Abstract
Background:
Abemaciclib is the first and only cyclin-dependent kinases 4 and 6 inhibitor approved for adjuvant treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−), node-positive, and high-risk early breast cancer (EBC), with indications varying by geography. Premenopausal patients with HR+, HER2− tumors may have different tumor biology and treatment response compared to postmenopausal patients.
Objectives:
We describe the efficacy and safety of abemaciclib plus endocrine therapy (ET) for the large subgroup of premenopausal patients with HR+, HER2− EBC in monarchE.
Design:
Randomized patients (1:1) received adjuvant ET with or without abemaciclib for 2 years plus at least 3 additional years of ET as clinically indicated.
Methods:
Patients were stratified by menopausal status (premenopausal versus postmenopausal) at diagnosis. Standard ET (tamoxifen or aromatase inhibitor) with or without gonadotropin-releasing hormone agonist was determined by physician’s choice. Invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) by menopausal status were assessed at data cutoff on 1 April 2021 (median follow-up of 27 months).
Results:
Among randomized patients, 2451 (43.5%) were premenopausal and 3181 (56.4%) were postmenopausal. The choice of ET for premenopausal patients varied considerably between countries. Treatment benefit was consistent across menopausal status, with a numerically greater effect size in premenopausal patients. For premenopausal patients, abemaciclib with ET resulted in a 42.2% and 40.3% reduction in the risk of developing IDFS and DRFS events, respectively. Absolute improvement at 3 years was 5.7% for IDFS and 4.4% for DRFS rates. Safety profile for premenopausal patients was consistent with the overall safety population.
Conclusion:
Abemaciclib with ET demonstrated clinically meaningful treatment benefit for IDFS and DRFS versus ET alone regardless of menopausal status and first ET, with a numerically greater benefit in the premenopausal compared to the postmenopausal population. Safety data in premenopausal patients are consistent with the overall safety profile of abemaciclib.
Keywords: abemaciclib, early breast cancer, high risk, monarchE, premenopausal
Introduction
In monarchE, adjuvant abemaciclib combined with endocrine therapy (ET) significantly improved invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−), node-positive, high-risk early breast cancer (EBC) while maintaining an acceptable safety profile.1 To date, abemaciclib is the first and only cyclin-dependent kinases 4 and 6 inhibitor approved in the adjuvant setting for certain types of HR+, HER2−, node-positive, high-risk EBC.
Premenopausal patients with HR+, HER2− tumors may have different tumor biology and treatment response compared to postmenopausal patients.2–6 Among new diagnoses of breast cancer (BC), approximately 29% occur in women under 55 years old7 and in some countries, particularly in Asia, there has been a notable increase in the incidence of premenopausal BC over recent decades8; therefore, there is a growing need for improved therapy. The benefit of abemaciclib and other cyclin-dependent kinase 4 and 6 inhibitors has been demonstrated in premenopausal and postmenopausal patients with metastatic BC.9 It is important to evaluate the benefit of abemaciclib in the large subgroup of premenopausal patients with HR+, HER2−, node-positive, high-risk EBC enrolled in monarchE.
Risk-adapted ET strategies such as tamoxifen alone or with ovarian function suppression (OFS) or aromatase inhibitors (AIs) with OFS have become standard of care for premenopausal patients and are recommended in many guidelines. This is supported by the Suppression of Ovarian Function Trial (SOFT) and Tamoxifen and The Exemestane Trial (TEXT), which demonstrated significant improvements in both disease-free and overall survival with exemestane plus OFS or tamoxifen plus OFS compared to tamoxifen alone in premenopausal women.10 However, there are limited prospective data on contemporary ET prescribing practices in this high-risk population since the publication of these practice changing studies.
In monarchE, ET choice was at the discretion of the investigator, and as such, the study can be analyzed to determine regional treatment patterns in clinical practice within one of the largest subgroups of premenopausal women with high-risk EBC since SOFT and TEXT.11,12 More importantly, there is a critical need to understand the benefit of adding abemaciclib to adjuvant therapy, specifically for premenopausal patients with HR+, HER2−, node-positive, high-risk EBC who may have received intensive (neo)adjuvant chemotherapy. Here, we present the treatment patterns and efficacy and safety results of abemaciclib plus ET versus ET alone for premenopausal patients in monarchE.
Methods
MonarchE study design and population
monarchE (NCT03155997) is an open-label, global, randomized phase III trial, evaluating patients with HR+, HER2−, node-positive EBC at high risk of recurrence. Patients were randomized 1:1 to receive standard of care ET with or without adjuvant abemaciclib for up to 2 years followed by ET for a total of at least 5 years. Abemaciclib dosing was 150 mg twice daily for the first 2 years (treatment period) or until a patient met criteria for discontinuation. The choice of ET and the use of OFS were at the discretion of the treating physician.
The detailed study design was previously published.13 Eligible patients must have ⩾4 positive axillary lymph nodes or 1 to 3 positive axillary lymph nodes and tumor size ⩾5 cm, Grade 3 disease, or central Ki-67 ⩾20%. Patients were stratified by prior chemotherapy, menopausal status (premenopausal versus postmenopausal), and geographic region. Menopausal status was determined by the investigator at the time of initial diagnosis, and the patients were stratified by menopausal status. Menopausal status after the initial diagnosis (i.e. after chemotherapy and/or prophylactic oophorectomy) was not captured.
Statistical analysis
The subgroup analysis by menopausal status was performed at an additional follow-up analysis (AFU1) with a data cutoff date of 1 April 2021, and median follow-up time of 27 months. Efficacy endpoints such as IDFS and DRFS have been previously described for the intention-to-treat population at this data cutoff point.1
To evaluate the treatment benefit of abemaciclib by menopausal status, IDFS and DRFS analyses were performed using unstratified Cox proportional hazard model and Kaplan–Meier method. A further exploratory analysis was conducted to characterize premenopausal patients based on physician’s choice of first ET (tamoxifen or AI, with or without OFS). Those subgroup analyses were exploratory and not alpha controlled for testing statistical significance. Safety data were assessed for premenopausal patients.
Results
Patients
Of the 5637 patients enrolled in monarchE, 2451 (43.5%) were premenopausal and 3181 (56.4%) were postmenopausal at initial diagnosis. Five patients (<0.1%) did not report menopausal status in the case report form. Premenopausal patients more commonly had larger tumors (⩾5 cm) than postmenopausal patients, and a higher percentage of premenopausal patients received neoadjuvant chemotherapy than postmenopausal patients (Table 1). In the Asia region, 59% of patients were premenopausal compared to 40.4% in North America/Europe and 37.7% in the rest of the world.
Table 1.
Patient demographics and disease characteristics.
| Demographics and characteristics | Premenopausal | Postmenopausal | ||
|---|---|---|---|---|
| Abemaciclib + ET, n = 1227 | ET alone, n = 1224 | Abemaciclib + ET, n = 1576 | ET alone, n = 1605 | |
| Age, years (range) | ||||
| Median | 44 (23–65) | 44 (22–60) | 59 (32–89) | 59 (27–86) |
| Region, n (%) | ||||
| Asia | 340 (27.7) | 342 (27.9) | 234 (14.8) | 240 (15.0) |
| NA/EU | 596 (48.6) | 595 (48.6) | 870 (55.2) | 884 (55.1) |
| Other | 291 (23.7) | 287 (23.4) | 472 (29.9) | 481 (30.0) |
| Prior chemotherapy, n (%) | ||||
| Neoadjuvant | 512 (41.7) | 516 (42.2) | 510 (32.4) | 515 (32.1) |
| Adjuvant | 685 (55.8) | 675 (55.1) | 945 (60.0) | 958 (59.7) |
| No chemotherapy | 30 (2.4) | 33 (2.7) | 121 (7.7) | 132 (8.2) |
| Number of positive lymph nodes, n (%) | ||||
| 0 | 7 (0.6) | 1 (0.1) | 0 (0.0) | 6 (0.4) |
| 1 to 3 | 501 (40.8) | 520 (42.5) | 615 (39.0) | 622 (38.8) |
| ⩾4 or more | 719 (58.6) | 703 (57.4) | 961 (61.0) | 977 (60.9) |
| Histological grade, n (%) | ||||
| Grade 1 | 94 (7.7) | 91 (7.4) | 115 (7.3) | 125 (7.8) |
| Grade 2 | 585 (47.7) | 585 (47.8) | 790 (50.1) | 810 (50.5) |
| Grade 3 | 474 (38.6) | 467 (38.2) | 610 (38.7) | 597 (37.2) |
| Radiologic tumor size at diagnosis, n (%) | ||||
| <2 cm | 328 (26.7) | 330 (27.0) | 457 (29.0) | 449 (28.0) |
| 2–5 cm | 592 (48.2) | 599 (48.9) | 815 (51.7) | 860 (53.6) |
| ⩾5 cm | 262 (21.4) | 256 (20.9) | 239 (15.2) | 223 (13.9) |
| Pathologic tumor size at surgery, n (%) | ||||
| <2 cm | 351 (28.6) | 342 (27.9) | 430 (27.3) | 425 (26.5) |
| 2–5 cm | 571 (46.5) | 576 (47.1) | 798 (50.6) | 843 (52.5) |
| ⩾5 cm | 279 (22.7) | 288 (23.5) | 327 (20.7) | 322 (20.1) |
| Central Ki-67, n (%) | ||||
| <20% | 376 (30.6) | 395 (32.3) | 575 (36.5) | 579 (36.1) |
| ⩾20% | 576 (46.9) | 561 (45.8) | 684 (43.4) | 675 (42.1) |
| Unavailable | 275 (22.4) | 268 (21.9) | 317 (20.1) | 351 (21.9) |
Where values do not add up to 100%, remaining data are missing, unavailable, or could not be assessed.
ET, endocrine therapy; EU, Europe; NA, North America.
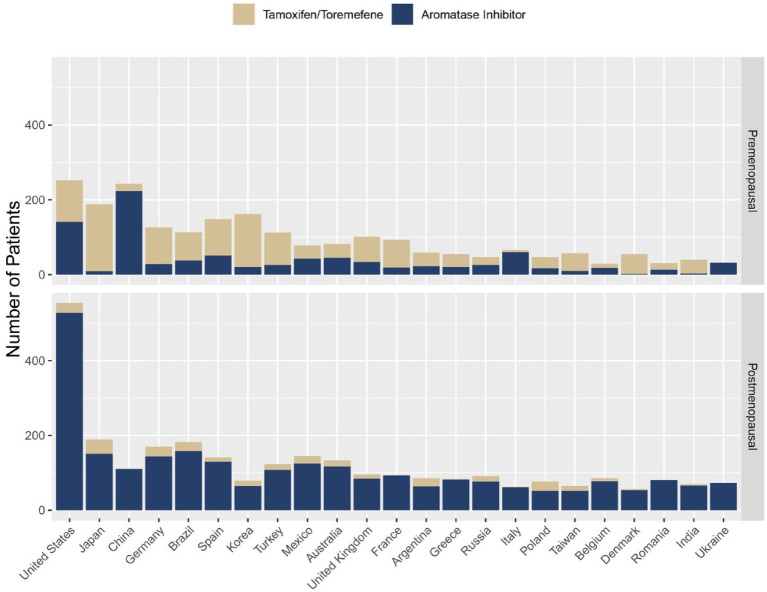
The choice of ET was well balanced between treatment arms for both premenopausal and postmenopausal patients. Most postmenopausal patients (89.3%, n = 2818) received AI as their first ET on study, while a substantial proportion of premenopausal patients (58.2%, n = 1415) received tamoxifen as their first ET; 989 (70%) of these premenopausal patients did not have a gonadotropin-releasing hormone (GnRH) agonist recorded in the database (Table 2). The first ET for premenopausal patients varied considerably between countries (Figure 1); for example, tamoxifen was received by 95% of patients from Japan, 78% of patients from Germany, 44% of patients from the United States, and 8% of patients from China.
Table 2.
Summary of initial ET by menopausal status.
| First ET received | Premenopausala | Postmenopausal | ||
|---|---|---|---|---|
| Abemaciclib + ET, n = 1222 | ET alone, n = 1209 | Abemaciclib + ET, n = 1565 | ET alone, n = 1591 | |
| Aromatase inhibitors, n (%) | 520 (42.6) | 481 (39.8) | 1407 (89.9) | 1411 (88.7) |
| With GnRH agonist, n | 389 | 358 | 23 | 36 |
| Without GnRH agonist, n | 131b | 123b | 1384 | 1375 |
| Tamoxifen, n (%) | 697 (57.0) | 718 (59.4) | 158 (10.1) | 180 (11.3) |
| With GnRH agonist, n | 195 | 231 | 5 | 6 |
| Without GnRH agonist, n | 502 | 487 | 153 | 174 |
Based on ET exposure data for treated patients at the primary outcome data cutoff, by treatment patients actually received.
ET and GnRH use was well balanced between treatment arms, as presented at ESMO 2021.
Median age 45 years, 94.9% patients received chemotherapy.
ET, endocrine therapy; GnRH, gonadotropin-releasing hormone.
Figure 1.
First ET by country in premenopausal and postmenopausal patientsa.
aCountries with enrollment ⩾100 patients.
ET, endocrine therapy.
Efficacy
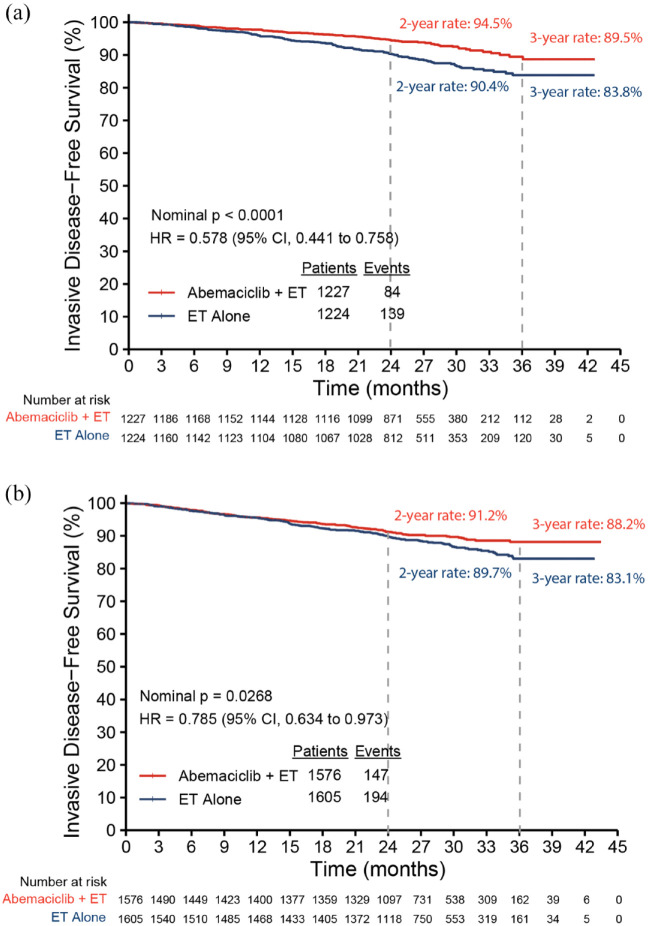
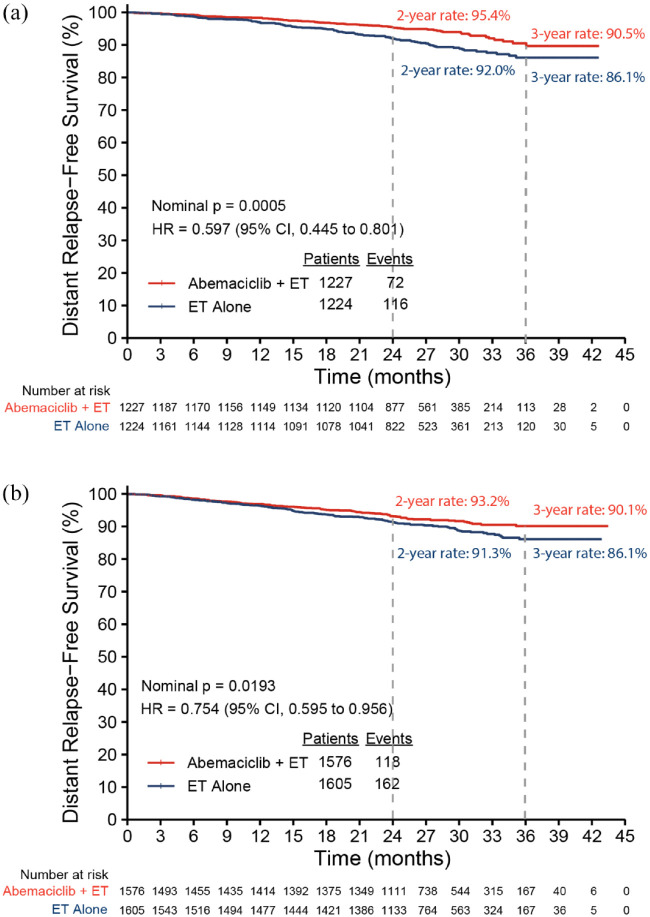
At the time of the AFU1 analysis, 223 IDFS events were identified in the subgroup of premenopausal patients, 84 in the abemaciclib plus ET arm, and 139 in the ET alone arm. Abemaciclib plus ET demonstrated a 42.2% reduction in the risk of developing an IDFS event compared to ET alone [hazard ratio (HR): 0.578, 95% confidence interval (CI): 0.441, 0.758; nominal p < 0.0001) in premenopausal patients. The 3-year IDFS rates reflected an absolute difference of 5.6% (89.5% in abemaciclib plus ET versus 83.8% in ET alone; Figure 2(a)). Most recurrences were distant metastatic disease (72 DRFS events in abemaciclib plus ET versus 116 in ET alone), and abemaciclib plus ET demonstrated a 40.3% reduction in the risk of developing distant recurrence or death (HR: 0.597, 95% CI: 0.445, 0.801; nominal p = 0.0005) in premenopausal patients. The 3-year DRFS rates showed an absolute difference of 4.4% (90.5% in abemaciclib plus ET versus 86.1% in ET alone; Figure 3(a)). For postmenopausal patients, consistent treatment benefit for IDFS (Figure 2(b); HR: 0.785, 95% CI: 0.634, 0.973; nominal p = 0.0268) and DRFS (Figure 3(b); HR: 0.754, 95% CI: 0.595, 0.956; nominal p = 0.0193) was observed, with a 3-year absolute improvement of 5.1% for IDFS and 4.0% for DRFS.
Figure 2.
Kaplan–Meier plot of IDFS by menopausal status: (a) premenopausal and (b) postmenopausal.
CI, confidence interval; ET, endocrine therapy; IDFS, invasive disease-free survival; HR, hazard ratio.
Figure 3.
Kaplan–Meier plot of DRFS by menopausal status: (a) premenopausal and (b) postmenopausal.
CI, confidence interval; DRFS, distant relapse-free survival; ET, endocrine therapy; HR, hazard ratio.
Within the premenopausal subgroup, treatment benefit was consistent for IDFS and DRFS regardless of the initial ET patients received (Table 3; interaction test p value: 0.350 for IDFS and 0.335 for DRFS). A numerically higher percentage of IDFS events was observed in patients receiving tamoxifen (13%) than in those receiving AI (9%) in the ET alone arm. For patients with tamoxifen as initial ET, abemaciclib plus ET demonstrated a 47.8% reduction in the risk of developing an IDFS event compared to ET alone (HR: 0.522, 95% CI: 0.370, 0.738), and for patients with AI as initial ET, the relative risk reduction in abemaciclib plus ET was 32.0% for IDFS (HR: 0.680, 95% CI: 0.435, 1.064).
Table 3.
Efficacy results for premenopausal patients by ET subgroups.
| Efficacy by ET subgroups | Abemaciclib + ET, n = 1227 | ET alone, n = 1224 | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| n | Events | 2-year survival rate (95% CI) | n | Events | 2-year survival rate (95% CI) | ||
| IDFS by initial ET | |||||||
| Tamoxifen | 697 | 49 | 94.4 (92.3, 95.9) | 718 | 94 | 89.6 (87.0,91.6) | 0.522 (0.37,0.74) |
| Aromatase inhibitor | 521 | 34 | 94.9 (92.5, 96.5) | 478 | 44 | 91.6 (88.6,93.8) | 0.680 (0.44,1.06) |
| DRFS by initial ET | |||||||
| Tamoxifen | 697 | 41 | 95.3 (93.4, 96.7) | 718 | 77 | 91.7 (89.4,93.6) | 0.536 (0.37,0.78) |
| Aromatase inhibitor | 521 | 31 | 95.5 (93.2,97.0) | 478 | 38 | 92.5 (89.6,94.6) | 0.719 (0.45,1.16) |
Based on ET exposure data for randomized patients at additional follow-up analysis data cutoff, by treatment to which patients were randomized. Interaction test p value: 0.350 for IDFS and 0.335 for DRFS.
CI, confidence interval; DRFS, distant relapse-free survival; ET, endocrine therapy; HR, hazard ratio; IDFS, invasive disease-free survival; yr, year.
Safety
A total of 2431 (n = 1222, abemaciclib + ET; n = 1209, ET alone) premenopausal patients received at least one dose of study treatment and were included in the safety analyses. Consistent with the previously reported safety profile in the safety population of monarchE, treatment-emergent adverse events (TEAEs) and any grade and Grade ⩾3 TEAEs were seen in more patients in the abemaciclib plus ET arm than in the ET alone arm. The most frequent TEAEs in the abemaciclib arm were diarrhea, neutropenia, leukopenia, abdominal pain, and fatigue. The most common Grade ⩾3 TEAEs were neutropenia and leukopenia. In the ET alone arm, the most frequent TEAEs were arthralgia, hot flushes, fatigue, and headache (Table 4).
Table 4.
TEAEs for premenopausal patients.
| TEAEs | Abemaciclib + ET, n = 1222, n (%) | ET alone, n = 1209, n (%) | ||||
|---|---|---|---|---|---|---|
| ⩾20% in either arm | Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 |
| Diarrhea | 1035 (84.7) | 71 (5.8) | 0 | 90 (7.4) | 4 (0.3) | 0 |
| Neutropenia | 591 (48.4) | 234 (19.1) | 9 (0.7) | 98 (8.1) | 12 (1.0) | 3 (0.2) |
| Leukopenia | 472 (38.6) | 146 (11.9) | 1 (0.1) | 105 (8.7) | 6 (0.5) | 0 |
| Abdominal paina | 469 (38.4) | 18 (1.5) | 0 | 126 (10.4) | 4 (0.3) | 0 |
| Fatiguea | 467 (38.2) | 22 (1.8) | 0 | 213 (17.6) | 1 (0.1) | 0 |
| Nauseaa | 339 (27.7) | 4 (0.3) | 0 | 106 (8.8) | 0 | 0 |
| Arthralgiaa | 301 (24.6) | 2 (0.2) | 0 | 416 (34.4) | 9 (0.7) | 0 |
| Anemia | 281 (23.0) | 11 (0.9) | 1 (0.1) | 47 (3.9) | 5 (0.4) | 0 |
| Headache | 266 (21.8) | 3 (0.2) | 0 | 213 (17.6) | 2 (0.2) | 0 |
| Hot flusha | 226 (18.5) | 2 (0.2) | 0 | 332 (27.5) | 6 (0.5) | 0 |
Patient has a maximum common terminology criteria for adverse events grade of 3.
ET, endocrine therapy; TEAEs, treatment-emergent adverse events.
Venous thromboembolic events (VTEs) were more frequent among premenopausal patients in the abemaciclib plus ET arm than among those in the ET alone arm regardless of ET choice (Table 5), and premenopausal patients taking tamoxifen had a higher incidence of VTE than those taking AI. There were no fatal VTE. Interstitial lung disease occurred more frequently in the abemaciclib arm regardless of menopausal status (3.6% versus 1.6% in premenopausal patients and 2.9% versus 1.1% in postmenopausal patients; Table 6).
Table 5.
VTEs by first ET in premenopausal patients.
| First ET received | Abemaciclib + ET, n = 697, n (%) | ET alone, n = 718, n (%) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Tamoxifena | ||||||
| VTEb | 19 (2.7) | 10 (1.4) | 1 (0.1) | 4 (0.6) | 3 (0.4) | 0 |
| Pulmonary embolism | 9 (1.3) | 9 (1.3) | 0 | 1 (0.1) | 1 (0.1) | 0 |
| Abemaciclib + ET, n = 519, n (%) | ET alone, n = 480, n (%) | |||||
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Aromatase inhibitora | ||||||
| VTEb | 5 (1.0) | 4 (0.8) | 0 | 2 (0.4) | 0 | 0 |
| Pulmonary embolism | 4 (0.8) | 4 (0.8) | 0 | 0 | 0 | 0 |
Based on ET exposure data for treated patients at additional follow-up analysis data cutoff, by treatment patients actually received.
Identified by selected terms in embolic and thrombotic events SMQ.
ET, endocrine therapy; VTEs, venous thromboembolic events.
Table 6.
Treatment-emergent interstitial lung disease events by menopausal status.
| Premenopausal | Abemaciclib + ET, n = 1222, n (%) | ET alone, n = 1209, n (%) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Interstitial lung diseasea | 44 (3.6) | 3 (0.2) | 0 | 19 (1.6) | 0 | 0 |
| Postmenopausal | Abemaciclib + ET, n = 1565, n (%) | ET alone, n = 1591, n (%) | ||||
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Interstitial lung diseasea | 45 (2.9) | 7 (0.4) | 0 | 18 (1.1) | 1 (0.1) | 0 |
Identified by interstitial lung disease events SMQ.
ET, endocrine therapy.
Discussion
The benefit of abemaciclib has been demonstrated in the metastatic BC setting regardless of menopausal status.14 These exploratory analyses present the efficacy and safety of adjuvant abemaciclib for premenopausal patients with HR+, HER2−, node-positive, high-risk EBC and show robust treatment benefit of abemaciclib plus ET compared to ET alone.
The monarchE trial enrolled a higher proportion of premenopausal women than that observed in a broad EBC patient population.15 The underlying reasons for this enrollment pattern are unknown but could include the possibilities that younger age is associated with less favorable disease characteristics or that investigators are more willing to enroll younger patients into adjuvant clinical trials testing new drugs. Among premenopausal patients, the IDFS and DRFS event rates in the control arm suggested a numerically poorer outcome in patients who received tamoxifen as their first ET than in those who received AI, noting that the majority of patients who received AI also received OFS. Although the number of events limits a robust comparison, the numerical difference between ET subgroups in the control arm was consistent with the findings in the SOFT and TEXT trials that demonstrated lower recurrence rates at 5 years among premenopausal women who received the AI exemestane plus OFS than among those who received tamoxifen with or without OFS.11 However, as there is no significant interaction effect demonstrated by the interaction p values, the benefit of adding abemaciclib to adjuvant ET was consistent regardless of initial ET.
Across menopausal status, the magnitude of relative risk reduction was greater in premenopausal patients than in postmenopausal patients. Notably, the Kaplan–Meier curves for both IDFS and DRFS in premenopausal patients separated earlier than those in postmenopausal patients, which suggests an early treatment effect. Overall, the treatment benefit of abemaciclib plus ET was generally consistent across menopausal status at initial diagnosis.
Importantly, the monarchE subgroup data reflect contemporary prescribing practices of ET in one of the largest groups of premenopausal patients since the SOFT and TEXT trials. The percentage of premenopausal patients who received their first ET of tamoxifen versus AI varied by region in the monarchE study. Tamoxifen has been widely used by patients in Japan, whereas in China, AIs have largely replaced tamoxifen. Variability was also notable across Europe and the United States with a large proportion of patients receiving tamoxifen as first ET.
While this study provides insights into the large geographic variability in ET choice, the addition of abemaciclib to ET demonstrated a consistent treatment benefit regardless of ET choice in premenopausal patients. Not all premenopausal patients received a GnRH agonist. Younger premenopausal patients (<40 years) were more likely to receive GnRH agonists in keeping with anticipated lower rates of chemotherapy-induced menopause or amenorrhea. Some patients who were premenopausal at initial diagnosis and did not receive a GnRH agonist may have been rendered postmenopausal after chemotherapy or may have undergone prophylactic oophorectomy. Differences in ET- and GnRH agonist-prescribing practices may be due to physician decision to prescribe, patient decision to receive therapy, local guidelines, reimbursement practices, or limitations in data capture.
Overall, this study demonstrates that geographic differences exist in the adoption of risk-adapted ET choice in premenopausal women. Further studies to evaluate the impact of these geographic differences may help to optimize the care of premenopausal women with BC.
The safety results in this premenopausal subgroup are consistent with the overall safety profile of abemaciclib. While the overall incidence of VTE is low, given the higher rates of VTE observed with tamoxifen use, physicians should discuss the benefits and risks of ET choice with patients.
These analyses have notable limitations. First, determination of menopausal status at the time of diagnosis rather than at randomization fails to capture changes in menopausal status induced by common medical interventions such as chemotherapy or oophorectomy. Notably, though assessing and measuring chemotherapy-induced amenorrhea is important, it is not always easy to capture, and serum testing of hormonal profiles to assess ovarian function can lead to inaccurate assessment of permanent menopause versus transient ovarian failure. Although AI without GnRH agonist is not a recommended treatment option for premenopausal women, 25% of premenopausal patients in the study who were treated with AI as first ET did not receive a GnRH agonist. The median age of these women at randomization was 45 years, and 95% had received prior chemotherapy. Thus, some of these women may have been rendered postmenopausal after chemotherapy but were categorized by menopausal status at diagnosis.
This exploratory analysis was not powered or alpha controlled for statistical testing but menopausal status was a prespecified subgroup previously reported as part of the forest plot to assess the consistency of treatment benefit across subgroups.13 At this analysis timepoint, there was a sufficient number of events within this large patient population to enable a robust statistical evaluation of the efficacy of abemaciclib plus ET by menopausal status, highlighting clinically meaningful treatment benefit for both premenopausal and postmenopausal patients. Notably, the benefit of abemaciclib in premenopausal patients was consistent regardless of initial ET. Beyond the notable efficacy of abemaciclib, this study provides insight into contemporary ET prescribing practices both generally and geographically. In summary, adjuvant abemaciclib combined with ET led to clinically meaningful treatment benefit in IDFS and DRFS regardless of menopausal status and ET choice for patients with HR+, HER2−, node-positive, high-risk EBC.
Acknowledgments
The authors and Eli Lilly and Company would like to thank the patients and their families/caregivers for participating in this trial. monarchE would not have been possible without the investigators and their support staff who participated in this work. Medical writing support was provided by Trish Huynh, employee of Eli Lilly and Company.
Footnotes
ORCID iD: Nadia Harbeck  https://orcid.org/0000-0002-9744-7372
https://orcid.org/0000-0002-9744-7372
Contributor Information
Shani Paluch-Shimon, Hadassah University Hospital & Faculty of Medicine Hebrew University, Jerusalem 91120, Israel.
Patrick Neven, Universitaire Ziekenhuizen Leuven - Campus Gasthuisberg, Leuven, Belgium.
Jens Huober, Breast Center, University of Ulm, Ulm, Germany.
Irfan Cicin, Trakya University Faculty of Medicine, Edirne, Turkey.
Matthew P. Goetz, Department of Oncology, Mayo Clinic, Rochester, MN, USA
Chikako Shimizu, National Center for Global Health and Medicine, Tokyo, Japan.
Chiun-Sheng Huang, National Taiwan University Hospital, Taipei; National Taiwan University College of Medicine, Taipei.
Hans Joachim Lueck, Gynäkologisch-Onkologische Praxis Hannover, Hannover, Germany.
Jane Beith, Chris O’Brien Lifehouse, Camperdown, NSW, Australia.
Eriko Tokunaga, Department of Breast Oncology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan.
Jessica Reyes Contreras, Oncológico Potosino, San Luis Potosí, México.
Rosane Oliveira de Sant’Ana, Division of Clinical Oncology, Instituto do Câncer do Ceará, Fortaleza, Brazil; Universidade de Fortaleza, Fortaleza, Brazil.
Ran Wei, Eli Lilly and Company, Indianapolis, IN, USA.
Ashwin Shahir, Eli Lilly and Company, Indianapolis, IN, USA.
Sarah C. Nabinger, Eli Lilly and Company, Indianapolis, IN, USA
Tammy Forrester, Eli Lilly and Company, Indianapolis, IN, USA.
Stephen R. D. Johnston, Royal Marsden NHS Foundation Trust, London, UK
Nadia Harbeck, Breast Center, Department of Gynecology and Obstetrics and Comprehensive Cancer Center Munich, LMU University Hospital, Munich, Germany.
Declarations
Ethics approval and consent to participate: The study protocol and all amendments were approved by ethical institutional review boards before implementation, and all patients provided written informed consent. The study was performed in compliance with the Declaration of Helsinki.
Consent for publication: Not applicable.
Author contribution(s): Shani Paluch-Shimon: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing.
Patrick Neven: Formal analysis, Investigation, Writing – review & editing.
Jens Huober: Investigation, Writing – review & editing.
Irfan Cicin: Conceptualization, Investigation, Writing – review & editing.
Matthew P. Goetz: Formal analysis, Investigation, Writing – original draft, Writing – review & editing.
Chikako Shimizu: Investigation, Writing – review & editing.
Chiun-Sheng Huang: Investigation, Writing – review & editing.
Hans Joachim Lueck: Investigation, Writing – review & editing.
Jane Beith: Investigation, Writing – review & editing.
Eriko Tokunaga: Investigation, Writing – review & editing.
Jessica Reyes Contreras: Investigation, Writing – review & editing.
Rosane Oliveira de Sant’Ana: Investigation, Writing – review & editing.
Ran Wei: Formal analysis, Investigation, Writing – review & editing.
Ashwin Shahir: Formal analysis, Investigation, Writing – review & editing.
Sarah C. Nabinger: Formal analysis, Investigation, Writing – review & editing.
Tammy Forrester: Formal analysis, Investigation, Writing – review & editing.
Stephen R. D. Johnston: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing.
Nadia Harbeck: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All writing, editorial assistance, and statistical analysis were funded by Eli Lilly and Company. This work was supported by the sponsor (Eli Lilly and Company) and designed together with the study Executive Committee (no grant number).
SPS reports fees for from Pfizer for corporate-sponsored research; fees for speaker’s bureau, honoraria, consultancy from Roche, Novartis, Pfizer, and AstraZeneca; fees for consultancy from Eli Lilly and Company and Gilead; and fees for speaker’s bureau and consultancy from MSD, outside the submitted work. PN reports institutional fees for consultancy from Pfizer, Novartis, Eli Lilly and Company, Roche, and Astrazeneca; institutional fees for advisory board participation from Pfizer, Novartis, Eli Lilly and Company, Roche, and Sanofi; institutional fees for lecturing and attendance at scientific exchange meetings, and research funding from Kom op Tegan Kanker, outside the submitted work. JH receives fees for advisory board participation from Eli Lilly and Company, Novartis, Roche, Pfizer, Hexal, AstraZeneca, MSD, Celgene, and Abbvie; fees for corporate-sponsored research from Celgene, Novartis, Hexal, and Eli Lilly and Company; and fees for travel expenses from Roche, Pfizer, Novartis, Celgene, and Daiichi Sankyo, outside the submitted work; IC has nothing to disclose, outside the submitted work; MPG reports institutional fees for consultancy from Eli Lilly and Company, Biovica, Novartis, Sermonix, Context Pharm, Pfizer, AstraZeneca, Eagle Pharmaceuticals; personal fees from Genomic Health; institutional grant from Pfizer, Eli Lilly and Company, Sermonix; institutional honoraria for advisory board participation from ARC Therapeutics, Blueprint Medicines, Sanofi Genzyme, Biotheranostics; personal fees for CME presentation from Research to Practice, Clinical Education Alliance, Medscape; personal fees for session moderating from Curio Science; personal fees for serving as a panelist from Total Health Conferencing, outside the submitted work; CS reports grants for corporate-sponsored research from Eli Lilly and Company, outside the submitted work. CSH reports personal fees and grants for advisory board participation and speaker’s bureau from Eli Lilly and Company, Novartis, and Daiichi Sankyo; grants, personal fees, and non-financial support for advisory board participation, and speaker’s bureau from Pfizer, Roche and AstraZeneca; grants from EirGenix and OBI Pharma, grants and personal fees for speaker’s bureaus from MSD; outside the submitted work. HJL reports fees for corporate-sponsored research and travel support from Eli Lilly and Company, outside the submitted work. JB reports institution fees for advisory board participation from Eli Lilly and Company, Roche, and Pfizer, outside the submitted work. ET reports personal fees for lectures from Eli Lilly and Company, AstraZeneca and Daiichi Sankyo, outside the submitted work. JRC reports corporate-sponsored research fees from Eli Lilly and Company. ROS reports corporate-sponsored research fees from Eli Lilly and Company. RW, AS, SCN, TF are employees and stock shareholders of Eli Lilly and Company. SRDJ reports personal fees for consulting, advisory role, speaker’s bureau, and research funding from AstraZeneca, Novartis, Pfizer; personal fees for consulting, advisory role, research funding from Eli Lilly and Company, Puma Biotechnology; personal fees for speaker’s bureau from Eisai; and personal fees for speaker’s bureau, research funding from Roche/Genentech, outside the submitted work. NH reports personal fees for lectures and consulting from AstraZeneca, Eli Lilly and Company, Novartis, and Pfizer, outside the submitted work.
Availability of data and materials: Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available on request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 2021; 32: 1571–1581. [DOI] [PubMed] [Google Scholar]
- 2. Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016; 34: 3308–3314. [DOI] [PubMed] [Google Scholar]
- 3. Fu J, Zhong C, Wu L, et al. Young patients with hormone receptor-positive breast cancer have a higher long-term risk of breast cancer specific death. J Breast Cancer 2019; 22: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong W, Tan L, Jiang WG, et al. Effect of younger age on survival outcomes in T1N0M0 breast cancer: a propensity score matching analysis. J Surg Oncol 2019; 119: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 5. Kan Z, Ding Y, Kim J, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun 2018; 9: 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan JW, Zabidi MMA, Ng PS, et al. The molecular landscape of Asian breast cancers reveals clinically relevant population-specific differences. Nat Commun 2020; 11: 6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Cancer Institute. Surveillance E, and end results program. Cancer stat facts: female breast cancer. SEER 22 2015–2019, all races, females, https://seer.cancer.gov/statfacts/html/breast.html (accessed 2022).
- 8. Lin CH, Yap YS, Lee KH, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst 2019; 111: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neven P, Rugo HS, Tolaney SM, et al. Abemaciclib plus fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in premenopausal women: subgroup analysis from the MONARCH 2 trial. Breast Cancer Res 2021; 23: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015; 372: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018; 379: 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020; 38: 3987–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neven P, Rugo HS, Tolaney SM, et al. Abemaciclib for pre/perimenopausal women with HR+, HER2- advanced breast cancer. J Clin Oncol 2018; 36: 1002. [Google Scholar]
- 15. Heer E, Harper A, Escandor N, et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Global Health 2020; 8: e1027–e1037. [DOI] [PubMed] [Google Scholar]