Abstract
Objective:
Most patients with major depressive disorder (MDD) require second-step treatments to achieve remission. The TReatment with Exercise Augmentation for Depression (TREAD) study was designed to test the efficacy of aerobic exercise as an augmentation treatment for MDD patients who had not remitted with antidepressant treatment.
Method:
Eligible participants in this randomized controlled trial were sedentary individuals (men and women aged 18–70 years) diagnosed with DSM-IV nonpsychotic MDD who had not remitted with selective serotonin reuptake inhibitor (SSRI) treatment. Participants were recruited through physician referrals and advertisements. A total of 126 participants were randomized to augmentation treatment with either 16 kcal per kg per week (KKW) or 4 KKW of exercise expenditure for 12 weeks while SSRI treatment was held constant. Supervised ses sions were conducted at The Cooper Institute, Dallas, Texas, with additional home-based sessions as needed to fulfill the weekly exercise prescription. The primary outcome was remission (as determined by a score ≤ 12 on the Inventory of Depressive Symptomatology, Clinician-Rated). The study took place between August 2003 and August 2007.
Results:
There were significant improvements over time for both groups combined (F1,121 = 39.9, P < .0001), without differential group effect (group effect: F1,134 = 3.2, P = .07; group-by-time effect: F1,119 = 3.8, P = .06). Adjusted remission rates at week 12 were 28.3% versus 15.5% for the 16-KKW and 4-KKW groups, respectively, leading to a number needed to treat (NNT) of 7.8 for 16 KKW versus 4 KKW. Men, regardless of family history of mental illness, and women without a family history of mental illness had higher remission rates by week 12 with higher-dose (women, 39.0%; men, 85.4%) than with lower-dose exercise (women, 5.6%; men, 0.1%) (women: t95 = 2.1, P = .04; men: t88 = 5.4, P < .0001) (NNT: women, 3.0; men, 1.2).
Conclusions:
There was a trend for higher remission rates in the higher-dose exercise group (P < .06), with a clinically meaningful NNT of 7.8 in favor of the high exercise dose. Significant differences between groups were found when the moderating effects of gender and family history of mental illness were taken into account and suggest that higher-dose exercise may be better for all men and for women without a family history of mental illness.
Most depressed patients require 2 or more treatments to achieve remission.1–3 Studies such as the National Institute of Mental Health–sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial have shown that subsequent treatments yield only an additional 20%–30% increase in remission rates.3–6 For patients with some response to their initial treatment (but not remission), augmentation was the preferred next step.7
Pharmacologic augmentation agents such as lithium, triiodothyronine (T3), buspirone, and atypical antipsychotics4,5,8–10 have improved depression outcomes, but none have proven to be universally effective, and they are associated with side effects and inconvenience (eg, the need for blood monitoring8). Disadvantages of using pharmacologic augmentation strategies include increased cost and risk of interactions. Nonpharmacologic augmentation strategies such as psychotherapy and exercise may provide an effective alternative to medications.
Results of studies using psychotherapy as an augmentation strategy are variable,1,11–13 and therapy can be difficult to implement.14 In STAR*D, no obvious advantage was seen for any specific augmentation (including cognitive-behavioral therapy), and it is difficult to retain patients through multiple steps.6,15 Therefore, identifying alternative augmentation strategies, as well as identifying which treatments are best for which patients, is critical to the advancement of the treatment of depression. Unfortunately, previous trials have primarily been designed to look at the efficacy of an intervention and have not been designed to evaluate moderating variables.16,17 Unless moderators are identified for antidepressant treatments, overall response rates will remain low. Therefore, it is critical that studies also be designed to evaluate moderators.
Exercise is a plausible nonpharmacologic augmentation treatment, due to its demonstrated efficacy as a monotherapy18,19 as well as in combination with other depression treatments.20–25 There are also plausible neurobiological mechanisms for the efficacy of exercise as a treatment for depression, such as changes in serotonergic and noradrenergic neuromodulatory function similar to those observed with antidepressant treatments26–28 and increased hippocampal neurogenesis.29 However, the role of exercise as an augmentation to currently unsuccessful antidepressant treatment has not been well studied. The objective of the TReatment with Exercise Augmentation for Depression (TREAD) study was to evaluate the efficacy of 2 doses of aerobic exercise and to determine if there are clinical and demographic moderators of these 2 treatments. While no specific moderators have been identified for exercise as an augmentation, a number of candidates have been found to relate to treatment response and were evaluated in this study.
METHOD
The research protocol for this randomized controlled trial (clinicaltrials.gov Identifier: NCT00076258) was approved and monitored by the institutional review boards of The University of Texas Southwestern Medical Center at Dallas and The Cooper Institute, Dallas, Texas, and by a Data and Safety Monitoring Board composed of experts who were not a part of the study team (board members were affiliated with The University of Texas Southwestern Medical Center at Dallas or with the Epidemiology Data Center, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania). Study activities commenced after potential participants provided informed consent following explanation and discussion of study procedures. The study took place between August 2003 and August 2007.
Participants from the Dallas/Fort Worth area were recruited through physician referrals and advertisements. Inclusion criteria were as follows: (1) men and women aged 18–70 years; (2) diagnosis of nonpsychotic major depressive disorder (MDD) based on the Structured Clinical Interview for DSM-IV Axis I Disorders30 and confirmed by a psychiatrist; (3) completion of > 2 and < 6 months of treatment with a selective serotonin reuptake inhibitor (SSRI), with at least 6 weeks at an adequate dose (ie, 20 mg/d of escitalopram; 40 mg/d of citalopram, paroxetine, or fluoxetine; 25 mg/d of paroxetine controlled-release; or 150 mg/d of sertraline) prior to entry; (4) report of subjective improvement from SSRI monotherapy with at least moderate residual depressive symptomatology, quantified by a 17-item Hamilton Depression Rating Scale (HDRS17)31 score ≥ 14; (5) not engaged in regular exercise (defined as an expenditure of < 35 kcal/kg/d or exercise < 3 days/wk for 20 minutes or less per session over the past month); (6) capable of exercise; and (7) able and willing to provide informed consent.
Exclusion criteria included (1) history, physical examination, or laboratory results indicating a significant medical condition; (2) depression due to a comorbid psychiatric disorder; (3) current pharmacologic or formal psychotherapeutic treatment other than SSRI; (4) treatment resistance, defined as failure of 2 or more pharmacologic treatments of adequate dose and duration during the current depressive episode (as determined by a psychiatrist and by review of the Antidepressant Treatment History Form32,33); and (5) pregnancy or planning a pregnancy in the next year. All participants received a maximal exercise test to determine cardiorespiratory fitness (maximal oxygen consumption, or VO2 max, calculated as liters of oxygen per minute), to screen for abnormal blood pressure and electrocardiogram responses, and to aid in the generation of exercise prescriptions.
Participants were randomized to augmentation treatment using block randomization to ensure that the sample would remain relatively balanced throughout the course of the trial. Randomization numbers were generated using SAS (SAS Institute Inc, Cary, North Carolina). Personnel who collected clinician-rated measures and study physicians who provided medication management and clinical care were blind to augmentation treatment assignment.
Acute-Phase Exercise Intervention
Participants received augmentation with either a higher-end public health dose or a lower-end public health dose of exercise. The 2 exercise doses required total weekly energy expenditures of 4 or 16 kcal per kg per week (KKW). The dose of 16 KKW was selected on the basis of public health physical activity recommendations34 and would require walking at ≈ 4.0 mph for 210 minutes per week. The 4-KKW dose is equivalent to walking at 3.0 mph for approximately 75 minutes per week and reflects minimal activity by public health standards.34
The acute phase lasted 12 weeks. A combined supervised and home-based monitored exercise protocol allowed participants scheduling flexibility. Supervised sessions were conducted at The Cooper Institute, with additional home-based sessions as needed to fulfill the weekly exercise prescription. Participants received 3 supervised sessions the first week and 2 the second week. Beginning with the third week, participants reported to The Cooper Institute each week to be weighed, complete 1 exercise session, and address any exercise-related concerns with The Cooper Institute staff. For participants randomized to 16 KKW, prescribed energy expenditure was 10 KKW the first week, 13 KKW the second week, and 16 KKW in weeks 3–12.
Participants exercised using treadmills, cycle ergometers, or a combination of both in the exercise laboratory with self-selected training intensity. Participants maintained an online diary of the frequency and duration of all exercise sessions via the study Web site. Items recorded during exercise sessions included location (laboratory or home), exercise modality, energy expenditure, duration, heart rates (preexercise and postexercise), and perceived exertion. In addition, precise data from each home-based exercise session were recorded on a heart-rate monitor (Polar SI610; Polar Electro Inc, Lake Success, New York) and downloaded during visits to The Cooper Institute. A behavioral intervention program that incorporated 7 empirically validated strategies optimized participant exercise adherence (see Trivedi et al35).
Medication Management
Participants met with a psychiatrist throughout the study to assess symptomatology and medication safety and tolerability. The dose of SSRI was kept constant. Participants exhibiting a significant exacerbation of symptoms or intolerance to their medication were withdrawn (n = 5).
Assessment Visits and Outcome Measures
Personnel who were conducting research assessments received comprehensive training in research with human subjects, Health Insurance Portability and Accountability Act regulations, and the specific assessment tools. Interrater reliability sessions were conducted quarterly, and established safety procedures were maintained.
Primary clinician-rated measures included the 30-item Inventory of Depressive Symptomatology, Clinician-Rated (IDS-C30)36 and the HDRS17. Remission (defined as an IDS-C30 score ≤ 12) served as the primary outcome measure. The self-report version of the IDS (IDS-SR30) was completed weekly. Note that we have also reported the 16-item Quick Inventory of Depressive Symptomatology, Clinician-Rated (QIDS-C16)37 and Self-Report (QIDS-SR16)37 since they provide a more succinct measure of depressive symptoms. Psychosocial measures, including the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36),38 the Social Adjustment Scale, Self-Report (SAS-SR),39 and the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q),40 were administered at baseline and weeks 6 and 12. Outcome measures were collected prior to the first exercise session of the week.
Statistical Methods
We evaluated all participants who had a baseline visit and at least 1 postbaseline visit. Baseline characteristics were compared between exercise groups using t tests or χ2 tests. Median energy expenditure, minutes of exercise, and adherence to the prescribed amount of exercise over weeks 3 to 12 were compared by Wilcoxon 2-sample test.
The change over time in probability of remission (IDS-C30 score ≤ 12) was compared between groups using a generalized linear mixed model (GLMM)41 as implemented in SAS (Proc Glimmix; SAS Institute Inc, Cary, North Carolina). To identify specific moderators of outcomes17 for the 2 doses of exercise, 24 specific clinical and demographic variables that have been associated with outcomes in previous research were used.3,14,42–44 Since it was impractical to construct an a priori model including all 24 potential preselected covariates along with all possible interactions, the following procedures were used. Continuous and ordinal variables were dichotomized by estimating cut points that best predicted remission. Eight variables were eliminated from further consideration as they predicted remission less accurately than random chance on the basis of a receiver operating characteristic curve analysis. Logistic regression models to predict remission were fit for all subsets45,46 of the 16 remaining variables and their interactions with exercise group and were ranked by goodness of fit as measured by the generalized Akaike information criteria.47 The best-fitting model was selected. By this method, the QIDS-C16 scores, derived from the IDS-C30 scores, and the SF-36 mental scores were selected along with African American race, family history of mental illness, recurrent MDD episode status, gender, and the interactions of all these with exercise group.
Time effects could not be explicitly modeled by the logistic regression; thus, covariate interactions with time could not be selected. However, moderator effects, if present, would be observed in a significant 3-way interaction of covariate by group by time. Therefore, to examine the possible moderator effects of the selected covariates, the covariate-by–exercise group–by-time interaction terms (along with all 2-way interactions) for QIDS-C16 score, SF-36 mental score, race, family history of mental illness, recurrent MDD episode status, and gender were included if significant at the 0.10 level. Moderator effects were examined by estimating exercise-group effects within moderator-defined subgroups. Parameter estimates from the adjusted GLMM for all participants were used to compute the group (initial) effect and group-by-visit interaction (growth) effect for each moderator subgroup after adjusting for mean values of the other covariates. Time was log-transformed to provide a more linear relationship with outcome. Effect sizes were defined as the number needed to treat (NNT)48 to obtain 1 additional remission based on the probability of remission at week 12, estimated from the GLMM. The NNTs are being provided as a general guide to the size of the effect obtained since analyses conducted on subgroups resulted in the use of relatively small samples in comparison to those normally used in depression trials and are not specifically provided as an additional statistical test.
RESULTS
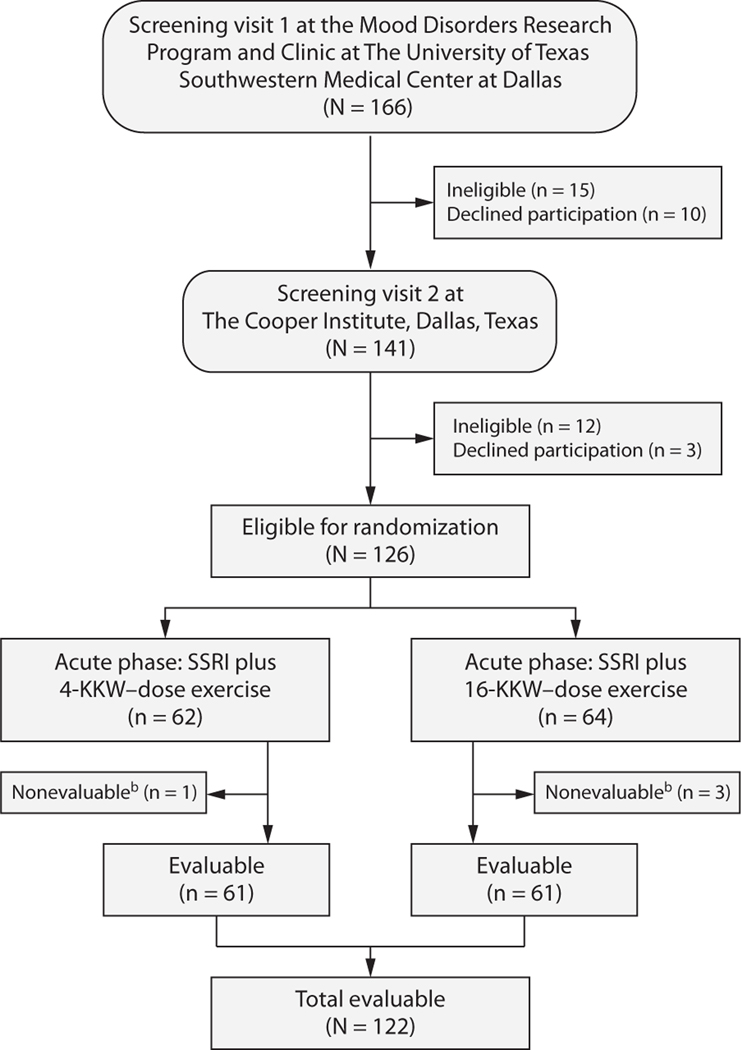
Figure 1 presents the flow of participants through the TREAD study. Of 126 randomized patients, 122 (97%) were evaluable (3 higher-dose subjects and 1 lower-dose subject did not provide postbaseline data and were not evaluable). Participants were predominantly white (86%), female (82%), and married/living together (51%), with a mean age of 47.0 years (SD = 10.0 years) and a mean body mass index (calculated as kg/m2) of 30.9 (SD = 6.2) (Table 1). The mean age at depression onset was 27.4 years (SD = 11.3 years), with the mean length of current episode being 80.4 months (SD = 96.0 months; median = 45 months). Escitalopram was the most commonly used SSRI (73%), followed by sertraline (15%). Baseline symptom severity was consistent with moderate depression. No between-group differences were found.
Figure 1. Flow Diagram of Participants in the TReatment with Exercise Augmentation for Depression (TREAD) Studya.
aNote that 25 participants exited the study before completing the full 12 weeks of the acute phase for the following reasons: 2 for adverse events (both in the 16-KKW group), 10 due to inconvenience or time constraints (2 in the 4-KKW group, 8 in the 16-KKW group), 5 because they had worsening symptoms and/or desired or required a different treatment (3 in the 4-KKW group, 2 in the 16-KKW group), 7 due to noncompliance (3 in the 4-KKW group, 4 in the 16-KKW group), and 1 who was lost to follow-up (in the 4-KKW group). However, all of these participants, with the exception of the 4 nonevaluable participants who had no postbaseline data, are included in the analyses.
bNo postbaseline data available.
Abbreviation: SSRI = selective serotonin reuptake inhibitor.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants (N = 122)
| Baseline Characteristic | 16-KKW Exercise Group (n = 61) | 4-KKW Exercise Group (n = 61) | t or χ2 | df | P Value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, mean (SD), y | 45.6 (10.4) | 48.5 (9.4) | 1.6 | 119 | .11 |
| Sex, female, n (%) | 52 (85.3) | 48 (78.7) | 0.9 | 1 | .35 |
| Race, n (%) | 2.2 | 3 | .53 | ||
| White | 51 (83.6) | 54 (88.5) | |||
| Black | 9 (14.8) | 5 (8.2) | |||
| Hispanic | 0 (0.0) | 1 (1.6) | |||
| Other | 1 (1.6) | 1 (1.6) | |||
| Weight and fitness, mean (SD) | |||||
| Weight, kg | 87.0 (23.6) | 87.8 (17.7) | 0.2 | 111 | .82 |
| Body mass index | 30.3 (6.8) | 31.4 (5.5) | 1.0 | 113 | .34 |
| VO2 max, L/min | 1.7 (0.5) | 1.7 (0.7) | 0.8 | 103 | .45 |
| Depression, mean (SD) | |||||
| Age at onset, y | 27.8 (10.7) | 27.1 (12.0) | −0.4 | 119 | .72 |
| Length of current episode, mo | 71.2 (93.2) | 89.6 (98.5) | 1.1 | 120 | .29 |
| Weeks of adequate dose of SSRI | 8.8 (8.3) | 6.7 (4.1) | −1.6 | 78 | .10 |
| SSRI, n (%) | 4.2 | 5 | .52 | ||
| Citalopram | 3 (4.9) | 2 (3.3) | |||
| Escitalopram | 45 (73.8) | 44 (72.1) | |||
| Fluoxetine | 1 (1.6) | 3 (4.9) | |||
| Paroxetine controlled-release | 3 (4.9) | 1 (1.6) | |||
| Paroxetine | 0 (0.0) | 2 (3.3) | |||
| Sertraline | 9 (14.8) | 9 (14.8) | |||
| Baseline symptom severity, mean (SD) | |||||
| HDRS17 | 17.8 (3.8) | 18.1 (3.8) | 0.5 | 120 | .65 |
| IDS-C30 | 33.3 (7.1) | 34.7 (7.8) | 1.0 | 119 | .30 |
| QIDS-C16 | 13.9 (2.6) | 14.0 (2.6) | 0.3 | 120 | .76 |
| IDS-SR30 | 31.7 (8.2) | 33.1 (10.9) | 0.8 | 109 | .44 |
| QIDS-SR16 | 12.2 (3.6) | 13.0 (4.3) | 1.2 | 113 | .23 |
| Functional assessment, mean (SD) | |||||
| SAS-SR | 2.5 (0.4) | 2.4 (0.4) | −0.6 | 117 | .58 |
| SF-36 physical | 79.5 (20.5) | 80.3 (20.7) | 0.2 | 116 | .83 |
| SF-36 mental | 49.8 (14.5) | 49.0 (15.8) | −0.3 | 117 | .77 |
| Q-LES-Q general activities | 59.0 (10.8) | 60.7 (10.7) | 0.8 | 115 | .40 |
Abbreviations: HDRS17 = 17-Item Hamilton Depression Rating Scale; IDS-C30 = 30-Item Inventory of Depressive Symptomatology, Clinician-Rated; IDS-SR30 = 30-Item Inventory of Depressive Symptomatology, Self-Report; KKW = kcal/kg/wk; QIDS-C16 = 16-Item Quick Inventory of Depressive Symptomatology, Clinician-Rated; QIDS-SR16 = 16-Item Quick Inventory of Depressive Symptomatology, Self-Report; Q-LES-Q = Quality of Life Enjoyment and Satisfaction Questionnaire; SAS-SR = Social Adjustment Scale, Self-Report; SF-36 = Medical Outcomes Study 36-Item Short-Form Health Survey; SSRI = selective serotonin reuptake inhibitor; VO2 max = maximal oxygen consumption.
Exercise
Table 2 shows median kcal expended, minutes of exercise, and degree of adherence to the prescribed amount of exercise by week. Median kcal expended per week during weeks 3–12 were significantly greater in the 16-KKW group than in the 4-KKW group (824 kcal vs 290 kcal; P < .0001), as were minutes of exercise per week (132 minutes vs 60 minutes; P < .0001). The median number of exercise sessions per week was not significantly different between groups (P = .19), with 3 sessions/wk in the 16-KKW group and 2 sessions/wk in the 4-KKW group. Median adherence rates were significantly greater (P = .0005) in the 4-KKW group (99.4%) than in the 16-KKW group (63.8%).
Table 2.
Median Exercise and Adherence Data by Treatment Group by Week (N = 122)
| Week | Treatment Group, n |
kcal Expenditure per Week, Median |
Duration of Exercise, Minutes per Week, Median |
Percent Adherencea per Week, Median |
||||
|---|---|---|---|---|---|---|---|---|
| 4 KKW | 16 KKW | 4 KKW | 16 KKW | 4 KKW | 16 KKW | 4 KKW | 16 KKW | |
| 1 | 61 | 60 | 345.0 | 781.0 | 71.0 | 157.0 | 100.0 | 99.9 |
| 2 | 59 | 58 | 314.0 | 864.5 | 65.0 | 163.0 | 99.7 | 92.1 |
| 3 | 61 | 53 | 309.0 | 1,072.0 | 64.0 | 193.0 | 99.6 | 84.4 |
| 4 | 58 | 50 | 303.0 | 976.0 | 62.0 | 179.5 | 99.6 | 82.1 |
| 5 | 58 | 48 | 295.0 | 915.5 | 61.5 | 175.5 | 99.3 | 83.0 |
| 6 | 58 | 50 | 303.5 | 872.0 | 63.5 | 152.5 | 100.0 | 70.5 |
| 7 | 55 | 48 | 302.0 | 796.0 | 61.0 | 126.5 | 99.5 | 65.0 |
| 8 | 54 | 46 | 306.0 | 928.5 | 61.0 | 142.0 | 99.2 | 61.0 |
| 9 | 54 | 46 | 302.0 | 542.5 | 57.5 | 93.0 | 99.4 | 41.3 |
| 10 | 49 | 44 | 300.0 | 886.0 | 58.0 | 132.0 | 99.6 | 64.8 |
| 11 | 50 | 37 | 233.0 | 736.0 | 47.5 | 120.0 | 67.8 | 60.0 |
| 12 | 53 | 48 | 144.0 | 379.0 | 30.0 | 75.5 | 44.2 | 33.1 |
Percent adherence = (actual kcal/prescribed kcal) × 100. As an example, a person weighing 70 kg would need to expend 1,120 kcal per week at the 16-KKW dose or 280 kcal per week at the 4-KKW dose, assuming 100% adherence. Abbreviation: KKW = kcal/kg/wk.
Both groups had slight mean weight increases from baseline to exit (4-KKW group: mean = 0.67 kg [SD = 2.69 kg]; P = .06), although the increase was significant only in the 16-KKW group (mean = 1.05 kg [SD = 2.84 kg]; P = .005). While the 16-KKW group significantly increased from baseline in VO2 max (mean = 0.13 L/min [SD = 0.21 L/min]; P < .001), the 4-KKW group did not (mean = 0.10 L/min [SD = 0.68 L/min]; P = .76).
Remission
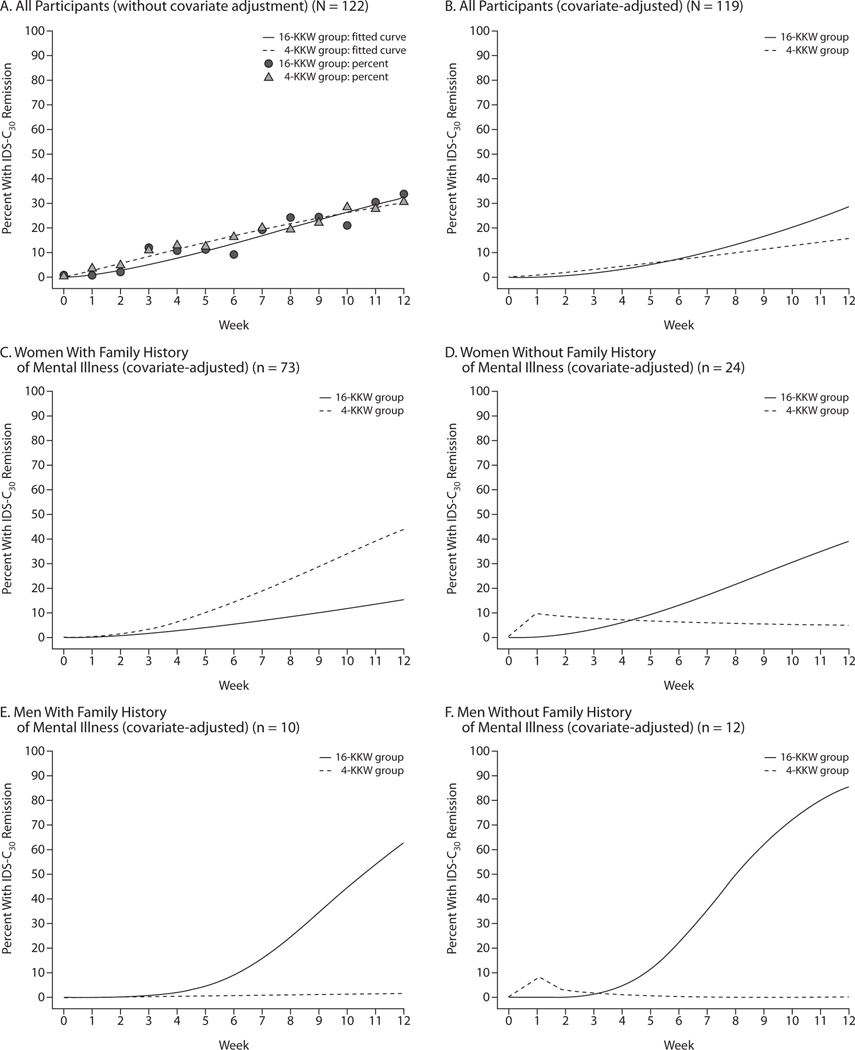
Exit remission rates were identical at 29.5% (18 of 61) for both exercise groups. Figure 2A shows unadjusted percents remitted and GLMM fitted curves. Estimated remission rates improved significantly during the study for all participants (F1,1118 = 49.4, P < .0001). The difference between groups was not significant (group effect: F1,1118 = 1.9, P = .17; group-by-time effect: F1,1118 = 1.6, P = .20).
Figure 2. Probability of Remissiona Estimated From a Generalized Linear Mixed Model.
aRemission defined as a 30-Item Inventory of Depressive Symptomatology, Clinician-Rated (IDS-C30) score ≤ 12.
The covariate-adjusted GLMM (Figure 2B) also showed significant improvement over time for both groups combined (F1,121 = 39.9, P < .0001), although the group effect showed only a trend toward significance (group effect: F1,134 = 3.2, P = .07; group-by-time effect: F1,119 = 3.8, P = .06). Adjusted remission rates at week 12 were 15.5% versus 28.3% for the 4-KKW and 16-KKW groups, respectively (t92 = 1.3, P = .18), leading to an NNT of 7.8 for 16 KKW versus 4 KKW.
Gender and family history of mental illness were identified as treatment moderators due to a significant gender-by-group-by-time interaction (F1,90 = 6.6, P = .01) and family history–by-group-by-time interaction (F1,95 = 5.6, P = .02). To better understand the moderating effects of gender and family history, the group (initial) and group-by-visit interaction (growth) effects for all 4 subgroups (women with a family history of mental illness, women with no family history, men with a family history, and men with no family history) were estimated from the GLMM (Figure 2C–2F).
Women with a family history of mental illness exhibited numerically greater growth in remission rates in the 4-KKW group than in the 16-KKW group (Figure 2C), but the difference was not significant (t131 = 0.5, P = .59). At week 12, estimated remission rates in this subgroup were 43.7% and 15.5% for the 4-KKW and 16-KKW groups, respectively (t93 = 2.0, P = .04) (NNT = 3.5). Among women and men with no family history of mental illness (Figure 2D and 2F, respectively), the efficacy for the groups was reversed, with growth in remission rates being significantly greater for the 16-KKW group than the 4-KKW group (t83 = 2.4,P = .02; and t85 = 4.5, P ≤ .0001, respectively). Remission rates at week 12 for women with no family history were 39.0% for the 16-KKW group and 5.6% for the 4-KKW group (t95 = 2.1, P = .04) (NNT = 3.0), while for men with no family history, rates were 85.4% and 0.1% for the higher-dose and lower-dose groups, respectively (t88 = 5.4, P < .0001) (NNT = 1.2). Men with a family history of mental illness (Figure 2E) also experienced significantly greater remission-rate growth in the higher-dose versus lower-dose group (t95 = 2.3, P = .02), resulting in week 12 remission rates of 62.6% and 1.7% in the 16-KKW and 4-KKW groups, respectively (t82 = 2.9, P = .005) (NNT = 1.6).
DISCUSSION
Covariate-adjusted remission rates were 15.5% for lower-dose and 28.3% for higher-dose exercise augmentation, leading to an NNT of 7.8, well within the range of findings that warrant change in clinical practice (ie, the recommendation of exercise as an augmentation treatment for depression). Evaluation of moderating variables indicates that men with or without a family history of mental illness and women with no family history of mental illness had higher remission rates with higher-dose exercise compared to lower-dose exercise.
Studies6,10,42 of treatment augmentation generally yield effect sizes that are equivalent to or smaller than those observed in this trial. Recent data4,5 examining aripiprazole as an augmentation treatment show that, after an adequate first step with an antidepressant, differences in remission rates between the treatments and placebo are similar to this study—approximately 10%.
The unadjusted remission rates for exercise augmentation were identical at 29.5% (18 of 61) for both treatment groups. These rates are comparable to those of level 2 in STAR*D (ie, citalopram augmentation with bupropion or buspirone, which yielded remission rates of 29.7% and 30.1%, respectively, based on the HDRS17).6 This finding suggests that exercise is a reasonable alternative to medication augmentation for patients who prefer a nonpharmacologic option.
These results also demonstrate the importance of examining moderating variables,16,17 as specific moderator effects revealed important, clinically relevant findings that were not otherwise evident. Participants with no family history of mental illness were significantly more likely to achieve remission with the higher dose of exercise as opposed to the lower dose. Men with a family history of mental illness were also more likely to achieve remission with higher-dose exercise, whereas women with a family history of mental illness appeared to benefit more from lower-dose exercise, although these effects did not reach significance. While both doses appeared to yield a benefit with SSRI treatment, the higher dose appeared superior to the lower dose for men in general and in women with no family history of mental illness. Conversely, the lower dose may be sufficient for women with a history of mental illness.
A review by Teychenne et al49 reported that less than 90 minutes of weekly physical activity in depressed individuals resulted in effective reductions in the likelihood of depression in participants. These findings illustrate why it is important to examine moderating variables, as high-dose exercise may be more effective for some, while low-dose exercise is more effective for others. This study also provides a method for future research to explore the use of moderating variables to develop treatments that can be tailored to the individual. Personalizing treatment for depression should optimize outcomes for each individual.
A limitation of the current study is that participants assigned to the 4-KKW exercise group showed better adherence than those assigned to the 16-KKW exercise group, although there was a clear difference in KKW expenditure between the groups. However, the greater adherence in the 4-KKW group provides evidence that low-dose exercise may be more tolerable for depressed patients and indicates an intervention that may be more acceptable to a wider spectrum of patients. Another limitation of the study is the absence of a true control group. While unadjusted remission rates were identical between groups (29.5%), adjusted remission rates were numerically, although not statistically, different (15.5% for the 4-KKW group versus 28.3% for the 16-KKW group). Future research should be designed to either include an inert control or be conducted with sufficient samples to conduct equivalency analyses. Furthermore, research is needed to examine longer-term outcomes. It may also be useful to examine improvements in fitness and other outcomes such as self-efficacy, as well as how these factors may mediate treatment response.
CONCLUSIONS
This study illustrates 3 important points: (1) exercise is a viable augmentation strategy for depressed patients who are nonresponsive to SSRIs, (2) depressed patients are willing and able to complete a structured exercise program with behavioral support, and (3) exercise treatment dose should be tailored to the individual.
Acknowledgments:
The authors thank the following individuals for their contributions to this project: Several persons assisted with trial implementation, including Tyson M. Bain, MS; Heather O. Chambliss, PhD, FACSM; Alexander N. Jordan, MS; Heather Kitzman-Ulrich, PhD; Jennifer Kupper, MS; Lucille Marcoux, ANP, MSN, RN; Erin L. Sinclair, MA, LPC (all affiliated with The Cooper Institute, Dallas, Texas, at the time of their participation); Ella Daly, MB, MRCPsych (Dr. Daly is currently a full-time employee of Johnson & Johnson Pharmaceutical Research & Development and completed work on this study while she was on the faculty at The University of Texas Southwestern Medical Center at Dallas); Mariam Andersen, MA; Shailesh Jain, MD, MPH; Beverly Kleiber, PhD; David W. Morris, PhD; Anne Marie Jones, MS; and Michelle Rivas, MS, CCRC (all affiliated with The University of Texas Southwestern Medical Center at Dallas at the time of their participation). For assistance with database management, we thank Bradley Witte, BS (The University of Texas Southwestern Medical Center at Dallas), and Beth Wright, MS; Carrie E. Finley, MS; Mei Sui, MD, MPH; and Carolyn E. Barlow, MS (all affiliated with The Cooper Institute, Dallas, Texas). For assistance with statistical analyses (development of moderator selection methodology), we thank Richard M. Golden, PhD, MSEE, BSEE (University of Texas at Dallas) and T. Michael Kashner, PhD, JD, MPH (The University of Texas Southwestern Medical Center at Dallas). We thank A. John Rush, MD, for providing comments on the study design and the manuscript (Dr. Rush was affiliated with The University of Texas Southwestern Medical Center at Dallas at the time of his contribution to the study; he has received consulting fees from the University of Michigan and Brain Resource; has received speaker fees from Otsuka Pharmaceuticals; has received author royalties from Guilford Publications and The University of Texas Southwestern Medical Center at Dallas; and has received research support from the National Institute of Mental Health). We are very grateful to all of the study participants who contributed to this project. We also thank Eric Nestler, MD, PhD, and Carol A. Tamminga, MD, both of the Department of Psychiatry, The University of Texas Southwestern Medical Center, Dallas, for administrative support. None of the acknowledged individuals have potential conflicts of interests or disclosures to report except as noted above.
Funding/support:
This work was supported by NIMH (1-R01-MH067692-01; principal investigator, Dr Trivedi) and was supported in part by a NARSAD Independent Investigator Award (Dr Trivedi), NARSAD Young Investigator Awards (Drs Greer and Galper), and the National Cancer Institute (R44CA139607; principal investigator, Dr Henley).
Potential conflicts of interest:
Dr Trivedi has received research support from the Agency for Healthcare Research and Quality, Corcept Therapeutics, Cyberonics, Merck, NARSAD, National Institute of Mental Health (NIMH), National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix (EPIX), Solvay, and Targacept and has received consulting and speaker fees from Abbott, Abdi Ibrahim, Akzo (Organon), AstraZeneca, Axon Advisors, Bristol-Myers Squibb, Cephalon, CME Institute of Physicians Postgraduate Press, Eli Lilly, Evotec, Fabre-Kramer, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, Meade Johnson, MedAvante, Medtronic, Neuronetics, Otsuka, Pamlab, Parke-Davis, Pfizer, PGxHealth, Rexahn, Sepracor, Shire Development, Takeda, VantagePoint, and Wyeth-Ayerst. Dr Greer has received grant/ research support from NARSAD and the National Institutes of Health. Dr Carmody has been a consultant for Cyberonics. Dr Galper has received grant/research support from NIMH and NARSAD and has received an honorarium from The Cooper Institute. Mr Henley has received research support from the National Cancer Institute. Drs Church, Dunn, Earnest, Sunderajan, and Blair and Mr Grannemann have no potential conflicts of interests to disclose relative to the subject of this article.
Disclaimer:
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Footnotes
Drug names: aripiprazole (Abilify), bupropion (Wellbutrin, Aplenzin, and others), buspirone (BuSpar and others), citalopram (Celexa and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), lithium (Lithobid and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others).
Trial Registration: clinicaltrials.gov Identifier: NCT00076258
REFERENCES
- 1.Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry. 2002;159(8):1354–1360. [DOI] [PubMed] [Google Scholar]
- 2.Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry. 2001;62(11):869–877. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, et al. ; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. [DOI] [PubMed] [Google Scholar]
- 4.Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843–853. [DOI] [PubMed] [Google Scholar]
- 5.Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156–165. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi MH, Fava M, Wisniewski SR, et al. ; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–1252. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski SR, Fava M, Trivedi MH, et al. Acceptability of second-step treatments to depressed outpatients: a STAR*D report. Am J Psychiatry. 2007;164(5):753–760. [DOI] [PubMed] [Google Scholar]
- 8.Augmentation Fava M. and combination strategies in treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 18):4–11. [PubMed] [Google Scholar]
- 9.Nelson JC. Augmentation strategies in depression 2000. J Clin Psychiatry. 2000;61(suppl 2):13–19. [PubMed] [Google Scholar]
- 10.Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T3 augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519–1530, quiz 1665. [DOI] [PubMed] [Google Scholar]
- 11.Kocsis JH, Gelenberg AJ, Rothbaum BO, et al. ; REVAMP Investigators. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009; 66(11):1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829–835. [DOI] [PubMed] [Google Scholar]
- 13.Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739–752. [DOI] [PubMed] [Google Scholar]
- 14.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409–416. [DOI] [PubMed] [Google Scholar]
- 15.Rush AJ, Trivedi MH, Wisniewski SR, et al. ; STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231–1242. [DOI] [PubMed] [Google Scholar]
- 16.Kraemer HC, Wilson GT, Fairburn CG, et al. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. [DOI] [PubMed] [Google Scholar]
- 17.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296(10): 1286–1289. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1–8. [DOI] [PubMed] [Google Scholar]
- 20.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. [DOI] [PubMed] [Google Scholar]
- 21.Dimeo F, Bauer M, Varahram I, et al. Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med. 2001; 35(2):114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinsen EW, Medhus A, Sandvik L. Effects of aerobic exercise on depression: a controlled study. Br Med J (Clin Res Ed). 1985;291(6488):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mather AS, Rodriguez C, Guthrie MF, et al. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry. 2002;180(5):411–415. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006; 12(4):205–213. [DOI] [PubMed] [Google Scholar]
- 25.Veale D, Le Fevre K, Pantelis C, et al. Aerobic exercise in the adjunctive treatment of depression: a randomized controlled trial. J R Soc Med. 1992; 85(9):541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29(1):63–74. [DOI] [PubMed] [Google Scholar]
- 27.Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20(3):160–188. [DOI] [PubMed] [Google Scholar]
- 28.Greer TL, Trivedi MH. Exercise in the treatment of depression. Curr Psychiatry Rep. 2009;11(6):466–472. [DOI] [PubMed] [Google Scholar]
- 29.Yau SY, Lau BW, So KF. Adult hippocampal neurogenesis: a possible way how physical exercise counteracts stress [published online ahead of print September 30, 2010]. Cell Transplant. 2010. [DOI] [PubMed]
- 30.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). New York, NY: Biometrics Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 31.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. [DOI] [PubMed] [Google Scholar]
- 32.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 33.Sackeim HA, Prudic J, Devanand DP, et al. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. J Clin Psychopharmacol. 1990;10(2):96–104. [DOI] [PubMed] [Google Scholar]
- 34.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 35.Trivedi MH, Greer TL, Grannemann BD, et al. TREAD: TReatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials. 2006;3(3):291–305. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996; 26(3):477–486. [DOI] [PubMed] [Google Scholar]
- 37.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36), 1: Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 39.Weissman MM, Prusoff BA, Thompson WD, et al. Social adjustment by self-report in a community sample and in psychiatric outpatients. J Nerv Ment Dis. 1978;166(5):317–326. [DOI] [PubMed] [Google Scholar]
- 40.Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 41.Wolfinger R, O’Connell M. Generalized linear mixed models: a pseudo-likelihood approach. J Statist Comput Simulation. 1993; 48(3):233–243. [Google Scholar]
- 42.Fournier JC, DeRubeis RJ, Shelton RC, et al. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol. 2009;77(4):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlis RH, Alpert J, Nierenberg AA, et al. Clinical and sociodemographic predictors of response to augmentation, or dose increase among depressed outpatients resistant to fluoxetine 20 mg/day. Acta Psychiatr Scand. 2003;108(6):432–438. [DOI] [PubMed] [Google Scholar]
- 44.Rush AJ, Wisniewski SR, Warden D, et al. Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry. 2008;65(8):870–880. [DOI] [PubMed] [Google Scholar]
- 45.Furnival GM. All possible regressions with less computation. Technometrics. 1971;13(2):403–408. [Google Scholar]
- 46.Miller A. Subset Selection in Regression. London, England: Chapman and Hall; 2002. [Google Scholar]
- 47.Bozdogan H. Akaike’s information criterion and recent developments in information complexity. J Math Psychol. 2000;44(1):62–91. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. [DOI] [PubMed] [Google Scholar]
- 49.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46(5):397–411. [DOI] [PubMed] [Google Scholar]