Abstract
Background
Coronary artery stenosis (CAS) ≥50% often coexists in patients with ischemic stroke, which leads to a significant increase in the occurrence of major vascular events after stroke. This study aimed to develop a nomogram for diagnosing the presence of ≥50% asymptomatic CAS in patients with ischemic stroke.
Methods
A primary cohort was established that included 275 non-cardioembolic ischemic stroke patients who were admitted from January 2011 to April 2013 to a teaching hospital in southern China. The preoperative data were used to construct two models by the best subset regression and the forward stepwise regression methods, and a nomogram between these models was established. The assessment of the nomogram was carried out by discrimination and calibration in an internal cohort.
Results
Out of the two models, model 1 contained eight clinical-related variables and exhibited the lowest Akaike Information Criterion value (322.26) and highest concordance index 0.716 (95% CI, 0.654-0.778). The nomogram showed good calibration and significant clinical benefit according to calibration curves and the decision curve analysis.
Conclusion
The nomogram, composed of age, sex, NIHSS score on admission, hypertension history, fast glucose level, HDL cholesterol level, LDL cholesterol level, and presence of ≥50% cervicocephalic artery stenosis, can be used for prediction of ≥50% asymptomatic coronary artery disease (CAD). Further studies are needed to validate the effectiveness of this nomogram in other populations.
Keywords: Acute ischemic stroke, coronary artery disease, coronary computed tomography angiography, cerebral angiography, nomogram
1. INTRODUCTION
Ischemic heart disease and stroke are the leading causes of mortality among adults aged 50 and over around the world [1] Patients suffering a transient ischemic attack (TIA) or ischemic stroke were found to be at high risk of coronary artery disease (CAD) [2] CAD is strongly associated with a high risk of long-term myocardial infarction (MI) after ischemic stroke or TIA, [3, 4] long-term recurrent ischemic stroke, [5] and is the leading cause of long-term mortality in patients with stroke [6]. Compared to patients with ischemic stroke with no coronary artery stenosis (CAS), the risk for 2-year combined major vascular events was 4.36× higher for those with asymptomatic CAS ≥50% [7]. Invasive coronary angiography cannot be routinely performed in patients with cerebral artery atherosclerosis [8]. Therefore, several studies have begun to explore predictive models to screen patients with ischemic stroke who are at high risk of presence of ≥50% asymptomatic CAD [2, 9-11]
The traditional statistical strategies only adopted the variables which were significant on univariate analysis to establish the final prediction models, which led to model overfitting, thus showing poor results [12]. Several advanced statistical methodologies have been developed to minimize this limitation, such as the best subsets regression (BSR) and the forward stepwise regression (FSR) methods [13]. The nomogram is a graphical statistical instrument that incorporates variables to develop a continuous scoring system and calculates the precise risk probability of a particular outcome for an individual patient [14]. This instrument is an important component of modern medical decision making and has been used in an extensive array of applications including cancer, stroke, surgery, and other specialties [13, 15-17]. To date, a nomogram model with adequate power to detect the probability of asymptomatic CAD in ischemic stroke patients has not yet been designed.
Therefore, our study aimed to develop an effective nomogram for prediction of asymptomatic CAD in non-cardioembolic stroke patients by using preexisting vascular risk factors, feasible baseline measurements, and adopting advanced statistical analysis.
2. PATIENTS AND METHODS
2.1. Patient Selection
This was a single-center, cross-sectional study. Data from patients admitted to the stroke unit of the second affiliated hospital of Guangzhou Medical University from January 2011 to April 2013 were collected for this study if they meet the following selection criteria: at least 18 years old; experienced an ischemic stroke within 14 days after the onset of symptoms; no prior history of CAD (i.e., angiographically confirmed CAD, unstable angina, coronary artery stent, or angioplasty/coronary artery bypass graft); suspected to have non-atherosclerotic arterial stenosis, such as arterial dissection and vasculitis, patients who undergone revascularization procedures, or undergone radiotherapy for nasopharyngeal cancer, patients without both cervicocephalic digital subtract angiography (DSA) and coronary multi-detector computed tomography angiography (MDCTA), were not eligible. The study was approved by the ethics committee of the Second Affiliated Hospital of Guangzhou Medical University (No. 2021-hs-27) and was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The informed consent of patients was waived because of its retrospective design.
2.2. Demographics and Clinical Characteristics
Demographic data and data of vascular risk factors (hypertension history, diabetes mellitus history, hyperlipidemia, ischemic stroke history, CAD, active or past smoking) were collected from medical records. Routine blood tests, fast blood glucose, and lipid profile were measured within 48 h after admission. Systolic and diastolic blood pressures were measured in the supine position upon admission. All patients were subjected to brain magnetic resonance imaging with spin-echo diffusion-weighted imaging or computed tomography scan and 12-lead electrocardiography.
2.3. Cervicocephalic Atherosclerosis Assessment
Imaging of cervical and intracranial arteries consisted of cervical and intracranial DSA in all patients. Cervicocephalic atherosclerosis was assessed using standardized methods [9]. The cervicocephalic arteries were divided into nine segments: common carotid, extracranial carotid, intracranial carotid, middle cerebral, anterior cerebral, extracranial vertebral, intracranial vertebral, basilar, and posterior vertebral arteries. The existence of cervicocephalic artery disease (CVD) was confirmed when there was stenosis ≥50% in any of the abovementioned arteries [9].
2.4. Coronary Stenosis Measurement
The presence of coronary artery atherosclerosis was assessed using 64-section CT coronary angiography. All 64-section CT coronary angiographies were reviewed by two experienced radiologists blinded to the clinical data and results of cervicocephalic atherosclerosis assessment. The existence of CAS was confirmed when there was stenosis ≥50% in at least one segment at the four main coronary branches, which are the left main, left anterior descending, left circumflex, and right coronary arteries [18].
2.5. Selection of Variables
To construct the nomogram, we used two methods to select the significant predictors of asymptomatic CAD. In order to avoid over-fitting or under-fitting of the model, three advanced statistical methods, best subset regression (BSR) and forward stepwise regression, were adopted to select variables in the primary cohort. The criteria of variable selection for the BSR and the forward stepwise regressions were determined by the Bayesian information criterion (BIC) [13].
2.6. Model Development
CAD-related predictive models were established in the primary cohort based on the selected variables by adopting binary logistic regression. Eventually, we developed two models: (a) model 1 consisted of eight variables according to the BSR, (b) model 2 consisted of six variables according to the forward stepwise regression. The final model was determined by the Akaike Information Criterion (AIC), [19] the receiver operating characteristic (ROC) curves, and the Harrell concordance index (C-index). The nomogram was derived from the final model.
2.7. Performance of the Nomogram
The model was internally validated using all data of the training cohort by 10-fold cross-validation. Discriminative performance was measured by C-index. Calibration was tested using a calibration plot with bootstraps of 500 resamples, which described the degree of fit between actual and nomogram-predicted CAD.
2.8. Clinical Usage
The decision curve analysis (DCA) was used to assess the clinical usage of nomogram. Detailed descriptions of the DCA have been previously reported [15]. Results were considered statistically significant at p < 0.05.
2.9. Statistical Analysis
Categorical variables were expressed as numbers (percentage) and continuous variables as medians (quartile). Differences in baseline characteristics between groups for continuous variables were assessed using Mann-Whitney U test, and the Chi-squared test or Fisher’s exact test was used for categorical variables according to their sample size. Proportions were calculated for categorical variables, dividing the number of events by the total number after excluding missing/unknown cases.
Differences between groups were considered statistically significant at p < 0.05. SPSS 21.0 (IBM Corp., Armonk, NY, USA), R statistical software (http://www.R-project.org, The R Foundation), and Free Statistics software version 1.4 was used for statistical analysis.
3. RESULTS
3.1. Clinical Characteristics
The flow chart outlining the patient inclusion process is shown in Fig. (1). A total of 275 patients (200 males and 75 females) were included in the study. The mean age of enrolled subjects was 63.3±10.1 years. A total of 88 acute stroke patients had manifestations of CAS, giving a prevalence rate of 32.0%.
Fig. (1).
Flowchart of patients’ enrollment.
The risk factors for CAD and the comparisons between groups with and without CAD are shown in Table 1. Age, hypertension history, diabetes mellitus history, National Institutes of Health Stroke Scale Score (NIHSS) on admission, fast blood glucose level, high-density lipoprotein cholesterol (HDL-C) level, and presence of ≥50% stenosis in the cervicocephalic artery were significantly enriched in the group with CAD compared to the groups without CAD (all p < 0.05). All risk factors shown in Table 1 were adopted in the variables selection for prediction model development.
Table 1.
Risk factors for ≥50% asymptomatic coronary artery disease (Univariate Analysis).
| Variables |
Total
(n = 275) |
Non-CAD
(n = 187) |
CAD
(n = 88) |
p-value |
|---|---|---|---|---|
| Male, n (%) | 200 (72.7) | 132 (70.2) | 71 (78) | 0.310 |
| Age, mean (SD) | 63.3 (10.1) | 62.4 (10.2) | 65.2 (9.5) | 0.030 |
| Ischemic stroke history, n (%) | 55 (20.0) | 32 (17.1) | 23 (26.1) | 0.113 |
| Hypertension, n (%) | 207 (75.3) | 128 (68.4) | 79 (89.8) | < 0.001 |
| Diabetes mellitus, n (%) | 85 (30.9) | 48 (25.7) | 37 (42.0) | 0.009 |
| Hyperlipidemia, n (%) | 142 (51.6) | 94 (50.3) | 48 (54.5) | 0.594 |
| Smoking (active or past), n (%) | 93 (33.8) | 61 (32.6) | 32 (36.4) | 0.634 |
| NIHSS on admission (median, IQR) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 4.0 (2.0, 7.0) | 0.013 |
| Glucose, mean (SD) | 5.9 (2.4) | 5.6 (2.1) | 6.5 (2.8) | 0.006 |
| Triglycerides, median (IQR) | 1.4 (1.0, 1.8) | 1.3 (1.0, 1.8) | 1.4 (1.2, 1.9) | 0.102 |
| HDL-C, mean (SD) | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.2) | 0.007 |
| LDL-C, mean (SD) | 2.8 (0.9) | 2.8 (0.8) | 2.9 (1.0) | 0.238 |
| Presence of CVA ≥50% stenosis, n (%) | 144 (52.4) | 87 (46.5) | 57 (64.8) | 0.007 |
Note: CAD, coronary artery disease; NIHSS, National Institutes of Health Stroke Scale; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; CVA, cervicocephalic artery.
3.2. Selection of Variables Using the BSR and the Stepwise Regression
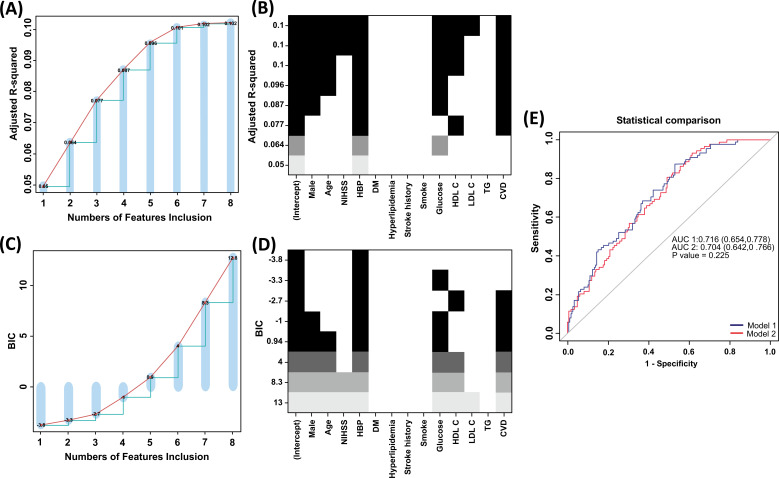
The BSR method showed great benefits in variable selection because all possible combinations of variables were calculated and the final selected combination was considered optimal based on the max adjusted R2 (Fig. 2A and B) or minimum BIC (Fig. 2C and D). As shown in Fig. (2A and B), the adjusted R2 method selected eight parameters, including male, age, hypertension, NIHSS at admission, fast blood glucose level, HDL-C level, low-density lipoprotein cholesterol (LDL-C) level, and CVD. However, only one parameter was selected by minimum BIC: hypertension history. We also used the stepwise regression to choose combinations of six potential predictors, including sex, age, hypertension, fast blood glucose level, HDL level, and CVD (Supplementary Table 1 (291.8KB, pdf) ). Because it is inappropriate to use a single variable to develop the prediction model, we excluded the result from minimum BIC for further analysis. The choice of final prediction model was determined by the ROC curve and the C-index, which were also used to examine the efficiency of the two models, with model 1 based on adjusted R2 and model 2 based on stepwise regression (Fig. 2E).
Fig. (2).
Variable selection methods. (A and B) The selection of variables using the BSR method (Adjusted R2). (C and D) The selection of variables using the BSR method (BIC). (E) The comparison between ROC curves of CAD in primary cohort. BIC, Bayesian information criterion; BSR, best subsets regression; NIHSS, National Institutes of Health Stroke Scale; HBP, hypertension; DM, Diabetes mellitus; LDL-C, low density lipoprotein cholesterol; HDL-C, highdensity lipoprotein cholesterol; CVD, cervicocephalic artery disease.
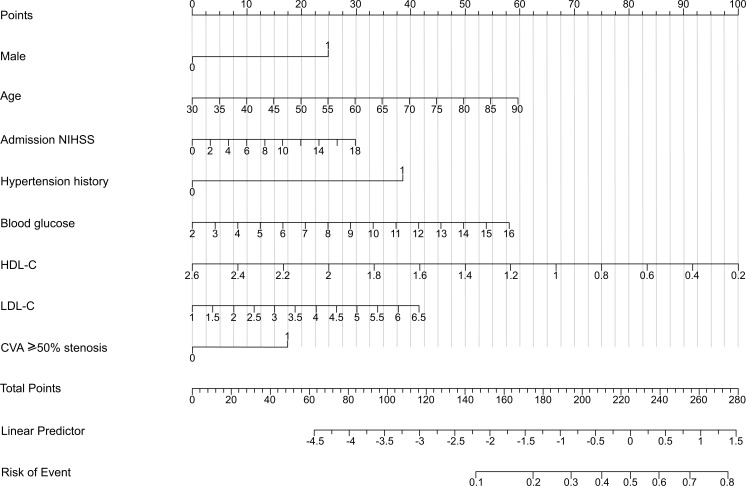
The nomogram obtained from the final model was optimal (Fig. 3). Higher total points based on the sum of the assigned number of points for each predictor in the nomogram were associated with an increased risk of mortality. For example, a male patient aged 70 years with a baseline NIHSS score of 14, hypertension history, fast blood glucose level 8.0mmol/L, HDL-C level 1.0mmol/L, LDL-C level 4.0mmol/L and presence of CVA ≥50% stenosis would have a total of 258 points (25 points for male sex, 40 points for age, 23 points for baseline NIHSS, 38 points for hypertension history, 25 points for blood glucose level, 67points for HDL-C level, 22.5 points for LDL-C level, and 17.5 points for presence of CVA ≥50% stenosis). The predicted CAD is 70.0% for this patient.
Fig. (3).
CAD-related nomogram prediction score. CAD-related nomogram was constructed to predict asymptomatic CAD for stroke patients, with the male, age, NIHSS on admission, hypertension history, blood glucose level, HDL-C level, LDL-C level and CVA≥50% stenosis. The “total points” are calculated as the sum of the individual score of each of the 8 variables included in the nomogram. NIHSS, National Institutes of Health Stroke Scale; HDL-C, highdensity lipoprotein cholesterol; LDL-C, low lipoprotein cholesterol; CVA, cervicocephalic artery.
3.3. Performance of Nomogram
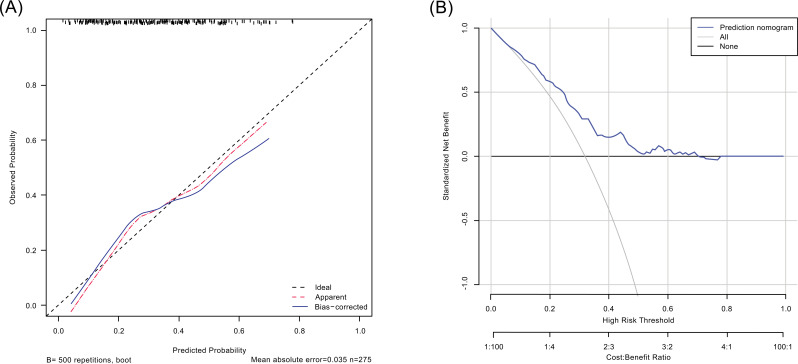
Prediction potential of the nomogram was measured by calculating the C-index, which was 0.716 (95% CI, 0.654-0.778), indicating relatively good predictive power. Fig. (4a) shows a calibration plot, which compares the nomogram prediction and actual observation of CAD. The calibration plot revealed good predictive accuracy of the nomogram.
Fig. (4).
Calibration plot (A) and decision curve analysis (B) of the nomogram. (A) The dotted line represents the performance of the nomogram, whereas the solid line corrects for any bias in the nomogram. The dashed line represents the reference line where an ideal nomogram would lie. (B) The x-axis indicates the threshold probability. The y-axis measures the net benefit. The gray line displays the net benefit of the strategy of treating all patients. The black line illustrates the net benefit of the strategy of treating no patients. The blue line indicates the nomogram. Decision curve analysis is a specific method developed for evaluating the prognostic value of nomogram strategies. This nomogram was developed to assess the probability of the asymptomatic CAD of a given patient. A stroke patient with a high risk of asymptomatic CAD may need“further treatment,” such as intensified lipid-lowering therapy or percutaneous coronary intervention; a patient with a low risk of asymptomatic CAD may not need“further treatment.” Distinguishing patients with a high and low risk of asymptomatic CAD is the main purpose of this nomogram. In the present study, the reference risk was calculated by assuming that all patients need further treatment for preventing CAD, whereas zero net benefit was defined as no patients needing further therapy. The threshold probability is when the expected benefit of further therapy is equal to the expected benefit of avoiding further therapy. For any given probability threshold, the nomogram with the greatest net benefit would be the most preferred model. CAD indicates coronary artery disease.
DCA can estimate the net benefit of a model based on the difference between the number of true- and false-positive results and is widely used in assessing whether the nomogram-assisted decision would improve patient outcomes. As shown in Fig. (4b), the DCA indicated that when the threshold probabilities ranged between 2.1% and 78.0% in the training cohort, the use of the nomogram to predict CAD provided a greater net benefit than the “treat all” or “treat none” strategies, which indicates the clinical usefulness of the nomogram.
4. DISCUSSION
In this study, we developed a precise nomogram, based on age, sex, NIHSS score at admission, hypertension history, blood glucose level, HDL cholesterol level, LDL cholesterol level, and presence of stenosis ≥50% in the cervicocephalic artery to predict the probability of asymptomatic CAD in patients with acute ischemic stroke. The calibration of the nomogram and its ability to predict CAD were demonstrated in the developing cohort. Given that all parameters were evaluated within 48 h of admission, this nomogram could be applied in the early stage of stroke for predicting asymptomatic CAD.
Our study shows the incidence of asymptomatic CAD was 32.0%, which is within the range reported by recent studies (18.0% to 52%) [9-11, 18]. Current models for predicting asymptomatic CAD in stroke have limitations. Both PRECORIS study [9] and the study by Choi and colleagues [18] developed models based on cut-off values of discrete variables, such as age, sex, and LDL cholesterol level. However, this may reduce predictive accuracy, given that they do not fully utilize within-category information. By converting the total score into a continuum of individual scores through a logarithmic formula, we developed a relatively precise nomogram (C-index, 0.716) for predicting probability from 2.1% to 78.0% of asymptomatic CAD in acute ischemic stroke patients. Patients with CAD can be referred early to a cardiologist for further diagnosis and treatment, which may reduce the incidence of serious coronary events in short-term [20] and long-term [3].
Advanced age, male sex, and hypertension history have previously been reported to be associated with the incidence of asymptomatic CAD in acute ischemic stroke patients [9]. Consistent with previous reports, age, sex, and hypertension history in our nomogram were significant predictors of asymptomatic CAD. However, NIHSS was also a significant predictor of asymptomatic CAD in our study but has seldomly been reported in previous similar studies [9-11, 18]. Because higher NIHSS is strongly associated with large artery disease and poor outcome of ischemic stroke, [20] and the coronary and cervicocephalic arteries are often simultaneously affected by similar vascular risk factors, [22] it is necessary to consider NIHSS as a predictor of CAD in ischemic stroke patients.
In addition to nonmodifiable variables (age, sex, high blood pressure, and baseline NIHSS score) of the individual patient, the levels of fasted glucose, HDL-C, and LDL-C upon admission were also predictors in the nomogram. Hyperglycemia shares many common mechanisms with other atherogenic factors, such as endothelial activation and inflammation, mitochondrial oxidative stress, changes in extracellular matrix components, and disruption of cellular defense systems [23]. In diabetic patients, each 1-standard deviation increase in fasting plasma glucose conveyed 2.11-fold higher risks of significant coronary stenosis after adjustment for other conventional cardiovascular risk factors [24]. In the prediabetic state, the severity of angiographic CAD also increased along with the increasing fast plasma glucose levels [25]. Studies of LDL-C in stroke patients with asymptomatic coronary heart disease are limited and results are inconsistent [9, 11]. One study has shown the association between higher LDL-C and stroke combined with asymptomatic CAS, [9] but another study has shown a non-significant correlation [11]. Our study shows that high LDL-C is significantly associated with asymptomatic CAD and is independent of blood glucose level and HDL-C level. A prospective community-based cohort study showed an interaction between triglyceride (TG), HDL-C, and LDL-C levels in the occurrence of CAD. High TG and low HDL-C levels were associated with risk of CAD, which was a phenomenon seen in people with high LDL-C levels but not observed in those with lower LDL-C levels [26]. Recent meta-analysis showed that a high HDL-C level is associated with reduced risk of total stroke and total ischemic stroke [27]. Another prospective community-based study has shown that HDL-C is not associated with a reduction in the occurrence of stroke with large vessel subtype in cerebral infarction, but a reduction in the occurrence of lacunar infarct subtype in cerebral infarction, suggesting that the mechanism of HDL-C in reducing the occurrence of cerebral infarction is not related to its protective function against lipid-rich atherosclerosis [28].
The presence of ≥50% cervicocephalic artery stenosis has been proved to be strongly associated with asymptomatic CAD in nondisabled stroke patients [2, 9]. In our study, we found that the presence of ≥50% cervicocephalic artery stenosis is related to the presence of asymptomatic CAD in all noncardioembolic ischemic stroke patients, which extends the use of this result in a broader stroke population. Some characteristics, such as smoking habit and previous incidence of stroke, were not found to be associated with CAD, which is probably attributable to the differences in sample size, study population, and study methods. A recent study suggests that patients with TIA/stroke with cardiovascular disease are at high risk of recurrent ischemic events despite the use of antithrombotic and lipid-lowering therapy. Thus, intensive lipid-lowering therapy may be justified. However, increased antithrombotic therapy will increase the risk of extracranial hemorrhage, thereby offsetting its benefits [5].
Several limitations should be addressed when interpreting the results of this study. First, we only included patients of Asian ethnicity, which may limit the generalizability of the results. Second, this is a retrospective study. The findings of our study need to be corroborated by prospective studies and randomized controlled trials. Third, large-scale and multi-center clinical trials are needed to be performed to validate and modify the model. Despite these limitations, our analysis has a few strengths. We developed a novel and relatively accurate nomogram. In addition, the nomogram was based on variables that can be easily abstracted and used in a real-world setting.
CONCLUSION
In conclusion, approximately one-third of noncardioembolic ischemic stroke patients have ≥50% asymptomatic CAD. In addition, our study developed a nomogram, composed of age, sex, NIHSS score on admission, hypertension history, fast blood glucose level, HDL cholesterol level, LDL cholesterol level, and presence of ≥50% cervicocephalic artery stenosis, which could be used for prediction of ≥50% asymptomatic CAD. Further studies are warranted to validate the effectiveness of this nomogram in other populations.
ACKNOWLEDGEMENTS
We thank Ke Liu and Qinmei Li from the Department of Radiology for their help in the measurement of the severity of coronary stenosis on MDCTA.
LIST OF ABBREVIATIONS
- CAD
coronary artery disease
- CAS
coronary artery stenosis
- TIA
transient ischemic attack
AUTHORS’ CONTRIBUTIONS
Jie Yang: Design, data collection, analyses, manuscript preparation. Xinguang Yang: Data collection, cervicocephalic atherosclerosis assessment; Jun Wen: Data collection, manuscript preparation. Jiayi Huang: Data collection, manuscript preparation. Lihong Jiang: data collection, manuscript preparation. Sha Liao: data collection, manuscript preparation. Chun Lian: Data collection, manuscript preparation. Haiyan Yao: Data collection, manuscript preparation. Li Huang: Data collection, manuscript preparation. Youming Long: Study design, data collection, critical revisions of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethical review committee of the Second Affiliated Hospital of Guangzhou Medical University (No. 2021-hs-27) (Guangzhou, China).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Written informed consent was obtained from all individual participants included in the study.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The dataset that support the results and findings of this research are available from the corresponding author [YL], upon reasonable request.
FUNDING
The work was supported by the Guangzhou Health Science and technology project (20211A011081). The funder had no influence in the design of this trial nor in the writing of the manuscript and presenting the study outcomes, and also had no role in collection, analysis and interpretation of data.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's website along with the published article.
REFERENCES
- 1.Vos T., Lim S.S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvet D., Song D., Yoo J., et al. Predicting asymptomatic coronary artery disease in patients with ischemic stroke and transient ischemic attack: The PRECORIS score. Stroke. 2014;45(1):82–86. doi: 10.1161/STROKEAHA.113.003414. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger M., Béjot Y., Rothwell P.M., Touzé E. Long-term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: Systematic review and meta-analysis. J. Am. Heart Assoc. 2018;7(2):e007267. doi: 10.1161/JAHA.117.007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K.J., Kim S.E., Kim J.Y., et al. Five-year risk of acute myocardial infarction after acute ischemic stroke in korea. J. Am. Heart Assoc. 2021;10(1):e018807. doi: 10.1161/JAHA.120.018807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulanger M., Li L., Lyons S., et al. Effect of coexisting vascular disease on long-term risk of recurrent events after TIA or stroke. Neurology. 2019;93(7):e695–e707. doi: 10.1212/WNL.0000000000007935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunnoo T., Hasan N., Khan M.S., Slark J., Bentley P., Sharma P. Quantifying the risk of heart disease following acute ischaemic stroke: A meta-analysis of over 50,000 participants. BMJ Open. 2016;6(1):e009535. doi: 10.1136/bmjopen-2015-009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amarenco P., Lavallée P.C., Labreuche J., et al. Coronary artery disease and risk of major vascular events after cerebral infarction. Stroke. 2013;44(6):1505–1511. doi: 10.1161/STROKEAHA.111.000142. [DOI] [PubMed] [Google Scholar]
- 8.Roh J.W., Kwon B.J., Ihm S.H., et al. Predictors of significant coronary artery disease in patients with cerebral artery atherosclerosis. Cerebrovasc. Dis. 2019;48(3-6):226–235. doi: 10.1159/000504927. [DOI] [PubMed] [Google Scholar]
- 9.Calvet D., Touzé E., Varenne O., Sablayrolles J.L., Weber S., Mas J.L. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010;121(14):1623–1629. doi: 10.1161/CIRCULATIONAHA.109.906958. [DOI] [PubMed] [Google Scholar]
- 10.Amarenco P., Lavallée P.C., Labreuche J., et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011;42(1):22–29. doi: 10.1161/STROKEAHA.110.584086. [DOI] [PubMed] [Google Scholar]
- 11.Yoo J., Yang J.H., Choi B.W., et al. The frequency and risk of preclinical coronary artery disease detected using multichannel cardiac computed tomography in patients with ischemic stroke. Cerebrovasc. Dis. 2012;33(3):286–294. doi: 10.1159/000334980. [DOI] [PubMed] [Google Scholar]
- 12.Harrell F.E., Jr, Lee K.L., Matchar D.B., Reichert T.A. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat. Rep. 1985;69(10):1071–1077. [PubMed] [Google Scholar]
- 13.Wu W., Deng Z., Alafate W., et al. Preoperative prediction nomogram based on integrated profiling for glioblastoma multiforme in glioma patients. Front. Oncol. 2020;10:1750. doi: 10.3389/fonc.2020.01750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams S.T., Leveson S.H. Clinical prediction rules. BMJ. 2012;344(1):d8312. doi: 10.1136/bmj.d8312. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Yuan K., Wang H., et al. Nomogram to predict mortality of endovascular thrombectomy for ischemic stroke despite successful recanalization. J. Am. Heart Assoc. 2020;9(3):e014899. doi: 10.1161/JAHA.119.014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuang X., Cao D., Yang D., et al. Association of diabetic retinopathy and diabetic macular oedema with renal function in southern Chinese patients with type 2 diabetes mellitus: a single-centre observational study. BMJ Open. 2019;9(9):e031194. doi: 10.1136/bmjopen-2019-031194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jehi L., Yardi R., Chagin K., et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283–290. doi: 10.1016/S1474-4422(14)70325-4. [DOI] [PubMed] [Google Scholar]
- 18.Choi H.Y., Shin S.J., Yoo J., et al. Coronary calcium score for the prediction of asymptomatic coronary artery disease in patients with ischemic stroke. Front. Neurol. 2020;11:206. doi: 10.3389/fneur.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozet E., Ziemons E., Marini R.D., Hubert P. Usefulness of information criteria for the selection of calibration curves. Anal. Chem. 2013;85(13):6327–6335. doi: 10.1021/ac400630k. [DOI] [PubMed] [Google Scholar]
- 20.Alkhachroum A.M., Miller B., Chami T., Tatsuoka C., Sila C. A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: Type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. J. Clin. Neurosci. 2019;64:83–88. doi: 10.1016/j.jocn.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Adams H.P., Jr, Davis P.H., Leira E.C., et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53(1):126–131. doi: 10.1212/WNL.53.1.126. [DOI] [PubMed] [Google Scholar]
- 22.Adams R.J., Chimowitz M.I., Alpert J.S., et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke council and the council on clinical cardiology of the american heart association/american stroke association. Stroke. 2003;34(9):2310–2322. doi: 10.1161/01.STR.0000090125.28466.E2. [DOI] [PubMed] [Google Scholar]
- 23.La Sala L., Prattichizzo F., Ceriello A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2019;26(2) Suppl.:15–24. doi: 10.1177/2047487319878373. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y., Bi Y., Li M., et al. Significant coronary stenosis in asymptomatic Chinese with different glycemic status. Diabetes Care. 2013;36(6):1687–1694. doi: 10.2337/dc12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gui M.H., Li X., Lu Z.Q., Gao X. Fasting plasma glucose correlates with angiographic coronary artery disease prevalence and severity in Chinese patients without known diabetes. Acta Diabetol. 2013;50(3):333–340. doi: 10.1007/s00592-012-0405-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.S., Chang P.Y., Zhang Y., Kizer J.R., Best L.G., Howard B.V. Triglyceride and hdl-c dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: The strong heart study. Diabetes Care. 2017;40(4):529–537. doi: 10.2337/dc16-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qie R, Liu L, Zhang D, et al. Dose–response association between high-density lipoprotein cholesterol and stroke: A systematic review and meta-analysis of prospective cohort studies. Prev Chronic Dis. 2021;18E45 doi: 10.5888/pcd18.200278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito I., Yamagishi K., Kokubo Y., et al. Association of high-density lipoprotein cholesterol concentration with different types of stroke and coronary heart disease: The Japan public health center-based prospective (JPHC) study. Atherosclerosis. 2017;265:147–154. doi: 10.1016/j.atherosclerosis.2017.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher's website along with the published article.
Data Availability Statement
The dataset that support the results and findings of this research are available from the corresponding author [YL], upon reasonable request.