Summary
Surface-enhanced Raman spectroscopy (SERS) is a label-free, non-destructive technique for rapid identification of molecules with the interest of public safety and forensics. In the current work, we present a detailed protocol for designing a SERS-active substrate comprising Au-nanoparticles-decorated Ag nano-dendrites for the trace detection of explosives, biomolecules, dye, and pesticides. We elaborate the procedure for studying near-field enhancements in plasmonic structures. This protocol also addresses some of the challenges faced in SERS experiments and the potential solutions to overcome them.
For complete details on the use and execution of this protocol, please refer to Vendamani et al. (2022).1
Subject areas: Surface Plasmon Resonance (SPR), Physics, Material Sciences
Graphical abstract

Highlights
-
•
Electroless etching for AgNDs formation and immersion bath for decoration of AuNPs
-
•
Rapid detection of biomolecules, dye, explosives, and pesticides using SERS
-
•
Simulations of near-field enhancement on plasmonic structures using COMSOL 5.4
-
•
Demonstrated the versatility, durability, and reproducibility of our SERS substrates
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Surface-enhanced Raman spectroscopy (SERS) is a label-free, non-destructive technique for rapid identification of molecules with the interest of public safety and forensics. In the current work, we present a detailed protocol for designing a SERS-active substrate comprising Au-nanoparticles-decorated Ag nano-dendrites for the trace detection of explosives, biomolecules, dye, and pesticides. We elaborate the procedure for studying near-field enhancements in plasmonic structures. This protocol also addresses some of the challenges faced in SERS experiments and the potential solutions to overcome them.
Before you begin
Surface-enhanced Raman spectroscopy is one of the versatile vibrational techniques to diagnose the molecules down to trace level concentration. Fabrication of bio-compatible Ag/Au bimetallic SERS-active substrates is highly motivated and potential for molecular diagnosis. Silver nano-dendrites (AgND) are known to enhance the SERS signal strongly whereas the AuNPs offer the dual benefit of prevention of rapid oxidation and additional enhancement.2,3,4
The fabricated AgNDs structure holds fractals with multi-level branches and trunks, which can produce enhanced localized near-field enhancements.3,4 The decoration of AuNPs on AgNDs is introduced to fabricate bimetallic Au/Ag nanostructures to attain superior molecular detection. Recently, it has been demonstrated that the synergistic effects of both AuNPs chemical stability and the AgNDs strong plasmonic effects have considerably improved the SERS activity and durability.5,6 As compared to our previous work7 where we have worked only on the Ag dendrites with Ammonium Nitrate (1 μM) as a probe molecules, this samples showed superior performance (100 nM). The step-by-step protocol in upcoming segments describes the handling of a wide range of molecules and optimizing critical parameters (laser power, acquisition time, focusing objective, etc.), which are essential to achieve versatility of substrate and maximum signal enhancement respectively.
Fabrication of gold nanoparticles decorated silver dendrites on Si substrate and its pre-treatment
Timing: <5 h
In the present protocol, we have used commercially available 3″ p-type, Boron doped (100) oriented Si wafers as a reducing agent for the preparation of silver dendrites by adopting electroless etching because of its multi-functionality and bio-compatibility.
-
1.
Place the silicon wafer in an ultrasonic bath of ethanol for 5 min.
-
2.
Place the silicon wafer in an ultrasonic bath of acetone for 10 min.
Note: These steps ensure to remove organic residues from the sample surface.
-
3.
Clean the silicon wafer with deionized water several times.
-
4.
Dip the Si wafer in 10% HF for 5 min.
Note: This step removed native oxide, and makes the surface hydrogenated.
-
5.
Immediately immerse the hydrogenated Si wafers into 30 mM AgNO3 and 4.6 M HF (48%) composed electrolytic solution for silver dendrite (AgNDs) formation at 30°C.
Note: Highly symmetrical dendrites with 8 μm primary and 6 μm secondary branches are attained at 15 min of etching time.
CRITICAL: Back side of Si wafer was also etched during AgNDs formation. This has been ruled out by sealing the Si wafer’s wrong (rough) side with acid-resistant tape and removed once after the etching.
-
6.
Perform decoration/passivation of gold nanoparticles (AuNPs) on AgNDs by Galvanic Displacement (GD) process at various molar concentrations of gold salt (HAuCl4.3H2O; 99.9%) to improve the durability of AgNDs considering natural oxidation. The density of AuNPs at 1 mM concentration was increased at various deposition times (i.e., 30 min, 1 h, 2 h, and 3 h). The deposition at 3 h illustrated homogeneously distributed AuNPs over the entire AgNDs surface.
The impact of high-density hot spots (using bimetallic structure) in molecular detection was evident in the SERS signal.
Note: We performed decoration/passivation of gold nanoparticles (AuNPs) on AgNDs at various molar concentrations (i.e., 0.05 mM, 0.1 mM, 0.5 mM, 1 mM, 1.5 mM, 3 mM, 5 mM, 7 mM, respectively) of gold salt to improve the durability of AgNDs considering natural oxidation.
CRITICAL: We have noticed that the distribution of AuNPs completely concealed the nature of the dendritic structure at elevated molar concentrations of Au salt. The optimized threshold concentration is about 1 mM, which produced an isolated and uniform distribution of AuNPs on AgNDs.
Note: We prepared AuNPs decorated AgNDs on a 3″ wafer with optimized AgNDs (30 mM AgNO3::4.6 MHF) and AuNPs (1 mM at 3 h) conditions. Subsequently, the 3″ AuNPs decorated AgNDs substrate was fragmented into 1 cm2 for further investigation like FESEM, TEM, XRD, and XPS.
-
7.
Figure 1 presents the AuNPs (1 mM at 3 h deposition time) decorated AgNDs. This sample was labeled as AuNPs@AgNDs-3h for convenience in the discussion.
Figure 1.
FESEM image of AuNPs decorated AgNDs at 3 h of deposition time
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Silver salt (AgNO3) | Finar, India | CAS No. 7783-90-6 |
| Ethanol | Supelco, India | CAS No. 64-17-5 |
| Hydrofluoric acid | Sigma-Aldrich, India | CAS No. 7664-39-3 |
| HAuCl4.3H2O | Sigma-Aldrich, India | CAS No. 16961-25-4 |
| Antibiotics (penicillin G, kanamycin, ampicillin) | Sigma-Aldrich, India | CAS No. 113-98-4; 64013-70-3; 7177-48-2 |
| DNA bases (adenine, cytosine) | Sigma-Aldrich, India | CAS No. 73-24-5; 71-30-7 |
| Ammonium nitrate | HEMRL, Pune, India | NA |
| Thiram | Sigma-Aldrich, India | CAS No. 137-26-8 |
| Software and algorithms | ||
| Origin | www.originlab.com | Origin 2018 |
| COMSOL | www.comsol.com | COMSOL 5.3 |
| Gatan DM3 | www.gatan.com | Gatan Microscopy Suite 3.x |
| Other | ||
| Si wafers (1–10 Ω-cm, p-type) | Macwin India Ltd. | NA |
| Field emission scanning electron microscope | Carl ZEISS, Ultra 55 | https://www.felmi-zfe.at/instrumentation/sem/zeiss-ultra-55/ |
| Transmission electron microscope | Technai | https://www.fei.com/products/tem/tecnai-g2-spirit-for-life-sciences/#gsc.tab=0 |
| X-ray Diffractometer | Bruker D8 advance | https://www.bruker.com/en/products-and-solutions/diffractometers-and-scattering-systems/x-ray-diffractometers/d8-advance-family/d8-advance.html |
| UV-Visible spectrophotometer | Jasco V-670 | https://www.jasco.de/en/content/V-670/∼nm.13∼nc.407/V-670-UV-VIS-NIR-Spectrophotometer.html |
| X-ray photoelectron microscope | Thermo Scientific | https://www.thermofisher.com/in/en/home/electron-microscopy/products/xps-instruments/k-alpha.html |
| Micro-Raman spectrometer | Horiba LabRAM | https://www.horiba.com/ind/scientific/products/detail/action/show/Product/labram-hr-evolution-1083/ |
Materials and equipment
Materials and reagents used in the present protocol have been listed in key resources table. This protocol used a wide range of molecular solutions as the initial stock solutions for the successive diluted molecular solutions as shown below.
| Reagent | Amount/Vol. of solvent | Final concentration | |
|---|---|---|---|
| Dye | Crystal violet | 40.8 mg/5 mL | 100 μM |
| DNA bases | Adenine | 16.9 mg/5 mL | 25 mM |
| Cytosine | 16.7 mg/5 mL | 30 mM | |
| Antibiotics | Penicillin G | 33.4 mg/5 mL | 20 mM |
| Ampicillin | 34.9 mg/5 mL | 20 mM | |
| Kanamycin | 36.3 mg/5 mL | 15 mM | |
| Explosive | Ammonium Nitrate | 18.0 mg/5 mL | 500 μM |
| Pesticide | Thiram | 36.1 mg/5 mL | 30 mM |
CRITICAL: In the present work, throughout the experiment, there were no chemical signatures of impurities traced in the spectral region of our interest. Preparing stock solutions of all the analyte molecules was done in the fume hood. The glassware and pipettes used in the preparation of the stock solution were appropriately cleaned with acetone and ethanol for 30 min before use. The prepared stock solutions of all the molecules were handled carefully and stored in a vacuum desiccator for further use.
Preparation of analyte solutions
Timing: <1 h for solution preparation
Timing: <30 min for preparationof each analyte molecule
In order to test the versatility of the substrate, we have chosen different classes of probe molecules. For all the procedures below, use an appropriate pipette (10 μL, 100 μL, or 1,000 μL) to measure the solvent during the dilution of the solution for accuracy.
-
•
Soak all glassware in acetone for 30 min.
-
•
Subsequently, soak glassware in ethanol for another 30 min.
-
•
Clean glassware twice with Milli-Q water.
-
•
Dry thoroughly in the oven at approximately 70°C for 12 h before the sample preparation.
-
•
In a fume hood, prepare a 100 μM stock solution of crystal violet.
Dilution of CV stock solution to make 2 mL of each concentration.-
•Make serial dilutions of stock solution to make 10 μM, 5 μM, 1 μM, 100 nM, 10 nM and 1 nM solutions respectively.
-
•
-
•
Prepare DNA bases (adenine/cytosine) of 25 mM /30 mM stock solution in Milli-Q water at 50°C–60°C for better solute dissolution in the solvent.
Dilution of adenine at various concentrations to make 2 mL of each concentration.-
•Make serial dilutions of stock solution to make 1 mM, 100 μM, 50 μM, 10 μM and 100 nM solutions respectively.Dilution of cytosine molecules at various concentrations to make 2 mL of each concentration.
-
•Make serial dilutions of stock solution to make 1 mM, 100 μM, 50 μM, 10 μM, 100 nM and 10 nM solutions respectively.
-
•
-
•
Antibiotics (penicillin G/ampicillin/kanamycin) of 20 mM/20 mM/15 mM stock prepared in Milli-Q water.
Dilution of penicillin G stock solution to make 2 mL of each concentration.-
•Make serial dilutions of stock solution to make 1 mM, 100 μM, 50 μM, 10 μM, 100 nM and 10 nM solutions respectively.Dilution of ampicillin stock solution to make 2 mL of each concentration.
-
•Make serial dilutions of stock solution to make 1 mM, 100 μM, 50 μM, 10 μM, 100 nM and 10 nM solutions respectively.Dilution of kanamycin stock solution to make 2 mL of each concentration.
-
•Make serial dilutions of stock solution to make 1 mM, 100 μM, 50 μM, 10 μM and 100 nM solutions respectively.
-
•
-
•
Ammonium nitrate of 500 μM and thiram of 30 mM stock solutions prepared in Milli-Q water.
Dilution of AN stock solution to make 2 mL of each concentration.-
•Make serial dilutions of stock solution to make 50 μM, 10 μM, 5 μM, 1 μM and 100 nM solutions respectively.Dilution of Thiram stock solution to make 2 mL of each concentration.
-
•Make serial dilutions of stock solution to make 10 μM, 5 μM, 300 nM, 100 nM and 10 nM solutions respectively.Note: Glass vials are sealed with built-in caps and parafilm to avoid leakage. The micropipette was cleaned with acetone and a fresh tip was used for every usage.
-
•
Step-by-step method details
Surface-enhanced Raman detection with the Micro-Raman setup
Timing: <10 min for preparation and <1 min per measurement
-
1.
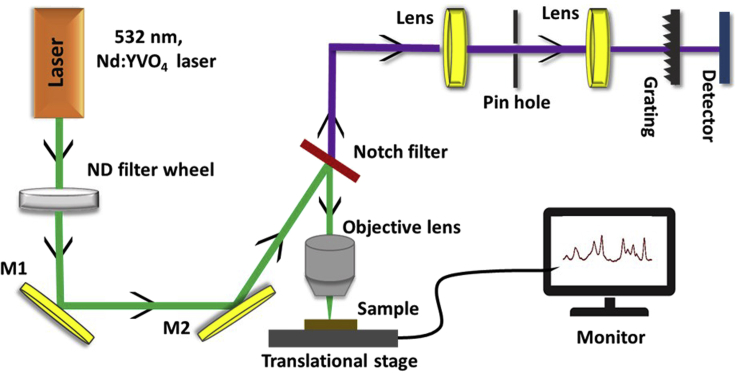
Raman spectra (in the reflection mode) of all the molecules were collected by Horiba LabRam Raman spectrometer with frequency-doubled Nd-YAG, 532 nm laser excitation with 25 mW laser power. The Raman system attached to a microscope with 50× objective and 5 s acquisition time was used for the detection of all the analytes in the present work. Figure 2 shows the schematic representation of Raman setup.
CRITICAL: The sample was focused on using the optical image generated in the LabRam software on the monitor screen.
-
2.
20 μL of interested molecules with desired concentration were drop-casted onto the sliced AuNPs @ AgNDs (∼ 0.5 cm2) substrate for SERS studies.
Note: A different substrate was used for each measurement.
-
3.
A random large area (45 × 45 μm2 for CV, and 100 × 100 μm2 for cytosine) was chosen for mapping, followed by a small area (20 × 20 μm2 for CV, and cytosine) mapping for the analyte CV and cytosine.
Note: 918 cm-1 peak for CV and 792 cm-1 peak for Cytosine were selected, and a mapping window was set to generate a SERS intensity profile image of the selected area.
Figure 2.
Schematic representation of conceptual Raman set-up
All the measurements were performed under the below conditions.
| Instrument | |
| Grating | 1,800 grooves/mm |
| Wavelength of excitation | 532 nm |
| Object of focusing | 50× (for SERS); 10× (for mapping) |
| Detector | CCD detector |
| Spectral acquisition parameter | |
| Acquisition time | 10 s (for SERS); 3 s (for mapping) |
| Time of exposure | 10 s |
| Excitation power | ∼25 mW |
| Beam spot | ∼1.5 μm |
| Sample size | 5 mm2 |
| Detection wavelength range | 400–1,800 cm-1 |
COMSOL simulation for near-field enhancement around AuNP decorated AgNDs substrate
Timing: <3 h per calculation
-
4.
COMSOL version of 5.4 was used for simulation of near field enhancement around AuNPs@AgNDs-3h. Step-by-step implementation of the model on COMSOL is summarized in Figure 3. We have used the RF module with electromagnetic waves and frequency domain physics.
-
5.
Geometry of the AuNPs@AgNDs-3h was created, taking inspiration from the FESEM images. A perfectly matching layer (PML) was defined to truncate the simulation geometry and create an absorbing and non-reflecting layer. This is followed by defining material properties for the Au, Ag, and surrounding medium.
Note: The surrounding medium was defined as air (ε = 1), and the dielectric functions for Au (∼5.27 at 532 nm) and Ag (∼ 11.8 at 532 nm) have been taken from Johnson and Christy’s work.8
-
6.
An incident plane wave polarized in X-direction and propagating in Y-direction with an excitation wavelength of 532 nm was defined. Scattering boundary conditions were defined at the outer layer of the PML.
-
7.
A default physics-controlled mesh in COMSOL was used to describe the geometry, and was further edited to mesh the edges finely. Default solvers in COMSOL was used to solve Maxwell’s equations for the given conditions.
-
8.
Computation time for the model was ∼3 h for the given meshing conditions. The solutions were plotted as a surface plot to visualize the near-field enhancement.
Figure 3.
Schematic of workflow for the COMSOL Simulations performed in this work
Expected outcomes
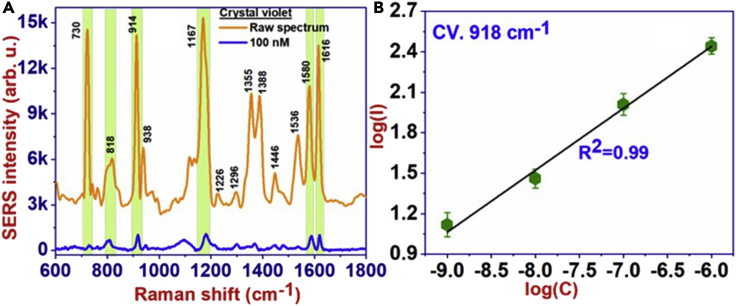
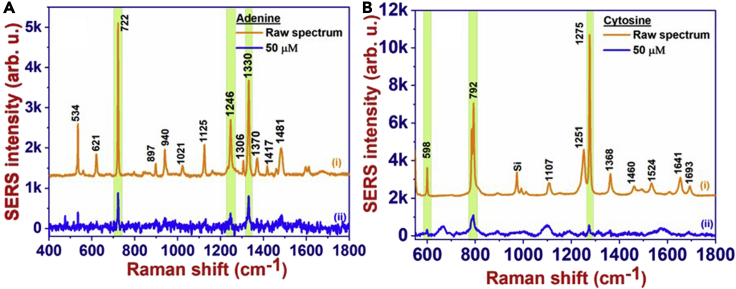
Primarily AuNP@AgNDs-3h substrate (AuNPs decorated at 3 h of deposition) was tested with a CV molecule, which clearly shows the ability to detect CV at concentrations as low as 100 nM (shown in Figure 4A). The observed peaks are assigned to different vibrational modes9 presented in Table 1. This investigation demonstrates the impact and functionality of Ag and Au bimetallic plasmonic metals on SERS signals. Figure 4B illustrates the log (intensity) versus the log (concentration) data with an R2 value of 0.99. Similar detection capabilities were tested for DNA bases (adenine, cytosine) and antibiotics et al. (penicillin G, ampicillin, and kanamycin). Figures 5A and 5B depict the sensitivity of 50 μM concentration compared to the raw (powder/bulk) spectrum of adenine and cytosine, respectively. The linear profiles of adenine and cytosine depicted an R2 value of 0.99. The observed peaks are attributed to different vibrational modes,10 which are presented in Table 1. Furthermore, in the extended detection of antibiotics at a concentration of 50 μM, the modes are compared with the corresponding bulk spectrum shown in Figures 6A–6C. The consistent peak assignments confirmed from the references11,12 are depicted in Table 1.
Figure 4.
SERS sensitivity studies of CV
(A and B) (A) SERS sensitivity of CV at 50 μM concentrations in compared to raw spectrum of CV, and (B) Linearity plot of log(concentration) versus log (intensity). Error bars are added at each concentration of the analyte by counting the standard deviation of intensity variation at 10–15 random positions of the substrate.
Table 1.
Assignment to vibrations of the various molecules used in the present study
| Analytes | Peak position (cm-1) | Assignments |
|---|---|---|
| Crystal violet9 | 918 | Ring skeletal vibration |
| 1181 | Ring C−H bending a | |
| 1379 | N-phenyl stretching | |
| 1530 and 1621 | Ring C−C stretching | |
| Adenine10 | 534 | Wagging C2-H, N9-H13 |
| 621 | Ring deformation | |
| 722 | Ring breath | |
| 1125 | Stretching C8-N9, bending N9-H, C8-H | |
| 1246 | Rocking NH2, stretching C5-N7, N1-C2, C2-N3 | |
| 1330 | Stretching C5-N7, N1-C2, C3-N3, C5-C6, bending C2/8-H | |
| 1481 | Stretching C2-N3, N1-C6, bending C2-H, scissoring NH2 | |
| Cytosine10 | 604 | Bending C2=O |
| 792 | Ring breathing | |
| 1115 | C=O | |
| 1251 | Ring stretching C-N | |
| 1368 | Bending N1-H, C5-H, C6-H | |
| 1524 | Stretching N3-C4-C5 | |
| 1641 | Stretching C2=O | |
| Penicillin-G11,12 | 985 | β-lactam ring |
| 1586 | Stretching of C-O | |
| 1668 | C=O |
Figure 5.
SERS sensitivity studies of DNA-bases
(A and B) SERS sensitivity of 50 μM concentrations as compared to the bulk spectrum of (A) Adenine, and (B) Cytosine.
Figure 6.
SERS sensitivity data of antibiotics
(A–C) SERS sensitivity of 50 μM concentrations as compared to the bulk spectrum of (A) Penicillin G, (B) Ampicillin, and (C) Kanamycin.
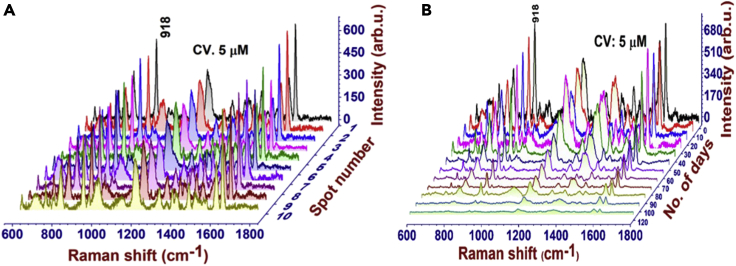
Reproducibility and durability are essential parameters to evaluate the substrate efficacy in molecular detection. As shown in Figure 7A, the spectral reproducibility was tested by performing the SERS measurements at 10 random spots on the sample surface, which shows spectral reproducibility with lower Relative Standard Deviation (RSD) values (∼ 8% for cytosine, 7% for CV). The durability of the substrate was tested in due course of 120 days of ambient exposure (shown in Figure 7B). We exposed 10 samples to an ambient environment; each sample was utilized for analyzing CV at various intervals of time. Finally compared the spectra of CV at each interval of time to examine the stability of the substrate. The quality of the substrate was keenly monitored during the time of exposure by estimating the signal strength of CV. It is observed that there is no intensity drop up to four-five weeks of exposure. After that, a gradual intensity drop was observed for a prolonged duration.
Figure 7.
Reproducibility and stability data of the substrate
(A and B) (A) Spectra reproducibility of CV molecule at random spots of a sample surface, and (B) Time stability (durability) of substrate tested for 120 days by CV detection.
The homogeneity of the overall SERS substrate was tested by performing Raman mapping of Cytosine at a randomly chosen large area of 20 × 20 μm2 with 4 μm spacing with a 10× microscope objective, 3 s acquisition time. The SERS mapping image is generated by assigning a false color to the intensity of the selected mapping Raman mode of the analyte. This experiment (shown in Figure 8) shows better signal reproducibility of the substrate over the large surface area.
Figure 8.
SERS substrate mapping studies
(A–C) (A) Selected large-area Raman mapping (45 × 45 μm2) of SERS substrate with 532 nm laser excitation, 10× microscope objective, 25 mW laser power with CV as probe molecule for 918 cm-1 Raman mode with 3 s acquisition time, averaged over 3 spectra (B) Small area (20 × 20 μm2) SERS mapping of CV, and (C) corresponding representative spectra of the mapping region.
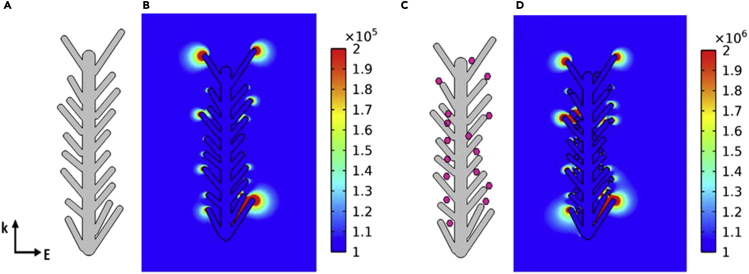
Further, the near-field enhancement generated on the fabricated AuNPs@AgNDs-3h structure was extracted by performing COMSOL simulations. The details are illustrated in Figure 9. The detailed COMSOL simulation results are shown in Figure 9A [geometry of Ag dendrites constructed], 9(b) [near-field simulation of the Ag dendrites showing enhancement at the tips], 9(c) [geometry of AuNPs@Ag nano dendrites constructed], and 9(d) [near-field simulation of AuNPs@AgNDs-3h clearly showing additional enhancement relative to the plain Ag dendrites].
Figure 9.
Results of COMSOL simulation showing
(A–D) (A) Geometry of Ag dendrites constructed, (B) Near-field simulation of the AgNDs showing enhancement at the tips, (C) Geometry of AuNPs@AgNDs-3h constructed and (D) Near-field simulation of AuNPs@AgNDs-3h clearly showing additional enhancement relative to the plain AgNDs.
Quantitative analysis of SERS signals
The relationship between intensity and analyte concentration is non-linear. We plotted log (intensity) versus log (concentration) plots, which show better R2 values following the lower concentration levels.
At each concentration, the error bars are included [shown in Figure 4B]. The added error bar denotes the standard deviations of intensity variations measured at 10–15 random positions of a substrate for all the concentrations. The peak intensity at each concentration has been determined using the Lorentz fitting. We found that the deviations in the spectrum are under 9%.
CRITICAL: We have drop-casted 20 μL of analyte on the AuNPs@AgNDs-3h substrate. Here, it is emphasized that not all the molecules under the laser spot contribute to the resultant Raman signal. Only those molecules near the hot-spot regions are responsible for signal enhancement.
Limitations
SERS is sensitive to experimental conditions like laser power, acquisition time, focusing conditions, distribution of molecules on the sample surface and inhomogeneous distribution of hotspots. This leads to inherent signal fluctuations causing poor reproducibility.
Preparation of AuNPs Capped Silver dendrites is sensitive to ambient pressure, temperature and humidity.
Drop casting could lead to different patterns depending on the solvent, the evaporation rate and temperature. This often leads to the coffee ring effect which has to be carefully used to the advantage in SERS.
In SERS investigation, the excitation at 532 nm excitation tends to damage the sample surface and also induce fluorescence competing with the SERS signal.
Troubleshooting
Problem 1
During the formation of AgNDs on the Si wafer, the back side of the Si wafer could also have been etched/reacted, which makes difficult to evaluate the density/volume fraction of AgNDs formation at a particular AgNO3 concentration (step-3).
Potential solution
The back side of the Si wafer has been covered with acid resistant tape. The formation of AgNDs is considerably low on tape.
Problem 2
The decoration of AuNPs on AgNDs at various molar concentrations (i.e., 0.05 mM, 0.1 mM, 0.5 mM, 1 mM, 1.5 mM, 3 mM, 5 mM, 7 mM, respectively) of gold seed solution, which will increase the density of AuNPs distribution. At a higher molar concentration of about 7 mM, the distribution of AuNPs completely concealed the nature of the dendritic structure, which will not support the SERS sensing suppressing the Ag performance (step-5).
Potential solution
After analyzing the data at all molar concentrations of Au seed solution, we deliberately chose the optimized AuNPs deposition at 1 mM concentration with isolated and uniform distribution.
Problem 3
The prepared silver nano-dendrites get oxidized easily during prolonged storage (∼120 days) under an ambient environment. The AgNDs substrate turns to deep silver when it gets strong oxidation, which will affect the enhancement of the Raman signal (step-6).
Potential solution
The stability of AgNDs could be improved firmly by storing the samples in a high vacuum desiccator immediately after the preparation. Moreover, the oxidation effect can be controlled or overruled by passivating the AgNDs with Au nanoparticles or other 2D materials. In the present protocol, we found that oxidation was significantly controlled by decorating a high density of AuNPs.
Problem 4
We faced difficulty detecting the analyte at trace-level concentrations due to the sparse distribution of molecules distributed over the sample surface. Basically, the trace level concentration is defined as a measurement below one ppm (parts per million) while using this term in another way to describe the lower concentration of analyte detected (i.e., which is difficult to detect), in the present work. We detected the analytes in nM (trace) concentrations.
Potential solution
Initially, proceed with real-time display analysis (RTD) for 5 s to find the signature peak. Optimistically with the help of RTD parameters, we tried to get the Raman features of the detected analytes.
Resource availability
Lead contact
Added information/clarification about the protocol, requests for resources, and material should be directed and will be accomplished by prof. Soma Venugopal Rao (soma_venu@uohyd.ac.in) and Dr. V.S. Vendamani (mani.vsvp@gmail.com).
Materials availability
This work has not released any new products.
Acknowledgments
V.S.V. thanks UGC, New Delhi, for fellowship in the form of DS Kothari postdoctoral fellowship (sanction order no. F.4-2/2006 [BSR]/PH/19-20/0008). We acknowledge Prof. V.V. Ravi Kanth Kumar, Department of Physics, Pondicherry University for support and help in the analysis and measurements of XPS. V.R.S. thanks DRDO, India, for financial support (# ERIP/ER/1501138/M/01/319/D [R&D]). V.R.S. and S.V.S.N.R. thank the University of Hyderabad (UoH) for financial support from the Institute of Eminence (IoE) with a project (no. UOH/IOE/RC1/RC1-20-016). The IoE scheme was granted to the University by the Ministry of Education, Government of India, vide MHRD notification F11/9/2019-U3(A). V.R.S. thanks the Director, ACRHEM for his support and encouragement during various discussions.
Author contributions
V.S.V. planned the work, prepared the samples, performed the Raman and other characteristic investigations, and wrote the original draft. R.B. performed the Raman mapping and COMSOL simulations while writing and editing the manuscript. M.M.N. helped with XPS measurements and analysis of the data. S.V.S.N.R. provided an idea on conceptualization and reviewed/edited the article. V.R.S. conceived the project idea, conceptualized and supervised this work, participated in discussions of the obtained results, corrected the original draft, and acquired funding support.
Declaration of interests
The authors declare no competing interests.
Data and code availability
The data reported in the present manuscript can be shared by the corresponding author upon reasonable request.
The codes used in this work can be shared upon reasonable request.
References
- 1.Vendamani V.S., Beeram R., Neethish M.M., Rao S.V.S.N., Rao S.V. Wafer-scale silver nanodendrites with homogeneous distribution of gold nanoparticles for biomolecules detection. iScience. 2022;25:104849. doi: 10.1016/j.isci.2022.104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandarenka H.V., Khinevich N.V., Burko A.A., Redko S.V., Zavatski S.A., Shapel U.A., Mamatkulov K.Z., Vorobyeva M.Y., Arzumanyan G.M. 3D silver dendrites for single-molecule imaging by surface-enhanced Raman spectroscopy. ChemNanoMat. 2021;7:141–149. doi: 10.1002/cnma.202000521. [DOI] [Google Scholar]
- 3.Cheng Z.Q., Li Z.L., Luo X., Shi H.Q., Luo C.L., Liu Z.M., Nan F. Enhanced second harmonic generation by double plasmon resonances in mesoscale flower-like silver particles. Appl. Phys. Lett. 2019;114:011901. doi: 10.1063/1.5079241. [DOI] [Google Scholar]
- 4.Wang X., Liu C., Gao C., Yao K., Masouleh S.S.M., Berté R., Ren H., Menezes L.D.S., Cortés E., Bicket I.C., et al. Self-constructed multiple plasmonic hotspots on an individual fractal to amplify broadband hot electron generation. ACS Nano. 2021;15:10553–10564. doi: 10.1021/acsnano.1c03218. [DOI] [PubMed] [Google Scholar]
- 5.Huang J., Ma D., Chen F., Bai M., Xu K., Zhao Y. Ag nanoparticles decorated cactus-like Ag dendrites/Si nanoneedles as highly efficient 3D surface-enhanced Raman scattering substrates toward sensitive sensing. Anal. Chem. 2015;87:10527–10534. doi: 10.1021/acs.analchem.5b02788. [DOI] [PubMed] [Google Scholar]
- 6.Yin H.J., Chen Z.Y., Zhao Y.M., Lv M.Y., Shi C.A., Wu Z.L., Zhang X., Liu L., Wang M.L., Xu H.J. Ag@Au core-shell dendrites: a stable, reusable and sensitive surface enhanced Raman scattering substrate. Sci. Rep. 2015;5:14502–14509. doi: 10.1038/srep14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vendamani V.S., Rao S.V.S.N., Pathak A.P., Soma V.R. Robust and cost-effective silver dendritic nanostructures for SERS-based trace detection of RDX and ammonium nitrate. RSC Adv. 2020;10:44747–44755. doi: 10.1039/d0ra08834j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson P.B., Christy R.W. Optical constant of the nobel metals. Phys. Rev. B. 1972;6:4370–4379. doi: 10.1103/PhysRevB.6.4370. [DOI] [Google Scholar]
- 9.Fateixa S., Nogueira H.I.S., Trindade T. Surface-enhanced Raman scattering spectral imaging for the attomolar range detection of crystal violet in contaminated water. ACS Omega. 2018;3:4331–4341. doi: 10.1021/acsomega.7b01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madzharova F., Heiner Z., Gühlke M., Kneipp J. Surface-enhanced hyper-Raman spectra of adenine, guanine, cytosine, thymine, and uracil. J. Phys. Chem. C Nanomater. Interfaces. 2016;120:15415–15423. doi: 10.1021/acs.jpcc.6b02753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wali L.A., Hasan K.K., Alwan A.M. Rapid and highly efficient detection of ultra-low concentration of penicillin G by gold nanoparticles/porous silicon SERS active substrate. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019;206:31–36. doi: 10.1016/j.saa.2018.07.103. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Li X., Yang M., Yang L., Han X., Jiang X., Zhao B. High sensitive detection of penicillin G residues in milk by surface-enhanced Raman scattering. Talanta. 2017;167:236–241. doi: 10.1016/j.talanta.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Wiórkiewicz-Kuczera J., Karplus M. Ab initio study of the vibrational spectra of N9-H and N7-H adenine and 9-methyladenine. J. Am. Chem. Soc. 1990;112:5324–5340. doi: 10.1021/ja00169a045. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data reported in the present manuscript can be shared by the corresponding author upon reasonable request.
The codes used in this work can be shared upon reasonable request.