Abstract
ALG6-CDG is a rare, but second most common, type 1 congenital disorder of glycosylation (CDG) caused by a defect in the α-1-3-glucosyltransferase (ALG6) enzyme in the N-glycan assembly pathway. Many mutations have been identified and inherited in an autosomal recessive pattern. There are less than 100 ALG6-CDG cases reported, all sharing the phenotype of hypotonia and developmental delay. The majority (perhaps >70%) have seizures, but a minority have intractable epilepsy or epileptic encephalopathy. We report the clinical course, EEG findings, and neuroimaging of a child found to have compound heterozygous alleles c.257 + 5G > A and c.680G > A (p.G227E) who developed explosive onset of intractable epilepsy and epileptic encephalopathy. Initially, CDG was not suspected due to its rarity and lack of multi-organ system involvement, but rapid whole exam sequence (8-day turnaround) revealed the specific diagnosis quickly.
Keywords: congenital disorders of glycosylation, epileptic encephalopathy, epilepsy, developmental delay, EEG, inborn errors of metabolism
Introduction
Congenital disorders of glycosylation (CDG) are a broad category of metabolic disorders (approximately 100) caused by defects in various steps along the glycan synthesis and modification pathways. Most proteins and lipids in the body are modified by this process; CDGs tend to have multi-system involvement.1–3 More than 80% of known CDGs have neurological manifestations such as developmental delay, intellectual disability, neuropathy, hypotonia, and seizures.1 This case report will focus specifically on ALG6-CDG (OMIM #603147), which is due to a deficiency in the ALG6 enzyme. We will present the clinical phenotype, EEG, neuroimaging, and specific genetic mutations in the patient, and compare these findings with other documented cases of ALG6-CDG. A review of literature reveals less than 100 total reported cases of ALG6-CDG1,4–9 with a wide range of clinical phenotypes.
ALG6-CDG (AKA CDG-Ic) is a type-I CDG that is inherited in an autosomal recessive fashion and is the second most frequently observed CDG behind PMM2-CDG (CDG-Ia). ALG6-CDG results from a defect in the ALG6 gene, which encodes an α−1,3-glucosyltransferase (glucosyltransferase 1) that adds glucose to lipid-linked oligosaccharides in the endoplasmic reticulum in the N-glycan assembly pathway. Patients primarily present with feeding problems, neurological problems (psychomotor retardation, axial hypotonia, seizures, ataxia, and strabismus), low serum cholesterol, endocrine abnormalities, and clotting factor deficiencis.9–11
Case Presentation
WS was born full term with an uncomplicated pregnancy and delivery. She required a NICU stay for respiratory issues that resolved. She was born with congenital microcephaly (OFC 30.5 cm, <first percentile) and remained microcephalic with OFC of 43.2 cm (first percentile) at 18-month follow-up. There is a distant family history of epilepsy but otherwise no known neurological disease or developmental delay.
She presented at 6 months old for a first-time seizure, described as focal tonic that self-resolved. Her initial interictal routine EEG at the time demonstrated age-appropriate organization but was notable for frequent left posterior spikes and sharp waves (Figure 1), prompting initiation of levetiracetam. At the time of presentation, parents noted difficulties at 3 months of age due to delay in endogenous smile, but no other delays.
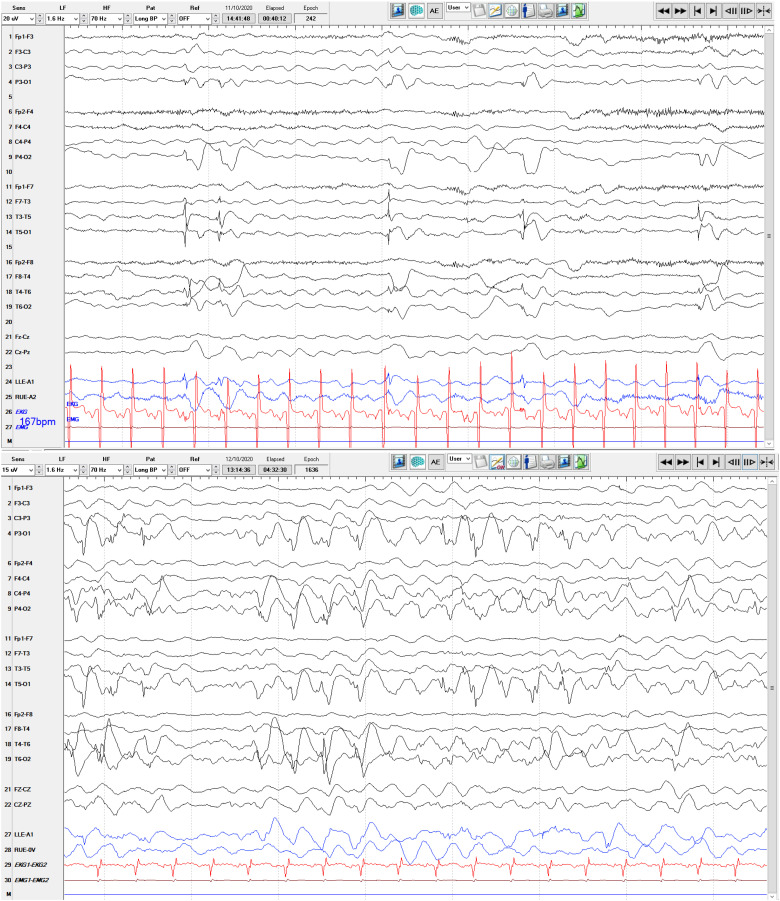
Figure 1.
Top: Routine interictal EEG at 6 months old in a 10–20 scalp placement longitudinal montage with left sided posterior sharp waves visualized best in T3-T5 and T5-O1 electrode readings. Bottom: Long term ictal EEG at 7 months old in a 10–20 scalp placement longitudinal montage showing a segment of an electrographic seizure, demonstrating moderate amplitude polyspike/spike and wave complexes best appreciated posteriorly, with 1–3 Hz frequency.
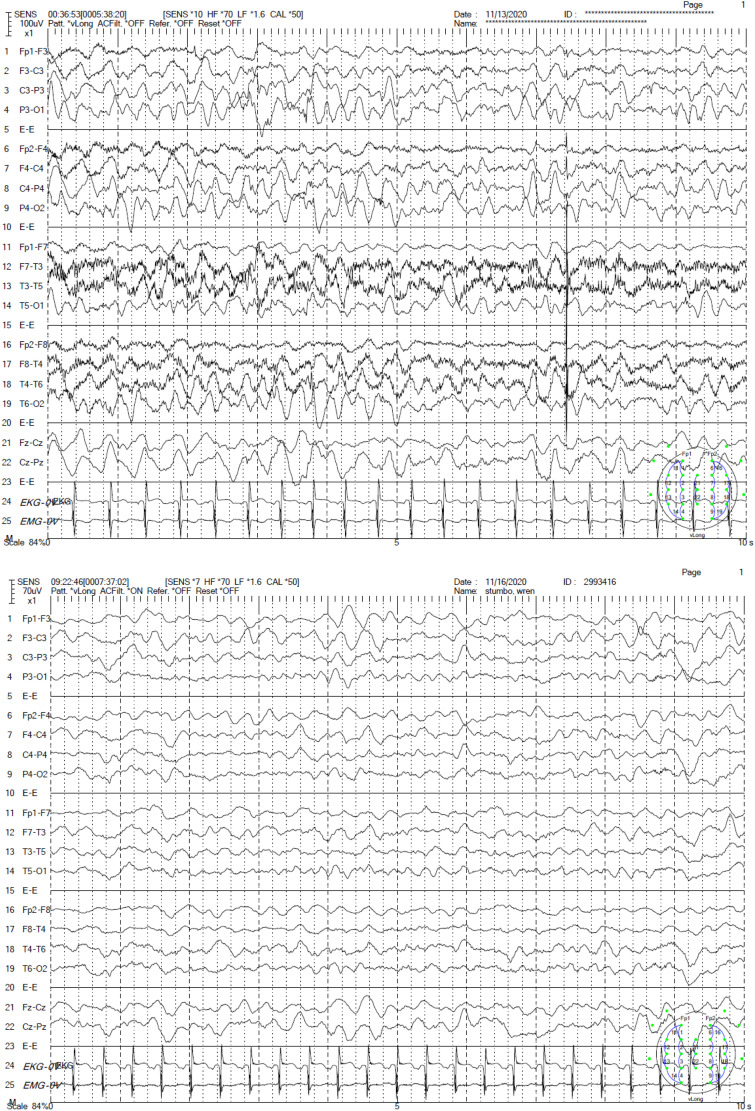
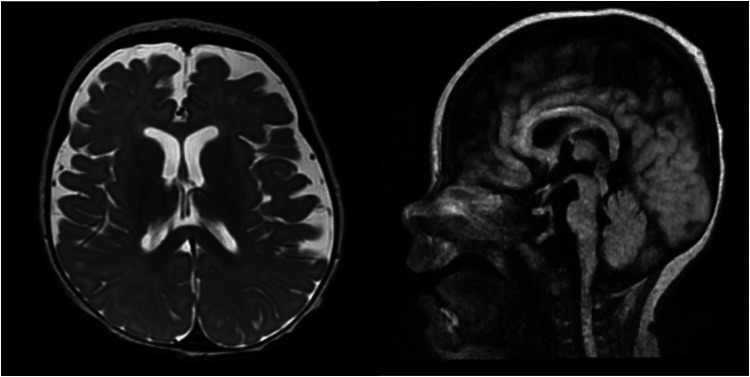
Within 2 days, she developed frequent clinical seizures. Continuous EEG captured multiple electroclinical seizures beginning with background attenuation, followed by rhythmic theta frequency, with shifting laterality. The seizure would then build in amplitude, slowing to 4 Hz and spreading biposteriorly as sharply contoured waves. These seizures prompted rapid titration of levetiracetam, fosphenytoin and lacosamide. At this time, her background on EEG continued to be normal. Approximately 4 weeks after initial presentation, she deteriorated clinically. Her interictal EEG had nearly continuous polyspike and spike wave discharges involving both occipital regions, lack of organization, and disappearance of posterior-dominant rhythm indicating an epileptic encephalopathy. She had 3 separate stereotyped electrographic seizures (1) bi-occipital fast polyspikes spreading temporally with better evolution on the right, (2) left centro-temporal 8 Hz activity spreading to the right centro-temporal areas, and (3) focal right occipital fast polyspikes that evolved in amplitude and frequency (Figure 1). After initiation of phenobarbital, there was a dramatic electroclinical improvement (Figure 2). An MRI was obtained, which demonstrated mild to moderate atrophy, thinning of the brainstem, abnormal myelination for age with no migration abnormalities appreciated (Figure 3).
Figure 2.
EEG prior to (top) and after (bottom) initiation of phenobarbital.
Figure 3.
Left: MRI obtained at 6 months old T2 weighted imaging axial cut demonstrating mild atrophy without migration abnormalities. Right: Sagittal cut T2 FLAIR weighted imaging demonstrating thinning brainstem.
At this point her physical exam was significant for diffuse hypotonia and lack of visual tracking. Her clinical deterioration, explosive onset of intractable seizures, and evolution to epileptic encephalopathy raised concern for a genetic metabolic disorder or genetic infantile epileptic encephalopathy syndrome. Ammonia, lactate, acylcarnitine profile, urine organic acids, serum amino acids, and Ohio newborn screen were unremarkable. An in-house (Nationwide Children's Hospital) rapid whole exome assay (returning results within 8 days) revealed an ALG6 gene mutation. This finding was confirmed with a send-out rapid whole exome assay (Baylor Genetics, results returned 2 weeks after in-house results were available) that revealed compound heterozygous variants of c.257 + 5G > A and c.680G > A (p.G227E) in the ALG6 gene, associated with ALG6-CDG. No other potentially pathologic variations were detected. Mitochondrial DNA analysis was unremarkable. Carbohydrate deficient transferrin (Mayo Clinic) had a CDG type-I profile (abnormal mono-oligosaccharide/di-oligosaccharide transferrin and a-oligosaccharide/di-oligosaccharide transferrin ratios). Apolipoprotein C-III profile was normal. Tri-sialo/di-oligosaccharide transferrin ratio was normal which effectively ruled out CDG type-II. Repeat testing 2 months later revealed a similar profile. Together, these tests confirmed the diagnosis of ALG6-CDG.
Her epilepsy continued to be medically intractable. She was trialed on the ketogenic diet, topiramate, clobazam, phenobarbital, and felbamate without success. At 18 month follow up she had a baseline of near daily seizures lasting 5–30 s each. Her anti-seizure medication regimen included levetiracetam, topiramate, felbamate, clonazepam, and phenobarbital.
Developmentally, her social smile was not achieved until 9 months. Babbling was noted to occur by 5 months but remained nonverbal by 18 months. Additionally, she could not sit unsupported, lift head when laying prone, or pass objects from hand to hand.
On physical examination at 18 months, she had a weak cry, no blink to threat, nor visual tracking. She had right esotropia and severe axial hypotonia with appendicular hypertonicity. She did not have any facial, skull, or ear dysmorphisms.
Discussion
The most recent summary of ALG6-CDG case reports comes from Morava et al who obtained clinical data from 41 patients registered in the Euroglycan database from 1995 to 20139 and noted that a common allele was c.257 + 5G > A. There were 4 patients who shared the exact compound heterozygous variant as our patient (c.257 + 5G > A and p.G227E) and all were of Caucasian/European descent, as was our patient. These 4 children all had hypotonia, visual loss, and global brain atrophy, three had seizures that were refractory, two had feeding issues, two had speech delay, and none had muscle weakness. Dercksen et. al describes 4 cases with the same genotype as our patient in a semi-isolated Caucasian community in South Africa. These children had severe epilepsy, hypotonia, mild to severe cerebral atrophy on neuroimaging, poor feeding, and recurrent infections.
Of the total ALG6-CDG cohort from Morava et al, most (70%) developed seizures, though only a minority (22%) were intractable. Eleven of the 41 died during childhood (including 2 of the 4 sharing our patient's alleles) while 13 were alive in teens to age 36 years at the time of the study. Patients surviving past puberty had stagnation of psychomotor development but no regression. Encephalopathy appeared to be static. One adult patient could take steps independently. Severe multisystem involvement was the cause of most morbidity and mortality including protein losing enteropathy, hypoalbuminemia, hypoglycemia, and frequent infections.
In a study from Fiumara et al looking at electroclinical features of early-onset epileptic encephalopathy in CDG, seizures were the chief presenting complaint in CDG subtypes other than PMM2-CDG.12 These patients went on to develop early-onset epileptic encephalopathy (EOEE). One patient had ALG6-CDG (homozygous p.A84 T mutation) and developed early myoclonic epilepsy of infancy (EMEI). Unlike our patient, this child presented in the first week of life with large myoclonic jerks during sleep. Like our patient, this child had prolonged apnea (up to 15 min) following seizure. At age 6 months this child began to have daily tonic seizures that did not respond to phenobarbital, vigabatrin, or valproate. A partial response was observed with levetiracetam. Psychomotor development was severely delayed and vision severely impaired. Pereira et al reported a case of ALG6-CDG with epileptic spasms and generalized tonic-clonic seizures (onset at age 6 months) without hypsarrhythmia (but with abundant posterior interictal spikes and sharp waves).13
To date, there are less than 100 ALG6-CDG cases reported in the literature. All share the phenotype of hypotonia and developmental delay, and the majority (perhaps >70%) have seizures,12 but a minority have medically intractable epilepsy. Few reports provide detail regarding specific seizure types, specific EEG features, and presence of epileptic encephalopathy. From an epilepsy viewpoint, there is clinical heterogeneity within ALG6-CDG depending on gene variants that are present. This report documents the rapid evolution to medically intractable epileptic encephalopathy in a patient who has compound heterozygous variants of c.257 + 5G > A and c.680G > A (p.G227E) in the ALG6 gene and serves to expand the known clinical phenotypes of these patients.
Furthermore, this report highlights a situation where rapid exome sequencing helped make a relatively quick diagnosis (8 days into hospitalization) of a very rare condition. CDGs in general are rare and the most common CDG (PMM2-CDG) does not usually present with intractable epilepsy. Additionally, CDGs will often have other features such as dysmorphisms and multi-organ dysfunction which were not present in our patient. It is unlikely we would have ordered transferrin assays looking specifically for CDG when she initially presented. With the widespread availability of next generation sequencing, the algorithm for working up intractable idiopathic epilepsy has pushed towards early genetic testing.14 In this case we chose whole exome sequencing due to its higher rate of identifying pathogenic variants, particularly in the case of epileptic encephalopathies.15,16 Additionally, our institution runs a rapid whole exome sequencing assay that has an 8-day turn-around time, which identified her specific rare condition (ALG6-CDG) quickly in a scenario when the broader category of CDG in general was of low suspicion. We were able to establish her diagnosis and confirm it with transferrin assays weeks before it would have been made with a commercial genetic epilepsy panel, saving valuable time in an effort to help a critically ill infant.
Abbreviations
- EEG
electroencephalogram
- CDG
congenital disorder of glycosylation
- IEF
isoelectric focusing
- ICU
intensive care unit
- MRI
magnetic resonance imaging
- LTM
long term EEG monitoring
- EMEI
early myoclonic epilepsy of infancy
- EOEE
early-onset epileptic encephalopathy
Footnotes
Author Contributions: Clark performed literature review, case review, and manuscript preparation. Drees performed literature review and manuscript preparation. Murray performed case review and manuscript preparation. Kulkarni performed literature review, case review, and manuscript preparation.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics and Informed Consent: Our institution does not require ethical approval for reporting individual cases or case series. Written informed consent was obtained from a legally authorized representative for anonymized patient information to be published in this article.
Data Availability: The datasets generated and/or analyzed during the current study are not publicly available due patient confidentiality but are available from the corresponding author on reasonable request.
ORCID iDs: Daniel James Clark https://orcid.org/0000-0002-4633-7886
Thomas Murray https://orcid.org/0000-0002-5298-3649
Neil Kulkarni https://orcid.org/0000-0002-8360-164X
References
- 1.Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11(5):453-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurological aspects of human glycosylation disorders. Annu Rev Neurosci. 2015;38(1):105-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paprocka J, Jezela-Stanek A, Tylki-Szymańska A, Grunewald S. Congenital disorders of glycosylation from a neurological perspective. Brain Sci. 2021;11(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westphal V, Schottstädt C, Marquardt T, Freeze HH. Analysis of multiple mutations in the hALG6 gene in a patient with congenital disorder of glycosylation Ic. Mol Genet Metab. 2000;70(3):219-223. [DOI] [PubMed] [Google Scholar]
- 5.Eklund EA, Sun L, Yang SP, Pasion RM, Thorland EC, Freeze HH. Congenital disorder of glycosylation Ic due to a de novo deletion and an hALG-6 mutation. Biochem Biophys Res Commun. 2006;339(3):755-760. [DOI] [PubMed] [Google Scholar]
- 6.Imbach T, Grunewald S, Schenk B, et al. Multi-allelic origin of congenital disorder of glycosylation (CDG)-ic. Hum Genet. 2000;106(5):538-545. [DOI] [PubMed] [Google Scholar]
- 7.Dercksen M, Crutchley AC, Honey EM, et al. ALG6-CDG in South Africa: genotype-phenotype description of five novel patients. JIMD Rep. 2012;8:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeken J, Lefeber D, Matthijs G. Clinical utility gene card for: ALG6 defective congenital disorder of glycosylation. Eur J Hum Genet. 2015;23(2):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morava E, Tiemes V, Thiel C, et al. ALG6-CDG: a recognizable phenotype with epilepsy, proximal muscle weakness, ataxia and behavioral and limb anomalies. J Inherit Metab Dis. 2016;39(5):713-723. [DOI] [PubMed] [Google Scholar]
- 10.Grünewald S, Imbach T, Huijben K, et al. Clinical and biochemical characteristics of congenital disorder of glycosylation type Ic, the first recognized endoplasmic reticulum defect in N-glycan synthesis. Ann Neurol. 2000;47(6):776-781. [PubMed] [Google Scholar]
- 11.Newell JW, Seo N, Enns GM, McCraken M, Mantovani JF, Freeze HH. Congenital disorder of glycosylation Ic in patients of Indian origin. Mol Genet Metab. 2003;79(3):221-228. [DOI] [PubMed] [Google Scholar]
- 12.Fiumara A, Barone R, Del Campo G, Striano P, Jaeken J. Early-onset epileptic encephalopathy in infants with different forms of congenital disorders of glycosylation (CDG). Brain Dev. 2017;39(4):366-367. [DOI] [PubMed] [Google Scholar]
- 13.Pereira AG, Bahi-Buisson N, Barnerias C, et al. Epileptic spasms in congenital disorders of glycosylation. Epileptic Disord. 2017;19(1):15-23. [DOI] [PubMed] [Google Scholar]
- 14.Ritter DM, Holland K. Genetic testing in epilepsy. Semin Neurol. 2020;40(6):730-738. [DOI] [PubMed] [Google Scholar]
- 15.Helbig KL, Farwell Hagman KD, Shinde DN, et al. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med. 2016;18(9):898-905. [DOI] [PubMed] [Google Scholar]
- 16.Butler KM, da Silva C, Alexander JJ, Hegde M, Escayg A. Diagnostic yield from 339 epilepsy patients screened on a clinical gene panel. Pediatr Neurol. 2017;77:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]