Abstract
The remarkable elastic properties of polymers are ultimately due to their molecular structure, but the relation between the macroscopic and molecular properties is often difficult to establish, in particular for (bio)polymers that contain hydrogen bonds, which can easily rearrange upon mechanical deformation. Here we show that two-dimensional infrared spectroscopy on polymer films in a miniature stress tester sheds new light on how the hydrogen-bond structure of a polymer is related to its viscoelastic response. We study thermoplastic polyurethane, a block copolymer consisting of hard segments of hydrogen-bonded urethane groups embedded in a soft matrix of polyether chains. The conventional infrared spectrum shows that, upon deformation, the number of hydrogen bonds increases, a process that is largely reversible. However, the 2DIR spectrum reveals that the distribution of hydrogen-bond strengths becomes slightly narrower after a deformation cycle, due to the disruption of weak hydrogen bonds, an effect that could explain the strain-cycle induced softening (Mullins effect) of polyurethane. These results show how rheo-2DIR spectroscopy can bridge the gap between the molecular structure and the macroscopic elastic properties of (bio)polymers.
The unique strength and resilience of polymeric materials such as plastics and biopolymer networks finds its origin in the molecular-scale structural changes that the polymer chains undergo when subjected to deformation. A detailed understanding of the connection between the macroscopic and microscopic properties of polymers is essential, not only for predicting the mechanical properties of synthetic polymers but also for understanding the molecular origin of dysfunctional polymer systems, such as occur in for instance collagen-related diseases. The most straightforward way to investigate the molecular origin of polymer elastic response is to directly observe the changes in molecular structure induced by externally applied strain. Such experiments have used a range of different structural probing methods, notably X-ray diffraction1−3 but also Raman, infrared (IR), and visible spectroscopy.4−11
In the case of hydrogen-bonded (H-bonded) polymer networks, the strain-induced structural changes generally involve rearrangement of the hydrogen-bonds (H-bonds) between molecular groups of adjacent (bio)polymer chains. Among the above-mentioned techniques, IR spectroscopy is very suitable to investigate such rearrangements, since the frequencies and line shapes of the vibrational transitions contain detailed information on the hydrogen-bond structure.12 Combining rheology with infrared spectroscopy (rheo-IR) to study the molecular changes in polymers under applied strain (Figure 1A) has provided a detailed molecular picture of the strain-induced molecular rearrangements in a broad range of polymers.4−9 However, infrared absorption spectra are often rather congested, and it can be difficult to disentangle overlapping vibrational bands. Although the absorption frequency is often a good indicator of the local structure and/or environment, it is generally difficult to derive unambiguous conclusions from the frequencies alone, since they are determined by more than one effect (e.g., the conformation and solvent interactions). Furthermore, with conventional IR spectroscopy, inhomogeneous spectral broadening (due to a distribution of transition frequencies) cannot be easily separated from homogeneous spectral broadening (caused by fast frequency fluctuations of individual vibrations).
Figure 1.
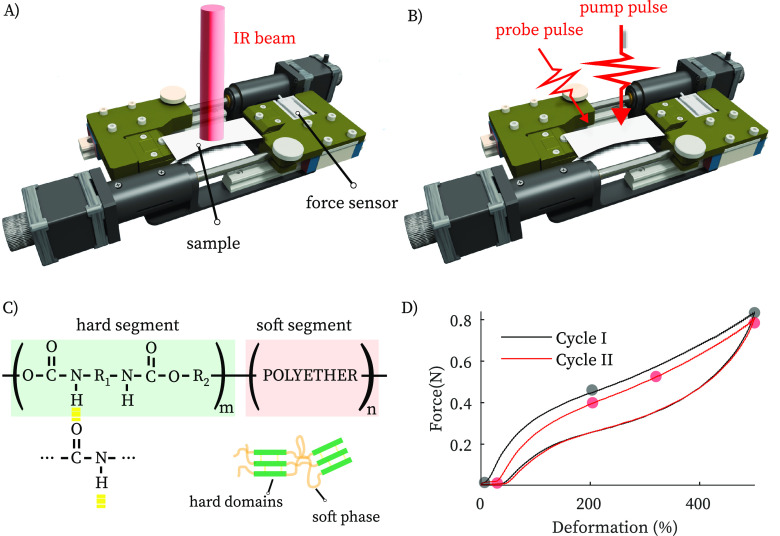
(A, B) Schematic of rheo-IR and rheo-2DIR. (C) Structure of polyurethane, a block copolymer composed of soft and hard segments. The urethane groups in the hard segments form strong hydrogen bonds that act as physical cross-links. Because of the different polarity and chemical nature, the soft and hard segments separate, leading to the formation of hard domains embedded in a soft phase. (D) Stress–strain curve of a TPU film at a strain rate of 8 s–1 with a strain step-size of 3%. The stress–strain curve in the second cycle is more compliant than in the first cycle (strain softening). Filled circles indicate the deformations at which rheo-2DIR was performed.
These challenges can be addressed by means of two-dimensional infrared (2DIR) spectroscopy, which makes it possible to separate overlapping spectral bands, measure vibrational couplings, and separate the homogeneous and inhomogeneous contributions to the line broadening.13 Inspired by rheo-IR spectroscopy, we here combine two-dimensional infrared spectroscopy with rheometry by inserting a miniature universal stress tester into a 2DIR setup (rheo-2DIR). We use this new method to investigate strain-induced changes in the H-bond distribution of thermal polyurethane, one of the most commonly used polymers, that exhibits strain-cycle induced softening behavior that is believed to be related to changes in the H-bond structure.2
A detailed description of the 2DIR setup can be found in refs (14) and (15). Briefly, we use an amplified Ti:sapphire laser combined with an optical parametric amplifier and difference-frequency generation to generate tunable mid-IR pulses (∼20 μJ, ∼ 6100 nm) with a spectral width of 150 cm–1 (fwhm) at 1 kHz repetition rate. The IR beam is split into a probe and reference beam (each 5%), and a pump beam (90%) that is aligned through a Fabry-Pérot interferometer. The pump and probe beams are overlapped in the sample (∼250 μm focal diameter), and the transmitted spectra of the probe pulse in the presence and absence of the pump pulse are recorded with a 32-pixel mercury cadmium telluride array. The pump and probe polarizations are at the magic angle (54.5°) to obtain polarization-independent spectra.13 In the rheo-2DIR setup (Figure 1B), the polymer films are clamped on both sides in a miniature home-built stress tester inserted in the 2DIR setup, and controlled deformations are applied by moving the two clamps in opposite directions with 2 μm precision, using steppermotors (Physik Instrumente). The force is measured with a precision of 0.001 N using a force sensor (KD34s, ME-Systeme) positioned on one of the clamps. Polyurethane films were purchased in the form of polyurethane condoms from Protex (France) and Sagami (Japan). To remove the lubricant, the samples were wiped with a clean tissue and subsequently dried using a nitrogen flow. The tissue was partially wet with ethanol to ensure a complete removal of the lubricant; no experimental dependence on the cleaning procedure was found (see Supporting Information). The film thickness was 31 ± 5 μm (Protex 002) and 18 ± 5 μm (Sagami 001).
Thermoplastic polyurethane (TPU) is a block copolymer composed of urethane- and polyether-based segments (Figure 1C). At room temperature, the polyether (“soft”) domains are above their glass transition temperature, and give TPU its rubber-like behavior; the polyurethane (“hard”) domains are below their glass or melt transition temperature and are believed to give rise to the hysteresis, permanent deformation, the modulus and tensile strength of a particularTPU formulation.16 The mechanical response to deformation of TPU involves changes in the arrangement and strength of the H-bonds formed between the urethane links within hard segments and between the urethane links of hard and soft segments. In particular, the stress-softening behavior upon recovery of zero-stress condition after deformation (Mullins effect) is believed to be connected to the disruption of weak H-bonds between hard and soft domains.2
Figure 1D shows stress–strain measurements where we deform a thin polyurethane film up to a final strain of 500%. We observe an elastically rigid response up to 100% (elastic regime) and then a more compliant response at higher strain (strain softening), which are consistent with descriptions based on rubber elasticity theory.17 Above 400%, the polyurethane again stiffens (strain hardening), due to the limited extensibility of polymer chains as it approaches the fracture point. The mechanical response of the sample during the second cycle is much more compliant compared to the first cycle. This strain-softening behavior (the Mullins effect)16 has been extensively investigated, but its molecular origins are still not completely understood.1,2 Here, we show that rheo-2DIR spectroscopy can shed new light on this phenomenon by revealing how the H-bond distribution changes upon deformation.
Figure 2A shows the conventional IR spectrum of the polyurethane film (Sagami 001) in the carbonyl-stretch region. We observe two intense bands at 1703 and 1733 cm–1, which are due to the hydrogen-bonded (1703 cm–1) and free (1733 cm–1) carbonyl groups.18−22 As we mentioned above, since these materials are poly(ether–urethane)s, H-bonds of different strength can form between urethane groups and urethane N–H and ether groups. Clearly, however, ether groups cannot H-bond to the urethane carbonyls. The lowering of the CO-stretch frequency upon H-bond formation is a well-known effect, that can be used as a sensitive probe of the H-bond structure. The peak of the hydrogen-bonded carbonyl groups is much broader than that of the free CO groups, and its shape reflects the distribution of H-bond strengths in the sample (convoluted with the homogeneous line shape). Figure 2B shows IR spectra of polyurethane film at different strains (the spectra have been normalized to the spectral area to correct for sample thinning due to stretching). Going from 0 to 200% strain, the intensity of the free-carbonyl peak decreases by ∼10%, while the intensity of the hydrogen-bonded carbonyl increases by a similar amount. At higher (500%) deformation, the hydrogen-bonded carbonyl peak slightly decreases in intensity and broadens, while the free carbonyl does not show a significant change with respect to 200%. This is more clearly visible in Figure 2C, where we plot the normalized intensity as a function of frequency and strain. We observe that the intensity of the hydrogen-bonded carbonyl increases when TPU enters the strain-softening regime, while it decreases when approaching the strain-hardening regime. In the strain-softening regime, stress is relieved by the conformational rearrangement of the chains, leading to a strain-induced ordering in the network. The increase in the number of hydrogen bonds at moderate strain reflects this increased ordering because the enhanced alignment of the polyurethane chains facilitates H-bond formation between urethane groups (this behavior is similar to strain-induced crystallization in natural rubber20,21,23,24). At higher strain, the finite extensibility of the chains leads to an upturn of the stress–strain curve. When approaching the maximum extension of the chain, hydrogen bonds are weakened and broken, leading to a decrease in intensity of the hydrogen-bonded carbonyl vibration in the strain-hardening region.25 To summarize, rheo-IR shows that in the strain-softening regime, the number of H-bond increases because of an enhanced alignment of the chains, while it decreases in the strain-hardening regime because hydrogen bonds are weakened and broken while resisting extension.
Figure 2.
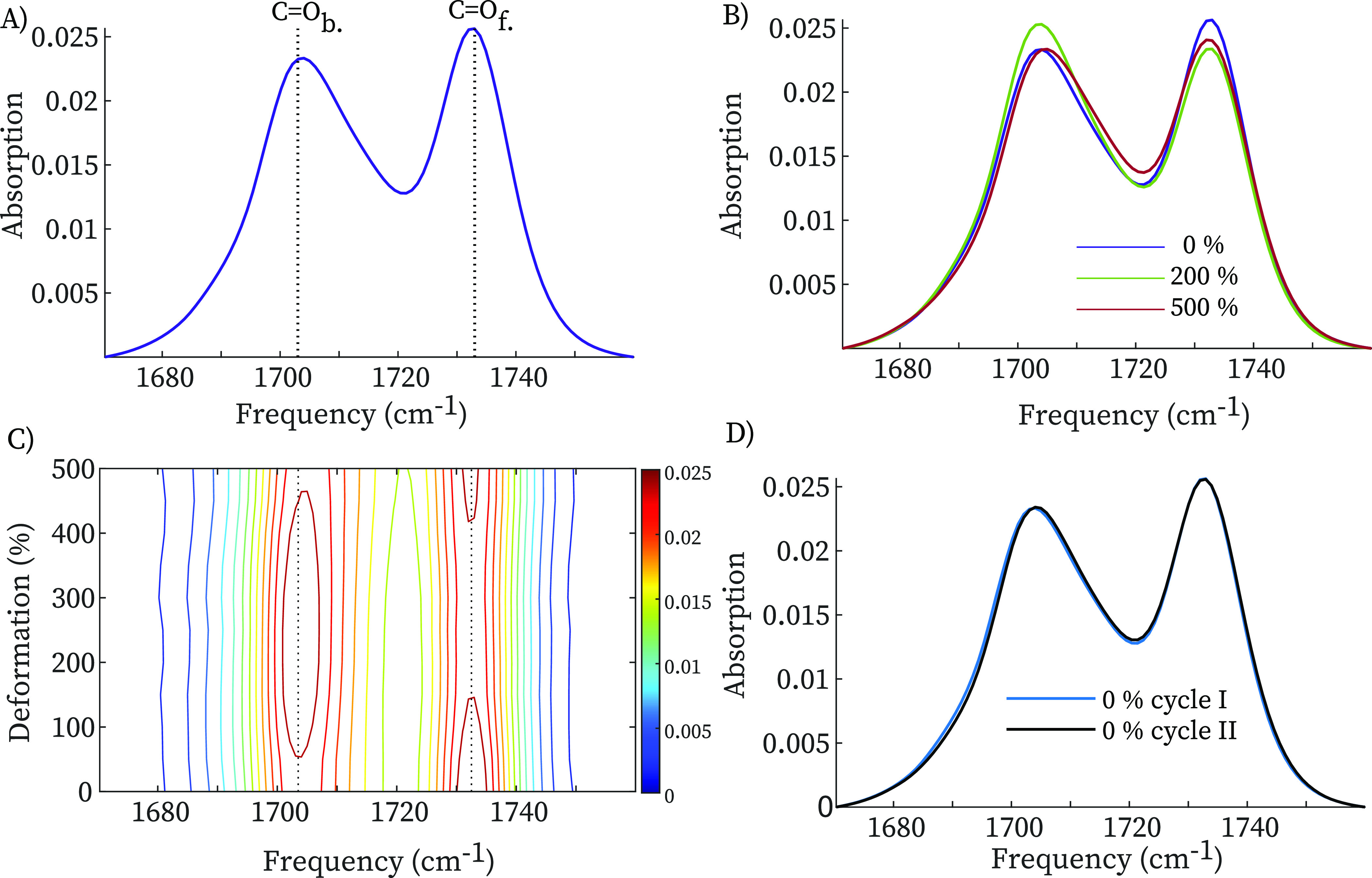
(A) IR absorption spectra of TPU film at 0% deformation. (B) IR spectra of TPU at 0, 200, 500% deformation normalized with respect to the respective total spectrum area to compensate for thinning effect. (C) 2D gradient FT-IR map with respect to the deformation percentage. (D) IR spectra of before deformation and upon recovery of the zero-stress condition.
After unloading the sample, the infrared spectrum is very similar to the one observed before the deformation cycle (Figure 2D) with the relative intensity between the two carbonyl bands returning to its value before deformation. This is somewhat surprising given that the macroscopic elastic properties have changed significantly (Figure 1D). However, subtle changes in the vibrational lineshapes are generally difficult to observe in the linear IR spectrum, because the wings of the absorption bands are difficult to distinguish from the background absorption; and for strained polymer samples the situation is worsened since the deformation cycle causes changes in the background spectrum. To investigate strain-induced changes in the line shape in detail, we therefore use 2DIR spectroscopy. Two important advantages of 2DIR with respect to conventional IR spectroscopy are (1) the homogeneous and inhomogeneous contributions to the line shape are observed separately and (2) the background absorption does not contribute to the 2DIR signal (since the 2DIR response scales as μ4, while conventional IR scales as μ2, where μ is the transition dipole moment of the vibrational transition).13Figure 3A shows the 2DIR spectrum of the hydrogen-bonded carbonyl groups at 0% strain. In pump–probe 2DIR spectroscopy, we use a tunable narrow-band (10 cm–1 fwhm) pump pulse to excite molecular vibrations (in this case the CO-stretch vibration) at a specific frequency νpump, and measure the pump-induced change in absorption ΔA at all frequencies using a broad-band probing pulse that is detected in frequency-resolved manner. The pump polarization is set at an angle of ∼54.5◦ with respect to the strain direction and the probe polarization. Plotting ΔA(νprobe, νpump) we obtain two-dimensional IR spectra at a specific time delay, Tw, between pump and probe pulses.13 In Figure 3A, positive ΔA is plotted as red contour areas and negative ΔA as blue contour areas.
Figure 3.
(A) 2DIR spectrum of TPU at zero stress condition at a Tw = 1 ps. (B) 2DIR spectra and nodal line slopes at zero stress and upon recovery of zero-stress condition after a deformation up to 500% at a Tw = 1 ps. The nodal line slope at zero stress is shifted along the probe axis in the right 2DIR spectrum for comparison.
The resonant excitation of the v = 0 → 1 transition by the pump pulse causes a decrease in absorption at the v = 0 → 1 transition (due to depletion of the v = 0 state and v = 1 → 0 stimulated emission) and an increase in absorption at the v = 1 → 2 transition frequency.13 The dependence of the 2DIR response on the pump frequency is a measure of the inhomogeneous broadening of the IR band, and the two-dimensional line shape can be used to disentangle inhomogeneous and homogeneous contributions to the line broadening.13 Since the inhomogeneous line shape reflects the distribution of H-bond strengths, we can thus investigate changes in this distribution caused by the strain cycle. The simplest and most robust parameter to characterize the extent of inhomogeneous broadening is the inverse value of the slope (“nodal line slope”, NLS) of the 2DIR contours:26,27 in the limiting case of purely homogeneous broadening, the ΔA contours are vertically aligned (zero nodal line slope, no dependence of the response on the pump frequency except for overall amplitude), whereas in the case of purely inhomogeneous broadening the slope is 1.
Figure 4A shows the nodal line slope values during the deformation cycle, which were measured at a Tw = 1 ps (no time-dependence of the NLS was observed, see Supporting Information). Samples were strained up to desired deformation and allowed to relax for 10–15 min before measuring (measurement time 1–2 h). In Figure 3B we compare the 2DIR spectra at zero stress and upon recovery of zero-stress after a deformation up to 500%, where we indicate the nodal line slopes as determined from global least-squares fits. After the deformation cycle, the nodal line slope has decreased, indicating a decrease in the inhomogeneous width. To be certain that the observed subtle change in slope is significant, we have repeated several experiments on different TPU samples (Figure 4B). The NLS at zero-stress condition decreases from 0.49 ± 0.01 to 0.43 ± 0.03 upon deformation, where values and errors represent the mean and standard deviations obtained over four independent experiments on different samples. Similar decrease is observed in the central line slope, CLS,27 which represents an alternative observable of the H-bond inhomogeneity (see Supporting Information). When comparing experiments on Protex 002 and Sagami 001, we find that the decrease of the slope after the deformation cycle is reproducible, although the values of the nodal line slopes show small variations. Repeating the deformation cycle on the same sample does not further change the slope (see Supporting Information), similar to the stress–strain curve, which also tends to stabilize, with most of the softening occurring in the first deformation cycle. The decrease of the NLS indicates that the H-bond distribution has become narrower after a deformation cycle, suggesting that some of the H-bonds that are broken during loading are not reformed upon recovery of zero-stress condition. This is confirmed by Figure 4C, which shows slices of the 2DIR spectrum taken along the diagonal at which the negative (ΔA < 0) signal is maximal. It is difficult to observe this narrowing in the conventional IR spectrum because of the changes in the background absorption upon deformation (whereas in the 2DIR spectrum the background contribution is eliminated because the signal scales nonlinearly with the absorption cross section). After the deformation cycle, the diagonal carbonyl peak has become narrower, mostly due to disappearance of intensity at the high-frequency side. Thus, the narrowing of the H-bond distribution after a deformation cycle is due to the disappearance of weak hydrogen bonds.
Figure 4.

(A) Nodal line slope during a deformation cycle. (B) Nodal line slopes before and after a deformation cycle in 5 independent experiments. In the thicker samples (Protex 002), we can only measure 2DIR spectra at 200% strain (for lower deformation the IR absorption is too high). (C) Comparison of bleach diagonal slices extracted by 2DIR spectra in 3B. Error bars represent +/– one standard deviation.
The rheo-2DIR results show that a strain cycle causes an irreversible reduction in the number of weak hydrogen bonds in TPU. These weak H-bonds are found mostly in the amorphous regions between the hard and soft segments, where the urethane NH groups form hydrogen bonds mostly to polyether instead of carbonyl groups.28 Recent X-ray studies have attributed the strain-softening behavior of TPU to strain-induced softening of interfacial “fuzzy” regions between the hard and soft domains.1,2 Our results seem to confirm this idea, and they provide a molecular-level explanation of the strain-softening of the fuzzy regions. This picture is confirmed by the lower nodal line slope observed in 2DIR spectra recorded after deforming the sample at high (∼100 °C) temperature, which probably also destroys the weak hydrogen bonds in the unordered regions (see Supporting Information). The presence and the amount of the fuzzy interface will likely depend on the degree of mixing between the hard and soft phases, which is in turn determined by different experimental parameters, such as segments length, chemical composition, and thermal treatment.29−32 A different degree of mixing will thus affect the initial inhomogeneity and the amplitude of the decrease upon deformation, which probably explains the differences between the examined samples (Sagami 001 and Protex 002).
To conclude, rheo-2DIR spectroscopy sheds new light on the molecular processes that underlie the Mullin effect in polyurethane. Based on these first experiments, we believe that rheo-2DIR can be a valuable addition to the existing physical methods for studying the elastic properties of materials. In particular, comparing the relative amplitudes of the rheo-IR and rheo-2DIR spectra33 can provide direct information on the degree of interchain coupling as a function of strain in biopolymer networks, where the mechanical properties are modulated by the formation and/or disruption of H-bonds.34−37 Rheo-2DIR can thus help to improve our understanding of the molecular origin of the elastic response of not only synthetic but also, in particular, biological polymer-based materials.
Acknowledgments
This publication is part of the project (with Project Number VI.Veni.212.240) of the research programme NWO Talent Programme Veni 2021, which is financed by the Dutch Research Council (NWO). F.C. acknowledges financial support from The Netherlands Organization for Scientific Research (NWO) (Grant Number: 680-91-13). The authors thank the Tecnology Center of the Universiy of Amsterdam for technical support and the reviewers of the article for their useful comments and suggestions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.2c03109.
Sample characterization, time dependence of NLS, CLS, effect of cleaning procedures, IR spectrum of Protex 002, IR spectra of Sagami 001, NLS cycles on Protex 002, and temperature effect on nodal line slope (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sui T.; Baimpas N.; Dolbnya I. P.; Prisacariu C.; Korsunsky A. M. Multiple-length-scale deformation analysis in a thermoplastic polyurethane. Nat. Commun. 2015, 6, 1–9. 10.1038/ncomms7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui T.; Salvati E.; Ying S.; Sun G.; Dolbnya I. P.; Dragnevski K.; Prisacariu C.; Korsunsky A. M. Strain softening of nano-scale fuzzy interfaces causes Mullins effect in thermoplastic polyurethane. Sci. Rep. 2017, 7, 916. 10.1038/s41598-017-00904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Wang H.; Xiong B.; Pöselt E.; Eling B.; Men Y. Destruction and Reorganization of Physically Cross-Linked Network of Thermoplastic Polyurethane Depending on Its Glass Transition Temperature. ACS Appl. Polym. Mater. 2019, 1, 3074–3083. 10.1021/acsapm.9b00729. [DOI] [Google Scholar]
- Siesler H. W.Analysis/Networks/Peptides; Springer Berlin Heidelberg: Berlin and Heidelberg, Germany, 1984; pp 1–77. [Google Scholar]
- Hofmann G. R.; Sevegney M. S.; Kannan R. M. A Rheo-Optical FTIR Spectrometer for Investigating Molecular Orientation and Viscoelastic Behavior in Polymers. International Journal of Polymer Analysis and Characterization 2004, 9, 245–274. 10.1080/10236660490920237. [DOI] [Google Scholar]
- Fischer W. B.; Pötschke P.; Pompe G.; Eichhorn K.-J.; Siesler H. W. Rheo-optical fourier transform infrared spectroscopy of polyurethanes and their blends with polyolefins. Macromol. Chem. Phys. 1997, 198, 2057–2072. 10.1002/macp.1997.021980705. [DOI] [Google Scholar]
- Radebe N. W.; Fengler C.; Klein C. O.; Figuli R.; Wilhelm M. Rheo-IR: A combined setup for correlating chemical changes via FTIR spectroscopy and rheological properties in a strain-controlled rheometer. J. Rheol. 2021, 65, 681–693. 10.1122/8.0000251. [DOI] [Google Scholar]
- Boulet-Audet M.; Vollrath F.; Holland C. Rheo-attenuated total reflectance infrared spectroscopy: a new tool to study biopolymers. Phys. Chem. Chem. Phys. 2011, 13, 3979–3984. 10.1039/c0cp02599b. [DOI] [PubMed] [Google Scholar]
- Papadopoulos P.; Sölter J.; Kremer F. Structure-property relationships in major ampullate spider silk as deduced from polarized FTIR spectroscopy. Eur. Phys. J. E 2007, 24, 193–199. 10.1140/epje/i2007-10229-9. [DOI] [PubMed] [Google Scholar]
- Varol H. S.; Meng F.; Hosseinkhani B.; Malm C.; Bonn D.; Bonn M.; Zaccone A.; Parekh S. H. Nanoparticle amount, and not size, determines chain alignment and nonlinear hardening in polymer nanocomposites. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E3170. 10.1073/pnas.1617069114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filonenko G. A.; Lugger J. A. M.; Liu C.; vanHeeswijk E. P. A.; Hendrix M. M. R. M.; Weber M.; Müller C.; Hensen E. J. M.; Sijbesma R. P.; Pidko E. A. Tracking Local Mechanical Impact in Heterogeneous Polymers with Direct Optical Imaging. Angew. Chem., Int. Ed. 2018, 57, 16385–16390. 10.1002/anie.201809108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A.; Zscherp C. What vibrations tell us about proteins. Q. Rev. Biophys. 2002, 35, 369–430. 10.1017/S0033583502003815. [DOI] [PubMed] [Google Scholar]
- Hamm P.; Zanni M.. Concepts and Methods of 2D Infrared Spectroscopy; Cambridge University Press: 2011; pp 635–636. [Google Scholar]
- Huerta-Viga A.; Shaw D. J.; Woutersen S. pH Dependence of the Conformation of Small Peptides Investigated with Two-Dimensional Vibrational Spectroscopy. J. Phys. Chem. B 2010, 114, 15212–15220. 10.1021/jp105133r. [DOI] [PubMed] [Google Scholar]
- Roeters S. J.; Sawall M.; Eskildsen C. E.; Panman M. R.; Tordai G.; Koeman M.; Neymeyr K.; Jansen J.; Smilde A. K.; Woutersen S. Unraveling VEALYL Amyloid Formation Using Advanced Vibrational Spectroscopy and Microscopy Author links open overlay panel. Biophys. J. 2020, 119, 87–98. 10.1016/j.bpj.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H.; Boyce M. Stress–strain behavior of thermoplastic polyurethanes. Mech. Mater. 2005, 37, 817–839. 10.1016/j.mechmat.2004.08.001. [DOI] [Google Scholar]
- Laity P. R.; Taylor J. E.; Wong S. S.; Khunkamchoo P.; Cable M.; Andrews G. T.; Johnson A. F.; Cameron R. E. The Effect of Polyurethane Composition and Processing History on Mechanical Properties. Journal of Macromolecular Science, Part B 2005, 44, 261–287. 10.1081/MB-200050487. [DOI] [Google Scholar]
- Srichatrapimuk V. W.; Cooper S. L. Infrared thermal analysis of polyurethane block polymers. Journal of Macromolecular Science, Part B 1978, 15, 267–311. 10.1080/00222347808212599. [DOI] [Google Scholar]
- Wang H.; Graff D. K.; Schoonover J. R.; Palmer R. A. Static and Dynamic Infrared Linear Dichroic Study of a Polyester/Polyurethane Copolymer Using Step-Scan FT-IR and a Photoelastic Modulator. Appl. Spectrosc. 1999, 53, 687–696. 10.1366/0003702991947126. [DOI] [Google Scholar]
- Cao H.; Qi F.; Liu R.; Wang F.; Zhang C.; Zhang X.; Chai Y.; Zhai L. The influence of hydrogen bonding on N-methyldiethanolamine-extended polyurethane solid–solid phase change materials for energy storage. RSC Adv. 2017, 7, 11244–11252. 10.1039/C7RA00405B. [DOI] [Google Scholar]
- Harthcock M. Probing the complex hydrogen bonding structure of urethane block copolymers and various acid containing copolymers using infra-red spectroscopy. Polymer 1989, 30, 1234–1242. 10.1016/0032-3861(89)90041-4. [DOI] [Google Scholar]
- Pongkitwitoon S.; Hernández R.; Weksler J.; Padsalgikar A.; Choi T.; Runt J. Temperature dependent microphase mixing of model polyurethanes with different intersegment compatibilities. Polymer 2009, 50, 6305–6311. 10.1016/j.polymer.2009.10.067. [DOI] [Google Scholar]
- Niemczyk A.; Piegat A.; Sonseca Olalla Á.; El Fray M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. 10.1016/j.eurpolymj.2017.05.046. [DOI] [Google Scholar]
- Eom Y.; Kim S. M.; Lee M.; Jeon H.; Park J.; Lee E. S.; Hwang S. Y.; Park J.; Oh D. X. Mechano-responsive hydrogen-bonding array of thermoplastic polyurethane elastomer captures both strength and self-healing. Nat. Commun. 2021, 12, 1–11. 10.1038/s41467-021-20931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama M.; Kawauchi T.; Kotani T.; Nomura S.; Matsuda T. Structure and Properties of Fatigued Segmented Poly(urethaneurea) I. Segment Orientation Mechanism due to Fatigue. Polym. J. 1986, 18, 719–733. 10.1295/polymj.18.719. [DOI] [Google Scholar]
- Lazonder K.; Pshenichnikov M. S.; Wiersma D. A. Easy interpretation of optical two-dimensional correlation spectra. Opt. Lett. 2006, 31, 3354–3356. 10.1364/OL.31.003354. [DOI] [PubMed] [Google Scholar]
- Guo Q.; Pagano P.; Li Y.-L.; Kohen A.; Cheatum C. M. Line shape analysis of two-dimensional infrared spectra. J. Chem. Phys. 2015, 142, 212427. 10.1063/1.4918350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilgör E.; Yurtsever E.; Yilgör I. Hydrogen bonding and polyurethane morphology. II. Spectroscopic, thermal and crystallization behavior of polyether blends with 1,3-dimethylurea and a model urethane compound. Polymer 2002, 43, 6561–6568. 10.1016/S0032-3861(02)00566-9. [DOI] [Google Scholar]
- Castagna A. M.; Pangon A.; Dillon G. P.; Runt J. Effect of Thermal History on the Microstructure of a Poly(tetramethylene oxide)-Based Polyurea. Macromolecules 2013, 46, 6520–6527. 10.1021/ma400856w. [DOI] [Google Scholar]
- Laity P.; Taylor J.; Wong S.; Khunkamchoo P.; Norris K.; Cable M.; Andrews G.; Johnson A.; Cameron R. A 2-dimensional small-angle X-ray scattering study of the microphase-separated morphology exhibited by thermoplastic polyurethanes and its response to deformation. Polymer 2004, 45, 5215–5232. 10.1016/j.polymer.2004.05.032. [DOI] [Google Scholar]
- Saiani A.; Novak A.; Rodier L.; Eeckhaut G.; Leenslag J.-W.; Higgins J. S. Origin of Multiple Melting Endotherms in a High Hard Block Content Polyurethane: Effect of Annealing Temperature. Macromolecules 2007, 40, 7252–7262. 10.1021/ma070332p. [DOI] [Google Scholar]
- Fernández-d’Arlas B.; Baumann R. P.; Pöselt E.; Müller A. J. Influence of composition on the isothermal crystallisation of segmented thermoplastic polyurethanes. CrystEngComm 2017, 19, 4720–4733. 10.1039/C7CE01028A. [DOI] [Google Scholar]
- Dunkelberger E. B.; Grechko M.; Zanni M. T. Transition Dipoles from 1D and 2D Infrared Spectroscopy Help Reveal the Secondary Structures of Proteins: Application to Amyloids. J. Phys. Chem. B 2015, 119, 14065–14075. 10.1021/acs.jpcb.5b07706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keten S.; Buehler M. J. Geometric Confinement Governs the Rupture Strength of H-bond Assemblies at a Critical Length Scale. Nano Lett. 2008, 8, 743–748. 10.1021/nl0731670. [DOI] [PubMed] [Google Scholar]
- Giubertoni G.; Burla F.; Bakker H. J.; Koenderink G. H. Connecting the Stimuli-Responsive Rheology of Biopolymer Hydrogels to Underlying Hydrogen-Bonding Interactions. Macromolecules 2020, 53, 10503–10513. 10.1021/acs.macromol.0c01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantner G. E.; Oroudjev E.; Schitter G.; Golde L. S.; Thurner P.; Finch M. M.; Turner P.; Gutsmann T.; Morse D. E.; Hansma H.; Hansma P. K. Sacrificial Bonds and Hidden Length: Unraveling Molecular Mesostructures in Tough Materials. Biophys. J. 2006, 90, 1411–1418. 10.1529/biophysj.105.069344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.; Wang B.; Li D.; Xu X.; Liu Y.; Huang Y.; Hu Z. Toughening shape-memory epoxy resins via sacrificial hydrogen bonds. Polym. Chem. 2022, 13, 1130–1139. 10.1039/D1PY01625C. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.