Abstract
Background
Tranexamic acid (TXA) reduces rates of blood transfusion for total hip arthroplasty (THA) and total knee arthroplasty (TKA). Although the use of oral TXA rather than intravenous (i.v.) TXA might improve safety and reduce cost, it is not clear whether oral administration is as effective.
Methods
This noninferiority trial randomly assigned consecutive patients undergoing primary THA or TKA under neuraxial anaesthesia to either one preoperative dose of oral TXA or one preoperative dose of i.v. TXA. The primary outcome was calculated blood loss on postoperative day 1. Secondary outcomes were transfusions and complications within 30 days of surgery.
Results
Four hundred participants were randomised (200 THA and 200 TKA). The final analysis included 196 THA patients (98 oral, 98 i.v.) and 191 TKA patients (93 oral, 98 i.v.). Oral TXA was non-inferior to i.v. TXA in terms of calculated blood loss for both THA (effect size=–18.2 ml; 95% confidence interval [CI], –113 to 76.3; P<0.001) and TKA (effect size=–79.7 ml; 95% CI, –178.9 to 19.6; P<0.001). One patient in the i.v. TXA group received a postoperative transfusion. Complication rates were similar between the two groups (5/191 [2.6%] oral vs 5/196 [2.6%] i.v.; P=1.00).
Conclusions
Oral TXA can be administered in the preoperative setting before THA or TKA and performs similarly to i.v. TXA with respect to blood loss and transfusion rates. Switching from i.v. to oral TXA in this setting has the potential to improve patient safety and decrease costs.
Keywords: bleeding, blood loss, complications, cost effectiveness, total hip arthroplasty, total knee arthroplasty, tranexamic acid, transfusion
Editor's key points.
-
•
Intravenous tranexamic acid has blood-sparing properties in surgery
-
•
An oral formulation of tranexamic acid might have convenience and safety benefits for preoperative prophylaxis
-
•
This non-inferiority trial found that an oral formulation was as good as the i.v. preparation of tranexamic acid in reducing blood loss and preventing transfusions in primary hip and knee arthroplasty
As combined annual volume for total hip arthroplasty (THA) and total knee arthroplasty (TKA) is projected to reach more than 1.5 million in the UK by 2035,1,2 reducing both the risk of complications and cost associated with these procedures should be a public health priority. Blood transfusion for acute blood loss in the setting of THA and TKA is associated with increased length of stay (LOS), cost of care, and risk for adverse events.3, 4, 5, 6 Although the perioperative use of tranexamic acid (TXA) helps to reduce transfusion rates in THA and TKA7 and thereby reduce costs,8 it is not clear which formulation of the drug provides the optimal combination of effectiveness, cost, and ease of dosing.
The authors performed a randomised non-inferiority trial, hypothesising that the use of one preoperative dose of oral TXA would be non-inferior to the use of i.v. TXA for patients undergoing primary THA or TKA, leading to similar calculated blood loss (CBL) and transfusion rates.
Methods
Study design and participants
This was an investigator-initiated randomised non-inferiority trial comparing the efficacy of oral vs i.v. TXA in reducing blood loss and preventing transfusion in the setting of THA and TKA. Eight fellowship-trained attending arthroplasty surgeons and 12 attending anaesthesiologists enrolled consecutive patients undergoing unilateral primary THA or TKA at a single, urban orthopaedic hospital in the USA. Eligible participants were aged 18–80 yr. Patients were excluded if they had a history of venous thromboembolism, a myocardial infarction or stroke within 1 yr of surgery, preoperative thrombocytopenia (platelets <100 μl−1), a BMI >40 kg m−2, or prior major surgery on the operative joint. Enrolled patients gave written consent to participate in the study.
To standardise the anaesthetic, only patients having neuraxial anaesthesia with sedation were included. In addition, patients on anticoagulant or antiplatelet agents (except for a daily dose of aspirin 81 mg) were excluded, as were those with new-onset or active atrial fibrillation. For THA cases, only patients undergoing surgery with a posterolateral approach were included. All TKA approaches were performed with the use of a tourniquet and a medial parapatellar arthrotomy. Baseline demographic and descriptive data were recorded for all patients, including age, sex, and American Society of Anesthesiologists (ASA) physical status. Study data were collected and managed using REDCap (Research Electronic Data Capture).9,10
The protocol was approved by the local Institutional Review Board, and the trial was registered at ClinicalTrials.gov (NCT04089865). The study protocol is available in Appendix A. The Consolidated Standards of Reporting Trials (CONSORT) guidelines11 and the rules in the Declaration of Helsinki12 were followed throughout the study. A planned interim analysis was performed when enrolment reached 200 subjects; this was done to ensure neither drug was associated with increased CBL, transfusion rate, or complication rate.
Enrolment and randomisation
Patients scheduled for primary THA or TKA and appearing to meet inclusion criteria by review of the electronic medical record were approached by research coordinators on the day of surgery. If the patient was confirmed to meet the criteria and consented (verbally and written) to enrolment, they were randomised to the oral or i.v. TXA arm of the study according to a confidential list accessed by the research coordinator. The randomisation sequences, which were separate for THA and TKA subjects, were structured 1:1 with a block size of two. The trial was not fully blinded.
Procedures
Preoperative laboratory data (within 1 month of surgery) were reviewed to determine baseline haematocrit for each patient. In the oral arm of the study, subjects received TXA 1950 mg p.o. (three 650 mg pills) in the holding area 2 h before surgery. In the i.v. arm of the study, subjects received i.v. TXA 1000 mg i.v. in the operating room before and within 30 min of incision. Surgical approaches were consistent throughout the study, as outlined above. Drain use and wound closure methods were not standardised. A postoperative complete blood count (CBC) was sent upon arrival to the PACU and again on the morning of postoperative day (POD) 1 to measure haematocrit. Subjects underwent standard postoperative physical therapy (PT) while inpatient. Pharmacologic and mechanical prophylaxis against deep vein thrombosis was prescribed postoperatively. Standard hospital criteria for transfusion included a postoperative haemoglobin <7 g dl−1 or a haemoglobin <8 g dl−1 in a subjects with symptomatic anaemia or prior cardiac history. Complete blood counts were only ordered after POD1 if clinically indicated.
Outcomes
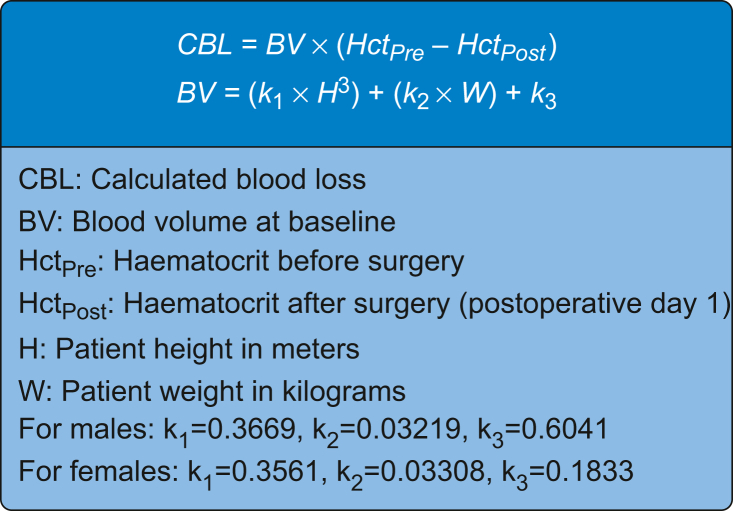
Calculated blood loss was considered the primary outcome variable in this study and was calculated based on baseline and POD1 haematocrit using the Gross formula13 (Fig 1). Blood transfusion (intraoperative or postoperative) was considered a secondary outcome, as were LOS and time to discharge from inpatient PT. Complications were recorded throughout the study and classified as cardiac, neurological, pulmonary, renal, infectious, allergic (to TXA), or ‘other’ (e.g. periprosthetic fracture) via medical record review for up to 30 days after surgery. The timing of TXA dosing (with respect to incision) was also recorded to ensure appropriate dosing.
Fig 1.
The Gross equation for calculated blood loss (CBL).
Statistical analysis
With expected procedural blood loss in the i.v. group estimated at 1173 ml based on the results from a previous study at our institution,14 a conservative non-inferiority margin (NIM) was set at 20% of this value based on author group consensus, or 235 ml. Using this NIM, an a priori power analysis based on a one-sided t-test with alpha set to 0.025 indicated that 336 patients (168 THA and 168 TKA) would be needed to achieve a power of 80% in discerning non-inferiority based on CBL. To ensure adequate power, it was decided to enrol 400 patients in total (200 THA and 200 TKA).
Baseline demographic information was compared by calculating standardised differences and using a threshold of 0.28.15 For non-inferiority testing, CBL values were compared using one-sided two-sample t-tests after assessing normality. The a priori NIM of 235 ml was used, with an alpha level of 0.025. Rates of transfusion and complications were compared using Fisher's exact tests. Continuous secondary outcomes were compared using two-sample independent t-tests or Wilcoxon rank sum tests, depending on normality testing. Categorical secondary outcomes were analysed using χ2 or Fisher's exact tests. Transfusion rates were compared using a critical alpha level of 0.025. Other secondary outcomes and complications were analysed with a critical alpha level of 0.05. Differences were assessed based on an intention-to-treat framework. CBL values for patients discharged POD0 (who had no POD1 haematocrit measures) were treated as missing values in the main analysis. Post hoc analyses were done, as described in Appendix B, exploring the impact of missing values and protocol deviations.
Results
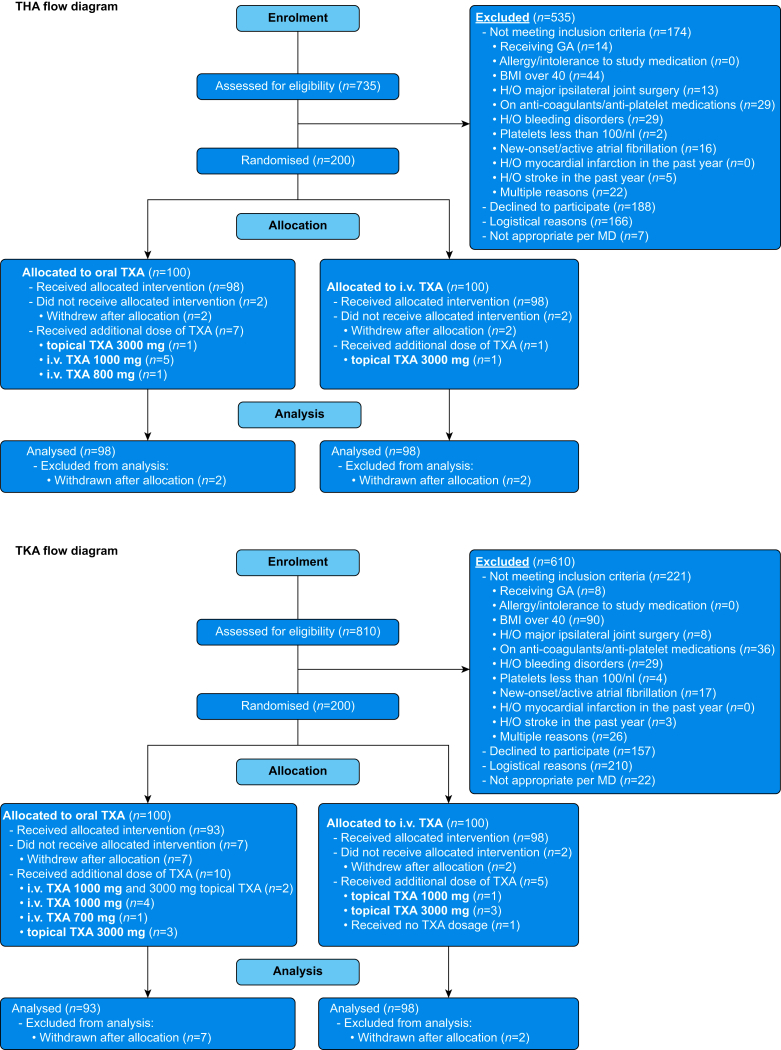
Enrolment began on September 17, 2019. Interim analysis in January 2021 showed no difference in safety profiles between the two arms,16 so enrolment continued to goal, which was achieved on November 10, 2021. In total, 400 patients were enrolled (200 THA and 200 TKA; Fig 2).11 Thirteen patients (four THA, nine TKA) were excluded from the study and subsequent analysis because they withdrew consent, including three patients who withdrew owing to the large size of the oral TXA tablets. This left 387 subjects for the intention-to-treat analysis. Table 1 shows baseline data for the oral and i.v. arms of the study. All subjects underwent neuraxial anaesthesia with sedation (no general anaesthesia). subjects undergoing TKA routinely also had regional anaesthesia blocks performed. Some subjects had periarticular injections performed intraoperatively, but this was done based on surgeon preference and was not standardised.
Fig 2.
CONSORT diagram showing patient allocation. CONSORT, Consolidated Standards of Reporting Trials; THA, total hip arthroplasty; TKA, total knee arthroplasty; TXA, tranexamic acid; H/O, history of.
Table 1.
subject characteristics and intraoperative factors. Normal continuous variables are presented as mean (standard deviation). Non-normal continuous variables presented as median [inter-quartile range]. Categorical data are presented as n (%). ∗Absolute values of standardised difference below 0.28 represent satisfactory balance for that demographic. †P-values 0.05 represent no statistical difference between the groups in the associated factor.
|
Patient characteristics | |||
|---|---|---|---|
|
Total hip arthroplasty | |||
| Oral (n = 98) | Intravenous (n = 98) | Standardised difference∗ | |
| Baseline factors | |||
| Age (yr) | 65 [57, 70] | 65 [61, 70] | −0.155 |
| Female | 55 (56) | 42 (43) | 0.268 |
| Race | 0.210 | ||
| White | 92 (94) | 87 (89) | |
| Non-White | 6 (6) | 11 (11) | |
| Ethnicity | 0.254 | ||
| Non-Hispanic | 90 (91) | 92 (94) | |
| Hispanic | 8 (9) | 6 (6) | |
| ASA physical status | 0.222 | ||
| 1 | 3 (3) | 5 (5) | |
| 2 | 82 (84) | 86 (88) | |
| 3 | 13 (13) | 7 (7) | |
| BMI (kg m−2) | 28.7 (5.1) | 29.6 (4.3) | 0.196 |
| Perioperative factors | |||
| Oral | Intravenous | P-value† | |
| Surgical time (min) | 76 [65, 95] | 83 [69, 95] | 0.097 |
| Drain use | 1 (1) | 1 (1) | 1.000 |
| Total knee arthroplasty | |||
| Baseline factors | |||
| Oral (n = 93) | Intravenous (n = 98) | Standardised difference∗ | |
| Age (yr) | 66 [60, 71] | 66 [61, 71] | 0.015 |
| Female | 56 (60) | 56 (57) | 0.062 |
| Race | 0.228 | ||
| White | 75 (81) | 81 (83) | |
| Non-White | 18 (19) | 17 (17) | |
| Ethnicity | 0.024 | ||
| Non-Hispanic | 85 (91) | 89 (91) | |
| Hispanic | 8 (9) | 9 (9) | |
| ASA physical status | 0.136 | ||
| 1 | 3 (3) | 2 (2) | |
| 2 | 76 (82) | 84 (87) | |
| 3 | 14 (15) | 11 (11) | |
| BMI (kg m−2) | 29.4 [26.9, 33.0] | 30.8 [26.0, 34.3] | −0.075 |
| Perioperative factors | |||
| Oral | I.V. | P-value† | |
| Surgical time (min) | 95 [79, 116] | 91 [76, 105] | 0.161 |
| Tourniquet time (min) | 49 [39, 65] | 48 [37, 60] | 0.197 |
| Drain use | 11 (12) | 6 (6) | 0.180 |
With respect to CBL, oral TXA was non-inferior to i.v. TXA for both THA (effect size=–18.2 ml; 95% confidence interval [CI], –113 to 76.3; P<0.001) and TKA (effect size=–79.7 ml; 95% CI, –179 to 19.6; P<0.001). The mean CBL values were 842 vs 861 ml for THA and 798 vs 878 ml for TKA in the oral and i.v. arms, respectively (Table 2). There was one postoperative red blood cell transfusion event recorded in the study, occurring in a subject in the i.v. TXA/TKA subgroup. No other blood products were transfused. There were no transfusions in the oral arm of the study (P=1.000).
Table 2.
Calculated blood loss between baseline and postoperative day 1. CBL presented as mean (standard deviation). Non-inferiority margin=235 ml, alpha=0.025. ∗The n values reported reflect patients excluded from the analysis (see Fig 2) and missing values from same-day discharge cases (see Appendix B). †The null hypothesis was that CBL would be at least 235 ml higher for cases in which oral TXA was used compared with cases were i.v. TXA was used. These results indicate non-inferiority for oral TXA use. CBL, calculated blood loss; TXA, tranexamic acid.
| CBL (ml) |
Effect size (ml) (95% confidence interval) |
P-value for non-inferiority | ||
|---|---|---|---|---|
| Oral TXA | I.V. TXA | |||
| Total hip arthroplasty | 842 (328) n=89∗ | 860 (313) n=90∗ | –18.2 (–113, 76.3) | <0.001† |
| Total knee arthroplasty | 799 (334) n=90∗ | 878 (348) n=96∗ | –79.65 (–179, 19.6) | <0.001† |
Oral TXA was dosed at a mean of 1.6 (0.6) h before incision among subjects undergoing THA and at a median of 2.0 h (Q1: 1.0 h, Q3: 2.0 h) before incision among subjects undergoing TKA. Intravenous TXA was dosed at a median of 18 min (Q1: 12 min, Q3: 27 min) before incision among subjects undergoing THA and at a mean of 19 (9) min before incision among subjects undergoing TKA.
Ten subjects experienced a complication, including one infection and one stroke (Table 3). No difference in complication rates was found between the two arms (P=0.73 for THA, P=0.50 for TKA). No instances of venous thromboembolism or cardiac event were found. There were no pulmonary or allergic complications. The case of infection (i.v. TXA/TKA subgroup) involved a stitch abscess that resolved with oral antibiotics. The stroke (i.v. TXA/THA subgroup) was diagnosed in a subjects who presented to the emergency department 4 days after surgery complaining of new right-hand numbness. This subjects was treated with clopidogrel and was noted to have near-full recovery from the stroke by 6 weeks postoperatively.
Table 3.
Complications recorded in the study. CVA, cerebrovascular accident.
| Study arm | Oral | Intravenous |
|---|---|---|
| Total hip arthroplasty | ||
| Complications |
Other Small right hip greater trochanter avulsion fracture, noted 2 weeks postoperatively. |
Neurological (CVA/stroke) New onset right hand numbness and weakness at 4 days postoperatively. Diagnosed with a stroke at the emergency room and started on Clopidogrel. |
|
Other Some swelling in both lower extremities. |
Other Three syncopal episodes in 1 day. Went to emergency room. Issues resolved spontaneously. |
|
|
Other Bilateral lower extremity weeping oedema. |
Other Intraoperative calcar crack treated with prophylactic cerclage cabling. |
|
|
Other Hyperkalaemia postoperatively. |
||
|
Other Posterior dislocation (twice). Closed reduced in the emergency room. |
||
| Total knee arthroplasty | ||
| Complications |
Infection Stitch abscess ‘deep’ in the most distal aspect of the incision. The ‘abscess’ completely resolved with a course of oral antibiotics. |
|
|
Other Hyponatraemia postoperatively. |
||
Median LOS for THA cases was 1.3 days in the oral TXA arm and 1.2 days in the i.v. TXA arm (P=0.087). Median LOS for TKA cases was 1.9 days in the oral TXA arm and 2.0 days in the i.v. TXA arm (P=0.18). In addition, the length of time from hospital admission to discharge from inpatient PT was not different between the two arms (P=0.30 for THA, P=0.49 for TKA).
The results of post hoc analyses are detailed in Appendix B.
Discussion
The present data provide strong evidence of non-inferiority for oral TXA vs i.v. TXA. CBL was similar in the two arms of the study, with effect size 95% CIs of –113 to 76.3 ml (favouring oral TXA) and –179 to 19.6 ml (favouring oral TXA) in the THA and TKA subgroups, respectively. The single transfusion among enrolled patients occurred in the i.v. arm of the study. Post hoc analyses supported the finding of non-inferiority. No difference in incidence of complications, including venous thromboembolism or cardiac event, was found. Secondary outcomes such as LOS did not differ between the oral and i.v. TXA arms.
A recent meta-analysis of Level 1 studies comparing the use of oral vs i.v. TXA in THA and TKA was performed by Sun and colleagues,17 finding no statistically significant difference between oral and i.v. TXA with respect to haemoglobin decrease, blood loss, transfusion rate, LOS, and other outcomes. This analysis included 10 studies, each of which focused on either THA or TKA but not both. Dosing amounts and procedures also varied considerably among the studies included. Statistical comparisons in this meta-analysis were designed as direct, two-tailed tests rather than one-tailed non-inferiority tests. In addition, only one of these studies had more than 60 patients in a comparison group, in contrast to the four groups (oral TXA/THA, i.v. TXA/THA, oral TXA/TKA, and i.v. TXA/TKA) of approximately 90 patients each in the current study. Sun and colleagues17 noted that using oral TXA, because of its ease of access and administration, may streamline perioperative workflows. However, they pointed out that more high-quality randomised trials, such as that presented here, were necessary to reach a definitive conclusion.
Another earlier trial by Fillingham and colleagues18 also suggested that oral and i.v. TXA had similar effectiveness and safety profiles in TKA. These authors noted that a switch from i.v. to oral TXA in only TKA would have potentially saved US$23–67 million for the USA healthcare system in 2014 alone, when there were about 700 000 TKAs performed annually. This was based on a cost savings of US$33–94 per patient. Meanwhile, work by Sloan and colleagues19 has estimated that the annual volume of primary TKA and THA in the US will reach 1.9 million by the year 2030. Extrapolating the findings of Fillingham and colleagues18 using these volume projections suggests that a switch from i.v. to oral TXA for both THA and TKA could save US$60–180 million in the USA annually by the year 2030.
The use of TXA has revolutionised blood management protocols for THA and TKA, severely reducing the frequency of perioperative transfusions. At the authors' institution, the routine self-directed donation of blood in the week before surgery has since been eliminated. Clearly, reducing the frequency of infusions can reduce cost and improve patient safety. The authors of this paper posit that the administration of TXA in the preoperative area, in oral form, could further improve patient safety by decreasing the risk of lapses in the dosing of the medication. In addition, this could facilitate the removal of liquid TXA formulations from the operating room proper, perhaps moving it to centralised medication dispensers for the cases where the i.v. formulation may still be needed perioperatively. This logistical change could potentially prevent TXA-related medication errors in the operating theatre, including inadvertent and wrong-route dosing. Accidental spinal dosing of TXA is of particular concern in the orthopaedic operating room and is a potentially catastrophic event, with a recent study detailing 21 cases of accidental spinal administration of TXA, which led to 10 deaths and several other severe injuries.20
There are several limitations of this trial. First, the a priori NIM was set at 20% of expected CBL values, but it ended up being about 25–30% of observed POD1 CBLs, which were lower than expected. Understanding that more patients were enrolled and analysed than the a priori sample size calculation called for, the authors noted that accounting for this extra enrolment could have reduced the a priori NIM to 191 ml with no change to the study's conclusions. As this 191 ml threshold is about 20% of the study's observed CBL means, the authors were satisfied with the powering of the study.
Also, the dosages of TXA, which were based on recommended pharmacologic data, were different in the two arms. Although the raw dose of oral TXA was 1.95 times that of the i.v. dose, oral TXA has only 34% bioavailability,21 meaning that the effective TXA dose with oral administration was less than that with i.v. administration. Although oral TXA was given about 2 h before surgery compared to within 30 min of incision for i.v. TXA, the authors expect that differences in dose timing had no significant effect on the study outcomes, as plasma drug concentration after oral TXA dosing peaks around 2.5 h after administration.21
Although several different equations for CBL exist, the Gross equation was used in this study, as it is straightforward, based on blood concentrations, and is the most often used CBL equation in orthopaedic trauma research.22 Regardless of the chosen CBL equation, any systematic error from the calculation method would be expected to affect both study arms similarly and, therefore, not negate the conclusions. In addition, the 13 patients who withdrew consent were excluded from the analysis. As they received i.v. TXA according to typical hospital protocol, including them would have biased toward a finding of non-inferiority (away from the null). Although perioperative fluid management was not standardised in this study, patients without significant cardiac disease undergoing THA or TKA are generally given approximately 1 L of crystalloid intraoperatively with no routine postoperative fluid administration. It is not expected that there was any differential fluid administration between the oral and i.v. TXA arms in this study that might have biased the CBL estimates through a dilutional mechanism.
Mechanical venous thrombosis prophylaxis with sequential compression devices was used routinely in-hospital postoperatively. The postoperative medication used for pharmacologic prophylaxis against venous thrombosis was not standardised in the study, because the choice of medication was not usually known before surgery, and this would have severely complicated enrolment. That being noted, the reader should know that most patients receive aspirin for prophylaxis at the host institution unless other factors justifying escalation of pharmacologic prophylaxis are present.
It should also be noted that the patients, anaesthesiologists, and surgeons were not blinded to the intervention. However, the research assistants and statisticians that collected and analysed the resulting data were blinded to intervention. In addition, this study did not have a control arm with no TXA dosing, as the use of TXA is considered standard of care. Despite a temporary pause in enrolment during the height of the COVID-19 pandemic, it is not expected that the pandemic had any differential effect on the two study arms. After the pause, all patients had a negative COVID-19 polymerase chain reaction (PCR) test within the 5 days prior to surgery.
This study excluded patients undergoing revision surgery and bilateral surgery, and anyone using preoperative antiplatelet or anticoagulant drugs and those with certain preoperative conditions. These exclusions may limit the study's generalisability. Furthermore, this trial was performed at a single specialised centre in the USA that performs tens of thousands of joint replacements annually. The host institution has developed formal and informal care standards for THA and TKA patients, including the use of neuraxial and regional anaesthesia over general anaesthesia, early diet administration postoperatively, mobilisation on the day of surgery, and other parts of enhanced recovery protocols. The utilisation of such protocols at a specialised centre may further limit the study's generalisability. Because the anterior hip approach accounts for a minority of THA cases at the study institution, the decision was made to keep the utilised hip approach consistent and enrol only THA cases with the posterior approach. The authors recognise that this may again limit the generalisability of the results but suggest that the concepts proven remain valid. It is also acknowledged that this study included very few cases of same-day discharge (only 22 out of 387), which some readers may consider a further limitation to generalisability. However, the authors contend that postoperative patient recovery location should not change perioperative blood loss, making any conclusions regarding the utility of TXA in perioperative blood management valid regardless of same-day discharge status. In addition – although this was not an issue in this study – the authors concede that staffing and logistical challenges in the holding areas of some hospitals may make it difficult for some institutions to switch to oral TXA dosing in the preoperative setting.
In conclusion, this is the largest randomised trial showing that oral TXA is non-inferior to i.v. TXA in reducing blood loss and transfusions in the setting of THA and TKA. The authors believe that routine use of a single preoperative dose of oral TXA 1950 mg before primary THA or TKA in patients having no contraindications can potentially reduce cost and improve patient safety while maintaining the standard of care. Intravenous and topical dosing should be retained as adjuncts for certain patients, such as those with poor enteric drug absorption or intolerance of the oral tablets.
Authors' contributions
Conceptualisation: CJD, LAR, JS, SGM
Investigation: AGDV, AR, MC, PKS, SH, DK, DM, MK, KJE, EMS, KK, JB, MF, AI, SG, MA, KD, SGM
Methodology: CJD, JFR, EG, MP, CF, EMS, DD, AS, SGM
Data curation: JFR, EG, MP, CF, MW, DD, AS, HZ
Project administration: JFR, EG, MP, CF, MW, DD, AS
Formal analysis: JFR, EG, MP, CF, MW, DD, HZ
Visualisation and original draft preparation: CJD, JFR, EG, MP
Study supervision: CJD, SGM
Funding acquisition: SGM
All authors were responsible for final review and editing of the manuscript.
Declarations of interest
We declare no competing interests. Please see the authors' separate declaration of interests form for a detailed list of authors' relationships and interests.
Data sharing
Deidentified patient data will be available via weblink to researchers for further work. Requests for data access should be sent to reichelj@hss.edu. Data will be available beginning November 1, 2022 until December 31, 2024.
Funding
Department of Anesthesiology, Critical Care & Pain Management Research and Education Fund at Hospital for Special Surgery; REDCap access funded by National Center for Advancing Translational Science (NCATS) of the National Institute of Health (NIH; award number UL1 TR002384).
Handling editor: Paul Myles
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.11.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Culliford D., Maskell J., Judge A., Cooper C., Prieto-Alhambra D., Arden N.K. Future projections of total hip and knee arthroplasty in the UK: results from the UK Clinical Practice Research Datalink. Osteoarthr Cartil. 2015;23:594–600. doi: 10.1016/j.joca.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg N., Kirkley S.A., Heal J.M. A cost analysis of autologous and allogeneic transfusions in hip-replacement surgery. Am J Surg. 1996;171:324–330. doi: 10.1016/S0002-9610(97)89635-3. [DOI] [PubMed] [Google Scholar]
- 4.Husted H., Holm G., Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79:168–173. doi: 10.1080/17453670710014941. [DOI] [PubMed] [Google Scholar]
- 5.Bong M.R., Patel V., Chang E., Issack P.S., Hebert R., Cesare P.E. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19:281–287. doi: 10.1016/j.arth.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.L., Park J., Han S., Cho I.Y., Jang K. Allogeneic blood transfusion is a significant risk factor for surgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty. 2017;32:320–325. doi: 10.1016/j.arth.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Gwam C.U., Mistry J.B., Etcheson J.I., et al. Decline in allogeneic blood transfusion usage in total hip arthroplasty patients: National Inpatient Sample 2009 to 2013. Hip Int. 2018;28:382–390. doi: 10.5301/hipint.5000590. [DOI] [PubMed] [Google Scholar]
- 8.Gillette B.P., Maradit Kremers H., Duncan C.M., et al. Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J Arthroplasty. 2013;28:137–139. doi: 10.1016/j.arth.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Gross J.B. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–280. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Jules-Elysee K.M., Tseng A., Sculco T.P., et al. Comparison of topical and intravenous tranexamic acid for total knee replacement: a randomized double-blinded controlled study of effects on tranexamic acid levels and thrombogenic and inflammatory marker levels. J Bone Joint Surg Am. 2019;101:2120–2128. doi: 10.2106/JBJS.19.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat B Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 16.Reichel J.F., Popovic M., DeMeo D., et al. May 13–15, 2021. The effect of oral versus intravenous tranexamic acid on perioperative blood loss in joint arthroplasty patients. Presented at the 46th annual regional Anesthesiology and acute Pain medicine meeting of the American society of regional anesthesia and Pain medicine (ASRA) Orlando, FL. [Google Scholar]
- 17.Sun C., Zhang X., Chen L., et al. Comparison of oral versus intravenous tranexamic acid in total knee and hip arthroplasty: a GRADE analysis and meta-analysis. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillingham Y.A., Kayupov E., Plummer D.R., Moric M., Gerlinger T.L., Della Valle C.J. The James A. Rand Young Investigator's award: a randomized controlled trial of oral and intravenous tranexamic acid in total knee arthroplasty: the same efficacy at lower cost? J Arthroplasty. 2016;31:26–30. doi: 10.1016/j.arth.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 19.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 20.Patel S., Robertson B., McConachie I. Catastrophic drug errors involving tranexamic acid administered during spinal anaesthesia. Anaesthesia. 2019;74:904–914. doi: 10.1111/anae.14662. [DOI] [PubMed] [Google Scholar]
- 21.Pilbrant A., Schannong M., Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20:65–72. doi: 10.1007/BF00554669. [DOI] [PubMed] [Google Scholar]
- 22.McKibben N.S., Lindsay S.E., Friess D.M., Zusman N.L., Working Z.M. Methods of quantifying intraoperative blood loss in orthopaedic trauma surgery: a systematic review. J Orthop Trauma. 2022;36:e215–e226. doi: 10.1097/BOT.0000000000002313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.