Abstract
Angiolipomatous hamartoma is a benign mesenchymal proliferation of unknown aetiology. Only a few cases have been documented in the published literature. This current case report describes a 57-year-old female patient who was hospitalized for an assessment of a previously radiologically-verified splenic lesion and further treatment. The patient had been surgically treated 10 years previously; a lobectomy of the superior left pulmonary lobe had been performed in order to remove a verified tumour lesion. A complete radiological examination was undertaken, which verified a spleen of a size that was within the physiological range, with a centrally-located lobular tumour lesion. Given the risk of splenic rupture, as well as the fact that the lesion’s aetiology was still undetermined, and finally the fact that differential diagnostics indicated the possibility of a metastasis, the patient was treated surgically. Laparoscopic splenectomy, in the treatment of splenic diseases, even rare ones such as this, is not a novelty. Indeed, it needs to be applied as the standard approach, with the well-known benefits that the minimalized approach offers.
Keywords: Spleen, splenic tumour, laparoscopic splenectomy, angiolipoma, angliolipomatous hamartoma
Introduction
Angiolipomatous hamartoma (ALH) is a benign mesenchymal proliferation of unknown aetiology.1 Only a few cases have been documented in the current literature.2 ALHs are mainly detected in cervical, mediastinal and retroperitoneal lymph nodes,1 although there have also been reports of extranodal localization.2This current case report presents the first case of isolated primary ALH in the spleen and a review of the relevant literature.
Case report
In October 2021, a 57-year-old female patient was hospitalized in the Department for Hepato-Pancreato-Biliary Surgery, University Clinic for Digestive Surgery, University Clinical Centre of Serbia, Belgrade, Serbia or an assessment of a previously radiologically-verified splenic lesion and further treatment. A review of her medical records revealed that the patient had been surgically treated 10 years previously. This previous surgery included a lobectomy of the superior left pulmonary lobe to remove a verified tumour lesion, which upon pathohistological analysis, was classified as a pulmonary adenocarcinoma, acinar type, stage T2aN1MX. The patient had a family history of pulmonary carcinoma (her father suffered from the disease). After the aforementioned operation, the patient was regularly followed up and monitored by an oncologist. A year ago, within the post-surgery oncological follow-up, a 3-cm lesion of undetermined aetiology was discovered in the central region of the spleen as an incidental finding. Subsequent abdominal computed tomography (CT) examinations demonstrated a progressive enlargement of the splenic tumour. The density of the mass detected was –50.3 Hounsfield units. Given the risk of splenic rupture, as well as the fact that the lesion’s aetiology remained undetermined, and the fact that differential diagnostics indicated the possibility of metastasis, the oncology council decided to surgically treat the patient.
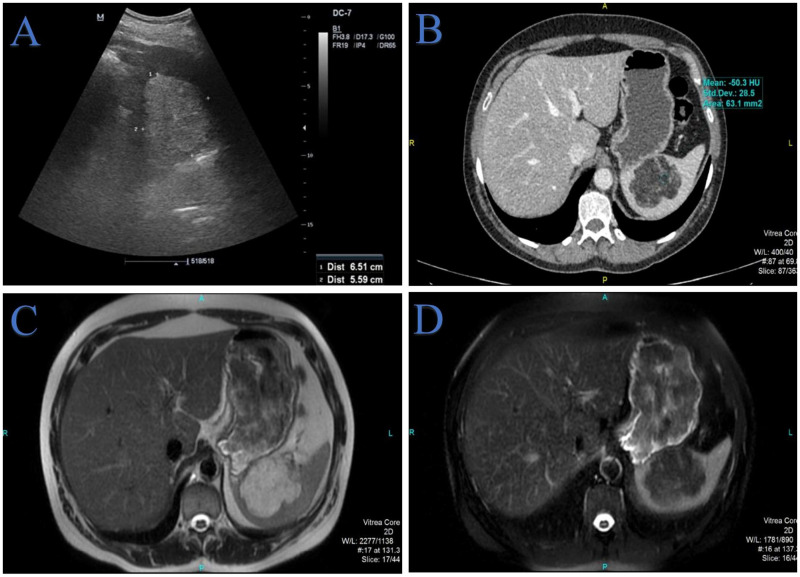
Upon admission, a complete radiological examination was undertaken, which verified a spleen of a size that was within the physiological range, with a centrally-located lobular tumour lesion with an irregular shape. The measurements of the aforementioned tumour lesion, in terms of the anteroposterior, latero-lateral and craniocaudal diameters, were 55 × 65 × 59 mm, respectively (Figure 1). Considering the previous malignancy, a positron emission tomography/computed tomography (PET/CT) scan was undertaken. The finding did not show an accumulation of the radiopharmaceutical agent in the spleen. The patient was without any complaints. She denied smoking cigarettes, consuming alcohol or using illegal drugs. Laboratory markers were within the normal reference range. The tumour markers cancer antigen 19-9 and carcinoembryonic antigen were within the normal ranges.
Figure 1.
Preoperative radiological examination of a 57-year-old female patient who was hospitalized for an assessment of a previously radiologically-verified splenic lesion and further treatment: (a) an abdominal ultrasound examination showed a 6.5-cm splenic mass that was markedly hyperechoic relative to the parenchyma and accompanied by acoustic shadowing; (b) an axial multidetector computed tomography scan of the abdomen in the portal-venous phase of the examination clearly showed a hypodense, lobulated and clearly demarcated mass in the spleen. The measured density of –50.3 Hounsfield units corresponds to the fatty component of the lesion with well-enhancing vessels intralesionally; (c) an axial T2-weighted magnetic resonance (MR) image showed a hyperintense intraparenchymal lesion of the spleen due to the large amount of lipomatous content and (d) an axial fat-suppressed T2-weighted MR image clearly showed suppression of the fat.
Laparoscopic splenectomy was performed under general endotracheal anaesthesia. Before performing the procedure, the surgical team placed a urethral catheter, nasogastric suction and an elastic bandage on the lower extremities to prevent deep vein thrombosis. The patient was placed in the right lateral decubitus position, with the left arm extended, on the operating table, which was rotated to the right and flexed by approximately 40 degrees. The surgeon and the camera operator stood on the right side of the patient, and the scrub nurse on the left side. Through a small incision immediately under the umbilicus, by the use of a Veress needle, an artificial pneumoperitoneum was created with a pressure of 12 mmHg. The first 10-mm port was placed through the same incision and the laparoscope (Karl Storz Endoscope; Karl Storz, Tuttlingen, Germany) was introduced into the abdominal cavity. Thereafter, under direct laparoscopic vision, three more working ports were deployed at typical sites on the left subcostal arch. Approximately 5 cm on the line from the umbilicus to the left costal margin, a 12-mm working port was placed. Two 5-mm trocars were placed below to left subcostal margin, one subxiphoid, and one along the anterior axillary line (Figure 2a). A laparoscopic harmonic scalpel (Ethicon Endo-Surgery, Blue Ash, OH, USA) was used to release the spleen from its ligaments and the short gastric vessels (Figure 2b). The splenic hilum was meticulously dissected with identification of the splenic artery and vein. Vessels were ligated with hem-o-lock clips and dissected (Figure 2c). A splenic specimen was placed in an Endobag™ (Medtronic, Minneapolis, MN, USA) (Figure 2d). The 12-mm incision was enlarged somewhat to allow for easy passage of a ring forceps into the bag, the specimen was fragmented with the forceps, removed from the abdomen and sent for histopathological examination.
Figure 2.
Laparoscopic splenectomy information for a 57-year-old female patient who was hospitalized for an assessment of a previously radiologically-verified splenic lesion and further treatment: (a) port sites for the laparoscopic splenectomy; (b) intraoperative presentation of the splenic tumour (arrow). Short gastric vessels were divided using a laparoscopic harmonic scalpel; (c) vessels were ligated with hem-o-lock clips and (d) splenic specimen placed into a nylon Endobag™.
The patient was discharged from the hospital on the third day after the procedure, with prescribed antibiotic prophylaxis and necessary post-splenectomy immunization, in line with the current guidelines in the literature.3
After the surgical procedure and the initial histopathological examination, the differential diagnosis offered only the options of angiomyolipoma and well-differentiated liposarcoma. Pathohistological examination of the spleen tissue showed non-circumscribed benign mesenchymal proliferation of haphazard muscular blood vessels of varying sizes and mature fatty tissue (Figure 3), which was consistent with diagnosis of ALH. A lack of other mesenchymal tissue types, such as smooth muscle, excluded a diagnosis of (extrarenal) angiomyolipoma (AML). AML is a sharply demarcated tumour, but the current lesion looked more like a hamartoma than a real neoplasm. Immunohistochemistry also showed an absence of melanocytic markers (HMB-45, Melan A, S-100) and muscle specific actin (HHF35) in fat and blood vessel components, which was consistent with a diagnosis of ALH. Endothelial cells of the vascular component of the ALH were cluster of differentiation (CD)31, CD34 and factor VIII positive, CD8 negative, without any atypia or mitoses. Loss of CD8 demonstrated that the spleen architecture was being replaced by a space-occupying lesion. Immunohistochemical staining for smooth muscle actin was positive just in the vessel walls. There were no thrombi within the vascular channels. Adipose tissue (S-100+) was mature, with no necrosis, dense sclerosis, lipoblasts or marked cytologic atypia, which distinguishes ALH from well-differentiated liposarcoma.
Figure 3.
Pathohistological examination of a splenic tissue sample from a 57-year-old female patient who was hospitalized for an assessment of a previously radiologically-verified splenic lesion and further treatment: (a) non-circumscribed benign mesenchymal proliferation with vascular and fatty components, surrounded by residual splenic tissue (haematoxylin & eosin; scale bar 5000 µm); (b) mature adipose tissue was more predominant in some parts of lesion, with no necrosis, dense sclerosis, lipoblasts or marked cytologic atypia (haematoxylin & eosin; scale bar 2000 µm); (c) cluster of differentiation (CD)31 immunohistochemical staining of haphazard thick-walled muscular vessels (scale bar 500 µm) and (d) CD34 immunohistochemical staining of vessels of varying sizes inside the adipose tissue (scale bar 500 µm).
Written informed consent was obtained from the patient for publication of this case report. This study conforms to the CARE guidelines.4
Discussion
Angiolipomatous hamartoma is a rare disease of unknown aetiology, which can be categorized more as a pseudotumour rather than a typical neoplasm.1 It most commonly presents in the form of yellow-coloured unencapsulated lymph nodes with a size of approximately 15 mm.2 They can be found as individual or multiple tumours.1
A search of the electronic databases (Pubmed®, EMBASE, Medline, Scopus and Google Scholar) using ‘angiolipomatous hamartoma and spleen’ in English did not identify a single documented case of the presence of this type of neoplasm in the spleen. A further search of the same electronic databases yielded five case reports describing this type of neoplasm in different anatomical localizations, which are presented in Table 1.5–9
Table 1.
The majority of splenic lesions are identified by CT scan or ultrasonography. In the case of finding an isolated splenic lesion >1 cm during an oncological follow-up, splenic metastases should be suspected.10 Radiological diagnostics can mainly provide approximate data, which can be useful in further diagnostics and treatment, although, in most cases of this type, a definitive diagnosis is established after histopathological verification.11–13 Studies have shown that PET/CT can be used to help discriminate benign from malignant splenic masses.14,15 Moreover, PET/CT can identify additional unsuspected sites of disease.14,15 Although PET/CT is superior to other radiological techniques, there are still a certain number of false-positive or false-negative findings.15
It is considered by some authors that splenic biopsy can be a reasonable and useful diagnostic method for diagnosing lesions of an uncertain nature.16,17 Apart from the risk of possible splenic rupture and bleeding, considering the coexistence with other changes, a splenic biopsy might not be reliable. Also, possible peritoneal dissemination is another reason why this biopsy is not advisable.18 In our opinion, splenectomy, either classical or laparoscopic, is at the same time the best diagnostic and therapeutic method.
Preoperative abdominal CT and magnetic resonance imaging showed that the described lesion displayed characteristic solid areas rich in extracellular lipids, with postcontrast opacification of the sparse internal stroma of the lesion. Considering our surgical department’s years of experience in treating rare diseases and tumours of the spleen, as well as the fact that the lesion was positioned centrally in the spleen, a less radical procedure was not an option, which is why a splenectomy was undertaken in the current case.13,19,20
In conclusion, an isolated ALH of the spleen is an extremely rare finding that, as yet, has never been documented in the current literature. Given the small number of documented cases of this type of neoplasm with different anatomical localizations, each new case must be approached individually and in a multidisciplinary fashion in order to gain new insights and to draw new conclusions. Laparoscopic splenectomy, in the treatment of splenic diseases, even in rare ones such as this, is not a novelty, rather it needs to be applied as the standard approach, with the well-known benefits that the minimalized approach offers. Also, one must keep in mind that, in cases such as these, splenectomy is performed both as a therapeutic and as a diagnostic procedure.
Research Data
Supplemental material, sj-pdf-1-imr-10.1177_03000605231153767 for Laparoscopic approach in the treatment of splenic angiolipomatous hamartoma: the first report of a case by Vladimir M. Milosavljevic, Boris S. Tadic, Nikola M. Grubor, Milica D. Mitrovic, Miljan S. Ceranic, Borislav L. Toskovic and Tatjana T. Terzic in Journal of International Medical Research
Acknowledgements
We thank Dr Bogdan Crnokrak for assistance in improving images and language style; and Dr Marija Zdravkovic for comments that greatly improved the manuscript.
Footnotes
Author contributions: Conceptualization: V.M.M. and B.S.T.; methodology: N.M.G. and V.M.M.; resources: B.L.T., N.M.G. and M.D.M.; writing the original draft: M.D.M., B.S.T and V.M.M.; writing – review and editing: M.S.C., B.L.T. and M.D.M.; supervision: B.T. and T.T.T.
The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Vladimir M. Milosavljevic https://orcid.org/0000-0002-6998-2116
Boris S. Tadic https://orcid.org/0000-0001-5400-1015
References
- 1.Tsang P. Angiolipomatous hamartoma. PathologyOutlines.com website, https://www.pathologyoutlines.com/topic/lymphnodesangiolipomatoushyperplasia.html (2013, accessed 19 January 2023).
- 2.Bisceglia M, Spagnolo D, Galliani C, et al. Tumoral, quasitumoral and pseudotumoral lesions of the superficial and somatic soft tissue: new entities and new variants of old entities recorded during the last 25 years. Part XII: appendix. Pathologica 2006; 98: 239–298. 2006/12/21. [PubMed] [Google Scholar]
- 3.Bonanni P, Grazzini M, Niccolai G, et al. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum Vaccin Immunother 2017; 13: 359–368. 2016/12/09. DOI: 10.1080/21645515.2017.1264797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013; 2: 38–43. 2014/01/15. DOI: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madero S, Onate JM, Garzon A. Giant lymph node hyperplasia in an angiolipomatous mediastinal mass. Arch Pathol Lab Med 1986; 110: 853–855. 1986/09/01. [PubMed] [Google Scholar]
- 6.Ide F, Shimoyama T, Horie N. Vascular transformation of sinuses in bilateral cervical lymph nodes. Head Neck 1999; 21: 366–369. 1999/06/22. DOI: 10.1002/(sici)1097-0347(199907)21:4<366::aid-hed12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Kakiuchi C, Ishida T, Sato H, et al. Secretion of interleukin-6 and vascular endothelial growth factor by spindle cell sarcoma complicating Castleman's disease (so-called ‘vascular neoplasia'). J Pathol 2002; 197: 264–271. 2002/05/17. DOI: 10.1002/path.1110. [DOI] [PubMed] [Google Scholar]
- 8.White NJ, Cochrane DD, Beauchamp R. Paraparesis caused by an angiolipomatous hamartoma in an adolescent with Proteus syndrome and scoliosis. J Neurosurg 2005; 103: 282–284. 2005/10/22. DOI: 10.3171/ped.2005.103.3.0282. [DOI] [PubMed] [Google Scholar]
- 9.Demir R, Schmid A, Hohenberger W, et al. Angiolipomatous mesenchymal hamartoma (angiolipomatosis) of the sigmoid mesocolon. Int J Clin Exp Pathol 2011; 4: 210–214. 2011/02/18. [PMC free article] [PubMed] [Google Scholar]
- 10.Heller MT, Harisinghani M, Neitlich JD, et al. Managing incidental findings on abdominal and pelvic CT and MRI, part 3: white paper of the ACR Incidental Findings Committee II on splenic and nodal findings. J Am Coll Radiol 2013; 10: 833–839. 2013/11/05. DOI: 10.1016/j.jacr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Smoot RL, Truty MJ, Nagorney DM. Splenectomy for Conditions Other Than Trauma. Shackelford's Surgery of the Alimentary Tract 2019; 2: 1635–1653. [Google Scholar]
- 12.Kaza RK, Azar S, Al-Hawary MM, et al. Primary and secondary neoplasms of the spleen. Cancer Imaging 2010; 10: 173–182. 2010/08/18. DOI: 10.1102/1470-7330.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milosavljevic V, Tadic B, Gonzalez-Urquijo M, et al. Myoid Angioendothelioma: A Rare Benign Splenic Tumor. Balkan Med J 2021; 38: 190–191. 2021/02/18. DOI: 10.5152/balkanmedj.2021.20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji T, Kuang A. 18F-FDG PET/CT Findings in a Splenic Lymphangioma. Clin Nucl Med 2015; 40: e375–e377. 2015/05/29. DOI: 10.1097/RLU.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 15.Metser U, Miller E, Kessler A, et al. Solid splenic masses: evaluation with 18F-FDG PET/CT. J Nucl Med 2005; 46: 52–59. 2005/01/06. [PubMed] [Google Scholar]
- 16.Gonzalez-Urquijo M, Rodarte-Shade M, Gil-Galindo G. Splenic Primary Solid Tumors: Does a Preoperative Histopathology Diagnosis Really Matter? Am Surg 2021; 87: 316–320. 2020/09/17. DOI: 10.1177/0003134820951480. [DOI] [PubMed] [Google Scholar]
- 17.Weinreb I, Bailey D, Battaglia D, et al. CD30 and Epstein-Barr virus RNA expression in sclerosing angiomatoid nodular transformation of spleen. Virchows Arch 2007; 451: 73–79. 2007/05/12. DOI: 10.1007/s00428-007-0422-7. [DOI] [PubMed] [Google Scholar]
- 18.Keogan MT, Freed KS, Paulson EK, et al. Imaging-guided percutaneous biopsy of focal splenic lesions: update on safety and effectiveness. AJR Am J Roentgenol 1999; 172: 933–937. 1999/12/10. DOI: 10.2214/ajr.172.4.10587123. [DOI] [PubMed] [Google Scholar]
- 19.Milosavljević V, Tadić B, Grubor N, et al. Laparoscopic Vs. Open Surgery in Management of Benign Neoplasms of Spleen – Single Institution Experience. Indian Journal of Surgery 2019; 82: 355–359. DOI: 10.1007/s12262-019-01974-5. [Google Scholar]
- 20.Reljic M, Tadic B, Stosic K, et al. Isolated Splenic Metastasis of Primary Lung Cancer Presented as Metachronous Oligometastatic Disease – A Case Report. Diagnostics (Basel) 2022; 12: 209. 2022/01/22. DOI: 10.3390/diagnostics12010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231153767 for Laparoscopic approach in the treatment of splenic angiolipomatous hamartoma: the first report of a case by Vladimir M. Milosavljevic, Boris S. Tadic, Nikola M. Grubor, Milica D. Mitrovic, Miljan S. Ceranic, Borislav L. Toskovic and Tatjana T. Terzic in Journal of International Medical Research