Abstract
There is an urgent need in aquaculture to develop microbial control strategies, since disease outbreaks are recognized as important constraints to aquaculture production and trade and since the development of antibiotic resistance has become a matter of growing concern. One of the alternatives to antimicrobials in disease control could be the use of probiotic bacteria as microbial control agents. This review describes the state of the art of probiotic research in the culture of fish, crustaceans, mollusks, and live food, with an evaluation of the results obtained so far. A new definition of probiotics, also applicable to aquatic environments, is proposed, and a detailed description is given of their possible modes of action, i.e., production of compounds that are inhibitory toward pathogens, competition with harmful microorganisms for nutrients and energy, competition with deleterious species for adhesion sites, enhancement of the immune response of the animal, improvement of water quality, and interaction with phytoplankton. A rationale is proposed for the multistep and multidisciplinary process required for the development of effective and safe probiotics for commercial application in aquaculture. Finally, directions for further research are discussed.
Aquaculture of finfish, crustaceans, mollusks, and algal plants is one of the fastest-growing food-producing sectors, having grown at an annual rate of almost 10% from 1984 to 1995 compared with 3% for livestock meat and 1.6% for capture fisheries production (97).
Disease outbreaks are being increasingly recognized as a significant constraint on aquaculture production and trade, affecting the economic development of the sector in many countries. For instance, disease is now considered to be the limiting factor in the shrimp culture subsector (65, 124). So far, conventional approaches, such as the use of disinfectants and antimicrobial drugs, have had limited success in the prevention or cure of aquatic disease (124). Furthermore, there is a growing concern about the use and, particularly, the abuse of antimicrobial drugs not only in human medicine and agriculture but also in aquaculture. The massive use of antimicrobials for disease control and growth promotion in animals increases the selective pressure exerted on the microbial world and encourages the natural emergence of bacterial resistance (World Health Organization antimicrobial resistance fact sheet 194, http://www.who.int/inf-fs/en/fact194.html). Not only can resistant bacteria proliferate after an antibiotic has killed off the other bacteria, but also they can transfer their resistance genes to other bacteria that have never been exposed to the antibiotic. The subtherapeutic (prophylactic) use of antibiotics related to those used in human medicine or the use of any antimicrobial agent known to select for cross-resistance to antimicrobials used in human medicine could pose a particularly significant hazard to human health (146).
According to the World Health Organization (fact sheet 194 web site), much needs to be done to reduce the overuse and inappropriate use of antimicrobials. The emphasis in disease management should be on prevention, which is likely to be more cost-effective than cure. This may lead to less reliance on the use of chemicals (antimicrobials, disinfectants, and pesticides), which largely treat the symptoms of the problem and not the cause (92).
Several alternative strategies to the use of antimicrobials in disease control have been proposed and have already been applied very successfully in aquaculture. The use of antimicrobial drugs in a major producing country such as Norway has dropped from approximately 50 metric tons per year in 1987 to 746.5 kg in 1997, measured as active components. During the same time, the production of farmed fish in Norway increased approximately from 5 × 104 to 3.5 × 105 metric tons. The dramatic decrease observed in the consumption of antimicrobial agents is mainly due to the development of effective vaccines (66, 124), which illustrates very well the potential effectiveness of the procedure. Enhancing the nonspecific defense mechanisms of the host by immunostimulants, alone or in combination with vaccines, is another very promising approach (96, 111). Third, Yasuda and Taga (148) already anticipated in 1980 that bacteria would be found to be useful both as food and as biological control agents of fish disease and activators of the rate of nutrient regeneration in aquaculture. Vibrio alginolyticus has been employed as a probiotic in many Ecuadoran shrimp hatcheries since late 1992 (49). As a result, hatchery down time was reduced from approximately 7 days per month to less than 21 days annually, while production volumes increased by 35%. The overall antibiotic use was decreased by 94% between 1991 and 1994. The addition of probiotics is now also common practice in commercial shrimp hatcheries in Mexico (101). According to Browdy (14), one of the most significant technologies that has evolved in response to disease control problems is the use of probiotics. Considering the recent successes of these alternative approaches, the Food and Agriculture Organization of the United Nations (124) defined the development of affordable yet efficient vaccines, the use of immunostimulants and nonspecific immune enhancers, and the use of probiotics and bioaugmentation for the improvement of aquatic environmental quality as major areas for further research in disease control in aquaculture. The results of this research will undoubtedly help to reduce chemical and drug use in aquaculture and will make aquaculture products more acceptable to consumers.
This review aims to provide an overview of the work done on bacteria as biological control agents for aquaculture environments, with a critical evaluation of the results obtained so far and a detailed description of the possible modes of action involved. Furthermore, a rationale for the search for probiotics is presented and directions for further research are proposed.
DEFINITION OF PROBIOTICS
As new findings emerged, several definitions of probiotics have been proposed. Fuller (30) gave a precise definition of probiotics which is still widely referred to, i.e., a live microbial feed supplement which beneficially affects the host animal by improving its intestinal balance.
Historically, the interest has centered on terrestrial organisms, and the term “probiotic” inevitably referred to gram-positive bacteria associated with the genus Lactobacillus. The application of the definition proposed by Fuller (30) in aquaculture, however, requires some considerations. Similarly to humans and terrestrial animals (56), it can be assumed in aquaculture that the intestinal microbiota does not exist as entity by itself but that there is a constant interaction with the environment and the host functions. Many researchers have already investigated the relationship of the intestinal microbiota to the aquatic habitat or food. Cahill (17) summarized the results of these investigations on fishes, giving evidence that the bacteria present in the aquatic environment influence the composition of the gut microbiota and vice versa. The genera present in the intestinal tract generally seem to be those from the environment or the diet which can survive and multiply in the intestinal tract (17). However, it can be claimed that in aquaculture systems the immediate ambient environment has a much larger influence on the health status than with terrestrial animals or humans.
Indeed, the host-microbe interactions are often qualitatively as well as quantitatively different for aquatic and terrestrial species. In the aquatic environment, hosts and microorganisms share the ecosystem. By contrast, in most terrestrial systems, the gut represents a moist habitat in an otherwise water-limitted environment. In some sense, microbes in an aquatic environment have the choice of living in association with the potential host (intestinal tract, gills, or skin) or not, while in the terrestrial environment, appreciable activity may be limited to aquatic niches such as those provided by the guts of host animals (53).
Much more than terrestrial animals, aquatic farmed animals are surrounded by an environment that supports their pathogens independently of the host animals, and so (opportunistic) pathogens can reach high densities around the animal (73). Surrounding bacteria are continuously ingested either with the feed or when the host is drinking. This is especially the case with filter feeders, which ingest bacteria at a high rate from the culture water, causing a natural interaction between the microbiota of the ambient environment and the live food.
While probiotic research in aquaculture focused in the beginning on fish juveniles, more attention has recently been given to larvae of fish and shellfish and to live food organisms (Table 1). Terrestrial animals (mammals) inherit an important part of the initially colonizing bacteria through contact with the mother, while aquatic species usually spawn axenic eggs in the water, without further contact with the parents. This allows ambient bacteria to colonize the egg surface. Furthermore, freshly hatched larvae or newborn animals do not have a fully developed intestinal system and have no microbial community in the intestinal tract, on the gills, or on the skin. Because the early stages of aquatic larvae depend for their primary microbiota partly on the water in which they are reared (17, 50, 102), the properties of the bacteria in the ambient water are of the utmost importance (115).
TABLE 1.
Overview of literature reports dealing with probiotics as biological control agents in aquaculture
| Putative probiotic | Origina | Observations | Method of administrationa | Suggested mode of actiona | Reference(s) |
|---|---|---|---|---|---|
| Fish eggs and Larvae | |||||
| Several strains (unidentified) | Eggs of cod and halibut | Failure of the strains to prevent the adherence of environmental bacteria to cod eggs | Bathing in bacterial suspension | Antagonism | 51 |
| Vibrio salmonicida-like and Lactobacillus plantarum | Fish | Increase of survival of halibut larvae 2 weeks after hatching | Addition to culture water | Immunostimulation | 84 |
| Bacillus strain IP5832 spores (Paciflor 9) | ? | Increase of weight of turbot larvae when fed spore-fed rotifers; decrease of mortality when challenged with an opportunistic Vibrionaceae member | Addition to rotifer diet | Antagonism and/or improved nutritional value of the rotifers | 35 |
| Streptococcus lactis and Lactobacillus bulgaricus | ? | Increase of survival of turbot larvae 17 days after hatching | Enrichment of rotifers and Artemia | ? | 32 |
| Lactobacillus or Carnobacterium | Rotifers (Brachionus plicatilis) | Decrease of mortality of turbot larvae challenged with a pathogenic Vibrio sp. | Enrichment of rotifers | Antagonism and/or improved nutritional value of the rotifers | 37 |
| Vibrio pelagius | Copepod-fed turbot larvae | Decrease (?) of mortality of turbot larvae challenged with A. caviae | Addition to culture water | ? | 104 |
| Strain E (Vibrio alginolyticus-alike) | Healthy turbot larvae | Decrease of mortality of turbot larvae challenged with a pathogenic Vibrio strain P; also, the growth rate of the larvae may be increased | Enrichment of rotifers | Competition for iron | 38 |
| Microbially matured water | — | Increase of initial growth rate of turbot and halibut larvae | As culture water | ? | 115, 135 |
| Fish juveniles and Adults | |||||
| Lyophilized Carnobacterium divergens | Atlantic salmon intestines | Increase (!) of mortality of Atlantic salmon fry challenged with cohabitants infected with A. salmonicida | Addition to diet | — | 43 |
| Lyophilized Carnobacterium divergens | Atlantic salmon intestines | Decrease of mortality of Atlantic cod fry when challenged with a pathogenic V. anguillarum strain | Addition to diet | ? | 45 |
| Lyophilized Carnobacterium divergens | Atlantic salmon intestines | Decrease of mortality of Atlantic cod fry challenged with a pathogenic V. anguillarum strain 12 days after the infection; 4wk after the infection, however, the same mortality as in the control was reached | Additon to diet | Antagonism | 44 |
| Carnobacterium strain K1 | Atlantic salmon intestines | Growth inhibition of V. anguillarum and A. salmonicida in fish intestinal mucus and fecal extracts (no in vivo test) | — | Antagonism | 59 |
| Carnobacterium | Atlantic salmon intestines | Growth inhibition of V. anguillarum in turbot fecal extracts (no in vivo test) | — | Antagonism | 85 |
| Fluorescent pseudomonad F19/3 | Fish mucus | Decrease of mortality of Atlantic salmon presmolts challenged with stress-inducible A. salmonicida infection | Bathing in bacterial suspension | Competition for iron | 117 |
| Pseudomonas fluorescens AH2 | Iced Lake Victorian Nile perch | Decrease of mortality of rainbow trout juveniles challenged with a pathogenic V. anguillarum | Addition to culture water and/or bathing in bacterial suspension | Competition for iron | 48 |
| Vibrio alginolyticus | Commercial shrimp hatchery in Ecuador | Decrease of mortality of Atlantic salmon juveniles challenged with a pathogenic A. salmonicida, V. anguillarum, and V. ordalii | Bathing in bacterial suspension | Antagonism | 5 |
| Bacillus megaterium, B. polymyxa, B. licheniformis, 2 strains of B. subtilis (Biostart) | ? | Increase of survival and net production of channel catfish | Addition to pond water | ? | 95 |
| Spray-dried Tetraselmis suecica (unicellular alga) | ? | Decrease of mortality of Atlantic salmon juveniles challenged with several pathogens; alga was effective prophylactically as well as therapeutically | Addition to diet | Antagonism | 2 |
| Crustaceans | |||||
| Bacillus strain S11 | Penaeus monodon or mud and water from shrimp ponds | Increase of mean weight and survival of P. monodon larvae and postlarvae; decrease of mortality after challenge with the pathogen V. harveyi D331 | Addition to diet | Antagonism | 99; Rengpipat and Rukpratanporn, abstract |
| Vibrio alginolyticus | Pacific Ocean seawater | Increase of survival and weight of L. vannamei postlarvae; decreased observation of V. parahaemolyticus in the shrimps | Addition to culture water | Antagonism | 33 |
| Bacillus | ? | Increase of survival of penacid shrimps; decrease of luminous Vibrio densities | Addition to pond water | Antagonism | 73, 74 |
| Strain PM-4 and/or NS-110 | Soil | Increase of survival of P. monodon and P. trituberculatus larvae; decrease of Vibrio densities | Addition to culture water | Antagonism/food source for larvae | 67–69, 83 |
| Strain BY-9 | Coastal seawater | Increase of survival of P. monodon larvae; decrease of Vibrio densities | Addition to culture water | ? | Sugama and Tsumura, abstract |
| Bivalve mollusks | |||||
| Vibrio strain 11 | Microalgae in a scallop hatchery | Decrease of mortality of scallop larvae challenged with a pathogenic V. anguillarum-like strain | Bathing in bacterial suspension | Antagonism | 105 |
| Aeromonas media A 199 | ? | Decrease of mortality and suppression of the pathogen of Pacific oyster larvae when challenged with a pathogenic V. tubiashii | Addition to culture water | Antagonism | 42 |
| Live food—Algae | |||||
| Several strains | Turbot larvae | Growth stimulation of P. lutheri | Addition to culture water | ? | 75 |
| Flavobacterium sp. | Chaetoceros gracilis mass culture | Improved growth characteristics of C. gracilis, I. galbana, and P. lutheri | Addition to culture water | ? | 128 |
| Strain SK-05 | Skeletonema costatum culture | Inhibition of the growth of V. alginolyticus in Skeletonema costatum culture | Addition to culture water | Competition for resources | 101 |
| Live food—Rotifers | |||||
| Lactobacillus plantarum | ? | Inhibition of the growth of a fortuitous A. salmonicida strain in rotifer culture | Addition to diet | Antagonism | 36 |
| Lactococcus lactis AR21 | Rotifer culture | Counteraction of rotifer growth inhibition when challenged with V. anguillarum | Addition to culture water | ? | 54 |
| Several strains | Rotifer culture | Increase of the specific growth rate of rotifer culture | Addition to culture water | 108 | |
| Live food—Artemia | |||||
| Vibrio alginolyticus C14 | ? | Decrease of mortality of Artemia nauplii when challenged with V. parahaemolyticus | Addition to culture water | ? | 47 |
| Several strains | Artemia culture | Decrease of mortality of Artemia juveniles when challenged with V. proteolyticus | 141 |
—, not relevant; ?, not specified.
It was stated above that the interaction between the microbiota, including probiotics, and the host is not limited to the intestinal tract. Probiotic bacteria could also be active on the gills or the skin of the host but also in its ambient environment. The intensive interaction between the culture environment and the host in aquaculture implies that a lot of probiotics are obtained from the culture environment and not directly from feed, as stipulated by the definition of Fuller (30).
Therefore, the following modified definition is proposed, which allows a broader application of the term “probiotic” and addresses to the objections made earlier. A probiotic is defined as a live microbial adjunct which has a beneficial effect on the host by modifying the host-associated or ambient microbial community, by ensuring improved use of the feed or enhancing its nutritional value, by enhancing the host response towards disease, or by improving the quality of its ambient environment.
Based on this definition, probiotics may include microbial adjuncts that prevent pathogens from proliferating in the intestinal tract, on the superficial structures, and in the culture environment of the cultured species, that secure optimal use of the feed by aiding in its digestion, that improve water quality, or that stimulate the immune system of the host. Bacteria delivering essential nutrients to the host (single-cell protein) without being active in the host or without interacting with other bacteria, with the environment of the host, or with the host itself are not included in the definition. Although probiotics may also contribute substantially to the health and zootechnical performance in a nutritional way and although it is sometimes impossible to separate feeding of aquatic organisms from environmental control, this review is limited to the use of probiotics as biological control agents in aquaculture.
FUNDAMENTAL QUESTION: IS IT POSSIBLE TO MANIPULATE MICROBIAL COMMUNITIES?
Aquaculture practices such as discontinuous culture cycles, disinfection or cleaning of ponds or tanks prior to stocking, and sudden increases in nutrients due to exogenous feeding generally do not provide appropriate environments for the establishment of stable microbial communities. Therefore, it is very unlikely that under intensive rearing conditions a stable microbial community can be achieved (116). In the development of these microbial communities, one should consider both deterministic and stochastic factors (74, 139). Deterministic factors have a well-defined dose-response relationship. For a given value of a stochastic factor, a probability range of responses can occur. Deterministic factors influencing the microbial development in aquaculture systems include salinity, temperature, oxygen concentration, and quantity and quality of the feed. These combined environmental factors create a habitat in which a selected and well-defined range of microbes is able to proliferate (“the environment selects” axiom). The development of a microbial community in aquaculture systems is, however, also influenced by stochastic phenomena: chance favors organisms which happen to be in the right place at the right time to enter the habitat and to proliferate if the conditions are suitable (74).
This theoretical concept has been experimentally supported by Verschuere et al. (139), who monitored the community-level physiological profiles of the emerging microbial communities in the culture water of Artemia juveniles in three identical culture series. Although completely identical from the zootechnical point of view, the culture water of the three series showed clearly distinct microbial communities developing in the first days of the experiment. The same concept may be valid for the microbial communities developing in the culture water and on the inner and outer surfaces of eggs and larval organisms. Obviously, due to the heterogeneity of the microbial distribution in the air and water, in feeds, and on surfaces, the stochastic factors are very important in the colonization of aquacultural environments.
The idea that both environmental conditions and chance influence the emergence of microbial communities opens opportunities for the concept of probiotics as biological conditioning and control agents. Instead of allowing spontaneous primary colonization of the rearing water by bacteria accidentally present, the water could be preemptively colonized by the addition of probiotic bacteria, since it is generally recognized that preemptive colonization may extend the reign of pioneer organisms (1). It is suggested that in the case of preemptive colonization of rearing environments with emerging microbial communities, a single addition of a probiotic culture may suffice to achieve colonization and persistence in the host and/or in its ambient environment, provided that the probiotic cultures are well adapted to the prevailing environmental conditions. When the host or its environment already carries a well-established and stable microbial community, it is much more probable that the probiotic will have to be supplied on a regular basis to achieve and maintain its artificial dominance.
It is therefore a pertinent question whether it is possible to modify the composition of a microbial community in the field by the exogenous addition of a probiotic. This is particularly important when a long-term exposure is required for the probiotic effect. It is not easy to answer this question, since the literature does not provide real evidence for this in aquacultural practices. Nevertheless, some assumptions can be made when referring to work done with lactic acid bacteria (103). Although lactic acid bacteria are not dominant in the normal intestinal microbiota of larval or growing fish (103), several trials have been done to induce an artificial dominance of lactic acid bacteria in fish fry (32, 37, 43, 44, 59, 123). The addition of high doses of lactic acid bacteria to established microbial communities of fish juveniles provoked a temporary change in the composition of the intestinal microbial community. Within a few days after the intake had stopped, however, the added strains showed a sharp decrease and were lost from the gastrointestinal tract in most of the fish (59, 103). Several reports describe bacteria firmly attached to the intestinal mucosa (102, 112, 122), and it is now accepted that fish contain a specific intestinal microbiota that becomes established at the juvenile stage or after metamorphosis. Unless the host has been exposed to a limited range of microorganisms in its development, it is improbable that a single exogenous addition of a probiotic to an established microbial community will result in long-term dominant colonization. This seems to be particularly the case when bacterial species are used which do not belong to the normal dominant intestinal microbiota of the cultured species or its particular development stage. In such cases, it is necessary to supply the probiotic on a regular basis if a continuous colonization at high densities is required.
RECENT FINDINGS
General Considerations
It was suggested in 1980 by Yasuda and Taga (148) that bacteria would be found to be useful not only as food but also as biological controllers of fish disease and activators of nutrient regeneration. Only in the late 1980s did the first publications on biological control in aquaculture emerge, and since then the research effort has continually increased. Generally, probiotics are applied in the feed or added to the culture tank or pond as preventive agents against infection by pathogenic bacteria, although nutritional effects are also often attributed to probiotics, especially for filter feeders.
Most probiotics proposed as biological control agents in aquaculture belong to the lactic acid bacteria (Lactobacillus, Carnobacterium, etc.), to the genus Vibrio (Vibrio alginolyticus, etc.), to the genus Bacillus, or to the genus Pseudomonas, although other genera or species have also been mentioned. An overview of the published results is given in Table 1.
Fish Eggs and Larvae
There is an urgent need to control the microbiota in hatching incubators by alternative means, since the use of antibiotics has to be minimal. In fact, the use of antibiotics does not allow microbial control and may result in an unfavorable alteration of the microbiota. Since it assumed that uncontrolled development of the microbial communities in hatcheries is one of the major reasons for the unpredictable and often variable results, the introduction of microbial control practices by means of probiotics may have a beneficial effect on the cultures in hatcheries. A relatively dense, nonpathogenic, and diverse adherent microbiota present on the eggs would probably be an effective barrier against colony formation by pathogens on fish eggs (52, 84). This rationale has been tried with cod eggs. Hansen and Olafsen (51) attempted to manipulate the egg microbiota of cod (Gadus morhua) by incubating gnotobiotic eggs in cultures of defined inhibitory bacterial strains; however, these strains failed to prevent colonization of the eggs by the microbiota naturally present in the incubator. Nevertheless, it should be noted that the choice of strains is very important. As discussed below, the screening and preselection of potential or putative probiotics should be based on extensive experimental work performed in vivo. Therefore, it would be better if the experimental setup described by Hansen and Olafsen (51) was used as a preselection tool, not to verify the effect of selected bacteria, as is often done now.
The establishment of a normal gut microbiota may be regarded as complementary to the establishment of the digestive system, and under normal conditions it serves as a barrier against invading pathogens. Larvae may ingest substantial amounts of bacteria by grazing on suspended particles and egg debris (9). It is therefore obvious that the egg microbiota will affect the primary colonization of the fish larvae.
It has been observed that survival of halibut (Hippoglossus hippoglossus) larvae in the first 2 weeks after hatching is affected by incubation with indigenous bacteria isolated from fish (unpublished data cited in reference 84). Larval survival in the presence of Vibrio salmonicida-like strains and Lactobacillus plantarum amounted to 95%, wheras Vibrio iliopiscarius reduced survival to 63% compared to the control group (81%).
During initial feeding, it is possible to induce an artificial dominance of a certain group of bacteria in the fish-associated microbiota by adding a strain to the rearing water (see, e.g., reference 123) or to the culture medium of the live food (see, e.g., reference 37). Gatesoupe (37) was able to improve the survival rate of larval turbot (Scophthalmus maximus) by daily addition of lactic acid bacteria to the enrichment medium of the rotifers used as live food for the turbot larvae. The added lactic acid bacteria could be retrieved in large amounts from the turbot larvae, and a significant reduction of larval mortality was observed when the larvae were challenged with a pathogenic Vibrio on day 9. The hypothesis was that the lactic acid bacteria would act as a microbial barrier against the pathogenic Vibrio and might curb the invasion of turbot larvae by the pathogen. Similarly, García de la Banda et al. (32) added lactic acid bacteria (Streptococcus lactis and Lactobacillus bulgaricus) to Brachionus and Artemia used in turbot larva feeding. In a single experiment without replicates, 55% survival was found on day 17 when living lactic acid bacteria had been added and 66% survival was found with disabled ones, as opposed to 34% in the control group. Apparently, the bacterial cells, alive or disabled, provoked improved survival of the turbot larvae.
Bacillus strain IP5832 spores have been introduced into the culture medium of rotifers, which were fed to turbot larvae (35). A decrease in the proportion of members of the Vibrionaceae in the rotifers was observed, and the mean weight of the turbot larvae on day 10 was significantly improved with the spore-fed rotifers. When an experimental infection was performed with an opportunistic Vibrio sp., mortality was observed in all treatments but the mean survival of the spore-fed rotifers on day 10 was significantly higher than that of the control group (31 and 10%, respectively). Although the author suggested that the likeliest mode of action is the production of antibiotics, it is not clear whether an improvement of the nutritional status of the larvae could also contribute to the increased resistance to infection.
Attention has also been focused on siderophore production and the probiotic effect of Vibrio type E on turbot larvae (38). The main effect of rotifer enrichment with this strain was to improve the survival of the larval turbot after a 48-h challenge with the pathogenic Vibrio type P.
Fish Juveniles and Adults
In experiments performed by Gildberg et al. (43), Atlantic salmon (Salmo salar) fry given a diet supplemented with a lactic acid bacterium (related to Lactobacillus plantarum but later reclassified as Carnobacterium divergens) was challenged with cohabitant fishes infected with Aeromonas salmonicida through intraperitoneal injection. Mortality was recorded during the next 4 weeks. It was shown that lactic acid bacteria given as supplements in the dry feed could colonize the intestine, but no protection against A. salmonicida infection could be detected. Contrary to the expectations, the highest mortality was recorded with fish given the diet containing lactic acid bacteria.
Atlantic cod fry fed on dry feed containing lactic acid bacteria (Carnobacterium divergens) was exposed to a virulent strain of Vibrio anguillarum. An improved disease resistance was obtained, and 3 weeks after the challenge lactic acid bacteria dominated the intestinal microbiota of the surviving fish given feed supplemented with C. divergens (45). Similarly, two strains of C. divergens were isolated from the intestine of Atlantic cod and Atlantic salmon and were added to a commercial dry feed and administered for 3 weeks to Atlantic cod fry (44). Twelve days after the infection with the same virulent V. anguillarum strain, reduced cumulative mortality was recorded, but 4 weeks after the infection, the same cumulative mortality was reached in all groups. Thus, the probiotic treatment only delayed the mortality of the cod fry in the infection trials. The authors argued, however, that this observation does not exclude the considerable importance of such methods under normal rearing conditions in the presence of moderate levels of opportunistic bacteria.
Jöborn et al. (59) studied the ability of Carnobacterium strain K1 to colonize the intestinal tract of rainbow trout (Oncorynchus mykiss) (13 to 16 cm long) and to inhibit two common fish pathogens, V. anguillarum and A. salmonicida, in mucus and fecal extracts of rainbow trout. The production of growth inhibitors against both pathogens was demonstrated in vitro in both the mucus and the fecal extracts. Furthermore, the Carnobacterium cells remained viable in the intestinal tract, since considerable densities (105 CFU/g) were found in the fecal pellets at least until 4 days after the last feeding. However, a sharp decrease of 3 log units was observed after 3 days once the treatment was stopped. Unfortunately, these in vitro observations have not been confirmed by published in vivo data. Olsson et al. (85) found that the growth of V. anguillarum in fecal extracts from turbot juveniles was inhibited by Carnobacterium cells. It was concluded that the turbot intestinal tract and feces can serve as an enrichment site for V. anguillarum and that the use of intestinal bacteria with inhibitory activity against Vibrio spp. might be used to decrease the load of fish-pathogenic Vibrio spp. in turbot hatcheries.
Several strains of siderophore-producing Pseudomonas fluorescens have been successfully applied as biological control agents. They were able to exclude a pathogenic A. salmonicida strain from Atlantic salmon presmolts with stress-inducible furunculosis infection (117) and to limit the mortality of rainbow trout (40 g) infected with V. anguillarum (48). Short-term bathing of the fishes in a bacterial suspension of the probiotic (48, 117), long-term exposure in the rearing water (48), or a combination of the two treatments (48) led to a significant decrease in the mortality after the challenge trial. In both studies, a good correlation was found between the production of siderophores and the protective action of P. fluorescens, suggesting that competition for free iron is involved in the mode of action. Smith and Davey (117) concluded that P. fluorescens exerted its effect from the host's exterior, since the strain did not significantly invade the fish following bath treatment.
A V. alginolyticus strain used as a probiotic in a commercial shrimp hatchery in Ecuador has been applied in a bath treatment to Atlantic salmon (21 g) maintained in freshwater (5). V. alginolyticus was found in the intestine up to 21 days after the initial probiotic application. The challenge experiments revealed that the application of the probiont to Atlantic salmon led to a reduction in mortality after exposure to A. salmonicida and to a lesser extent after exposure to V. anguillarum and V. ordalii.
Crustaceans
Penaeid shrimps.
In contrast to the already broad application of probiotics in commercial penaeid shrimp hatcheries (33, 73; D. R. W. Griffith, Abstr. Larvi'95 Fish Shellfish Larvicult. Symp. p. 478, 1995); relatively few in-depth studies have been published on this subject. Maeda and Liao (68) reported the use of a soil bacterial strain, PM-4, that promoted the growth of Penaeus monodon nauplii, probably acting as a food source. This strain also showed an in vitro inhibitory effect against a V. anguillarum strain. When added to tanks inoculated with diatoms and rotifers, the strain resulted in 57% survival of the larvae after 13 days, while without the bacterium all the larvae had died after 5 days (67). Maeda and Liao (69) produced similar data in another study, but the effect was attributed to strain NS-110.
A V. alginolyticus strain, which was selected based on its apparent lack of pathogenicity, was inoculated daily into 25- and 60-ton larval rearing tanks containing Litopenaeus vannamei postlarvae (33). The average survival and wet weight were higher in the tanks containing shrimps that had undergone bacterial inoculation compared to shrimps receiving prophylactic doses of oxytetracycline and the control group. Tanks of shrimps receiving bacterial inoculation showed no presence of V. parahaemolyticus in any of the samples taken from the shrimp's microbiota, while control tanks and those containing shrimps receiving antibiotics had V. parahaemolyticus in approximately 10% of the samples.
Rengpipat and Rukpratanporn (S. Rengpipat and S. Rukpratanporn, Abstr. Fifth Asian Fisheries Forum, p.193, 1998) reported the use of Bacillus strain S11 as a probiotic administered in enriched Artemia to larvae of the black tiger shrimp (Penaeus monodon). It was found that the P. monodon larvae fed the Bacillus-fortified Artemia had significantly shorter development times and fewer disease problems than did larvae reared without the Bacillus strain. After being fed for 100 days with the Bacillus strain S11-supplemented feed, P. monodon postlarvae were challenged with a pathogenic V. harveyi strain, D331, by immersion of the shrimps. Ten days later, all the groups treated with Bacillus strain S11 showed 100% survival whereas the control group had only 26% survival (99). Although one has to be cautious when comparing the performance of different aquaculture farms, Moriarty (73) concluded, based on his studies in Indonesia, that the use of several Bacillus cultures in penaeid culture ponds allowed the culture of the shrimps for over 160 days without problems whereas the farms that did not use the Bacillus cultures experienced almost complete failure in all ponds, with luminescent Vibrio disease killing the shrimps before 80 days of culture was reached. A cost-benefit analysis of the use of Bacillus cultures was done for a particular farm in Thailand, and under the given circumstances there was a clear benefit to the producer (74).
Strain BY-9 was produced on large scale and inoculated at 106 CFU/ml into an 18-ton larval rearing tank for mass production of P. monodon larvae. The strain was confirmed to inhibit the in vitro growth of V. harveyi. The results showed that BY-9 inoculation gave a lower Vibrio density and a higher survival rate (46.1 and 10.6%, respectively) than those of the control of larvae cultured up to the tenth postlarval stage (PL-10) (H. K. Sugama and S. Tsumura, Abstr. Fifth Asian Fish. Forum, p. 74, 1998).
Crabs.
After an inoculation of diatoms and rotifers, the bacterial strain PM-4 was daily introduced for 7 days in 200-m3 tanks containing crab (Portunus trituberculatus) larvae and was also inoculated with diatoms and rotifers (67). There was a negative correlation between the presence of PM-4 and the densities of Vibrio spp. In seven trials, the average survival of the crab larvae was 27.2% with strain PM-4. In six of nine trials without PM-4, no larvae grew into adults, resulting in an average survival of only 6.8%. These results were reported in three independent experiments (67, 69, 83).
Bivalve Mollusks
Several studies have focused on the nutritional contribution of probiotics to mollusk larvae; however, no indication was given of their potential biological control abilities (26, 27; J. Torrie and P. Neima, Abstr. Larvi'95 Fish Shellfish Larvicult. Symp., p. 489, 1995). A bacterial strain isolated from the gonads of Chilean scallop (Argopecten purpuratus) broodstock and characterized as Alteromonas haloplanktis displayed in vitro inhibitory activity against the known pathogens V. ordalii, V. parahaemolyticus, V. anguillarum, V. alginolyticus, and Aeromonas hydrophila (106). This A. haloplanktis and a Vibrio strain 11 that showed in vitro inhibition of a V. anguillarum-related pathogen protected the scallop larvae against the V. anguillarum-related pathogen in an experimental infection (105).
Aeromonas media A199 was found to be inhibitory in vitro to 89 strains of aeromonads and Vibrio and could prevent the death of oyster (Crassostrea gigas) larvae when they were challenged in vivo with Vibrio tubiashii (42). Administration of the probiotic strain to the larvae fed with algae caused a spectacular decrease of the pathogen densities in the larvae compared to those in the larvae treated with V. tubiashii only. However, A. media A199 could no longer be detected on the host only 4 days after the probiotic treatment, indicating that it would be necessary to introduce the probiotic at regular intervals if a prolonged protective effect is required.
Live Food
Unicellular algae.
Unicellular algae are often given as a first food or are included in the culture system as a food for rotifers and Artemia. Bacteria increase the growth rate and yield of algae (29, 75, 128). However, since bacteria may also inhibit algal growth (75), careful screening may be necessary when bacteria are to be used as probiotics in larval rearing or in the green-water technique. Rico-Mora et al. (101) selected a strain (SK-05) for its active growth in organic-poor substrates and inoculated it into a Skeletonema costatum culture. When all cultures had reached the late exponential phase, Vibrio alginolyticus was inoculated as a typical contaminant. After 48 h it was shown that strain SK-05 prevented the proliferation of V. alginolyticus, although it exerted no in vitro inhibitory action against V. alginolyticus. It was suggested that the protective effect was due to competitive exclusion, since only strain SK-05 was able to utilize the exudates of S. costatum.
Rotifers.
Several studies have been published on the nutritional contribution of lactic acid bacteria (see e.g., references 34, 36, 39, and 55) and other bacteria (see e.g., references 50, and 108; P. Bogaert, M. Dehasque, and P. Sorgeloos, Abstr. World Aquacult. '93, p. 186, 1993) to the production rate of the rotifer Brachionus plicatilis. The control of the microbiota of rotifer cultures has received less attention. Under optimal culture conditions, no increase of the growth rate of the rotifers was observed after addition of Lactococcus lactis AR21 to the diet. Under a suboptimal feeding regime where the amount of food was reduced by 55%, L. lactis AR21 counteracted the growth inhibition of the rotifers due to V. anguillarum in two of the three experiments performed. It was not possible, however, to recover either L. lactis AR21 or V. anguillarum from the rotifers after 24 h (54). Live food such as rotifers is often suspected of being a vector for bacterial infections of the predatory organisms (76, 81, 89, 129). It is therefore surprising that studies dealing with the proliferation of larval pathogens in rotifer cultures are so scarce. Only Gatesoupe (36) reported that the proliferation of Aeromonas salmonicida, accidentally appearing in the experimental rotifer culture, was inhibited by the presence of Lactococcus plantarum as a supplement.
Artemia.
The capacity of V. alginolyticus C14 to prevent mortality in Artemia nauplii was demonstrated by Gomez-Gil et al. (47); i.e., inoculation of strain C14 before the challenge prevented Artemia nauplii from dying after being experimentally infected with V. parahaemolyticus HL58, although strain HL58 was still able to colonize the nauplii (47).
Verschuere et al. (140) selected nine bacterial strains that positively influenced the growth and/or survival of juveniles of the brine shrimp Artemia cultured as a live food for other species. All nine strains were able to delay the death of the Artemia when experimentally infected with the pathogenic V. proteolyticus CW8T2, although large differences were found among the strains. While all Artemia in the axenic control died within 2 days after the infection, the survival rates of the Artemia cultures inoculated beforehand with strain LVS8 or a mixture of the nine strains showed more than 80% survival after 4 days (141). Furthermore, the growth of V. proteolyticus CW8T2 in the Artemia culture water was considerably slowed in the presence of strain LVS8.
Microbially Matured Water
Although it is not strictly a probiotic treatment, attempts have been made to optimize the rearing water for larvae of several marine species by so-called microbial maturation (115, 135). Microbial maturation of seawater prepared by transient maintenance in a maturation tank with a biofilter led to a significantly higher initial growth rate of turbot larvae than in membrane-filtered water. Proliferation of opportunistic bacteria was observed in the rearing water after hatching of the turbot eggs, but it occurred to a lesser extent in the microbially matured water (115). Also, clear differences in survival of halibut yolk sac larvae were observed (135). The experiments supported the hypothesis that microbial maturation selects for nonopportunistic bacteria that protect the marine larvae from the proliferation of detrimental opportunistic bacteria (115).
Interaction with Nutritional Effects
As mentioned above, it is sometimes impossible to separate feeding aquatic organisms from environmental control. Addition of bacteria to the rearing water of filter feeders such as rotifers, bivalve larvae or adults, and crustacean larvae may result in massive uptake of these bacteria, possibly acting as a (complementary) food source or contributing to the digestion of the food, even if the main goal of the probiotic application was, e.g., suppression of a pathogen in the culture water. It is sometimes unclear whether the probiotic effect is attributed to suppression of a pathogen or if it is a direct or indirect consequence of the nutritional effect of the probiotic.
This duality has been addressed by Riquelme et al. (105) and Verschuere et al. (140, 141). Riquelme et al. (105) evaluated the effect of 11 bacterial strains that were inhibitory in vitro toward a V. anguillarum-related pathogen on the survival of Chilean scallop larvae when they were fed only the different inhibitory bacterial strains for 14 days. Only two strains (Vibrio strains 11 and 334) had no detectable effects on the scallop larvae, while the other strains resulted in high mortality. In an in vivo challenge test, only Vibrio strain 11 had a protective effect against infection with the V. anguillarum-related pathogen. Verschuere et al. (140, 141) inverted the approach and selected nine bacterial strains that enhanced to the nutritional value of the dry food for Artemia juveniles. The in vivo antagonism of the nine strains for the pathogen V. proteolyticus CW8T2 was examined in experimental-infection trials, and they showed various degrees of protection. These results indicate that the two aspects must be examined separately, but it is conceivable that a combination of nutritional contribution and disease control yields the best probiotic effect.
POSSIBLE MODES OF ACTION
Although many publications about probiotics in aquaculture have emerged during the last decade, the approach was generally empirical and the arguments with regard to the mode of action were often circumstantial. The exact modes of action of the probiotics were rarely completely elucidated. In human and agriculture application, probiotic research has enjoyed much more attention through history and several modes of action have been supported by unambiguous experimental data (see the review by Fuller [30]). It is clear that the experience obtained with terrestrial animals has been used in aquaculture, especially with regard to the use of lactic acid bacteria. Also, the suppression of plant root pathogens by fluorescent Pseudomonas spp. has been examined in detail (see the review by O'Sullivan and O'Gara [88]). Although each of these disciplines has its specific characteristics, several of the microbial-ecological mechanisms involved are similar, allowing some transfer of experimental evidence.
Considering the possible probiotic effect in vivo, one has to make a distinction between the intrinsic ability of the strain to positively influence the host and its ability to reach and maintain itself in the location where the effect is to be exerted. For instance, the production of siderophores or inhibitory compounds in sufficient amounts and even under the conditions prevailing in the gut is of no relevance if the strain is not ingested by the host. This is important, since Prieur (93) has demonstrated both selective ingestion and digestion of microbes by the bivalve Mytilus edulis. Similarly, if the candidate probiotic is not capable of efficient proliferation in the gut after being ingested, it is improbable that it will exert strong effects unless it is added regularly through the diet. Hence, the possible modes of action require implicitely that the candidate probiotics be able to reach the location where their probiotic effect is required. Those modes are as follows: production of inhibitory compounds; competition for chemicals or available energy; competition for adhesion sites; enhancement of the immune response; improvement of water quality; interaction with phytoplankton; source of macro- and micronutrients; and enzymatic contribution to digestion. The last two of these are not dealt with in this review. The possible modes of action are discussed in more detail in the following sections.
Production of Inhibitory Compounds
General aspects.
Microbial populations may release chemical substances that have a bactericidal or bacteriostatic effect on other microbial populations, which can alter interpopulation relationships by influencing the outcome of competition for chemicals or available energy (28, 62, 94). The presence of bacteria producing inhibitory substances in the intestine of the host, on its surface, or in its culture medium is thought to constitute a barrier against the proliferation of (opportunistic) pathogens.
In general, the antibacterial effect of bacteria is due to the following factors, either singly or in combination: production of antibiotics (145), bacteriocins (15, 94, 136), siderophores (which are dealt with in a later section), lysozymes, proteases, and/or hydrogen peroxide and the alteration of pH values by the production of organic acids (126). Vandenbergh (136) added the formation of ammonia and diacetyl to this list.
Lactic acid bacteria are known to produce compounds such as bacteriocins that inhibit the growth of other microorganisms (136). There are many reports of the inhibitory activity of lactic acid bacteria mediated by bacteriocins, mostly but not exclusively against gram-positive bacteria (reviewed by Piard and Desmazeaud [91]) (121). However, almost all the pathogens involved in aquaculture are gram negative. The possible involvement of lactic acid bacteria as probiotics in aquaculture is discussed by Ringø and Gatesoupe (103). Since lactic acid bacteria normally account for only a small part of the gut microbiota of fish and since they are generally considered to be nonpathogenic (with some exceptions), it can be questioned if the inhibition of closely related species by bacteriocins produced by lactic acid bacteria can effectively contribute to the health status of the higher organism.
Compounds other than bacteriocins and antibiotics have been suggested to play a role in the amensalism that may occur between bacterial species. Nair et al. (79) showed that a large proportion of marine bacteria produced bacteriolytic enzymes against V. parahaemolyticus. Furthermore, Imada et al. (57, 58) isolated and characterized Alteromonas sp. strain B-10-31, isolated from nearshore seawater of Japan, which produces an alkaline protease inhibitor called monastatin. In an in vitro assay, the purified and concentrated monastatin showed inhibitory activity against a protease from Aeromonas hydrophila and a thiol protease from V. anguillarum, both pathogenic to fish.
Many authors assign the inhibitory effects detected in in vitro antagonism tests to bacteriocins or antibiotics without looking for any other causes. It has been argued that observed growth inhibition can, in many cases, be accounted for by primary metabolites or simply by a decrease of the pH (6, 131). Unless the inhibitory compounds have been identified, in this review we use the expression “inhibitory compounds” rather than antibiotics or bacteriocins.
Production in aquaculture.
Many studies have demonstrated the presence of bacterial strains showing in vitro inhibition toward pathogens known to occur in aquaculture (3, 5, 6, 24, 43–45, 50, 54, 59, 63, 83, 86, 99, 105, 110, 126, 127, 130, 143). This shows that the ability to inhibit other bacteria is not uncommon for bacteria found in aquaculture environments. However, it has not been demonstrated that production of such inhibitory compounds occurs under in vivo conditions, and the ecological relevance of the production of inhibitory compounds toward other bacteria is still unclear.
The in vitro production of inhibitory compounds toward known pathogens for the considered species has often been used in the selection of putative probiotic strains (see, e.g., references 42, 51, 99, and 105). At this stage, however, the association between amensalistic activity and in vivo probiotic activity is very weak and circumstantial. Typically, a correlation is made between the in vitro ability of the probiotics to inhibit pathogens and the in vivo protection of the cultured aquatic species, but in none of the studies published so far has it been shown unequivocally that the production of inhibitory compounds is the cause of the observed in vivo probiotic activity of the strains. Hence, future research in this field is required. Similar research has already been performed in the field of plant disease suppression, where the production of inhibitory compounds by some fluorescent Pseudomonas spp. is now recognized as an important factor in the disease suppression ability of these strains (see the review by O'Sullivan and O'Gara [88]).
Competition for Chemicals or Available Energy
General aspects.
Competition for chemicals or available energy may determine how different microbial populations coexist in the same ecosystem (28). On theoretical grounds, it seems likely that competition for nutrients will operate in the mammalian gut, but the evidence for its occurrence in humans and terrestrial animals is not good (30). Competition for nutrients can theoretically play an important role in the composition of the microbiota of the intestinal tract or ambient environment of cultured aquatic species, but to date there have been no comprehensive studies on this subject (103). Hence, successful application of the principles of competition to natural situations is not easy and remains a major task for microbial ecologists.
The microbial ecosystem in aquaculture environments is generally dominated by heterotrophs competing for organic substrates as both carbon and energy sources. Specific knowledge of the factors governing the composition of the microbiota in aquaculture systems is required to manipulate it. This knowledge, however, is generally not available, and one must therefore rely on an empirical approach. Nevertheless, Rico-Mora et al. (101) selected a bacterial strain for its active growth in organic-poor substrates and inoculated it into a diatom culture, where it prevented the establishment of an introduced V. alginolyticus strain. Since the inoculated strain had no in vitro inhibitory effect on V. alginolyticus, it was suggested that the strain was able to outcompete V. alginolyticus due to its ability to utilize the exudates of the diatom.
Verschuere et al. (140) selected several strains with a positive effect on the survival and growth of Artemia juveniles. The in vitro antagonism tests and filtrate experiments showed that no extracellular inhibitory compounds were involved in the protective action of these strains against V. proteolytics CW8T2 but that living cells were required to protect Artemia against the pathogen. It was suggested that the selected bacteria exerted their protective action by competing with the pathogen for chemicals and available energy (141).
Competition for iron.
Virtually all microorganisms require iron for growth (98). Siderophores are low-molecular-weight (<1,500), ferric ion-specific chelating agents (80) which can dissolve precipitated iron and make it available for microbial growth. The ecological significance of siderophores resides in their capacity to scavenge an essential nutrient from the environment and deprive competitors of it. Successful bacterial pathogens are able to compete successfully for iron in the highly iron-stressed environment of the tissues and body fluids of the host (147). The ecological significance of siderophores in soils as important tools for iron acquisition by microorganisms and plants and their involvement in suppression of plant root pathogens have been established (12, 88).
The omission of iron from the diet of early-weaned seabass (Dicentrarchus labrax) larvae had no detrimental effect on the survival or the growth rate of the fish but significantly limited the bacterial load of the larvae and increased the diversity of the microbiota (40). The requirement for iron is high for many pathogens, including V. anguillarum. In a challenge test with this bacterium, salmon mortality increased linearly with dietary iron content (40, 109). Both observations indicate the importance and the biological role of ferric iron in the establishment of the microbiota associated with cultured aquatic species.
Harmless bacteria which can produce siderophores could be used as probiotics to compete with pathogens whose pathogenicity is known to be due to siderophore production and competition for iron or to outcompete all kind of organisms requiring ferric iron from solution. The possible effectiveness of siderophore-producing probiotics can be illustrated by the study of Gatesoupe (38), in which the addition of the bacterial siderophore deferoxamine to live food (rotifers) increased the resistance of turbot larvae challenged with the pathogenic Vibrio strain P. The addition of a siderophore-producing Vibrio strain E protected the turbot larvae slightly more.
Pybus et al. (94) tested thirty strains of V. anguillarum as potential probiotics against the salmon pathogen V. ordalii by the deferred-antagonism test. Only one strain (V. anguillarum VL4335) inhibited strains of V. ordalii in vitro, and this effect was blocked when iron salts were added to the medium, indicating that the growth inhibition was linked to iron deficiency. Using the chrome-azurol sulfate assay to measure siderophore production, V. anguillarum VL4335 yielded significantly higher values than other V. anguillarum strains.
Smith and Davey (117) showed that the fluorescent pseudomonad F19/3 is capable of inhibiting the growth of Aeromonas salmonicida in culture media and that this inhibition is due to competition for free iron. Strain F19/3 was also capable of excluding A. salmonicida from Atlantic salmon (Salmo salar) presmolts with stress-inducible infections.
Gram et al. (48) examined the applicability of the siderophore-producing Pseudomonas fluorescens AH2 isolated from frozen freshwater fish. This latter strain is inhibitory to several gram-positive and gram-negative bacteria, particularly when iron availability is limited. In vitro tests revealed that the growth of V. anguillarum was inhibited by the filter-sterilized supernatans from iron-limited cultures of P. fluorescens AH2 but not from iron-replete cultures. During coculture, P. fluorescens AH2 inhibited the growth of V. anguillarum independently of the iron concentration, when the initial level of the siderophore producer was 100 to 1000 times greater than the level of the fish pathogen. The in vitro tests were confirmed by an in vivo antagonism test, in which the mortality of rainbow trout juveniles due to V. anguillarum infection was decreased by 46% when the culture was treated with P. fluorescens AH2.
Siderophore production by fluorescent pseudomonads in soil is known to be influenced by a great variety of factors (12, 88). Therefore, detection of in vitro production of siderophores does not necessarily mean that they are produced in significant amounts in vivo in order to have a significant biological control effect. Similar to the production of inhibitory compounds, the evidence for the participation of competition for chemicals or available energy and, more specifically, of free iron or siderophores in the mode of action of probiotics is still circumstantial.
Competition for Adhesion Sites
One possible mechanism for preventing colonization by pathogens is competition for adhesion sites on gut or other tissue surfaces. It is known that the ability to adhere to enteric mucus and wall surfaces is necessary for bacteria to become established in fish intestines (86, 87, 143). Since bacterial adhesion to tissue surface is important during the initial stages of pathogenic infection (61), competition for adhesion receptors with pathogens might be the first probiotic effect (72). In human medicine, adherent strains are key candidates for probiotic therapy (113).
Adhesion can be nonspecific, based on physicochemical factors, or specific, involving adhesin molecules on the surface of adherent bacteria and receptor molecules on epithelial cells (113). Inhibition of the adhesion of pathogens to human or other mammal cells has already been demonstrated in vitro by several authors (8, 10, 19, 21). Competitive exclusion resulting from preemptive colonization has been shown for the cecal walls of chickens, which kept their effect after the ceca were washed four times in buffered saline (120). As far as is known, similar research approaches have not yet been followed with aquatic species, and competition for adhesion sites as a mode of probiotic action is still hypothetical.
Adhesion capacity and growth on or in intestinal or external mucus has been demonstrated in vitro for fish pathogens like V. anguillarum and A. hydrophila (31, 61) and for candidate probiotics such as Carnobacterium strain K1 (59) and several isolates inhibitory to V. anguillarum (86). In one of these studies, the aim was to measure the in vitro capacity of the strains to adhere to and grow in turbot intestinal mucus in order to investigate their potential to colonize farmed turbot as a means of protecting the host from infection by V. anguillarum (86). The intestinal isolates generally adhered much better to a film of turbot intestinal mucus, skin mucus, and bovine serum albumin than did V. anguillarum, indicating that they could compete effectively with the pathogen for adhesion sites on the mucosal intestinal surface.
Adhesion of probiotics to the gut wall or other tissues does not necessarily imply competition for adhesion sites as the (only) mode of action for the probiotic effect. It is conceivable that bacteria are able to colonize, for example, the intestinal gut wall of a fish and exert their protective action against a pathogen by excreting inhibitory compounds.
Enhancement of the Immune Response
Immunostimulants are chemical compounds that activate the immune systems of animals and render them more resistant to infections by viruses, bacteria, fungi, and parasites (96). Fish larvae, shrimps, and other invertebrates have immune systems that are less well developed than adult fish and are dependent primarily on nonspecific immune responses for their resistance to infection (118).
Observations obtained in experiments with warm-blooded animals indicate that probiotic (lactic acid) bacteria administered orally may induce increased resistance to enteric infections (56). There are many reports that bacterial compounds act as an immunostimulant in fish and shrimp, as has been reviewed recently (111), but only specific cell compounds or nonliving cells were used in these studies. It has also been suggested that ingestion of bacteria and subsequent endocytosis in cod and herring larvae are involved in stimulation of the developing immune system (84). However, at present it is not clear whether bacteria administrered as probiotics could have a beneficial effect on the immune response of cultured aquatic species, but such a mode of action cannot a priori be excluded.
Improvement of Water Quality
In several studies, water quality has been recorded during the addition of the probiotics, especially Bacillus spp. The rationale is that gram-positive Bacillus spp. are generally more efficient in converting organic matter back to CO2 than are gram-negative bacteria, which would convert a greater percentage of organic carbon to bacterial biomass or slime (119). It is reasoned that by maintaining higher levels of these gram-positive bacteria in the production pond, farmers can minimize the buildup of dissolved and particulate organic carbon during the culture cycle while promoting more stable phytoplankton blooms through the increased production of CO2 (114). However, several studies utilizing one or more bacterial species such as Bacillus, Nitrobacter, Pseudomonas, Enterobacter, Cellulomonas, and Rhodopseudomonas spp. in the culture of shrimps (65, 99) or channel catfish (13, 20, 95, 133) could not confirm this hypothesis. This shows that the published evidence for water quality improvement is poor, except with regard to nitrification.
The start-up of biofilters by transferring medium from an existing filter is a common practice in aquaculture. However, the use of nitrifying cultures as inocula to speed up the establishment of nitrification is not so common. A lot of bacterial cultures containing nitrifying bacteria to control the ammonia level in culture water are available commercially and are aimed especially at aquarium hobbyists. Nitrifiers are responsible for the oxidation of ammonia to nitrite and subsequently to nitrate. Seeding of biological filters with nitrifying bacteria is effective in reducing the activation time of new biofilters (18). Perfettini and Bianchi (90) used inocula consisting of frozen cells to accelerate the conditioning of new closed seawater culture systems, and the time to establish nitrification was shortened by about 30%. In a commercial semiclosed water recirculation system, the start-up of a nitrifying biofiter was reduced from 3–4 weeks to 10 days by inoculation of a very active liquid culture of nitrifiers (I. Van Hauteghen, G. Rombaut, and W. Verstraete, Abstr. Int. Grf. AQUA 2000, p. 338, 2000).
Nitrifying cultures could also be added to the ponds or the tanks when an incidental increase of ammonia or nitrite levels is observed. Besides ammonia, nitrite toxicity is a common problem in fish culture (64), for example in pond rearing of catfish (134).
Interaction with Phytoplankton
Recent reports demonstrate that many bacterial strains may have a significant algicidal effect on many species of microalgae, particularly of red tide plankton (29). Of 41 bacterial strains tested, 23 inhibited the growth of the unicellular alga Pavlova lutheri to various degrees (75). Bacteria antagonistic toward algae would be undesirable in larval rearing where unicellular algae are added (e.g., the green-water technique) but would be advantageous when undesired algal species develop in the culture pond.
Claims for bacterial amendments include a decrease of the proportion of cyanobacteria. One such bacterial suspension consisted of Bacillus, Nitrobacter, Pseudomonas, Enterobacter, Cellulomonas, and Rhodopseudomonas spp. and was applied to channel catfish ponds (13). However, the percentages of cyanobacteria did not differ between the treatment and the control on any of the sampling dates.
Positive effects of bacteria on cultured microalgae have also been observed (29, 75, 101, 128) (see above). It is conceivable that bacteria can indirectly influence the health or the zootechnical performance of the cultured aquatic animals through their effect on the microalgae used as food or in the green-water technique. When probiotic bacteria are selected to be used in a culture environment comprising algae, their possible interaction with these unicellular algae must be taken into consideration when the mode of action is being investigated.
RATIONALE FOR SELECTING AND DEVELOPING PROBIOTICS IN AQUACULTURE
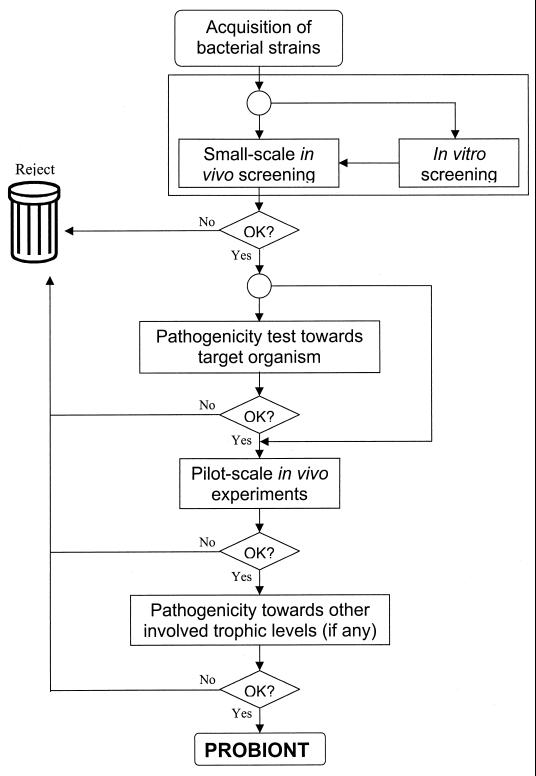
The development of probiotics applicable to commercial use in aquaculture is a multistep and multidisciplinary process requiring both empirical and fundamental research, full-scale trials, and an economic assessment of its use. This section discusses the different crucial phases which must be covered in order to develop effective and safe probiotics. In Fig. 1 this rationale is presented schematically.
FIG. 1.
Rationale for the research and development of probiotics as biological control agents in aquaculture.
Acquisition of Background Information
Before research and development activities are begun, bottlenecks in culture practices or in economic development of the aquaculture farm or industry should be identified, taking into account possible restricting or directing legislative decisions. A critical review of the available scientific literature and a profound knowledge of the rearing practices used in aquaculture farms should determine whether a probiotic approach would be feasible and is worth further examination. The abiotic and biotic environment inhabited by the cultured organisms should be well characterized, with particular attention being paid to the microbiota, the relationships between the microbiota (pathogens and others) and the host, and the relationships between the microbiota and the carrying capacity of the culture environment.
Acquisition of Putative Probiotics
The acquisition of a good pool of candidate probiotics is of major importance in this process. It is vital in this phase that the choice of strains is made as a function of the possible role of the probiotics to be developed, although there is no unequivocal indication that putative probiotics isolated from the host or from their ambient environment perform better than isolates completely alien to the cultured species or originating from a very different habitat (Table 1). There are several reports of the use in aquaculture of probiotics developed for humans or terrestrial animals (34, 35, 37; Bogaert et al., Abstr. World Aquacult. '93). However, there is an elegant logic in isolating putative probiotics from the host or the environment in which the bacteria are supposed to exert their probiotic effect. It is assumed that strains showing a dominant colonization of, e.g., the intestinal mucus of fish are good candidates to competitively exclude pathogens from the adhesion sites of the gut wall. Similarly, the presence of a dominant bacterial strain in high densities in culture water indicates its ability to grow successfully under the prevailing conditions, and one can expect that this strain will compete efficiently for nutrients with possibly deleterious strains. Identification of the isolates at this stage is not essential.
Screening and Preselection of Putative Probiotics
Generally at the end of the previous phase, one ends up with a pool of isolates that must be screened and preselected to obtain a restricted number of isolates for further examination. Several approaches followed in literature are discussed below. The application of in vitro tests to screen the acquired bacterial strains presupposes a well-known mode of action to select the appropriate in vitro test. Since the evidence about the possible modes of action of probiotics is still equivocal, preference should be given to in vivo tests in the search for probiotics. The use of the target organism in the screening procedure provides a stronger basis.
In vitro antagonism tests.
A common way to screen the candidate probiotics is to perform in vitro antagonism tests, in which pathogens are exposed to the candidate probiotics or their extracellular products in liquid (43, 45, 48) or solid (2, 4, 6, 24, 42, 48, 63, 71, 83, 86, 105, 126, 127, 143) medium. Depending on the exact arrangement of the tests, candidate probiotics can be selected based on the production of inhibitory compounds (43, 48, 59, 63, 86, 117) or siderophores, or on the competition for nutrients (24).
The results of in vitro antagonism tests should be interpreted with great caution. Olsson et al. (86) concluded that growth in marine broth or on marine agar produced fewer detectable inhibitory metabolites than did growth in tryptic soy broth. This is in agreement with the findings of Mayer-Harting et al. (71), who noted that the composition of the medium might affect the amount of bacteriocin produced or the amount released into the medium. In an attempt to overcome this problem, Jöborn et al. (59) performed in vitro antagonism tests between Carnobacterium strain K1 and pathogenic V. anguillarum and A. salmonicida in intestinal mucus and fecal extracts of rainbow trout. Furthermore, growth inhibition may not always be a consequence of the production of inhibitory substances like antibiotics, but inhibition caused by primary metabolites or changes in pH is also possible (6, 131).
The preselection of candidate probionts based on these in vitro antagonism tests has often led to the finding of effective probiotics (see, e.g., reference 42). It is unclear, however, whether the finding of a good probiotic and the criterion for the preselection are connected. In other words, it has not been shown that the isolates that did not inhibit the pathogens in the in vitro antagonism test were less effective in protecting the host against pathogenic effects in vivo. It has not been demonstrated unequivocally that there is a representative in vitro assay which can provide an extra criterion in the selection process. According to Ringø and Gatesoupe (103), neither a positive nor a negative in vitro test may predict the actual effect in vivo.
Colonization and adhesion.
It is stated above that a candidate probiotic should either be supplied on a regular basis or be able to colonize and persist in the host or in its ambient environment. The ability of a strain to colonize the gut or an external surface of the host and adhere to the mucus layer may be a good criterion for preselection among the putative probiotics. This involves the viability of the potential probiotic within the host and/or within its culture environment, adherence to host surfaces, and the ability to prevent the establishment of potentially pathogenic bacteria. Examination of adhesion properties using intestinal cells has become a standard procedure for selecting new probiotic strains for human application (113), but it is less common in aquaculture (59, 86).
Small-scale tests, with particular attention to monoxenic cultures.
(i) Small-scale tests (xenic).
Sea scallop (Placopecten magellanicus) larvae, 6 to 8 days old, were cultured in the presence of several bacterial isolates. After 4 days, the health of the larvae was measured by comparing their sizes, swimming patterns, and feeding behaviors. From this, bacteria were identified as neutral, potential pathogens, or possible probionts (Torrie and Neima, Abstr. Larvi '95). The rearing of marine fish and mollusk larvae on a small scale allows the screening of many candidate probiotics in the presence of the target organism. Small-scale in vivo antagonism tests were performed in plastic multiwell dishes during the yolk sack stage of turbot and halibut larvae, allowing representative and reproducible examination of putative probiotics (Ø. Bergh and L. Torkildsen, Abstr. Eighth Int. Symp. Microb. Ecol., p. 104, 1998).
(ii) Monoxenic cultures.
To determine the effects of a specific bacterial strain on a cultured organism, the elimination of other microbes from the culture system may be necessary to avoid microbial interactions (16). For example, several authors have reported that the nutritional value of the food of Artemia depends partly on the spontaneous colonization of the food particles by harmless bacteria (22, 25).
A first selection of candidate probiotics can be performed by culturing the fish species under monoxenic conditions, i.e., only in the presence of the putative probiotic as a bacterial species. This approach has already been used to study the effects of several bacterial strains on cultures of unicellular algae (29, 75, 128), the rotifer Brachionus plicatilis (82, 108), the brine shrimp Artemia (140), turbot larvae (75, 82), and larvae of the oyster Crassostrea gigas (27, 82). Generally these cultures are performed on a small scale, where all inputs (culture water, feed, etc.) are sterile and where the eggs, larvae, or unicellular algae are harvested under sterile conditions or disinfected before the experiment. The limiting factor in this approach, however, is the availability of large numbers of axenic organisms. Therefore, this approach has been used only with live food organisms (unicellular algae, rotifers, and Artemia) or larvae of mollusks or fishes.
Evaluation of Pathogenicity of Selected Strains
Before a culture can be used as a probiotic, it is necessary to confirm that no pathogenic effects can occur in the host. Therefore, the target species should be challenged with the candidate probiotic, under normal or stress conditions. This can be done by injection challenges, by bathing the host in a suspension of the candidate probiotic, or by adding the probiotic to the culture. Garriques and Arevalo (33) examined the pathogenicity of three bacterial isolates toward Litopenaeus vannamei by adding these to the nauplii cultures. Mortality was recorded for up to 4 days, and the animals were monitored for phototactic response. Austin et al. (5) injected 21-g Atlantic salmon intramuscularly or intraperitoneally with a suspension containing the candidate probiotic. The fish were monitored for 7 days, after which the survivors were sacrificed and examined for disease symptoms by examining the kidneys, spleen and muscles.
This phase in the search for probiotics can be combined with the previous one when small-scale in vivo tests are performed, but it should preferably be done under monoxenic conditions to eliminate every interaction with the already established microbiota. Other trophic levels involved in the culture of the target organism should also be taken into account in the research and development process. When probiotics are being developed to biologically control and/or increase live-food production, pathogenicity toward the predator larvae or juveniles should also be considered. To test this, Verschuere et al. (L. Verschuere, G. Rombaut, R. Robles, Y. Yaodong, P. Sorgeloos, and W. Verstraete, Abstr. Eighth Int. Symp. Microb. Ecol., p. 338, 1998) injected 125-day-old juveniles of Litopenaeus vannamei with a mixture of nine probiotic strains for Artemia juveniles. One day after injection, survival was measured and samples of hemolymph were plated on marine agar to see whether the defense systems of the shrimps were able to cope with the intrusion. Similarly, unicellular algae may be inhibited or even stimulated by bacterial isolates from, e.g., turbot larvae (75). When probiotics are selected for larval rearing by the green-water technique, their possible interaction with algae should be considered.
In Vivo Evaluation of Potential Probiotic Effects on the Host
The effect of candidate probiotics should be tested in vivo as well. When the probiotic effect is supposed to be nutritional, the candidate probiotics could be added to the culture of the aquatic species and their effect on growth and/or survival parameters could be assessed. However, when biological control of the microbiota is desired, representative in vivo challenge tests seem to be the appropriate tool to evaluate the potential effect of the candidate probiotic on the host.
Mode of application of the putative probiotic.
The putative probiotic can be added to the host or its ambient environment in several ways: (i) addition to the artificial diet, (ii) addition to the culture water (140), (iii) bathing (5, 117), and (iv) addition via live food (23, 46, 107).
Experimental infections—in vivo antagonism tests.
The next important step in the in vivo challenge test involves experimental infection with a representative pathogen. Pathogens or opportunistic pathogens can be administered via the diet (live food or artificial), through immersion, or via the culture water, similarly to the probiotics. In experiments performed by Gildberg et al. (43), Atlantic salmon fry was challenged with cohabitant fishes which were previously infected with Aeromonas salmonicida through intraperitoneal injection. The mortality of the challenged fishes due to acute furunculosis was monitored over time.
In vivo challenge tests to detect probiotic effects should be performed with great caution. The long-term effects should be studied to determine how the pathogen is evolving. It is important to observe whether the growth or the activity of the pathogen in the location where the antagonism of the probiotic is expected is really suppressed or if it is only delayed, maybe simply due to some competition for nutrients. This can be illustrated by the work of Gildberg and Mikkelsen (44). At twelve days after infection of Atlantic cod fry with a pathogenic V. anguillarum strain, reduced cumulative mortality was observed in fish given feed supplemented with Chaetoceros divergens. At 4 weeks after the infection, however, the same cumulative mortality as in the control treatment was reached. The authors argued that “this does not exclude the possibility though, that such methods may be of considerable importance under normal rearing conditions in the presence of moderate levels of opportunistic bacteria.” In contrast, Gibson et al. (42) observed a clear decrease in the level of the pathogen V. tubiashii in a culture of oysters when Aeromonas media was added, although the probiotic strain itself could no longer be detected in the culture after 4 days. This shows the necessity to record mortality or disease over a long period after experimental infection. Moreover, the pathogen should be quantified regularly in the culture.
Mass Production, Economic Evaluation, and Evaluation of Compliance with legislation
The approach outlined above should result in a set of strains with a well-established probiotic effect on the target organism without affecting other possibly involved trophic levels. Comparative pilot experiments under hatchery or growout conditions in the farms should be performed to estimate the economic consequences of the probiotic application. An important factor in the economic evaluation is the mass production of the probiont. Also, effective legislation, if any, should be taken into account before commercial application is begun. Finally, a cost-benefit analysis will determine whether the probiotics could be applied in practice or not.
Development of Monitoring Tools
Once it is decided to apply probiotics on a large scale, it is necessary to develop monitoring tools to control the production and application of the bacterial cultures. Production of large amounts of bacterial biomass requires appropriate quality control to avoid contamination by other bacteria. Attention should be paid to the genetic stability of the produced bacterial biomass, since spontaneous or induced mutations could affect the probiotic activity of the bacterial strain(s). Monitoring should also be performed following the administration of the probiotic cultures to the cultured aquatic species. Monitoring the microbiota in the culture system should become part of the routine practice in hatcheries. Molecular tools may be most appropriate for this kind of application.
CONCLUSIONS AND FURTHER DIRECTIONS
This review shows that experimental evidence is accumulating that the health and zootechnical performances of cultured aquatic species can be improved by the prophylactic use of probiotics. Progress has been made in the culture of live food, crustacea, mollusks, and fish. The use of probiotics as biological control agents should be considered to be a kind of risk insurance that may not provide any notable benefit when the culture is performing under optimal conditions and in the absence of (opportunistic) pathogens but that will be very helpful if infectious diseases break out.
The scarcity of data about their effective implementation in practice makes the practical relevance of probiotics in aquaculture hazy as yet. Furthermore, there is still a lack of knowledge about the exact modes of action involved in probiotic effects. Typically, a correlation is made between, for instance, the in vitro observations that a pathogen is inhibited by the production of an antimicrobial or bacteriostatic agent or by competition for nutrients (e.g., production of a siderophore) and the in vivo probiotic effect of the same bacterial strain. It can be argued that it is unlikely from an evolutionary point of view that bacteria spend a great deal of energy and resources if this effort is not beneficial to the organism in one way or another, but the evidence is still circumstantial and not conclusive. More in-depth studies of the competitive processes between bacteria must be carried out, and the ecological relevance of the different processes in situ remains to be elucidated. Also, the interaction between the cultured aquatic species and its associated microbiota deserves further research into possible immunostimulative effects.
Most of the literature references on the use of probiotics report on probiotics consisting of single bacterial strains (Table 1). It stands to reason that probiotics based on a single strain are less effective than mixed-culture probiotics when microbial control is desired. The approach should be systemic, i.e., based on a mixture of versatile strains capable of acting and interacting under a variety of conditions and able to maintain themselves in a dynamic way. It has been argued above that in aquaculture the microbial habitat undergoes continuous alterations, allowing constant changes in the structural composition and the functions of the microbial community. It is unlikely that a single bacterial species will be able to remain dominant in a continuously changing environment. The probability that a beneficial bacterium will dominate the associated microbiota is higher when several bacteria are administered then when only one probiotic strain is involved.
At present, for a sample or situation, it is not feasible to examine the extent to which all niches are filled in or, alternatively, how many opportunities are left for putative pathogens to grow and become a threat. Hence, one useful approach is to carefully monitor shifts in the overall microbial communities. Methods which rapidly provide insights into how a community of microbial species evolves are of utmost value. They may signal instability and potential evolution of unwanted microbial associations. Examples of such approaches are the monitoring of the Biolog pattern and the denaturing gradient gel electrophores (DGGE) pattern (41, 60, 77, 78, 137). These approaches, coupled with appropiate information technology, may permit the microbial ecology of aquaculture systems to be monitored in the near future.
Techniques to indicate probiotic strains, such as the use of green fluorescent protein or immunoassay procedure or 16S rDNA probes (70, 137), may also be useful in distinguishing an exogenous probiotc strain in a mixed microbial community, in order to better locate and quantify the probiotic strain. From a practical point of view, however, comparison of the spectrum of activity and the duration of the effect with those of antimicrobials, vaccines, and immunostimulants should also be made.
In order to become of practical significance, probiotics applicable in large-scale aquaculture will have to be produced and formulated under industrial conditions that conform to quality control guidelines. Much effort obviously still has to be invested in terms of the production of such multispecies inocula and the methods needed to preserve them and to validate their quality.
REFERENCES
- 1.Atlas R M, Bartha R. Microbial ecology—fundamentals and applications. Menlo Park, Calif: Benjamin/Cummings Science Publishing; 1997. [Google Scholar]
- 2.Austin B, Baudet E, Stobie M. Inhibition of bacterial fish pathogens by Tetraselmis suecica. J Fish Dis. 1992;15:55–61. [Google Scholar]
- 3.Austin B, Billaud A C. Inhibition of the fish pathogen, Serratia liquefaciens, by an antibiotic-producing isolate of Planococcus recovered from seawater. J Fish Dis. 1990;13:553–556. [Google Scholar]
- 4.Austin B, Day J G. Inhibition of prawn pathogenic Vibrio sp. by a commercial spray-dried preparation of Tetraselmis suecica. Aquaculture. 1990;90:389–392. [Google Scholar]
- 5.Austin B, Stuckey L F, Robertson P A W, Effendi I, Griffith D R W. A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonas salmonicida, Vibrio anguillarum and Vibrio ordalii. J Fish Dis. 1995;18:93–96. [Google Scholar]
- 6.Bergh Ø. Bacteria associated with early life stages of halibut, Hippoglossus hippoglossus L., inhibit growth of a pathogenic Vibrio sp. J Fish Dis. 1995;18:31–40. [Google Scholar]
- 7.Reference deleted.
- 8.Bernet M F, Brassart D, Neeser J R, Servin A L. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cells and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beveridge M C M, Begum M, Frerichs G N, Millar S. The ingestion of bacteria in suspension by the tilapia Oreochromis niloticus. Aquaculture. 1989;81:373–378. [Google Scholar]
- 10.Blomberg L, Henriksson A, Conway P L. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Appl Environ Microbiol. 1993;59:34–39. doi: 10.1128/aem.59.1.34-39.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Bossier P, Höfte M, Verstraete W. Ecological significance of siderophores in soil. Adv Microb Ecol. 1988;10:358–414. [Google Scholar]
- 13.Boyd C D, Hollerman W D, Plum J A, Saeed M. Effect of treatment with a commercial bacterial suspension on water quality of channel catfish pond. Prog Fish-Cult. 1984;46:36–40. [Google Scholar]
- 14.Browdy C. Recent developments in penaeid broodstock and seed production technologies: improving the outlook for superior captive stocks. Aquaculture. 1998;164:3–21. [Google Scholar]
- 15.Bruno M E C, Montville T J. Common mechanistic action of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1993;59:3003–3010. doi: 10.1128/aem.59.9.3003-3010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull A T, Slater J H. Microbial interactions and communities. New York, N.Y: Academic Press, Inc.; 1982. [Google Scholar]
- 17.Cahill M M. Bacterial flora of fishes: a review. Microb Ecol. 1990;19:21–41. doi: 10.1007/BF02015051. [DOI] [PubMed] [Google Scholar]
- 18.Carmignani G M, Bennett J P. Rapid start-up of a biological filter in a closed aquaculture system. Aquaculture. 1977;11:85–88. [Google Scholar]
- 19.Chan R C Y, Reid G, Irvin R T, Bruce A W, Costerton J. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985;47:84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiayuvareesajja S, Boyd C D. Effect of zeolite, formalin, bacterial augmentation and aeration on total ammonia nitrogen concentrations. Aquaculture. 1993;116:33–45. [Google Scholar]
- 21.Chauvière G, Coconnier M H, Kerneis S, Darfeuille-Michaud A, Joly B, Servin A L. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol Lett. 1992;91:213–218. doi: 10.1016/0378-1097(92)90700-x. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino A. The vital requirements of Artemia: physiology and nutrition. In: Persoone G, Sorgeloos P, Roels O, Jaspers E, editors. The brine shrimp Artemia—ecology, culture, use in aquaculture. Vol. 2. Wetteren, Belgium: Universa Press; 1980. pp. 55–82. [Google Scholar]
- 23.Dhert P, Lavens P, Duray M, Sorgeloos, P P. Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using omega-3-HUFA-enriched live food. Aquaculture. 1990;90:63–74. [Google Scholar]
- 24.Dopazo C P, Lemos M L, Lodeiros C, Bolinches J, Barja J L, Toranzo A E. Inibitory activity of antibiotic-producing marine bacteria against fish pathogens. J Appl Bacteriol. 1988;65:97–101. doi: 10.1111/j.1365-2672.1988.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 25.Douillet P. Effect of bacteria on the nutrition of the brine shrimp Artemia fed on dried diets. In: Sorgeloos P, Bengtson D A, Decleir W, Jaspers E, editors. Artemia research and its applications—ecology, culturing, use in aquaculture. Vol. 3. Wetteren, Belgium: Universa Press; 1987. pp. 295–308. [Google Scholar]
- 26.Douillet P, Langdon C J. Use of a probiotic for the culture of larvae of the Pacific oyster (Crassostrea gigas Thunberg) Aquaculture. 1994;119:25–40. [Google Scholar]
- 27.Douillet P, Langdon C J. Effects of marine bacteria on the culture of axenic oyster Crassostrea gigas (Thunberg) larvae. Biol Bull. 1993;184:36–51. doi: 10.2307/1542378. [DOI] [PubMed] [Google Scholar]
- 28.Fredrickson A G, Stephanopoulos G. Microbial competition. Science. 1981;213:972–979. doi: 10.1126/science.7268409. [DOI] [PubMed] [Google Scholar]
- 29.Fukami K, Nishijima T, Ishida Y. Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia. 1997;358:185–191. [Google Scholar]
- 30.Fuller R. A review: probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 31.Garcia T, Otto K, Kjelleberg S, Nelson D R. Growth of Vibrio anguillarum in salmon intestinal mucus. Appl Environ Microbiol. 1997;63:1034–1039. doi: 10.1128/aem.63.3.1034-1039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García de la Banda I, Chereguini O, Rasines I. Influencia de la adición de bacteria lácticas en el cultivo larvario del rodaballo (Scophthalmus maximus L.) Bol Inst Esp Oceanogr. 1992;8:247–254. [Google Scholar]
- 33.Garriques D, Arevalo G. An evaluation of the production and use of a live bacterial isolate to manipulate the microbial flora in the commercial production of Penaeus vannamei postlarvae in Ecuador. In: Browdy C L, Hopkins J S, editors. Swimming through troubled water. Proceedings of the Special Session on Shrimp Farming, Aquaculture '95. Baton Rouge, La: World Aquaculture Society; 1995. pp. 53–59. [Google Scholar]
- 34.Gatesoupe F J. The continuous feeding of turbot larvae, Scophthalmus maximus, and control of the bacterial environment of rotifers. Aquaculture. 1990;89:139–148. [Google Scholar]
- 35.Gatesoupe F J. Bacillus sp. spores as food additive for the rotifer Brachionus plicatilis: improvement of their bacterial environment and their dietary value for larval turbot, Scophthalmus maximus L. In: Kaushik S, editor. Fish nutrition in practice. Proceedings of the 4th International Symposium on Fish Nutrition and Feeding. Paris, France: Institut National de la Recherche Agronomique; 1991. pp. 561–568. [Google Scholar]
- 36.Gatesoupe F J. The effect of three strains of lactic bacteria on the production rate of rotifers, Brachionus plicatilis, and their dietary value for larval turbot, Scophthalmus maximus. Aquaculture. 1991;96:335–342. [Google Scholar]
- 37.Gatesoupe F J. Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic Vibrio. Aquat Living Resour. 1994;7:277–282. [Google Scholar]
- 38.Gatesoupe F J. Siderophore production and probiotic effect of Vibrio sp. associated with turbot larvae, Scophthalmus maximus. Aquat Living Resour. 1997;10:239–246. [Google Scholar]
- 39.Gatesoupe F J, Arakawa T, Watanabe T. The effect of bacterial additives on the production rate and dietary value of rotifers as food for Japanese flounder, Paralichthys olivaceus. Aquaculture. 1989;83:39–44. [Google Scholar]
- 40.Gatesoupe F J, Zambonino Infante J L, Cahu C, Quazuguel P. Early weaning of seabass larvae, Dicentrarchus labrax: the effect on microbiota, with particular attention to iron supply and exoenzymes. Aquaculture. 1997;158:117–127. [Google Scholar]
- 41.Gejman P V, Cao Q H, Guedj F, Sommer S. The sensitivity of denaturing gradient gel electrophoresis: a blinded analysis. Mutat Res Genomics. 1998;382:109–114. doi: 10.1016/s1383-5726(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 42.Gibson L, Woodworth J, George A. Probiotic activity of Aeromonas media on the Pacific oyster, Crassostrea gigas, when challenged with Vibrio tubiashii. Aquaculture. 1998;169:111–120. [Google Scholar]
- 43.Gildberg A, Johansen A, Bogwald J. Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquaculture. 1995;138:23–34. [Google Scholar]
- 44.Gildberg A, Mikkelsen H. Effects of supplementing the feed of Atlantic cod (Gadus morhua) fry with lactic acid bacteria and immunostimulating peptides during a challenge trial with Vibrio anguillarum. Aquaculture. 1998;167:103–113. [Google Scholar]
- 45.Gildberg A, Mikkelsen H, Sandaker E, Ringø E. Probiotic effect of lactic acid bacteria in the feed on growth and survival of fry of Atlantic cod (Gadus morhua) Hydrobiologia. 1997;352:279–285. [Google Scholar]
- 46.Gomez-Gil B, Herrera-Vega M A, Abreu-Grobois F A, Roque A. Bioencapsulation of two different Vibrio species in nauplii of the brine shrimp (Artemia franciscana) Appl Environ Microbiol. 1998;64:2318–22. doi: 10.1128/aem.64.6.2318-2322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Gil B, Roque A, Turnbull J F, Inglis V. Reduction in Artemia nauplii mortality with the use of a probiotic bacterium. In: Kane A S, Poynton S L, editors. Proceedings of the Third International Symposium on Aquatic Animal Health. Baltimore, Md: APC Press; 1998. p. 116. [Google Scholar]
- 48.Gram L, Melchiorsen J, Spanggaard B, Huber I, Nielsen T. Inhibition of Vibrio anguillarum by Pseudomonas fluorescens strain AH2—a possible probiotic treatment of fish. Appl Environ Microbiol. 1999;65:969–973. doi: 10.1128/aem.65.3.969-973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reference deleted.
- 50.Hagiwara A, Hamada K, Hori S, Hirayama K. Increased sexual reproduction in Brachionus plicatilis (Rotifera) with the addition of bacteria and rotifer extracts. J Exp Mar Biol Ecol. 1994;181:1–8. [Google Scholar]
- 51.Hansen G H, Olafsen J A. Bacterial colonization of cod (Gadus morhua L.) and halibut (Hippoglossus hippoglossus) eggs in marine aquaculture. Appl Environ Microbiol. 1989;55:1435–1446. doi: 10.1128/aem.55.6.1435-1446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen G H, Strøm E, Olafsen J A. Effect of different holding regimens on the intestinal microflora of herring (Clupea harengus) larvae. Appl Environ Microbiol. 1992;58:461–470. doi: 10.1128/aem.58.2.461-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris J M. The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microb Ecol. 1993;25:195–231. doi: 10.1007/BF00171889. [DOI] [PubMed] [Google Scholar]
- 54.Harzevili A R S, Van Duffel H, Dhert P, Swings J, Sorgeloos P. Use of a potential probiotic Lactococcus lactis AR21 strain for the enhancement of growth in the rotifer Brachionus plicatilis (Muller) Aquacult Res. 1998;29:411–417. [Google Scholar]
- 55.Hirayama K, Funamoto H. Supplementary effect of several nutrients on nutritive deficiency of baker's yeast for population growth of the rotifer Brachionus plicatilis. Bull Jpn Soc Sci Fish. 1983;49:505–510. [Google Scholar]
- 56.Holzapfel W H, Haberer P, Snel J, Schillinger U, Huis in't Veld J. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 57.Imada C, Maeda M, Taga N. Purification and characterization of the protease inhibitor “monastatin” from a marine Alteromonas sp. with reference to inhibition of the protease produced by a bacterium pathogenic to fish. Can J Microbiol. 1985;31:1089–1094. [Google Scholar]
- 58.Imada C, Simidu U, Taga N. Isolation and characterization of marine bacteria producing alkaline protease inhibitor. Bull Jpn Soc Sci Fish. 1985;51:799–803. [Google Scholar]
- 59.Jöborn A, Olsson J C, Westerdahl A, Conway P L, Kjelleberg S. Colonization in the fish intestinal tract and production of inhibitory substances in intestinal mucus and faecal extracts by Carnobacterium sp. strain Kl. J Fish Dis. 1997;20:383–392. [Google Scholar]
- 60.Kim J S, Sakai M, Hosoda A, Matsuguchi T. Application of DGGE analysis to the study of bacterial community structure in plant roots and in nonrhizosphere soil. Soil Sci Plant Nutr. 1999;45:493–497. [Google Scholar]
- 61.Krovacek K, Faris A, Ahne W, Mansson I. Adhesion of Aeromonas hydrophila and Vibrio anguillarum to fish cells and to mucus-coated glass slides. FEMS Microbiol Lett. 1987;42:85–89. [Google Scholar]
- 62.Lemos M L, Dopazo C P, Toranzo A E, Barja J L. Competitive dominance of antibiotic-producing marine bacteria in mixed cultures. J Appl Bacteriol. 1991;71:228–232. doi: 10.1111/j.1365-2672.1991.tb04452.x. [DOI] [PubMed] [Google Scholar]
- 63.Lemos M L, Toranzo A E, Barja J L. Antibiotic activity of epiphytic bacteria isolated from intertidal seaweeds. Microb Ecol. 1985;11:149–163. doi: 10.1007/BF02010487. [DOI] [PubMed] [Google Scholar]
- 64.Lewis W M, Morris D P. Toxicity of nitrite to fish: a review. Trans Am Fish Soc. 1986;115:183–195. [Google Scholar]
- 65.Lin C K. Progression of intensive marine shrimp culture in Thailand. In: Browdy C L, Hopkins J S, editors. Swimming through troubled water. Proceedings of the Special Session on Shrimp Farming, Aquaculture '95. Baton Rouge, La: World Aquaculture Society; 1995. pp. 13–23. [Google Scholar]
- 66.Lunestad B T. Notes of the Workshop on Aquaculture/Environment Interactions: impacts on microbial ecology. Halifax, Nova Scotia, Canada: Canadian Aquaculture Society; 1998. Impact of farmed fish on the environment, presentation by representative of Norway. [Google Scholar]
- 67.Maeda M. Biocontrol of the larvae rearing biotope in aquaculture. Bull Natl Res Inst Aquacult. 1994;1:71–74. [Google Scholar]
- 68.Maeda M, Liao I C. Effect of bacterial population on the growth of a prawn larva, Penaeus monodon. Bull Natl Res Inst Aquacult. 1992;21:25–29. [Google Scholar]
- 69.Maeda M, Liao I C. Microbial processes in aquaculture environment and their importance for increasing crustacean production. Jpn Int Res Cent Agricult Sci. 1994;28:283–288. [Google Scholar]
- 70.Martinez-Picado J, Blanch A R, Jofre J. Rapid detection and identification of Vibrio anguillarum by using a specific oligonucleotide probe complementary to 16S rRNA. Appl Environ Microbiol. 1994;60:732–737. doi: 10.1128/aem.60.2.732-737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayer-Harting A, Hedges A J, Berkeley R C W. Methods for studying bacteriocins. Methods Microbiol. 1972;7A:315–422. [Google Scholar]
- 72.Montes A J, Pugh D G. The use of probiotics in food-animal practice. Vet Med. 1993;88:282–288. [Google Scholar]
- 73.Moriarty D. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture. 1998;164:351–358. [Google Scholar]
- 74.Moriarty D J W, Body A G C. Proceedings of Fish Asia '95 Conference: 2nd Asian Aquaculture and Fisheries Exhibition and Conference. Singapore, Singapore: RAI Exhibitions; 1995. Modifying microbial ecology in ponds: the key to sustainable aquaculture; pp. 1–10. [Google Scholar]
- 75.Munro P D, Barbour A, Birkbeck T H. Comparison of the growth and survival of larval turbot in the absence of culturable bacteria with those in the presence of Vibrio anguillarum, Vibrio alginolyticus, or a marine Aeromonas sp. Appl Environ Microbiol. 1995;61:4425–4428. doi: 10.1128/aem.61.12.4425-4428.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muroga K, Higashi M, Keitoku H. The isolation of intestinal microflora of farmed red seabream (Pagrus major) and black seabream (Acanthopagrus schlegeli) at larval and juvenile stages. Aquaculture. 1987;65:79–88. [Google Scholar]
- 77.Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek Int J Gen Mol Microbiol. 1998;73:127–141. doi: 10.1023/a:1000669317571. [DOI] [PubMed] [Google Scholar]
- 78.Muyzer G. DGGE/TGGE a method for identifying genes from natural ecosystems. Curr Opin Microbiol. 1999;2:317–322. doi: 10.1016/S1369-5274(99)80055-1. [DOI] [PubMed] [Google Scholar]
- 79.Nair S, Tsukamoto K, Shimidu U. Distribution of bacteriolytic bacteria in the coastal marine environments of Japan. Bull Jpn Soc Sci Fish. 1985;51:1469–1473. [Google Scholar]
- 80.Neilands J B. Iron absorption and transport in microorganisms. Ann Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- 81.Nicolas J L, Robic E, Ansquer D. Bacterial flora associated with a trophic chain consisting of micro-algae, rotifers and turbot larvae: influence of bacteria on larval survival. Aquaculture. 1989;83:237–248. [Google Scholar]
- 82.Nicolas J L, Ansquer D, Besse B. Influence of bacterial flora on performances of larval rearings in marine aquaculture. In: Lésel R, editor. Microbiology in poecilotherms. Proceedings of the International Symposium on Microbiology in Poecilotherms. Paris, France: Elsevier Science Publishers B.V.; 1990. pp. 177–180. [Google Scholar]
- 83.Nogami K, Maeda M. Bacteria as biocontrol agents for rearing larvae of the crab Portunus trituberculatus. Can J Fish Aquacult Soc. 1992;49:2373–2376. [Google Scholar]
- 84.Olafsen J A. Proceedings from the US-EC Workshop on Marine Microorganisms: Research Issues for Biotechnology. Brussels, Belgium: European Commission; 1998. Interactions between hosts and bacteria in aquaculture; pp. 127–145. [Google Scholar]
- 85.Olsson J C, Jöborn A, Westerdahl A, Blomberg L, Kjelleberg S, Conway P L. Survival, persistence and proliferation of Vibrio anguillarum in juvenile turbot, Scophthalmus maximus (L.), intestine and faeces. J Fish Dis. 1998;21:1–9. doi: 10.1046/j.1365-2761.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- 86.Olsson J C, Westerdahl A, Conway P, Kjelleberg S. Intestinal colonization potential of turbot (Scophthalmus maximus)- and dab (Limanda limanda)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1992;58:551–556. doi: 10.1128/aem.58.2.551-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Onarheim A M, Raa J. Characteristics and possible biological significance of an autochthonous flora in the intestinal mucosa in sea-water fish. In: Lésel R, editor. Microbiology in poecilotherms. Proceedings of the International Symposium on Microbiology in Poecilotherms. Paris, France: Elsevier Science Publishers B.V.; 1990. pp. 197–201. [Google Scholar]
- 88.O'Sullivan D J, O'Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev. 1992;56:662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Benavente G, Gatesoupe F J. Bacteria associated with cultured rotifers and Artemia are detrimental to larval turbot, Scophthalmus maximus L. Aquacult Eng. 1988;118:289–293. [Google Scholar]
- 90.Perfettini J, Bianchi M. The comparison of two simple protocols designed to initiate and stimulate ammonia oxidation in closed aquaculture systems. Aquaculture. 1990;88:179–188. [Google Scholar]
- 91.Piard J C, Desmazeaud M. Inhibiting factors produced by lactic acid bacteria. 2. Bacteriocins and other antibacterial substances. Lait. 1992;72:113–142. [Google Scholar]
- 92.Planas M, Cunha I. Larviculture of marine fish: problems and perspectives. Aquaculture. 1999;177:171–190. [Google Scholar]
- 93.Prieur D. Experimental studies of trophic relationships between bacteria and bivalve molluscs. Kiel Meeresforsch Sonderh. 1981;5:376–383. [Google Scholar]
- 94.Pybus V, Loutit M W, Lamont I L, Tagg J R. Growth inhibition of the salmon pathogen Vibrio ordalii by a siderophore produced by Vibrio anguillarum strain VL4335. J Fish Dis. 1994;17:311–324. [Google Scholar]
- 95.Queiroz J, Boyd C. Effects of a bacterial inoculum in channel catfish ponds. J World Aquacult Soc. 1998;29:67–73. [Google Scholar]
- 96.Raa J. The use of immunostimulatory substances in fish and shellfish farming. Rev Fish Sc. 1996;4:229–288. [Google Scholar]
- 97.Rana K J. Review of the State of the World Aquaculture. FAO Fisheries circular no. 886. Rome, Italy: Food and Agriculture Organization of the United Nations; 1997. Status of global production and production trends; pp. 3–16. [Google Scholar]
- 98.Reid R T, Live D H, Faulkner D J, Butler A. A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature. 1993;366:455–458. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- 99.Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture. 1998;167:301–313. [Google Scholar]
- 100.Reference deleted.
- 101.Rico-Mora R, Voltolina D, Villaescusa-Celaya J A. Biological control of Vibrio alginolyticus in Skeletonema costatum (Bacillariophyceae) cultures. Aquacult Eng. 1998;19:1–6. [Google Scholar]
- 102.Ringø E, Birkbeck T H. Intestinal microflora of fish larvae and fry. Aquacult Res. 1999;30:73–93. [Google Scholar]
- 103.Ringø E, Gatesoupe F J. Lactic acid bacteria in fish: a review. Aquaculture. 1998;160:177–203. [Google Scholar]
- 104.Ringø E, Vadstein O. Colonization of Vibrio pelagius and Aeromonas caviae in early developing turbot (Scophthalmus maximus L.) larvae. J Appl Microbiol. 1998;84:227–233. doi: 10.1046/j.1365-2672.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- 105.Riquelme C, Araya R, Vergara N, Rojas A, Guaita M, Candia M. Potential probiotic strains in the culture of the Chilean scallop Argopecten purpuratus (Lamarck, 1819) Aquaculture. 1997;154:17–26. [Google Scholar]
- 106.Riquelme C, Hayashida G, Araya R, Uchida A, Satomi M, Ishida Y. Isolation of a native bacterial strain from the scallop Argopecten purpuratus with inhibitory effects against pathogenic Vibrios. J Shellfish Res. 1996;15:369–374. [Google Scholar]
- 107.Robles R, Sorgeloos P, Van Duffel H, Nelis H. Progress in biomedication using live foods. J Appl Ichthyol. 1998;14:207–212. [Google Scholar]
- 108.Rombaut G, Dhert P, Vandenberghe J, Verschuere L, Sorgeloos P, Verstraete W. Selection of bacteria enhancing the growth rate of axenically hatched rotifers (Brachionus plicatilis) Aquaculture. 1999;176:195–207. [Google Scholar]
- 109.Rørvik K A, Salte R, Bentsen H B, Thomassen M. Proceedings of the Fifth International Conference of the European Association of Fish Pathologists. Budapest, Hungary: European Association of Fish Pathologists; 1991. Effects of dietary iron and n-3 unsaturated fatty acids (omega-3) on health and immunological parameters in farmed salmon; p. 86. [Google Scholar]
- 110.Ruiz C M, Roman G, Sanchez J L. A marine bacterial strain effective in producing antagonisms to other bacteria. Aquacult Int. 1996;4:289–291. [Google Scholar]
- 111.Sakai M. Current research status of fish immunostimulants. Aquaculture. 1999;172:63–92. [Google Scholar]
- 112.Sakata T. Microflora in the digestive tract of fish and shellfish. In: Lésel R, editor. Microbiology in poecilotherms. Proceedings of the International Symposium on Microbiology in Poecilotherms. Paris, France: Elsevier Science Publishers B.V.; 1990. pp. 171–176. [Google Scholar]
- 113.Salminen S, Isolauri E, Salminen E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: succesful strains for future challenges. Antonie van Leeuwenhoek. 1996;70:347–358. doi: 10.1007/BF00395941. [DOI] [PubMed] [Google Scholar]
- 114.Scura E D. Dry seasons production problems on shrimp farms in Central America and the Caribbean Basin. In: Browdy C L, Hopkins J S, editors. Swimming through troubled water. Proceedings of the Special Session on Shrimp Farming, Aquaculture '95. Baton Rouge, La: World Aquaculture Society; 1995. pp. 200–213. [Google Scholar]
- 115.Skjermo J, Salvesen I, Oie G, Olsen Y, Vadstein O. Microbially matured water: a technique for selection of a non-opportunistic bacterial flora in water that may improve performance of marine larvae. Aquacult Int. 1997;5:13–28. [Google Scholar]
- 116.Skjermo J, Vadstein O. Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture. 1999;177:333–343. [Google Scholar]
- 117.Smith P, Davey S. Evidence for the competitive exclusion of Aeromonas salmonicida from fish with stress-inducible furunculosis by a fluorescent pseudomonad. J Fish Dis. 1993;16:521–524. [Google Scholar]
- 118.Söderhall K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 119.Stanier R Y, Doudoroff M, Adelberg E A. The microbial world. Englewood Cliffs, N.J: Prentice-Hall Inc.; 1963. [Google Scholar]
- 120.Stavric S, Gleeson T M, Blanchfield B, Pivnick P. Role of adhering microflora in competitive exclusion of Salmonella from young chicks. J Food Prot. 1987;50:928–932. doi: 10.4315/0362-028X-50.11.928. [DOI] [PubMed] [Google Scholar]
- 121.Stoffels G, Nes I F, Gudmundsdottir A. Isolation and properties of a bacteriocin-producing Carnobacterium piscicola isolated from fish. J Appl Bacteriol. 1992;73:309–326. doi: 10.1111/j.1365-2672.1992.tb04982.x. [DOI] [PubMed] [Google Scholar]
- 122.Strøm E, Olafsen J A. The indigenous microflora of wild-captured juvenile cod in net-pen rearing. In: Lésel R, editor. Microbiology in poecilotherms. Proceedings of the International Symposium on Microbiology in Poecilotherms. Paris, France: Elsevier Science Publishers B.V.; 1990. pp. 181–185. [Google Scholar]
- 123.Strøm E, Ringø E. Changes in the bacterial composition of early developing cod, Gadus morhua (L.) larvae following inoculation of Lactobacillus plantarum into the water. In: Walther B T, Fyhn H J, editors. Physiological and biochemical aspects of fish development. Bergen, Norway: University of Bergen; 1993. pp. 226–228. [Google Scholar]
- 124.Subasinghe R. Review of the State of the World Aquaculture. FAO Fisheries circular no. 886. Rome, Italy: Food and Agriculture Organization of the United Nations; 1997. Fish health and quarantine; pp. 45–49. [Google Scholar]
- 125.Reference deleted.
- 126.Sugita H, Matsuo N, Hirose Y, Iwato M, Deguchi Y. Vibrio sp. strain NM10, isolated from the intestine of a Japanese coastal fish, has an inhibitory effect against Pasteurella piscida. Appl Environ Microbiol. 1997;63:4986–4989. doi: 10.1128/aem.63.12.4986-4989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sugita H, Shibuya K, Shimooka H, Deguchi Y. Antibacterial activities of intestinal bacteria in freshwater cultured fish. Aquaculture. 1996;145:195–203. [Google Scholar]
- 128.Suminto K, Hirayama K. Application of a growth-promoting bacterium for stable mass culture of three marine microalgae. Hydrobiologia. 1997;358:223–230. [Google Scholar]
- 129.Tanasomwang V, Muroga K. Intestinal microflora of larval and juvenile stages in Japanese flounder (Paralichthys olivaceus) Fish Pathol. 1988;23:77–83. [Google Scholar]
- 130.Tanasomwang V, Nakai T, Nishimura Y, Muroga K. Vibrio-inhibiting marine bacteria isolated from black tiger shrimp hatchery. Fish Pathol. 1998;33:459–466. [Google Scholar]
- 131.Ten Brink B, Minekus M, Bol J, Huis in 't Veld J. Production of antibacterial compounds by lactobacilli. FEMS Microbiol Rev. 1987;46:64. [Google Scholar]
- 132.Reference deleted.
- 133.Tucker C S, Lloyd S W. Evaluation of a commercial bacterial ammendment for improving water quality in channel catfish ponds. Research report no. 10. Mississippi Agricultural and Forestry Experiment Station, Mississippi State University; 1985. pp. 1–4. [Google Scholar]
- 134.Tucker C S, Francis-Floyd R, Beleau M H. Nitrite-induced anemia in channel catfish, Ictularus punctatus Rafinesque. Bull Environ Contam Toxicol. 1989;43:295–310. doi: 10.1007/BF01701761. [DOI] [PubMed] [Google Scholar]
- 135.Vadstein O, Oie G, Olsen Y, Salvesen I, Skjermo J, Skjak-Braek G. A strategy to obtain microbial control during larval development of marine fish. In: Reinertsen H, Dahle L A, Jørgensen L, Tvinnereim K, editors. Proceedings of the First International Conference on Fish Farming Technology. Rotterdam, The Netherlands: Balkema; 1993. pp. 69–75. [Google Scholar]
- 136.Vandenbergh P. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev. 1993;12:221–238. [Google Scholar]
- 137.van Elsas J D, Duarte G F, Rosado A S, Smalla K. Microbiological and molecular biological methods for monitoring microbial inoculants and their effects in the soil environment. J Microbiol Methods. 1998;32:133–154. [Google Scholar]
- 138.Reference deleted.
- 139.Verschuere L, Dhont J, Sorgeloos P, Verstraete W. Monitoring Biolog patterns ans r/K-strategists in the intensive culture of Artemia juveniles. J Appl Microbiol. 1997;83:603–612. [Google Scholar]
- 140.Verschuere L, Rombaut G, Huys G, Dhont J, Sorgeloos P, Verstraete W. Microbial control of the culture of Artemia juveniles through pre-emptive colonization by selected bacterial strains. Appl Environ Microbiol. 1999;65:2527–2533. doi: 10.1128/aem.65.6.2527-2533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verschuere L, Heang H, Criel G, Dafnis S, Sorgeloos P, Verstraete W. Protection of Artemia against the pathogenic effects of Vibrio proteolyticus CW8T2 by selected bacterial strains. Appl Environ Microbiol. 2000;66:1139–1146. doi: 10.1128/aem.66.3.1139-1146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reference deleted.
- 143.Westerdahl A, Olsson C, Kjelleberg S, Conway P. Isolation and characterization of turbot (Scophthalmus maximus)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1991;57:2223–2228. doi: 10.1128/aem.57.8.2223-2228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reference deleted.
- 145.Williams S T, Vickers J C. The ecology of antibiotic production. Microb Ecol. 1986;12:43–52. doi: 10.1007/BF02153221. [DOI] [PubMed] [Google Scholar]
- 146.Witte W, Klare I, Werner G. Selective pressure by antibiotics as feed additives. Infection. 1999;27(Suppl. 2):35–38. doi: 10.1007/BF02561669. [DOI] [PubMed] [Google Scholar]
- 147.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 148.Yasuda K, Taga N. A mass culture method for Artemia salina using bacteria as food. Mer. 1980;18:53–62. [Google Scholar]