Abstract
Xenobiotic receptors, such as the pregnane X receptor, regulate multiple host physiologic pathways including xenobiotic metabolism, certain aspects of cellular metabolism, and innate immunity. These ligand-dependent nuclear factors regulate gene expression via genomic recognition of specific promoters and transcriptional activation of the gene. Natural or endogenous ligands are not commonly associated with this class of receptors; however, since these receptors are expressed in a cell-type specific manner in the liver and intestines, there has been significant recent effort to characterize microbially derived metabolites as ligands for these receptors. In general, these metabolites are thought to be weak micromolar affinity ligands. This journal anniversary minireview focuses on recent efforts to derive potentially nontoxic microbial metabolite chemical mimics that could one day be developed as drugs combating xenobiotic receptor–modifying pathophysiology. The review will include our perspective on the field and recommend certain directions for future research.
SIGNIFICANCE STATEMENT
Xenobiotic receptors (XRs) regulate host drug metabolism, cellular metabolism, and immunity. Their presence in host intestines allows them to function not only as xenosensors but also as a response to the complex metabolic environment present in the intestines. Specifically, this review focuses on describing microbial metabolite–XR interactions and the translation of these findings toward discovery of novel chemical mimics as potential drugs of the future for diseases such as inflammatory bowel disease.
Introduction
Xenobiotic receptors or, more appropriately, xenobiotic nuclear receptors [NRs; specifically, the arylhydrocarbon receptor (AHR), the farnesoid X receptor (FXR), the liver X receptor (LXR), the constitutive androstane receptor (CAR), the pregnane X receptor (PXR), the retinoid X receptor (RXR), and their functionally related receptors] are promiscuous ligand-binding proteins that function as sensors for the presence of foreign chemicals, either in their native or metabolized state, and regulate their metabolic clearance from the body (Xie and Evans, 2001; Wallace and Redinbo, 2013). These receptors function as ligand-regulated transcription factors resulting in the induction of gene promoter-specific transcription. Recent studies have demonstrated that these xenobiotic receptors can bind to microbially derived metabolites and that these receptors function beyond metabolism in regulating inflammation, immunity, and other aspects of host physiology (Nieves et al., 2022). The goal of this minireview is to update new findings of microbial metabolite–NR interactions with an emphasis on intestinal physiology, (Guan et al., 2021) and, with this knowledge, demonstrate recent advances in developing potent microbial metabolite mimics as potential drugs of the future (Nuzzo and Brown, 2020). It is essential to point out that the authors have previously published reviews on this topic (Dvořák, Klapholz et al., 2020; Dvořák, Sokol et al., 2020, Dvořák et al., 2021); however, here, we will only provide new updates to this field of research (Li et al., 2020).
Brief Historical Perspective
Gastrointestinal immune balance significantly depends on the function of NRs (Schmidt and Mangelsdorf, 2008; Klepsch et al., 2019). The major xenobiotic receptors are expressed abundantly in the intestinal epithelium and the microenvironment (including the immune cell compartment), AHR (epithelium and mucosal immune cells), FXR (epithelium), CAR (epithelium and immune cells), LXR (epithelium and immune cells), PXR (epithelium > immune cells), and RXR (epithelium and immune cells). Related receptors such as peroxisome proliferator-activated receptor (PPAR) will only be mentioned where relevant concerning microbial metabolite ligands (Modica et al., 2010).
Arylhydrocarbon Receptor
The mammalian AHR is a transcription factor belonging to the basic helix-loop-helix (bHLH)-PAS family of transcription factors. AHR activation is ligand-dependent. Upon binding with specific ligands, AHR translocates from the cytoplasm to the nucleus, being chaperoned by proteins such as heat shock protein 90, hepatitis B virus X-associated protein, and p23. In the nucleus, it dimerizes with AHR nuclear translocator. The latter is required for DNA binding and AHR transcriptional activity (Stockinger et al., 2021). Historically, AHR is an evolutionarily conserved environmental sensor and a critical pathway facilitating the toxic effects of environmental toxins. However, more recently, this viewpoint has expanded to include a two-faced receptor with perhaps beneficial functions seen in the context of immune cells and “nontoxic” natural ligands. Under homeostatic conditions, AHR involvement might be subtle, but its role becomes important during pathology (e.g., regeneration following tissue injury) (Stockinger et al., 2021). Microbial indoles are known ligands of AHR (Zelante et al., 2013; Pernomian et al., 2020; Scott et al., 2020; Dvořák et al., 2021; Gasaly et al., 2021; Wang et al., 2022).
Farnesoid X Receptor
The FXR was first identified by Forman et al. (1995). Bile acids (e.g., chenodeoxycholic acid) serve as their physiologic ligands (Gustafsson 1999; Kliewer et al., 1999). FXR is also a ligand-driven transcription factor that heterodimerizes with RXRα and drives gene/promoter-specific transcription. Bile acids, via FXR, induce fibroblast growth factor (FGF) 15/FGF19. The latter is a hormone secreted by the intestinal epithelial cells into the enterohepatic circulation. FGF15/FGF19 activates hepatic FGF receptor 4-β-klotho receptor complexes. In this manner, FXR signaling regulates gene expression involved in cholesterol, bile acid, and lipid metabolism, as well as that regulating cell proliferation. Agonists for FXR and analogs for FGF15/19 are currently being developed as drugs combating metabolic syndrome and cholestatic diseases (Katafuchi and Makishima, 2022; Panzitt et al., 2022). Bacterial metabolites, although not chemically characterized, serve as FXR ligands (Zhang et al., 2015). More recently, microbial bile acids have been shown to function as FXR ligands (Schoeler and Caesar, 2019; Guzior and Quinn, 2021; Xiang et al., 2021; Yan et al., 2021; Cai et al., 2022), and this pathway is relevant to humans with inflammatory bowel disease (Wilson et al., 2020). Microbial bile acids also regulate the levels of colonic retinoic acid receptor-related orphan receptor γ+ regulatory T (Treg) cells, which reduces intestinal inflammation in appropriate colitis models (Song et al., 2020). Other microbial metabolites serving as ligands for FXR include altenusin, a nonsteroidal fungal metabolite (Zheng et al., 2017).
Liver X Receptor
The LXR, assigned initially as an orphan receptor, was later deorphanized with the discovery of oxygenated cholesterol derivatives such as oxysterols (Janowski et al., 1996). This receptor is a master regulator of cholesterol metabolism (Peet et al., 1998). More recently, the receptor has had significant pharmacologic effects on various diseases, including obesity, diabetes, and autoimmunity, to name a few (Viennois et al., 2011). LXR regulates intestinal immunity, modulating receptor-related orphan receptor γt+ Treg and Th17 cells in the mesenteric lymph nodes through distinct mechanisms (Jakobsson et al., 2014; Parigi et al., 2021). Steroidal and triterpenoidal fungal metabolites are LXR ligands (Ondeyka et al., 2005). Hydroxy and oxo fatty acids, produced by microbes (e.g., lactobacillus) metabolizing unsaturated fatty acids, serve as efficient LXR ligands (Nanthirudjanar et al., 2015). Microbial metabolites of citrus pectin oligosaccharides are also LXR ligands and have been shown to exhibit anti-inflammatory properties (Hu et al., 2021).
Constitutive Androstane Receptor
The CAR is a constitutive transactivator and undergoes ligand-mediated translocation to the nucleus to exert full genomic effects. In addition, CAR has ligand-independent effects (e.g., via kinase signaling) that can also result in gene transcription (Mackowiak and Wang, 2016). It is expressed in the liver and small intestines (Qatanani and Moore, 2005). CAR is also a regulator of drug metabolism but has a more comprehensive role in modulating intestinal inflammation (Qatanani and Moore, 2005). Multiple microbial indoles can activate CAR (Venkatesh et al., 2014), but the significance of these interactions remains unknown.
Pregnane X Receptor
The PXR, a master regulator of xenobiotic metabolism (Kliewer, 2015), is now an established (agonist) target for the prevention of colitis and colitis-induced colon cancer (Cheng et al., 2012). This receptor forms a complex with its obligate heterodimeric partner, the RXR (NR2B1). The receptor complex binds response elements in the promoters and enhancers, usually in the form of direct (DR3, DR4) or averted (ER6) repeats of the consensus motif AG(G/T)TC(A/C) (Goodwin et al., 1999). Transcriptional induction follows the recruitment of the p160 family and other coactivators but with a complex interplay with other regulators, such as co-repressors. In terms of its canonical role in drug metabolism, PXR was discovered in 1998 (Bertilsson et al., 1998; Blumberg and Evans, 1998; Kliewer et al., 1998) and described as a master regulator of drug metabolism in that PXR can enhance the clearance of its own xenobiotic ligands via enzymatic (phases I and II) and transporter-mediated (phase III transporters) effects. In addition, PXR is an important mediator of drug–drug interactions when multiple drugs are coadministered (Goodwin et al., 1999; Wang et al., 2014; Shehu et al., 2016; Nicolussi et al., 2020, Wang et al., 2020; Hall et al., 2021). PXR also has a role in bile acid metabolism, acting on feedback and feed-forward mechanisms to alter bile acid production from cholesterol (Handschin and Meyer, 2005). PXR has a very promiscuous ligand binding pocket, allowing for various xenobiotic interactions (Orans et al., 2005). Specific interactions of interest include the effect of the antitubercular drug rifampicin on isoniazid-mediated hepatotoxicity via the accumulation of the endogenous hepatotoxin protoporphyrin IX in the liver (Li, Lu et al., 2013). PXR may prognosticate for survival in patients undergoing liver transplantation (Amer et al., 2019), perhaps via its effects on tacrolimus metabolism (Stifft et al., 2018). In addition, recent work has highlighted new roles for PXR as a factor promoting radio resistance in cancer (Niu et al., 2022) and drug sensitivity or resistance (Creusot et al., 2020; Sári et al., 2020; Matheux et al., 2021; Ozawa et al., 2021) and promotes striated and smooth muscle metabolism and physiology (Swales et al., 2012; Zhang et al., 2019; Ortiz-Flores et al., 2020). Our prior work demonstrated that gut microbe-produced indole metabolites of L-tryptophan (e.g., indole and indole propionic acid) are ligands of PXR (Venkatesh et al., 2014). To develop nontoxic yet “nature”-inspired agonists for PXR, we developed synthetic analogs mimicking the binding of indole pharmacophore on PXR. These PXR-selective leads, FKK5 and FKK6, are potent inhibitors of intestinal inflammation in organoids and mice (Dvořák, Kopp et al., 2020). We termed this concept microbial metabolite mimicry, which has been reviewed as a new approach to the drug discovery (Nuzzo and Brown, 2020). These compounds are, however, synthetic intermediates in the chemistry toward a dual indole structure (a requirement called for by the simultaneous binding of multiple indoles on PXR). It is important to note, however, that, to date, no crystal structure studies have been published with microbial metabolites or their mimics and PXR. This contrasts with the availability of structures demonstrating synergistic binding of select endocrine-disrupting molecules within the PXR ligand-binding domain (Delfosse et al., 2021). Other microbially derived metabolites of bile acid (lithocholic acid) and vitamin metabolism (vitamin K2) are PXR ligands and drive metabolic maturation of pluripotent stem cells and fetal hepatocytes (Avior et al., 2015).
The FKK5 and FKK6 compounds at the schedule and concentrations reported do not seem toxic to mice; however, these results are unpublished and would need confirmation in larger animals and other rodent models. To this end, it is known that systemic PXR activation can result in deleterious consequences such as hyperlipidemia and adverse changes in blood pressure and glucose metabolism (Rysä et al., 2013; Hassani-Nezhad-Gashti et al., 2020; Rahunen et al., 2022). In this context, PXR is expressed at the protein and mRNA level in microdissected human intestines (epithelial mucosa) and the liver (Miki et al., 2005; Godoy et al., 2013). Interestingly, even in total liver mRNA analysis, PXR was the most abundant compared with related receptors, AHR, and CAR (Liu, Lu et al., 2021). Others have reported cell-specific XR signatures in the intestines (Modica et al., 2010). On a functional level, it is clear that PXR is present in the liver as potent ligands such as hyperforin have resulted in drug interactions in humans (Nicolussi et al., 2020). PXR can also modulate the drug hepatotoxicity (Shehu et al., 2021). Thus, our goals are to formulate FKK5 and FKK6 for locoregional (colonic) delivery with limited systemic bioavailability.
Since the publication of our original paper on microbial metabolites and PXR (Venkatesh et al., 2014), several groups have independently replicated our findings concerning indole 3-propionic acid (IPA) and its effects on mouse intestinal barrier function (Dodd et al., 2017) and have found that IPA abrogates intestinal inflammation (Konopelski and Mogilnicka, 2022), IPA action partly depends on PXR (Pulakazhi Venu et al., 2019; Xiao et al., 2020; Du et al., 2021), PXR inversely regulates toll-like receptor (TLR) 4 (Esposito et al., 2016; Huang et al., 2018; Erickson et al., 2020; Lu et al., 2021; Su et al., 2022; Yuan et al., 2022), epithelial TLR4 regulates inflammation and tumors (Fukata et al., 2011; Sodhi et al., 2012; Burgueño et al., 2021), as well as the discovery of additional indole microbial metabolites (e.g., indole acetamide) with weak effects on PXR (Illés et al., 2020). Two reports from the same investigators challenge the notion that epithelial TLR4 is functional (Crame et al., 2021; Tam et al., 2022); however, further work is needed to clarify this discrepancy as these observations are likely to be context- and model-specific (Gribar et al., 2008; Takahashi et al., 2009; Soliman et al., 2010; Nanthakumar et al., 2011; Sodhi et al., 2012, Li et al., 2014; Belmonte et al., 2016; Dheer et al., 2016; Kim et al., 2016; Coleman and Haller, 2017; Inoue et al., 2017; Hu et al., 2018; Latorre et al., 2018; Price et al., 2018; Nighot et al., 2019; Stephens and von der Weid, 2020; Burgueño et al., 2021; Chen, Zhang et al., 2021; Kayisoglu et al., 2021; Qi-Xiang et al., 2022; Sodhi et al., 2022; Zhou et al., 2022). IPA is a weak human PXR (steroid and xenobiotic receptor ligand) (Venkatesh et al., 2014); in keeping with this, intercellular permeability is not altered in the human caco-2 cells (Scott et al., 2020). Another downstream effector of intestinal inflammation is dysregulated NF-κB signaling in both immune and epithelial cells. In this context, epithelial NF-κB signaling is thought to be essential for the maintenance of epithelial self-renewal and the maintenance of a proper balance between Paneth and goblet cells (Brischetto et al., 2021). In our context, however, PXR inhibits NF-κB signaling (Shah et al., 2007; Bautista-Olivier and Elizondo, 2022), creating an apparent contradiction. However, it is known that noncanonical epithelial NF-κB via the supplementation of RelA dimers to the canonical NF-κB modules exacerbates intestinal inflammation (McDaniel et al., 2016; Ke et al., 2019; Ramakrishnan et al., 2019; Chawla et al., 2021). Hence, it is conjectured that PXR may inhibit the noncanonical NF-κB or normalize epithelial NF-κB signaling pathways within the in vivo context of intestinal inflammation (Guma et al., 2011; Kaci et al., 2011). Indeed, since PXR activation also prevents colitis-induced cancer, PXR may be involved in inhibiting the IKK2/NF-κB signaling pathway in epithelial cells (Vlantis et al., 2011).
Microbial indole metabolite biomimicry has been successful for receptors beyond PXR (Dvořák, Klapholz et al., 2020, Dvořák, Sokol et al., 2020, Li et al., 2020). Field (49, 111) has discovered complementary host targets of IPA, which further asserts the premise that IPA inhibits inflammation. In this context, indole acts to mitigate cytotoxicity by Klebsiella spp. via suppression of toxin production, enhanced conversion of tilimycin (cytotoxin) to tilivalline (less potent cytotoxin) and activation of PXR (Ledala et al., 2022). Indeed, IPA may promote nerve regeneration by an unknown mechanism partly dependent on PXR (Serger et al., 2022). Additionally, PXR activation is expressed in platelets. It inhibits platelet-mediated thrombosis in mice expressing the human PXR transgene, perhaps via nongenomic inhibition of the Src family of kinases (Flora et al., 2019).
Retinoid X Receptor
The RXR integrates signaling via several NRs by heterodimerization. RXR dimers signal via binding to specific DNA sequences on gene promoters. RXR interacts with ligands that are specific to the receptor (e.g., 9-cis-13,14-dihydroretinoic acid), and these ligands are endogenous or exogenous xenobiotics (e.g., organotins). However, to date, microbial metabolite ligands that specifically bind RXR have not been described, although it is conceivable that low-affinity promiscuous ligands are present in the microbial metabolome with RXR binding properties (Brtko and Dvořák, 2020). However, a dietary microbial metabolite of chlorophyl A, phytol, and phytol’s, phytanic and pristanic acids are ligands of RXR and one heterodimeric partner, PPARα and PPARγ (Bobe et al., 2020). Other known microbially derived ligands to PPARγ include conjugated linoleic acid (Bassaganya-Riera et al., 2012) and indole metabolites (Venkatesh et al., 2014; Tsukidate et al., 2020). More recently, inosine, a gut microbial metabolite, has been described as a PPARγ signaling activator that attenuates colitis in mice (Li et al., 2021).
Endogenous Microbially Derived Ligands for XRs and Their Use as Potential Therapeutic Agents
Various microbially derived metabolites serve as ligands to xenobiotic NRs. Microbial metabolites with host protective properties (e.g., intestinal inflammation/obesity and short-chain fatty acids, indole metabolites), per se, have been proposed as sources for developing new drugs (Giddings and Newman, 2013; Saha et al., 2016; Stringlis et al., 2018; Descamps et al., 2019; Raihan et al., 2021; Ramírez-Rendon et al., 2022). The use of purified microbial metabolites as drugs has had mixed results. Considerable caution is advocated in this approach, especially with high or chronically administered doses of the microbial metabolite in question (Lee, Ecton et al., 2020; Konopelski et al., 2021; Liu, Sun et al., 2021; Sehgal et al., 2021; Chen et al., 2022; Paeslack et al., 2022; Teng et al., 2022; Zhang, Jiang et al., 2022). For example, several microbial metabolites have been proposed to partly function as antioxidants, such as microbial “green tea” metabolites (Pérez-Burillo et al., 2021). However, not all molecules with antioxidant function remain beneficial when used in large doses. Vitamin C (ascorbic acid) has been proposed as a potent anti-infective and antisepsis agent (Kuhn et al., 2018; Moskowitz et al., 2018; May et al., 2021). Yet, recent randomized clinical studies have questioned such benefits (Assouline et al., 2021; Sevransky et al., 2021) and demonstrated an even higher risk of death from intravenous ascorbic acid (Lamontagne et al., 2022). It is likely that indiscriminate applications of antioxidants may result in a similar fate and should be approached with caution (Ye et al., 2022). With that said, one must be cautious the other way in interpreting all indole metabolites, even uremic toxins, as uniformly injurious to health (Vanholder et al., 2022).
Key Recent Advances
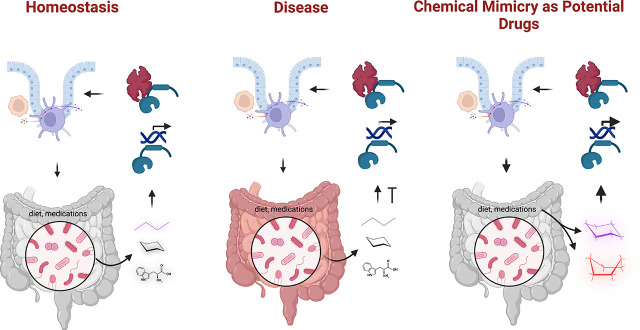
One alternative approach to the therapeutic use of endogenous microbial metabolites requires that microbial ligands bind xenobiotic receptors (XR). The metabolite should be amenable to easy chemical analog synthesis. The metabolite should serve as potent XR ligands and affect host physiology in the desired manner. These microbial metabolite mimics could be developed into new drugs. The requisite here is that the mimics must be nontoxic to the host. Thus, not all ligand–receptor pairs would be amenable to such an approach. We have termed this approach microbial metabolite mimicry (Dvořák, Sokol et al., 2020).
Arylhydrocarbon Receptor
Microbially derived ligands of AHR include short chain fatty acids (e.g., butyrate) are products of the gut microbiome (Marinelli et al., 2019). By contrast, however, butyrate was found only to induce AHR expression but not to serve as a direct ligand (Jin et al., 2017; Jourova et al., 2022; Modoux et al., 2022). The overall result in an intact host may be additive or synergistic; AHRs, while induced by short chain fatty acids, are activated by resident ligands that are also derived from the microbiota (Modoux et al., 2022). For example, indoles derived from the tryptophan metabolism are efficient AHR ligands (Rothhammer et al., 2016). The indole scaffold is a promising chemical moiety for drug mimicry (Dvořák, Klapholz et al., 2020). This scaffold is built on the observation that the microbial metabolite, indole, and its metabolites are endogenous AHR ligands (Hubbard et al., 2015; Lamas et al., 2016). Indeed, potent drug leads, PY109 and PY108, were developed for AHR using indole as the primary chemical scaffold (Chen et al., 2020). Other AHR mimics include the triarylmethanes (Goya-Jorge et al., 2020). More recently, it was shown that a chemically stable form of Thermosporothrix hazakensis metabolite 2-(1'H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester, 2-(1H-indole-3-carbonyl)-N-methyl thiazole-4-carboxamide is a nanomolar potency AHR activator (Grycová et al., 2022). Recently, a 2.85 Å cryo-EM crystal structure was submitted for binding the heat shock protein 90–hepatitis B virus X-associated protein–AHR complex, which provides the first structural visualization of the PAS B domain attached to indirubin (J. Gruszczyk et al., preprint, DOI: https://doi.org/10.1101/2022.05.17.491947). These developments should promote the discovery and characterization of microbial metabolites that serve as AHR ligands. Another recent advance, at least conceptually, calls for studying microbial metabolites combined at their physiologically relevant concentrations. Groestlinger et al. (2022) show that combining urolithin A, alternariol, and deoxynivalenol yields different effects on gut barrier function when each is used alone. Indeed, newer tryptophan microbial metabolites are being evaluated as AHR ligands and inducers; indole 3- carboxaldehyde is an AHR ligand (Vyhlídalová et al., 2020) with selective benefit in epithelial cell proliferation rather than intestinal barrier function in piglets (Zhang, Huang et al., 2022). This raises the possibility that different activators/inducers may augment receptor bias regarding which phenotypes might be most affected. In this context, in a mouse model of immune cancer checkpoint–induced colitis, indole 3- carboxaldehyde alleviated colitis without affecting the antitumor efficacy of the immune cancer checkpoint (Renga et al., 2022). Enteric formulated indole 3-carboxaldehyde has been characterized for therapeutic use in murine metabolic syndrome (Puccetti et al., 2021). However, microbial metabolites, specifically indoles, can also drive cancer growth via AHR (Hezaveh et al., 2022). Ellagitannin-containing foods are partially converted to urolithin A, an AHR ligand (Shen et al., 2021). Urolithin A analog, UAS03, is efficient in reducing colonic inflammation and enhancing barrier function in vivo (Singh et al., 2019). It is important to note that not all microbial metabolite ligands of AHR will result in a typical phenotype (indole), and indole 3-carboxaldehyde (AHR ligand) protects against E.coli-induced endometritis in dysbiotic mice. Still, indole propionic acid does not (Zhao, Bao et al., 2022). Yet it is abundantly clear that in the intestines, IPA protects from inflammation (Konopelski and Mogilnicka, 2022). Thus, context is a crucial issue when evaluating metabolites as XR ligands.
Farnesoid X Receptor
More recently, intestinal commensal fungi, specifically Penicillium oxalicum SL2 produce metabolites that are ligands of FXR [e.g., (6R)3,7-dimethyl-6,7-dihydroxy-2(Z)-octanoic acid] (Zhao, Luan et al., 2022). Novel amino acid conjugations of host bile acids producing phenylalanocholic acid, tyrosocholic acid, and leucocholic acid have been described as novel FXR agonists. Still, they are enriched in patients with inflammatory bowel disease (Quinn et al., 2020). Newer computational models for ligand docking and binding have been published that could aid in biomimicry and synthesis of new microbial mimics (Jiang et al., 2021). Isoform- or tissue-specific ligands might tease out beneficial functions from nonbeneficial ones (van Zutphen et al., 2019; Ramos Pittol et al., 2020). Microbial bile acids may target receptors beyond FXR and PXR, as a recent study shows that NR4A1 (NUR77) is targeted by isoallo-lithocholic acid, which enhances Treg function (Li, Hang et al., 2021).
Constitutive Androstane Receptor
New observations for the CAR in the intestines show that CAR acts within T cells in the small intestine to detoxify bile acids and diminish inflammation (Chen, Huang et al., 2021). However, to date, no microbial metabolites have been found as ligands to CAR. For the other receptors, LXR, PXR, PPAR, and RXR, there need to be further discovery efforts on biomimicry.
Current Challenges and Knowledge Gaps
The current challenge is to address the true impact of microbial metabolites on NR targets. First, we need to look at all 48 human receptors. Context-specific approaches are required to validate receptor-ligand relationships. In general, the microbial metabolites would be weak ligands of a given receptor; however, chemical mimics may be designed to improve potency based on their interactions with the receptor. Modest potency mimics may suffice in conditions where the chemical mimic may induce receptor abundance or be combined with microbial metabolites that function as such. In this current challenge, a better understanding of NR crosstalk would help to elucidate microbial metabolite effects on overall phenotype. For example, it is now established that there is a fine cross-sharing of promoter occupancy by multiple NR(s) such that the absence of one or more receptors could be partially compensated for by the presence of other receptors (Pascussi et al., 2004; Kumar et al., 2010; Zhai et al., 2010; Oladimeji et al., 2016; Pavek 2016; Bwayi et al., 2022). Suppose potency differences exist in activating the different NRs by a given microbial metabolite(s). In that case, the receptor interplay could have profound effects on the final phenotype of the host.
Second, we need to characterize crystal structures of microbial metabolites bound to xenobiotic receptors. This is not an easy task as many of the metabolites have significant solubility issues. More innovative approaches toward crystal structure determination would help move the field forward (Delfosse et al., 2021). It is also possible that weak ligands will not be amenable to structural resolution when bound to receptors.
Third, we need to define a system’s view of how different microbial metabolites interact with a given xenobiotic receptor, for example, by performing synergy or antagonist studies of a combinatorial effect of different indole metabolites on a given xenobiotic receptor to determine what may result in synergy, additivity, or antagonism.
Fourth, we must characterize concentration-dependent effects of a microbial metabolite on different proteins/enzymes and receptor systems and present a hierarchical receptor structure that informs the scientific community of how the metabolite might function in vivo. For example, microbial metabolites also bind G protein-coupled receptors and interact with histone-modifying enzymes (e.g., short-chain fatty acids). In a system’s view, this knowledge should ideally be combined with other effects the metabolite may have on other receptor systems (e.g., AHR). There is also a paucity of knowledge regarding the alternatively spliced isoforms of each NR. For example, PXR has at least three isoforms with variable expression across tissues and at least one isoform with dominant negative function (Tompkins et al., 2008; Lin et al., 2009; Breuker et al., 2014; Brewer and Chen, 2016). Thus, knowing which isoforms are targeted by the metabolite and in which tissues these interactions dominate would be a critical knowledge gap to cover.
Fifth, we need to assess whether specific diets or medications could alter metabolites significantly and how that affects receptor physiology and phenotypes in an intact host. This is a very challenging aspect of discovery. It is now established that in the human diet, for example, fiber or fat consumption markedly changes the microbial structure and the metabolome in the feces and gut mucosa (Claesson et al., 2012; Wan et al., 2019; Dahl et al., 2020; Tanes et al., 2021). The use of medications significantly modulates the fecal microbiome and its metabolome (Weersma et al., 2020; Kumari et al., 2022). Additionally, variability introduced by ethnicity and geography also impacts a healthy gut microbiome (Shanahan et al., 2021). Carefully controlled human studies are required from which fecal metabolomes are tested against various XRs. These should also be modeled in appropriate animal models of the disease where possible.
Sixth, we should assess for xenobiotic receptor selectivity and categorize single versus dual versus multireceptor engaging mimics, noting which receptors, when engaged, are prone to be off-target and contribute to toxicity of the compound.
Seventh, we must define ligands/mimics with other binding modes, especially orthosteric versus allosteric antagonism of receptors (Biswas et al., 2009; Chai et al., 2020).
Finally, we need to understand how microbial metabolite mimics may be developed in the context of the known fact that since these XRs are expressed in both the intestines and liver, there is a potential for unpredictable drug–drug or metabolic adverse interactions. For example, a potent PXR ligand could induce high blood cholesterol (Meng et al., 2019; Sui et al., 2021) or even drug interactions with coadministered drugs by the induction of cytochrome [CYP enzymes and phase III transporters (e.g., MDR1)]. One strategy might be to consider compounds with colonic delivery to bypass systemic delivery via absorption in the small intestines. This could also bypass drug–drug effects that in the intestines might most likely occur during small intestinal CYP and transporter induction. However, this aspect requires proof-of-concept and validation before they are adopted within the therapeutic platform.
Perspective on Future Directions
Discovery of Natural Microbial Metabolite Agonists of XRs
With the advent of pulldown approaches and insights into deconvolution of metabolites by mass spectrometry (Kim et al., 2011), the Krause laboratory has had significant success in delineating novel ligands to XRs such as PPARα (Liu et al., 2022). A similar effort is needed to capture fecal and tissue homogenates from rodents and humans, especially using a solid phase microextraction (Huang et al., 2019; Sajid et al., 2019; Kataoka, 2021). Mining the literature for new microbially produced ligands (e.g., N-methylserotonin) (Han et al., 2022), could also be screened for ligand activation across multiple xenobiotic receptors. Careful and context-driven applications of these metabolites should be fully assessed. In addition, defining all the modifications of the metabolite in the host would be relevant to how host metabolism (e.g., via CYP450s) alters how the metabolite interacts with a particular NR. For example, baicalein efficiently interacts with PXR, but its glucuronide metabolite is a weak interactor (Dou et al., 2012). Similarly, microbial metabolites in a host could be heavily modified by host metabolism resulting in mixtures of the parent metabolite and its metabolism by-products. In addition, microbial metabolites could interact with their cognate receptors (e.g., mouse PXR ligand pregnenolone carbonitrile does not activate human PXR, and similarly, rifampicin, a human PXR ligand does not activate mouse PXR). So species-specific interaction rodent models should be employed to study the in vivo aspects of metabolite–XR interactions and phenotypes.
Discovery of Natural Microbial Metabolite Antagonists of XRs
To date, very few microbial metabolite antagonists of XRs have been described. One study shows that mice treated with tempol have reduced Lactobacillus and bile salt hydrolase in the gut, and this leads to an increase in Tauro-β-muricholic acid, which is an FXR antagonist (Li, Jiang et al., 2013). Indeed, several other bile acid conjugates, Tauro-conjugated beta, and alpha-muricholic acids have also been identified as FXR antagonists (Sayin et al., 2013; Degirolamo et al., 2014; Gonzalez et al., 2016). Other examples include combinatorial concentrations of indoles that, in some combinations, act to antagonize the AHR (Jin et al., 2014). Emphasis must be placed on finding novel natural microbial metabolites as antagonists for XRs. As previously mentioned, mining the literature for novel microbially produced ligands with well-defined host phenotypes (e.g., imidazole propionate) (Koh et al., 2018) can guide the assessment of ligand-XR interactions.
Discovery of Physiologically Relevant Interactions of Metabolites on Receptor Systems
A more comprehensive review of the different receptor systems that are engaged by microbial metabolites has been published (Zheng et al., 2022). Here we call for a detailed analysis of receptor dynamics using combinatorial concentrations of microbial metabolites that may be present in a context-dependent manner in a host. There could be significant concentration dependencies of different metabolites on different receptors, and knowledge of how this could be integrated would significantly advance our understanding of host–microbiome relationships.
Systems Study of Multiple Metabolite–XR Interactions on Host Physiology or Pathophysiology
In an intact host, multiple microbial metabolites are contextually present and can drive phenotypes. For example, tryptophan loading increases indole metabolites of tryptophan (Wikoff et al., 2009), and in this manner, diet could influence the metabolite repertoire considerably. In the context of host pathophysiology, during intestinal inflammation, indole metabolites decrease (Alexeev et al., 2018), and depending on the diet, these levels could replete or vary considerably (Li, Illés et al., 2021). The net outcome could vary on several levels, one of which is dietary constituents. In the future, more controlled systematic studies of metabolite XR interactions are needed as they relate to specific host physiologies or pathophysiological conditions.
Conclusions
We are honored to contribute a minireview to the Xenobiotic Receptors special section in honor of Drug Metabolism and Disposition’s 50th anniversary. The 50th anniversary celebrates Professor Wen Xie’s contributions to the field and congratulates him on the well-deserved honor of being the recipient of the Richard Akita Award in DMD. Following Professor Xie’s landmark paper in Nature describing the first humanized steroid and xenobiotic receptor mice in 2000 (Xie et al., 2000), the entire orphan NR field, and acknowledging the seminal contribution of other investigators and receptor systems, has exploded with discoveries and novel pathways regulated by these receptors. We anxiously await new treatment paradigms built on a new fund of knowledge of how these xenobiotic receptors contextually contribute to disease.
Abbreviations
- AHR
arylhydrocarbon receptor
- CAR
constitutive androstane receptor
- CYP
cytochrome
- FGF
fibroblast growth factor
- FXR
farnesoid X receptor
- IPA
indole 3-propionic acid
- LXR
liver X receptor
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NR
nuclear receptor
- PPAR
peroxisome proliferator-activated receptors
- PXR
pregnane X receptor
- RXR
retinoid X receptor
- TLR
toll-like receptor
- XR
xenobiotic receptor
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Mani, Li, Dvořák.
Footnotes
Some of the work referenced in this article was funded by Czech Science Foundation [Grant 22-00355S] (to Z.D.) and National Institutes of Health [Grant R01 CA 222469-01] (to S.M.).
A patent has been submitted by the Albert Einstein College of Medicine (S.M.) in conjunction with Palacký University (Z.D.) and the Drexel University College of Medicine to the US Patent and Trademark Office about microbial metabolite mimicry and indoles and gut barrier function. The authors have no other conflicts of interest to report.
References
- Alexeev EELanis JMKao DJCampbell ELKelly CJBattista KDGerich MEJenkins BRWalk STKominsky DJ, et al. (2018) Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol 188:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A, McColl K, Bouayyad S, Kanwar A, Sen G, French JJ, Wilson CH, Manas DM, Wright MC, White SA (2019) The association of pregnane X receptor activation with outcomes after liver transplantation-A retrospective study. Clin Transplant 33:e13734. [DOI] [PubMed] [Google Scholar]
- Assouline BFaivre AVerissimo TSangla FBerchtold LGiraud RBendjelid KSgardello SElia NPugin J, et al. (2021) Thiamine, ascorbic acid, and hydrocortisone as a metabolic resuscitation cocktail in sepsis: a meta-analysis of randomized controlled trials with trial sequential analysis. Crit Care Med 49:2112–2120. [DOI] [PubMed] [Google Scholar]
- Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, Sadeh R, Moussaieff A, Cohen M, Itskovitz-Eldor J, Nahmias Y (2015) Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology 62:265–278. [DOI] [PubMed] [Google Scholar]
- Bassaganya-Riera JViladomiu MPedragosa MDe Simone CCarbo AShaykhutdinov RJobin CArthur JCCorl BAVogel H, et al. (2012) Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS One 7:e31238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Olivier CD, Elizondo G (2022) PXR as the tipping point between innate immune response, microbial infections, and drug metabolism. Biochem Pharmacol 202:115147. [DOI] [PubMed] [Google Scholar]
- Belmonte LAchamrah NNobis SGuérin CRiou GBôle-Feysot CBoyer ORichard VRego JCDéchelotte P, et al. (2016) A role for intestinal TLR4-driven inflammatory response during activity-based anorexia. Sci Rep 6:35813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A (1998) Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA 95:12208–12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A, Mani S, Redinbo MR, Krasowski MD, Li H, Ekins S (2009) Elucidating the ‘Jekyll and Hyde’ nature of PXR: the case for discovering antagonists or allosteric antagonists. Pharm Res 26:1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Evans RM (1998) Orphan nuclear receptors--new ligands and new possibilities. Genes Dev 12:3149–3155. [DOI] [PubMed] [Google Scholar]
- Bobe G, Zhang Z, Kopp R, Garzotto M, Shannon J, Takata Y (2020) Phytol and its metabolites phytanic and pristanic acids for risk of cancer: current evidence and future directions. Eur J Cancer Prev 29:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuker CPlanque CRajabi FNault JCCouchy GZucman-Rossi JEvrard AKantar JChevet EBioulac-Sage P, et al. (2014) Characterization of a novel PXR isoform with potential dominant-negative properties. J Hepatol 61:609–616. [DOI] [PubMed] [Google Scholar]
- Brewer CT, Chen T (2016) PXR variants: the impact on drug metabolism and therapeutic responses. Acta Pharm Sin B 6:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischetto C, Krieger K, Klotz C, Krahn I, Kunz S, Kolesnichenko M, Mucka P, Heuberger J, Scheidereit C, Schmidt-Ullrich R (2021) NF-κB determines Paneth versus goblet cell fate decision in the small intestine. Development 148:dev199683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brtko J, Dvořák Z (2020) Natural and synthetic retinoid X receptor ligands and their role in selected nuclear receptor action. Biochimie 179:157–168. [DOI] [PubMed] [Google Scholar]
- Burgueño JFFritsch JGonzález EELandau KSSantander AMFernández IHazime HDavies JMSantaolalla RPhillips MC, et al. (2021) Epithelial TLR4 signaling activates DUOX2 to induce microbiota-driven tumorigenesis. Gastroenterology 160:797–808.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwayi MNGarcia-Maldonado EChai SCXie BChodankar SHuber ADWu JAnnu KWright WCLee HM, et al. (2022) Molecular basis of crosstalk in nuclear receptors: heterodimerization between PXR and CAR and the implication in gene regulation. Nucleic Acids Res 50:3254–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Sun L, Gonzalez FJ (2022) Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SC, Wright WC, Chen T (2020) Strategies for developing pregnane X receptor antagonists: Implications from metabolism to cancer. Med Res Rev 40:1061–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MMukherjee TDeka AChatterjee BSarkar UASingh AKKedia SLum JDhillon MKBanoth B, et al. (2021) An epithelial Nfkb2 pathway exacerbates intestinal inflammation by supplementing latent RelA dimers to the canonical NF-κB module. Proc Natl Acad Sci USA 118:e2024828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JHaller CAJernigan FEKoerner SKWong DJWang YCheong JEKosaraju RKwan JPark DD, et al. (2020) Modulation of lymphocyte-mediated tissue repair by rational design of heterocyclic aryl hydrocarbon receptor agonists. Sci Adv 6:eaay8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MLHuang XWang HHegner CLiu YShang JEliason ADiao HPark HFrey B, et al. (2021) CAR directs T cell adaptation to bile acids in the small intestine. Nature 593:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SZhang YNiu XMohyuddin SGWen JBao MYu TWu LHu CYong Y, et al. (2021) Coral-derived endophytic fungal product, butyrolactone-I, alleviates Lps induced intestinal epithelial cell inflammatory response through TLR4/NF-κB and MAPK signaling pathways: an in vitro and in vivo studies. Front Nutr 8:748118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Chen CC, Liao HY, Wu YW, Liou JM, Wu MS, Kuo CH, Lin CH (2022) Alteration of gut microbial metabolites in the systemic circulation of patients with parkinson’s disease. J Parkinsons Dis 12:1219–1230. [DOI] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Gonzalez FJ (2012) Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci 33:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJJeffery IBConde SPower SEO’Connor EMCusack SHarris HMCoakley MLakshminarayanan BO’Sullivan O, et al. (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488:178–184. [DOI] [PubMed] [Google Scholar]
- Coleman OI, Haller D (2017) Bacterial signaling at the intestinal epithelial interface in inflammation and cancer. Front Immunol 8:1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crame EE, Bowen JM, Secombe KR, Coller JK, François M, Leifert W, Wardill HR (2021) Epithelial-specific TLR4 knockout challenges current evidence of tlr4 homeostatic control of gut permeability. Inflamm Intest Dis 6:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creusot NGassiot MAlaterre EChiavarina BGrimaldi MBoulahtouf AToporova LGerbal-Chaloin SDaujat-Chavanieu MMatheux A, et al. (2020) The anti-cancer drug dabrafenib is a potent activator of the human pregnane X receptor. Cells 9:1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl WJ, Rivero Mendoza D, Lambert JM (2020) Diet, nutrients and the microbiome. Prog Mol Biol Transl Sci 171:237–263. [DOI] [PubMed] [Google Scholar]
- Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A (2014) Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 7:12–18. [DOI] [PubMed] [Google Scholar]
- Delfosse VHuet THarrus DGranell MBourguet MGardia-Parège CChiavarina BGrimaldi MLe Mével SBlanc P, et al. (2021) Mechanistic insights into the synergistic activation of the RXR-PXR heterodimer by endocrine disruptor mixtures. Proc Natl Acad Sci USA 118:e2020551118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps HC, Herrmann B, Wiredu D, Thaiss CA (2019) The path toward using microbial metabolites as therapies. EBioMedicine 44:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheer R, Santaolalla R, Davies JM, Lang JK, Phillips MC, Pastorini C, Vazquez-Pertejo MT, Abreu MT (2016) Intestinal epithelial Toll-like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infect Immun 84:798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd DSpitzer MHVan Treuren WMerrill BDHryckowian AJHigginbottom SKLe ACowan TMNolan GPFischbach MA, et al. (2017) A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou WMukherjee SLi HVenkatesh MWang HKortagere SPeleg AChilimuri SSWang ZTFeng Y, et al. (2012) Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One 7:e36075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Qi R, Wang J, Liu Z, Wu Z (2021) Indole-3-propionic acid, a functional metabolite of Clostridium sporogenes, promotes muscle tissue development and reduces muscle cell inflammation. Int J Mol Sci 22:12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák ZKlapholz MBurris TPWilling BPGioiello APellicciari RGalli FMarch JO’Keefe SJSartor RB, et al. (2020) Weak microbial metabolites: a treasure trove for using biomimicry to discover and optimize drugs. Mol Pharmacol 98:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák ZKopp FCostello CMKemp JSLi HVrzalová AŠtěpánková MBartoňková IJiskrová EPoulíková K, et al. (2020) Targeting the pregnane X receptor using microbial metabolite mimicry. EMBO Mol Med 12:e11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák Z, Poulíková K, Mani S (2021) Indole scaffolds as a promising class of the aryl hydrocarbon receptor ligands. Eur J Med Chem 215:113231. [DOI] [PubMed] [Google Scholar]
- Dvořák Z, Sokol H, Mani S (2020) Drug mimicry: promiscuous receptors PXR and AhR, and microbial metabolite interactions in the intestine. Trends Pharmacol Sci 41:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, Alston L, Nieves K, Chang TKH, Mani S, Flannigan KL, Hirota SA (2020) The xenobiotic sensing pregnane X receptor regulates tissue damage and inflammation triggered by C difficile toxins. FASEB J 34:2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito GNobile NGigli SSeguella LPesce Md’Alessandro ABruzzese ECapoccia ESteardo LCuomo R, et al. (2016) Rifaximin improves clostridium difficile toxin A-induced toxicity in Caco-2 cells by the PXR-dependent TLR4/MyD88/NF-κB pathway. Front Pharmacol 7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora GD, Sahli KA, Sasikumar P, Holbrook L-M, Stainer AR, AlOuda SK, Crescente M, Sage T, Unsworth AJ, Gibbins JM (2019) Non-genomic effects of the Pregnane X Receptor negatively regulate platelet functions, thrombosis and haemostasis. Sci Rep 9:17210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BMGoode EChen JOro AEBradley DJPerlmann TNoonan DJBurka LTMcMorris TLamph WW, et al. (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693. [DOI] [PubMed] [Google Scholar]
- Fukata MShang LSantaolalla RSotolongo JPastorini CEspaña CUngaro RHarpaz NCooper HSElson G, et al. (2011) Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis 17:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasaly N, de Vos P, Hermoso MA (2021) Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol 12:658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings LA, Newman DJ (2013) Microbial natural products: molecular blueprints for antitumor drugs. J Ind Microbiol Biotechnol 40:1181–1210. [DOI] [PubMed] [Google Scholar]
- Godoy PHewitt NJAlbrecht UAndersen MEAnsari NBhattacharya SBode JGBolleyn JBorner CBöttger J, et al. (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87:1315–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ, Jiang C, Patterson AD (2016) An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151:845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C (1999) The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 56:1329–1339. [DOI] [PubMed] [Google Scholar]
- Goya-Jorge E, Rampal C, Loones N, Barigye SJ, Carpio LE, Gozalbes R, Ferroud C, Sylla-Iyarreta Veitía M, Giner RM (2020) Targeting the aryl hydrocarbon receptor with a novel set of triarylmethanes. Eur J Med Chem 207:112777. [DOI] [PubMed] [Google Scholar]
- Gribar SC, Anand RJ, Sodhi CP, Hackam DJ (2008) The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol 83:493–498. [DOI] [PubMed] [Google Scholar]
- Groestlinger J, Seidl C, Varga E, Del Favero G, Marko D (2022) Combinatory exposure to urolithin A, alternariol, and deoxynivalenol affects colon cancer metabolism and epithelial barrier integrity in vitro. Front Nutr 9:882222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grycová AJoo HMaier VIllés PVyhlídalová BPoulíková KSládeková LNádvorník PVrzal RZemánková L, et al. (2022) Targeting the Aryl hydrocarbon receptor with microbial metabolite mimics alleviates experimental colitis in mice. J Med Chem 65:6859–6868. [DOI] [PubMed] [Google Scholar]
- Guan XJ, Zhang YY, Zheng X, Hao HP (2021) Drug discovery inspired from nuclear receptor sensing of microbial signals. Trends Mol Med 27:624–626. [DOI] [PubMed] [Google Scholar]
- Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M (2011) Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med 208:1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JA (1999) Seeking ligands for lonely orphan receptors. Science 284:1285–1286. [DOI] [PubMed] [Google Scholar]
- Guzior DV, Quinn RA (2021) Review: microbial transformations of human bile acids. Microbiome 9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Chanteux H, Ménochet K, Ledecq M, Schulze MED (2021) Designing out PXR activity on drug discovery projects: a review of structure-based methods, empirical and computational approaches. J Med Chem 64:6413–6522. [DOI] [PubMed] [Google Scholar]
- Han NDCheng JDelannoy-Bruno OWebber DTerrapon NHenrissat BRodionov DAArzamasov AAOsterman ALHayashi DK, et al. (2022) Microbial liberation of N-methylserotonin from orange fiber in gnotobiotic mice and humans. Cell 185:2495–2509.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Meyer UA (2005) Regulatory network of lipid-sensing nuclear receptors: roles for CAR, PXR, LXR, and FXR. Arch Biochem Biophys 433:387–396. [DOI] [PubMed] [Google Scholar]
- Hassani-Nezhad-Gashti F, Salonurmi T, Hautajärvi H, Rysä J, Hakkola J, Hukkanen J (2020) Pregnane X receptor activator rifampin increases blood pressure and stimulates plasma renin activity. Clin Pharmacol Ther 108:856–865. [DOI] [PubMed] [Google Scholar]
- Hezaveh KShinde RSKlötgen AHalaby MJLamorte SCiudad MTQuevedo RNeufeld LLiu ZQJin R, et al. (2022) Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55:324–340.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Zhang S, Pan S (2021) Characterization of citrus pectin oligosaccharides and their microbial metabolites as modulators of immunometabolism on macrophages. J Agric Food Chem 69:8403–8414. [DOI] [PubMed] [Google Scholar]
- Hu ZC, Tan YL, Huang SG, Pan P, Liu XB, Wang J, Guo WL (2018) Molecular imaging of Toll-like receptor 4 detects ischemia-reperfusion injury during intussusception. Oncotarget 9:7882–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Mukherjee S, DesMarais V, Albanese JM, Rafti E, Draghi Ii A, Maher LA, Khanna KM, Mani S, Matson AP (2018) Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr Res 83:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Chen G, Ye N, Kou X, Zhu F, Shen J, Ouyang G (2019) Solid-phase microextraction: An appealing alternative for the determination of endogenous substances - A review. Anal Chim Acta 1077:67–86. [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Perdew GH (2015) Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab Dispos 43:1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illés P, Krasulová K, Vyhlídalová B, Poulíková K, Marcalíková A, Pečinková P, Sirotová N, Vrzal R, Mani S, Dvořák Z (2020) Indole microbial intestinal metabolites expand the repertoire of ligands and agonists of the human pregnane X receptor. Toxicol Lett 334:87–93. [DOI] [PubMed] [Google Scholar]
- Inoue R, Yajima T, Tsukahara T (2017) Expression of TLR2 and TLR4 in murine small intestine during postnatal development. Biosci Biotechnol Biochem 81:350–358. [DOI] [PubMed] [Google Scholar]
- Jakobsson T, Vedin LL, Hassan T, Venteclef N, Greco D, D’Amato M, Treuter E, Gustafsson JÅ, Steffensen KR (2014) The oxysterol receptor LXRβ protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunol 7:1416–1428. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383:728–731. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zhang H, Xiao D, Wei H, Chen Y (2021) Farnesoid X receptor (FXR): structures and ligands. Comput Struct Biotechnol J 19:2148–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UHCheng YPark HDavidson LACallaway ESChapkin RSJayaraman AAsante AAllred CWeaver EA, et al. (2017) Short chain fatty acids enhance aryl hydrocarbon (Ah) responsiveness in mouse colonocytes and Caco-2 human colon cancer cells. Sci Rep 7:10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 85:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourova L, Anzenbacherova E, Dostal Z, Anzenbacher P, Briolotti P, Rigal E, Daujat-Chavanieu M, Gerbal-Chaloin S (2022) Butyrate, a typical product of gut microbiome, affects function of the AhR gene, being a possible agent of crosstalk between gut microbiome, and hepatic drug metabolism. J Nutr Biochem 107:109042. [DOI] [PubMed] [Google Scholar]
- Kaci G, Lakhdari O, Doré J, Ehrlich SD, Renault P, Blottière HM, Delorme C (2011) Inhibition of the NF-kappaB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl Environ Microbiol 77:4681–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katafuchi T, Makishima M (2022) Molecular basis of bile acid-FXR-FGF15/19 signaling axis. Int J Mol Sci 23:6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H (2021) In-tube solid-phase microextraction: current trends and future perspectives. J Chromatogr A 1636:461787. [DOI] [PubMed] [Google Scholar]
- Kayisoglu O, Weiss F, Niklas C, Pierotti I, Pompaiah M, Wallaschek N, Germer CT, Wiegering A, Bartfeld S (2021) Location-specific cell identity rather than exposure to GI microbiota defines many innate immune signalling cascades in the gut epithelium. Gut 70:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke K, Chen TH, Arra M, Mbalaviele G, Swarnkar G, Abu-Amer Y (2019) Attenuation of NF-κB in intestinal epithelial cells is sufficient to mitigate the bone loss comorbidity of experimental mouse colitis. J Bone Miner Res 34:1880–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Li H, Collins JJ, Ingber DE (2016) Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA 113:E7–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-G, Lou AC, Saghatelian A (2011) A metabolomics strategy for detecting protein-metabolite interactions to identify natural nuclear receptor ligands. Mol Biosyst 7:1046–1049. [DOI] [PubMed] [Google Scholar]
- Klepsch V, Moschen AR, Tilg H, Baier G, Hermann-Kleiter N (2019) Nuclear receptors regulate intestinal inflammation in the context of IBD. Front Immunol 10:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA (2015) Nuclear receptor PXR: discovery of a pharmaceutical anti-target. J Clin Invest 125:1388–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lehmann JM, Willson TM (1999) Orphan nuclear receptors: shifting endocrinology into reverse. Science 284:757–760. [DOI] [PubMed] [Google Scholar]
- Kliewer SAMoore JTWade LStaudinger JLWatson MAJones SAMcKee DDOliver BBWillson TMZetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82. [DOI] [PubMed] [Google Scholar]
- Koh AMolinaro AStåhlman MKhan MTSchmidt CMannerås-Holm LWu HCarreras AJeong HOlofsson LE, et al. (2018) microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 175:947–961.e17. [DOI] [PubMed] [Google Scholar]
- Konopelski P, Chabowski D, Aleksandrowicz M, Kozniewska E, Podsadni P, Szczepanska A, Ufnal M (2021) Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, increases blood pressure via cardiac and vascular mechanisms in rats. Am J Physiol Regul Integr Comp Physiol 321:R969–R981. [DOI] [PubMed] [Google Scholar]
- Konopelski P, Mogilnicka I (2022) Biological effects of indole-3-propionic acid, a gut microbiota-derived metabolite, and its precursor tryptophan in mammals’ health and disease. Int J Mol Sci 23:1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn SO, Meissner K, Mayes LM, Bartels K (2018) Vitamin C in sepsis. Curr Opin Anaesthesiol 31:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Jaiswal B, Kumar S, Negi S, Tyagi RK (2010) Cross-talk between androgen receptor and pregnane and xenobiotic receptor reveals existence of a novel modulatory action of anti-androgenic drugs. Biochem Pharmacol 80:964–976. [DOI] [PubMed] [Google Scholar]
- Kumari R, Yadav Y, Misra R, Das U, Das Adhikari U, Malakar P, Dubey GP (2022) Emerging frontiers of antibiotics use and their impacts on the human gut microbiome. Microbiol Res 263:127127. [DOI] [PubMed] [Google Scholar]
- Lamas BRichard MLLeducq VPham HPMichel MLDa Costa GBridonneau CJegou SHoffmann TWNatividad JM, et al. (2016) CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne FMasse MHMenard JSprague SPinto RHeyland DKCook DJBattista MCDay AGGuyatt GH, et al. ; LOVIT Investigators and the Canadian Critical Care Trials Group (2022) Intravenous vitamin C in adults with sepsis in the intensive care unit. N Engl J Med 386:2387–2398. [DOI] [PubMed] [Google Scholar]
- Latorre E, Layunta E, Grasa L, Pardo J, García S, Alcalde AI, Mesonero JE (2018) Toll-like receptors 2 and 4 modulate intestinal IL-10 differently in ileum and colon. United European Gastroenterol J 6:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledala NMalik MRezaul KPaveglio SProvatas AKiel ACaimano MZhou YLindgren JKrasulova K, et al. (2022) Bacterial indole as a multifunctional regulator of klebsiella oxytoca complex enterotoxicity. MBio 13:e0375221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DM, Ecton KE, Trikha SRJ, Wrigley SD, Thomas KN, Battson ML, Wei Y, Johnson SA, Weir TL, Gentile CL (2020) Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am J Physiol Gastrointest Liver Physiol 319:G51–G62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Feng Y, Tian M, Ji J, Hu X, Chen F (2021) Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ (2013) Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lu J, Cheng J, Wang L, Matsubara T, Csanaky IL, Klaassen CD, Gonzalez FJ, Ma X (2013) Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat Med 19:418–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HIllés PKarunaratne CVNordstrøm LULuo XYang AQiu YKurland IJLukin DJChen W, et al. (2021) Deciphering structural bases of intestinal and hepatic selectivity in targeting pregnane X receptor with indole-based microbial mimics. Bioorg Chem 109:104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ranhotra HS, Mani S, Dvořák Z, Sokol H, Müller R (2020) Human microbial metabolite mimicry as a strategy to expand the chemical space of potential drugs. Drug Discov Today 25:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WHang SFang YBae SZhang YZhang MWang GMcCurry MDBae MPaik D, et al. (2021) A bacterial bile acid metabolite modulates Treg activity through the nuclear hormone receptor NR4A1. Cell Host Microbe 29:1366–1377.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Teo WL, Low MJ, Meijer L, Sanderson I, Pettersson S, Greicius G (2014) Constitutive TLR4 signalling in intestinal epithelium reduces tumor load by increasing apoptosis in APC(Min/+) mice. Oncogene 33:369–377. [DOI] [PubMed] [Google Scholar]
- Lin YSYasuda KAssem MCline CBarber JLi CWKholodovych VAi NChen JDWelsh WJ, et al. (2009) The major human pregnane X receptor (PXR) splice variant, PXR.2, exhibits significantly diminished ligand-activated transcriptional regulation. Drug Metab Dispos 37:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Sun C, Chen Y, Du F, Yang Y, Wu G (2021) Indole-3-propionic acid-aggravated CCl4-induced liver fibrosis via the TGF-β1/Smads signaling pathway. J Clin Transl Hepatol 9:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu YF, Corton JC, Klaassen CD (2021) Expression of cytochrome P450 isozyme transcripts and activities in human livers. Xenobiotica 51:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JSahin CAhmad SMagomedova LZhang MJia ZMetherel AHOrellana APoda GRBazinet PL, et al. (2022). The omega-3 hydroxy fatty acid 7(S)-HDHA is a high-affinity PPARα ligand that regulates brain neuronal morphology. Sci Signal 15:eabo1857. [DOI] [PubMed] [Google Scholar]
- Lu QJiang CHou JQian HChu FZhang WYe MChen ZLiu JYao H, et al. (2021) Patchouli alcohol modulates the pregnancy X receptor/Toll-like receptor 4/nuclear factor kappa B axis to suppress osteoclastogenesis. Front Pharmacol 12:684976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak B, Wang H (2016) Mechanisms of xenobiotic receptor activation: direct vs. indirect. Biochim Biophys Acta 1859:1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Martin-Gallausiaux C, Bourhis J-M, Béguet-Crespel F, Blottière HM, Lapaque N (2019) Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep 9:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheux AGassiot MFromont GLeenhardt FBoulahtouf AFabbrizio EMarchive CGarcin AAgherbi HCombès E, et al. (2021) PXR modulates the prostate cancer cell response to afatinib by regulating the expression of the monocarboxylate transporter SLC16A1. Cancers (Basel) 13:3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CN, Bellomo R, Lankadeva YR (2021) Therapeutic potential of megadose vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br J Pharmacol 178:3864–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel DK, Eden K, Ringel VM, Allen IC (2016) emerging roles for noncanonical NF-κB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm Bowel Dis 22:2265–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Gwag T, Sui Y, Park SH, Zhou X, Zhou C (2019) The atypical antipsychotic quetiapine induces hyperlipidemia by activating intestinal PXR signaling. JCI Insight 4:e125657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Tazawa C, Blumberg B, Sasano H (2005) Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol 231:75–85. [DOI] [PubMed] [Google Scholar]
- Modica SGofflot FMurzilli SD’Orazio ASalvatore LPellegrini FNicolucci ATognoni GCopetti MValanzano R, et al. (2010) The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology 138:636–648, 648.e1–648.e12. [DOI] [PubMed] [Google Scholar]
- Modoux MRolhion NLefevre JHOeuvray CNádvorník PIlles PEmond PParc YMani SDvořák Z, et al. (2022) Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes 14:2105637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz AAndersen LWHuang DTBerg KMGrossestreuer AVMarik PESherwin RLHou PCBecker LBCocchi MN, et al. (2018) Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care 22:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA (2011) The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 6:e17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanthirudjanar TFurumoto HZheng JKim YIGoto TTakahashi NKawada TPark SBHirata AKitamura N, et al. (2015) Gut microbial fatty acid metabolites reduce triacylglycerol levels in hepatocytes. Lipids 50:1093–1102. [DOI] [PubMed] [Google Scholar]
- Nicolussi S, Drewe J, Butterweck V, Meyer Zu Schwabedissen HE (2020) Clinical relevance of St. John’s wort drug interactions revisited. Br J Pharmacol 177:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves KM, Hirota SA, Flannigan KL (2022) Xenobiotic receptors and the regulation of intestinal homeostasis: harnessing the chemical output of the intestinal microbiota. Am J Physiol Gastrointest Liver Physiol 322:G268–G281. [DOI] [PubMed] [Google Scholar]
- Nighot M, Rawat M, Al-Sadi R, Castillo EF, Nighot P, Ma TY (2019) Lipopolysaccharide-induced increase in intestinal permeability is mediated by TAK-1 activation of IKK and MLCK/MYLK gene. Am J Pathol 189:797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Cui H, Gu X, Wu T, Sun M, Zhou C, Ma M (2022) Nuclear receptor PXR confers irradiation resistance by promoting DNA damage response through stabilization of ATF3. Front Oncol 12:837980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo A, Brown JR (2020) Microbiome metabolite mimics accelerate drug discovery. Trends Mol Med 26:435–437. [DOI] [PubMed] [Google Scholar]
- Oladimeji P, Cui H, Zhang C, Chen T (2016) Regulation of PXR and CAR by protein-protein interaction and signaling crosstalk. Expert Opin Drug Metab Toxicol 12:997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondeyka JGJayasuriya HHerath KBGuan ZSchulman MCollado JDombrowski AWKwon SSMcCallum CSharma N, et al. (2005) Steroidal and triterpenoidal fungal metabolites as ligands of liver X receptors. J Antibiot (Tokyo) 58:559–565. [DOI] [PubMed] [Google Scholar]
- Orans J, Teotico DG, Redinbo MR (2005) The nuclear xenobiotic receptor pregnane X receptor: recent insights and new challenges. Mol Endocrinol 19:2891–2900. [DOI] [PubMed] [Google Scholar]
- Ortiz-Flores M, Portilla-Martínez A, Cabrera-Pérez F, Nájera N, Meaney E, Villarreal F, Pérez-Durán J, Ceballos G (2020) PXR is a target of (-)-epicatechin in skeletal muscle. Heliyon 6:e05357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Miura T, Terashima J, Habano W (2021) Cellular irinotecan resistance in colorectal cancer and overcoming irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: a review. Cancer Drug Resist 4:946–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeslack N, Mimmler M, Becker S, Gao Z, Khuu MP, Mann A, Malinarich F, Regen T, Reinhardt C (2022) Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids DOI: 10.1007/s00726-022-03161-5 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzitt K, Zollner G, Marschall HU, Wagner M (2022) Recent advances on FXR-targeting therapeutics. Mol Cell Endocrinol 552:111678. [DOI] [PubMed] [Google Scholar]
- Parigi SMDas SFrede ACardoso RFTripathi KPDoñas CHu YOOAntonson PEngstrand LGustafsson JÅ, et al. (2021) Liver X receptor regulates Th17 and RORγt+ Treg cells by distinct mechanisms. Mucosal Immunol 14:411–419. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Drocourt L, Assénat E, Larrey D, Pichard-Garcia L, Vilarem MJ, Maurel P (2004) Cross-talk between xenobiotic detoxication and other signalling pathways: clinical and toxicological consequences. Xenobiotica 34:633–664. [DOI] [PubMed] [Google Scholar]
- Pavek P (2016) Pregnane X receptor (PXR)-mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions. Front Pharmacol 7:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ (1998) Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 93:693–704. [DOI] [PubMed] [Google Scholar]
- Pérez-Burillo S, Navajas-Porras B, López-Maldonado A, Hinojosa-Nogueira D, Pastoriza S, Rufián-Henares JÁ (2021) Green tea and its relation to human gut microbiome. Molecules 26:3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernomian L, Duarte-Silva M, de Barros Cardoso CR (2020) The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol 59:382–390. [DOI] [PubMed] [Google Scholar]
- Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, Barton GM (2018) A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity 49:560–575.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti M, Pariano M, Borghi M, Barola C, Moretti S, Galarini R, Mosci P, Ricci M, Costantini C, Giovagnoli S (2021) Enteric formulated indole-3-carboxaldehyde targets the aryl hydrocarbon receptor for protection in a murine model of metabolic syndrome. Int J Pharm 602:120610. [DOI] [PubMed] [Google Scholar]
- Pulakazhi Venu VK, Saifeddine M, Mihara K, Tsai YC, Nieves K, Alston L, Mani S, McCoy KD, Hollenberg MD, Hirota SA (2019) The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am J Physiol Endocrinol Metab 317:E350–E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatanani M, Moore DD (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 6:329–339. [DOI] [PubMed] [Google Scholar]
- Qi-Xiang M, Yang F, Ze-Hua H, Nuo-Ming Y, Rui-Long W, Bin-Qiang X, Jun-Jie F, Chun-Lan H, Yue Z (2022) Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut Microbes 14:2112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn RAMelnik AVVrbanac AFu TPatras KAChristy MPBodai ZBelda-Ferre PTripathi AChung LK, et al. (2020) Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahunen R, Kummu O, Koivukangas V, Hautajärvi H, Hakkola J, Rysä J, Hukkanen J (2022) Pregnane X receptor‒4β-hydroxycholesterol axis in the regulation of overweight- and obesity-induced hypertension. J Am Heart Assoc 11:e023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raihan T, Rabbee MF, Roy P, Choudhury S, Baek KH, Azad AK (2021) Microbial metabolites: the emerging hotspot of antiviral compounds as potential candidates to avert viral pandemic alike COVID-19. Front Mol Biosci 8:732256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan SKZhang HMa XJung ISchwartz AJTriner DDevenport SNDas NKXue XZeng MY, et al. (2019) Intestinal non-canonical NFκB signaling shapes the local and systemic immune response. Nat Commun 10:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Rendon D, Passari AK, Ruiz-Villafán B, Rodríguez-Sanoja R, Sánchez S, Demain AL (2022) Impact of novel microbial secondary metabolites on the pharma industry. Appl Microbiol Biotechnol 106:1855–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Pittol, J. M., Milona A., Morris I., Willemsen E. C. L., van der Veen S. W., Kalkhoven E., van Mil S. W. C. (2020). FXR isoforms control different metabolic functions in liver cells via binding to specific DNA motifs. Gastroenterology 159:1853–1865.e1810. [DOI] [PubMed] [Google Scholar]
- Renga GNunzi EPariano MPuccetti MBellet MMPieraccini GD’Onofrio FSantarelli IStincardini CAversa F, et al. (2022) Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J Immunother Cancer 10:e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer VMascanfroni IDBunse LTakenaka MCKenison JEMayo LChao CCPatel BYan RBlain M, et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysä J, Buler M, Savolainen MJ, Ruskoaho H, Hakkola J, Hukkanen J (2013) Pregnane X receptor agonists impair postprandial glucose tolerance. Clin Pharmacol Ther 93:556–563. [DOI] [PubMed] [Google Scholar]
- Saha S, Rajpal DK, Brown JR (2016) Human microbial metabolites as a source of new drugs. Drug Discov Today 21:692–698. [DOI] [PubMed] [Google Scholar]
- Sajid M, Khaled Nazal M, Rutkowska M, Szczepańska N, Namieśnik J, Płotka-Wasylka J (2019) Solid phase microextraction: apparatus, sorbent materials, and application. Crit Rev Anal Chem 49:271–288. [DOI] [PubMed] [Google Scholar]
- Sári ZMikó EKovács TJankó LCsonka TLente GSebő ÉTóth JTóth DÁrkosy P, et al. (2020) Indolepropionic acid, a metabolite of the microbiome, has cytostatic properties in breast cancer by activating AHR and PXR receptors and inducing oxidative stress. Cancers (Basel) 12:2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235. [DOI] [PubMed] [Google Scholar]
- Schmidt DR, Mangelsdorf DJ (2008) Nuclear receptors of the enteric tract: guarding the frontier. Nutr Rev 66 (10, Suppl 2):S88–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler M, Caesar R (2019) Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord 20:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Fu J, Chang PV (2020) Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 117:19376–19387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal RIlha MVaittinen MKaminska DMännistö VKärjä VTuomainen MHanhineva KRomeo SPajukanta P, et al. (2021) Indole-3-propionic acid, a gut-derived tryptophan metabolite, associates with hepatic fibrosis. Nutrients 13:3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serger ELuengo-Gutierrez LChadwick JSKong GZhou LCrawford GDanzi MCMyridakis ABrandis ABello AT, et al. (2022) The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 607:585–592. [DOI] [PubMed] [Google Scholar]
- Sevransky JERothman REHager DNBernard GRBrown SMBuchman TGBusse LWCoopersmith CMDeWilde CEly EW, et al. ; VICTAS Investigators (2021) Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA 325:742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ (2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol 292:G1114–G1122. [DOI] [PubMed] [Google Scholar]
- Shanahan F, Ghosh TS, O’Toole PW (2021) The healthy microbiome—what is the definition of a healthy gut microbiome? Gastroenterology 160:483–494. [DOI] [PubMed] [Google Scholar]
- Shehu AI, Li G, Xie W, Ma X (2016) The pregnane X receptor in tuberculosis therapeutics. Expert Opin Drug Metab Toxicol 12:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu AI, Zhu J, Li J, Lu J, McMahon D, Xie W, Gonzalez FJ, Ma X (2021) Targeting xenobiotic nuclear receptors PXR and CAR to prevent cobicistat hepatotoxicity. Toxicol Sci 181:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen PXLi XDeng SYZhao LZhang YYDeng XHan BYu JLi YWang ZZ, et al. (2021) Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine 64:103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RChandrashekharappa SBodduluri SRBaby BVHegde BKotla NGHiwale AASaiyed TPatel PVijay-Kumar M, et al. (2019) Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi CPAhmad RJia HFulton WBLopez CGonzalez Salazar AJIshiyama ASampah MSteinway SWang S, et al. (2022) The administration of amnion-derived multipotent cell secretome ST266 protects against necrotizing enterocolitis in mice and piglets. Am J Physiol Gastrointest Liver Physiol 323:G265–G282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi CPNeal MDSiggers RSho SMa CBranca MFPrindle T JrRusso AMAfrazi AGood M, et al. (2012) Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143:708–718.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AMichelsen KSKarahashi HLu JMeng FJQu XCrother TRRabizadeh SChen SCaplan MS, et al. (2010) Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PLoS One 5:e15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XSun XOh SFWu MZhang YZheng WGeva-Zatorsky NJupp RMathis DBenoist C, et al. (2020) Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, von der Weid PY (2020) Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 11:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifft F, van Kuijk SMJ, Bekers O, Christiaans MHL (2018) Increase in tacrolimus exposure after steroid tapering is influenced by CYP3A5 and pregnane X receptor genetic polymorphisms in renal transplant recipients. Nephrol Dial Transplant 33:1668–1675. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Shah K, Wincent E (2021) AHR in the intestinal microenvironment: safeguarding barrier function. Nat Rev Gastroenterol Hepatol 18:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringlis IA, Zhang H, Pieterse CMJ, Bolton MD, de Jonge R (2018) Microbial small molecules - weapons of plant subversion. Nat Prod Rep 35:410–433. [DOI] [PubMed] [Google Scholar]
- Su XZhang MQi HGao YYang YYun HZhang QYang XZhang YHe J, et al. (2022) Gut microbiota-derived metabolite 3-idoleacetic acid together with LPS induces IL-35+ B cell generation. Microbiome 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Meng Z, Chen J, Liu J, Hernandez R, Gonzales MB, Gwag T, Morris AJ, Zhou C (2021) Effects of dicyclohexyl phthalate exposure on PXR activation and lipid homeostasis in mice. Environ Health Perspect 129:127001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales KE, Moore R, Truss NJ, Tucker A, Warner TD, Negishi M, Bishop-Bailey D (2012) Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress. Cardiovasc Res 93:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Sugi Y, Hosono A, Kaminogawa S (2009) Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol 183:6522–6529. [DOI] [PubMed] [Google Scholar]
- Tam JSY, Coller JK, Prestidge CA, Bowen JM (2022) Investigation of TLR4 antagonists for prevention of intestinal inflammation. Inflammation DOI: 10.1007/s10753-022-01714-0 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanes CBittinger KGao YFriedman ESNessel LPaladhi URChau LPanfen EFischbach MABraun J, et al. (2021) Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 29:394–407.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YMu JXu FZhang XSriwastva MKLiu QMLi XLei CSundaram KHu X, et al. (2022) Gut bacterial isoamylamine promotes age-related cognitive dysfunction by promoting microglial cell death. Cell Host Microbe 30:944–960.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins LM, Sit TL, Wallace AD (2008) Unique transcription start sites and distinct promoter regions differentiate the pregnane X receptor (PXR) isoforms PXR 1 and PXR 2. Drug Metab Dispos 36:923–929. [DOI] [PubMed] [Google Scholar]
- Tsukidate T, Li Q, Hang HC (2020) Nuclear receptor chemical reporter enables domain-specific analysis of ligands in mammalian cells. ACS Chem Biol 15:2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen T, Bertolini A, de Vries HD, Bloks VW, de Boer JF, Jonker JW, Kuipers F (2019) Potential of intestine-selective FXR modulation for treatment of metabolic disease. Handb Exp Pharmacol 256:207–234. [DOI] [PubMed] [Google Scholar]
- Vanholder R, Nigam SK, Burtey S, Glorieux G (2022) What if not all metabolites from the uremic toxin generating pathways are toxic? A hypothesis. Toxins (Basel) 14:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh MMukherjee SWang HLi HSun KBenechet APQiu ZMaher LRedinbo MRPhillips RS, et al. (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennois E, Pommier AJ, Mouzat K, Oumeddour A, El Hajjaji FZ, Dufour J, Caira F, Volle DH, Baron S, Lobaccaro JM (2011) Targeting liver X receptors in human health: deadlock or promising trail? Expert Opin Ther Targets 15:219–232. [DOI] [PubMed] [Google Scholar]
- Vlantis K, Wullaert A, Sasaki Y, Schmidt-Supprian M, Rajewsky K, Roskams T, Pasparakis M (2011) Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest 121:2781–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyhlídalová BKrasulová KPečinková PMarcalíková AVrzal RZemánková LVančo JTrávníček ZVondráček JKarasová M, et al. (2020) Gut microbial catabolites of tryptophan are ligands and agonists of the aryl hydrocarbon receptor: a detailed characterization. Int J Mol Sci 21:E2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BD, Redinbo MR (2013) Xenobiotic-sensing nuclear receptors involved in drug metabolism: a structural perspective. Drug Metab Rev 45:79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YWang FYuan JLi JJiang DZhang JLi HWang RTang JHuang T, et al. (2019) Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68:1417–1429. [DOI] [PubMed] [Google Scholar]
- Wang J, Bwayi M, Gee RRF, Chen T (2020) PXR-mediated idiosyncratic drug-induced liver injury: mechanistic insights and targeting approaches. Expert Opin Drug Metab Toxicol 16:711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dai S, Guo Y, Xie W, Zhai Y (2014) Biology of PXR: role in drug-hormone interactions. EXCLI J 13:728–739. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yin L, Qi Y, Zhang J, Zhu H, Tang J (2022) Intestinal flora-derived kynurenic acid protects against intestinal damage caused by Candida albicans infection via activation of aryl hydrocarbon receptor. Front Microbiol 13:934786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weersma RK, Zhernakova A, Fu J (2020) Interaction between drugs and the gut microbiome. Gut 69:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106:3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Almousa A, Teft WA, Kim RB (2020) Attenuation of bile acid-mediated FXR and PXR activation in patients with Crohn’s disease. Sci Rep 10:1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H, Che Q, Guo J, Su Z (2021) Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes 13:1949095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HWCui MLi YDong JLZhang SQZhu CCJiang MZhu TWang BWang HC, et al. (2020) Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM (2000) Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406:435–439. [DOI] [PubMed] [Google Scholar]
- Xie W, Evans RM (2001) Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem 276:37739–37742. [DOI] [PubMed] [Google Scholar]
- Yan T, Yan N, Wang H, Yagai T, Luo Y, Takahashi S, Zhao M, Krausz KW, Wang G, Hao H, et al. (2021) FXR-deoxycholic acid-TNF-α axis modulates acetaminophen-induced hepatotoxicity. Toxicol Sci 181:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Li H, Anjum K, Zhong X, Miao S, Zheng G, Liu W, Li L (2022) Dual role of indoles derived from intestinal microbiota on human health. Front Immunol 13:903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TLv SZhang WTang YChang HHu ZFang LDu JWu SYang X, et al. (2022) PF-PLC micelles ameliorate cholestatic liver injury via regulating TLR4/MyD88/NF-κB and PXR/CAR/UGT1A1 signaling pathways in EE-induced rats. Int J Pharm 615:121480. [DOI] [PubMed] [Google Scholar]
- Zelante TIannitti RGCunha CDe Luca AGiovannini GPieraccini GZecchi RD’Angelo CMassi-Benedetti CFallarino F, et al. (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Wada T, Zhang B, Khadem S, Ren S, Kuruba R, Li S, Xie W (2010) A functional cross-talk between liver X receptor-α and constitutive androstane receptor links lipogenesis and xenobiotic responses. Mol Pharmacol 78:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Jiang M, Zhao J, Song Y, Du W, Shi J (2022) The mechanism underlying the influence of indole-3-propionic acid: a relevance to metabolic disorders. Front Endocrinol (Lausanne) 13:841703 Lausanne. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang Z, Xie X, Yao Q, Liu J, Lai B, Xiao L, Wang N (2019) Xenobiotic pregnane X receptor promotes neointimal formation in balloon-injured rat carotid arteries. J Cell Physiol 234:4342–4351. [DOI] [PubMed] [Google Scholar]
- Zhang R, Huang G, Ren Y, Wang H, Ye Y, Guo J, Wang M, Zhu W, Yu K (2022) Effects of dietary indole-3-carboxaldehyde supplementation on growth performance, intestinal epithelial function, and intestinal microbial composition in weaned piglets. Front Nutr 9:896815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Osaka T, Tsuneda S (2015) Bacterial metabolites directly modulate farnesoid X receptor activity. Nutr Metab (Lond) 12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CBao LQiu MFeng LChen LLiu ZDuan SZhao YWu KZhang N, et al. (2022) Dietary tryptophan-mediated aryl hydrocarbon receptor activation by the gut microbiota alleviates Escherichia coli-induced endometritis in mice. Microbiol Spectr 10:e0081122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WY, Luan ZL, Sun CP, Zhang BJ, Jin LL, Deng S, Zhang HL, Yu ZL, Wang C, Ma XC (2022) Metabolites isolated from the human intestinal fungus Penicillium oxalicum SL2 and their agonistic effects on PXR and FXR. Phytochemistry 193:112974. [DOI] [PubMed] [Google Scholar]
- Zheng X, Cai X, Hao H (2022) Emerging targetome and signalome landscape of gut microbial metabolites. Cell Metab 34:35–58. [DOI] [PubMed] [Google Scholar]
- Zheng ZZhao ZLi SLu XJiang MLin JAn YXie YXu MShen W, et al. (2017) Altenusin, a nonsteroidal microbial metabolite, attenuates nonalcoholic fatty liver disease by activating the farnesoid X receptor. Mol Pharmacol 92:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zou Y, Zhang Y, Teng S, Ye K (2022) Involvement of CCN1 protein and TLR2/4 signaling pathways in intestinal epithelial cells response to Listeria monocytogenes. Int J Mol Sci 23:2739. [DOI] [PMC free article] [PubMed] [Google Scholar]