The U.S. Food and Drug Administration (FDA) regulates the commercial availability of novel therapeutics, though does not restrict physician prescribing. While FDA approves a product for clinical use based on a risk-benefit assessment for a specific indication, physicians may legally prescribe approved agents for unapproved indications (i.e. off-label). Within ophthalmology, use of previously approved therapeutics for off-label indications is common, including intravitreal antibiotics for endophthalmitis and bevacizumab for neovascular age-related macular degeneration. However, little is understood about the accumulation of evidence to support use of novel therapeutics for unapproved indications, which has implications for current practice and adoption of approved drugs for new uses. Prior work has demonstrated associations between off-label use and adverse events, suggesting potential gaps in evidence supporting safety.1 To better elucidate the clinical evidence base supporting off-label use, we characterized the frequency, accrual and quality of on-label and off-label prospective studies of novel FDA-approved ophthalmic therapeutics.
We used the publicly-available Drugs@FDA Database to identify novel therapeutics receiving original FDA approval for ophthalmologic indications between January 1, 2008 and December 31, 2012.2 We defined novel therapeutics as new molecular entities or biologics not previously FDA-approved, specifically excluding approvals of generics, diagnostics, and reformulations or combination therapies comprising previously-approved agents. We employed a multi-pronged search integrating ClinicalTrials.gov, PubMed, and FDA documents to identify pre-approval and post-approval prospective clinical studies, including interventional and observational studies, of sample therapeutics through December 31, 2018. From FDA documents, ClinicalTrials.gov registrations, and publications, we abstracted indication, enrollment information, sponsor, and study design characteristics. Indications were categorized as follows: original FDA approved indication, supplemental FDA approved indication, or off-label indication. For additional details, please see Supplementary Methods, available at www.aaojournal.org.
Using descriptive statistics, we characterized the proportion of studies across indication types, overall, and stratified by therapeutic and study characteristics. We used Chi-squared testing to assess differences between studies of FDA-approved and off-label indications and between industry and non-industry funded off-label studies. Hypothesis testing was performed using a 2-sided type I error of 0.006, corrected for multiple comparisons.
Between 2008 and 2012, FDA approved seven novel therapeutics (2 biologics and 5 new molecules) for ophthalmologic indications (Supplementary Table 1 available at www.aaojournal.org): aflibercept (Eylea), alcaftadine (Lastacaft), bepotastine (Bepreve), besifloxacin (Besivance), difluprednate (Durezol), ocriplasmin (Jetrea), and tafluprost (Zioptan), for which we identified 469 prospective clinical studies. Eighty-two percent (n=386) of studies were interventional and 56.7% (n=266) were funded by industry. Studies of aflibercept were most common, accounting for 67.0% (n=314).
Among these 469 prospective studies, 19% (n=89) were conducted pre-approval and 81.0% (n=380) post-approval; 65% (n=237) of the 380 post-approval studies accrued in the first 4 years after original FDA approval (Figure). Median pre-approval and post-approval studies per therapeutic were 11 (interquartile range [IQR], 4–18) and 13 (IQR, 9–24), respectively.
Figure.
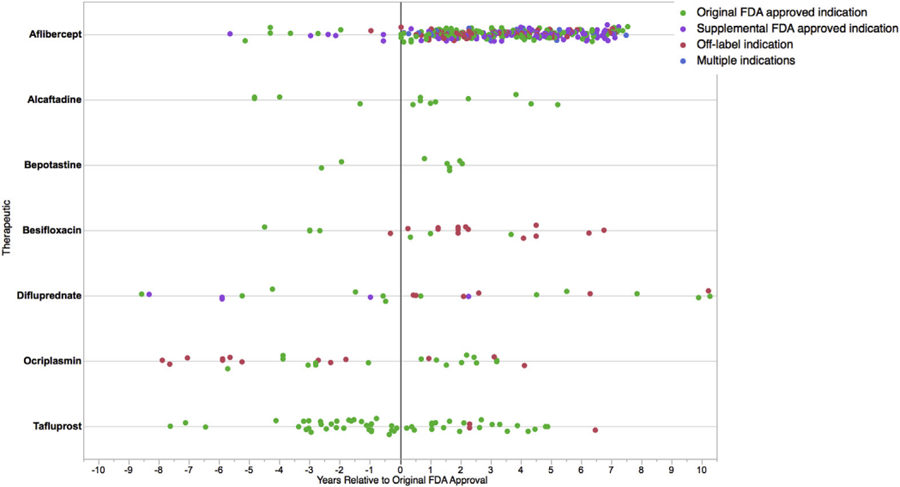
Distribution of prospective clinical studies evaluating novel therapeutics originally approved by FDA for ophthalmologic indications between 2008 and 2012 relative to date of original FDA approval, by therapeutic and study indication type.
Fifty percent of the 380 post-approval studies (n=190) evaluated the original FDA approved indication, while 29.7% (n=113) and 26.6% (n=101) evaluated supplemental FDA approved and off-label indications, respectively. Original FDA approved indications were supported by a median of 13 (IQR 9–55) prospective studies, while median prospective studies per off-label indication was 1 (IQR, 1–2; range 1–19). Median off-label indications evaluated per therapeutic was 4 (IQR, 0–12; range 0–26).
Compared to studies of FDA-approved indications, off-label indication studies were smaller (median enrollment=30 [IQR, 17.8–54.5] vs 60 [IQR, 32–189]; p<0.0001) and less frequently used an active comparator (44.4% vs 68.1%; p=0.002). Industry-funded off-label studies were were more frequently interventional, randomized, controlled, masked, and larger than non-infustry funded off-label studies, though these differences were not statistically significant (Supplementary Table 2 available at www.aaojournal.org).
As medical science innovates new uses for marketed therapeutics, familiarity with evidence supporting new and existing indications becomes increasingly important. Our study of seven novel ophthalmic therapeutics originally FDA-approved between 2008 and 2012 found that approximately a dozen prospective studies of any indication are conducted on average after FDA approval. Further, while studies of off-label indications were overall frequent, most off-label indications were evaluated by just one prospective study, raising concerns about potential limitations to the evidence supporting off-label use of these medications.
Post-approval studies serve several purposes, including assessing long-term effectiveness, identifying rare adverse events, and evaluating drug performance in real-world populations. Prior work evaluating 69 novel drugs approved across medical specialities between 2005 and 2010 observed a median of 55 (IQR, 33–119) post-approval studies per drug, of which two-thirds studied the original indication.3 Among ophthalmic therapeutics, we observed far fewer studies across a comparable timespan, fewer of which evaluated the original indication. Recent evidence suggests that most industry-sponsored post-approval studies evaluate new indications rather than bolster evidence around already-approved uses.4
While studies of off-label indications were frequent, for many ophthalmic therapeutics, the collection of studies supporting any single off-label indication was narrow. Off-label indications may carry different safety and effectivness profiles than indications originally reviewed by FDA. Poorly-substantiated off-label use may expose patients to questionable benefits with uncertain risks. Prior work has estimated the incidence of adverse events with off-label use to be 1.5-fold greater than with on-label use, further augmented with off-label use lacking strong evidence.1 Dangers of extrapolating benefits of FDA approved indications have been observed in cardiology, where off-label use of certain drug-eluting stents has been associated with higher rates of stent thrombosis.5 Physicians are often unaware which indications for a drug are FDA-approved, highlighting a need for improved understanding of data supporting off-label use.6
This study has limitations. First, our search included studies only through 2018, though selection of this timeframe allowed at least six years post-FDA approval for study accrual, consistent with prior work.7 Second, while ours is the first to characterize the frequency and quality of clinical studies evaluating approved and off-label indications in ophthalmology, we cannot comment on real-world frequency of off-label use or ascertain the impact of studies on clinical practice.
In conclusion, ophthalmologists should recognize that, for some therapeutics, prospective data guiding off-label use may be limited. Expanding post-approval evaluation of FDA-approved and off-label indications may mitigate uncertainities and aid ophthalmologists in optimally assessing the decision to adopt approved therapeutics for off-label use.
Supplementary Material
Financial Support:
This project was not supported by any external grants or funds. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
Footnotes
Conflicts of Interest: In the past 36 months, J.D.W. received research support through Yale University from the Laura and John Arnold Foundation and the Food and Drug Administration (U01FD005938). J.S.R received research support through Yale from Johnson and Johnson, from Medtronic, Inc. and the Food and Drug Administration (FDA) (U01FD004585), from the Centers of Medicare and Medicaid Services (CMS), from the FDA (U01FD005938), from the Medical Devices Innovation Consortium, from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HS025164, R01HL144644), and from the Laura and John Arnold Foundation. C.D.R has received research grant support and consulting payments from Allergan, Genentech, Kodiak, Novartis, and Regeneron.
Supplementary Material: 1 document and 2 tables
References
- 1.Eguale T, Buckeridge DL, Verma A, et al. Association of Off-label Drug Use and Adverse Drug Events in an Adult Population. JAMA Intern Med 2016;176(1):55–63. [DOI] [PubMed] [Google Scholar]
- 2.Drugs@FDA: FDA-Approved Drugs 2019; Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed March 1, 2019, 2019.
- 3.Zeitoun JD, Ross JS, Atal I, et al. Postmarketing studies for novel drugs approved by both the FDA and EMA between 2005 and 2010: a cross-sectional study. BMJ open 2017;7(12):e018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skydel JJ, Luxkaranayagam AT, Dhruva SS, Ross JS, Wallach JD. Analysis of Postapproval Clinical Trials of Therapeutics Approved by the US Food and Drug Administration Without Clinical Postmarketing Requirements or Commitments. JAMA Netw Open 2019;2(5):e193410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Win HK, Caldera AE, Maresh K, et al. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. Jama 2007;297(18):2001–2009. [DOI] [PubMed] [Google Scholar]
- 6.Chen DT, Wynia MK, Moloney RM, Alexander GC. U.S. physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. Pharmacoepidemiology and drug safety 2009;18(11):1094–1100. [DOI] [PubMed] [Google Scholar]
- 7.Wallach JD, Egilman AC, Dhruva SS, et al. Postmarket studies required by the US Food and Drug Administration for new drugs and biologics approved between 2009 and 2012: cross sectional analysis. BMJ (Clinical research ed.) 2018;361:k2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


