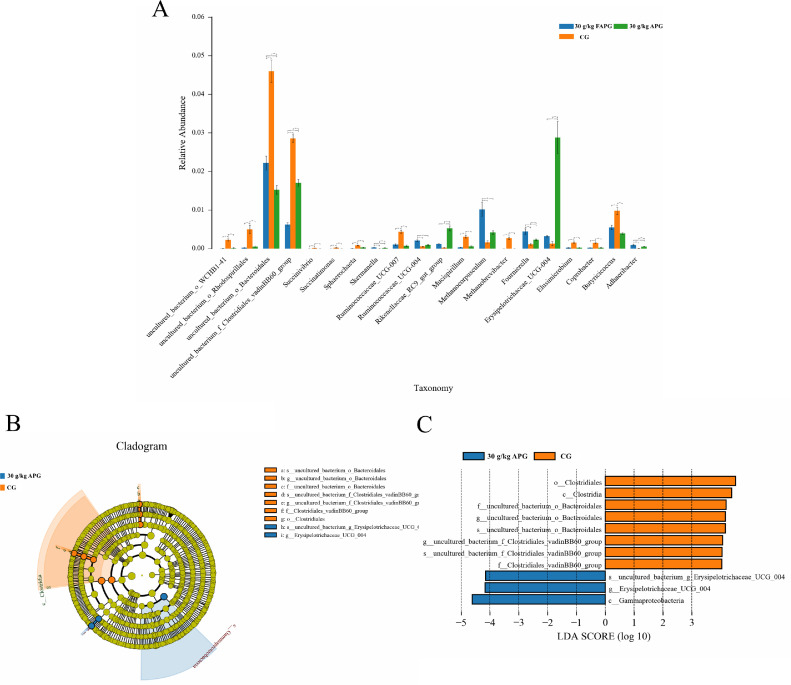
Differences in microbial composition between the experimental groups are shown in Figure 3. The addition of 30 g/kg of unfermented and fermented A. paniculata to the diet (30 g/kg APG) significant decreased the relative abundance of uncultured-bacterium-o-WCHB-41, uncultured-bacterium-o-Rhodospirillales, uncultured-bacterium-o-Bacteroidales, uncultured-bacterium-f-Clostridiales-vadinBB60-group, Succinivibrio, Succinatimonas, Sphaerochaeta, Ruminococcaceae-UCG-007, Mucispirillum, Methanobrevibacter, Elusimicrobium, Coprobacter, and Butyricicoccus, but increased the abundance of Skermanella, Ruminococcaceae-UCG-004, Rikenellaceae-RC9-gut-group, Methanocorpusculum, Fournierella, Erysipelotrichaceae-UCG-004, and Adhaeribacter (P < 0.05) compared with CG (Figure 3A).
Figure 3.
Analysis of differences between groups. (A) Histogram of ANOVA between groups. (B) Cladogram based on LEfSe analysis (C) LDA value distribution histogram.
LEfSe analysis showed that s-uncultured-bacterium-o-Bacteroidales, g-uncultured-bacterium-o-Bacteroidales, f-uncultured-bacterium-o-Bacteroidales, s-uncultured-bacterium-f-Clostridiales-vadinBB60-group, g-uncultured-bacterium-f-Clostridiales-vadinBB60-group, f-Clostridiales-vadinBB60-group, and o-Clostridiales were enriched in CG, whereas s-uncultured-bacterium-g-Erysipelotrichaceae-UCG-004 and g-Erysipelotrichaceae-UCG-004 were enriched in 30 g/kg APG (Figure 3B).
The length of the histogram in Figure 3C represents the impact of different species. The results showed that the most influential microorganisms in CG are o-Clostridiales, c-Clostridia, f-uncultured-bacterium-o-Bacteroidales, g-uncultured-bacterium-o-Bacteroidales, s-uncultured-bacterium-o-Bacteroidales, g-uncultured-bacterium-f-Clostridiales-vadinBB60-group and s-uncultured-bacterium-f-Clostridiales-vadinBB60-group. In 30 g/kg APG, the most influential microorganisms were s-uncultured-bacterium-g-Erysipelotrichaceae-UCG-004, g-Erysipelotrichaceae-UCG-004 and c-Gammaproteobacteroa.
Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment of Intestinal Microbes
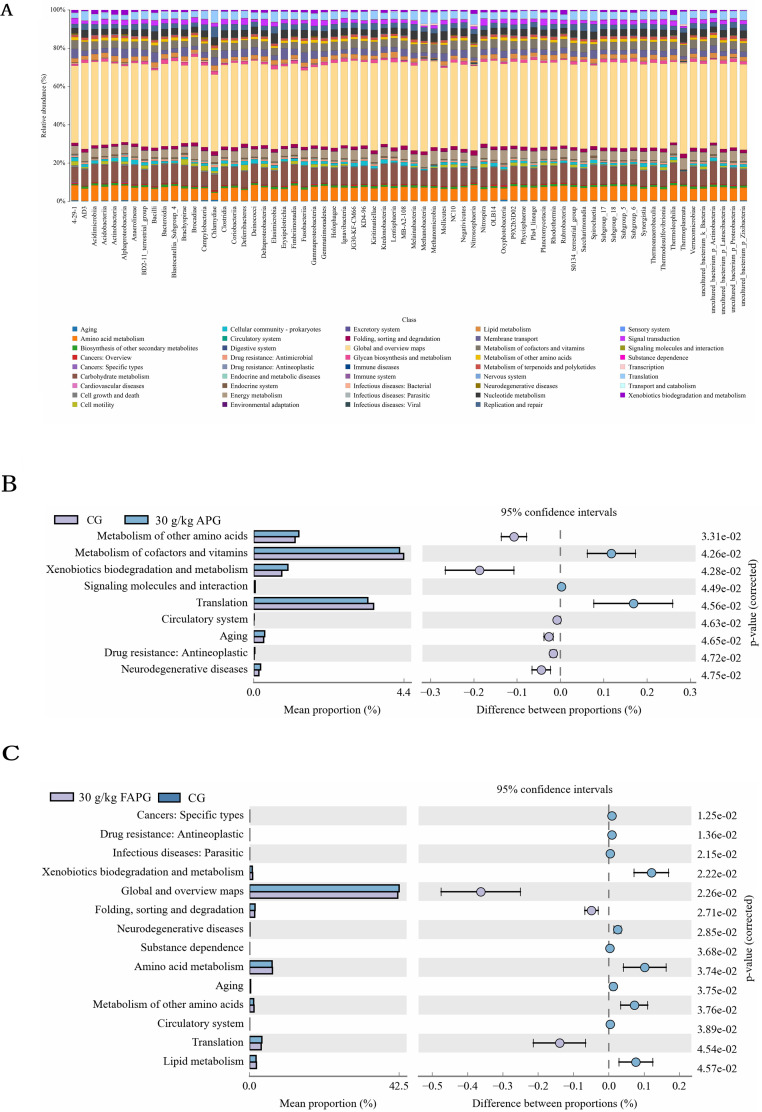
As shown in Figure 4, in 30 g/kg APG, pathways of Metabolism of other amino acids, Xenobiotics biodegradation and metabolism, Aging, Drug resistance: Antineoplastic, Neurodegenerative diseases, and Circulatory system were upregulated (P < 0.05), but pathways of Metabolism of cofactors and vitamins, Translation and Signaling molecules and interaction were downregulated (P < 0.05) compared with CG. In 30 g/kg FAPG, pathways of Cancers: Specific types, Drug resistance: Antineoplastic, Infectious diseases: Parasitic, Xenobiotics biodegradation and metabolism, Neurodegenerative diseases, Substance dependence, Amino acid metabolism, Aging, Metabolism of other amino acids, Circulatory system and Lipid metabolism were upregulated (P < 0.05), but pathways of Global and overview maps, Folding, sorting and degradation and Translation were downregulated (P < 0.05) compared with CG.
Figure 4.
KEGG pathway enrichment analysis. (A) Histogram of KEGG pathways. (B) KEGG metabolic pathways difference between the control and 30 g/kg unfermented A. paniculata group (C) KEGG metabolic pathways difference between control and 30 g/kg fermented A. paniculate group.
COG Analysis
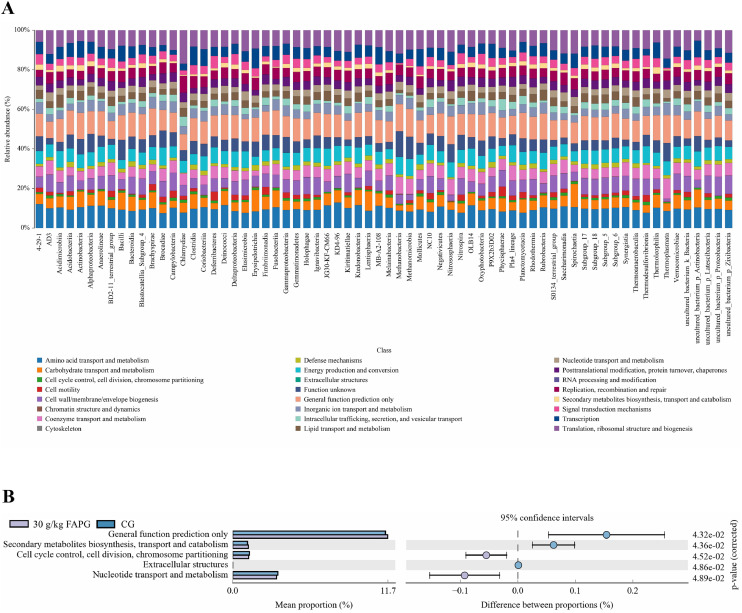
COG (Clusters of Orthologous Groups of proteins) is a commonly used function classification database of proteins in prokaryotes. In 30 g/kg FAPG, Gerneral function prediction only, Secondary metabolites biosynthesis, transport and catabolism, Extracellular structures were upregulated (P < 0.05; Figure 5), but Cell cycle control, cell division, chromosome partitioning and Nucleotide transport and metabolism were downregulated (P < 0.05) compared with the CG.
Figure 5.
COG pathways. (A) Histogram of COG pathways. (B) Statistical graph of COG function classification.
DISCUSSION
In traditional herbal medicine, A. paniculata has been reported to possess antihepatotoxic, antibiotic, antimalarial, antihepatic, antithrombogenic, anti-inflammatory, antivenom, and antipyretic properties, in addition to its use as an immunostimulant (Kumar et al., 2021). When used as a feed additive, A. paniculata has the potential to improve the nutritional composition of feed, increase the body weight of poultry, and reduce mortality of domestically raised poultry (Jahja et al., 2022).
The enhancement of ADG and reduction of FCR (ADFI/ADG) has been shown to improve in growth performance. Consistent with these findings, our results showed that both unfermented and fermented A. paniculata promoted growth, with a more distinct effect observed using fermented A. paniculata. However, we found that Muscovy duck body weight significantly reduced following consumption of feed containing 50 g/kg of fermented A. paniculata. This may be attributed to the presence of toxic factors and bitter taste of A. paniculata at a large dose. Moreover, excessive introduction of live bacteria into the animal intestines through the intake of fermented products may affect nutrient absorption and utilization and growth in the host animals.
Carcass traits are indicators of meat quality (Gungor et al., 2020). It is generally acknowledged that meat quality is optimal when the dressed percentage and percentage of eviscerated yield are over 80% and 60%, respectively. The meat quality within our study was considered “excellent”, as the dressed percentage and percentage of eviscerated yield exceeded the aforementioned standards. Additionally, the marked increase in other carcass traits, including dressed percentage of 10 g/kg FAPG, percentage of breast muscle yield of 30 g/kg APG, lung index of 30 g/kg APG, 30 g/kg, and 50 g/kg FAPG, indicated an improvement in the productive performance of the experimental Muscovy duck groups compared with CG.
The immune organ indices are measures of the immune function of the body in poultry (Zhang et al., 2021). The spleen is the largest peripheral immune organ in poultry and is involved in humoral and cellular immunity (Madej et al., 2020). Bursa of Fabricius, containing various bursal-derived peptides, is a unique humoral immune central organ in poultry (Feng et al., 2012). The thymus is the main site of T cell production, and its immune function plays an important role in anti-infection, antitumor, and autoimmune effects (Amirghofran et al., 2012). Our results showed that supplementation with 30 g/kg fermented A. paniculata significantly increased the bursa index by 90.79% compared with CG. Additionally, supplementation with 30 g/kg unfermented A. paniculata and fermented A. paniculata clearly increased the thymus index of the ducks by 60.23% and 106.25%, respectively, compared with CG. These results indicated that both unfermented and fermented A. paniculata improved the immune organ indices of Muscovy ducks; however, fermented A. paniculata had a more distinct effect on the 2 organ immune indices.
In the present study, we found that supplementation with fermented A. paniculata significantly increased serum lysozyme activity in Muscovy ducks, consistent with Deng et al. (2016) who found that using the ultra-fine powder of A. paniculata as a diet supplement increased serum lysozyme activity in Sanhuang chickens. Lysozyme is an alkaline enzyme that can hydrolyze polysaccharides on the surface of pathogenic bacteria, and possesses anti-infective, antiviral, and immune-enhancing effects (Khlyustova et al., 2022). Additionally, serum lysozyme has shown to be an important indicator of nonspecific immunity in the body. Therefore, our results suggest that fermented A. paniculata supplementation may have improved innate immunity in the Muscovy ducks by increasing serum lysozyme activity.
Most of the peripheral blood lymphocytes of Muscovy ducks are T cells, which are important in mediating cellular immunity (Wu et al., 2019). After stimulation with nonspecific antigen ConA in vitro, they can transform into lymphoblasts for lymphocyte proliferation (Alekseeva et al., 2021). Therefore, the degree of T lymphocyte transformation and proliferation reflects the level of cellular immunity in the body (Hafez et al., 2020). In the present study, supplementation with unfermented and fermented A. paniculata observably increased the conversion rate of peripheral blood lymphocytes in Muscovy ducks. This indicated that A. paniculata can improve the cellular immunity of the body, which was consistent with the findings of Hu et al. (2006). Notably, fermented A. paniculata had a considerably better effect than unfermented A. paniculata, which may be due to the further release of active substances that occurs following fermentation.
The growth performance of poultry is closely related to the morphological structure of the intestinal mucosa, and the integrity of its structure and function is important for poultry health (Wang et al., 2021). In the present study, supplementation with both fermented and unfermented A. paniculata significantly improved the villi height and villi surface area of the ducks, indicating that both unfermented and fermented A. paniculata can improve intestinal function of digestive absorption. However, supplementation with 30 g/kg fermented A. paniculata had the best effects on villi surface area and villi width, indicating that fermented A. paniculata is preferable for improving intestinal health in Muscovy ducks. These results could explain the observed improvement in growth performance of Muscovy ducks fed fermented A. paniculata compared with those fed unfermented A. paniculata. Intestine thickness is closely related to small intestinal motility, which directly affects the tonic contraction, rhythmic segmental motility, and peristalsis of the small intestine (Maged et al., 2022). The thicker the intestinal walls, the stronger the intestinal peristaltic function, and the faster the excretion of pathogenic microorganisms, such as bacteria and viruses. In the present study, supplementation with both 30 g/kg of unfermented and fermented A. paniculata significantly increased the intestine thickness of Muscovy ducks, which further improved intestinal function and health in Muscovy ducks. Additionally, intestinal mucosal immune-related cells, such as intraepithelial lymphocytes, play an important role in maintaining the integrity of intestinal epithelial cells and immune responses, and acts as the body's first defense barrier against pathogens (Tian et al., 2021). The local immune function of the intestine can be reflected by the changes in the number of these immune-related cells (Wang et al., 2019). In the present study, supplementation with either 30 g/kg of unfermented or fermented A. paniculata improved intestinal immune function, in addition to intestine thickness, which improved intestinal function and health in the Muscovy ducks. These results indicate that A. paniculata could be used as a potential alternative to antibiotic growth promoters.
The effect of A. paniculata on the intestinal microbial composition and structure was examined and the richness and diversity of intestinal bacteria in the cecum of the Muscovy ducks were assessed using α-diversity indices, including Shannon index (Zengin et al., 2022). Supplementation with either 30 g/kg unfermented or 30 g/kg fermented A. paniculata significantly increased the Shannon index, indicating that both treatments can improve species richness and diversity in the cecum of Muscovy ducks. Additionally, β-analysis showed that the experimental and control groups form 2 distinct clusters, suggesting that both fermented and unfermented A. paniculata had considerable effects on intestinal microbiota composition and structure.
Trillions of bacteria inhabit the gastrointestinal tract of animals. In most hosts, these symbionts play a large role in promoting microbe-host internal environmental balance (Wang et al., 2022). Among them, the main metabolic end products of Succinivibrio and Succinatimonas are acetic acid and succinic acid (Li et al., 2020). Succinic acid is harmful to the environment to some extent and can cause pollution to water bodies and the atmosphere. Sphaerochaeta has a helical morphology and motility conferred by flagella around the axial cytosol, and has some pathogenicity (Bidzhieva et al., 2020). Mucispirillum is a spiral, flagellated, gram-negative bacteria, and obligate anaerobes, which live in the intestinal mucus layer, and is associated with intestinal inflammation development (Loy et al., 2017). In the present study, supplementation with unfermented and fermented A. paniculata decreased the abundance of Succinivibrio, Succinatimonas, Sphaerochaeta, and Mucispirillum, indicating the animal and environmental health promoting effects of A. paniculata.
In the present study, supplementation with unfermented and fermented A. paniculata increased the abundance of Skermanella. Skermanella possess antimony resistance properties (Luo et al., 2012); therefore, contributing to improved heavy metal tolerance, health status, and meat quality in Muscovy ducks. We observed an increase Ruminococcaceae abundance, which are the main microorganisms that convert primary bile acids into secondary bile acids, and thus play an important role in lipid digestion and absorption (Gu et al., 2022). Therefore, these results indicate that A. paniculata can promote lipid digestion and absorption in Muscovy ducks. We observed an increase in the abundance of the Rikenellaceae, Methanocorpusculum, and Fournierella genera. Rikenellaceae reduces the negative associated effects of IBD enteritis (Huang et al., 2019), Methanocorpusculum is involved in the mediation of abdominal fat deposition (Dong et al., 2019), and Fournierella is positively associated with muscle and bone health (Farkas et al., 2022). Therefore, the increased abundance of these 3 genera indicated that both unfermented and fermented A. paniculata can improve intestinal health, reduce body fat levels, and improve carcass quality and the health status of Muscovy ducks.
Overall, supplementation with unfermented and fermented A. paniculata inhibited the abundance of harmful bacteria (Succinivibrio, Succinatimonas, Sphaerochaeta, and Mucispirillum) and increased the abundance of beneficial bacteria (Rikenellaceae, Methanocorpusculum, Fournierella). We found that supplementation with unfermented and fermented A. paniculata increased Adhaeribacter abundance, which could reduce antibiotic residues and promote chlortetracycline degradation (REF). The transfer of antibiotics through the food chain pose considerable threats to human health; thus, the use of A. paniculata as a natural alternative to antibiotics provides important implications for reducing the risks to human health from poultry consumption.
Based on KEGG pathway enrichment analysis database, supplementation with unfermented and fermented A. paniculata upregulated the pathways of Metabolism of other amino acids, Xenobiotics biodegradation and metabolism, Aging, Drug resistance: Antineoplastic, Neurodegenerative diseases, and Circulatory system. Metabolism of other amino acids is associated with the metabolism of amino acids, such as taurine, hypotaurine, phosphonate, hypophosphite, selenoamino acids, and cyanoamino acids (Hu et al., 2003). Xenobiotics biodegradation and metabolism are associated with benzoic acid aminobenzoic acid, orthofluorobenzoic acid, chloride paraffin, chloroalkene, methylbenzene (Nakov et al., 2020). Additionally, supplementation with 30 g/kg fermented A. paniculata promoted pathways related to amino acid and lipid metabolism, of which Amino acid metabolism pathways are related to the metabolism of alanine, aspartic acid, glutamic acid, glycine, serine, threonine, valine, leucine, lysine, and isoleucine, as well as the biosynthesis of valine, leucine, isoleucine, lysine, and arginine (Cui et al., 2020). Lipid metabolism pathways are related to biosynthesis of fatty acids, elongation of fatty acid, synthesis and degradation of ketones, biosynthesis of cork and wax, steroids, primary bile acid, secondary bile acid, and steroid hormone, and metabolism of glycerides, glycerophospholipids, ether lipid, sphingoglycolipid, arachidonic acid, linoleic acid, and linolenic acid (Russell, 2018). Overall, compared with 30 g/kg unfermented A. paniculata, 30 g/kg fermented A. paniculata had a stronger effect on the metabolism and degradation of amino acids and lipids in Muscovy ducks.
COG analysis showed that supplementation with 30 g/kg fermented A. paniculata improved secondary metabolites biosynthesis, transport, and catabolism. Secondary metabolites are a class of nonessential small organic compounds necessary for the normal operation of cell activities, growth, and development of body (Adebayo et al., 2019). Therefore, our results suggest that fermented A. paniculata supplementation may play an important role in promoting the growth and development of Muscovy ducks through improving secondary metabolite biosynthesis.
Overall, these results suggest that fermented A. paniculata can maintain normal body activities, promote protein synthesis, and maintain the extracellular structure of eukaryotic cells. Moreover, COG and KEGG enrichment analysis of differentially expressed genes among the 3 groups confirmed that unfermented and fermented A. paniculata can regulate microbial diversity, abundance, and structure. However, fermented A. paniculata had considerably stronger effects than unfermented A. paniculata on pathways associated with amino acid and lipid metabolism, and secondary metabolites biosynthesis, transport, and catabolism. Therefore, it was speculated fermented A. paniculata may improve nutrient metabolism by bacteria, improve their adaptability to the environment, and regulate the immune and digestive system by regulating the genes in these pathways.
These results indicate that the observed improvement in duck growth performance, immune status, and intestinal morphology can be attributed to the consumption of diets supplemented with fermented A. paniculata; however, further studies are necessary to elucidate the specific molecular mechanism of fermented A. paniculata.
CONCLUSIONS
In summary, supplementation with fermented A. paniculata improved growth performance, immune status, intestinal morphology, and gut microbiota composition and structure in Muscovy ducks. Therefore, fermented A. paniculata have potential application in the production of Muscovy ducks, and could be used as potential alternative for antibiotics.
ACKNOWLEDGMENTS
This study was funded in part by the grant from Municipal Party Committee Talent Project of Ganzhou City (Grant Number: JXXTCXQN202009), Science and Technology Project of Ganzhou City (Grant Number: 2019GSKF) and Technical System of Poultry Industry in Jiangxi Province (Grant Number: JXARS-09).
DISCLOSURES
All authors declare they have no conflicts of interest.
REFERENCES
- Adebayo B.J., Isah O.A., Omoniyi L.A. Nutritional composition and secondary metabolites of some selected forages consumed by small ruminants. Niger. J. Anim. Sci. 2019;21:195–201. [Google Scholar]
- Alekseeva I.V., Nikenina E.V., Abramova A.Y., Kozlov A.Y., Koplik E.V., Pertsov S.S. Lymphocyte index of peripheral blood in rats at different stages of the post-stress period under conditions of antigenic exposure and injection of lipopolysaccharide. Bull. Exp. Biol. Med. 2021;172:113–116. doi: 10.1007/s10517-021-05345-7. [DOI] [PubMed] [Google Scholar]
- Amirghofran Z., Ahmadi H., Karimi M.H. Immunomodulatory activity of the water extract of Thymus vulgaris, Thymus daenensis, and Zataria multiflora on dendritic cells and T cells responses. J. Immunoassay Immunochem. 2012;33:388–402. doi: 10.1080/15321819.2012.655822. [DOI] [PubMed] [Google Scholar]
- Aneesh A., George A.J., Krishna B.D., Abraham M.J., Kariyil B.J. Hepatoprotective and nephroprotective effect of Aegle marmelos and A. paniculata in aflatoxicosis of broiler chicken. Indian. J. Anim. Res. 2021;55:1242–1245. [Google Scholar]
- Arify T., Valavan S.E., Varun A., Sundaresan A., Manimaran K. Effect of garlic (Allium sativum) and nilavembu (A. aniculate) on growth performance and cost effectiveness of broiler chicken. Indian J. Anim. Sci. 2019;89:347–352. [Google Scholar]
- Bidzhieva S.K., Sokolova D.S., Grouzdev D.S., Kostrikina N.A., Poltaraus A.B., Tourova T.P., Shcherbakova V.A., Troshina O.Y., Nazina T.N. Sphaerochaeta halotolerans sp. nov., a novel spherical halotolerant spirochete from a Russian heavy oil reservoir, emended description of the genus Sphaerochaeta, reclassification of Sphaerochaeta coccoides to a new genus Parasphaerochaeta gen. nov. as Parasphaerochaeta coccoides comb. nov. and proposal of Sphaerochaetaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2020;70:4748–4759. doi: 10.1099/ijsem.0.004340. [DOI] [PubMed] [Google Scholar]
- Cui Y., Han C., Li S., Geng Y., Wei Y., Shi W., Bao Y. High-throughput sequencing-based analysis of the intestinal microbiota of broiler chickens fed compound small peptide of Chinese medicine. Poult. Sci. 2020;100 doi: 10.1016/j.psj.2020.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W.Q., Ma Y.F., Li J., Deng B.X., Huang Y.F. Ultramicro-pulverised powder of Andrographis paniculata to improve antioxidant and immune function of Sanhuang broiler. J. Fujian Agric. Forest. Univers. 2016;45:104–106. [Google Scholar]
- Dong L.F., Zhang T.T., Diao Q.Y. Effect of dietary supplementation of moringa oleifera on the production performance and fecal methanogenic community of lactating dairy cows. Animals (Basel) 2019;9:262. doi: 10.3390/ani9050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V., Csitári G., Menyhárt L., Such N., Pál L., Husvéth F., Rawash M.A., Mezőlaki Á., Dublecz K. Microbiota composition of mucosa and interactions between the microbes of the different gut segments could be a factor to modulate the growth rate of broiler chickens. Animals (Basel) 2022;12:1296. doi: 10.3390/ani12101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X.-L., Liu Q.T., Cao R.B., Zhou B., Zhang Y.P., Liu K., Liu X.D., Wei J.C., Li X.F., Chen P.Y. Characterization and immunomodulatory function comparison of various bursal-derived peptides isolated from the humoral central immune organ. Peptides. 2012;33:258–264. doi: 10.1016/j.peptides.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Gu T.T., Duan M.C., Zhang R.K., Zeng T., Xu W.W., Feng W.F., Jiang C.Q., Tian Y., Chen L., Lu L.Z. Probiotic fermented feed alleviates liver fat deposition in shaoxing ducks via modulating gut microbiota. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.928670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor E., Erener G. Effect of dietary raw and fermented sour cherry kernel (Prunus cerasus L.) on growth performance, carcass traits, and meat quality in broiler chickens. Poult. Sci. 2020;99:301–309. doi: 10.3382/ps/pez490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez A., Nassef E., Fahmy M., Elsabagh M., Bakr A., Hegazi E. Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ. Sci. Pollut. Res. Int. 2020;27:19108–19114. doi: 10.1007/s11356-019-04344-6. [DOI] [PubMed] [Google Scholar]
- Hu X.H., Galili G. Increased lysine synthesis coupled with a knockout of its catabolism synergistically boosts lysine content and also transregulates the metabolism of other amino acids in Arabidopsis seeds. The Plant Cell. 2003;15:845–853. doi: 10.1105/tpc.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.L., Liao X.B., Li J.H. Effects of Andrographispaniculata(Burm. f.) Nees on lymphocyte activity and phagocytic function of leucocytes in broilers. J Chin. Vet. Med. 2006;5:17–20. [Google Scholar]
- Huang K.Y., Dong W., Liu W.Y., Yan Y.M., Wan P., Peng Y.J., Xu Y.J., Zeng X.X., Cao Y.L. 2- o-β-d-glucopyranosyl-l-ascorbic acid, an ascorbic acid derivative isolated from the fruits of lycium barbarum l., modulates gut microbiota and palliates colitis in dextran sodium sulfate-induced colitis in mice. J. Agric. Food Chem. 2019;67:11408–11419. doi: 10.1021/acs.jafc.9b04411. [DOI] [PubMed] [Google Scholar]
- Khlyustova A., Kirsch M., Ma X.J., Cheng Y.F., Yang R. Surfaces with antifouling-antimicrobial dual function via immobilization of lysozyme on zwitterionic polymer thin films. J. Mater. Chem. B. 2022;10:2728–2739. doi: 10.1039/d1tb02597j. [DOI] [PubMed] [Google Scholar]
- Kumar S., Bikarma S., Vikas B. Andrographis paniculata (Burm.f.) Nees: traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J Ethnopharmacol. 2021;275 doi: 10.1016/j.jep.2021.114054. 114054-114054. [DOI] [PubMed] [Google Scholar]
- Jahja E., Yuliana R., Simanjuntak W.T., Fitriy N., Rahmawati A., Yulinah E. Potency of Origanum vulgare and Andrographis paniculata extracts on growth performance in poultry. Vet. Anim. Sci. 2022;19 doi: 10.1016/j.vas.2022.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.Y., Sheng F.Y., Zhang Z., Ma X., Gao T.H., Fu C.M., Li P. Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113954. [DOI] [PubMed] [Google Scholar]
- Julaton T., Taclendo A., Oyong G., Rempillo O., Galvez M.C., Vallar E. In silico insights on the pro-inflammatory potential of polycyclic aromatic hydrocarbons and the prospective anti-inflammatory capacity of andrographis paniculata phytocompounds. Int. J. Environ. Res. Public. Health. 2022;19:8588. doi: 10.3390/ijerph19148588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.P., Shen J.S., Xu Y.X., Zhu W.Y. Metagenomic analysis reveals significant differences in microbiome and metabolic profiles in the rumen of sheep fed low N diet with increased urea supplementation. FEMS Microbiol. Ecol. 2020;96:fiaa117. doi: 10.1093/femsec/fiaa117. [DOI] [PubMed] [Google Scholar]
- Lin Z., Ye L., Li Z., Huang X., Lu Z., Yang Y., Xing H., Bai J., Ying Z. Chinese herb feed additives improved the growth performance, meat quality, and nutrient digestibility parameters of pigs. Anim. Model Exp. Med. 2020;3:47–54. doi: 10.1002/ame2.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.H., Wu Y.F., Wu J., Li X., Yu L.L., Xie K., Zhang M.Y., Ren L.L., Ji Y.L., Li Y.H. Exposure to veterinary antibiotics via food chain disrupts gut microbiota and drives increased Escherichia coli virulence and drug resistance in young adults. Pathogens. 2020;11:1062. doi: 10.3390/pathogens11091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy A., Pfann C., Steinberger M., Hanson B., Herp S., Brugiroux S., Neto J.C.G., Boekschoten M.V, Schwab C., Urich T., Tait A.E R., Rattei T., Stecher B., Berry D. Lifestyle and horizontal gene transfer-mediated evolution of mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems. 2017;2 doi: 10.1128/mSystems.00171-16. e00171-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G.S., Shi Z.J., Wang H., Wang G.J. Skermanella stibiiresistens sp. nov., a highly antimony-resistant bacterium isolated from coal-mining soil, and emended description of the genus Skermanella. Int. J. Syst. Evol. Microbiol. 2012;62(Pt 6):1271–1276. doi: 10.1099/ijs.0.033746-0. [DOI] [PubMed] [Google Scholar]
- Madej J.P., Skonieczna J., Siwek M., Kowalczyk A., Łukaszewicz E., Slawinska A. Genotype-dependent development of cellular and humoral immunity in the spleen and cecal tonsils of chickens stimulated in ovo with bioactive compounds. Poult. Sci. 2020;99:4343–4350. doi: 10.1016/j.psj.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maged A., Hani H.A., Alqhtani A.H. Growth performance, histological changes and functional tests of broiler chickens fed diets supplemented with tribulus terrestris powder. Animals (Basel) 2022;12:1930. doi: 10.3390/ani12151930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakov R., Velikova T. Chemical metabolism of xenobiotics by gut microbiota. Curr. Drug Metab. 2020;21:260–269. doi: 10.2174/1389200221666200303113830. [DOI] [PubMed] [Google Scholar]
- Russell A.D. Significance and regulation of lipid metabolism. Semin. Cell. Dev. Biol. 2018;81:97. doi: 10.1016/j.semcdb.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Tian F., Shao C.Y., Wang Y.Y, Liu X.L., Ma Y.F., Han D.P. Dietary Lactobacillus casei can be used to influence intraepithelial lymphocyte migration and modulate mucosal immunity in chicks. Br. Poult. Sci. 2021;62:492–498. doi: 10.1080/00071668.2021.1889464. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang B., Du H., Zhang H., Li H., Wang F., Zhao X. Effects of Diutina rugosa SD-17 on growth performance, intestine morphology, and immune status of chickens. Poult. Sci. 2019;98:6311–6318. doi: 10.3382/ps/pez428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.K., Gong L., Zhou Y.H., Tang L., Zeng Z.H., Wang Q., Zou P., Yu D.Y., Li W.F. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021;7:829–840. doi: 10.1016/j.aninu.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.X., Tang Y.F., Long L.N., Zhang H.H. Effects of dietary l-theanine on growth performance, antioxidation, meat quality, and intestinal microflora in white feather broilers with acute oxidative stress. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.889485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.J., Liu Z.N., Zhu E.P., Li M.H., Jiang H.H.i., Luo Y., Wang Q.X., Wu X.P., Wu B.C. Changes in the small intestine mucosal immune barrier in Muscovy ducklings infected with Muscovy duck reovirus. Vet. Microbiol. 2019:85–92. doi: 10.1016/j.vetmic.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Zengin M., Sur A., İlhan Z., Azman M.A., Tavşanlı H., Esen S., Bacaksız O.K., Demir E. Effects of fermented distillers grains with solubles, partially replaced with soybean meal, on performance, blood parameters, meat quality, intestinal flora, and immune response in broiler. Res. Vet. Sci. 2022;150:58–64. doi: 10.1016/j.rvsc.2022.06.027. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Y., Zhang S., Cong G.L., Zhang Y.J., Madsen M.H., Tan B.J., Shi S.R. Effects of soy protein concentrate in starter phase diet on growth performance, blood biochemical indices, carcass traits, immune organ indices and meat quality of broilers. Animals (Basel) 2021;11:281. doi: 10.3390/ani11020281. [DOI] [PMC free article] [PubMed] [Google Scholar]