Abstract
As a result of global change, hosts and parasites (including pathogens) are experiencing shifts in their thermal environment. Despite the importance of heat stress tolerance for host population persistence, infection by parasites can impair a host's ability to cope with heat. Host–parasite eco-evolutionary dynamics will be affected if infection reduces host performance during heating. Theory predicts that within-host parasite burden (replication rate or number of infecting parasites per host), a key component of parasite fitness, should correlate positively with virulence—the harm caused to hosts during infection. Surprisingly, however, the relationship between within-host parasite burden and virulence during heating is often weak. Here, we describe the current evidence for the link between within-host parasite burden and host heat stress tolerance. We consider the biology of host–parasite systems that may explain the weak or absent link between these two important host and parasite traits during hot conditions. The processes that mediate the relationship between parasite burden and host fitness will be fundamental in ecological and evolutionary responses of host and parasites in a warming world.
This article is part of the theme issue ‘Infectious disease ecology and evolution in a changing world’.
Keywords: thermal tolerance, pathogen evolution, virulence, transmission, trade-off, disease burden
1. Introduction
Global climate change has triggered escalating thermal variability and increased periods of extreme warm temperatures [1]. How individuals and populations, particularly ectothermic organisms, respond to the magnitude and pace of thermal variation is key to their persistence [2–4]. Gradual changes in average temperatures are slow relative to most biological processes [5]. By contrast, thermal variability over shorter time scales, such as seasonal, diurnal or tidal changes, are relatively fast [2,6]. Rapid weather-driven warming—heatwaves—can arise over a matter of days or even hours [4,5,7,8]. Extreme heat may disproportionately interfere with population and community structure and constitute the greatest selective force on species under continuing global climate change [4,9,10].
Alongside shifts in temperature, the geographical distribution of many parasites (including microbial pathogens such as viruses, bacteria and fungi) and the severity of infection during disease outbreaks are changing (e.g. [11,12]). Many populations are therefore facing the simultaneous stresses of extreme heat and virulent infection [13,14]. Although these environmental conditions may influence all host–parasite interactions in some way [15], much of the work to date focuses on ectothermic hosts and their parasites (table 1). The fact that infection can significantly increase the sensitivity of hosts to heat stress amplifies the risk that many populations face (reviewed in [14,26]). Indeed, infection can alter a host's entire thermal performance curve, shifting lower and upper thermal limits, alongside thermal optima (e.g. [27–29]). Exposure to parasites in combination with rapid heating may propel many species to the brink of extinction [30].
Table 1.
Studies measuring the relationship between parasite burden and changes in host tolerance to extreme heat. Most studies find no relationship between host heat tolerance and parasite burdens (highlighted in bold). Studies were included if they quantified critical thermal maximum (CTmax) or survival times following heat shock alongside quantifying parasite burden in infected individuals. To find these studies, a non-formal search of the literature was conducted (using Google Scholar search terms: (infect* OR parasite* OR pathogen*) AND (burden OR load) AND Ctmax), and forward and backward searches on known papers). While the list may not be exhaustive, it is likely representative. Only 10 studies were found highlighting the general lack of data available.
| host type | host species | parasite type | parasite species | heat tolerance trait measured | type and duration of heat stress. | impact of infection on host heat tolerance | relationship between infection burden and host heat tolerance | notes on response | reference |
|---|---|---|---|---|---|---|---|---|---|
| honeybee | hybrid from A. m. ligustica and A. m. carnica | mite | Varroa destructor | TDT (thermal death time) | acclimation at 32°C or 38°C; static heat shock at 45, 47, 49 and 51°C | reduced TDT (38°C acclimation) or no difference (32°C acclimation) | no clear difference in TDT of bees infected with 1 or 2 Varroa mites | reduction only became apparent at warmer temperatures, in some cases a single mite infection increased TDT | Aldea-Sánchez et al. [16] |
| brown trout | Salmo trutta | myxozoan endoparasite | Tetracapsuloides bryosalmonae | CTmax (loss of righting) | 0.22°C/min temperature ramp | reduced CTmax | weak negative relationship | stronger negative relationship was found between CTmax and a symptom of infection (kidney hyperplasia) | Bruneaux et al. [17] |
| amphibian | Litoria spenceri | fungus | Batrachochytrium dendrobatidis | CTmax (loss of righting, or onset of spasms) | 1°C/min temperature ramp | reduced CTmax | no relationship | Greenspan et al. [18] | |
| freshwater crustacean | Daphnia magna | bacteria | Pasteuria ramosa | CTmax (mortality) | 0.06°C/min temperature ramp | reduced CTmax | weak negative & no relationship depending on genotype | the strength of the relationship was dependent on host and pathogen genotype combination. | Hector et al. [19] |
| freshwater fishes | Lepomis macrochirus & Lepomis megalotis | helminth endoparasite | fish were parasatized with up to seven different helminth species | CTmax (onset of spasms) | 1°C/min temperature ramp | no uninfected control to compare to | negative correlation | wild-caught fishes all with natural infections | Lutterschmidt et al. [20] |
| aphid | Acyrthosiphon pisum | fungus | Beauveria bassiana | CTmax (locomotion stopped) | 0.3°C/min temperature ramp | reduced CTmax | no relationship | there was no difference in the magnitude of changes in heat tolerance of hosts inoculated with a low or high parasite dose | Porras et al. [21] |
| beetle | Hippodamia convergens | fungus | Beauveria bassiana | CTmax (locomotion stopped) | 0.3°C/min temperature ramp | reduced CTmax | no relationship | ||
| amphibian | Notophthalmus viridescens | protist | Ichthyophonus | CTmax (onset of spasms) | 1°C/min temperature ramp | reduced CTmax | no relationship | lesions on skin were measured as an index of infection load | Sherman [22] |
| mosquito | Aedes aegypti | virus | dengue virus | knockdown time | static heat shock at 42°C | reduced host knockdown times | no relationship | Ware-Gilmore et al. [23] | |
| amphipod crustacean | Corophium volutator | trematode parasite | metacercaria | LT50: temperature causing 50% mortality | static heat shock for 10 min at 36, 36.5 or 38.5°C | marginal reduction in LT50 | no relationship | infection burdens varied between approximately 1–20 parasites | Meißner et al. [24] |
| freshwater crustacean | Daphnia magna | bacteria | Pasteuria ramosa | knockdown time | static heat shock at 37°C | reduced host knockdown times | positive, negative, and no relationship depending on genotype | direction and strength of relationship depended on the host–parasite genotype combination, but there was no interaction with host sex | Laidlaw et al. [25] |
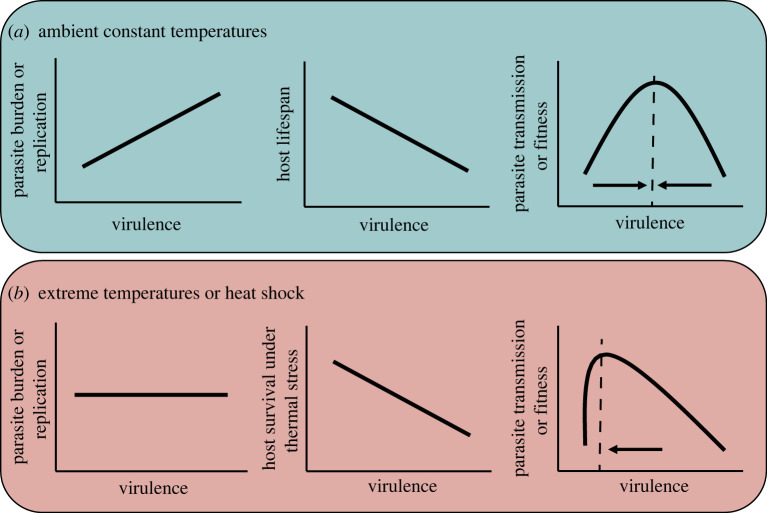
Parasites can significantly contract host heat tolerance during extreme warming events (e.g. [18,19,21,23]). Reduced tolerance to heating is therefore an intrinsic component of the virulence—harm caused by infection (e.g. host mortality and reduced reproductive output)—experienced by a host. Conventional wisdom predicts that parasites should experience a trade-off between virulence and transmission, driven in part by a positive relationship between virulence and parasite replication ([31,32]; box 1; figure 1a). However, within-host parasite burden, a common proxy for parasite replication and fitness, is rarely found to correlate with the harm hosts experience during heat stress (table 1). The general lack of a relationship between parasite burden and host heat tolerance is therefore puzzling. It suggests that host heat tolerance is influenced by interactions between several host and parasite processes. Heatwaves and rapid heating may therefore constitute a condition that negates the assumed link between parasite replication and virulence (e.g. [55]; box 1). Disentangling the link between host and parasite performance in the face of extreme warming is crucial as these processes will fundamentally shape the eco-evolutionary responses of parasites to global change [13,14].
Box 1. Virulence–transmission trade-off and parasite evolution.
Virulence can be defined as the harm parasites cause to a host during infection (reviewed by [33]). Virulence is highly variable across parasite species [33,34], with some species causing little harm to their host (e.g. Henipavirus infections in grey-headed fruit bats [35]) and some death (e.g. Batrachochytrium dendrobatids in amphibians [36,37]). Parasites are often dependent on their host for survival, but to be successful, parasites must transmit between new hosts [38]. For parasites, a trade-off is predicted to occur between within-host replication and between-host transmission [39–42]. To replicate, parasites consume host resources, which increases the harm caused to the host, reduces the host's lifespan and increases the risk of host mortality. The increased host mortality associated with replication is hypothesized to decrease parasite transmission [39–43], resulting in conflict between within-host growth and between-host transmission.
Overall, theory predicts that optimal virulence is that which maximizes transmission [33,39]. The virulence–transmission trade-off may be influenced by transmission mode [44]. For environmentally transmitted parasites, where transmission may be airborne or use vectors (indirectly transmitted [44]), high virulence is predicted to evolve as parasites do not rely on hosts for transmission [40,42,44,45]. Alternatively, parasites transmitted through direct contact between hosts (direct transmission [44]) rely on a live host for transmission. Thus, as these parasites rely on their host to successfully transmit to new hosts, theory predicts that extreme virulence is less likely to evolve [44,46].
Although empirical studies across a range of species find support for the virulence–transmission trade-off (e.g. [47–50]), other studies find contradicting evidence [31,50,51]. For example, if most of the virulence stems from toxin production and not pathogen replication, and there is a multiplicity of infection in the host, a clear relationship between growth and virulence would not be observed [32,52]. Infection by opportunistic parasites may also not yield this relationship as the interaction is not under natural selection [53]. Thus, the broad relevance and application of the virulence–transmission trade-off have been extensively debated and challenged [31,32,51]. A recent meta-analysis across empirical studies found strong support for a positive correlation between within-host replication and virulence and between within-host replication and transmission [43]. The authors also highlighted the need for further studies to be conducted to more accurately assess the relationship [43].
Figure 1.
Virulence–transmission trade-off under ambient and stressful conditions. (a) Theoretical predictions from the trade-off hypothesis for the relationships between virulence and within-host parasite burden, host lifespan and parasite fitness—R0 (box 1). (b) Under extreme heat, the relationship between virulence and within-host parasite burden disappears, which has the potential to shift the relationship between virulence and parasite transmission. Without a positive relationship between parasite burden/replication and virulence, one consequence could be for lower virulence to become optimal (particularly when demographic change is considered [54]). However, if virulence is not associated with within-host parasite burden (or at least fitness), parasite evolution could instead be constrained or dampened due to the random removal of genetic variation within the population. Note that these hypothetical predictions do not account for important complexities of host–parasite systems, such as transmission mode, which mediate the relationships between host and parasite fitness traits ([40,42,44,45]; see main text and box 1 for a discussion of the generality of the trade-off hypothesis).
Here, we aim to understand the disconnect between within-host parasite burden and host performance during heating events. We will describe how the characteristics of host–parasite interactions, which vary widely across systems, may regulate the relationship between within-host infection burden and host heat tolerance. We propose a variety of mechanisms that could be operating when an infected host experiences excessive heat. It is a matter of urgency that we understand how changing global temperature patterns will influence the ecology and evolution of infectious diseases [56]. By bringing together knowledge from disease ecology and evolution, we hope to encourage the formation of predictions under extreme warming.
2. Host thermal performance as a component of parasite virulence
Evolutionary theory predicts a strong association between parasite virulence (usually modelled as parasite-induced mortality) and transmission (figure 1a; for virulence–transmission trade-off primer and a discussion of its general relevance see box 1). Optimal virulence is predicted to result from a trade-off between within-host replication and transmission duration (box 1; figure 1a) [33,39]. If we consider the reduction in host heat tolerance (e.g. increased host mortality during heat stress) as a component of parasite virulence, we may therefore predict a negative correlation with within-host infection burden (or replication rate). Despite this prediction, the relationship between host heat tolerance and infection burden, across a diversity of systems, appears to be weak—if not absent (table 1). Under extreme warming, therefore, the optimal strategy for a parasite may shift (figure 1b; [54]). It is potentially challenging to make predictions on parasite virulence evolution during heating based on the trade-off hypothesis (see box 1 for some caveats relating to the trade-off hypothesis).
Translating virulence into component traits can be tricky. One difficulty arises because virulence, as formalized in theory, is formidable to accurately measure as it relies on another abstract trait: fitness [31]. It is also often unclear which host–parasite traits interact to determine experienced virulence ([31]; box 1). Whether we consider virulence as a population or individual trait can also add confusion, particularly when traversing the fields of ecology and evolution. Parasites impact individual hosts, but evolution is a population-level process. So, it is necessary to translate processes which occur at the level of the individual into population-level responses [57].
3. The disconnect between host heat tolerance and infection burden
The extent to which infection can modify host performance during heat stress is vast. Infection can reduce host upper thermal limits, in some systems up to 8°C, and on average 2–3°C (Reviewed in [26]; see references in table 1). Within-host parasite burden, however, has rarely been found to correlate with the reduction in host heat tolerance caused by infection (table 1; box 2). In the mosquito Aedes aegypti, no correlation was found between reduced host heat tolerance and dengue virus burden, despite within-host variation in viral loads spanning two orders of magnitude [23]. The lack of a clear link between parasite burden and reductions in host heat tolerance demonstrates that other components of host–parasite interactions are key in regulating this aspect of virulence (see also [14,26]).
Box 2. Obligate killers allow us to accurately link within-host parasite burden, parasite replication, virulence and transmission.
For obligately killing parasites, host mortality is an essential component of their transmission [58]. Selection on these parasites should maintain virulence that promotes host death at a time that maximizes transmission (see box 1; [55,59,60]), although trade-offs and classic assumptions may still apply [61]. For our interests, obligate killers are interesting because all parasite propagules remain within the host until after death. The relationships between parasite replication, parasite burden, virulence and transmission are much easier to characterize compared to non-obligately killing parasites. While obligate parasites may continue to replicate within a dead host, such replication would be significantly resource limited, and of minor concern in experimental studies in which hosts are frozen shortly after death. Obligately killing parasites thus present a powerful system for exploring the factors driving the relationship between within-host parasite replication and host heat tolerance.
Pasteuria ramosa is an obligate bacterial parasite of the freshwater crustacean, Daphnia magna [62]. Pasteuria spores exist in the environment and are picked up by the host during filter feeding. After entering a host, P. ramosa replicates, manipulates host physiology (causing castration and gigantism), reduces host fecundity and eventually causes early host death [63,64]. At the point of host death, millions of parasite transmission spores are released into the environment where they can persist until they reach the next host [60,63,64].
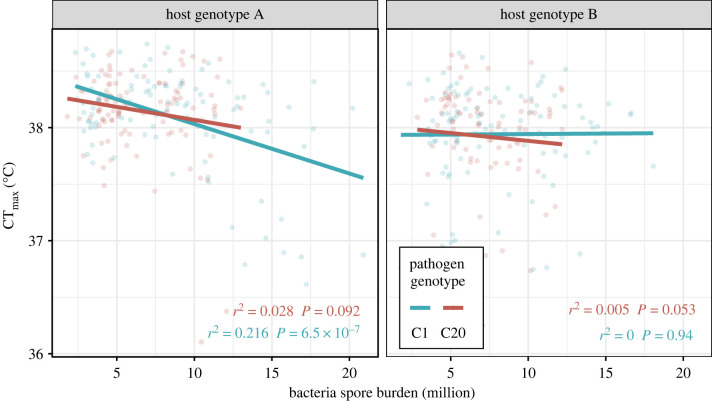
Pasteuria ramosa imposes high levels of virulence under warming via substantial reductions in host heat tolerance [19,25]. Once infection becomes fully developed hosts can experience a reduction in their heat tolerance of up to 2°C. Within-host infection burdens spanning an order of magnitude (2–20 million) explained only a portion of this reduction (figure 2). A clear negative relationship was found in one host–parasite genotype combination linking these two traits (host genotype A + parasite C1; figure 2). Variation across host and parasite genotypes (figure 2) reveals how parasite burden (and replication) alone cannot fully explain reduced host heat tolerance during infection.
Host heat tolerance was also found associated with host body size and the age of an infection in this host–parasite system [19]. Host body size and infection age both represent highly quantitative traits. Interactions between host traits, in addition to genotype–genotype interactions, confirm that both the host and parasite are fundamental in driving host responses to heat stress.
Host–parasite systems are fascinatingly diverse [58]. This diversity, however, can restrict our ability to make broad generalizations. The language used to describe infection burdens varies considerably as a result (e.g. disease burden, spore loads, viral titre, infection intensity, etc.). For our purposes, we define burden as the number of parasite individuals infecting a host (although this definition itself has ‘grey areas’, e.g. box 3). Often these language differences indicate differences in host–parasite life histories and transmission strategies that will influence the relationship between host and parasite fitness traits.
Box 3. When parasite replication and within-host burden are decoupled.
How parasite burden relates to changes in heat tolerance could depend on the extent of resource use by the parasite. In some metazoan parasites, reproduction and replication can be decoupled from parasite burden. For example, in long-lived metazoan parasites, the adult parasite may stay within a host for many years, producing many broods of parasite larvae (e.g. [65,66]). In this case, the parasite burden might be measured as the number of adult parasites within the host, and parasite replication the number of larvae the host is able to produce over a lifetime of infection. One example comes from rhizocephalan parasites that infect crab hosts [65]. While most infections are by a single parasite, in some systems there can be multiple infections by the same species within a host [67–69]. Co-infections can be visually detected by the existence of multiple reproductive organs, called externa (figure 3). It should be noted that not all multiple externa in rhizocephalans come from multiple individuals. In some species, a single individual can produce multiple externa in a single host [65].
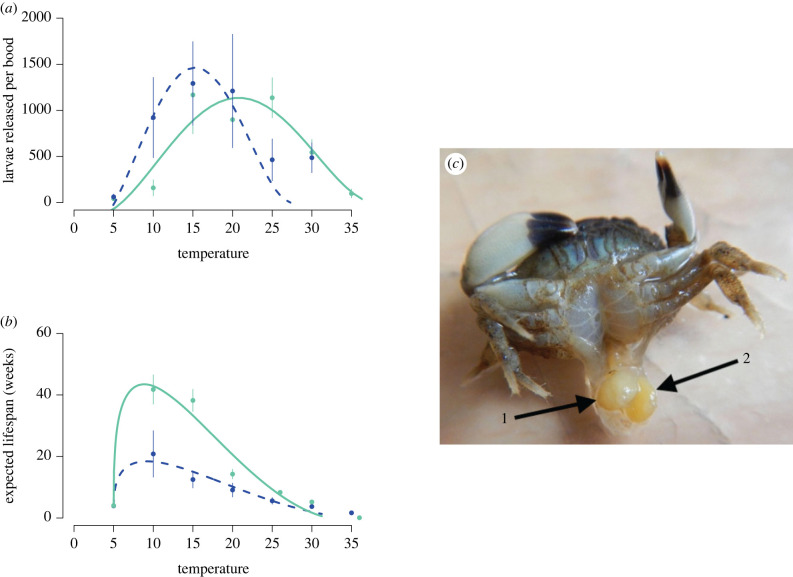
Whether co-infection by multiple parasite genotypes leads to increased reproductive output, and how co-infection influences the thermal performance of the host and its parasites, remain open questions. The mud crab Eurypanopeus depressus, infected by the rhizocephalan Loxothylacus panopaei, can have co-infections [67,69], and single infection can lower host heat tolerance [27]. In addition to the experiments described in [27], parallel unpublished experiments with hosts with two externa were run (figure 3). The methods for hosts with multiple externa are the same as those for single externa [27]. Co-infections were naturally occurring in the field. Intriguingly, it appears that an increase in infection burden from one to two externas lowers the thermal performance optima and upper limit for parasite reproduction (figure 3). Infection by two externa also decreased the absolute rate of survival, but did not change the thermal dependence of survival (e.g. the shape of the survival response across temperature, figure 3). Despite that change in thermal performance, the overall total number of larvae produced by crabs infected with two externa was similar to hosts with single externa. Thus, co-infections led to a decrease in the per externa number of larvae. These results suggest there is a host-derived limit to the number of parasite larvae that can be produced. While we didn't see any increase in the relationship between temperature and survival between the one and two externa, the marked decline in expected survival suggests that the increase in incidence of infection has a direct cost. There is little benefit, and potentially a cost, to survival from co-infections, which could explain their relative rarity in the field [27].
For many unicellular parasites, such as bacteria and viruses, replication produces ‘offspring’ that can impact their host in a similar way to the ‘parent’ (e.g. by extracting resources, developing, replicating or interacting with the host's biology) [58]. In this group of parasites, within-host parasite burden could be reasonably proportional to virulence and transmission when parasite populations are large—particularly for parasites that transmit after host death (box 2; [60,70–73]). By contrast, some parasites transmit continually or in pulses while the host is alive (including both unicellular and multicellular parasites). If a host experiences lasting symptoms of infection from parasites after transmission, the negative impact on host heat tolerance may not be directly proportional to the parasite burden at the point of death (e.g. [17]). Similarly, for some multicellular parasites, replication and the parasite burden experienced by the host can be decoupled ([27], box 3).
Host responses to infection and heat stress may also play a vital role in mediating the relationship between parasite burden and thermal performance. Host heat tolerance might be governed by physiological or genetic trade-offs between responses to heat stress and to infection [74]. Across the sexes, genotypes and populations, hosts that exhibit the highest innate heat tolerance can suffer the greatest declines once infected (reviewed in [26]). In Drosophila melanogaster, immune activation alone was sufficient to reduce host heating tolerance, and this effect was most pronounced in heat-tolerant populations [75]. Infection has also been found to diminish sexual dimorphism by reducing heat tolerance of the more heat-tolerant sex [25]. Underlying genetic trade-offs for hosts could therefore mask any direct relationship between parasite burden and host tolerance to extreme heat [50].
Genetic variation in disease tolerance could also help explain the lack of a relationship between parasite burden and host heat tolerance. Rather than actively reducing parasite burdens (resistance strategy), a host may compensate for the fitness costs resulting from infection (i.e. disease tolerance; [76–81]). In the host Daphnia magna, the extent to which increases in Pasteuria ramosa parasite burdens reduced host heat tolerance varies across host genotypes. This pattern suggests genetic variation in host disease tolerance (box 2). The ability of a host to modulate its immune response between resistance and tolerance could also be thermally dependent. Indeed, thermal regulation itself has been suggested as a mechanism of disease tolerance [82]. From a parasite's perspective, virulence can be determined not only by total burden, but also by pathogenicity per parasite [83]. For some parasites, virulence is linked more closely to toxin production than replication [52], which may itself be mediated by parasite genetics and temperature [84]. Symptoms of infection (whether host- or parasite-induced) may become particularly potent under extreme heat [17]. Any relationship between infection burden and host heat tolerance could therefore be obscured if extreme heat promotes the expression of genetic variation for host disease tolerance, parasite pathogenicity or other drivers of virulence.
Infections progress through stages involving various host and parasite processes. Following initial infection by one or a few parasites, infections often develop via replication, use of host resources, host manipulation and extended periods of host defense [58,85]. The timing of heat stress relative to the stage of infection may determine which host or parasite infection processes are most influential to host heat tolerance. Some parasites may cause substantial costs to their host very early in infection by eliciting costly host defenses, causing immunopathology, or by some other physiological manipulation [75,86–89]. Opportunistic or recently emerged parasites, which may not rely on a specific host for transmission or may be maladapted, can in some cases impose high levels of virulence or illicit harmful immune responses [53,90]. In such cases, hosts could experience reduced heat tolerance simply as a result of initial infection, rather than any subsequent parasite replication.
Lastly, host heat tolerance may be less impacted by infection burden if temperature alters the colonization resistance that beneficial bacteria can provide to hosts. Microbes colonizing a host can confer protection against infection [91] and reduce parasite loads via direct or host-immune-mediated mechanisms (table 1 in [92]). An increase in the inhibitory effects of bacterial symbionts on parasites at higher temperatures has been shown in bumblebees [93] and mosquito vectors of malaria [94]. This pattern can extend to the protective effects of the host microbiota (community of host-colonizing microbial symbionts). Northern cricket frogs were found to be more resistant to infection by the invasive B. dendrobatidis at higher temperatures as these conditions promoted the persistence of the antifungal bacterium, Stenotrophomonas maltophilia on the skin microbiome [95]. The relationship between protection and temperature is not necessarily linear. In D. melanogaster, high temperatures resulted in greater Drosophila C virus replication and in lower Wolbachia-mediated protection [96]. However, in this system, temperatures during host development from egg to adult mediated bacteria-mediated protection. Higher temperatures (25°C) drove stronger protection, but protection was found to be lower or absent when flies developed at 18°C. Temperature-modulated manipulation of beneficial bacteria could be one explanation for a lack of a consistent relationship between parasite burden and host heat tolerance.
4. Host heat tolerance during infection: what mechanisms could be at work?
Across systems, host heat tolerance can be influenced by infection status (table 1). The underlying mechanisms driving these changes remain largely unexplored [14,21]. Given the wide range of host–parasite interactions that have similar responses, there could be multiple underlying mechanisms. Changes to host heat tolerance could occur as a result of the parasite's biological response (e.g. parasite resource extraction from the host), the host's biological response (e.g. immune response) or an interaction between the two species (e.g. tissue or mechanical damage to the host by parasite infection). To move from quantifying patterns to predicting future outcomes of heating events, we must understand which aspects of parasite infection can drive shifts in host thermal performance.
It is possible that changes in parasite resource extraction from its host could drive changes in host heat tolerance. The resources available to a parasite are directly linked to the pool of resources available to its host. How much host resource is given to the parasite can vary, depending on a range of factors, including the stage of infection, population density, host sex and manipulation of host physiology [97–102]. A host could increase its resource intake to accommodate the additional stress of infection (e.g. [103–105]). Interestingly, evidence from anthropogenic supplemental feeding on host–parasite interactions suggests that supplemental feeding can influence disease outcomes through individual and population-level changes in behaviour [106,107]. However, if an infection leads to a diseased state, then host energy intake could at times be reduced, potentially exacerbating the impact of infection burden on host performance (e.g. [108]).
The increased burden of providing energy for both parasite functions and its own can lead to an overall increased host energy use and metabolic rate (e.g. [99]). For example, a crab infected by a mature rhizocephalan can have double the metabolic rate of the uninfected host [109]. Similarly, in flies infected by ectothermic mites, there was an increase in metabolic rate that scaled with intensity of infestation [110]. Given a limit to a host's maximum metabolic rate [111], parasite-driven increases in host metabolic rate could cause hosts to reach their maximum rate at lower temperatures than uninfected hosts. The impact of infection on host metabolic rate will be a key determinant of host thermal performance. However, the relationship between within-host parasite burden and host metabolic rate may not always be linear [98,112,113], which could blur the link between parasite burden and host heat tolerance.
The strongest effect of infection on host performance can come around the thermal optima for parasite reproduction (e.g. [27]). Therefore, the increase in extraction of host resources used for parasite reproduction could be an underlying driver of reduced host performance. In the rhizocephalan-infected crab described above, the reduction in metabolic rate was only found in mature, reproductively active infections [109]. Additionally, in another species of rhizocephalan-infected crab, the strongest difference in the thermal performance between host and parasite was found at the optimal temperature for parasite reproduction, similar to that for uninfected host survival [27]. Where parasite reproductive output influences host thermal performance, infected host survival would be negatively related to the thermal dependence of parasite reproduction—which may not be proportional to within-host infection burden (see box 3). In systems where parasite burden is equivalent to parasite reproductive output, we may expect a stronger relationship between parasite burden and altered host–parasite heat tolerance (box 2).
Host immune response itself can be modulated by temperature and can have nonlinear relationships with temperature. For example, in an abalone and shrimp, there was a rapid decline in immune response at or above 32°C, making the host most susceptible to vibrio infections at high temperature [114,115]. In some marine invertebrates, immune response can increase with temperature [116], and yet the opposite is also true. Activation of the immune response itself can lead to changes in host heat tolerance [75]. In some cases of long-term macroparasite infections, there is evidence that some can inhibit or bypass the host's immune response [117]. Host heat tolerance could therefore be determined by potentially complex interactions between a host's own immune response and their current infection burden.
Beyond just the physiological tolerances of host and parasite, there are behaviours and physiological responses to infection that directly alter the experienced temperature for the system. In endotherms, fever can help to trigger immune responses [118] and generally have a strong influence on the host's thermal response. A fever response in endotherms, however, may limit their ability to regulate internal body temperatures during extreme heat events. In July 1995, 57% of patients admitted for near-fatal heat stroke were also carrying pathogenic infections [119,120]. In some ectotherms, behavioural changes, such as moving towards hotter micro-climates, can alter the host's body temperature to combat parasite infection, known as behavioural fever [121]. Interestingly, some species manipulate parasite reproduction through febrile behaviour by plastically regulating their temperature to below the parasite's optimum [122]. In longer-lived parasites that can manipulate host behaviour—for example, trematode worm infections in snail hosts—heat tolerance of infected hosts can be parasite species-dependent [123] and can lead hosts to select for thermal niches that may benefit the parasite [124].
Heating may also drive a disconnect between parasite burden and virulence by interacting with host and parasite acclimation and size differences. The capacity of organisms to increase their tolerance to heat stress via thermal acclimation is often size-dependent [74,125,126]. In addition, warmer temperatures drive increased rates of reproduction, but generally result in smaller offspring ([127–130]; see [13]). In some parasites, small cells can cause higher virulence as they more effectively evade immune responses [131], which may in part explain the observation that some parasites become more infectious following development at warmer temperatures [132]. The relationship between parasite burden and host heat tolerance could therefore be skewed across temperatures by size-dependent virulence, as well as the interaction between host and parasite acclimation capacity (e.g. [16,18]).
5. Parasite transmission strategy may direct the eco-evolutionary consequences of extreme heating
The transmission strategy of a parasite will shape its eco-evolutionary response to heating by regulating the relationship between parasite burden and virulence. Parasites can be broadly grouped by how they transmit between hosts. Important transmission strategies include parasites that transmit directly between alive hosts or via vectors, parasites that kill their host to transmit or have environmental transmission stages, and opportunistic parasites not dependent on a host [53,55,58,59]. To begin to understand the eco-evolutionary consequences of heating across the diversity of parasite strategies, it is useful to consider predictions formulated for other drivers of disease dynamics.
Both seasonality and predation share similarities with extreme heating, often involving rapid changes in host and parasite population sizes and asymmetrical mortality across healthy and uninfected hosts [133–140]. It seems likely, however, that extreme heating will impose a far more intense selective force than that of most seasonal changes and predation [4,9,10]. The pressures put upon populations by extreme heat and infection will be multifaceted, not only contracting population sizes due to excessive mortality [1,141–144] but also impacting subsequent population growth via reductions in reproduction and fertility [145–148]. The dynamics here may match closely to those seen in mosquito vector systems under extreme daily temperature fluctuations [149–152]. We highlight the need for the development of specific eco-evolutionary theory addressing the interaction between infection dynamics and parasite virulence under extreme heat [13,14,56].
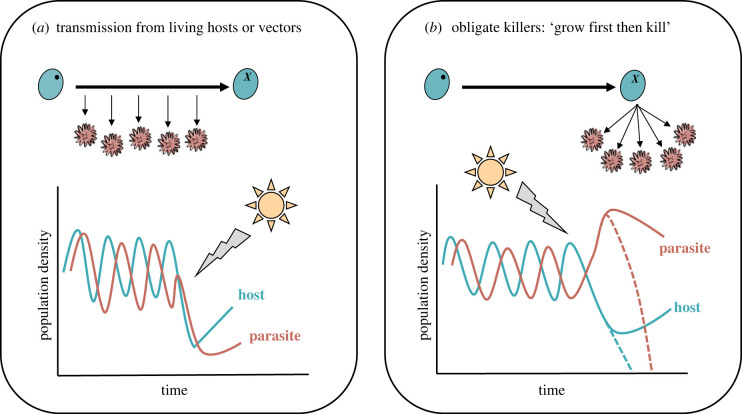
Ecologically, for parasites that transmit between living hosts (directly or via vectors), heating may tightly constrain their ongoing transmission (figure 4a). Disproportionate declines in the number of infected hosts due to heat stress, alongside the death of a portion of the susceptible host population (e.g. [141–144]), will shrink the susceptible and infected population sizes. As a result of extensive host mortality, the parasite population size (i.e. infected hosts) will also contract. Ongoing transmission following an extreme heat event could therefore be limited by both small host and parasite population sizes (figure 4a; e.g. [149,150,154]). Over time, host populations may slowly recover, with a lag in parasite transmission, potentially resulting in slow joint epidemiological dynamics as populations reestablish (figure 4a). However, if host populations (both infected and uninfected) are reduced too much they risk extinction, and transmission may fall below a threshold for parasite persistence [154].
Figure 2.
The impact of within-host Pasteuria ramosa spore burdens on the critical thermal limit (CTmax) of Daphnia magna from Hector et al. [19]. CTmax is the temperature causing mortality during a 0.06°C/min heating ramp from ambient temperature and was measured on two host genotypes (A or B) infected with one of two parasite genotypes (C1 or C20). The strength of the relationship depended on the specific host–parasite genotype combination—a clear negative relationship is only apparent for one genotype pair.
Figure 3.
(a) The mean number of parasite larvae (i.e. nauplii and cyprids) released by brood from Eurypanopeus depressus infected by Loxothylacus panopaei with either one (solid line, estimate +/– s.e. of minimum temperature = 6.01 + /– 2.3, and maximum temperature = 38.03 + /– 2.34) or two externa (dashed line, estimate + /–s.e. of minimum temperature = 5.14 + /– 1.23 and maximum = 27.47 + /– 0.07) within a two-week period, after having been acclimated to temperature treatments over 11 days and held at experimental temperature for a week (mean + /– s.e.). Two-week period for comparison was selected because at 20°C, L. panopaei will release approximately 1 brood a week. (b) The expected lifespan in weeks of E. depressus infected by L. panopaei with either one (green, estimate + /– s.e. of minimum temperature 4.99, and maximum temperature 32.10 [27]) or two externa (dashed line, estimate + /–s.e. of minimum temperature = 4.99 + /– 0.02 and maximum temperature = 35.05 + /– 2.95). Expected lifespan was calculated from a fit survival object, methods available in [27]. Due to logistical constraints, replication in the two externa groups was not equal across temperatures (replication at 5°C = 3, 10°C = 5, 15°C = 4, 20°C = 3, 25°C = 3, 30°C = 4, 35°C = 3). Additional information about experimental design and methods in [27]. Animals with co-infections were kept in the same conditions as those with single infections. (c) An E. depressus with two attached L. panopaei externa (arrows indicate the two different externa). (Online version in colour.)
Figure 4.
Ecological relationship between parasite transmission strategy and host–parasite population densities under extreme heating. (a) Direct transmission between living hosts or via vectors. (b) Transmission depends on host death or via environmental stages. Blue and red lines represent the dynamics of a hypothetical host and parasite population, respectively. Across time, we may see fluctuating host–parasite population dynamics depending on the system [153]—although our general point does not depend on the exact nature of these dynamics. After an extreme heat event (sun and arrow) population dynamics are interrupted, with subsequent dynamics depending on the host–parasite system: (a) excessive host mortality causes both host and parasite populations to shrink, with parasite reestablishment lagging behind any host population recovery; (b) excessive host mortality results in an overabundance of environmental parasite transmission stages, which could suppress host population growth or cause local extinction.
In obligate killers and parasites that can persist in the environment extreme heat will cause high mortality in the infected host population (figure 4b). In contrast with other transmission strategies, however, the environment may become saturated with viable environmental parasite transmission stages. Based on theory from seasonal population disturbances, we expect that the subsequent ecological dynamics will depend on factors including the population sizes of both the surviving parasites and susceptible hosts, and the R0 of the parasite [38,133,155]. An overabundance of parasites in the environment, especially if they are highly virulent or transmit quickly, could either overwhelm the remaining host population causing local extinction, or suppress host populations preventing fast host population growth (figure 4b; [133,145,155–157]).
The evolutionary consequence for parasites with different transmission strategies may vary because of the distinct ecological dynamics discussed above (figure 4). In systems with direct or vectored transmission, both host and parasite populations may experience a bottleneck following extreme heating (figure 4a; [135]). Host and parasite evolution may simply be constrained by low genetic variation—without any shift in the parasite population's mean trait values. However, if heat extremes cause higher mortality for hosts infected with more virulent parasite genotypes (e.g. [19]), low virulence infections may continue to transmit, and selection could favour the evolution of reduced virulence (e.g. [137,–139,158]). Indeed, the demographic impact of heating may increase selection on virulence relative to transmission, driving the evolution of reduced virulence [54]. Importantly, if virulence experienced during heat stress is unrelated to within-host parasite burden, selection on virulence, replication and transmission could be decoupled [55].
When host populations infected with obligate killers face extreme heat, it is likely that ongoing transmission will be predominantly host limited [156]. The relatively large proportion of infected hosts that die will contract host population size and cause a large release of viable parasites into the environment [156]. Less virulent parasite genotypes whose hosts survive heat stress may have a fitness advantage as they continue to replicate. However, there will also be an accumulation of more virulent genotypes in the environment. Virulence evolution will therefore depend on the capacity of surviving hosts to reestablish their population [145,156] and the relative fitness advantage for parasites replicating in surviving hosts versus those already in the environment [55,60]. If virulence is unrelated to parasite burden selection on parasite fitness traits may become decoupled. The less virulent parasite genotypes whose hosts survive heat stress may not be constrained by a virulence–transmission trade-off (or selection on virulence may dominate, e.g. [54]). As a result, evolutionary dynamics for these parasites may not follow dynamics predicted from current theory (e.g. [60]).
6. Conclusion
While we are beginning to understand the consequences of infection for hosts during heating, the eco-evolutionary consequences for parasites remain unclear [13,14,27,56]. Trade-off theory predicts a strong relationship between within-host parasite burden and virulence (box 1). Under extreme heat, there is often only a weak relationship, if any (table 1). Considering the diversity of host–parasite systems, a broad definition of parasite burden is difficult, let alone establishing general explanations for the missing link between parasite burden and host heat tolerance. More empirical studies are needed, across a wider diversity of systems, to make broad predictions about host and parasites under heating. At a fundamental level, extreme heat may present a condition under which relationships assumed by general theory simply do not hold (box 1).
Ongoing transmission—except for obligate killers—during an infection may affect our capacity to measure the cumulative parasite burden that a host has experienced. Obligate killers present us with the opportunity to directly relate parasite burden to replication, virulence and transmission. In these systems, a relationship between parasite burden and host heat tolerance has been documented, showing that parasite replication is an important driver of host sensitivity to heat stress (box 2). It is clear, however, that other aspects of host–parasite interactions are also crucial. Interactions between host and parasite processes at the genetic, physiological and ecological levels will regulate the eco-evolutionary relationship between parasite burden and host performance [12–14,26,27,56]. Escalating heating events are going to be a driving force for the evolution of hosts and parasites.
There is a pressing need to extend our understanding about how heating will drive host–parasite eco-evolutionary dynamics [13,14,56]. The development of theory will help guide experimental efforts, allowing us to make more grounded and detailed predictions about the ramifications of heating for parasite evolution. To predict evolutionary responses to heating, the link between individual and population-level eco-evolutionary processes will need to be considered [50,57,60,140,159]. The relationships between parasite virulence and transmission under heating may be mediated by interactions between the genetics, physiology and transmission strategy of hosts and parasites [26]. How within-host interactions translate into population-level processes, such as host mortality rates, population dynamics and parasite fitness, will in turn drive evolutionary change to extreme heat [54,57,60,140,159]. Heating may also shift genetic trade-offs within and between parasite fitness traits, and the strength of selection on virulence and transmission, shaping parasite evolutionary potential [50,54,60]. Predicting parasite evolution will also be aided by a deeper understanding of the mechanisms underlying infection-related declines in host heat tolerance (e.g. CTmax). Our discussion on mechanisms is predominantly speculative. Without understanding the mechanisms at play, it is difficult to know where our theoretical and experimental efforts should lie (e.g. [160]). Finally, to support modelling efforts, approaches such as experimental evolution of parasites across host populations in the laboratory could help to solidify evolutionary predictions.
Present predictions for disease transmission and distributional changes under global change do not incorporate the capacity for parasites to evolve (e.g. [12,27,161]). That under heating, key parasite infection traits do not align with conventionally predicted relationships (i.e. there is little relationship between parasite burden and virulence under heat stress), suggests current methods for prediction may be challenging to apply. A deeper understanding of these complex processes will be valuable for addressing the impacts of parasite evolution for host species persistence, host–parasite distributions and even the potential for zoonotic spill-overs into human and wildlife populations.
Data accessibility
Data presented in box 2 can be found at https://doi.org/10.1111/gcb.14713. Unpublished data presented in box 3 have been provided as electronic supplementary material. Data from Gehman et al. [27] can be found at https://github.com/alyssamina/Thermal-ecology-disease.
The data are provided in electronic supplementary material [162].
Authors' contributions
T.E.H.: conceptualization, visualization, writing---original draft, writing---review and editing; A.L.M.G.: conceptualization, visualization, writing---original draft, writing---review and editing; K.C.K.: conceptualization, writing---original draft, writing---review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
K.C.K. thanks the Natural Environment Research Council UK (NE/X000540/1) and a Philip Leverhulme Prize for funding.
References
- 1.Ummenhofer CC, Meehl GA. 2017. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135. ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingsolver JG, Diamond SE, Buckley LB. 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol 27, 1415-1423. ( 10.1111/1365-2435.12145) [DOI] [Google Scholar]
- 3.Bush A, Mokany K, Catullo R, Hoffmann A, Kellermann V, Sgrò C, McEvey S, Ferrier S. 2016. Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol. Lett. 19, 1468-1478. ( 10.1111/ele.12696) [DOI] [PubMed] [Google Scholar]
- 4.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147. ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheldon KS, Dillon ME. 2016. Beyond the mean: biological impacts of cryptic temperature change. Integr. Comp. Biol. 56, 110-119. ( 10.1093/icb/icw005) [DOI] [PubMed] [Google Scholar]
- 6.Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. 2013. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373-2380. ( 10.1111/gcb.12240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley LB, Huey RB. 2016. Temperature extremes: geographic patterns, recent changes, and implications for organismal vulnerabilities. Glob. Change Biol. 22, 3829-3842. ( 10.1111/gcb.13313) [DOI] [PubMed] [Google Scholar]
- 8.Buckley LB, Kingsolver JG. 2019. Environmental variability shapes evolution, plasticity and biogeographic responses to climate change. Glob. Ecol. Biogeogr. 28, 1456-1468. ( 10.1111/geb.12953) [DOI] [Google Scholar]
- 9.Gutschick VP, BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 160, 21-42. ( 10.1046/j.1469-8137.2003.00866.x) [DOI] [PubMed] [Google Scholar]
- 10.Grant PR, Grant BR, Huey RB, Johnson MTJ, Knoll AH, Schmitt J. 2017. Evolution caused by extreme events. Phil. Trans. R. Soc. B 372, 20160146. ( 10.1098/rstb.2016.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 13, e0007213. ( 10.1371/journal.pntd.0007213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JM, Sauer EL, Santiago O, Spencer S, Rohr JR. 2020. Divergent impacts of warming weather on wildlife disease risk across climates. Science 370, eabb1702. ( 10.1126/science.abb1702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claar DC, Wood CL. 2020. Pulse heat stress and parasitism in warming world. Trends Ecol. Evol. 35, 704-715. ( 10.1016/j.tree.2020.04.002) [DOI] [PubMed] [Google Scholar]
- 14.Hector TE, Hoang KL, Li J, King KC. 2022. Symbiosis and host responses to heating. Trends Ecol. Evol. 37, 611-624. ( 10.1016/j.tree.2022.03.011) [DOI] [PubMed] [Google Scholar]
- 15.Wolinkska K, King KC. 2009. Environment alters selection in host–parasite interactions. Trends Parasitol 25, 236-244. ( 10.1016/j.pt.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 16.Aldea-Sánchez P, Ramírez-Cáceres GE, Rezende EL, Bozinovic F. 2021. Heat tolerance, energetics, and thermal treatments of honeybees parasitized with Varroa. Front. Ecol. Evol. 9, 656504. ( 10.3389/fevo.2021.656504) [DOI] [Google Scholar]
- 17.Bruneaux M, Visse M, Gross R, Pukk L, Saks L, Vasemägi A. 2017. Parasite infection and decreased thermal tolerance: impact of proliferative kidney disease on a wild salmonid fish in the context of climate change. Funct. Ecol. 31, 216-226. ( 10.1111/1365-2435.12701) [DOI] [Google Scholar]
- 18.Greenspan SE, Bower DS, Roznik EA, Pike DA, Marantelli G, Alford RA, Schwarzkopf L, Scheffers BR. 2017. Infection increases vulnerability to climate change via effects on host thermal tolerance. Sci. Rep. 7, 9349. ( 10.1038/s41598-017-09950-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hector TE, Sgrò CM, Hall MD. 2019. Pathogen exposure disrupts an organism's ability to cope with thermal stress. Glob. Change Biol. 25, 3893-3905. ( 10.1111/gcb.14713) [DOI] [PubMed] [Google Scholar]
- 20.Lutterschmidt WI, Schaefer JF, Fiorillo RA. 2007. The ecological significance of Helminth endoparasites on the physiological performance of two sympatric fishes. Comp. Parasitol 74, 194-203. ( 10.1654/4248.1) [DOI] [Google Scholar]
- 21.Porras MF, Agudelo-Cantero GA, Santiago-Martínez MG, Navas CA, Loeschcke V, Sørensen JG, Rajotte EG. 2021. Fungal infections lead to shifts in thermal tolerance and voluntary exposure to extreme temperatures in both prey and predator insects. Sci. Rep. 11, 21710. ( 10.1038/s41598-021-00248-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman E. 2008. Thermal biology of newts (Notophthalmus viridescens) chronically infected with a naturally occurring pathogen. J. Therm. Biol. 33, 27-31. ( 10.1016/j.jtherbio.2007.09.005) [DOI] [Google Scholar]
- 23.Ware-Gilmore F, Sgrò CM, Xi Z, Dutra HLC, Jones MJ, Shea K, Hall MD, Thomas MB, McGraw EA. 2021. Microbes increase thermal sensitivity in the mosquito Aedes aegypti, with the potential to change disease distributions. PLoS Negl. Trop. Dis. 15, e0009548. ( 10.1371/journal.pntd.0009548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meißner K, Schaarschmidt T. 2000. Ecophysiological studies of Corophium volutator (Amphipoda) infested by microphallid trematodes. Mar. Ecol. Prog. Ser. 202, 143-151. ( 10.3354/meps202143) [DOI] [Google Scholar]
- 25.Laidlaw T, Hector TE, Sgrò CM, Hall MD. 2020. Pathogen exposure reduces sexual dimorphism in a host's upper thermal limits. Ecol. Evol. 10, 12 851-12 859. ( 10.1002/ece3.6828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hector TE, Sgrò CM, Hall MD. 2021. Thermal limits in the face of infectious disease: how important are pathogens? Glob. Change Biol. 27, 4469-4480. ( 10.1111/gcb.15761) [DOI] [PubMed] [Google Scholar]
- 27.Gehman ALM, Hall RJ, Byers JE. 2018. Host and parasite thermal ecology jointly determine the effect of climate warming on epidemic dynamics. Proc. Natl Acad. Sci. USA 115, 744-749. ( 10.1073/pnas.1705067115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padfield D, Castledine M, Buckling A. 2020. Temperature-dependent changes to host–parasite interactions alter the thermal performance of a bacterial host. ISME J. 14, 389-398. ( 10.1038/s41396-019-0526-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunze C, Luijckx P, Jackson AL, Donohue I. 2022. Alternate patterns of temperature variation bring about very different disease outcomes at different mean temperatures. eLife 11, e72861. ( 10.7554/eLife.72861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen JM, Civitello DJ, Venesky MD, McMahon TA, Rohr JR. 2019. An interaction between climate change and infectious disease drove widespread amphibian declines. Glob. Change Biol. 25, 927-937. ( 10.1111/gcb.14489) [DOI] [PubMed] [Google Scholar]
- 31.Alizon S, Hurford A, Mideo N, van Baalen M. 2009. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245-259. ( 10.1111/j.1420-9101.2008.01658.x) [DOI] [PubMed] [Google Scholar]
- 32.Leggett HC, Buckling A, Long GH, Boots M. 2013. Generalism and the evolution of parasite virulence. Trends Ecol. Evol. 28, 592-596. ( 10.1016/j.tree.2013.07.002) [DOI] [PubMed] [Google Scholar]
- 33.Frank SA. 1996. Models of parasite virulence. Q. Rev. Biol. 71, 37-78. ( 10.1086/419267) [DOI] [PubMed] [Google Scholar]
- 34.Ewald PW. 1994. Evolution of infectious disease. New York, NY: Oxford University Press. [Google Scholar]
- 35.Middleton DJ, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Westbury HA, Halpin K, Daniels PW. 2007. Experimental nipah virus infection in pteropid bats (Pteropus poliocephalus). J. Comp. Pathol. 136, 266-272. ( 10.1016/j.jcpa.2007.03.002) [DOI] [PubMed] [Google Scholar]
- 36.Voyles J, et al. 2009. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582. ( 10.1126/science.1176765) [DOI] [PubMed] [Google Scholar]
- 37.Wilber MQ, Knapp RA, Toothman M, Briggs CJ. 2017. Resistance, tolerance and environmental transmission dynamics determine host extinction risk in a load-dependent amphibian disease. Ecol. Lett. 20, 1169-1181. (( 10.1111/ele.12814) [DOI] [PubMed] [Google Scholar]
- 38.Anderson RM, May RM. 1986. The invasion, persistence and spread of infectious diseases within animal and plant communities. Phil. Trans. R. Soc. Lond. B 314, 533-570. ( 10.1098/rstb.1986.0072) [DOI] [PubMed] [Google Scholar]
- 39.Anderson R, May RM. 1982. Coevolution of hosts and parasites. Parasitology 85, 411-426. ( 10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- 40.Ewald PW. 1983. Host-parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 14, 465-485. ( 10.1146/annurev.es.14.110183.002341) [DOI] [Google Scholar]
- 41.Koella JC, Antia R. 1995. Optimal pattern of replication and transmission for parasites with two stages in their life cycle. Theor. Popul. Biol. 47, 277-291. ( 10.1006/tpbi.1995.1012) [DOI] [Google Scholar]
- 42.Walther BA, Ewald PW. 2004. Pathogen survival in the external environment and the evolution of virulence. Biol. Rev. 79, 849-869. ( 10.1017/S1464793104006475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acevedo MA, Dillemuth FP, Flick AJ, Faldyn MJ, Elderd BD. et al. 2019. Virulence-driven trade-offs in disease transmission: a meta-analysis. Evolution 73, 636-647. ( 10.1111/evo.13692) [DOI] [PubMed] [Google Scholar]
- 44.Antonovics J, et al. 2017. The evolution of transmission mode. Phil. Trans. R. Soc. B 372, 20160083. ( 10.1098/rstb.2016.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rafaluk-Mohr C. 2019. The relationship between parasite virulence and environmental persistence: a meta-analysis. Parasitology 146, 897-902. ( 10.1017/S0031182019000015) [DOI] [PubMed] [Google Scholar]
- 46.Boldin B, Kisdi E. 2012. On the evolutionary dynamics of pathogens with direct and environmental transmission. Evolution 66, 2514-2527. ( 10.1111/j.1558-5646.2012.01613.x) [DOI] [PubMed] [Google Scholar]
- 47.Messenger SL, Molineux IJ, Bull JJ. 1999. Virulence evolution in a virus obeys a trade-off. Proc. R. Soc. B 266, 397-404. ( 10.1098/rspb.1999.0651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen KH, Little T, Skorping A, Ebert D. 2006. Empirical support for optimal virulence in a castrating parasite. PLoS Biol. 4, e197. ( 10.1371/journal.pbio.0040197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Roode JC, Yates AJ, Altizer S. 2008. Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489. ( 10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mideo N, Nelson WA, Reece SE, Bell AS, Read AF, Day T. 2011. Bridging scales in the evolution of infectious disease life histories: application. Evolution 65, 3298-3310. ( 10.1111/j.1558-5646.2011.01382.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebert D, Bull JJ. 2003. Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends Microbiol. 11, 15-20. ( 10.1016/S0966-842X(02)00003-3) [DOI] [PubMed] [Google Scholar]
- 52.Leggett HC, Brown SP, Reece SE. 2014. War and peace: social interactions in infections. Phil. Trans. R. Soc. B 369, 20130365. ( 10.1098/rstb.2013.0365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown SP, Cornforth DM, Mideo N. 2012. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 20, 336-342. ( 10.1016/j.tim.2012.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Day T, Parsons T, Lambert A, Gandon S. 2020. The Price equation and evolutionary epidemiology. Phil. Trans. R. Soc. B 375, 20190357. ( 10.1098/rstb.2019.0357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day T. 2002. Virulence evolution via host exploitation and toxin production in spore-producing pathogens. Ecol. Lett. 5, 471-476. ( 10.1046/j.1461-0248.2002.00342.x) [DOI] [Google Scholar]
- 56.Rohr JR, Cohen JM. 2020. Understanding how temperature shifts could impact infectious disease. PLoS Biol. 18, e3000938. ( 10.1371/journal.pbio.3000938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mideo N, Alizon S, Day T. 2008. Linking within- and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol. Evol. 23, 511-517. ( 10.1016/j.tree.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 58.Schmid-Hempel P. 2011. Evolutionary parasitology. Oxford, UK: Oxford University Press. [Google Scholar]
- 59.Ebert D, Weisser WW. 1997. Optimal killing for obligate killers: the evolution of life histories and virulence of semelparous parasites. Proc. R. Soc. Lond. B 264, 985-991. ( 10.1098/rspb.1997.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall MD, Mideo N. 2018. Linking sex differences to the evolution of infectious disease life-histories. Phil. Trans. R. Soc. B 373, 20170431. ( 10.1098/rstb.2017.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Roode JC, Altizer S. 2010. Host–parasite genetic interactions and virulence-transmission relationships in natural populations of monarch butterflies. Evolution 64, 502-514. ( 10.1111/j.1558-5646.2009.00845.x) [DOI] [PubMed] [Google Scholar]
- 62.Ebert D. 2008. Host–parasite coevolution: insights from the Daphnia–parasite model system. Curr. Opin. Microbiol. 11, 290-301. ( 10.1016/j.mib.2008.05.012) [DOI] [PubMed] [Google Scholar]
- 63.Clerc M, Ebert D, Hall MD. 2015. Expression of parasite genetic variation changes over the course of infection: implications of within-host dynamics for the evolution of virulence. Proc. R. Soc. B 282, 20142820. ( 10.1098/rspb.2014.2820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebert D, Duneau D, Hall MD, Luijckx P, Andras JP, Du Pasquier L, Ben-Ami F. 2016. A population biology perspective on the stepwise infection process of the bacterial pathogen Pasteuria ramosa in Daphnia. Adv. Parasitol. 91, 265-310. ( 10.1016/bs.apar.2015.10.001) [DOI] [PubMed] [Google Scholar]
- 65.Hoeg JT. 1995. The biology and life cycle of the rhizocephala (Cirripedia). J. Mar. Biol. Assoc. UK 75, 517-550. ( 10.1017/S0025315400038996) [DOI] [Google Scholar]
- 66.Rosenkranz M, Lagrue C, Poulin R, Selbach C. 2018. Small snails, high productivity? Larval output of parasites from an abundant host. Freshw. Biol. 63, 1602-1609. ( 10.1111/fwb.13189 [DOI] [Google Scholar]
- 67.O'Shaughnessy KA, Harding JM, Burge EJ. 2014. Ecological effects of the invasive parasite Loxothylacus panopaei on the flatback mud crab Eurypanopeus depressus with implications for estuarine communities. Bullet. Mar. Sci. 90, 611-621. ( 10.5343/bms.2013.1060) [DOI] [Google Scholar]
- 68.Rees D, Glenner H. 2014. Control region sequences indicate that multiple externae represent multiple infections by Sacculina carcini (Cirripedia: Rhizocephala). Ecol. Evol. 4, 3290-3297. ( 10.1002/ece3.1177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker G, Clare A, Rittschof D, Mensching D. 1992. Aspects of the life-cycle of Loxothylacus panopaei (Gissler), a sacculinid parasite of the mud crab Rhithropanopeus harrisii (Gould): a laboratory study. J. Exp. Mar. Biol. Ecol. 157, 181-193. ( 10.1016/0022-0981(92)90161-3) [DOI] [Google Scholar]
- 70.Day T. 2001. Parasite transmission modes and the evolution of virulence. Evolution 55, 2389-2400. ( 10.1111/j.0014-3820.2001.tb00754.x) [DOI] [PubMed] [Google Scholar]
- 71.Gilchrist MA, Sasaki A. 2002. Modeling host–parasite coevolution: a nested approach based on mechanistic models. J. Theor. Biol. 218, 289-308. ( 10.1006/jtbi.2002.3076) [DOI] [PubMed] [Google Scholar]
- 72.André JB, Ferdy JB, Godelle B. 2003. Within-host parasite dynamics, emerging trade-off, and evolution of virulence with immune system. Evolution 57, 1489-1497. ( 10.1111/j.0014-3820.2003.tb00357.x) [DOI] [PubMed] [Google Scholar]
- 73.Alizon S, van Baalen M. 2005. Emergence of a convex trade-off between transmission and virulence. Am. Nat. 165, E155-E167. ( 10.1086/430053) [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann AA, Chown SL, Clusella-Trullas S. 2012. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934-949. ( 10.1111/j.1365-2435.2012.02036.x) [DOI] [Google Scholar]
- 75.Hector TE, Sgrò CM, Hall MD. 2020. The influence of immune activation on thermal tolerance along a latitudinal cline. J. Evol. Biol. 99, e52613. ( 10.1111/jeb.13663) [DOI] [PubMed] [Google Scholar]
- 76.Miller MR, White A, Boots M. 2006. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution 60, 945-956. [PubMed] [Google Scholar]
- 77.Best A, White A, Boots M. 2008. Maintenance of host variation in tolerance to pathogens and parasites. Proc. Natl Acad. Sci. USA 105, 20 786-20 791. ( 10.1073/pnas.0809558105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Råberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37-49. ( 10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Little TJ, Shuker DM, Colegrave N, Day T, Graham AL. 2010. The coevolution of virulence: tolerance in perspective. PLoS Pathog. 6, e1001006. ( 10.1371/journal.ppat.1001006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936-941. ( 10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soares MP, Teixeira L, Moita LF. 2017. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17, 83-96. ( 10.1038/nri.2016.136) [DOI] [PubMed] [Google Scholar]
- 82.Schieber AMP, Ayres JS. 2016. Thermoregulation as a disease tolerance defense strategy. Pathogens Dis. 74, ftw106. ( 10.1093/femspd/ftw106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Råberg L, Stjernman M. 2012. The evolutionary ecology of infectious disease virulence. In Ecoimmunology, pp. 548-578. New York, NY: Oxford University Press. [Google Scholar]
- 84.Drew GC, Stevens EJ, King KC. 2021. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 19, 623-638. ( 10.1038/s41579-021-00550-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall MD, Bento G, Ebert D. 2017. The evolutionary consequences of stepwise infection processes. Trends Ecol. Evol. 32, 612-623. ( 10.1016/j.tree.2017.05.009) [DOI] [PubMed] [Google Scholar]
- 86.Graham AL, Allen JE, Read AF. 2005. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 36, 373-397. ( 10.1146/annurev.ecolsys.36.102003.152622) [DOI] [Google Scholar]
- 87.Siva-Jothy MT, Moret Y, Rolff J. 2005. Insect immunity: an evolutionary ecology perspective. Adv. Insect Pysiol. 32, 1-48. ( 10.1016/S0065-2806(05)32001-7) [DOI] [Google Scholar]
- 88.Labbé P, Vale PF, Little TJ. 2010. Successfully resisting a pathogen is rarely costly in Daphnia magna. BMC Evol. Biol. 10, 355. ( 10.1186/1471-2148-10-355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cressler CE, Graham AL, Day T. 2015. Evolution of hosts paying manifold costs of defence. Proc. R. Soc. B 282, 20150065. ( 10.1098/rspb.2015.0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alizon S, Sofonea MT. 2021. SARS-CoV-2 virulence evolution: avirulence theory, immunity and trade-offs. J. Evol. Biol. 34, 1867-1877. ( 10.1111/jeb.13896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King KC. 2019. Defensive symbionts. Curr. Biol. 29, R78-R80. ( 10.1016/j.cub.2018.11.028) [DOI] [PubMed] [Google Scholar]
- 92.Ford SA, King KC. 2016. Harnessing the power of defensive microbes: evolutionary implications in nature and disease control. PLoS Pathog. 12, e1005465. ( 10.1371/journal.ppat.1005465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palmer-Young EC, Raffel TR, McFrederick QS. 2018. Temperature-mediated inhibition of a bumblebee parasite by an intestinal symbiont. Proc. R. Soc. B 285, 20182041. ( 10.1098/rspb.2018.2041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB. 2014. Temperature alters Plasmodium blocking by Wolbachia. Sci. Rep. 4, 1-6. ( 10.1038/srep03932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roback MJ, Richards-Zawacki CL. 2018. Temperature-dependent effects of cutaneous bacteria on a frog's tolerance of fungal infection. Front. Microbiol. 9, 410. ( 10.3389/fmicb.2018.00410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chrostek E, Martins N, Marialva MS, Teixeira L. 2021. Wolbachia-conferred antiviral protection is determined by developmental temperature. MBio 12, e02923-20. ( 10.1128/mBio.02923-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Civitello DJ, et al. 2022. Transmission potential of human schistosomes can be driven by resource competition among snail intermediate hosts. Proc. Natl Acad. Sci. USA 119, e2116512119. ( 10.1073/pnas.2116512119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gipson SAY, Pettersen AK, Heffernan L, Hall MD. 2022. Host sex modulates the energetics of pathogen proliferation and its dependence on environmental resources. Am. Nat. 199, E186-E196. ( 10.1086/718717) [DOI] [PubMed] [Google Scholar]
- 99.Nørgaard LS, Ghedini G, Phillips BL, Hall MD. 2021. Energetic scaling across different host densities and its consequences for pathogen proliferation. Funct. Ecol. 35, 475-484. ( 10.1111/1365-2435.13721) [DOI] [Google Scholar]
- 100.Hite JL, Cressler CE. 2018. Resource-driven changes to host population stability alter the evolution of virulence and transmission. Phil. Trans. R. Soc. B 373, 20170087. ( 10.1098/rstb.2017.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cressler CE, Nelson WA, Day T, McCauley E. 2014. Starvation reveals the cause of infection-induced castration and gigantism. Proc. R. Soc. B 281, 20141087. ( 10.1098/rspb.2014.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bashir-Tanoli S, Tinsley MC. 2014. Immune response costs are associated with changes in resource acquisition and not resource reallocation. Funct. Ecol. 28, 1011-1019. ( 10.1111/1365-2435.12236) [DOI] [Google Scholar]
- 103.Rivero A, Ferguson HM. 2003. The energetic budget of Anopheles stephensi infected with Plasmodium chabaudi: is energy depletion a mechanism for virulence? Proc. R. Soc. Lond. B 270, 1365-1371. ( 10.1098/rspb.2003.2389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nyasembe VO, Teal PEA, Sawa P, Tumlinson JH, Borgemeister C, Torto B. 2014. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr. Biol. 24, 217-221. ( 10.1016/j.cub.2013.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ponton F, Morimoto J, Robinson K, Kumar SS, Cotter SC, Wilson K, Simpson SJ. 2020. Macronutrients modulate survival to infection and immunity in Drosophila. J. Anim. Ecol. 89, 460-470. ( 10.1111/1365-2656.13126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Becker DJ, Streicker DG, Altizer S. 2015. Linking anthropogenic resources to wildlife–pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483-495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Becker DJ, Streicker DG, Altizer S. 2018. Using host species traits to understand the consequences of resource provisioning for host–parasite interactions. J. Anim. Ecol. 87, 511-525. ( 10.1111/1365-2656.12765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gardner E, Beli E, Clinthorne J, Duriancik D. 2010. Energy intake and response to infection with influenza. Annu. Rev. Nutr. 31, 353-367. ( 10.1146/annurev-nutr-081810-160812) [DOI] [PubMed] [Google Scholar]
- 109.Robles R, Alvarez F, Alcaraz G. 2002. Oxygen consumption of the crab Callinectes rathbunae parasitized by the rhizocephalan barnacle Loxothylacus texanus as a function of salinity. Mar. Ecol. Prog. Ser. 235, 189-194. [Google Scholar]
- 110.Brophy T, Luong LT. 2021. Ectoparasite-induced increase in Drosophila host metabolic rate. Physiol. Entomol. 46, 1-7. ( 10.1111/phen.12334) [DOI] [Google Scholar]
- 111.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690-692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 112.Cable JM, Enquist BJ, Moses ME. 2007. The allometry of host–pathogen interactions. PLoS ONE 2, e1130. ( 10.1371/journal.pone.0001130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hechinger RF. 2013. A metabolic and body-size scaling framework for parasite within-host abundance, biomass, and energy flux. Am. Nat. 182, 234-248. ( 10.1086/670820) [DOI] [PubMed] [Google Scholar]
- 114.Cheng W, Hsiao IS, Hsu CH, Chen JC. 2004. Change in water temperature on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Fish Shellfish Immunol. 17, 235-243. ( 10.1016/j.fsi.2004.03.007) [DOI] [PubMed] [Google Scholar]
- 115.Cheng WT, Wang LU, Chen JC. 2005. Effect of water temperature on the immune response of white shrimp Litopenaeus vannamei to Vibrio alginolyticus. Aquaculture 250, 592-601. ( 10.1016/j.aquaculture.2005.04.060) [DOI] [Google Scholar]
- 116.Liang S, Luo X, You W, Luo L, Ke C. 2014. The role of hybridization in improving the immune response and thermal tolerance of abalone. Fish Shellfish Immunol. 39, 69-77. ( 10.1016/j.fsi.2014.04.014) [DOI] [PubMed] [Google Scholar]
- 117.Rowley AF, Davies CE, Malkin SH, Bryan CC, Thomas JE, Batista FM, Coates CJ. 2020. Prevalence and histopathology of the parasitic barnacle, Sacculina carcini in shore crabs, Carcinus maenas. J. Invertebr. Pathol. 171, 107338. ( 10.1016/j.jip.2020.107338) [DOI] [PubMed] [Google Scholar]
- 118.Harper CV, et al. 2018. Temperature regulates NF-κB dynamics and function through timing of A20 transcription. Proc. Natl Acad. Sci. USA 115, E5243-E5249. ( 10.1073/pnas.1803609115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM. 1988. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann. Intern. Med. 1, 129. ( 10.7326/0003-4819-129-3-199808010-00001) [DOI] [PubMed] [Google Scholar]
- 120.Leon LR, Helwig BG. 2010. Heat stroke: role of the systemic inflammatory response. J. Appl. Physiol. 109, 1980-1988. ( 10.1152/japplphysiol.00301.2010) [DOI] [PubMed] [Google Scholar]
- 121.Elliot SI, Blanford S, Thomas MB. 2002. Host–pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proc. R. Soc. B 269, 1599-1607. ( 10.1098/rspb.2002.2067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Müller CB, Schmid-Hempel P. 1993. Exploitation of cold temperature as defence against parasitoids in bumblebees. Nature 363, 65-67. ( 10.1038/363065a0) [DOI] [Google Scholar]
- 123.Bates AE, Leiterer F, Wiedeback ML, Poulin R. 2011. Parasitized snails take the heat: a case of host manipulation? Oecologia 167, 613-621. ( 10.1007/s00442-011-2014-0) [DOI] [PubMed] [Google Scholar]
- 124.Wang SYS, Tattersall GJ, Koprivnikar J. 2019. Trematode parasite infection affects temperature selection in aquatic host snails. Physiol. Biochem. Zool. 92, 71-79. ( 10.1086/701236) [DOI] [PubMed] [Google Scholar]
- 125.Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI. 2018. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425-1439. ( 10.1111/ele.13107) [DOI] [PubMed] [Google Scholar]
- 126.Kellermann V, van Heerwaarden B. 2019. Terrestrial insects and climate change: adaptive responses in key traits. Physiol. Entomol. 44, 99-115. ( 10.1111/phen.12282) [DOI] [Google Scholar]
- 127.Yampolsky LY, Scheiner SM. 1996. Why larger offspring at lower temperatures? A demographic approach. Am. Nat. 147, 86-100. ( 10.1086/285841) [DOI] [Google Scholar]
- 128.Huete-Stauffer TM, Arandia-Gorostidi N, Alonso-Sáez L, Morán XAG. 2016. Experimental warming decreases the average size and nucleic acid content of marine bacterial communities. Front. Microbiol. 7, 730. (doi:10.3389/fmicb.2016.00730.eCollection 2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malerba ME, Marshall DJ. 2020. Testing the drivers of the temperature–size covariance using artificial selection. Evolution 74, 169-178. ( 10.1111/evo.13896) [DOI] [PubMed] [Google Scholar]
- 130.Verberk WCEP, Atkinson D, Hoefnagel KN, Hirst AG, Horne CR, Siepel H. 2021. Shrinking body sizes in response to warming: explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 96, 247-268. ( 10.1111/brv.12653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weiser JN. 2013. The battle with the host over microbial size. Curr. Opin. Microbiol. 16, 59-62. ( 10.1016/j.mib.2013.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shocket MS, Vergara D, Sickbert AJ, Walsman JM, Strauss AT, Hite JL, Duffy MA, Cáceres CE, Hall SR. 2018. Parasite rearing and infection temperatures jointly influence disease transmission and shape seasonality of epidemics. Ecology 99, 1975-1987. ( 10.1002/ecy.2430) [DOI] [PubMed] [Google Scholar]
- 133.Gubbins S, Gilligan CA. 1997. Persistence of host-parasite interactions in a disturbed environment. J. Theor. Biol. 188, 241-258. ( 10.1006/jtbi.1997.0466) [DOI] [Google Scholar]
- 134.Hall SR, Duffy MA, Cáceres CE. 2005. Selective predation and productivity jointly drive complex behavior in host-parasite systems. Am. Nat. 165, 70-81. ( 10.1086/426601) [DOI] [PubMed] [Google Scholar]
- 135.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467-484. ( 10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 136.Rapti Z, Cáceres CE. 2016. Effects of intrinsic and extrinsic host mortality on disease spread. Bull. Math. Biol. 78, 235-253. ( 10.1007/s11538-016-0141-9) [DOI] [PubMed] [Google Scholar]
- 137.Williams PD, Day T. 2001. Interactions between sources of mortality and the evolution of parasite virulence. Proc. R. Soc. Lond. B 268, 2331-2337. ( 10.1098/rspb.2001.1795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choo K, Williams PD, Day T. 2003. Host mortality, predation and the evolution of parasite virulence. Ecol. Lett. 6, 310-315. ( 10.1046/j.1461-0248.2003.00425.x) [DOI] [Google Scholar]
- 139.Morozov A, Best A. 2012. Predation on infected host promotes evolutionary branching of virulence and pathogens' biodiversity. J. Theor. Biol. 307, 29-36. ( 10.1016/j.jtbi.2012.04.023) [DOI] [PubMed] [Google Scholar]
- 140.Kirk D, Luijckz P, Jones N, Krichel L, Pencer C, Molnár P, Krkošek M. 2020. Experimental evidence of warming-induced disease emergence and its prediction by a trait-based mechanistic mode. Proc. R. Soc. B 287, 20201526. ( 10.1098/rspb.2020.1526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lister BC, Garcia A. 2018. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl Acad. Sci. USA 115, E10397-E10406. ( 10.1073/pnas.1722477115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abarca M, Larsen EA, Ries L. 2019. Heatwaves and novel host consumption increase overwinter mortality of an imperiled wetland butterfly. Front. Ecol. Evol. 7, 193. ( 10.3389/fevo.2019.00193) [DOI] [Google Scholar]
- 143.Ratnayake HU, Kearney MR, Govekar P, Karoly D, Welbergen JA. 2019. Forecasting wildlife die-offs from extreme heat events. Anim. Conserv. 22, 386-395. ( 10.1111/acv.12476) [DOI] [Google Scholar]
- 144.Conradie SR, Woodborne SM, Wolf BO, Pessato A, Mariette MM, McKechnie AE. 2020. Avian mortality risk during heat waves will increase greatly in arid Australia during the 21st century. Conserv. Physiol. 8, coaa048. ( 10.1093/conphys/coaa048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ebert D, Lipsitch M, Mangin KL. 2000. The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. Am. Nat. 156, 459-477. ( 10.1086/303404) [DOI] [PubMed] [Google Scholar]
- 146.Sales K, et al. 2018. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 9, 4771. ( 10.1038/s41467-018-07273-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Walsh BS, Parratt SR, Hoffmann AA, Atkinson D, Snook RR, Bretman A, Price TAR. 2019. The impact of climate change on fertility. Trends Ecol. Evol. 34, 249-259. ( 10.1016/j.tree.2018.12.002) [DOI] [PubMed] [Google Scholar]
- 148.van Heerwaarden B, Sgrò CM. 2021. Male fertility thermal limits predict vulnerability to climate warming. Nat. Commun. 12, 2214. ( 10.1038/s41467-021-22546-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. 2010. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl Acad. Sci. USA 107, 15 135-15 139. ( 10.1073/pnas.1006422107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. 2011. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl Acad. Sci. USA 108, 7460-7465. ( 10.1073/pnas.1101377108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carrington LB, Armijos MV, Lambrechts L, Barker CM, Scott TW. 2013. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes Aegypti life-history traits. PLoS ONE 8, e58824. ( 10.1371/journal.pone.0058824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON. 2017. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R. Soc. Open Sci. 4, 160969. ( 10.1098/rsos.160969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Papkou A, Gokhale CS, Traulsen A, Schulenburg H. 2016. Host–parasite coevolution: why changing population size matters. Zoology 119, 330-338. ( 10.1016/j.zool.2016.02.001) [DOI] [PubMed] [Google Scholar]
- 154.Bacaër N, Guernaoui S. 2006. The epidemic threshold of vector-borne diseases with seasonality: the case of cutaneous leishmaniasis in Chichaoua, Morocco. J. Math. Biol. 53, 421-436. ( 10.1007/s00285-006-0015-0) [DOI] [PubMed] [Google Scholar]
- 155.van den Berg F, Bacaer N, Metz JAJ, Lannou C, van den Bosch F. 2011. Periodic host absence can select for higher or lower parasite transmission rates. Evol. Ecol. 25, 121-137. ( 10.1007/s10682-010-9387-0) [DOI] [Google Scholar]
- 156.Fenton A, Fairbairn JP, Norman R, Hudson PJ. 2002. Parasite transmission: reconciling theory and reality. J. Anim. Ecol. 71, 893-905. ( 10.1046/j.1365-2656.2002.00656.x) [DOI] [Google Scholar]
- 157.Auld SKJR, Hall SR, Housley Ochs J, Sebastian M, Duffy MA. 2014. Predators and patterns of within-host growth can mediate both among-host competition and evolution of transmission potential of parasites. Am. Nat. 184, S77-S90. ( 10.1086/676927) [DOI] [PubMed] [Google Scholar]
- 158.Kisdi E, Geritz SAH, Boldin B. 2013. Evolution of pathogen virulence under selective predation: a construction method to find eco-evolutionary cycles. J. Theor. Biol. 339, 140-150. ( 10.1016/j.jtbi.2013.05.023) [DOI] [PubMed] [Google Scholar]
- 159.Kirk D, et al. 2018. Empirical evidence that metabolic theory describes the temperature dependency of within-host parasite dynamics. PLoS Biol. 16, e2004608. ( 10.1371/journal.pbio.2004608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Metcalf CJE, Walter KS, Wesolowski A, Buckee CO, Shevliakova E, Tatem AJ, Boos WR, Weinberger DM, Pitzer VE. 2017. Identifying climate drivers of infectious disease dynamics: recent advances and challenges ahead. Proc. R. Soc. B 284, 20170901. ( 10.1098/rspb.2017.0901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mordecai EA, et al. 2019. Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690-1708. ( 10.1111/ele.13335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hector TE, Gehman ALM, King KC. 2023. Infection burdens and virulence under heat stress: ecological and evolutionary considerations. Figshare. ( 10.6084/m9.figshare.c.6404298) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hector TE, Gehman ALM, King KC. 2023. Infection burdens and virulence under heat stress: ecological and evolutionary considerations. Figshare. ( 10.6084/m9.figshare.c.6404298) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data presented in box 2 can be found at https://doi.org/10.1111/gcb.14713. Unpublished data presented in box 3 have been provided as electronic supplementary material. Data from Gehman et al. [27] can be found at https://github.com/alyssamina/Thermal-ecology-disease.
The data are provided in electronic supplementary material [162].