Abstract
Neuroretinitis is a condition typically characterized by unilateral optic neuropathy and is most commonly a sequelae of cat scratch disease (CSD) due to infection with Bartonella henselae. Ophthalmologic examination will reveal a swollen optic nerve and may eventually reveal a canonical macular star; optical coherence tomography (OCT) will reveal flattening of the fovea, a thickened neurosensory retina, and subretinal fluid accumulation. Although CSD rarely presents with isolated neuorretinitis, it should be considered in patients presenting with unilateral visual changes. The differential diagnosis for neuroretinitis includes optic neuritis, inflammatory optic neuropathies (sarcoid, para-infectious, autoimmune), compressive, toxic, and more. We describe a pediatric patient presenting with visual changes that were initially concerning for optic neuritis and the diagnostic workup that ultimately led to a diagnosis of CSD neuroretinitis.
Keywords: Neuroretinitis, cat scratch disease, Bartonella, visual changes
Case Report
A 13-year-old girl with a past medical history of postural orthostatic tachycardia syndrome presented with a 3-day history of worsening central vision in the right eye accompanied by decreased color vision and pain with extraocular movements. Neurologic examination revealed normal mental status, sensorimotor function, coordination, and reflexes. Cranial nerve examination redemonstrated pain with extraocular movements and a subtle right relative afferent pupillary defect (RAPD). Other cranial nerve tests were normal. Visual field testing revealed a cecocentral scotoma of the right eye. Corrected visual acuity was 20/250 on the right and 20/20 on the left. Upon ophthalmologic examination, the patient confirmed loss of color vision in the right eye.
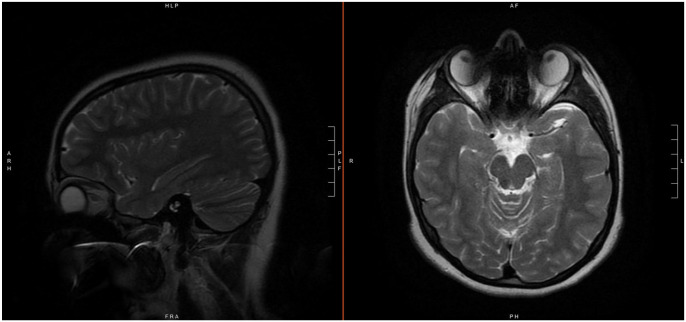
Clinical suspicion was initially high for optic neuritis. An orbital magnetic resonance imaging (MRI) with and without contrast was obtained and was negative, albeit limited due to artifact from the patient’s braces. Axial fluid-attenuated inversion recovery (FLAIR) imaging is shown (Figure 1). The brain was simultaneously imaged and was read as normal; T2 non-contrast images are shown (Figure 2). Despite normal MRI findings, cerebrospinal fluid (CSF) studies were collected, and inpatient steroids were begun due to a high suspicion for optic neuritis. CSF studies were unremarkable, showing a red blood cell count of 0, a white blood cell count of 6, a lymphocyte percentage of 91, 2% variant lymphocyte, and a monocyte percentage of 7. CSF culture revealed no growth. CSF protein was normal at 30 mg/dL, as was glucose at 57 mg/dL. Broad meningitis polymerase chain reaction (PCR) testing of the CSF was negative for all tested pathogens, though Bartonella was not included in this panel. Oligoclonal band testing was not immediately available, but ultimately revealed no unique bands, constituting a negative result. The patient was evaluated by neurology, who had a low suspicion for optic neuritis due to negative MRI. The patient was subsequently evaluated by neuro-ophthalmology.
Figure 1.
Axial MRI FLAIR imaging.
Abbreviations: MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery.
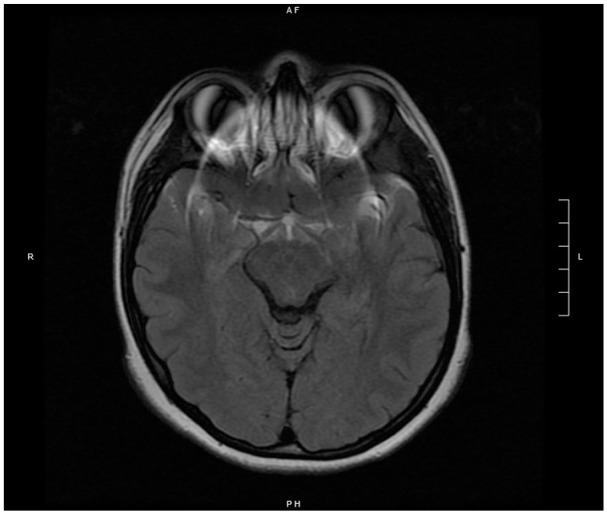
Figure 2.
Right parasagittal (left) and axial (right) T1 MRI imaging showing no visible intracranial or ocular pathology.
Abbreviation: MRI, magnetic resonance imaging.
Ophthalmologic evaluation revealed swelling of the right optic nerve, elevation of the macula, and early macular star (Figure 3). Optical coherence tomography (OCT) showed right intraretinal edema with serous retinal detachment (Figure 4). This was consistent with neuroretinitis. Furthermore, patient history was obtained and was remarkable for a cat bite 2 months prior raising concern for cat scratch disease (CSD). The patient was started on an empiric regimen of doxycycline, rifampin, and oral steroids. Before discharge with scheduled follow-up, an extensive serologic workup was initiated. Toxoplasma PCR, Lyme PCR, HIV, T Pallidum antibody testing were negative. IgG and IgM titers were negative for both Bartonella henselae and quintana, with titers below 1:320. A simultaneous autoimmune workup was performed. Angiotensin converting enzyme, anticardiolipin (IgG, IgM, and IgA), anti-nuclear antibody, and anti-neutrophil cytoplasmic antibody studies were obtained; all studies were unremarkable. The patient was tentatively diagnosed with serology negative CSD neuroretinitis.
Figure 3.
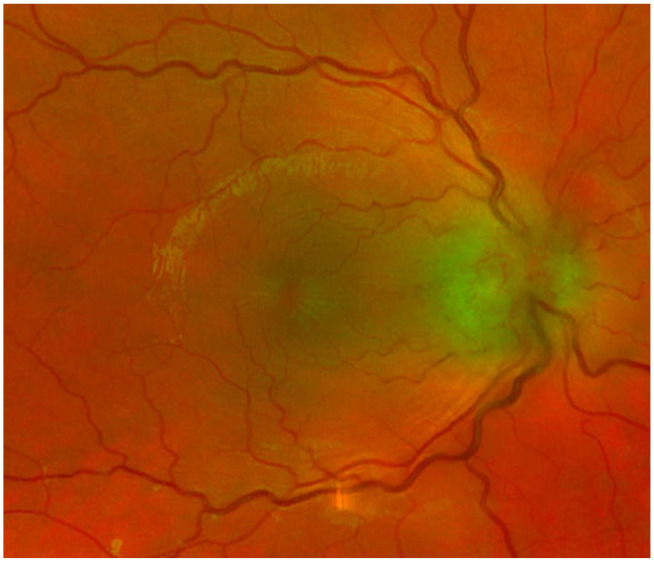
Fundoscopy demonstrating a macular star and edematous optic nerve.
Figure 4.
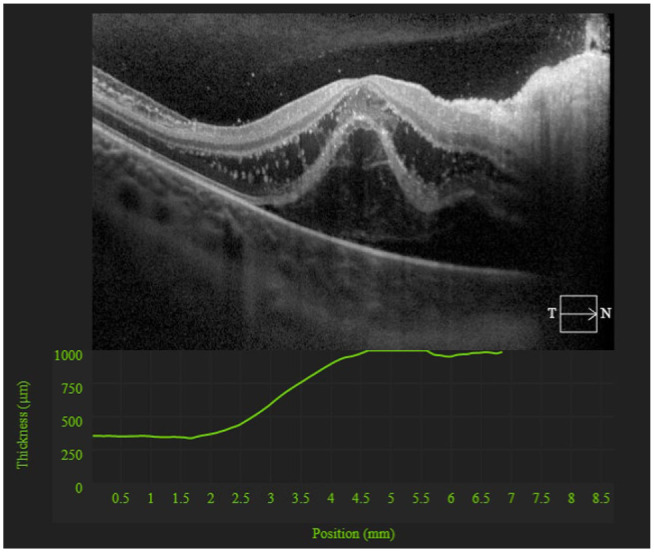
OCT of the macula demonstrating intraretinal edema, and subretinal fluid (serous retinal detachment).
Abbreviation: OCT, optical coherence tomography.
Repeat Bartonella testing was obtained due to the patient’s clinical history, and ultimately returned with Bartonella henselae IgG levels of 1:640, which constitutes a positive test, consistent with CSD neuorretinitis. At the 3-month timepoint, the patient’s visual acuity had returned to 20/20 with normal visual fields and intraretinal edema had resolved. Patient’s color vision is slowly recovering.
Discussion
Neuroretinitis is inflammation of the retina and optic disk which can stem from a variety of bacterial, viral, fungal, parasitic, and inflammatory etiologies. These include Mycobacterium tuberculosis, Zika virus, Histoplasmosis, Toxoplasmosis, and Vogt-Koyanagi-Harada syndrome.1 However, most often it is caused by infection with Bartonella henselae. Although CSD is the most common cause of neuroretinitis, neuroretinitis is a rare complication of CSD, occurring in only 1% to 2% of patients.2 Interestingly, this patient presented with isolated neuroretinitis with pain on extraocular movement (EOM) and did not display the typical signs and symptoms of CSD, which include a cutaneous lesion at the sight of inoculation and associated regional lymphadenopathy proximal to the inoculation site.3,4
Fundamentally, CSD neuroretinitis is an optic neuropathy, and thus is associated with many of the classic symptoms seen in this broad category of pathologies including central scotoma, dyschromatopsia, and RAPD. Notably, neuroretinitis is not often associated with pain on EOM, which typically helps to distinguish its presentation from that of optic neuritis.5 Fundoscopic findings consistent with neuroretinitis include optic disk edema and lipid exudation. Eventually, imaging will reveal a canonical “macular star” pattern which may be absent early in the disease process.6 OCT will reveal flattening of the fovea, a thickened neurosensory retina, and subretinal fluid accumulation.7 MRI findings are not consistently described in the pediatric literature.8
Diagnosis of Bartonella neuroretinitis is described by Ksiaa et al6 and includes clinical findings of young age and history of contact with cat, signs and symptoms of typical neuroretinitis (including positive fundoscopic and OCT findings), systemic symptoms, and positive serology (most reliably through Immunoglobulin G and Immunoglobulin M detection.)
While neuroretinitis is typically a self-resolving condition, treatment should be considered for high-risk patients, or in patients with risk of permanent vision loss.6 The mainstay of treatment includes antimicrobial therapy as well as corticosteroids. The antibiotic regimen of choice is typically doxycycline in combination with rifampin.9 Although the utility of adjunctive steroids was once debated, current literature supports their use.10
Conclusion
We believe that this case demonstrates the importance of including Bartonella-associated neuroretinitis on the differential diagnosis in patients presenting with scotoma, color vision changes, and an RAPD even in the absence of usual signs and symptoms of CSD. CSD is definitively diagnosed with serologic studies, although notably they may return false negative. However, if clinical suspicion is high, treatment with doxycycline, rifampin, and adjunctive corticosteroids should still be initiated.
Footnotes
Authors’ Note: This work has never been previously presented nor published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series of less than 3 cases.
Informed Consent: Verbal informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: David A. W. Sykes  https://orcid.org/0000-0002-8154-9921
https://orcid.org/0000-0002-8154-9921
References
- 1. Abdelhakim A, Rasool N. Neuroretinitis: a review. Curr Opin Ophthalmol. 2018;29:514-519. doi: 10.1097/icu.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 2. Mahjoub A, Bellazreg F, Ben Abdesslem N, et al. Cat scratch disease neuroretinitis: a case report. Ann Med Surg (Lond). 2021;69:102722. doi: 10.1016/j.amsu.2021.102722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moriarty RA, Margileth AM. Cat scratch disease. Infect Dis Clin North Am. 1987;1:575-590. [PubMed] [Google Scholar]
- 4. Carithers HA. Cat-scratch disease. An overview based on a study of 1,200 patients. Am J Dis Child. 1985;139(11):1124-1133. doi: 10.1001/archpedi.1985.02140130062031 [DOI] [PubMed] [Google Scholar]
- 5. Margolin E, Jeeva-Patel T. Neuroretinitis in a young woman. eNeurologicalSci. 2020;21:100280. doi: 10.1016/j.ensci.2020.100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ksiaa I, Abroug N, Mahmoud A, et al. Update on Bartonella neuroretinitis. J Curr Ophthalmol. 2019;31(3):254-261. doi: 10.1016/j.joco.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habot-Wilner Z, Zur D, Goldstein M, et al. Macular findings on optical coherence tomography in cat-scratch disease neuroretinitis. Eye (Lond). 2011;25(8):1064-1068. doi: 10.1038/eye.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy AK, Morriss MC, Ostrow GI, Stass-Isern M, Olitsky SE, Lowe LH. Utility of MR imaging in cat-scratch neuroretinitis. Pediatr Radiol. 2007;37(8):840-843. doi: 10.1007/s00247-007-0514-1 [DOI] [PubMed] [Google Scholar]
- 9. Reed JB, Scales DK, Wong MT, et al. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology. 1998;105:459-466. doi: 10.1016/s0161-6420(98)93028-7 [DOI] [PubMed] [Google Scholar]
- 10. Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96(4):e524-e532. doi: 10.1111/aos.13684 [DOI] [PubMed] [Google Scholar]