Abstract
Background:
Brain Derived Neurotrophic Factor (BDNF) has potential as a biomarker of depression treatment because serum BDNF in depressed human subjects is decreased and normalizes with treatment. The relationship between serum BDNF and exercise treatment of depression is not known. The Treatment with Exercise Augmentation for Depression (TREAD) study examined dosed exercise augmentation treatment of partial responders to antidepressants. Serum BDNF in TREAD subjects was analyzed to understand its relationship with exercise training.
Methods:
Subjects were randomized to high (16 kcal/kg/week or KKW) or low (4 KKW) energy expenditure exercise over 12 weeks. Actual kcal/week expended and IDS-C scores were collected weekly. One hundred four subjects in TREAD provided baseline blood samples; a subset of 70 subjects also provided week 12 samples. Serum BDNF was determined using ELISA. Correlations were examined between change in BDNF and 1) mean kcal/week expended, and 2) change in IDS-C score. Mixed-effects ANOVA examined the effect of baseline BDNF on outcome.
Results:
Resting serum BDNF was stable and did not correlate with energy expenditure (p = 0.15) or IDS-C improvement (p = 0.89). Subjects entering the study with higher BDNF improved more rapidly on the IDS-C (p = 0.003).
Limitations:
Serum may not be the most sensitive blood fraction in which to measure BDNF change. Pre-treatment with medication may mask exercise effect on BDNF.
Conclusions:
These results suggest that change in serum BDNF does not reflect efficacy of exercise augmentation treatment of MDD. Instead BDNF may function as an augmentation moderator. Pre-treatments that raise BDNF may improve the efficacy of exercise treatment of MDD.
Keywords: Major Depressive Disorder, Exercise training, Neurotrophin
1. Introduction
Several pharmacological and non-pharmacological treatments are available for Major Depressive Disorder (MDD); however, only about one-third of patients achieve remission following initial treatment (Corey-Lisle et al., 2004; Trivedi et al., 2006c). Augmentation and/or switching are commonly used strategies to achieve remission, with augmentation as the preferred option for those with initial partial response. Unfortunately, selection of specific antide-pressant treatments for a given patient remains a trial and error process. Therefore, identification of biological markers that can guide treatment selection is critical for personalized treatment of depression.
Exercise has demonstrated efficacy as a monotherapy (Blumenthal et al., 2007; Dunn et al., 2005) as well as in combination with other treatments for depression (Martinsen et al., 1985; Trivedi et al., 2006a) when used as a first step treatment. Exercise is associated with increases in hippocampal neurogenesis, monoamine neurotransmission and synaptic growth (Dishman et al., 2006; van Praag et al., 1999). These effects are similar to effects seen with medications and ECT, suggesting that a common physiological mechanism or pathway is involved. Brain Derived Neurotrophic Factor (BDNF) is thought to mediate exercise-related neuroplasticity (Griffin et al., 2009) and is a potential biological moderator or mediator of antidepressant response (Schmidt and Duman, 2007). In addition, animals treated with both exercise and antidepressants have enhanced BDNF expression and behavioral response compared to either treatment alone (Russo-Neustadt et al., 2001).
Although research correlating peripheral to central BDNF in humans has been limited by the scarcity of human brain tissue, there is some evidence that peripheral BDNF reflects net expression in the brain. For example, BDNF made in the rat brain in response to treatment with electroconvulsive shock correlates temporally with serum levels, suggesting that BDNF readily crosses the blood brain barrier and that peripheral levels may be used as a marker of central expression (Sartorius et al., 2009). Serum BDNF is lower in untreated depressed subjects when compared to controls (Karege et al., 2002; Sen et al., 2008) and level of BDNF is inversely correlated with depressive symptoms severity (Karege et al., 2002). Furthermore, level of serum BDNF recovers with antidepressant treatment (Shimizu et al., 2003).
Acutely (i.e. in min to h), serum BDNF levels increase in response to exercise in healthy (Rasmussen et al., 2009) as well as depressed subjects (Gustafsson et al., 2009). Changes in resting serum BDNF – measured before or at least 24 h after an exercise session – in response to exercise training have also been of interest. However, studies of resting BDNF in healthy subjects have produced mixed results. Two studies examining resting plasma level in male subjects have found that exercise training correlates with an increase in resting BDNF. However, another study in a male sample examining serum and plasma levels found BDNF was stable. In contrast to the acute- and short-term (over weeks) effects of exercise on BDNF, several studies agree that serum BDNF in healthy subjects who are long-term (i.e. over years) exercisers is lower than in those who are sedentary (Chan et al., 2008; Currie et al., 2009). The variability in these results is puzzling and reflects a need for more longitudinal data in BDNF studies of exercise.
The Treatment with Exercise Augmentation for Depression (TREAD) study provided an excellent opportunity to study the role of serum BDNF in exercise augmentation treatment of MDD. This study included patients who had partial response to ongoing SSRI treatment but continued to have significant residual symptoms. The TREAD study participants were treated with two doses of exercise to augment their current treatment. Data collected through the TREAD allowed us to determine if baseline levels of BDNF can be used to predict treatment response and if those effects are associated with other baseline characteristics such as Body Mass Index (BMI).
In this report, we address two important questions about the relationship among exercise, serum BDNF and depression. First, do resting BDNF levels change with exercise training in participants with MDD and are those levels associated with changes in depressive symptom severity – in other words, does BDNF mediate exercise treatment response? Second, do baseline levels of BDNF and other subject characteristics predict differential treatment response – in other words, does baseline BDNF level moderate treatment response?
2. Methods
2.1. Subjects
Subjects (n = 126) were depressed, sedentary adults, aged 18–70, who were recruited from the community via advertising. For this study 104 subjects provided blood samples of which 70 had samples available from both the baseline and twelve week visits. At study entry, all had been treated with an adequate dose of an SSRI for two to six months, and were partial responders or non-responders as defined by baseline Hamilton Depression Rating Scale (HDRS) scores ≥14. Confirmation of the diagnosis of MDD was done with the Structured Clinical Interview for DSM Axis I Disorders (SCID).
2.2. Procedures
A complete description of the TREAD study design and rationale is published elsewhere (Trivedi et al., 2006b). In brief, following consent and screening, subjects were randomized to either high or low energy expenditure, measured in kilocalories burned per kilogram of body weight per week (KKW). The high dose group had a target of 16 KKW, and the low dose group 4 KKW. During the 12-week acute phase of the study all subjects received personalized education and training in a dose-appropriate exercise regimen at the Cooper Institute in Dallas. Clinical data was gathered weekly, and included the Hamilton Depression Rating Scale (HDRS) and the Inventory of Depression Symptomatology (IDS). The IDS clinician rated version (IDS-C) was the primary outcome measure, although subjects also provided IDS self rated (IDS-SR) scores. Subjects’ exercise activities were closely tracked to obtain accurate estimates of actual calories expended. In addition to the symptom measures, maximum aerobic capacity (VO2 Max) was measured at baseline. Subjects also were assessed weekly with the Short Form Health Survey (SF-36), Social Adjustment Scale-Self Rated (SAS-SR), Quality of Life, Enjoyment and Satisfaction Questionnaire (QLES-Q), the Work and Social Adjustment Scale (WSAS), the Satisfaction with Life Scale (SWLS), the Motivation and Energy Inventory (MEI), the Snaith-Hamilton Pleasure Scale (SHAPS), and the Patient Perception of Benefits (PPB).
2.3. Sample collection and analysis
Resting blood samples were drawn in the morning; subjects fasted a minimum of 3 h prior to blood draw, and were at least 24 h from the last exercise session. 10 ml of peripheral venous blood was drawn into Falcon tubes. Samples were centrifuged at 900 rpm for 10 min at room temperature to separate the blood components. Serum was decanted and frozen at −70°C while awaiting analysis. Serum samples were analyzed according to manufacturer’s protocol using R&D Human BDNF Quantikine kits (R&D Systems, Minneapolis, MN, USA). This assay has an average recovery rate of 96% and the minimal detectable dose of BDNF is typically less than 20 pg/ml. Samples were run in triplicate.
2.4. Statistics
Mean BDNF levels at baseline and week 12 were compared by the Wilcoxon Signed-Rank test for all subjects and for those within the high and low exercise groups. Change in BDNF level over the 12-week exercise period was correlated with mean calories burnt per week and with change in depressive symptoms (IDS-C). Sub-analyses were performed within each exercise group. Kendall’s non-parametric correlations were used due to smaller numbers of subjects. Partial correlations including age and gender as covariates were not reported because the results did not differ from the primary analyses.
We assessed whether baseline level of BDNF along with other characteristics moderated weekly IDS-C treatment response using a mixed-effects repeated measures analysis (implemented in SAS Proc Mixed). Intercepts and slopes were random effects and all others were fixed effects. Each model contained terms for time, baseline IDS-C, baseline BDNF level, other covariates, and interactions among covariates and with time. The other covariates considered were: age, gender, race, marital status, family history of mental illness, single/recurrent MDD, onset age of MDD, length of current episode, number of adequate antidepressant trials, time on adequate dose of antidepressant, baseline VO2 Max, BMI, SF-36 Mental subscale, SF-36 Physical subscale, SAS-SR, Q-LES-Q, WSAS, SWLS, MEI, SHAPS, and PPB. These additional covariates were included in the model if they significantly improved the model fit. Time was log transformed to provide a more linear relationship with outcome. All subjects with at least one post baseline visit were included.
3. Results
One hundred four subjects provided baseline blood samples and of these 70 also completed 12 weeks of exercise and provided an end of study blood sample. Descriptive statistics for the sample of 104 are presented in Table 1. Demographics for the sub-sample of 70 completers did not differ from the 34 non-completers except for being older (completers: 49.4 ± 9.2 years, non-completers: 44.0 ± 9.0 years, t102 2.8, p a0.0061) so they are not presented separately. 52 subjects (50%) were randomized to each group. Mean age was 47.6 ± 9.4 years. The sample was 79.8% female. At baseline mean IDS-C was 34.2 ± 7.3. Mean IDS-SR was 32.8 ± 9.4. Mean final IDS-C was 20.5 ± 11.6 and IDS-SR was 19.3 ± 10.9. Subjects BMI fell in the obese range, with a mean of 30.9 ± 6.3.
Table 1.
Demographic and clinical characteristics.
| Variable | All N | All mean or % | All STD | 16 kcal N |
16 kcal Mean or % |
16 kcal STD |
4 kcal N |
4 kcal Mean or % |
4 kcal STD |
t or Chi-square | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 104 | 47.6 | 9.4 | 52 | 46.1 | 9.5 | 52 | 49.2 | 9.1 | 1.7 | 0.0851 |
| Female (%) | 104 | 79.8 | 52 | 84.6 | 52 | 75.0 | 1.5 | 0.2220 | |||
| BL IDS-C | 104 | 34.2 | 7.3 | 52 | 33.2 | 6.5 | 52 | 35.2 | 7.9 | 1.4 | 0.1748 |
| Week 12 IDS-C | 68 | 20.5 | 11.6 | 31 | 20.1 | 11.6 | 37 | 20.9 | 11.7 | 0.3 | 0.7715 |
| BL IDS-SR | 100 | 32.8 | 9.4 | 49 | 31.8 | 7.8 | 51 | 33.6 | 10.7 | 1.0 | 0.3404 |
| Week 12 IDS-SR | 67 | 19.3 | 10.9 | 31 | 19.0 | 10.1 | 36 | 19.6 | 11.7 | 0.2 | 0.8290 |
| BL BDNF | 104 | 19.1 | 6.2 | 52 | 18.7 | 5.9 | 52 | 19.4 | 6.6 | 0.5 | 0.6026 |
| BL BMI | 104 | 30.9 | 6.3 | 52 | 30.3 | 7.1 | 52 | 31.4 | 5.5 | 0.9 | 0.3824 |
Mean age of subjects randomized to the high dose group was 46.1; 84.6% were female. Subjects in the low dose group were 49.2 ± 9.1 years of age, and 75% were female. Baseline IDS-C scores were 33.2 ± 6.5 for the high dose group and 35.2 ± 7.9 for the low dose group and did not differ significantly (p = 0.1748). IDS-SR baseline scores also did not differ significantly (p = 0.3404) between the high and low dose groups with means of 31.8 ± 7.8 and 33.6 ± 10.7 respectively. High and low dose groups did not differ significantly in any measured demographic or clinical characteristic. The most common medication subjects took during the study was escitalopram, with 77 (74%) subjects, with a mean dose and dose standard deviation of 20.5 mg ± 3.2. The other medications were, in order of prevalence and given as name, n, mean dose in mg ± standard deviation were: sertraline, 14, 140.7 ± 34.7, citalopram, 5, 40.0 ± 0.0, paroxetine CR, 4, 28.8 ± 7.5, fluoxetine, 2, 40.0 ± 0.0 and paroxetine, 2, 40.0 ± 0.0.
The primary outcome results of TREAD are given in detail elsewhere (Trivedi et al., in press), but in sum, unadjusted remission rates at week 12 were identical (29.5%) for the high and low dose exercise groups. After adjustment for clinical and demographic variables, all subjects had a significant increase in remission rate over the study period (p < 0.0001), but the difference between groups was not significant (p = 0.06). Adjusted remission rates at week 12 were 15.5% versus 28.3% for the 4 and 16 KKW groups, respectively (p = 0.18) with an NNT of 7.8 in favor of high dose exercise.
Mean BDNF at baseline for all 104 subjects was 19.1 ± 6.2 ng/ml. The mean baseline BDNF among high dose subjects was 18.7 ± 5.9 and did not significantly differ from low dose subjects 19.4 ± 6.6 (p =0.6026). Mean BDNF values are shown in Table 2 for the 70 subjects with measurements at baseline and week 12. In this subset, BDNF at baseline for treatment completers was 19.1 ± 6.0 ng/ml. There was no change over the study period, with a final level of 19.2 ± 5.8 ng/ml (p = 0.8609). In the low dose group, initial BDNF level was 18.6 ± 6.5 ng/ml, and final 19.2 ± 5.7 ng/ml with no significant change (p = 0.4399). BDNF was also stable in the high dose group, with initial and final levels of 19.7 ± 5.4 and 19.3 ± 5.9 ng/ml respectively (p = 0.5457). Correlation between baseline BDNF and week 12 BDNF were 0.62 (p < 0.0001) for the whole sample (n = 70), 0.56 (p < 0.0001) for the low dose group and 0.64 (p < 0.0001) for the high dose group.
Table 2.
Mean (STD) BDNF (ng/ml) for all completers and by dose group.
| Group | n | Baseline | Week 12 | Change | p-value |
|---|---|---|---|---|---|
| All | 70 | 19.1(6.0) | 19.2(5.8) | 0.1 (3.5) | 0.8609 |
| 4 KKW | 38 | 18.6(6.5) | 19.2(5.7) | 0.5 (3.6) | 0.4399 |
| 16 KKW | 32 | 19.7(5.4) | 19.3(5.9) | −0.4 (3.3) | 0.5457 |
There was no significant correlation between caloric expenditure and serum BDNF in the entire sample (τ = −0.1176, p = 0.1499) (Table 3). There was also no significant correlation between change in BDNF and change in IDS-C score (τ = 0.0111, p = 0.8946). For the high dose group alone, there was no significant correlation with energy expenditure (τ = 0.0363, p = 0.7704) or change in IDS-C score (τ = 0.0348, p = 0.7853). Similar results were found for the low dose group for correlation with energy expenditure (τ = −0.1537, p = 0.1745) and change in IDS-C (τ = −0.0837, p = 0.4712). In addition, BDNF at baseline did not correlate significantly with IDS-C score at baseline (τ = 0.0825, p = 0.4124).
Table 3.
Correlations for all subjects and by group.
| Outcome | Group | n | Change in BDNF with outcome | p-value |
|---|---|---|---|---|
| Mean kcal/week | All | 70 | −0.1176 | 0.1499 |
| Mean kcal/week | 4 KKW | 38 | −0.1537 | 0.1745 |
| Mean kcal/week | 16 KKW | 32 | 0.0363 | 0.7704 |
| Change in IDS-C | All | 68 | −0.0111 | 0.8946 |
| Change in IDS-C | 4 KKW | 37 | −0.0837 | 0.4712 |
| Change in IDS-C | 16 KKW | 31 | 0.0348 | 0.7853 |
Results of the moderator analysis showed that BMI, marital status, and Q-LES-Q were all significant covariates in addition to BDNF level (Table 4). IDS-C scores decreased significantly over the course of the 12-week study period (time effect, p < 0.0001) and higher levels of baseline BDNF were associated with a steeper decrease (BDNF by time effect, p = 0.0046). Table 4 also shows that baseline BMI and BDNF were involved in a significant three-way interaction with time (BDNF by BMI by time effect, p = 0.0090). The nature of this interaction is illustrated in Figs. 1 and 2 which show the effect in IDS-C improvement trajectory by quartile of high versus low BDNF. Fig. 1 plots the hypothetical trajectories of subjects entering the study with BDNF values at the 25th and 75th percentile, but with average values for all other covariates. Baseline IDS-C score was included in the model but the interaction of baseline IDS-C and baseline BDNF did not significantly affect the model, so the model was adjusted for baseline IDS-C score and the mean baseline IDS-C value was used for the constructed trajectories. Fig. 2 demonstrates the interaction between baseline BDNF and BMI. Hypothetical subjects with the same BDNF level, but with BMI at the 75th percentile (Fig. 2B) diverge much more in IDS-C score over the study period than those with BMI at the 25th percentile (Fig. 2A). The high and low values of BDNF and BMI were determined by the 25th percentile (low BDNF 14.8, low BMI 26.0) and 75th percentile (high BDNF 22.8, high BMI 35.2) of the baseline distribution of these measures. It should be noted that since our sample was generally obese, even the “low” BMI trajectories use a value in the obese range of the continuous distribution of BMIs from study subjects.
Table 4.
Mixed-effects model results for IDS-C.
| Effect | Estimates | Standard error | Num df | Den df | F Stat | p-value |
|---|---|---|---|---|---|---|
| Intercept | −1.0013 | 0.91 | ||||
| Log (Week) | −4.1695 | 0.55 | 1 | 87 | 57.06 | <0.0001 |
| BL BDNF | 0.1096 | 0.15 | 1 | 90 | 0.56 | 0.4553 |
| BL BDNF*Log (Week) | −0.2569 | 0.09 | 1 | 85 | 8.47 | 0.0046 |
| BL IDS-C | −0.1056 | 0.13 | 1 | 104 | 0.63 | 0.4279 |
| BL IDS-C * Log (Week) | −0.1632 | 0.08 | 1 | 88 | 4.58 | 0.0351 |
| BL BMI | 0.0777 | 0.15 | 1 | 90 | 0.29 | 0.5945 |
| BL BMI * BL BDNF | 0.0257 | 0.03 | 1 | 89 | 1.04 | 0.3102 |
| BL BMI * Log (Week) | −0.0592 | 0.09 | 1 | 85 | 0.44 | 0.5067 |
| BL BMI * BL BDNF * Log (Week) | −0.0411 | 0.02 | 1 | 85 | 7.15 | 0.0090 |
| Marital status | 2.8396 | 1.18 | 1 | 94 | 5.83 | 0.0177 |
| BL Q-LES-Q GA | −0.1548 | 0.06 | 1 | 96 | 6.29 | 0.0138 |
Fig. 1.
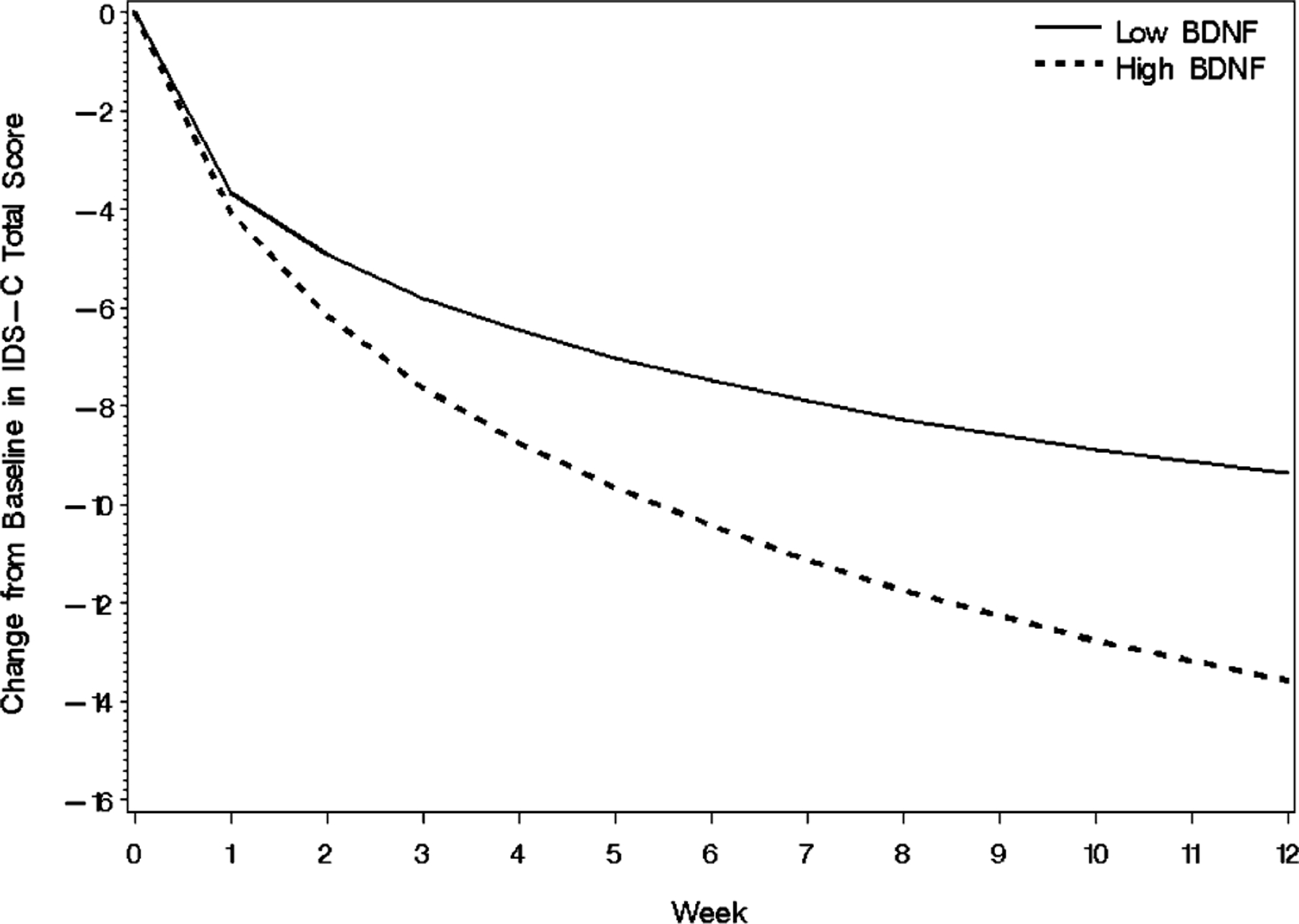
Mixed-effects model results on IDS-C trajectory by BDNF quartile. Lines represent the predicted outcome trajectories for hypothetical subjects who entered the study with BDNF values at the 25th and 75th percentile.
Fig. 2.
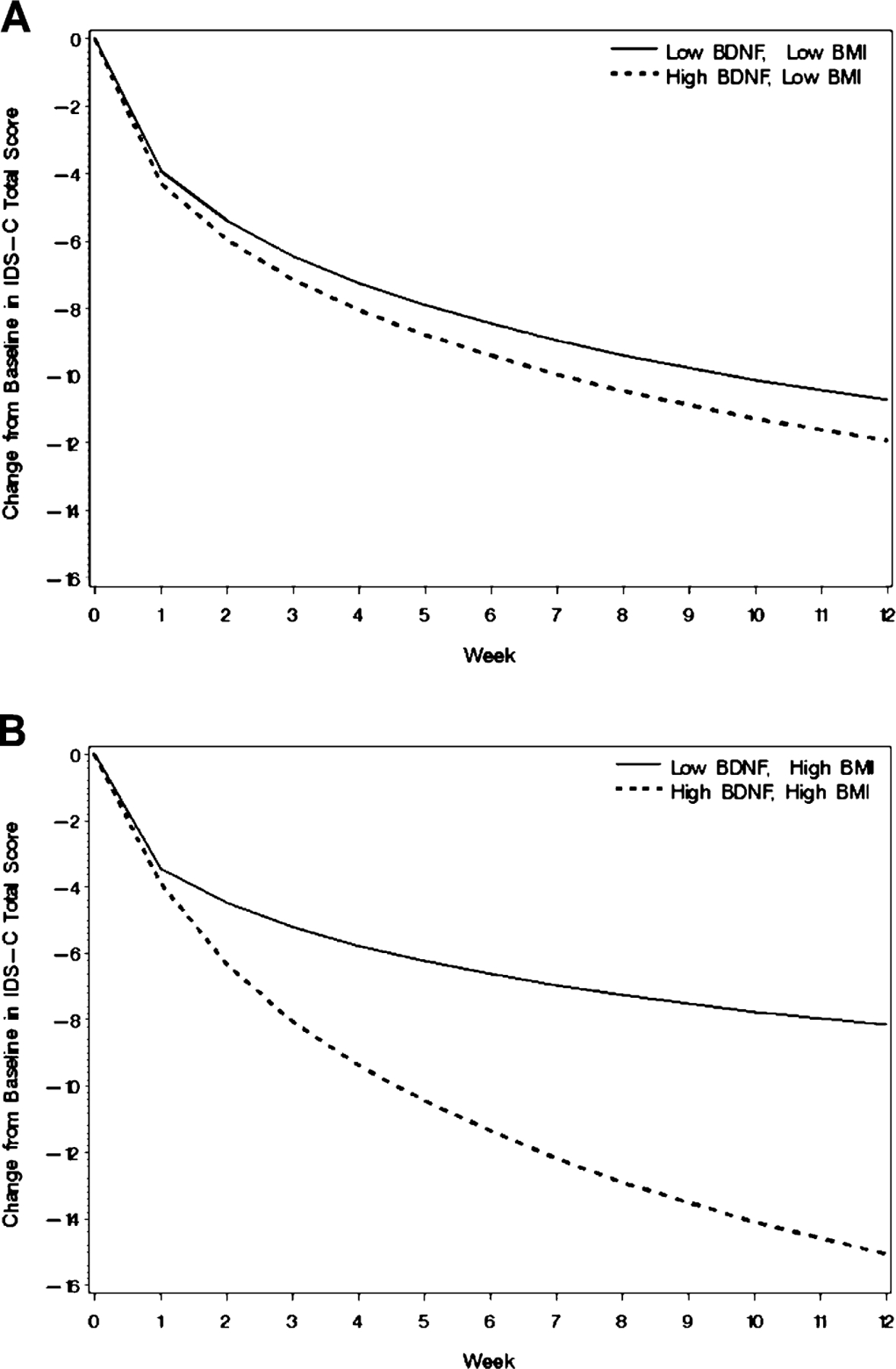
Mixed-effects model results on IDS-C trajectory by BDNF quartile for subjects in the lowest and highest BMI quartiles. Lines represent the (A) Outcome trajectory by quartile, low BMI subjects, (B) Outcome trajectory by quartile, high BDNF subjects.
4. Discussion
In this study we found that baseline BDNF levels moderate the effect of exercise augmentation in reducing depressive symptoms through an interaction with BMI. Subjects entering the study with higher resting BDNF experienced more rapid improvement in symptoms on the IDS-C. From a hypothesis generating standpoint, this finding is especially interesting because it suggests that exercise may work differently than other MDD treatments and may be superior as an augmentation treatment. In TREAD’s design, subjects entered with resting BDNF levels which were potentially already “corrected” by SSRI treatment. We can speculate that SSRIs may “prime” subjects to respond to exercise treatment which itself acts by a different mechanism. Support for differential action of exercise augmentation comes from a finding that plasma BDNF is increased by augmentation with medication (Yoshimura et al., 2010). Our findings are also consistent with the synergistic effect of medication and exercise found in animals (Russo-Neustadt et al., 2001) and suggest that exercise should be considered for patients with partial response to medications.
Interestingly, the effect of baseline resting BDNF level became much greater as subject BMI increased. Since BMI is itself correlated inversely with peripheral BDNF levels (El-Gharbawy et al., 2006; Han et al., 2010; Lommatzsch et al., 2005), the effect of the BDNF “boost” from pre-treatment may be even more important in those with high BMI. On the other hand, a possible confounder is that in TREAD exercise was dosed by weight so subjects with high BMI automatically had higher energy expenditure goals. However, there was no dose effect on symptom improvement, suggesting it is unlikely that the interaction between BMI and baseline BDNF is a dose effect. It may also be the case that high BDNF facilitated physiological changes related to exercise–weight loss and reversal of metabolic abnormalities. These are thought to independently predispose to mood symptoms and so their correction may have provided an additional impetus to recovery. That high BDNF at treatment onset may improve treatment efficacy in some cases is also supported by a study in which treatment resistant depressed subjects were treated with ECT, which found that high baseline BDNF was associated with remission (Piccinni et al., 2009).
In this analysis, we show that resting serum BDNF level remains stable during the course of exercise augmentation treatment of MDD. These results suggest a lack of general effect of change in BDNF with exercise in those already on an SSRI. However, as stated above, there is a strong relationship between BDNF level at baseline and change in symptoms. Aside from the possibility that exercise augmentation does not increase BDNF, this result could occur if, hypothetically, BDNF in the serum plateaus and thus does not reflect ongoing increase in central BDNF expression/action. Since little is known about the physiology of the relationship between peripheral and central BDNF, it is impossible to make a definitive interpretation. It is possible that measurement of another BDNF fraction may be more sensitive to changes in CNS expression. It is important to note, both studies reporting increases in BDNF following exercise training in healthy subjects measured plasma rather than serum BDNF. However, because serum BDNF is the standard measured fraction in depression literature and has more robust evidence that it is altered in MDD, we felt that it was the most appropriate for use in TREAD. In addition, these studies enrolled male subjects only – because the TREAD sample is mostly female it is difficult to draw a direct comparison.
Other factors related to the intervention, such as the type of exercise, the study duration, and the study design could also account for the differences in results from previously published studies. Additionally, in TREAD, our sample was composed primarily of obese subjects – this limits the applicability of the finding to the obese, and it may or may not been seen in depressed exercisers of normal weight. Finally, many factors act on BDNF release in addition to exercise and mood. While some of these are sufficiently understood – e.g. diet – to be at least partially controlled, others likely exert a tangible confounding on the results. Future studies designed specifically to examine the role of BDNF in exercise treatment of MDD are needed to clarify these issues.
Acknowledgments
TREAD was supported by NIMH grant #5-R01-MH067692 and by a NARSAD Independent Investigator Award (PI: Dr. Trivedi). Funding for the BDNF ELISAs was provided by the Sara M. and Charles E. Seay Center for Basic and Applied Research.
Role of funding source
Neither the NIMH, NARSAD, nor the Seay Center had further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Statement of financial conflict of interest
Marisa SP Toups does not have any interests to disclose.
Tracy L. Greer has received grant support from a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award and National Institutes of Health.
Benji T. Kurian has received research support from Targacept, Inc., and Pfizer.
Bruce D. Grannemann does not have any interests to disclose.
Thomas J. Carmody reports that he has received research support from Cyberonics, Inc.
Ryan Huebinger does not have any interests to disclose.
Chad Rethorst does not have any interests to disclose.
Madhukar H. Trivedi reports that he has received research support from the Agency for Healthcare Research and Quality (AHRQ), Corcept Therapeutics, Inc., Cyberonics, Inc., Merck, National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals (Epix), Solvay Pharmaceuticals, Inc., and Targacept. He has received consulting and speaker fees from Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Eli Lilly & Company, Meade Johnson, Medtronic, Neuronetics, Otsuka Pharmaceuticals, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., Sepracor, SHIRE Development, VantagePoint, and Wyeth-Ayerst Laboratories.
References
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic Medicine 2007;69:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Tong KY, Yip SP. Relationship of serum brain-derived neurotrophic factor (BDNF) and health-related lifestyle in healthy human subjects. Neuroscience Letters 2008;447:124–8. [DOI] [PubMed] [Google Scholar]
- Corey-Lisle PK, Nash R, Stang P, Swindle R. Response, partial response, and nonresponse in primary care treatment of depression. Archives of Internal Medicine 2004;164:1197–204. [DOI] [PubMed] [Google Scholar]
- Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neuroscience Letters 2009;451:152–5. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–56. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. American Journal of Preventive Medicine 2005;28:1–8. [DOI] [PubMed] [Google Scholar]
- El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. Journal of Clinical Endocrinology & Metabolism 2006;91:3548–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus 2009;19:973–80. [DOI] [PubMed] [Google Scholar]
- Gustafsson G, Lira CM, Johansson J, Wisen A, Wohlfart B, Ekman R, et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Research; 2009. [DOI] [PubMed]
- Han JC, Muehlbauer MJ, Cui HN, Newgard CB, Haqq AM. Lower brain-derived neurotrophic factor in patients with Prader-Willi syndrome compared to obese and lean control subjects. Journal of Clinical Endocrinology & Metabolism 2010;95:3532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Research 2002;109:143–8. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiology of Aging 2005;26:115–23. [DOI] [PubMed] [Google Scholar]
- Martinsen EW, Medhus A, Sandvik L. Effects of aerobic exercise on depression: a controlled study. British Medical Journal (Clinical Research Edition) 1985; 291:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni A, Del Debbio A, Medda P, Bianchi C, Roncaglia I, Veltri A, et al. Plasma Brain-Derived Neurotrophic Factor in treatment-resistant depressed patients receiving electroconvulsive therapy. European Neuropsychopharmacology 2009;19:349–55. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of BDNF from the brain during exercise. Experimental Physiology; 2009. [DOI] [PubMed]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activityeantidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behavioural Brain Research 2001;120:87–95. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 2009; 42:270–6. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behavioural Pharmacology 2007;18:391–418. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological Psychiatry 2008;64:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biological Psychiatry 2003;54:70–5. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, et al. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. Journal of Clinical Psychiatry, In press [DOI] [PMC free article] [PubMed]
- Trivedi MH, Greer TL, Grannemann BD, Chambliss HO, Jordan AN. Exercise as an augmentation strategy for treatment of major depression. Journal of Psychiatric Practice 2006a;12:205–13. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Galper DI, Sunderajan P, et al. TREAD: Treatment with Exercise Augmentation for Depression: study rationale and design. Clinical Trials 2006b;3:291–305. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry 2006c;163:28–40. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience 1999;2: 266–70. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Ikenouchi-Sugita A, Hori H, Umene-Nakano W, Katsuki A, Hayashi K, et al. Adding a low dose atypical antipsychotic drug to an antidepressant induced a rapid increase of plasma brain-derived neurotrophic factor levels in patients with treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:308–12. [DOI] [PubMed] [Google Scholar]


