Abstract
One of the mechanisms that bacteria utilize to evade the toxic effects of antibiotics is the active extrusion of structurally unrelated drugs from the cell. Both intrinsic and acquired multidrug transporters play an important role in antibiotic resistance of several pathogens, including Neisseria gonorrhoeae, Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Vibrio cholerae. Detailed knowledge of the molecular basis of drug recognition and transport by multidrug transport systems is required for the development of new antibiotics that are not extruded or of inhibitors which block the multidrug transporter and allow traditional antibiotics to be effective. This review gives an extensive overview of the currently known multidrug transporters in bacteria. Based on energetics and structural characteristics, the bacterial multidrug transporters can be classified into five distinct families. Functional reconstitution in liposomes of purified multidrug transport proteins from four families revealed that these proteins are capable of mediating the export of structurally unrelated drugs independent of accessory proteins or cytoplasmic components. On the basis of (i) mutations that affect the activity or the substrate specificity of multidrug transporters and (ii) the three-dimensional structure of the drug-binding domain of the regulatory protein BmrR, the substrate-binding site for cationic drugs is predicted to consist of a hydrophobic pocket with a buried negatively charged residue that interacts electrostatically with the positively charged substrate. The aromatic and hydrophobic amino acid residues which form the drug-binding pocket impose restrictions on the shape and size of the substrates. Kinetic analysis of drug transport by multidrug transporters provided evidence that these proteins may contain multiple substrate-binding sites.
The development and use of antibiotics has been one of the most important steps towards controlling infectious diseases in the 20th century. However, the subsequent appearance and spread of antibiotic resistance in pathogenic organisms have made many currently available antibiotics ineffective (160, 169). As a consequence, tuberculosis and other infectious diseases are reemerging, causing a serious public health problem (32, 38). To successfully fight the increasing numbers of drug-resistant and multidrug-resistant (MDR) bacteria, extensive knowledge of the molecular mechanisms underlying microbial antibiotic resistance is required.
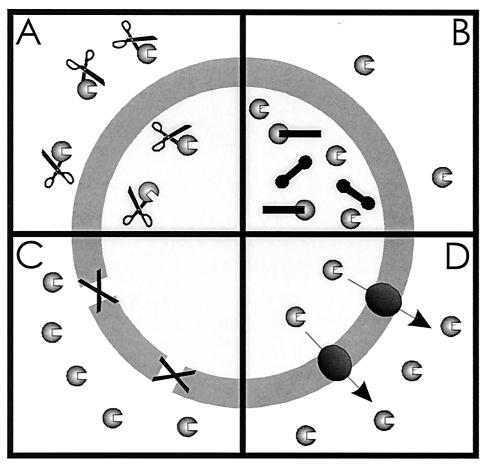
Microorganisms have developed various ways to resist the toxic effects of antibiotics and other drugs (Fig. 1) (83, 169). One of these mechanisms involves the production of enzymes that inactivate antibiotics by hydrolysis or the formation of inactive derivatives (Fig. 1A) (40). Well-known examples are β-lactamases (29, 206) and enzymes that phosphorylate, adenylate, or acetylate aminoglycoside antibiotics (234). A second mechanism of resistance is target alteration (Fig. 1B). Cellular targets can be altered by mutation or enzymatic modification in such a way that the affinity of the antibiotic for the target is reduced (95, 239, 265, 277). A third, more general mechanism of resistance is the inhibition of drug entry into the cell (Fig. 1C). Due to the low permeability of the outer membrane of gram-negative bacteria (175, 176) and the exceptionally efficient barrier of the gram-positive mycobacteria (101), drug diffusion across the cell envelope is reduced. The permeability of the outer membrane can be further decreased by the loss of porins (77, 157, 177). These barriers, however, cannot prevent the drugs from exerting their toxic action once they have entered the cell, and the active efflux of drugs (Fig. 1D) is essential to ensure significant levels of drug resistance (122, 177).
FIG. 1.
Resistance mechanisms in bacteria comprise (A) drug inactivation, (B) target alteration, (C) prevention of drug influx, and (D) active extrusion of drug from the cell.
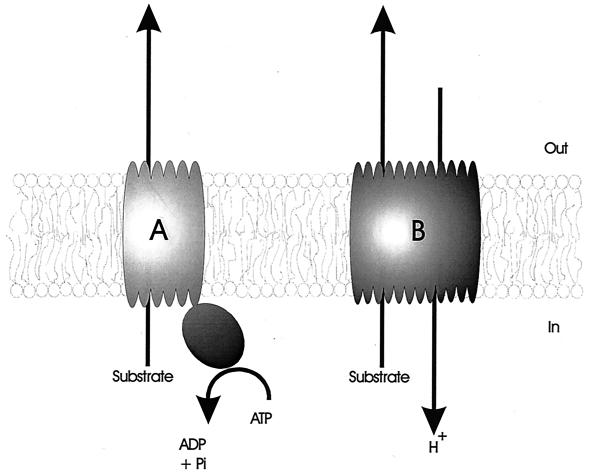
Some transporters, such as the tetracycline efflux proteins (209, 237), are dedicated systems which mediate the extrusion of a given drug or class of drugs. In contrast to these specific drug transporters, the so-called multidrug transporters can handle a wide variety of structurally unrelated compounds (127, 178, 190, 257). On the basis of bioenergetic and structural criteria, multidrug transporters can be divided into two major classes (Fig. 2). Secondary multidrug transporters utilize the transmembrane electrochemical gradient of protons or sodium ions to drive the extrusion of drugs from the cell. ATP-binding cassette (ABC)-type multidrug transporters use the free energy of ATP hydrolysis to pump drugs out of the cell.
FIG. 2.
Schematic representation of the two major classes of multidrug transporters. (A) ABC-type multidrug transporters utilize the free energy of ATP hydrolysis to pump drugs out of the cell. (B) Secondary multidrug transporters mediate the extrusion of structurally unrelated drugs in a coupled exchange with protons or sodium ions.
Here we present a comprehensive review describing the currently known multidrug transporters in bacteria. Important issues addressed are (i) the functional reconstitution of purified multidrug transporters into proteoliposomes, which allows detailed molecular characterization of the proteins, (ii) the transcriptional regulation of genes encoding multidrug efflux systems, (iii) the structure-function relationships that dictate drug recognition and transport, (iv) the presence of multiple drug-binding sites, and (v) the mechanism of transport of both ATP-driven and secondary multidrug transporters.
SECONDARY MULTIDRUG TRANSPORTERS
Most bacterial multidrug efflux systems known to date are sensitive to agents that dissipate the proton motive force (PMF), indicating that they mediate the extrusion of toxic compounds from the cells in a coupled exchange with protons (15, 42, 84, 133, 190). On the basis of size and similarities in the primary and secondary structure, these secondary multidrug transporters can be subdivided into distinct families of transport proteins: the major facilitator superfamily (MFS) (146), the small multidrug resistance (SMR) family (191), the resistance-nodulation-cell division (RND) family (216), and the multidrug and toxic compound extrusion (MATE) family (28). These families are not solely associated with multidrug export but include proteins involved in other PMF-dependent transport processes or other functions.
Major Facilitator Superfamily
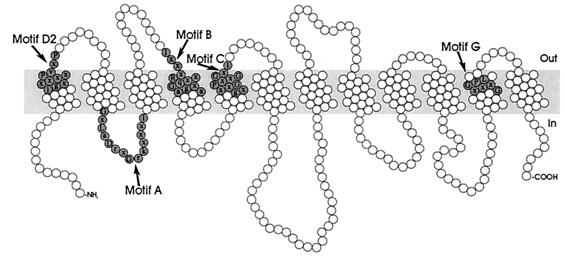
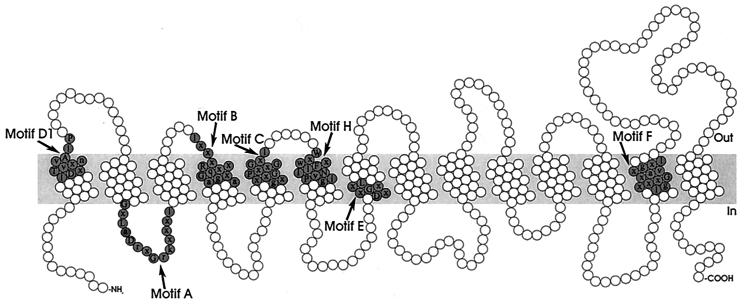
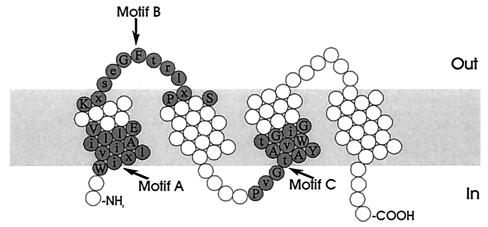
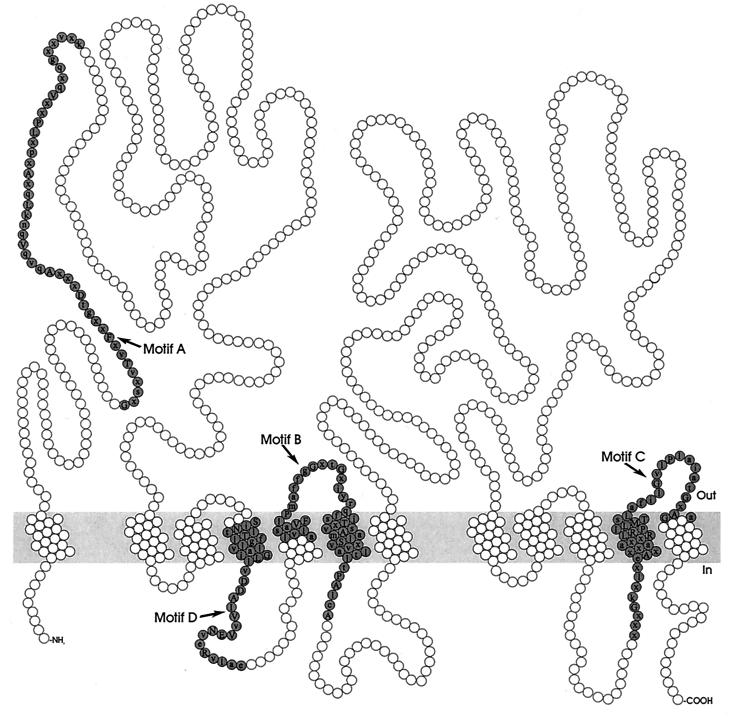
The MFS consists of membrane transport proteins which are found from bacteria to higher eukaryotes and are involved in the symport, antiport, or uniport of various substrates, such as sugars, Krebs cycle intermediates, phosphate esters, oligosaccharides, and antibiotics (146). Hydropathy analysis and alignment of conserved motifs of the resistance-conferring drug efflux proteins revealed that these proteins can be divided into two separate clusters, with either 12 or 14 transmembrane segments (TMS) (Fig. 3 and 4) (188). Table 1 summarizes the multidrug transporters from both the 12-TMS and 14-TMS clusters found in gram-positive and gram-negative bacteria.
FIG. 3.
Structural model for the 12-TMS multidrug transporters of the MFS. The residues constituting the conserved sequence motifs are shaded.
FIG. 4.
Structural model for the 14-TMS multidrug transporters of the MFS. The residues constituting the conserved sequence motifs are shaded.
TABLE 1.
MFS multidrug transporters
| Multidrug transporter | Organism | Accession no(s). | Reference(s) |
|---|---|---|---|
| 12-TMS cluster | |||
| Bcr | Escherichia coli | X63703 | 18 |
| Blt | Bacillus subtilis | L32599 | 4 |
| Bmr | Bacillus subtilis | M33768 | 170 |
| Cmr | Corynebacterium glutamicum | U43535 | 98 |
| EmrD | Escherichia coli | P31442 | 168 |
| LmrP | Lactococcus lactis | X89779 | 21 |
| MdfA (Cmr/CmlA) | Escherichia coli | Y08743, U44900 | 49, 179 |
| NorA | Staphylococcus aureus | D90119 | 278 |
| PmrA | Streptococcus pneumoniae | AJ007367 | 63 |
| Tap | Mycobacterium fortuitum, Mycobacterium tuberculosis | AJ000283 | 5 |
| 14-TMS cluster | |||
| Bmr3 | Bacillus subtilis | D50098 | 181 |
| EmrB | Escherichia coli | P27304 | 135 |
| LfrA | Mycobacterium smegmatis | U40487 | 244 |
| QacA | Staphylococcus aureus | X56628 | 213 |
| QacB | Staphylococcus aureus | U22531 | 191 |
| VceB | Vibrio cholerae | AF012101 | 37 |
The 12-TMS cluster of multidrug transporters.
The Staphylococcus aureus NorA protein was first discovered in a quinolone- and methicillin-resistant clinical isolate (251). Sequencing and characterization of the norA gene revealed that NorA is a 388-amino-acid membrane protein that confers resistance to hydrophilic compounds and only low or no resistance to hydrophobic drugs (271, 278). Studies on the substrate specificity demonstrated that NorA is a true multidrug transporter, mediating resistance to a range of structurally dissimilar drugs (171, 172).
A structural and functional homolog of NorA has been found in a Bacillus subtilis strain selected with increasing concentrations of rhodamine 6G and named Bmr for bacterial multidrug resistance (170). Ahmed et al. (4) described a second multidrug transporter of B. subtilis. This protein, named Blt, is highly homologous to Bmr and confers resistance to a similar range of drugs. Despite the similarities between Bmr and Blt, their expression patterns are different. Under standard cultivation conditions, Bmr is expressed while the expression of Blt is undetectable. The differences in regulation of transcription and the operon organization suggest that Bmr and Blt have distinct physiological functions and that the two transporters may be involved in the transport of different natural substrates (4).
PMF-dependent drug extrusion in Lactococcus lactis was first observed in strains exposed to increasing stepwise concentrations of daunomycin and rhodamine 6G (20). The gene responsible for this activity, lmrP, encodes an integral membrane protein that shows 19 and 24% homology with the multidrug transporters Bmr and NorA, respectively (21). Transport studies in whole cells and inside-out membrane vesicles demonstrated that LmrP mediates the electrogenic export of lipophilic drugs from the inner leaflet of the membrane to the aqueous phase (23, 203) (see below).
The Escherichia coli transporter Bcr confers resistance to the diketopiperazine antibiotic bicyclomycin (18). Vedantam-Gayatri et al. (261) revealed that the sulfathiazole resistance determinant sur is identical to bcr, indicating that Bcr is involved in resistance to structurally unrelated antibiotics. EmrD, an E. coli homolog of Bcr, is involved in a low-energy shock adaptive response. Expression of EmrD protects the cell against the uncouplers CCCP (carbonyl cyanide m-chlorophenylhydrazone) and tetrachlorosalicylanilide, but does not affect resistance to bicyclomycin, quinolones, or chloramphenicol, suggesting that EmrD has a fairly narrow substrate spectrum (167). Another MDR transporter found in E. coli is MdfA (49), also known as Cmr (179) and CmlA (208), which was initially described as a membrane-associated efflux pump for chloramphenicol. However, detailed analysis of the substrate spectrum revealed that MdfA confers resistance to a wide range of unrelated neutral and positively charged drugs. Substitutions of E26 in the first transmembrane segment demonstrated that a single membrane-embedded negatively charged residue is critical for the recognition of positively charged drugs by MdfA (50). Surprisingly, overexpression of MdfA causes decreased resistance to spectinomycin (19). Thus, the activity of a multidrug pump can result in resistance to several toxic compounds and increased sensitivity to certain others.
The multidrug transporter Cmr of the nonpathogenic organism Corynebacterium glutamicum significantly increases resistance to several structurally unrelated antibiotics when expressed in E. coli but does not affect antibiotic resistance when expressed in C. glutamicum. The inability of Cmr to confer resistance in its original host could result from a lower expression level of Cmr (98).
Mycobacteria cause several diseases, including tuberculosis and leprosy. The treatment of these infections is often difficult because mycobacteria are intrinsically resistant to most commonly used antibiotics. The Tap multidrug efflux pump in Mycobacterium fortuitum and Mycobacterium tuberculosis confers resistance to aminoglycosides and tetracycline and makes it more difficult to control mycobacterial infections (5). The number of TMS could not be unambiguously determined from the primary sequence and was estimated to be close to 10. However, Aínsa et al. (5) suggested, on the basis of the homology between Tap and other multidrug transporters of the MFS, that Tap is a 12-TMS transporter.
Several studies suggested that a reserpine-sensitive multidrug transporter is involved in fluoroquinolone resistance in Streptococcus pneumoniae (15, 25, 26, 279). Recently, Gill et al. (63) cloned and characterized the efflux pump PmrA of S. pneumoniae. Analogous to NorA, Bmr, and LmrP, PmrA is inhibited by the plant alkaloid reserpine. Expression of PmrA increases resistance to acriflavine, ethidium, and the fluoroquinolones ciprofloxacin and norfloxacin.
The 14-TMS cluster of multidrug transporters.
Resistance to antiseptic and disinfectant compounds, including intercalating dyes, quarternary ammonium compounds, and diamines, is widespread among multidrug-resistant strains of Staphylococcus aureus. One of the energy-dependent export mechanisms responsible for this resistance is encoded by the qacA gene (248). Hydropathy analysis (213) and topological analysis using alkaline phosphate and β-galactosidase fusions (192) indicated that the QacA protein contains 14 TMS. Whereas QacA confers resistance to monovalent and divalent organic cations, the closely related QacB only confers resistance to monovalent organic cations (192). It has been speculated that the extensive use in hospital environments of bacteriostatic divalent cations, such as chlorhexidine, resulted in the evolution of qacA from qacB (193).
Sequencing of an E. coli locus for multidrug resistance revealed the presence of two open reading frames, designated ermA and emrB (135). The emrB gene encodes a 513-amino-acid protein which is homologous to QacA. ErmA, a 42.7-kDa membrane fusion protein with a single TMS and a large C-terminal periplasmic domain, is thought to form a continuous channel between the inner and outer membrane (126, 135). EmrAB confers resistance to hydrophobic uncouplers and antibiotics (59, 135). Tanabe et al. (246) characterized the E. coli emrKY operon. Transcription of emrKY is growth phase dependent and induced by tetracycline. EmrK and EmrY are 50 and 63% identical to EmrA and EmrB, respectively, suggesting a similar function. However, the involvement of EmrKY in multidrug efflux remains to be demonstrated.
Bmr3, a third B. subtilis multidrug transporter, shows moderate homology to EmrB (25% identity). Bmr3 confers resistance to the antibiotics puromycin, tosufloxacin, and norfloxacin. Northern hybridization analysis revealed that expression of Bmr3 is decreased in the late log phase (181).
Shortly after the introduction of fluoroquinolones for the treatment of tuberculosis, resistant clinical isolates of M. tuberculosis were described. From the rapidly growing Mycobacterium smegmatis, which was used as a model genetic system for M. tuberculosis, the lfrA gene, which confers low-level fluoroquinolone resistance when present on a multicopy plasmid, was isolated. LfrA confers resistance to hydrophilic quinolones but not to the more hydrophobic quinolones (134). Hybridization analysis showed that LfrA homologs are present in M. tuberculosis and Mycobacterium avium (244).
Vibrio cholerae, an important gram-negative enteric pathogen, is the causative agent of the severe diarrheal disease cholera. Colmer et al. (37) identified a multidrug resistance pump, VceAB, that provides V. cholerae with resistance to several toxic compounds, including deoxycholate and the antibiotics nalidixic acid and chloramphenicol. VcreA and VceB show significant homology to EmrA and EmrB, respectively.
Small Multidrug Resistance Family
Multidrug transporters of the SMR family (Table 2, Fig. 5), the smallest secondary drug efflux proteins known, are typically about 107 amino acid residues in length. Hydropathy analysis (191), gene fusions with alkaline phosphatase and β-galactosidase (179), α-periodicity analysis (51), cysteine accessibility scanning (242), and Fourier transform infrared spectroscopy (11) support a model of a tightly packed four-helix antiparallel bundle. Due to the small size of the multidrug transporters of the SMR family, it has been proposed that they may function as homooligomeric complexes (187, 189).
TABLE 2.
SMR multidrug transporters
| Multidrug transporter | Organism | Accession no(s). | Reference(s) |
|---|---|---|---|
| EbrA | Bacillus subtilis | AB029306 | 154 |
| EbrB | Bacillus subtilis | AB029306 | 154 |
| EmrE (MvrC) | Escherichia coli | Z11877, M62732 | 162, 202 |
| Mmr | Mycobacterium tuberculosis | Z83866 | 42 |
| QacE | Gram-negative bacteria | X68232 | 187 |
| QacEΔ1 | Gram-negative + gram-positive bacteria | X68232 | 187 |
| QacG | Staphylococcus spp. | Y16944 | 85 |
| QacH | Staphylococcus saprophyticus | Y16945 | 84 |
| Smr (Ebr/QacC/QacD) | Staphylococcus aureus | X15574, M37888, M37889, M33479 | 71, 132, 221 |
| YkkC | Bacillus subtilis | P49856 | 97 |
| YkkD | Bacillus subtilis | AJ002571 | 97 |
FIG. 5.
Structural model for multidrug transporters of the SMR family. The residues constituting the conserved sequence motifs are shaded.
The first gene encoding a multidrug transporter of the SMR family was detected on both conjugative and nonconjugative plasmids from clinical isolates of S. aureus and other staphylococci (120, 121, 132). This gene, first described as qacC and also known as qacD (132) and ebr (221), has been renamed smr (71). Grinius and Goldberg (72) demonstrated that the purified and reconstituted Smr protein mediates electrogenic drug/proton antiport. In staphylococci isolated from the food industry, plasmids pST94 and p2H6 were found. Sequence analysis of these plasmids revealed two new members of the SMR family, designated QacG and QacH (84, 85). QacG and QacH have 69 and 76% identity to Smr, respectively. Both transporters confer resistance to ethidium bromide and quaternary ammonium compounds.
The E. coli multidrug transporter EmrE (also known as MvrC) has been identified and cloned on the basis of its ability to confer resistance to ethidium and methyl viologen (162, 202). Yerushalmi et al. (275) demonstrated that resistance is caused by PMF-dependent extrusion of drugs. Negative dominance studies indicated that EmrE functions as a homooligomer, probably composed of three monomers (276). Studies using single cysteine mutants of EmrE showed that none of the residues in the putative transmembrane helices react with N-ethyl-maleimide (NEM), a small membrane-permeant sulfhydryl reagent. As the reaction with NEM requires water, the results imply very tight packing of the protein without a continuous aqueous domain. Based on these findings, it was proposed that EmrE translocates its substrates through a hydrophobic pathway (242).
The antiseptic resistance genes qacE and qacEΔ1 are located on an integron, a potentially mobile element. As a consequence, these genes are widely spread among gram-negative bacteria (108). The qacEΔ1 gene but not the qacE gene was detected in clinical isolates of gram-positive bacteria (109). The qacE gene was originally found on the Klebsiella aerogenes plasmid R751 (187). The qacEΔ1 gene probably represents a disrupted form of qacE that evolved as a result of the insertion of a DNA segment near the 3′ end of the qacE gene. Ethidium transport experiments indicated that the mechanism of resistance mediated by QacE is active export driven by the PMF. Neither QacE nor QacΔE1 is inhibited by reserpine, a potent inhibitor of multidrug transporters such as Bmr, NorA, and LmrP (187).
The multidrug transporter Mmr of M. tuberculosis confers resistance to tetraphenyl phosphonium (TPP+), ethidium bromide, erythromycin, acriflavine, safarin O, and pyronin Y (42). TPP+ accumulation experiments showed that Mmr actively extrudes TPP+, using the PMF as the driving force. The presence of mmr-like genes in other Mycobacterium species (M. simiae, M. gordonae, M. marinum, and M. bovis) was demonstrated by Southern hybridization (42).
The Bacillus subtilis genome encodes seven SMR-type proteins (194). Surprisingly, six of these proteins are encoded from gene pairs in three distinct operons, ebrAB, ykkCD, and yvdRS. Masaoka et al. (154) demonstrated that neither EbrA nor EbrB is sufficient for conferring drug resistance. However, coexpression of the two proteins causes an MDR phenotype in both E. coli and B. subtilis. A similar result was obtained for the YkkCD pair. Only when both the ykkC and ykkD genes were expressed together was resistance observed against a broad range of drugs, including cationic dyes and neutral and anionic antibiotics (97). In contrast to the SMR-type multidrug transporters, which appear to function as homooligomers, EbrAB and YkkCD are composed of two dissimilar but homologous subunits. One member of each pair is short (105 to 106 amino acids), while the other is longer (111 to 117 amino acids). This difference is due to a C-terminal hydrophilic extension (97).
The tehA gene of E. coli, which was initially identified as part of an operon associated with resistance to potassium tellurite, encodes a 36-kDa protein with 10 TMS (247). Surprisingly, a region of TehA which includes TMS 2 through 5 was found to be homologous to members of the SMR family (250). Expression of TehA increased the resistance to monovalent cations, such as tetraphenylarsonium and ethidium bromide, but decreased the resistance to the divalent cations dequalinium and methyl viologen (250). Thus, like MdfA of E. coli, TehA confers resistance to one group of toxic compounds and hypersensitivity to another group. Since deletion of the C-terminal region of TehA (TMS 8 to 10) did not decrease transport significantly, TMS 2 to 5 may be primarily responsible for the activity of TehA (250). Although TehA contains only one of the three SMR signature sequences (191) and is larger than the reported SMR-type multidrug transporters, it may represent a distantly related member of the SMR family.
Resistance-Nodulation-Cell Division Family
Multidrug transporters belonging to the RND family (Table 3) interact with a membrane fusion protein (MFP) and an outer membrane protein to allow drug transport across both the inner and outer membrane of gram-negative bacteria. The secondary structure of RND-type efflux proteins (Fig. 6) was proposed to consists of 12 TMS, with two large loops between TMS 1 and 2 and TMS 7 and 8 (190, 216). Membrane topology analysis of the Pseudomonas aeruginosa RND protein MexB recently verified this model (75). The MFP proteins, which contain a single N-terminal TMS and a large C-terminal periplasmic domain, are thought to induce fusion of the inner and outer membrane (45, 216) or form a channel-like structure that spans the periplasmic space (281).
TABLE 3.
RND multidrug transporters
| Multidrug transporter | Organism | Accession no. | Reference |
|---|---|---|---|
| AmrB | Burkholderia pseudomallei | AF072887 | 161 |
| AcrB | Escherichia coli | U00734 | 141 |
| AcrF (EnvD) | Escherichia coli | X57948 | 110 |
| HI0895 | Haemophilus influenzae | L42023 | 57 |
| MexB | Pseudomonas aeruginosa | L11616 | 198 |
| MexD | Pseudomonas aeruginosa | U57969 | 200 |
| MexF | Pseudomonas aeruginosa | X99514 | 115 |
| MexY | Pseudomonas aeruginosa | AB015853 | 158 |
| MtrD | Neisseria gonorrhoeae | U60099 | 81 |
| YhiV | Escherichia coli | U00039 | 143 |
FIG. 6.
Structural model for multidrug transporters of the RND family. The residues constituting the conserved sequence motifs are shaded.
The acrA locus of E. coli has long been known to be involved in resistance to basic dyes, detergents, and antibiotics (166, 167). Characterization of this locus revealed the presence of two genes, acrA and acrB (formerly acrE), which are located within a single operon (141). AcrA is a highly asymmetric protein that belongs to the MFP family, while AcrB is a member of the RND family (45, 281). Insertion mutations showed that both AcrA and AcrB are required for drug resistance (143). A study by Fralick (58) suggested that the outer membrane protein TolC is the third component of the AcrAB efflux system. Recently, the 2.1-Å crystal structure of TolC has been determined (118). Three TolC promoters assemble to form a continuous, solvent-accessible “tunnel” that spans the outer membrane and periplasmic space. This tunnel may transiently open up upon the interaction with an energized inner membrane transporter and establish a conduit between the cytosol and the external environment. E. coli contains two AcrAB homologs, AcrEF (formerly known as EnvCD [110]) and YhiUV (formerly called OrfAB), as well as an AcrB homolog, AcrD, which is not associated with a gene for an MFP (142). Inactivation of AcrEF decreased the resistance to a broad range of drugs, including dyes, detergents, and various classes of clinically important antibiotics, indicating that AcrEF functions as a multidrug transporter (141). Mutations in the yhiUV genes result in drug hypersusceptibility, suggesting that YhiUV also plays a role in antibiotic resistance (142, 143). Direct multidrug transport, however, has not yet been demonstrated.
P. aeruginosa is a clinically important opportunistic pathogen characterized by relatively high intrinsic resistance to a variety of antimicrobial agents. This property is now recognized to result from the synergy between low outer membrane permeability and at least four RND efflux systems. The mexAB-oprM operon was first thought to play a role in the export of the siderophore pyoverdine (198). Further characterization of the operon, however, showed that overexpression of MexAB-OprM increased the resistance of P. aeruginosa to various antibiotics (199). A direct assay of drug accumulation showed that MexAB-OprM indeed pumps out various antibacterial agents in an energy-dependent fashion (129). MexA and MexB are members of the MFP and RND families, respectively. The third gene of the operon was originally identified as oprK, but Gotoh et al. (64) demonstrated that the outer membrane component of this efflux system is OprM rather than OprK. Results obtained by Evans et al. (52) suggested that the quorum-sensing-related homoserine lactone PAI-1 is a substrate for MexAB-OprM and that exclusion of PAI-1 limits the production of virulence factors, such as the blue-green pigment pyocyanin. Examination of P. aeruginosa strains showing widely different levels of intrinsic resistance suggested the presence of more than one endogenous multidrug efflux system (128). Indeed, Poole and coworkers (200) identified a second efflux operon in P. aeruginosa, designated MexCD-OprJ. The third antibiotic efflux system of P. aeruginosa is composed of the MFP MexE, the RND protein MexF, and the outer membrane protein OprN (115). Similar to MexAB-OprM, MexEF-OprN expression correlates inversely with the production of the virulence factor pyocyanin (115). In contrast to MexAB-OprM, MexCD-OprJ and MexEF-OprN do not confer resistance to β-lactam antibiotics (65, 115). Subunit swapping experiments demonstrated that the inner membrane efflux components of the MexAB-OprM transporter are responsible for the β-lactam specificity of the multidrug efflux pumps in P. aeruginosa (66, 155, 240). Recently, two new genes mediating resistance to quinolones, aminoglycosides, and macrolide antibiotics were cloned from the chromosome of P. aeruginosa (6, 158). These genes, designated mexX and mexY, show significant similarity to mexAB, mexCD, and mexEF. No open reading frame corresponding to an outer membrane protein was found downstream of mexXY. Mine et al. (158) demonstrated that TolC or OprM is required for MexXY activity in E. coli. It has been reported that OprM contributes to antibiotic resistance in P. aeruginosa independent of MexAB (283). The substrate specificity of this OprM-dependent and MexAB-independent system corresponds to that of the MexXY system, suggesting that OprM functions as the outer membrane protein of the MexXY drug efflux pump in P. aeruginosa (158).
The resistance of Neisseria gonorrhoeae to hydrophobic agents, including detergent-like fatty acids and bile salts, is conferred by the MtrRCDE efflux system. The mtrRCDE region of the gonococcal chromosome contains a transcriptional regulator gene (mtrR) and three tandemly linked genes (mtrC, mtrD, and mtrE), encoding proteins of the MFP, RND, and outer membrane protein families, respectively (80). Insertional inactivation of either the mtrC (80) or mtrE (41) gene resulted in a significant decrease in resistance to erythromycin, penicillin, Triton X-100, and fatty acids, suggesting that both proteins are required in the efflux process.
Genome sequencing of Haemophilus influenzae has identified a three-gene cluster, consisting of HI0893, HI0894, and HI0895, which is homologous to the acrRAB cluster of E. coli (57). Disruption of either HI0894 (an acrA homolog) or HI0895 (an acrB homolog) caused hypersusceptibility to erythromycin, rifampin, novobiocin, sodium dodecyl sulfate, and cationic dyes. Erythromycin accumulation experiments indicated that the HI0894 and HI0895 proteins contribute to the resistance of H. influenzae through PMF-dependent drug efflux (220).
Burkholderia pseudomallei, a motile gram-negative rod, is the causative agent of melioidosis. Successful treatment of melioidosis patients is difficult because B. pseudomallei is intrinsically resistant to a variety of antibiotics, including β-lactams, aminoglycosides, and macrolides. Recently, the amrAB-OprA operon was identified, which encodes a multidrug efflux system in B. pseudomallei which confers resistance to both aminoglycoside and macrolide antibiotics. AmrA and AmrB show strong homology to MexC and MexB, respectively, of P. aeruginosa (161).
Multidrug and Toxic Compound Extrusion Family
Norfloxacin accumulation experiments revealed that Vibrio parahaemolyticus contains an energy-dependent efflux system, designated NorM (163). NorM and its E. coli homolog YdhE mediate resistance to dyes, hydrophilic fluoroquinolones, and aminoglycosides. NorM contains 12 putative TMS, and on this basis it has been suggested to be a member of the MFS (163). NorM and YdhE, however, do not have sequence similarity with any members of the MFS and do not exhibit any of the signature sequences (see below). Brown et al. (28) found that NorM and YdhE are members of a previously unidentified family which contains more than 30 proteins, termed the multidrug and toxic compound extrusion (MATE) family. Phylogenetic analysis of the MATE family revealed the presence of three distinct clusters, of which the first includes NorM, YdhE, and hypothetical multidrug efflux proteins from H. influenzae and B. subtilis (28).
Electrogenic Drug/Proton Antiport
Drug transport by multidrug transporters of the MFS, SMR, RND, and MATE families is driven by the transmembrane electrochemical gradient of protons or possibly sodium ions. The PMF is composed of a chemical proton gradient (ΔpH, inside alkaline) and an electrical potential (ΔΨ, inside negative). The ionophores nigericin and valinomycin, which can selectively dissipate the ΔpH and ΔΨ, respectively, are valuable tools for the study of the energetics of secondary multidrug transporters. Ng et al. (174) demonstrated that NorA-mediated norfloxacin uptake in inside-out membrane vesicles was completely abolished by nigericin. In contrast, addition of valinomycin resulted in a slight increase in the uptake of norfloxacin. These results indicate that the ΔpH is the major driving force for NorA. Grinius et al. (73) reported that NorA-mediated drug transport in proteoliposomes is an electrogenic drug/proton antiport process. Transport experiments in the presence of valinomycin and/or nigericin showed that both the ΔpH and the ΔΨ are driving forces of LmrP-mediated transport of trimethyl ammonium-diphenyl hexatriene (TMA-DPH) (22) and Hoechst 33342 (203), consistent with an electrogenic exchange of one lipophilic cation for two or more protons. Mitchell et al. (159) investigated which component of the PMF is involved in energizing QacA-mediated drug efflux. It was found that both the ΔpH and the ΔΨ drive the electrogenic transport of monovalent and divalent cations by QacA. The involvement of both components of the PMF as the driving force in Smr-mediated drug transport was demonstrated by studies using purified Smr reconstituted into proteoliposomes. A ΔΨ (positive inside) superimposed on ΔpH (acidic inside) resulted in additional ethidium uptake via Smr (72). An electrogenic drug/proton antiport reaction by Smr was further supported by the observation that Smr increased the resistance of E. coli cells to ethidium in alkaline medium, when the PMF predominantly consists of a ΔΨ (72). The electrogenic drug/nH+ (n ≥ 2) antiport mechanism observed for LmrP, NorA, QacA, and Smr appears to be a general feature of multidrug transporters of the MFS and SMR families and may also be valid for the RND- and MATE-type multidrug transporters. The substrates used in the studies on the energetics of secondary multidrug transporters are monovalent cations. However, several multidrug transporters, such as QacA, MdfA, and YkkCD, also mediate resistance to divalent cations, neutral drugs, and anionic compounds. An analysis of the relative contribution of the ΔΨ and the ΔpH to the driving force for transport of these cations can provide further insight into the drug/proton ratio of the export process.
Conserved Sequences in Secondary Multidrug Transporters
Multiple-sequence analysis of transporters within the various clusters of solute and drug transporters of the MFS revealed a substantially larger sequence similarity in the N-terminal halves of these proteins than in their C-terminal halves (70, 146, 188, 213). It has been hypothesized that the N-terminal domain is involved in proton translocation, while the C-terminal domain of the transporters is primarily involved in determining the substrate specificity (70, 213). On the other hand, the significant sequence similarity observed between the N- and C-terminal halves of transporters of the MFS suggests that they have evolved from a duplication of a common ancestor with six TMS (122, 145, 215, 274). The 14-TMS cluster of the MFS may have evolved via a similar gene duplication event and the acquisition of two additional TMS (70, 188, 217). The homology between the first and second halves of RND proteins suggests that these proteins also may have arisen via an intragenic duplication event during evolution (75, 216).
The multiple alignments of amino acid sequences further revealed a number of strongly conserved amino acid sequence motifs (Table 4 and Fig. 3, 4, 5, and 6) throughout the multidrug transporters of the MFS, SMR, and RND families (188, 190, 191, 213, 216). The conservation of these motifs suggests that they play an important structural or functional role in the transporters. Motif A in the cytoplasmic loop between TMS 2 and 3 in transporters of the MFS, which is predicted to contain a β-turn structure, may be involved in the reversible conformational change required for the opening and closing of a transport channel (102, 188, 272, 273). The absolute conservation of the basic arginine residue suggests that motif B in TMS 4 of MFS transporters may be involved in proton transfer (188). Interestingly, motif C in TMS 5 is found in multidrug transporters and specific drug efflux pumps, but not in symporters of the MFS. This suggests that motif C dictates the direction of transport (70, 188). The GP dipeptide of this motif causes a bend in helix 5, and the repeating pattern of glycine residues forms a pocket devoid of side chains. These structures may determine the orientation of the unoccupied substrate-binding site(s) and, consequently, the direction of transport (261). The C-terminal motifs F and G represent partial duplications of motif C and may have a similar function. Site-directed mutagenesis studies can provide valuable information on the structure and mechanism of multidrug transporters of the MFS, RND, and SMR families. The conserved motifs may be directly involved in drug or proton translocation. The charged residue in motif B (Arg) and in motif D1 (Asp) in members of the MFS, in motif A (Glu) in SMR-type multidrug transporters, and in motif C (two Arg) in drug efflux systems of the RND family are predicted to be located in TMS. The presence of positively or negatively charged amino acid residues in the membrane is energetically unfavorable, suggesting that these motifs play a functional role in the binding and transport of charged drugs and/or translocation of protons. Alternatively, they may play a structural role in the interaction between the transporters of the RND family (motif A) and proteins of the MFP family, or in the formation of homo- and heterooligomers of SMR-type multidrug transporters. The conserved sequences are characteristic of proteins of the MFS, SMR, and RND families and can be used to identify new members of these families in bacterial genomes.
TABLE 4.
Consensus sequences of conserved motifs in transporters of the MFS, SMR, and RND families
| Motif | Consensus sequencea | Location |
|---|---|---|
| MFS, both 12- and 14-TMS cluster | ||
| A | G x L a D r x G r k x x (x) l | Loop between TMS 2 and 3 |
| B | l x x x R x x q G x g a a | TMS 4 |
| C | g x x x G P x x G G x l | End of TMS 5 |
| MFS, 12-TMS cluster | ||
| D2 | l g x x x x x P v x P | End of TMS 1 |
| G | G x x x G P L | End of TMS 11 |
| MFS, 14-TMS cluster | ||
| D1 | l D x T v x n v A l P | End of TMS 1 |
| E | D x x G x x L | TMS 7 |
| F | l g x x x G x a v x g x l | TMS 13 |
| H | W x w x F l l N v P i g | TMS 6 |
| SMR family | ||
| A | W i x l v i A i l l E V | TMS 1 |
| B | K x s e G F t r l x P S | Loop between TMS 1 and 2 |
| C | P v G t A Y A v W t G l G | Start of TMS 3 |
| RND family | ||
| A | G x s x v T v x F x x g t D x x x A q v q V q n k L q x A x p x L P x x V q x q g x x v x k | Loop between TMS 1 and 2 |
| B | a l v l s a V F l P m a f f g G x t G x i y r q f s i T x v s A m a l S v x v a l t l t P A l c A | TMS 6 |
| C | x x x G k x l x e A x x x a a x x R L R P l L M T s L a f i l G v l P l a i a t G x A G a | TMS 11 |
| D | S i N t l T l f g l v l a i G L l v D D A l V v V E N v e R v l a e | TMS 4 |
The motifs were identified by alignment of amino acid sequences (190, 191). The consensus sequences of the motifs are displayed as follows: x, any amino acid; capital letters, amino acid occurs in >70% of the examined sequences; lowercase letters, amino acid occurs in >40%; (x), amino acid not always present.
Résumé
The secondary multidrug transporters identified in bacteria (Tables 1 to 3) belong to one of four distinct families of proteins, MFS, SMR, RND, and MATE. Whereas multidrug transporters of the MFS and SMR families have been found in both gram-positive and gram-negative bacteria, RND- and MATE-type multidrug transporters have so far only been identified in gram-negative organisms. Table 5 indicates that bacteria can contain several multidrug transporters from different families. The presence of a variety of multidrug transporters with an overlapping substrate spectrum would seem redundant. However, the availability of an array of multidrug transporters, which are controlled by both global and specific regulatory proteins, enables the bacterium to fine tune the response to different environmental signals.
TABLE 5.
Number of sequenced and functionally characterized multidrug transporters of various families in selected bacteria
| Organism | MFS
|
SMR | RND | MATE | |
|---|---|---|---|---|---|
| 12-TMS cluster | 14-TMS cluster | ||||
| Bacillus subtilis | 2 | 1 | 2 | 0 | 0 |
| Escherichia coli | 3 | 1 | 1 | 3 | 1 |
| Staphylococcus aureus | 1 | 2 | 3 | 0 | 0 |
| Mycobacterium tuberculosis | 1 | 1 | 1 | 0 | 0 |
| Pseudomonas aeruginosa | 0 | 0 | 0 | 4 | 0 |
The most striking difference between multidrug transporters of the SMR family and drug efflux systems from the other families is their size. Whereas members of the MFS, RND, and MATE families consist of 12 or 14 TMS, SMR-type multidrug transporters only contain 4 TMS. These small multidrug efflux pumps are thought to function as homooligomers, presumably composed of three monomers. However, two new SMR-type multidrug transporters were recently identified in B. subtilis which require two homologous but dissimilar subunits for drug efflux. This observation raises the question of what molecular determinants dictate whether an SMR transporter functions as a homo- or heterooligomer.
Secondary multidrug transporters extrude drugs in exchange with protons (or sodium ions). Both components of the PMF can drive the extrusion of drugs by multidrug transporters from the MFS, SMR, and RND families, indicating that an electrogenic antiport mechanism is a general feature of secondary multidrug transporters.
Multiple sequence alignments of transporters within one family revealed the presence of three or more conserved motifs in multidrug transporters of the MFS, SMR, and RND families (Table 4). Hydropathy analysis, the recognition of family-specific conserved motifs, and the availability of the complete genomic sequences of 28 bacteria and 8 archaea (http://www.tigr.org/tdb/mdb) enable the identification of putative multidrug transporters. However, as multidrug transporters are frequently more homologous to substrate-specific transporters than to other multidrug transporters, the activity of newly identified proteins needs to be characterized.
ATP-DEPENDENT MULTIDRUG TRANSPORTERS
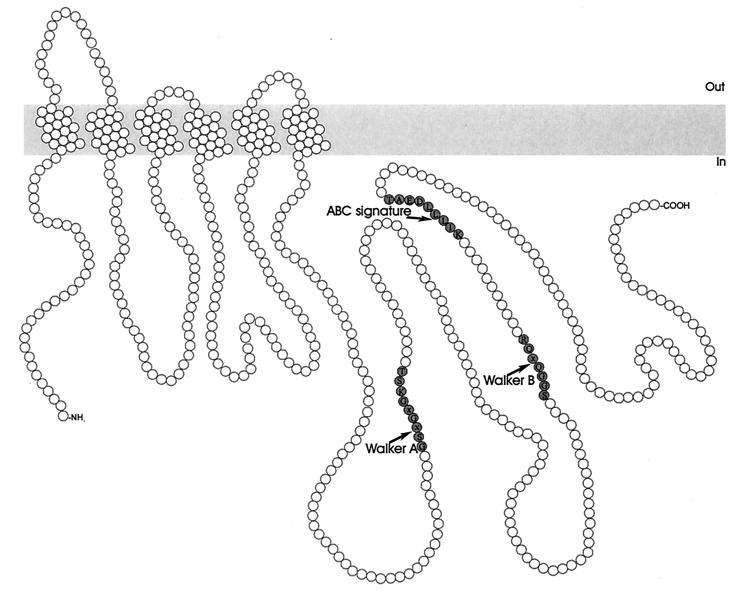
Although most bacterial multidrug drug transporters utilize the PMF (or sodium) for the extrusion of cytotoxic compounds, some drug efflux systems are driven by the free energy of ATP hydrolysis (Fig. 7). All ATP-dependent drug efflux proteins known to date are members of the ABC superfamily (87), also referred to as traffic ATPases (8). In general, ABC transporters require four distinct domains: two highly hydrophobic membrane domains, which usually consist of six putative transmembrane α-helices each, and two hydrophilic nucleotide-binding domains (NBDs), containing the Walker A and B motifs (263) and the ABC signature (96). The individual domains can be expressed as separate proteins or may be fused into multidomain polypeptides in a variety of ways (55, 87, 96).
FIG. 7.
Structural model for multidrug transporters of the ABC superfamily. Shown is the multidrug transporter LmrA, with six transmembrane helices and an NBD, containing the Walker A and B motifs and the ABC signature sequence. In view of the general four-domain organization, it may function as a homodimer. The Walker A and B motifs and the ABC signature sequence are shaded.
Most bacterial ABC drug transporters mediate the export of specific antibiotics (17, 55, 76, 130, 183, 197, 210, 212). The first true bacterial ABC multidrug transporter was found in Lactococcus lactis (20). The gene encoding this transporter, designated lmrA, encodes a 590-amino-acid membrane protein with an N-terminal hydrophobic domain with six putative transmembrane helices and a C-terminal hydrophilic domain, containing the ATP-binding cassette (256). LmrA is homologous to each of the two halves of the human multidrug transporter P-glycoprotein (256), suggesting that LmrA is functional as a homodimer. This suggestion was confirmed by studies on covalently linked dimers composed of wild-type LmrA units (designated KK), mutant LmrA units containing an inactivated NBD (designated MM), and combinations of wild-type and mutant units (designated KM and MK) (258). The ethidium transport activity and substrate-stimulated ATPase activity of the KK dimeric form of LmrA was comparable to that of wild-type LmrA. However, the KM, MK, and MM mutant forms of dimeric LmrA had lost all LmrA-associated ATPase activity and the ability to transport ethidium from the cell. These results are supported by reconstitution experiments which demonstrate the negative dominance of M units over K units in proteoliposomes and suggest that homodimeric LmrA is the minimal functional unit. Hence, functional crosstalk between two LmrA monomers is essential for activity (258).
When expressed in human lung fibroblast cells, LmrA is targeted to the membrane and confers multidrug resistance on these human cells (257). The pharmacological characteristics of LmrA and the human multidrug transporter P-glycoprotein (67) and the affinities of both proteins for vinblastine and Mg2+-ATP are very similar. Blockers of P-glycoprotein also inhibited LmrA-mediated resistance. These results demonstrate that LmrA and the human multidrug transporter P-glycoprotein are functionally interchangeable, indicating that this type of multidrug transporter is conserved from bacteria to humans (258).
Kinetic analysis of vinblastine dissociation from LmrA and characterization of transport of Hoechst 33342 or vinblastine in the presence of low concentrations of a second substrate revealed that LmrA contains at least two allosterically linked drug-binding sites with overlapping substrate specificities (see below) (257, 258). Purified LmrA reconstituted in proteoliposomes mediates ATP-dependent transport of Hoechst 33342 (147). Certain ABC transporters, including the LmrA homolog MsbA of E. coli (285), are able to translocate lipids. The specificity of reconstituted LmrA for C6-NBD-labeled phosphatidylethanolamine and phosphatidylcholine was investigated. Interestingly, LmrA catalyzes the transport of C6-NBD-labeled phosphatidylethanolamine but not of C6-NBD-labeled phosphatidylcholine. These results indicate that LmrA exhibits specificity for phospholipid headgroups and that LmrA may be involved in the translocation of specific lipid or lipid-linked precursors in L. lactis (147).
Hop-resistant lactobacilli can cause serious spoilage of beer. Sami et al. (218, 219) demonstrated that plasmid pRH45 of Lactobacillus brevis is involved in the high resistance to hop compounds (iso-α-acids). pRH45 contains the horA gene, which encodes a protein of the ABC superfamily. HorA is a structural homolog of LmrA, containing six putative TMS and a C-terminal ATP-binding domain. In addition to conferring hop resistance, HorA confers resistance to the structurally unrelated drugs novobiocin and ethidium (219). HorA has been heterologously expressed in Lactococcus lactis, purified, and functionally reconstituted in proteoliposomes. HorA-mediated Hoechst 33342 transport in proteoliposomes is inhibited by hop compounds (K. Sakamoto et al., submitted for publication).
Kaidoh et al. (105) described an ATP-dependent drug efflux system in the archaeon Haloferax volcanii. This efflux system mediates the transport of doxorubicin, vinblastine, vincristine, ethidium, and monensin. Expression of the transporter is induced in media containing a large excess of essential amino acids, glucose, or fructose. These observations suggest that this efflux system plays a role in the active extrusion of cytotoxic compounds or toxic metabolites generated in the cell. The gene encoding this ABC-type multidrug transporters remains to be cloned and sequenced (105). Genome sequencing revealed the presence of putative LmrA homologs in E. coli, B. subtilis, and the pathogens Helicobacter pylori, Mycobacterium genitalium, H. influenzae, and S. aureus (259). Functional characterization of these proteins is required to demonstrate their involvement in the ATP-dependent transport of structurally unrelated drugs.
Mutant analysis and photoaffinity labeling experiments have been used to identify essential residues and putative substrate-binding sites in the human multidrug transporter P-glycoprotein. The results demonstrated that, although some mutations leading to changes in specificity are located in other regions of the protein, TMS 5, 6, 11, and 12 and the loop between TMS 2 and 3 and TMS 11 and 12 are most important in drug binding and/or transport (39, 43, 68, 69, 82, 106, 137, 139, 164, 282). Interestingly, sequence conservation between the lactococcal multidrug transporter LmrA and P-glycoprotein includes parts of TMS 5 and 6 and the loop between TMS 2 and 3 (256), indicating that these particular regions may be important for substrate binding in both human and bacterial ABC-type multidrug transporters.
REGULATION
Several studies demonstrated that the expression levels of multidrug transporters such as Bmr, NorA, QacA, and P-glycoprotein can be enhanced by the addition of structurally diverse compounds which are exported by these pumps (3, 74, 104, 107). These observations indicate that the expression of multidrug transporters is controlled by regulatory proteins which, like the transporters, are capable of recognizing structurally diverse compounds. The activator and repressor proteins involved in the transcriptional regulation of multidrug transporter genes are summarized in Table 6.
TABLE 6.
Reported transcriptional regulators that control the expression of multidrug transporters in bacteria
| Multidrug transporter(s) | Regulator | Type | Reference |
|---|---|---|---|
| Blt | BltR | Activator | 4 |
| Blt, Bmr | Mta | Global activator | 16 |
| Bmr | BmrR | Activator | 3 |
| EmrB | EmrR | Repressor | 136 |
| QacA | QacR | Repressor | 74 |
| AcrB | MarA | Global activator | 182 |
| AcrB | AcrR | Repressor | 144 |
| AmrB | AmrR | Repressor | 161 |
| MexB | MexR | Repressor | 201 |
| MexD | NfxB | Repressor | 200 |
| MexF | MexT | Activator | 116 |
| MexZ | MexX | Repressor | 6 |
| MtrD | MtrA | Activator | 214 |
| MtrD | MtrR | Repressor | 185 |
The best-studied regulator of multidrug transport is BmrR, the transcriptional activator of the B. subtilis multidrug transporter Bmr. BmrR, which is encoded by a gene immediately downstream of bmr, belongs to the MerR family of regulators and has strong homology with other members of the family only in the N-terminal helix-turn-helix DNA-binding domain. Binding of one rhodamine 6G molecule to a dimer of BmrR increases the affinity of BmrR for the bmr promoter, resulting in enhanced transcription of the bmr gene (3). The individually expressed C-terminal region of BmrR forms dimers and binds a broad range of drugs with the same affinity as full-length BmrR (148, 150). High-resolution crystal structures of the BmrR C-terminal region and its complex with TPP+ revealed drug-induced unfolding and relocation of an α-helix, which exposes an internal drug-binding pocket. Residue Glu-134, which is completely buried in the unliganded structure, is the key to cation selectivity, while a number of nonpolar and aromatic side chains impose specific requirements on the size and shape of the substrate (284). The transcription of the blt gene is regulated by another member of the MerR family, BltR. The C-terminal domains of BmrR and BltR have no sequence similarity, which is consistent with the observation that Bmr and Blt are expressed in response to different inducers. This difference in inducer specificity may explain why Bmr and not Blt is normally expressed in wild-type B. subtilis (4).
The expression of the S. aureus multidrug efflux gene qacA is regulated by the divergently transcribed repressor protein QacR. QacR binds specifically to the qacA promoter, preventing transcription of the gene. Substrates of QacA, such as ethidium and rhodamine 6G, interact with QacR directly and inhibit the binding of QacR to the qacA promoter, resulting in derepression of qacA expression (74).
The EmrAB multidrug pump of E. coli is induced in the presence of CCCP, the weak acid salicylate, and a number of other structurally unrelated drugs. The derepression is controlled by the EmrR, a MarR type of repressor protein (136). EmrR was found to bind putative substrates of the EmrAB pump, such as 2,4-dinitrophenol, CCCP, and carbonyl cyanide p-(trifluoro-methoxy)-phenylhydrazone (FCCP), with micromolar affinity. Equilibrium dialysis experiments suggested one bound ligand per EmrR dimer (27).
Analysis of the sequence upstream of the mtrCDE multidrug efflux system of N. gonorrhoeae revealed the presence of the divergently transcribed repressor gene mtrR. MtrR binds to the 13-bp inverted repeat sequence between the mtrR and mtrC promoters, thereby inhibiting expression of the mtrRCDE gene complex (80, 185). Studies by Hagman and Shafer (79), however, showed that the MtrCDE efflux system is subject to both MtrR-dependent and MtrR-independent regulation. Recently, the transcriptional activator MtrA was shown to be involved in the modulation of mtrCDE gene expression (214).
The transcription of the acrAB operon in E. coli is regulated by the repressor AcrR (144). Gel mobility shift assays and lacZ transcriptional fusions suggested that the general-stress-enhanced transcription of acrAB (144) is primarily mediated by global regulatory pathways such as the mar regulon (see below) and that a major function of AcrR is that of a specific secondary modulator, which fine tunes the level of acrAB transcription (144).
The transcriptional repressor AmrR of Burkholderia pseudomallei shows strong similarity to the regulator MtrR. The expression of the AmrAB-OprA efflux system was not affected by the substrate streptomycin or by stress conditions (4% ethanol or 0.5% NaCl), and further studies are required to identify the inducers of this system (161).
The expression of the P. aeruginosa multidrug transporter MexAB-OprM is regulated by MexR, a MarR-type regulator (53, 201). The transcriptional repressor NfxB regulates a second MDR in P. aeruginosa, MexCD-OprJ, as well as its own expression (200). The mexEF-oprN efflux operon differs from the other P. aeruginosa efflux systems in that it is positively regulated by MexT, a protein belonging to the LysR family of transcriptional activators (115, 116). A third MDR in P. aeruginosa, MexXY, may be regulated by MexZ, which is homologous to the repressor proteins MtrR and AmrR (6).
In addition to the specific regulatory mechanisms that affect the expression of single multidrug transporters, induction of multidrug efflux systems can also result from global regulatory mechanisms. Multiple-antibiotic resistance (Mar) mutants of E. coli express elevated levels of resistance to a wide range of antibiotics. The alleles that affect the Mar phenotype are located in the marRAB operon (34). Expression of this operon is normally repressed by the transcriptional repressor MarR (9, 225) but can be derepressed by various compounds, such as tetracycline, chloramphenicol, and salicylates (35, 78). The marA gene encodes an AraC-type activator that is structurally and functionally similar to two other global regulators of E. coli, SoxS and Rob (60, 235, 270). The MarA, SoxS, and Rob proteins are transcriptional activators of at least a dozen promoters, the mar and soxRS regulons, that confer resistance to a variety of antibiotics and superoxide-generating agents (10, 62, 99, 100, 226). The observed resistance is partly due to increased micF transcription and the consequent decrease in expression of the outer membrane porin OmpF (33, 60). The RND-type multidrug transporter AcrAB-TolC plays a major role in the antibiotic and organic solvent resistance induced by MarA, SoxS, or Rob (182, 266). The mar operon is widespread among enteric bacteria, including the genera Salmonella, Shigella, Klebsiella, Citrobacter, Hafnia, and Enterobacter (36). Expression of E. coli MarA in Mycobacterium smegmatis resulted in increased resistance to multiple antimicrobial agents, suggesting that MarA functions in M. smegmatis and that a mar-like regulatory system exists in mycobacteria (156). Recently, Baranova et al. (16) identified a global MerR-type regulator in B. subtilis, termed Mta. The individually expressed N-terminal DNA-binding domain of Mta mimics the inducer-bound form and interacts directly with the promoters of bmr and blt, thereby inducing transcription of these genes. Additionally, this domain stimulates the expression of the mta gene and at least one more gene, ydfK, which encodes a hypothetical membrane protein (16).
RECONSTITUTION
Multidrug transporters have predominantly been studied in whole cells and membrane vesicles, in which other proteins and cellular components or processes may complicate the interpretation of the data. In order to elucidate the structure, function, and mechanism of multidrug transporters at the molecular level, methods have been developed for overexpression, purification, and functional reconstitution into liposomes (72, 73, 147, 203, 227, 275, 280). Although the overexpression of membrane proteins is often deleterious for the cells (48, 119, 203, 243), drug transporters could be overexpressed up to 5 to 30% of total membrane protein using homologous expression systems and inducible promoters, such as the T7 polymerase promoter (275), the lac promoter (236), and the nisA promoter (147, 203). The first step in the purification of membrane proteins involves extraction of the protein from the membrane. The E. coli multidrug transporter EmrE can be quantitatively and functionally extracted with a mixture of organic solvents, such as chloroform-methanol (275). However, solubilization of most other membrane proteins requires the use of detergents (115). The choice of detergent is crucial, since some detergents can irreversibly inactivate the protein (227; J. Knol, R. H. E. Friesen, and B. Poolman, submitted for publication). In addition, detergents are potential substrates for multidrug efflux proteins and can affect the transport activity (46, 203, 268). Retention of detergent during the reconstitution procedure is a serious problem which further restricts the choice of detergent for the solubilization and reconstitution of multidrug transporters (115). Affinity chromatography, a highly specific and efficient technique, allows the purification of several multidrug transporters to a high degree of purity (>90%) in a single step (72, 147, 203, 227, 280). After reconstitution in liposomes, Smr, P-glycoprotein, EmrE, NorA, LmrA, LmrP, AcrB, and HorA mediated the transport of multiple drugs in response to the imposition of an artificial proton gradient or the addition of ATP (72, 73, 147, 203, 227, 275, 280; K. Sakamoto, H. W. van Veen, and W. N. Konings, unpublished data). Taken together, these results demonstrate that multidrug transporters of the MFS, SMR, RND, and ABC families are capable of mediating drug transport independent of accessory proteins or cytosolic components.
SUBSTRATE BINDING
Both secondary and ATP-dependent multidrug transporters extrude a wide variety of toxic compounds. Although the substrates have very different structures, they share physical characteristics, such as high hydrophobicity, an amphiphilic nature, and a positive or neutral charge. In the absence of three-dimensional structural information, the identification of structure-function relationships in multidrug transporters has relied on amino acid sequence alignments and site-directed mutagenesis. The localization of a charged residue in the membrane-embedded part of the protein is normally considered energetically unfavorable, yet many multidrug transporters of the MFS and SMR families, such as MdfA, QacA, EmrE, and Smr, contain an acidic amino acid residue in the middle of TMS 1 (50). Furthermore, Guan et al. (75) identified three highly conserved negatively charged amino acid residues in transmembrane domains of efflux proteins of the RND family. Since the majority of the MDR substrates are positively charged, the negative charge of an intramembranous acidic residue may be involved in substrate binding. The role of the conserved Glu in TMS 1 of Smr has been investigated using site-directed mutagenesis. Even conservative substitutions, such as the replacement of Glu with Asp, effectively abolished the activity of the efflux system, indicating that this residue may be directly involved in the multidrug efflux process, potentially in substrate binding and/or the exchange of drug molecules for protons (72). Edgar and Bibi (50) demonstrated that a single membrane-embedded negative charge is critical for the recognition of positively charged drugs by the multidrug transporter MdfA of E. coli but not for transport activity. Replacement of the Glu residue in TMS 1 of EmrE abolished binding activity, but replacement with a negatively charged Asp created a mutant protein that retained its ability to bind substrate (165). Whereas QacA transports both monovalent and divalent cations, the closely related multidrug transporter QacB only transports monovalent cations. Sequence analysis and mutagenesis revealed that the presence of an acidic residue in TMS 10 (position 322 or 323) of these transporters is crucial for the recognition of divalent cations (192). Altogether, these results suggest that charge-charge interactions play an important role in the recognition and export of lipophilic cations by multidrug transporters.
Based on the observation that aromatic amino acid residues in transmembrane regions of the human multidrug transporter P-glycoprotein are highly conserved, Pawagi et al. (195) hypothesized that the side chains of these residues can participate in the initial binding and subsequent transport of a variety of ring-containing compounds. Indeed, Dougherty (47) indicated that cation-π interactions between cations, such as quarternary ammonium compounds, and aromatic side chains from the amino acids Phe, Tyr, and Trp are important in a variety of proteins that bind cationic substrates. The potential role of the conserved aromatic residues Tyr59 and Trp62 in the staphylococcal multidrug transporter Smr has been investigated by site-directed mutagenesis (189). Substitutions at these positions abolished the multidrug efflux capacities of the protein, indicating that these residues play a critical structural or functional role. Mutations of residues Phe143, Val286, and Phe306 of Bmr altered the sensitivity to reserpine and changed the substrate specificity, indicating that these residues are likely involved in substrate recognition (2, 111). Furthermore, mutations of Phe, Trp, and Tyr residues in TMS 6, 11, and 12 drastically altered the drug resistance profile of P-glycoprotein (82, 137). These results illustrate the importance of aromatic and nonpolar amino acid residues in drug binding.
The three-dimensional structure of the multidrug-binding domain of BmrR, the transcriptional activator of Bmr, supports a model in which a negatively charged amino acid residue as well as aromatic and nonpolar residues is involved in drug binding (284). The binding of TPP+ to Bmr induces the displacement of a short amphipatic α-helix to allow the drug to enter the drug-binding pocket. Inside the binding pocket, TPP+ forms a number of van der Waals' forces and stacking interactions with the surrounding hydrophobic and aromatic residues. The bottom of the binding pocket contains a buried glutamate residue, which makes an electrostatic interaction with the positively charged substrate (284). The substrate-binding site(s) of a multidrug transporter may have a similar organization. Most secondary multidrug transporters contain an essential membrane-embedded negatively charged amino acid residue. This residue could act like a magnet to attract positively charged substrates from the inner leaflet of the membrane. The surrounding hydrophobic residues would impose requirements on the size and shape of the substrate. In contrast to secondary multidrug transporters, the ABC multidrug transporters LmrA, P-glycoprotein, and others do not contain negatively charged residues in predicted TMS. It should also be noted that the binding of neutral or negatively charged substrates cannot be explained by the model described above.
There is increasing evidence for the presence of multiple drug-binding sites in both secondary and ATP-dependent multidrug transporters, rather than a single binding site, as suggested previously (126, 195). Tamai and Safa (245) indicated for the first time that the human multidrug transporter P-glycoprotein may contain at least two kinetically distinguishable drug-binding sites. Kinetic analysis of the drug-stimulated ATPase activity of P-glycoprotein showed that cyclosporin A competitively inhibits the verapamil-stimulated ATPase activity, whereas daunorubicin, gramicidin D, vinblastine, and colchicine give an allosteric inhibition. Interestingly, cooperative stimulation of the verapamil-induced ATPase activity was found with progesterone (131). Several other studies demonstrated a similar complex effect of pairs of substrates or modulators on the drug-induced ATPase activity of P-glycoprotein (30, 61, 153, 186). Noncompetitive inhibition of drug binding (44, 56, 153, 264) and drug transport by P-glycoprotein (13, 196, 230, 238) further support a model with multiple cooperating drug interaction sites.
Noncompetitive inhibition of rhodamine 6G transport by drugs such as quinidine, puromycin, and colchicine also suggested multiple cooperating drug interaction sites in the yeast multidrug transporter Pdr5p (117). Van Veen et al. (257) demonstrated that nicardipin interacts with the lactococcal multidrug transporter LmrA at a binding site distinct from but allosterically linked to the vinca alkaloid-binding site. Competition studies showed that QacA-mediated transport of ethidium is inhibited competitively by the monovalent cations benzalkonium and tetraphenylarsonium and noncompetitively by the divalent cations chlorhexidine and propamide. These observations also suggest that the S. aureus multidrug transporter QacA contains distinct binding sites for monovalent and divalent cations (159). Furthermore, kinetic analysis of LmrP-mediated Hoechst 33342 transport in inside-out membrane vesicles revealed a competitive inhibition by verapamil and quinine, a noncompetitive inhibition by nicardipin and vinblastine, and a noncompetitive inhibition by TPP+, implying that LmrP must contain at least two drug interaction sites (204). These results indicate that the presence of two or more interacting substrate-binding sites may be a general feature of multidrug efflux systems, including those of the SMR and RND families.
MECHANISM OF TRANSPORT
The common ability of MDR substrates to intercalate in the membrane led to the suggestion that multidrug transporters transport their substrates from the lipid bilayer (88, 207, 252) rather than from the cytoplasmic aqueous phase (7). Drug recognition within the membrane is suggested by a number of observations: (i) site-directed mutagenesis has identified specific residues implicated in substrate specificity, which are predominantly located within predicted TMS (2, 50, 65, 103, 184, 192), (ii) photoactive substrates label the human multidrug transporter P-glycoprotein in or near transmembrane helices (69, 207), and (iii) nonfluorescent acetoxymethyl esters of various fluorescent indicators, such as 2′,7′-bis-(2-carboxymethyl)-5-(and 6)-carboxy-fluorescein (BCECF) and calcein, are extruded from the membrane before they can be converted into fluorescent carboxylates by nonspecific esterases in the cytoplasm (23, 89, 91).
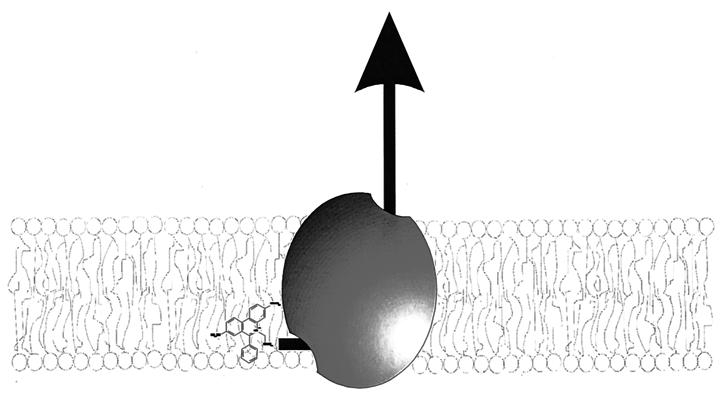
The most convincing evidence for drug efflux from the membrane to the aqueous phase, however, is provided by transport studies using the fluorescent compounds TMA-DPH, Hoechst 33342, and 2-{4-[4-(dimethylamino)phenyl]-1,3-butadienyl}-3-ethylbenzo-thiazolium perchlorate (LDS-751) which are strongly fluorescent in a hydrophobic environment but essentially nonfluorescent in an aqueous environment. Transport of these compounds from the membrane to the aqueous phase can be monitored as a decrease in fluorescence over time (22, 23, 180, 228, 229, 231). Shapiro et al. (229) demonstrated that the initial rate of P-glycoprotein-mediated Hoechst 33342 transport is directly proportional to the amount of Hoechst 33342 in the membrane, demonstrating that P-glycoprotein removes the drug from the lipid membrane. The mechanism of transport of LmrA (23), LmrP (22), MexAB-OprM (180), and QacA (159) was studied with TMA-DPH. TMA-DPH displays a biphasic interaction with bacterial membranes; a fast partitioning of the probe in the outer leaflet of the phospholipid bilayer, followed by a slower transbilayer movement of the probe from the outer to the inner leaflet (23, 159). The initial rate of TMA-DPH extrusion by the lactococcal multidrug transporter LmrA increases with the concentration of TMA-DPH in the inner leaflet, demonstrating that LmrA transports drugs from the inner leaflet of the membrane (23). A similar relationship between the initial rate of transport and the concentration of substrate in the inner leaflet of the membrane was observed for LmrP (22) and P-glycoprotein (229, 231). Also, the MDR transporters MexAB-OprM and QacA are capable of strongly reducing the accumulation of TMA-DPH in the inner leaflet of the bilayer (159, 180). Altogether, these results demonstrate that the multidrug transporters of the MFS, RND, and ABC families extrude toxic compounds from the inner leaflet of the cytoplasmic membrane to the aqueous extracellular medium (Fig. 8). Thus, despite differences in energy coupling, ATP- and PMF-dependent multidrug efflux pumps display a general mechanism of transport for hydrophobic drugs.
FIG. 8.
Extrusion of hydrophobic drugs by multidrug transporters. Drugs are expelled from the cytoplasmic leaflet of the membrane to the external medium.
Drug transport from the inner leaflet of the cytoplasmic membrane to the extracellular aqueous medium requires a high-affinity substrate-binding site that is accessible from the inner leaflet of the phospholipid bilayer. This high-affinity site is converted to a low-affinity outward-facing drug-binding site at the expense of ATP hydrolysis or proton translocation. Recently, drug transport cycles were described for the lactococcal ABC-type multidrug transporter LmrA (258) and the E. coli SMR-type multidrug transporter EmrE (165). Equilibrium binding experiments, photoaffinity labeling studies, and drug transport assays support the notion that homodimeric LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. The transporter possesses two drug-binding sites: a transport-competent site on the inner membrane surface and a drug release site on the outer membrane surface. The interconversion of these two sites is driven by the hydrolysis of ATP. Indeed, a combination of totalreflection Fourier transform infrared spectroscopy, 2H/H exchange, and fluorescence-quenching experiments indicated that LmrA undergoes a secondary-structure change and passes through three different conformational states during its drug transport cycle (262). The mechanism proposed for LmrA may also be relevant for P-glycoprotein and other ABC transporters (258). For P-glycoprotein, evidence has been obtained which supports a model of alternating catalytic sites (223). Both NBDs are able to bind and hydrolyze ATP (92, 138). The two nucleotide-binding sites interact strongly and cannot hydrolyze Mg2+-ATP simultaneously, suggesting that the two NBDs may alternate in catalysis (93, 224, 253, 254).
EmrE, which contains a membrane-embedded glutamate residue that is required for ligand binding and is proposed to function as a homotrimer, binds 1 mol of TPP+ per ∼3 mol of ErmE. From the observation that both binding and release of TPP+ from EmrE are strongly influenced by pH, Muth and Schuldiner (165) proposed a mechanism of transport by EmrE. As the substrate approaches the hydrophobic binding pocket, two protons are released from the negatively charged glutamate triplet. The positively charged substrate is bound through electrostatic interactions with the negatively charged carboxylate groups of the glutamates. Following a currently unknown conformational transition, the opening to the substrate-binding pocket becomes accessible to the external face of the membrane while being closed off from the internal face. The subsequent movement of two protons towards the binding pocket catalyzes the release of the bound substrate. EmrE then undergoes a conformational transition that converts the binding pocket accessibility back to the original membrane face. As most secondary multidrug transporters contain one or more negatively charged amino acid residues in TMS, a similar mechanism may be valid for other transporters. The available techniques for purification and functional reconstitution of the secondary multidrug transporters Smr, EmrE, NorA, LmrP, and AcrB enable structural studies that can reveal conformational changes during the catalytic cycle.
ANTIBIOTIC RESISTANCE
Bacterial multidrug efflux systems are a serious problem in the pharmacological treatment of patients with infectious diseases, since the substrate spectra of many multidrug transporters include clinically relevant antibiotics. Multidrug transporters are associated with both intrinsic and acquired resistance to antibiotics. Disruption of the genes encoding AcrAB, MexAB-OprM, HI0894/HI0895, NorA, and others causes increased sensitivity to several antibiotics, demonstrating that these multidrug transporters are expressed in wild-type strains and contribute to the intrinsic antibiotic resistance of E. coli, P. aeruginosa, H. influenzae, and S. aureus, respectively (143, 199, 220, 271). In addition, it has been shown that the expression of several genes encoding multidrug efflux pumps is inducible by the drugs to which they offer protection and that the expression of some multidrug transporters is modulated in response to environmental conditions. Acquired MDR can arise via three mechanisms: (i) amplification and mutations of genes encoding multidrug transporters, which change the expression level (174) or activity (113), (ii) mutations in specific or global regulatory genes which lead to the increased expression of multidrug transporters, exemplified by the mexR mutation in the OCR1 strain of P. aeruginosa (201), and (iii) intercellular transfer of resistance genes on transposons or plasmids, such as qacE (108).
The clinical importance of multidrug transporters strongly depends on the antibiotic spectrum to which they confer resistance. The antibiotic resistance profiles of the multidrug transporters known to date are summarized in Table 7. The data in Table 7 clearly show that the drug resistance profiles of the multidrug transporters vary strongly and family-specific profiles are not found. Resistance is observed against all important classes of antibiotics. MexAB-OprM of P. aeruginosa and the ABC-type multidrug transporter LmrA of L. lactis display very broad antibiotic specificity, demonstrating that both secondary and ATP-dependent multidrug transporters can seriously affect the efficacy of many antibiotics. LmrA and the human multidrug transporter P-glycoprotein are functionally interchangeable (257), suggesting that P-glycoprotein may also be involved in export of antibiotics. Indeed, several Steptomyces macrolide antibiotics have been reported to inhibit P-glycoprotein function (140). The plasmid-encoded multidrug transporter HorA confers resistance to a smaller range of antibiotics than the chromosomally encoded LmrA. However, since few antibiotic resistance data are available for other plasmid-encoded multidrug transporters, such as QacA, QacB, and Smr, it is not known whether a more restricted antibiotic resistance profile is a general feature of plasmid-encoded multidrug transporters. NorA and Bmr confer higher levels of resistance to hydrophilic quinolones, such as norfloxacin and ciprofloxacin, than to hydrophobic quinolones, such as sparfloxacin, ofloxacin, and nalidixic acid (104, 171, 271, 278). Similarly, norfloxacin transport by LfrA is inhibited by hydrophilic and not by hydrophobic quinolones (134). This specificity for hydrophilic quinolones was also observed for PmrA and NorM (63, 163). However, EmrB and VceB confer resistance only to hydrophobic quinolones, whereas LmrA and the multidrug transporters of the RND family transport both hydrophilic and hydrophobic quinolones (37, 66, 135, 158, 200, 205). In addition to conferring resistance, the overexpression of multidrug transporters can also cause increased drug sensitivity. The E. coli multidrug transporter MdfA confers resistance to the aminoglycosides neomycin and kanamycin but causes hypersensitivity to spectinomycin (19, 49). Overexpression of MexCD-OprJ results in hypersusceptibility to gentamicin and carbenicillin but not to ampicillin and penicillin (200, 240, 241). The mechanism of this increased drug susceptibility remains to be studied.
TABLE 7.
Reported drug resistance profiles of PMF- and ATP-dependent multidrug transportersa
| Drug | MFS
|
SMR
|
RND
|
MATE
|
ABC
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12-TMS cluster
|
14-TMS cluster
|
|||||||||||||||||||||||||||||||||||||||
| Bcr | Blt | Bmr | Cmr | EmrD | LmrP | MdfA | NorA | PmrA | Tap | Bmr3 | EmrB | LfrA | QacA | QacB | VceB | EbrAB | EmrE | Mmr | QacE | QacEΔ1 | QacG | QacH | Smr | TehA | YkkCD | AcrB | AcrF | AmrB | HI0895 | MexB | MexD | MexF | MexY | MtrD | YhiV | NorM | YdhE | HorA | LmrA | |
| Aminoglycosides | − | − | − | +b | + | − | − | − | + | − | + | − | −c | − | + | − | + | + | ||||||||||||||||||||||
| β-Lactams | ||||||||||||||||||||||||||||||||||||||||
| Carbapenems | − | − | − | + | − | − | ||||||||||||||||||||||||||||||||||
| Cephalosporins | − | − | − | − | + | + | ||||||||||||||||||||||||||||||||||
| Penicillins | − | + | + | − | − | + | −d | + | + | |||||||||||||||||||||||||||||||
| Chloramphenicol | + | − | − | − | + | + | − | − | − | + | + | − | − | − | + | + | − | − | + | + | + | − | − | + | ||||||||||||||||
| Glycopeptides | − | − | − | + | − | |||||||||||||||||||||||||||||||||||
| Lincosamides | + | − | − | + | ||||||||||||||||||||||||||||||||||||
| Macrolides | ||||||||||||||||||||||||||||||||||||||||
| 14-Membered | − | + | + | + | − | − | − | + | − | + | + | + | + | + | + | + | + | − | + | + | − | − | + | |||||||||||||||||
| 15-Membered | + | + | + | + | ||||||||||||||||||||||||||||||||||||
| 16-Membered | − | |||||||||||||||||||||||||||||||||||||||
| Novobiocin | + | − | + | + | + | + | + | + | ||||||||||||||||||||||||||||||||
| Quinolones | ||||||||||||||||||||||||||||||||||||||||
| Hydrophilic | + | +e | − | − | − | + | +e | + | − | + | − | + | − | − | − | + | − | − | + | + | + | + | − | + | + | |||||||||||||||
| Hydrophobic | +e | − | − | − | − | +e | − | − | − | + | − | + | + | − | + | + | + | − | + | |||||||||||||||||||||
| Rifampin | + | + | − | + | + | − | + | + | − | + | + | |||||||||||||||||||||||||||||
| Sulfonamides | + | − | + | |||||||||||||||||||||||||||||||||||||
| Tetracyclines | − | + | + | + | − | + | −f | + | − | − | + | − | − | + | + | + | − | + | + | + | + | − | − | + | ||||||||||||||||
| Trimethoprim | − | − | − | − | + | + | − | |||||||||||||||||||||||||||||||||
+, resistance; −, no resistance.
MdfA expression confers resistance to kanamycin and neomycin but hypersusceptibility to spectinomycin.
MexCD expression confers hypersusceptibility to carbenicillin but does not affect resistance to ampicillin and penicillin.
MexCD expression confers hypersusceptibility to gentamicin.
Bmr and NorA confer a higher level of resistance to hydrophilic quinolones than to hydrophobic quinolones.
LfrA confers resistance to tetracycline but not to minocycline and chlortetracycline.
PHYSIOLOGICAL FUNCTION
The efflux of a broad range of structurally unrelated toxic compounds can be the primary physiological function of multidrug transporters or, alternatively, can be merely a fortuitous side effect of the transport of an unidentified specific physiological substrate (173). The latter role of MDRs would be consistent with the observation that multidrug transporters belong to four distinct families which are frequently more homologous to substrate-specific transporters than to each other (126). More convincing evidence for a role of multidrug transporters in the efflux of a specific substrate comes from the B. subtilis multidrug transporter Blt. Woolridge et al. (269) demonstrated that overexpression of Blt results in an increased efflux of spermidine in the medium. The blt gene is cotranscribed with a downstream gene, btlD, which encodes a spermidine acetyltransferase (4, 269). This genetic arrangement suggests that the efflux of spermidine is the natural function of the Blt transporter, whereas multiple drugs may be recognized merely opportunistically. The ability of some MDR transporters to mediate lipids or fluorescent lipid derivatives may indicate that the physiological function of these multidrug transporters involves phospholipid translocation or transport of lipid-linked precursors of peptidoglycan (24, 90, 147, 254a, 280).
Alternatively, multidrug transporters may have evolved to protect cells from diverse environmental toxins. Schinkel et al. (222) demonstrated that disruption of the mouse mdr1a P-glycoprotein gene leads to increased sensitivity to drugs such as ivermectin and vinblastine. Similarly, increased antibiotic susceptibility was found in norA knockout mutants (94, 271). P-glycoprotein and the E. coli multidrug transporter AcrAB are induced in response to cellular damage (31) and stress conditions (143), respectively. Furthermore, the substrate spectra of several multidrug transporters include detergents (46, 203, 268), bile salts (80, 143, 249), organic solvents (266), and ionophores (54, 218, 233), suggesting that multidrug transporters play a role in protection of the membrane integrity or energy state of the cell.
CONCLUDING REMARKS
Multidrug transporters are a serious problem in the treatment of patients with hospital- or community-acquired infectious diseases, as they confer resistance to various of the antibiotics employed. A major force in the overexpression of endogenous multidrug transporters and spread of plasmid-encoded multidrug transporters is the huge consumption of antibiotics in human therapy, animal husbandry, and agriculture (12, 123). To restrain the evolution of antibiotic resistance, several strategies have been proposed to reduce the selective pressure of the presence of antibiotics (14, 123, 124, 267). The inappropriate use of antibiotics in household products, for the treatment of viral diseases, and as growth promoters in farm animals should be reduced. In addition, the development of new therapeutic agents is essential to fight the increasing number of multidrug-resistant microorganisms. One approach is to search for antibiotics with novel modes of action, which will not face previously selected resistance determinants. Alternatively, resistance-blocking agents can be identified and developed, allowing existing antibiotics to remain effective (125, 149). Several inhibitors of the multidrug transporters NorA and PmrA have been shown to increase the activity of fluoroquinolones against S. aureus and S. pneumoniae, respectively. Furthermore, these inhibitors dramatically suppress the in vitro emergence of resistant strains (1, 151, 152). However, since the known inhibitors, such as reserpine, are toxic to humans, the development of nontoxic inhibitors is required. Detailed information about the structure-function relationships that determine drug binding and transport by multidrug transporters should provide us with a rationale for these strategies.
Considerable progress has been made towards the identification of amino acid residues implicated in drug binding. Mutants of both ATP-dependent and secondary multidrug transporters demonstrated that aromatic, nonpolar, and negatively charged amino acid residues in predicted TMS are involved in substrate binding by multidrug transporters. Increasing evidence suggests the presence of multiple drug-binding sites, but the exact number and their three-dimensional organization are not known. Understanding the molecular mechanism of multidrug transport will ultimately require high-resolution structures of MDR proteins. As integral membrane proteins are generally very difficult to crystalize, structures of MDR regulator proteins which can bind a similar broad range of drugs may provide useful information on the structure-function relationships that dictate drug recognition. The low-resolution structure of P-glycoprotein (211) and the high-resolution (2.7 Å) three-dimensional structure of the drug-binding region of the regulator protein BmrR (284) are the first steps towards resolving the mystery of the broad substrate specificity of multidrug transporters.
REFERENCES
- 1.Aeschlimann J R, Dresser L D, Kaatz G W, Rybak M J. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:335–340. doi: 10.1128/aac.43.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed M, Borsch C M, Neyfakh A A, Schuldiner S. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensitive alkaloid reserpine. J Biol Chem. 1993;268:11086–11089. [PubMed] [Google Scholar]
- 3.Ahmed M, Borsch C M, Taylor S S, Vásquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–25813. [PubMed] [Google Scholar]
- 4.Ahmed M, Lyass L, Markham P N, Taylor S S, Vásquez-Laslop N, Neyfakh A A. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J Bacteriol. 1995;177:3904–3910. doi: 10.1128/jb.177.14.3904-3910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aínsa J A, Blokpoel M C J, Otal I, Young D B, De Smet K A L, Martín C. Molecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosis. J Bacteriol. 1998;180:5836–5843. doi: 10.1128/jb.180.22.5836-5843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aires J R, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altenberg G A, Vanoye C G, Horton J K, Reuss L. Unidirectional fluxes of rhodamine 123 in multidrug-resistant cells: evidence against direct drug extrusion from the plasma membrane. Proc Natl Acad Sci USA. 1994;91:4654–4657. doi: 10.1073/pnas.91.11.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ames G F-L, Mimura C S, Shyamala V. Bacterial periplasmic permeases belong to a family of transport proteins operating from E. coli to human: traffic ATPases. FEMS Microbiol Rev. 1990;75:429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 9.Ariza R R, Cohen S P, Bachhawat N, Levy S B, Demple B. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1994;176:143–148. doi: 10.1128/jb.176.1.143-148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariza R R, Li Z, Ringstad N, Demple B. Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J Bacteriol. 1995;177:1655–1661. doi: 10.1128/jb.177.7.1655-1661.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arkin I T, Russ W P, Lebendiker M, Schuldiner S. Determining the secondary structure and orientation of EmrE, a multi-drug transporter, indicates a transmembrane four-helix bundle. Biochemistry. 1996;35:7233–7238. doi: 10.1021/bi960094i. [DOI] [PubMed] [Google Scholar]
- 12.Austin D J, Kristinsson K G, Anderson R M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA. 1999;96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayesh S, Shao Y-M, Stein W D. Co-operative, competitive and non-competitive interactions between modulators of P-glycoprotein. Biochim Biophys Acta. 1996;1316:8–18. doi: 10.1016/0925-4439(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 14.Baquero F, Blázquez J. Evolution of antibiotic resistance. TREE. 1997;12:482–487. doi: 10.1016/s0169-5347(97)01223-8. [DOI] [PubMed] [Google Scholar]
- 15.Baranova N N, Neyfakh A A. Apparent involvement of a multidrug transporter in the fluoroquinolone resistance of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1396–1398. doi: 10.1128/aac.41.6.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baranova N N, Danchin A, Neyfakh A A. Mta, a global MerR-type regulator of the Bacillus subtilis multidrug-efflux transporters. Mol Microbiol. 1999;31:1549–1559. doi: 10.1046/j.1365-2958.1999.01301.x. [DOI] [PubMed] [Google Scholar]
- 17.Barrasa M I, Tercero J A, Lacalle R A, Jimenez A. The ard1 gene from Streptomyces capreolus encodes a polypeptide of the ABC-transporters superfamily which confers resistance to the aminonucleoside antibiotic A201A. Eur J Biochem. 1995;228:562–569. doi: 10.1111/j.1432-1033.1995.tb20295.x. [DOI] [PubMed] [Google Scholar]
- 18.Bentley J, Hyatt L S, Ainley K, Parish J H, Herbert R B, White G R. Cloning and sequence analysis of an Escherichia coli gene conferring bicyclomycin resistance. Gene. 1993;127:117–120. doi: 10.1016/0378-1119(93)90625-d. [DOI] [PubMed] [Google Scholar]
- 19.Bohn C, Bouloc P. The Escherichia coli cmlA gene encodes the multidrug efflux pump Cmr/MdfA and is responsible for isopropyl-β-d-thiogalactopyranoside exclusion and spectinomycin sensitivity. J Bacteriol. 1998;190:6072–6075. doi: 10.1128/jb.180.22.6072-6075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolhuis H, Molenaar D, Poelarends G, van Veen H W, Poolman B, Driessen A J M, Konings W N. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J Bacteriol. 1994;176:6957–6964. doi: 10.1128/jb.176.22.6957-6964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolhuis H, Poelarends G, van Veen H W, Poolman B, Driessen A J M, Konings W N. The lactococcal lmrP gene encodes a proton motive force-dependent drug transporter. J Biol Chem. 1995;270:26092–26098. doi: 10.1074/jbc.270.44.26092. [DOI] [PubMed] [Google Scholar]
- 22.Bolhuis H, van Veen H W, Brands J R, Putman M, Poolman B, Driessen A J M, Konings W N. Energetics and mechanism of drug transport mediated by the lactococcal multidrug transporter LmrP. J Biol Chem. 1996;271:24123–24128. doi: 10.1074/jbc.271.39.24123. [DOI] [PubMed] [Google Scholar]
- 23.Bolhuis H, van Veen H W, Molenaar D, Poolman B, Driessen A J M, Konings W N. Multidrug resistance in Lactococcus lactis: Evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J. 1996;15:4239–4245. [PMC free article] [PubMed] [Google Scholar]
- 24.Bosch I, Dunussi-Joannopoulos K, Wu R-L, Furlong S, Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–5694. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 25.Brenwald N P, Gill M J, Wise R. The effect of reserpine, an inhibitor of multi-drug efflux pumps, on the in-vitro susceptibilities of fluoroquinolone-resistant strains of Streptococcus pneumoniae to norfloxacin. J Antimicrob Chemother. 1997;40:458–460. doi: 10.1093/jac/40.3.458. [DOI] [PubMed] [Google Scholar]
- 26.Brenwald N P, Gill M J, Wise R. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2032–2035. doi: 10.1128/aac.42.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooun A, Tomashek J J, Lewis K. Purification and ligand binding of EmrE, a regulator of a multidrug transporter. J Bacteriol. 1999;181:5131–5133. doi: 10.1128/jb.181.16.5131-5133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown M H, Paulsen I T, Skurray R A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol. 1999;31:394–395. doi: 10.1046/j.1365-2958.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 29.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxbaum E. Co-operative binding sites for transported substrates in the multiple drug resistance transporter Mdr1. Eur J Biochem. 1999;265:64–70. doi: 10.1046/j.1432-1327.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary P M, Roninson I B. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993;85:632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- 32.Cohen M L. Epidemiology of drug resistance: implications for a postantimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S P, McMurry L M, Levy S B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988;170:5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen S P, Hächler H, Levy S B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen S P, Levy S B, Foulds J, Rosner J L. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J Bacteriol. 1993;175:7856–7862. doi: 10.1128/jb.175.24.7856-7862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S P, Yan W, Levy S B. A multidrug resistance regulatory chromosomal locus is widespread among enteric bacteria. J Infect Dis. 1993;168:484–488. doi: 10.1093/infdis/168.2.484. [DOI] [PubMed] [Google Scholar]
- 37.Colmer J A, Fralick J A, Hamood A N. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol Microbiol. 1998;27:63–72. doi: 10.1046/j.1365-2958.1998.00657.x. [DOI] [PubMed] [Google Scholar]
- 38.Culliton B J. Drug-resistant TB may bring epidemic. Nature. 1992;356:473. doi: 10.1038/356473a0. [DOI] [PubMed] [Google Scholar]
- 39.Currier S J, Kane S E, Willingham M C, Cardarelli C O, Pastan I, Gottesman M M. Identification of residues in the first cytoplasmic loop of P-glycoprotein involved in the function of chimeric human MDR1-MDR2 transporters. J Biol Chem. 1992;267:25153–25159. [PubMed] [Google Scholar]
- 40.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 41.Delahay R M, Robertson B D, Balthazar J T, Shafer W M, Ison C A. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology. 1997;143:2127–2133. doi: 10.1099/00221287-143-7-2127. [DOI] [PubMed] [Google Scholar]
- 42.De Rossi E, Branzoni M, Cantoni R, Milano A, Riccardi G, Ciferri O. mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J Bacteriol. 1998;180:6068–6071. doi: 10.1128/jb.180.22.6068-6071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devine S E, Ling V, Melera P W. Amino acid substitutions in the sixth transmembrane domain of P-glycoprotein alter multidrug resistance. Proc Natl Acad Sci USA. 1992;89:4564–4568. doi: 10.1073/pnas.89.10.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dey S, Ramachandra M, Pastan I, Gottesman M M, Ambudkar S V. Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc Natl Acad Sci USA. 1997;94:10594–10599. doi: 10.1073/pnas.94.20.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinh T, Paulsen I T, Saier M H. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doige C A, Yu X, Sharom F J. The effect of lipids and detergents on ATPase-active P-glycoprotein. Biochim Biophys Acta. 1993;1146:65–72. doi: 10.1016/0005-2736(93)90339-2. [DOI] [PubMed] [Google Scholar]
- 47.Dougherty D A. Cation-π interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science. 1996;271:163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- 48.Eckert B, Beck C F. Overproduction of transposon Tn10-encoded tetracycline resistance protein results in cell death and loss of membrane potential. J Bacteriol. 1989;171:3557–3559. doi: 10.1128/jb.171.6.3557-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar R, Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol. 1997;179:2274–2280. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgar R, Bibi E. A single membrane-embedded negative charge is critical for recognizing positively charged drugs by the Escherichia coli multidrug resistance protein MdfA. EMBO J. 1999;18:822–832. doi: 10.1093/emboj/18.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards R A, Turner R J. Alpha-periodicity analysis of small multidrug resistance (SMR) efflux transporters. Biochem Cell Biol. 1998;76:791–797. doi: 10.1139/bcb-76-5-791. [DOI] [PubMed] [Google Scholar]
- 52.Evans K, Passador I, Srikumar R, Tsang E, Nezezon J, Poole K. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1998;180:5443–5447. doi: 10.1128/jb.180.20.5443-5447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans K, Poole K. The MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa is growth-phase regulated. FEMS Microbiol Lett. 1999;173:35–39. doi: 10.1111/j.1574-6968.1999.tb13481.x. [DOI] [PubMed] [Google Scholar]
- 54.Eytan G D, Borgnia M J, Regev R, Assaraf Y G. Transport of polypeptide ionophores into proteoliposomes reconstituted with rat liver P-glycoprotein. J Biol Chem. 1994;269:26058–26065. [PubMed] [Google Scholar]
- 55.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferry D R, Russell M A, Cullen M H. P-glycoprotein possesses a 1,4-dihydropyridine-selective drug acceptor site which is allosterically coupled to a vinca-alkaloid-selective binding site. Biochem Biophys Res Commun. 1992;188:440–445. doi: 10.1016/0006-291x(92)92404-l. [DOI] [PubMed] [Google Scholar]
- 57.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Field C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelly J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrman J L, Geohagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 58.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furukawa H, Tsay J-T, Jackowski S, Takamura Y, Rock C O. Thiolactomycin resistance in Escherichia coli is associated with the multidrug resistance efflux pump encoded by emrAB. J Bacteriol. 1993;175:3723–3729. doi: 10.1128/jb.175.12.3723-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gambino L, Gracheck S J, Miller P F. Overexpression of the MarA positive regulator is sufficient to confer multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1993;175:2888–2894. doi: 10.1128/jb.175.10.2888-2894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garrigos M, Mir L M, Orlowski S. Competitive and non-competitive inhibition of the multidrug-resistance-associated P-glycoprotein ATPase; further experimental evidence for a multisite model. Eur J Biochem. 1997;244:664–673. doi: 10.1111/j.1432-1033.1997.00664.x. [DOI] [PubMed] [Google Scholar]
- 62.Gaudu P, Weiss B. SoxR, a [2Fe-2S] transcription factor, is active only in its oxidized form. Proc Natl Acad Sci USA. 1996;93:10094–10098. doi: 10.1073/pnas.93.19.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gill M J, Brenwald N P, Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:187–189. doi: 10.1128/aac.43.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotoh N, Tsujimoto H, Poole K, Yamagishi J-I, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–2569. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada A, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gotoh N, Tsujimoto H, Nomura A, Okamoto K, Tsuda M, Nishino T. Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1998;165:21–27. doi: 10.1111/j.1574-6968.1998.tb13122.x. [DOI] [PubMed] [Google Scholar]
- 67.Gottesman M M, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 68.Gottesman M M, Hrycyna C A, Schoenlein P V, Germann U A, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet. 1995;29:607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- 69.Greenberger L M. Major photoaffinity drug labeling sites for iodoaryl azidoprazosin in P-glycoprotein are within, or immediately C-terminal to, transmembrane domains 6 and 12. J Biol Chem. 1993;268:11417–11425. [PubMed] [Google Scholar]
- 70.Griffith J K, Baker M E, Rouch D A, Page M P G, Skurray R A, Paulsen I T, Chater K F, Baldwin S A, Henderson P J F. Membrane proteins: implications of sequence comparisons. Curr Opin Cell Biol. 1992;4:684–695. doi: 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 71.Grinius L, Dreguniene G, Goldberg E B, Liao C-H, Projan S J. A staphylococcal multidrug resistance gene product is a member of a new protein family. Gene. 1992;27:119–129. doi: 10.1016/0147-619x(92)90012-y. [DOI] [PubMed] [Google Scholar]
- 72.Grinius L, Goldberg E B. Bacterial multidrug resistance is due to a single membrane protein which functions as a drug pump. J Biol Chem. 1994;269:29998–30004. [PubMed] [Google Scholar]
- 73.Grinius L L, Siehnel R J, Morris C M. Quinolone efflux protein NorA is a proton-driven drug pump. FASEB J. 1997;11:A958. [Google Scholar]
- 74.Grkovic S, Brown M H, Roberts N J, Paulsen I T, Skurray R A. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J Biol Chem. 1998;273:18665–18673. doi: 10.1074/jbc.273.29.18665. [DOI] [PubMed] [Google Scholar]
- 75.Guan L, Ehrmann M, Yoneyama H, Nakae T. Membrane topology of the xenobiotic-exporting subunit, MexB, of the MexA,B-OprM Extrusion pump in Pseudomonas aeruginosa. J Biol Chem. 1999;274:10517–10522. doi: 10.1074/jbc.274.15.10517. [DOI] [PubMed] [Google Scholar]
- 76.Guilfoile P G, Hutchinson C R. A bacteriol analog of the mdr gene of mammalian tumor cells is present in Streptomyces peucetius, the producer of daunorubicin and doxorubicin. Proc Natl Acad Sci USA. 1991;88:8553–8557. doi: 10.1073/pnas.88.19.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gutmann L, Williamson R, Moreau N, Kitzis M-D, Collatz E, Acar J F, Goldstein F W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985;151:501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- 78.Hächler H, Cohen S P, Levy S B. marA, a regulated locus which controls expression of chromosomal multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1991;173:5532–5538. doi: 10.1128/jb.173.17.5532-5538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hagman K E, Shafer W M. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol. 1995;177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagman K E, Pan W, Spratt B G, Balthazar J T, Judd R C, Shafer W M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 81.Hagman K E, Lucas C E, Balthazar J T, Snyder L, Nilles M, Judd R C, Shafer W M. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology. 1997;143:2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 82.Hanna M, Brault M, Kwan T, Kast C, Gros P. Mutagenesis of transmembrane domain 11 of P-glycoprotein by alanine scanning. Biochemistry. 1996;35:3625–3635. doi: 10.1021/bi951333p. [DOI] [PubMed] [Google Scholar]
- 83.Hayes J D, Wolf C R. Molecular mechanisms of drug resistance. Biochem J. 1990;272:281–295. doi: 10.1042/bj2720281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heir E, Sundheim G, Holck A L. The Staphylococcus aureus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol Lett. 1998;163:49–56. doi: 10.1111/j.1574-6968.1998.tb13025.x. [DOI] [PubMed] [Google Scholar]
- 85.Heir E, Sundheim G, Holck A L. The qacG gene on plasmid pST94 confers resistance to quarternary ammonium compounds in staphylococci isolated from the food industry. J Appl Microbiol. 1999;86:378–388. doi: 10.1046/j.1365-2672.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- 86.Reference deleted.
- 87.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 88.Higgins C F, Gottesman M M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 89.Holló Z, Homolya L, Davis C W, Sarkadi B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim Biophys Acta. 1994;1191:384–388. doi: 10.1016/0005-2736(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 90.Höltje J-V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Homolya L, Holló Z, Germann U A, Pastan I, Gottesman M M, Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 92.Horio M, Gottesman M M, Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci USA. 1988;85:3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hrycyna C A, Ramachandra M, Ambudkar S V, Hee Ko Y, Pedersen P L, Pastan I, Gottesman M M. Mechanism of action of human P-glycoprotein ATPase activity; photochemical cleavage during a catalytic transition state using orthovanadate reveals cross-talk between the two ATP sites. J Biol Chem. 1998;273:16631–16634. doi: 10.1074/jbc.273.27.16631. [DOI] [PubMed] [Google Scholar]
- 94.Hsieh P-C, Siegel S A, Rogers B, Davis D, Lewis K. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc Natl Acad Sci USA. 1998;95:6602–6606. doi: 10.1073/pnas.95.12.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huovinen P, Sundström L, Swedberg G, Sköld O. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother. 1995;39:279–289. doi: 10.1128/aac.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hyde S C, Emsley P, Hartshorn M J, Mimmack M M, Gileady U, Pearce S R, Gallagher M P, Gill D R, Hubbard R E, Higgins C F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 97.Jack D L, Storms M L, Tchieu J H, Paulsen I T, Saier M H., Jr A broad-specificity multidrug efflux pump requiring a pair of homologous SMR-type proteins. J Bacteriol. 2000;182:2311–2313. doi: 10.1128/jb.182.8.2311-2313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jäger W, Kalinowski J, Pühler A. A Corynebacterium glutamicum gene conferring multidrug resistance in the heterologous host Escherichia coli. J Bacteriol. 1997;179:2449–2451. doi: 10.1128/jb.179.7.2449-2451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jair K-W, Martin R G, Rosner J L, Fujita N, Ishihama A, Wolf R E., Jr Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. J Bacteriol. 1995;177:7100–7104. doi: 10.1128/jb.177.24.7100-7104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jair K-W, Yu X, Skarstad K, Thöny B, Fujita N, Ishihama A, Wolf R E., Jr Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of chromosomal replication. J Bacteriol. 1996;178:2507–2513. doi: 10.1128/jb.178.9.2507-2513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jarlier V, Nikaido H. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett. 1994;123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 102.Jessen-Marshall A E, Paul N J, Brooker R J. The conserved motif, GXXX(D/E)(R/K)XG[X](R/K)(R/K), in hydrophilic loop 2/3 of the lactose permease. J Biol Chem. 1995;27:16251–16257. doi: 10.1074/jbc.270.27.16251. [DOI] [PubMed] [Google Scholar]
- 103.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaatz G W, Seo S M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaidoh K, Miyauchi S, Abe A, Tanabu S, Nara T, Kamo N. Rhodamine 123 efflux transporter in Haloferax volcanii is induced when cultured under ‘metabolic stress’ by amino acids: the efflux system resembles that in a doxorubicin-resistant mutant. Biochem J. 1996;314:355–359. doi: 10.1042/bj3140355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kajiji S, Talbot F, Grizzuti K, Van Dyke-Phillips V, Agresti M, Safa A R, Gros P. Functional analysis of P-glycoprotein mutants identifies predicted transmembrane domain 11 as a putative drug binding site. Biochemistry. 1993;32:4185–4194. doi: 10.1021/bi00067a005. [DOI] [PubMed] [Google Scholar]
- 107.Kato S, Nishimura J, Yufu Y, Ideguchi H, Umemura T, Nawata H. Modulation of expression of multidrug resistance gene (mdr-1) by adriamycin. FEBS Lett. 1992;308:175–178. doi: 10.1016/0014-5793(92)81269-r. [DOI] [PubMed] [Google Scholar]
- 108.Kazama H, Hamashima H, Sasatsu M, Arai T. Distribution of the antiseptic-resistance genes qacE and qacEΔ1 in Gram-negative bacteria. FEMS Microbiol Lett. 1998;159:173–178. doi: 10.1111/j.1574-6968.1998.tb12857.x. [DOI] [PubMed] [Google Scholar]
- 109.Kazama H, Hamashima H, Sasatsu M, Arai T. Distribution of the antiseptic-resistance gene qacEΔ1 in Gram-positive bacteria. FEMS Microbiol Lett. 1998;165:295–299. doi: 10.1111/j.1574-6968.1998.tb13160.x. [DOI] [PubMed] [Google Scholar]
- 110.Klein J R, Henrich B, Plapp R. Molecular analysis and nucleotide sequence of the envCD operon of Escherichia coli. Mol Gen Genet. 1991;230:230–240. doi: 10.1007/BF00290673. [DOI] [PubMed] [Google Scholar]
- 111.Klyachko K A, Schuldiner S, Neyfakh A A. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter Bmr. J Bacteriol. 1997;179:2189–2193. doi: 10.1128/jb.179.7.2189-2193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klyachko K A, Neyfakh A A. Paradoxical enhancement of the activity of a bacterial multidrug transporter caused by substitutions of a conserved residue. J Bacteriol. 1998;180:2817–2821. doi: 10.1128/jb.180.11.2817-2821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Knol J, Veenhoff L, Liang W-J, Henderson P J F, Leblanc G, Poolman B. Unidirectional reconstitution into detergent destabilized liposomes of the purified lactose transport systems of Streptococcus thermophilus. J Biol Chem. 1996;271:15358–15366. doi: 10.1074/jbc.271.26.15358. [DOI] [PubMed] [Google Scholar]
- 114.Reference deleted.
- 115.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Kocjancic Curty L, Pechère J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 116.Köhler T, Epp S F, Kocjancic Curty L, Pechère J-C. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J Bacteriol. 1999;181:6300–6305. doi: 10.1128/jb.181.20.6300-6305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kolaczkowski M, van der Rest M, Cybularz-Kolaczkowska A, Soumillion J-P, Konings W N, Goffeau A. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p. J Biol Chem. 1996;271:31543–31548. doi: 10.1074/jbc.271.49.31543. [DOI] [PubMed] [Google Scholar]
- 118.Kronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 119.Kurland C G, Dong H. Bacterial growth inhibition by overproduction of protein. Mol Microbiol. 1996;21:1–4. doi: 10.1046/j.1365-2958.1996.5901313.x. [DOI] [PubMed] [Google Scholar]
- 120.Leelaporn A, Paulsen I T, Tennent J M, Littlejohn T G, Skurray R A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J Med Microbiol. 1994;40:214–220. doi: 10.1099/00222615-40-3-214. [DOI] [PubMed] [Google Scholar]
- 121.Leelaporn A, Firth N, Paulsen I T, Hettiaratchi A, Skurray R A. Multidrug resistance plasmid pSK108 from coagulase-negative staphylococci: relationships to Staphylococcus aureus qacC plasmids. Plasmid. 1995;34:62–67. doi: 10.1006/plas.1995.1034. [DOI] [PubMed] [Google Scholar]
- 122.Levy S. Active efflux mechanisms for antibiotic resistance. Antimicrob Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levy S. The challenge of antibiotic resistance. Sci Am. 1998;278:32–39. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 124.Levy S. Multidrug resistance—a sign of the times. N Engl J Med. 1998;338:1376–1378. doi: 10.1056/NEJM199805073381909. [DOI] [PubMed] [Google Scholar]
- 125.Levy S, Nelson M. Reversing tetracycline resistance: a renaissance for the tetracycline family of antibiotics. In: Rosen B P, Mobashery S, editors. Resolving the antibiotic paradox. New York, N.Y: Kluwer Academic/Plenum Publishers; 1998. pp. 17–25. [PubMed] [Google Scholar]
- 126.Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 127.Lewis K, Hooper D C, Ouellette M. Multidrug resistance pumps provide broad defense. ASM News. 1997;63:605–610. [Google Scholar]
- 128.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Linton K J, Cooper H N, Hunter I S, Leadleay P F. An ABC-transporter from Streptomyces longisporoflavus confers resistance to the polyether-ionophore antibiotic tetronasin. Mol Microbiol. 1994;11:777–785. doi: 10.1111/j.1365-2958.1994.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 131.Litman T, Zeuthen T, Skovsgaard T, Stein W D. Competitive, non-competitive and cooperative ineractions between substrates of P-glycoprotein as measured by its ATPase activity. Biochim Biophys Acta. 1997;1361:169–176. doi: 10.1016/s0925-4439(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 132.Littlejohn T G, DiBerardino D, Messerotti L J, Spiers S J, Skurray R A. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene. 1991;101:59–66. doi: 10.1016/0378-1119(91)90224-y. [DOI] [PubMed] [Google Scholar]
- 133.Littlejohn T G, Paulsen I T, Gillespie M T, Tennent J A, Midgley M, Jones I G, Purewal A S, Skurray R A. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1992;95:259–266. doi: 10.1016/0378-1097(92)90439-u. [DOI] [PubMed] [Google Scholar]
- 134.Liu J, Takiff H E, Nikaido H. Active efflux of fluoroquinolones in Mycobacterium smegmatis mediated by LfrA, a multidrug efflux pump. J Bacteriol. 1996;178:3791–3795. doi: 10.1128/jb.178.13.3791-3795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lomovskaya O, Lewis K. emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lomovskaya O, Lewis K, Matin A. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J Bacteriol. 1995;177:2328–2334. doi: 10.1128/jb.177.9.2328-2334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loo T W, Clarke D M. Functional consequences of phenylalanine mutations in the predicted transmembrane domain of P-glycoprotein. J Biol Chem. 1993;268:19965–19972. [PubMed] [Google Scholar]
- 138.Loo T W, Clarke D M. Reconstitution of drug-stimulated ATPase activity following co-expression of each half of human P-glycoprotein as separate polypeptides. J Biol Chem. 1994;269:7750–7755. [PubMed] [Google Scholar]
- 139.Loo T W, Clarke D M. Mutation of amino acids located in predicted transmembrane domain segment 6 (TM6) modulate the activity and substrate specificity of human P-glycoprotein. Biochemistry. 1994;33:14049–14057. doi: 10.1021/bi00251a013. [DOI] [PubMed] [Google Scholar]
- 140.Loor F. Cyclosporins and related fungal products in the reversal of P-glycoprotein-mediated multidrug resistance. In: Gupta S, Tsuruo T, editors. Multidrug resistance in cancer cells: cellular, biochemical and biological aspects. Chichester, U.K: John Wiley & Sons Ltd; 1996. pp. 385–412. [Google Scholar]
- 141.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in Gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 143.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 144.Ma D, Alberti M, Lynch C, Nikaido H, Hearst J E. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 145.Maiden M C J, Davis E O, Baldwin S A, Moore D C M, Henderson P J F. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987;325:41–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- 146.Marger M, Saier M H., Jr A major superfamily of transmembrane facilitators that can catalyze uniport, symport and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 147.Margolles A, Putman M, van Veen H W, Konings W N. The purified and functionally reconstituted multidrug transporter LmrA of Lactococcus lactis mediates the transbilayer movement of specific fluorescent phospholipids. Biochemistry. 1999;38:16298–16306. doi: 10.1021/bi990855s. [DOI] [PubMed] [Google Scholar]
- 148.Markham P N, Ahmed M, Neyfakh A A. The drug-binding activity of the multidrug-responding transcriptional regulator BmrR resides in its C-terminal domain. J Bacteriol. 1996;178:1473–1475. doi: 10.1128/jb.178.5.1473-1475.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Markham P N, Neyfakh A A. Inhibition of the multidrug transporter NorA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:2673–2674. doi: 10.1128/aac.40.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Markham P N, LoGuidice J, Neyfakh A A. Broad ligand specificity of the transcriptional regulator of the Bacillus subtilis multidrug transporter Bmr. Biochem Biophys Res Commun. 1997;239:269–272. doi: 10.1006/bbrc.1997.7467. [DOI] [PubMed] [Google Scholar]
- 151.Markham P N. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug inhibitor reserpine. Antimicrob Agents Chemother. 1999;43:988–989. doi: 10.1128/aac.43.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Markham P N, Westhaus E, Klyachko K, Johnson M E, Neyfakh A A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404–2408. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin C, Berridge G, Higgins C F, Callaghan R. The multi-drug resistance reversal agent SR33557 and modulation of vinca alkaloid binding to P-glycoprotein by an allosteric interaction. Br J Pharmacol. 1997;122:765–771. doi: 10.1038/sj.bjp.0701429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Masaoka Y, Ueno Y, Morita Y, Kuroda T, Mizushima T, Tsuchiya T. A two-component multidrug efflux pump, EbrAB, in Bacillus subtilis. J Bacteriol. 2000;182:2307–2310. doi: 10.1128/jb.182.8.2307-2310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maseda H, Yoneyama H, Nakae T. Assignment of the substrate-selective subunits of the MexEF-OprN multidrug efflux pump of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:658–664. doi: 10.1128/aac.44.3.658-664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McDermott P F, White D G, Podglajen I, Aleksun M N, Levy S B. Multidrug resistance following expression of the Escherichia coli marA gene in Mycobacterium smegmatis. J Bacteriol. 1998;180:2992–2998. doi: 10.1128/jb.180.11.2995-2998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Medeiros A A, O'Brien T F, Rosenberg E Y, Nikaido H. Loss of OmpC porin in a strain of Salmonella typhimurium causes increased resistance to cephalosporins during therapy. J Infect Dis. 1987;156:751–757. doi: 10.1093/infdis/156.5.751. [DOI] [PubMed] [Google Scholar]
- 158.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mitchell B A, Paulsen I T, Brown M H, Skurray R A. Bioenergetics of the staphylococcal multidrug export protein QacA: identification of distinct binding sites for monovalent and divalent cations. J Biol Chem. 1999;274:3541–3548. doi: 10.1074/jbc.274.6.3541. [DOI] [PubMed] [Google Scholar]
- 160.Moellering R C., Jr Introduction: problems with antimicrobial resistance in Gram-positive cocci. Clin Infect Dis. 1998;16:1177–1178. doi: 10.1086/520288. [DOI] [PubMed] [Google Scholar]
- 161.Moore R A, DeShazer D, Reckseidler S, Weissman A, Woods D E. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother. 1999;43:465–470. doi: 10.1128/aac.43.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morimyo M, Hongo E, Hama-Inaba H, Machida I. Cloning and characterization of the mvrC gene of Escherichia coli K-12 which confers resistance against methyl viologen toxicity. Nucleic Acids Res. 1992;20:3159–3165. doi: 10.1093/nar/20.12.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Morris D I, Greenberger L M, Bruggemann E P, Cardarelli C, Gottesman M M, Pastan I, Seamon K B. Localization of the forskolin labeling sites to both halves of P-glycoprotein: similarity of the sites labeled by forskolin and prazosin. Mol Pharmacol. 1994;46:329–337. [PubMed] [Google Scholar]
- 165.Muth T R, Schuldiner S. A membrane-embedded glutamate is required for ligand binding to the multidrug transporter EmrE. EMBO J. 2000;19:232–240. doi: 10.1093/emboj/19.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nakamura H. Gene-controlled resistance to acriflavine and other basic dyes in Escherichia coli. J Bacteriol. 1965;90:8–14. doi: 10.1128/jb.90.1.8-14.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968;96:987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naroditskaya V, Schlosser M J, Fang N Y, Lewis K. An E. coli gene emrD is involved in adaptation to low energy shock. Biochem Biophys Res Commun. 1993;196:803–809. doi: 10.1006/bbrc.1993.2320. [DOI] [PubMed] [Google Scholar]
- 169.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 170.Neyfakh A A, Bidnenko V E, Chen L B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Neyfakh A A. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob Agents Chemother. 1992;36:484–485. doi: 10.1128/aac.36.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neyfakh A A, Borsch C M, Kaatz G W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux protein. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Neyfakh A A. Natural functions of bacterial multidrug transporters. Trends Microbiol. 1997;8:309–313. doi: 10.1016/S0966-842X(97)01064-0. [DOI] [PubMed] [Google Scholar]
- 174.Ng E Y W, Trucksis M, Hooper D C. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 178.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nilsen I W, Bakke I, Vader A, Olsvik Ø, El-Gewely M R. Isolation of cmr, a novel Escherichia coli chloramphenicol resistance gene encoding a putative efflux pump. J Bacteriol. 1996;178:3188–3193. doi: 10.1128/jb.178.11.3188-3193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ocaktan A, Yoneyama H, Nakae T. Use of fluorescent probes to monitor the subunit proteins of the MexA-MexB-OprM drug extrusion machinery in Pseudomonas aeruginosa. J Biol Chem. 1997;272:21964–21969. doi: 10.1074/jbc.272.35.21964. [DOI] [PubMed] [Google Scholar]
- 181.Ohki R, Murata M. bmr3, a third multidrug transporter gene of Bacillus subtilis. J Bacteriol. 1997;179:1423–1427. doi: 10.1128/jb.179.4.1423-1427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple antibiotic resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Olano C, Rodríguez A M, Méndez C, Salas J A. A second ABC transporter is involved in oleandomycin resistance and its secretion by Streptomyces antibioticus. Mol Microbiol. 1995;16:333–343. doi: 10.1111/j.1365-2958.1995.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 184.Oshita Y, Hiramatsu K, Yokota T. A point mutation in the norA gene is responsible for quinolone resistance in Staphylococcus aureus. Biochem Biophys Res Commun. 1990;172:1028–1034. doi: 10.1016/0006-291x(90)91549-8. [DOI] [PubMed] [Google Scholar]
- 185.Pan W, Spratt B G. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol. 1994;11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 186.Pascaud C, Garrigos M, Orlowski S. Multidrug resistance transporter P-glycoprotein has distinct but interacting binding sites for cytotoxic drugs and reversing agents. Biochem J. 1998;333:351–358. doi: 10.1042/bj3330351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paulsen I T, Littlejohn T G, Rådström P, Sundström L, Sköld O, Swedberg G, Skurray R A. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paulsen I T, Skurray R A. Topology, structure and evolution of two families of proteins involved in antibiotic and antiseptic resistance in eukaryotes and prokaryotes—an analysis. Gene. 1993;124:1–11. doi: 10.1016/0378-1119(93)90755-r. [DOI] [PubMed] [Google Scholar]
- 189.Paulsen I T, Brown M H, Dunstan S J, Skurray R A. Molecular characterization of the staphylococcal multidrug resistance export protein QacC. J Bacteriol. 1995;177:2827–2833. doi: 10.1128/jb.177.10.2827-2833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Paulsen I T, Skurray R A, Tam R, Saier M H, Jr, Turner R J, Weiner J H, Goldberg E B, Grinius L L. The SMR family: A novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 192.Paulsen I T, Brown M H, Littlejohn T G, Mitchell B A, Skurray R A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci USA. 1996c;93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Paulsen I T, Brown M H, Skurray R A. Characterization of the earliest known Staphylococcus aureus plasmid encoding a multidrug efflux system. J Bacteriol. 1998;180:3477–3479. doi: 10.1128/jb.180.13.3477-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 195.Pawagi A B, Wang J, Silverman M, Reithmeier R A F, Deber C M. Transmembrane aromatic amino acid distribution in P-glycoprotein: a functional role in broad substrate specificity. J Mol Biol. 1994;235:554–564. doi: 10.1006/jmbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 196.Pereira E, Borrel M N, Fiallo M, Garnier-Suillerot A. Noncompetitive inhibition of P-glycoprotein-associated efflux of THP-adriamycin by verapamil in living K562 leukemia cells. Biochim Biophys Acta. 1994;1225:209–216. doi: 10.1016/0925-4439(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 197.Podlesek Z, Comino A, Herzog-Velikonja B, Žgur-Bertok D, Komel R, Grabnar M. Bacillus licheniformis bacitracin-resistance ABC transporter: relationship to mammalian multidrug resistance. Mol Microbiol. 1995;16:969–976. doi: 10.1111/j.1365-2958.1995.tb02322.x. [DOI] [PubMed] [Google Scholar]
- 198.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 199.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfx-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 201.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs D E, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Purewal A S. Nucleotide sequence of the ethidium efflux gene from Escherichia coli. FEMS Microbiol Lett. 1991;82:229–232. doi: 10.1016/0378-1097(91)90338-b. [DOI] [PubMed] [Google Scholar]
- 203.Putman M, van Veen H W, Poolman B, Konings W N. Restrictive use of detergents in the functional reconstitution of the secondary multidrug transporter LmrP. Biochemistry. 1999;38:1002–1008. doi: 10.1021/bi981863w. [DOI] [PubMed] [Google Scholar]
- 204.Putman M, Koole L A, van Veen H W, Konings W N. The secondary multidrug transporter LmrP contains multiple drug interaction sites. Biochemistry. 1999;38:13900–13905. doi: 10.1021/bi991262k. [DOI] [PubMed] [Google Scholar]
- 205.Putman M, van Veen H W, Degener J E, Konings W N. Antibiotic resistance: era of the multidrug pump. Mol Microbiol. 2000;36:772–774. doi: 10.1046/j.1365-2958.2000.01871.x. [DOI] [PubMed] [Google Scholar]
- 206.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raviv Y, Pollard H B, Bruggemann E P, Pastan I, Gottesman M M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J Biol Chem. 1990;265:3975–3980. [PubMed] [Google Scholar]
- 208.Reeve E C, Suttie D R. Chromosomal location of a mutation causing chloramphenicol resistance in Escherichia coli K 12. Genet Res. 1968;11:97–104. doi: 10.1017/s0016672300011228. [DOI] [PubMed] [Google Scholar]
- 209.Roberts M C. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol Rev. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 210.Rodríguez A M, Olano C, Vilches C, Méndez C, Salas J A. Streptomyces antibioticus contains at least three oleandomycin-resistance determinants, one of which shows similarity with proteins of the ABC-transporter superfamily. Mol Microbiol. 1993;8:571–582. doi: 10.1111/j.1365-2958.1993.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 211.Rosenberg M F, Callaghan R, Ford R C, Higgins C F. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- 212.Ross J I, Eady E A, Cove J H, Cunliffe W J, Baumberg S, Wootton J C. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 213.Rouch D A, Cram D S, DiBerardino D, Littlejohn T G, Skurray R A. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol. 1990;4:2051–2062. doi: 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 214.Rouquette C, Harmon J B, Shafer W F. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol Microbiol. 1999;33:651–658. doi: 10.1046/j.1365-2958.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 215.Rubin R A, Levy S B, Heinrikson R L, Kétzy F J. Gene duplication in the evolution of the two complementing domains of Gram-negative bacterial tetracyclin efflux proteins. Gene. 1990;87:7–13. doi: 10.1016/0378-1119(90)90489-e. [DOI] [PubMed] [Google Scholar]
- 216.Saier M H, Jr, Tam R, Reizer A, Reizer J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 217.Saier M H, Jr, Paulsen I T, Sliwinski M K, Pao S S, Skurray R A, Nikaido H. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 1998;12:265–274. doi: 10.1096/fasebj.12.3.265. [DOI] [PubMed] [Google Scholar]
- 218.Sami M, Yamahita H, Hirono T, Kadokura H, Kitamoto K, Yoda K, Yamasaki M. Hop-resistant Lactobacillus brevis contains a novel plasmid harboring a multidrug resistance-like gene. J Ferment Bioeng. 1997;84:1–6. [Google Scholar]
- 219.Sami M, Suzuki K, Sakamoto K, Kadokura H, Kitamoto K, Yoda K. A plasmid pRH45 of Lactobacillus brevis confers hop resistance. J Gen Appl Microbiol. 1998;44:361–363. doi: 10.2323/jgam.44.361. [DOI] [PubMed] [Google Scholar]
- 220.Sánchez L, Pan W, Viñas M, Nikaido H. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J Bacteriol. 1997;179:6855–6857. doi: 10.1128/jb.179.21.6855-6857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sasatsu M, Shima K, Shibata Y, Kono M. Nucleotide sequence of a gene that encodes resistance to ethidium bromide from a transferable plasmid in Staphylococcus aureus. Nucleic Acids Res. 1989;17:10103. doi: 10.1093/nar/17.23.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schinkel A H, Smit J J M, van Trellingen O, Beijnen J H, Wagenaar E, van Deemter L, Mol C A A M, van der Valk M A, Robanus-Maandag E C, te Riele H P J, Berns A J M, Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 223.Senior A E, Al-Shawi M, Urbatsch I L. The catalytic cycle of P-glycoprotein. FEBS Lett. 1995;377:285–289. doi: 10.1016/0014-5793(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 224.Senior A E, Bhagat S. P-glycoprotein shows strong catalytic cooperativity between the two nucleotide sites. Biochemistry. 1998;37:831–836. doi: 10.1021/bi9719962. [DOI] [PubMed] [Google Scholar]
- 225.Seoane A S, Levy S B. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli. J Bacteriol. 1995;177:3414–3419. doi: 10.1128/jb.177.12.3414-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Seoane A S, Levy S B. Identification of new genes regulated by the marRAB operon in Escherichia coli. J Bacteriol. 1995;177:530–535. doi: 10.1128/jb.177.3.530-535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shapiro A B, Ling V. Reconstitution of drug transport by purified P-glycoprotein. J Biol Chem. 1995;270:16167–16175. doi: 10.1074/jbc.270.27.16167. [DOI] [PubMed] [Google Scholar]
- 228.Shapiro A B, Corder A B, Ling V. P-glycoprotein-mediated Hoechst 33342 transport out of the lipid bilayer. Eur J Biochem. 1997;250:115–121. doi: 10.1111/j.1432-1033.1997.00115.x. [DOI] [PubMed] [Google Scholar]
- 229.Shapiro A B, Ling V. Extraction of Hoechst 33342 from the cytoplasmic leaflet of the plasma membrane by P-glycoprotein. Eur J Biochem. 1997;250:122–129. doi: 10.1111/j.1432-1033.1997.00122.x. [DOI] [PubMed] [Google Scholar]
- 230.Shapiro A B, Ling V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur J Biochem. 1997;250:130–137. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 231.Shapiro A B, Ling V. Transport of LDS-751 from the cytoplasmic leaflet of the plasma membrane by the rhodamine-123-selective site of P-glycoprotein. Eur J Biochem. 1998;254:181–188. doi: 10.1046/j.1432-1327.1998.2540181.x. [DOI] [PubMed] [Google Scholar]
- 232.Shapiro A B, Fox K, Lam P, Ling V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone: evidence for a third drug-binding site. Eur J Biochem. 1999;259:841–850. doi: 10.1046/j.1432-1327.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 233.Sharom F J, DiDiodato G, Yu X, Ashbourne K J D. Interaction of the P-glycoprotein multidrug transporter with peptides and ionophores. J Biol Chem. 1995;270:10334–10341. doi: 10.1074/jbc.270.17.10334. [DOI] [PubMed] [Google Scholar]
- 234.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Skarstad K, Thöny B, Hwang D, Kornberg A. A novel binding protein of the origin of the Escherichia coli chromosome. J Biol Chem. 1993;268:5365–5370. [PubMed] [Google Scholar]
- 236.Someya Y, Moriyama Y, Futai M, Sawai T, Yamaguchi A. Reconstitution of the metal-tetracyclin/H+ antiporter of Escherichia coli in proteoliposomes including F0F1-ATPase. FEBS Lett. 1995;374:72–76. doi: 10.1016/0014-5793(95)01079-t. [DOI] [PubMed] [Google Scholar]
- 237.Speer B S, Shoemaker N B, Salyers A A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992;5:387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Spoelstra E C, Westerhoff H V, Pinedo H M, Dekker H, Lankelma J. The multidrug-resistance-reverser verapamil interferes with cellular P-glycoprotein-mediated pumping of daunomycin as a non-competitive substrate. Eur J Biochem. 1994;221:363–373. doi: 10.1111/j.1432-1033.1994.tb18748.x. [DOI] [PubMed] [Google Scholar]
- 239.Spratt B G. Resistance to antibiotics mediated by target alterations. Science. 1994;264:388–393. doi: 10.1126/science.8153626. [DOI] [PubMed] [Google Scholar]
- 240.Srikumar R, Li X-Z, Poole K. Inner membrane efflux components are responsible for β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Steiner Mordoch S, Granot D, Lebendiker M, Schuldiner S. Scanning cysteine accessibility of EmrE, an H+-coupled multidrug transporter from Escherichia coli, reveals a hydrophobic pathway for solutes. J Biol Chem. 1999;274:19480–19486. doi: 10.1074/jbc.274.27.19480. [DOI] [PubMed] [Google Scholar]
- 243.Sun L, Sreedharan S, Plummer K, Fisher L M. NorA plasmid resistance to fluoroquinolones: role of copy number and norA frameshift mutations. Antimicrob Agents Chemother. 1996;40:1665–1669. doi: 10.1128/aac.40.7.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Takiff H E, Cimino M, Musso M C, Weisbrod T, Martinez R, Delgado M B, Salazar L, Bloom B R, Jacobs W R., Jr Efflux pump of the proton antiporter family confers low level fluoroquinolone resistance in Mycobacterium smegmatis. Proc Natl Acad Sci USA. 1996;93:362–366. doi: 10.1073/pnas.93.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tamai I, Safa A R. Azidopine noncompetitively interacts with vinblastine and cyclosporin A binding to P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1991;266:16796–16800. [PubMed] [Google Scholar]
- 246.Tanabe H, Yamasaki K, Furue M, Yamamoto K, Katoh A, Yamamoto M, Yoshioka S, Tagami H, Aiba H, Utsumi R. Growth phase-dependent transcription of emrKY, a homolog of multidrug efflux emrAB genes of Escherichia coli, is induced by tetracycline. J Gen Appl Microbiol. 1997;43:257–263. doi: 10.2323/jgam.43.257. [DOI] [PubMed] [Google Scholar]
- 247.Taylor D E, Hou Y, Turner R J, Weiner J H. Location of a potassium tellurite resistance operon (tehA tehB) within the terminus of Escherichia coli K-12. J Bacteriol. 1994;166:2740–2742. doi: 10.1128/jb.176.9.2740-2742.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tennent J M, Lyon B R, Midgley M, Jones G, Purewal A S, Skurray R A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989;135:1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- 249.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Turner R J, Taylor D E, Weiner J H. Expression of Escherichia coli TehA gives resistance to antiseptics and disinfectants similar to that conferred by multidrug efflux pumps. Antimicrob Agents Chemother. 1997;41:440–444. doi: 10.1128/aac.41.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ubukata K, Itoh N-Y, Konno M. Cloning and expression of the norA gene for fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1989;33:1535–1539. doi: 10.1128/aac.33.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ueda K, Taguchi Y, Morishima M. How does P-glycoprotein recognize its substrates? Semin Cancer Biol. 1997;8:151–159. doi: 10.1006/scbi.1997.0066. [DOI] [PubMed] [Google Scholar]
- 253.Urbatsch I L, Sankaran B, Weber J, Senior A E. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J Biol Chem. 1995;270:19383–19390. doi: 10.1074/jbc.270.33.19383. [DOI] [PubMed] [Google Scholar]
- 254.Urbatsch I L, Sankaran B, Bhagat S, Senior A E. Both P-glycoprotein nucleotide-binding sites are catalytically active. J Biol Chem. 1995;270:26956–26961. doi: 10.1074/jbc.270.45.26956. [DOI] [PubMed] [Google Scholar]
- 254a.van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, H. A, Borst P, Vermeer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 255.van Veen H W, Konings W N. Multidrug transporters from bacteria to man: similarities in structure and function. Semin Cancer Biol. 1997;8:183–191. doi: 10.1006/scbi.1997.0064. [DOI] [PubMed] [Google Scholar]
- 256.van Veen H W, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen A J M, Konings W N. Multidrug resistance mediated by a bacterial homolog of the human drug transporter MDR1. Proc Natl Acad Sci USA. 1996;93:10668–10672. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.van Veen H W, Callaghan R, Soceneantu L, Sardini A, Konings W N, Higgins C F. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature. 1998;391:291–295. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 258.van Veen H W, Margolles A, Müller M, Higgins C F, Konings W N. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 2000;19:2503–2514. doi: 10.1093/emboj/19.11.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.van Veen H W, Konings W N. The ABC family of multidrug transporters in microorganisms. Biochim Biophys Acta. 1998;1365:31–36. doi: 10.1016/s0005-2728(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 260.Varela M F, Sansom C E, Griffith J K. Mutational analysis and molecular modelling of an amino acid sequence motif conserved in antiporters but not symporters in a transporter superfamily. Mol Membr Biol. 1995;12:313151–159. doi: 10.3109/09687689509072433. [DOI] [PubMed] [Google Scholar]
- 261.Vedantam G, Guay G G, Austria N A, Doktor S Z, Nichols B P. Characterization of mutations contributing to sulfathiazole resistance in Escherichia coli. Antimicrob Agents Chemother. 1998;42:88–93. doi: 10.1128/aac.42.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vigano C, Margolles A, van Veen H W, Konings W N, Ruysschaert J-M. Secondary and tertiary structure changes of reconstituted LmrA induced by nucleotide binding or hydrolysis; a Fourier transform attenuated total reflection infrared spectroscopy and tryptophan fluorescence quenching analysis. J Biol Chem. 2000;275:10962–10967. doi: 10.1074/jbc.275.15.10962. [DOI] [PubMed] [Google Scholar]
- 263.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang G, Pincheira R, Zhang J-T. Dissection of drug-binding-induced conformational changes in P-glycoprotein. Eur J Biochem. 1998;255:383–390. doi: 10.1046/j.1432-1327.1998.2550383.x. [DOI] [PubMed] [Google Scholar]
- 265.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Williams R J, Heymann D L. Containment of antibiotic resistance. Science. 1998;279:1153–1154. doi: 10.1126/science.279.5354.1153. [DOI] [PubMed] [Google Scholar]
- 268.Woodcock D M, Linsenmeyer M E, Chojnowski G, Kriegler A B, Nink V, Webster L K, Sawyer W H. Reversal of multidrug resistance by surfactants. Br J Cancer. 1992;66:62–68. doi: 10.1038/bjc.1992.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Woolridge D P, Vasquez-Laslop N, Markham P N, Chevalier M S, Gerner E W, Neyfakh A A. Efflux of the natural polyamine spermidine facilitated by the Bacillus subtilis multidrug transporter Blt. J Biol Chem. 1997;272:8864–8866. doi: 10.1074/jbc.272.14.8864. [DOI] [PubMed] [Google Scholar]
- 270.Wu J, Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991;173:2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yamada H, Kurose-Hamada S, Fukuda Y, Mitsuyama J, Takahata M, Minami S, Watanabe Y, Narita H. Quinolone susceptibility of norA-disrupted Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2308–2309. doi: 10.1128/aac.41.10.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yamaguchi A, Ono N, Akasaka T, Noumi T, Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon, Tn10: the role of the conserved dipeptide, Ser65-Asp66, in tetracycline transport. J Biol Chem. 1990;265:15525–15530. [PubMed] [Google Scholar]
- 273.Yamaguchi A, Nakatani M, Sawai T. Aspartic acid-66 is the only essential negatively charged residue in the putative hydrophilic loop region of the metal-tetracycline/H+ antiporter encoded by transposon Tn10 of Escherichia coli. Biochemistry. 1992;31:8344–8348. doi: 10.1021/bi00150a031. [DOI] [PubMed] [Google Scholar]
- 274.Yamaguchi A, Kimura T, Someya Y, Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10: the structural resemblance and functional difference in the role of the duplicated sequence motif between hydrophobic segments 2 and 3 and segments 8 and 9. J Biol Chem. 1993;268:6496–6504. [PubMed] [Google Scholar]
- 275.Yerushalmi H, Lebendiker M, Schuldiner S. EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J Biol Chem. 1995;270:6856–6863. doi: 10.1074/jbc.270.12.6856. [DOI] [PubMed] [Google Scholar]
- 276.Yerushalmi H, Lebendiker M, Schuldiner S. Negative dominance studies demonstrate the oligomeric structure of EmrE, a multidrug antiporter from Escherichia coli. J Biol Chem. 1996;271:31044–31048. doi: 10.1074/jbc.271.49.31044. [DOI] [PubMed] [Google Scholar]
- 277.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zeller V, Janoir C, Kitzis M, Gutmann L, Moreau N J. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1973–1978. doi: 10.1128/aac.41.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zgurskaya H I, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zgurskaya H I, Nikaido H. AcrA is a highly asymmetric protein capable of spanning the periplasm. J Mol Biol. 1999;285:409–420. doi: 10.1006/jmbi.1998.2313. [DOI] [PubMed] [Google Scholar]
- 282.Zhang X, Collins K I, Greenberger L M. Functional evidence that transmembrane 12 and the loop between transmembrane 11 and 12 form part of the drug-binding domain in P-glycoprotein encoded by MDR1. J Biol Chem. 1995;270:5441–5448. doi: 10.1074/jbc.270.10.5441. [DOI] [PubMed] [Google Scholar]
- 283.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of outer membrane protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zheleznova E E, Markham P N, Neyfakh A A, Brennan R G. Structural basis of multidrug recognition by BmrR, a transcriptional activator of a multidrug transporter. Cell. 1999;96:353–362. doi: 10.1016/s0092-8674(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 285.Zhou Z, White K A, Polissi A, Georgopoulos C, Raetz C R H. Function of Escherichia coli MsbA, an essential ABC transporter, in lipid A and phospholipid biosynthesis. J Biol Chem. 1998;273:12466–12475. doi: 10.1074/jbc.273.20.12466. [DOI] [PubMed] [Google Scholar]