Abstract
Background:
Heterogeneity in major depressive disorder (MDD) is well recognized but not well understood. Core depressive features are reward and emotional symptoms, which reflect dysfunctions in the positive valence (PV) and negative valence (NV) systems, respectively. This study assessed whether PV and NV systems (based on selected symptoms) were associated with different clinical features, antidepressant response and levels of immunomarkers in adults with MDD.
Methods:
These analyses used data from the Combining Medications to Enhance Depression Outcomes (CO-MED) study (N=665; n=166 for immunomarkers). PV and NV symptom scores were extracted from the clinician-rated 30-item Inventory of Depressive Symptomatology. Correlational analyses were conducted.
Results:
PV and NV symptom scores were substantially associated with different clinical features. PV symptoms (impaired motivation, impaired energy and anhedonia) were independently associated with female gender (p<.001), older age (p=.012), and higher cognitive and physical impairment (p<.001) according to the 7-item Cognitive and Physical Functioning Questionnaire. Conversely, NV symptoms (anxiety and interpersonal sensitivity) were independently associated with younger age (p=.013), more anxious comorbidities (p=.001 for GAD, p=.002 for social phobia) and other commonly associated non criterion symptoms (p<.001). Overall, PV symptoms were more responsive to antidepressants than NV symptoms (p<.0001; Cohen’s d=0.455). A PV symptom score was positively correlated with the concentration of three pro-inflammatory and one anti-inflammatory factors. In contrast, a NV symptom score was negatively associated with only one pro-inflammatory immunomarker.
Conclusions:
PV and NV system function appears to be reflected in selected clinical symptoms that differentially relate to other clinical features, treatment outcomes and immunological function.
Keywords: depression, major depressive disorder, RDoC, positive valence, negative valence, immunomarkers, biomarkers
1. Introduction
Despite the high prevalence and long history of research, heterogeneity of major depressive disorder (MDD) has not been fully understood. While several treatment options are available, selecting the “right” treatment for an individual has not been possible (Otte et al., 2016; Rush et al., 2009; Trivedi et al., 2016). Previous attempts to operationalize the different depressive presentations in clinical subtypes (melancholic, atypical, anxious) have had limited success (Arnow et al., 2015; Rush et al., 2009; Uher et al., 2011). The lack of biological markers either to support previous clinical classifications or by which to select treatment is also a major limitation (Insel et al., 2010; Rush & Ibrahim, 2018). Consequently, clinical practice presently relies on a try and try again approach to personalizing treatment selection (Gaynes et al., 2009; Rush & Ibrahim, 2018; Rush et al., 2009). Assessment of brain systems (alternatively referred to as functional domains) is a promising method for understanding the heterogeneity of mental disorders. The system-based approach is more consistent with the latent pathophysiology, has been well validated in basic science and can be readily applied to mood and other mental disorders (Insel et al., 2010; Woody & Gibb, 2015).
Emotional dysfunctions such as anhedonia and dysphoria are core symptoms of MDD (Otte et al., 2016; Russo & Nestler, 2013). Therefore, the systems responsible for reward and emotional responses [i.e. positive valence (PV) and negative valence (NV) systems] may be critical to understanding MDD (Woody & Gibb, 2015). PV systems modulate response to positive contexts or situations such as consummatory behavior, response to reward, motivation, engagement and interest (Craske, Meuret, Ritz, Treanor, & Dour, 2016; Cuthbert & Insel, 2013; Dillon et al., 2014; Morris & Cuthbert, 2012; Nusslock & Alloy, 2017), and engage the ventral tegmental area, nucleus accumbens, as well as fronto-striatal pathways (Craske et al., 2016; Dillon et al., 2014; Kringelbach, 2005; Nusslock & Alloy, 2017; Russo & Nestler, 2013). Conversely, NV systems are associated with responses to aversive and unpleasant contexts or situations such as anxiety, fear, phobia, and loss (Cuthbert & Insel, 2013; Dillon et al., 2014; Morris & Cuthbert, 2012; Nusslock & Alloy, 2017). NV systems involve the amygdala, insula and the striatum (Dillon et al., 2014; Nusslock & Alloy, 2017). Both the PV and NV systems can be studied from different perspectives such as genes, molecules, circuits, physiology, behavior, and self-report (Cuthbert & Insel, 2013; Morris & Cuthbert, 2012).
Functional neuroimaging studies suggest that both PV and NV systems operate differently in depressed individuals versus non-depressed individuals (J. P. Hamilton et al., 2012; Pizzagalli, 2014). Previous fMRI research has found that depressed patients had a lower activation of brain regions associated with PV systems during reward tasks (e.g. the nucleus accumbens, the ventral striatum and the orbitofrontal cortex) as compared to healthy controls (Pizzagalli, 2014). On the other hand, several studies have found greater baseline response on fMRI of neurological areas related to NV systems (such as the amygdala and the anterior insula) in depressed people when compared to non-depressed individuals (J. P. Hamilton et al., 2012).
Previous literature also suggests that some MDD patients present with a pro-inflammatory state and cytokines may selectively affect specific brain systems (Dowlati et al., 2010; Miller & Raison, 2016). Elevated levels of pro-inflammatory markers, such as interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), C-reactive protein (CRP), IL (interleukin) 1 beta (β), and IL-6 have all been linked to MDD (Dowlati et al., 2010; Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimaki, 2015). However, there are unresolved inconsistencies as other studies have failed to find an association between these same inflammatory cytokines and MDD (Dowlati et al., 2010; Haapakoski et al., 2015). Given the heterogeneity of depression, it is likely that only a subset of depressed individuals or only some selected symptoms are associated with the pro-inflammatory state (Jha, Minhajuddin, Gadad, Greer, et al., 2017; Miller & Raison, 2016; Uher et al., 2014).
PV symptoms may represent a symptom-dimension more closely associated with inflammation. Evidence from animal and human studies indicates that specific PV symptoms (such as anhedonia and motivation) and their respective brain circuits may be modulated by immunological processes (De La Garza, 2005; Eisenberger et al., 2010; Felger et al., 2016). In humans, the administration of low doses of bacterial endotoxin (a pro-inflammatory substance) was associated with a less pronounced functional response in the reward system (Eisenberger et al., 2010), and systemic inflammation (reflected by higher concentrations of peripheral immunomarkers) was correlated with anhedonia and lower connectivity in the reward circuit (Felger et al., 2016). The link between PV symptoms and immunological changes may be mediated by a decreased ventral-striatum response induced by pro-inflammatory cytokines (Felger et al., 2016). There is a significant body of work suggesting that inflammation would primarily impact dopaminergic pathways, and is “associated with a reorganization of motivational priorities” leading to environmental withdrawal, i.e. would primarily impact PV systems (Vichaya & Dantzer, 2018). Conversely, studies linking NV symptoms to a pro-inflammatory state are more scarce and equivocal (Michopoulos, Powers, Gillespie, Ressler, & Jovanovic, 2017; Toker, Shirom, Shapira, Berliner, & Melamed, 2005). In this context, it is possible that immunomarkers differently affect the emotional dimensions of depression (i.e. PV and NV). A differential association of these systems with systemic inflammation may guide therapies specifically targeting one symptom dimension such as the use of anti-inflammatory therapies to better manage PV symptoms.
This study addressed the heterogeneity of MDD by using selected self-reported symptoms to reflect brain systems and evaluating clinical and laboratory based correlates. Specifically, we investigated the relationship(s) between PV and NV system function (as assessed by symptom scores), other clinical features, treatment responsiveness and relationship with immunomarkers. Clinical data were assessed in 665 participants with MDD from the Combining Medications to Enhance Depression Outcomes (CO-MED) study (Rush et al., 2011), and immunomarkers were evaluated in the subset of 166 participants who participated in the optional biomarker study.
This study addressed the following questions:
Is it possible to operationalize the concepts of PV and NV symptoms in a concise and clinician-friendly manner?
Are either PV or NV total symptom scores associated with different clinical features?
Do PV and NV symptoms respond differently to antidepressant treatments?
At the molecular level, are PV and NV symptom scores differentially associated with immunological profiles?
2. Method
2.1. Participants and recruitment
CO-MED (clinicaltrial.gov identifier NCT00590863) was a single-blind, randomized, placebo-controlled clinical trial that investigated the relative efficacy of three pharmacological treatments for MDD: escitalopram + placebo, escitalopram + bupropion, and venlafaxine-XR + mirtazapine. Between March 2008 and September 2009, 665 outpatients with non-psychotic MDD were recruited from 15 sites (nine psychiatric and six primary care clinics). Inclusion criteria for the study were: 1) age between 18 and 75 years old; 2) DSM-IV-TR criteria for either chronic (current episode lasting 2 or more years) or recurrent MDD (with current episode lasting at least two months); 3) minimum score of 16 on the 17-item Hamilton Depression Rating Scale (HAM-D17) (M. Hamilton, 1960); and 4) no current use of psychotropic medications. The study protocol excluded individuals with any psychotic illness, lifetime bipolar disorder, current substance use disorder, need for hospitalization or presence of medical conditions that precluded the use of the study medications. The trial was approved by the Institutional Review Boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, each participating regional center, and all relevant clinics. Written informed consent was obtained from all subjects. See Rush et al. (2011) for further details.
In August 2008, a study examining blood-based biomarkers and treatment response was added to the original protocol. Participation in this add-on investigation was optional, and a second written informed consent was required. 166 participants consented and provided blood specimens at baseline. A previous report (Jha, Minhajuddin, Gadad, & Trivedi, 2017) detailed that individuals who did not provide blood (n = 499) were younger (mean age = 44.5 years versus 42.1; p = .030) and had a lower rate of statin use (20.5% versus 13.6% p = .034). However, no additional differences were found across demographic or clinical features.
2.2. Clinical measurements
MDD diagnosis, recurrent/chronic status and age at onset were examined by interview and confirmed by a research coordinator using the DSM-IV-based symptom checklist. We identified potential the items for PV and NV symptom scores a priori based on their face value and capacity to represent the concepts of PV and NV systems, while disregarding outcomes and other system function measurements. Items were identified from the Inventory of Depressive Symptomatology - Clinician Version (IDS-C30), which evaluates 30 MDD symptoms using item scores from 0 (less severe) to 3 (more severe) (IDS-QIDS, 2018; Mapi Research Trust, 2018; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). The items selection followed the NIMH definitions for PV and NV systems (Cuthbert & Insel, 2013; Morris & Cuthbert, 2012). Therefore, items that were chosen for the PV symptom score had to assess symptoms associated with “responses to positive motivational situations or contexts, such as reward seeking, consummatory behavior, and reward/habit learning”, and included the domains reward responsiveness (reward anticipation, initial response to reward, reward satiation), reward learning (probabilistic and reinforcement learning, reward prediction error, and habit), and reward valuation (reward probability, delay, effort). Similarly, items selected for the NV symptom score had to examine “responses to aversive situations or context, such as fear, anxiety, and loss” and included the domains acute threat (fear), potential threat (anxiety), sustained threat, loss, and frustrative nonreward.
Items included to reflect PV systems were: 1) (impaired) capacity for pleasure or enjoyment (excluding sex); 2) (impaired) general interest; 3) (impaired) response of mood to desired events (mood reactivity); 4) (impaired) energy level; and 5) (impaired) interest in sex. Items included to reflect NV systems were: 1) feeling anxious or tense; 2) panic/phobic symptoms; and 3) interpersonal sensitivity. We then created two scales (representing PV and NV symptom scores) by summing the individual symptom score for each item in the component. PV symptom score (based on five items), therefore, ranged from 0 to 15, whereas NV symptom score (based on three items) ranged from 0 to 9.
We assessed specific MDD symptoms and overall symptom severity with the HAM-D17, the 16-item Quick Inventory of Depressive Symptomatology Self Report Version (QIDS-SR16), and the 30-item Inventory of Depressive Symptomatology (IDS-C30), from which we extracted the 16-item Quick Inventory of Depressive Symptomatology Clinician Version (QIDS-C16) (IDS-QIDS, 2018; Mapi Research Trust, 2018; Rush et al., 2003). When analyses were performed between PV and NV symptom scores and severity of other depressive symptoms (e.g. depressed mood, cognitive impairment, neuro-vegetative symptoms, etc.), we used modified versions of the QIDS-C16 and IDS-C30 to assess severity. In these cases, any items assessing PV and NV were excluded (i.e. QIDS-C14 and IDS-C22). All severity assessments measured MDD symptoms over the past week.
We assessed MDD-associated symptoms (e.g., irritability, anxiety, mania, insomnia and panic) using the 16-item Concise Associated Symptoms Tracking (CAST) Scale – Clinician Version (Trivedi et al., 2011). In addition, participants completed the following self-report questionnaires: 1) the 5-item Work and Social Adjustment Scale to examine current functional impairment (Mundt, Marks, Shear, & Greist, 2002); 2) the 7-item Cognitive and Physical Functioning Questionnaire to investigate cognitive and executive performance in the past month (Fava, Iosifescu, Pedrelli, & Baer, 2009); 3) the 125-item Psychiatric Diagnostic Screening Questionnaire to assess current psychiatric comorbidities (Rush et al., 2005); and 4) the 36-item Self-Administered Comorbidity Questionnaire to investigate current medical comorbidities (Sangha, Stucki, Liang, Fossel, & Katz, 2003).
2.3. Biospecimen Collection and Processing
Whole blood was collected in EDTA-coated tubes and transported overnight at room temperature from the site of collection to the Biological Core of the National Institute of Mental Health Repository and Genomics Resource (NIMH RGR). Plasma was extracted via centrifugation (at 2500 rcf for 10 min) at room temperature and stored at −80°C at NIMH RGR. Upon request, plasma samples were transported on dry ice to the University of Texas Southwestern Medical Center and stored at −80°C until further assayed.
2.4. Immunomarkers
In samples from the subset of participants who provided blood (n = 166), we evaluated several immunomarkers previously demonstrated to be associated with MDD, including: CRP, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, and TNF-α (Dowlati et al., 2010; Haapakoski et al., 2015; Miller & Raison, 2016; Otte et al., 2016). Immunomarkers were categorized into one of two groups according to their inflammatory properties: 1) primarily pro-inflammatory; or 2) primarily anti-inflammatory (Cicchese et al., 2018; You et al., 2011).
Immunomarkers were measured in a blinded fashion with either the Bio-Plex Pro Human Acute Phase 4-plex kit (#171a4010m) or Bio-Plex Pro Human Cytokine Standard 27-plex kit (#m500kcaf0y), both acquired from Bio-Rad Laboratories, Hercules, CA, USA. A Bio-Rad Bioplex Magpix 200 machine equipped with Bioplex Manager software was used to quantify immunological factors. The details about all immunomarkers assessed by the kits including assay characteristics are available (Bio-Rad Laboratories, 2016). Intra and inter-assays variations were below 10%, and samples were within the detection ranges established by the manufacturer.
2.5. Statistical analyses
Correlation analyses between the total PV and NV symptom scores and IDS-C22 individual items determined whether we had inadvertently excluded items that were highly correlated with PV and NV symptom scores. Internal consistency was assessed with Cronbach’s alpha. A confirmatory factor analysis was done where the items selected for the PV and NV symptom scores was divided into 2 sets of items as defined for the PV and NV scores.
To explore the association between PV and NV symptom scores and other clinical features, univariate and multivariate analyses were performed. In the univariate analyses, t-tests and Pearso’s correlation coefficients were used for categorical and continuous variables, respectively. Analyses of covariance (ANCOVA) and Pearson’s partial correlation coefficients were used to control results for age and MDD overall severity (QIDS-C14), variables that tend to affect clinical presentation (Byers, Yaffe, Covinsky, Friedman, & Bruce, 2010; Kessler & Walters, 1998). With respect to the multivariate analyses, linear regressions were conducted to determine the variables that were independently associated with PV and NV symptom scores. In these procedures, we included demographics and clinical features with p < .10 in the univariate analysis after controlling for age and QIDS-C14. Effect sizes were reported as standardized regression coefficients.
We used a repeated measures mixed-effects model to measure improvement of PV, NV, and other depressive symptoms (IDS22). Both time and measure (PV, NV and other depressive symptoms) were repeated factors. Cohen’s d effect sizes were presented based on model estimated means at week 12. PV, NV and other depressive symptom scores were converted into z-scores (mean zero, standard deviation of 1 at baseline) for graphical presentation. Participants with no post-baseline data or with baseline PV and/or NV symptom score of zero were not included in the analyses.
With respect to the association between PV and NV symptoms scores and baseline levels of immunomarkers, we log-transformed baseline concentrations of immunomarkers due to non-normal distributions. We then used Pearson’s correlation coefficients to assess the relationship between PV and NV symptom scores and individual immunomarkers. Overall severity of MDD using IDS30, and severity of non-PV non-NV symptoms (i.e. other MDD symptoms) using IDS22 were included in the analyses to assess if the correlations with PV and NV symptoms were specific of the PV and NV symptom dimensions. Partial Pearso’s correlation coefficients were used to control for age, a variable with potential impact on concentrations of immunomarkers (Dowlati et al., 2010; Haapakoski et al., 2015). Since gender affects immune function (Afshan, Afzal, & Qureshi, 2012; Birur, Amrock, Shelton, & Li, 2017; Jha, Miller, Minhajuddin, & Trivedi, 2018), correlational analyses were repeated for males and females separately. Due to the exploratory nature of the analyses, no corrections for multiple comparisons were conducted.
3. Results
Operationalization of PV and NV symptoms
Based on the eight items extracted from the IDS-C30 (thought to best reflect the PV and NV systems), a confirmatory factor analysis was conducted which produced several goodness of fit statistics indicating the proposed division of items provided a good fit to the data, reinforcing that the items selected formed two distinct factors (Table 1). PV and NV symptoms had a modest positive correlation (Pearson’s correlation coefficient of .224, p < .001), as displayed in Supplemental Figure 1. Internal consistency as measured by Cronbach’s alpha was 0.66 for PV and 0.43 for NV.
Table 1.
Confirmatory factor analysis of the items selected for the positive valence and negative valence symptom scores.
| Index | Statistic | Interpretationa |
|---|---|---|
| Test of model fit | df=19, chi-square=27.8, p-value=0.087 | No significant lack of fit |
| RMSEA (root mean square error of approximation) | 0.026 95% CI (0.0, 0.046) | RMSEA between 0.05 and 0.02 indicates good fit |
| CFI (Comparative fit index) | .987 | CFI between 0.95 and 0.99 indicates very good fit |
| TLI (Tucker-Lewis index) | .980 | TLI between 0.95 and 0.99 indicates very good fit |
Longitudinal Structural Equation Modeling (Little, 2013).
None of the remaining 22 IDS-C30 symptoms had a correlation above .45 with either PV or NV symptom scales. Pearson’s correlation coefficient ranged between −.044 (distinct quality of mood) and .424 (depressed mood) for PV, and between −.057 (increased weight) and .286 (irritability) for NV (Supplemental Table 1). Consequently, no other items were added to either scale.
Sample demographics
For the 665 CO-MED participants, the mean scores for PV and NV scales were 8.7 (SD = 3.0) and 3.7 (SD = 1.8), respectively. The average age of the sample was 42.7 (SD = 13.0) and approximately two-thirds were female. The participants were moderately to severely depressed [mean score of 23.8 (SD = 4.8) for HAM-D17, 15.8 (SD = 3.4) for QIDS-C16, and 15.4 (SD = 4.3) for QIDS- SR16]. The mean number of weeks in treatment was 9.9 (SD = 3.9), and the number of post-baseline visits was 5.3 (SD = 2.2). For full baseline characteristics, see Rush et al. (2011).
Regarding valence symptom scores, women had significantly higher PV scores. Age was positively correlated with PV scores and negatively correlated to NV scores. There was a negative correlation between PV and educational level (Table 2).
Table 2.
Relationship between demographic/other depressive symptoms and positive/negative valence symptoms in individuals with major depressive disorder (MDD) (N=665).
| Variables | POSITIVE VALENCE SYMPTOMS |
NEGATIVE VALENCE SYMPTOMS |
||||
|---|---|---|---|---|---|---|
| Severity of positive valence symptoms | Statistical Significance | Statistical Significance | Severity of negative valence symptoms | Statistical significance | Statistical significance | |
|
| ||||||
| DEMOGRAPHICS | ||||||
| Categorical variables | Mean (SDa) | p b | Controlled p c | Mean (SDa) | p b | Controlled pc |
| Gender | ||||||
| Male (n=213) | 7.4 (3.0) | < .001 | < .001 | 3.4 (1.8) | .002 | .114 |
| Female (n=452) | 9.2 (2.8) | 3.9 (1.8) | ||||
| Race | ||||||
| Non-Black (n=485) | 8.6 (2.9) | .605 | .610 | 3.7 (1.8) | .845 | .541 |
| Black (n=180) | 8.7 (3.2) | 3.7 (1.8) | ||||
| Ethnicity | ||||||
| Hispanic (n=101) | 8.7 (3.2) | .939 | .201 | 3.5 (1.7) | .257 | .453 |
| Non-Hispanic (n=564) | 8.7 (2.9) | 3.7 (1.8) | ||||
| Employed | ||||||
| Yes (n=331) | 8.5 (2.9) | .098 | .979 | 3.7 (1.8) | .653 | .970 |
| No (n=334) | 8.8 (3.0) | 3.7 (1.8) | ||||
| Continuous variables | Correlation coefficient | p | Controlled p | Correlation coefficient | p | Controlled p |
| Age (years) | .022 | .571 | .027 d | −.161 | < .001 | < .001 d |
| Education (years) (n=642) | −.151 | < .001 | < .001 | .000 | .990 | .639 |
| Monthly household income (in dollars) (n=600) | −.070 | .088 | .314 | −.065 | .110 | .491 |
|
| ||||||
| OTHER DEPRESSIVE SYMPTOMSe | ||||||
| Continuous variables | Correlation coefficient | p | Controlled p | Correlation coefficient | p | Controlled p |
| QIDS-C14 | .528 | < .001 | < .001 d | .338 | < .001 | < .001 d |
| IDS-C22 | .505 | < .001 | < .001 d | .354 | < .001 | < .001 d |
SD = standard deviation
T-tests or ANOVA were used for categorical variables. Pearson’s correlation coefficients were used for continuous variables
Controlled p = scores controlled for age and overall MDD severity (QIDS-C14)
Age was controlled only for overall severity; other depressive symptoms were controlled only for age.
Other depressive symptoms = MDD symptoms except PV and NV symptoms; Bold = statistically significant (p < .05) after controlling for age and overall severity.
Associations with clinical features
PV and NV symptoms were substantially associated with different clinical features (Table 3).
Table 3.
Relationship between clinical features and positive/negative valence symptoms in individuals with major depressive disorder (MDD) (N=665).
| Clinical Variables | POSITIVE VALENCE SYMPTOMS |
NEGATIVE VALENCE SYMPTOMS |
||||
|---|---|---|---|---|---|---|
| Severity of positive valence symptoms | Statistical Significance | Statistical Significance | Severity of negative valence symptoms | Statistical Significance | Statistical Significance | |
|
| ||||||
| Categorical variables | Mean (SDa) | p b | Controlled pc | Mean (SDa) | p b | Controlled pc |
| Agoraphobia | ||||||
| Yes (n=69) | 9.3 (2.9) | .053 | .814 | 4.5 (1.6) | < .001 | .004 |
| No (n=596) | 8.6 (3.0) | 3.6 (1.8) | ||||
| Alcohol abuse | ||||||
| Yes (n=67) | 7.9 (3.2) | .038 | .072 | 3.9 (1.7) | .326 | .280 |
| No (n=597) | 8.7 (2.9) | 3.7 (1.8) | ||||
| Bulimia nervosa | ||||||
| Yes (n=78) | 8.6 (2.3) | .847 | .330 | 4.1 (1.7) | .065 | .311 |
| No (n=587) | 8.7 (3.0) | 3.7 (1.8) | ||||
| Drug abuse | ||||||
| Yes (n=35) | 8.4 (3.4) | .604 | .518 | 4.3 (1.9) | .052 | .052 |
| No (n=630) | 8.7 (2.9) | 3.7 (1.8) | ||||
| Generalized anxiety disorder | ||||||
| Yes (n=131) | 9.5 (2.9) | < .001 | .290 | 4.8 (1.7) | < .001 | < .001 |
| No (n=534) | 8.5 (3.0) | 3.5 (1.7) | ||||
| Hypochondriasis | ||||||
| Yes (n=29) | 10.2 (2.7) | .003 | .093 | 4.5 (2.4) | .092 | .178 |
| No (n=636) | 8.6 (2.9) | 3.7 (1.8) | ||||
| Obsessive-compulsive disorder | ||||||
| Yes (n=79) | 8.7 (3.3) | .857 | .111 | 4.6 (1.9) | < .001 | < .001 |
| No (n=586) | 8.6 (2.9) | 3.6 (1.7) | ||||
| Panic disorder | ||||||
| Yes (n=65) | 9.4 (2.7) | .044 | .993 | 4.8 (1.7) | < .001 | < .001 |
| No (n=600) | 8.6 (3.0) | 3.6 (1.8) | ||||
| Posttraumatic stress disorder | ||||||
| Yes (n=81) | 8.8 (2.9) | .745 | .073 | 4.6 (1.7) | < .001 | < .001 |
| No (n=584) | 8.6 (3.0) | 3.6 (1.8) | ||||
| Social Phobia | ||||||
| Yes (n=178) | 9.5 (2.7) | < .001 | .083 | 4.6 (1.8) | < .001 | < .001 |
| No (n=487) | 8.4 (3.0) | 3.4 (1.7) | ||||
| Somatoform disorder | ||||||
| Yes (n=21) | 10.0 (2.2) | .028 | .025 | 4.0 (2.0) | .532 | .799 |
| No (n=644) | 8.6 (3.0) | 3.7 (1.8) | ||||
| Continuous variables | Correlation coefficient | P | Controlled p | Correlation coefficient | p | Controlled p |
| Age at onset of first episode (years) | −.051 | .189 | .739 | −.144 | < .001 | .560 |
| Work and social adjustment | .408 | < .001 | < .001 | .236 | < .001 | .007 |
| Cognitive and physical functioning | .421 | < .001 | < .001 | .222 | < .001 | .029 |
| MDD-associated symptoms (CASTd total score) | .136 | < .001 | .334 | .395 | < .001 | < .001 |
| Number of psychiatric comorbidities | .115 | .003 | .850 | .331 | < .001 | < .001 |
| Number of medical comorbidities | .058 | .132 | .972 | .024 | .532 | .089 |
| Body mass index | .098 | .012 | .031 | −.060 | .120 | .078 |
SD = standard deviation
T-tests were used for categorical variables. Pearson’s correlation coefficients were used for continuous variables
Controlled p = scores controlled for age and overall MDD severity (QIDS-C14)
Concise Associated Symptoms Tracking Scale; Bold = statistically significant (p < .05) after controlling for age overall severity.
With respect to the multivariate analyses, PV score was independently and positively associated with 1) female gender [Β = 1.520, 95% CI (1.013, 1.937), t = 7.16, p < .001, standardized B = 0.240], 2) older age [Β = .019, 95% CI (.004, .034), t = 2.53, p = .012, standardized B = 0.084], 3) lower educational level [Β = −.121, 95% CI (−.186, −.056), t = −3.68, p < .001, standardized B = −0.122]; 4) worse work and social adjustment [Β = .085, 95% CI (.060, .109), t = 6.79, p < .001, standardized B = 0.255] and 5) worse cognitive and physical functioning [Β = .140, 95% CI (.103, .177), t = 7.46, p < .001, standardized B = 0.281]. Alcohol abuse, hypochondriasis, posttraumatic stress disorder, social phobia, somatoform disorder, and body mass index were excluded from the final model because they were not significant contributors. The model summary of the linear regression for PV symptom scores was R2 = .313, degrees of freedom = 5, F = 57.77, p < .001.
NV symptom score was independently and positively associated with 1) younger age [Β = −.012, 95% CI (−.022, −.002), t = −2.49, p = .013, standardized B = −0.002], 2) generalized anxiety disorder [Β = .555, 95% CI (.213, .897), t = 3.19, p = .002, standardized B = 0.897], 3) social phobia [Β = .504, 95% CI (.203, .806), t = 3.28, p = .001, standardized B = 0.806], 4) worse work and social adjustment [Β = .022, 95% CI (.008, .037), t = 3.02, p = .003, standardized B = 0.037] and 5) MDD-associated symptoms (CAST total score) [Β = .052, 95% CI (.038, .066), t = 7.15, p < .001, standardized B = 0.066]. The following variables were NOT independently associated with NV symptom scores: agoraphobia, drug abuse, obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, cognitive and physical functioning, number of psychiatric comorbidities, number of medical comorbidities, and body mass index. The model summary of the linear regression for NV scores was R2 = .219, degrees of freedom = 5, F = 36.90, p < .001.
Response to antidepressants
NV symptoms were less responsive to antidepressants than PV symptoms (mean difference [PV-NV] = 0.469; standard error = 0.041; df = 2007; p < .0001) (Figure 1). PV (p < .0001) and NV (p < .0001) symptoms responded less to antidepressants than other MDD symptoms. There were no statistical differences between the three individual treatment arms in terms of improvement in PV or NV symptoms.
Figure 1: Improvement pf positive (PV) symptom scores, negative valence (NV) symptom score and other depressive symptoms(ODS) over 12 weeks, using a repeated measures mixed-effects model.
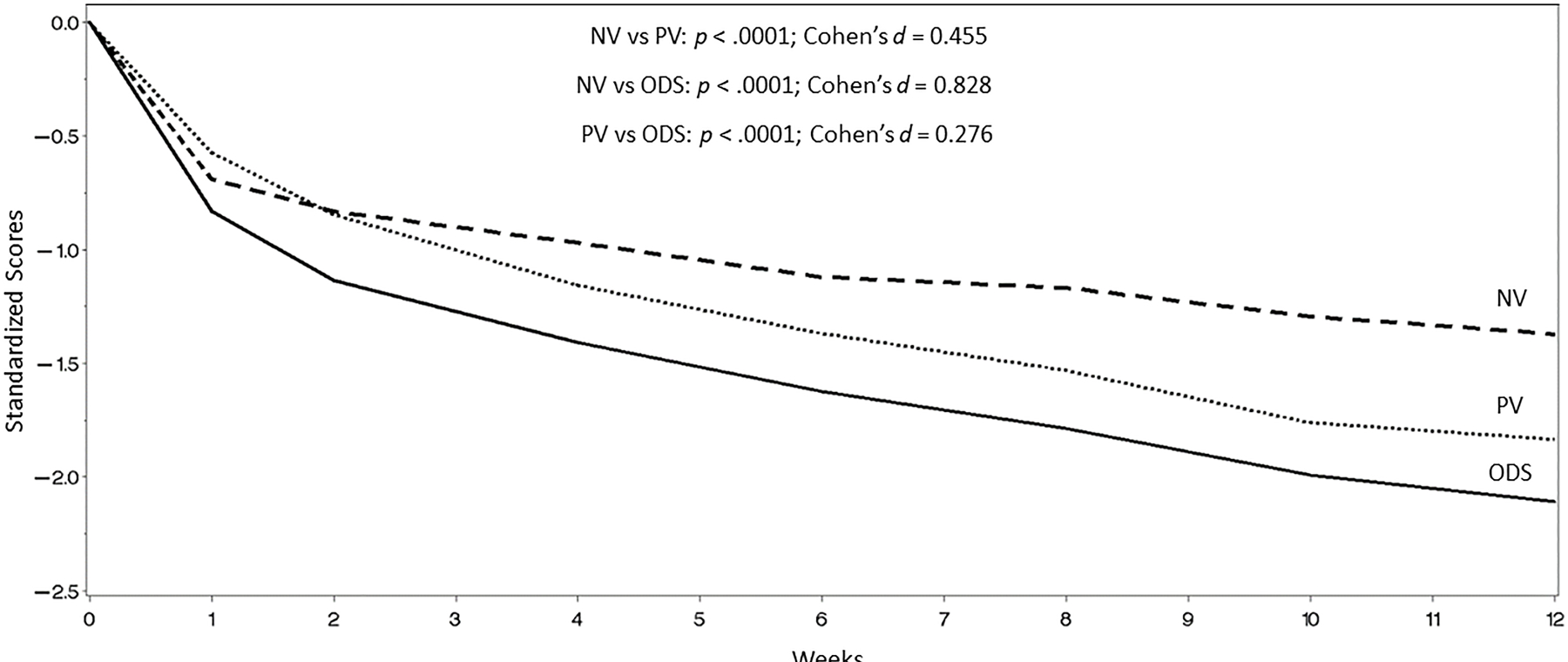
aOther symptoms consisted of the items of the Inventory of Depressive Symptomatology (IDS) that were not part of PV or NV symptom scores.
Associations with immunomarkers
At baseline, PV, but not NV, symptom scores showed statistically significant correlations with the level of three pro- and one anti-inflammatory immunomarkers (Table 4). Interestingly, most correlations between immunological factors (8/9) and PV symptom scores were positive, whereas most correlations between immunological factors (6/9) and NV symptoms scores were negative. In addition, the associations with levels of immunomarkers were stronger for PV symptoms than for overall MDD severity (IDS30) or for other MDD symptoms (IDS22). Overall, the correlations between inflammatory markers and PV and NV scores were numerically stronger in men than in women. Detection ranges and descriptions of the baseline levels of immunomarkers are displayed in Supplemental Table 2.
Table 4.
Relationship between immunomarkers, and symptoms of major depressive disorder (MDD), N=166.
| Variables | OVERALL SEVERITY (IDS30 TOTAL SCORE) |
POSITIVE VALENCE SYMPTOMS |
NEGATIVE VALENCE SYMPTOMS |
OTHER MDD SYMPTOMS (IDS22) |
||||
|---|---|---|---|---|---|---|---|---|
| r a | p a | r a | p a | r a | p a | r a | p a | |
| ALL SUBJECTS (n=166) | ||||||||
| Primarily pro-inflammatory factors | ||||||||
| Log c reactive protein (CRP) | −.030 | .784 | −.044 | .691 | −.136 | .219 | .020 | .859 |
| Log interferon-gamma | .226 | .039 | .293 | .007 | .046 | .676 | .163 | .140 |
| Log interleukin 1 beta | .002 | .986 | .225 | .040 | −.009 | .937 | −.097 | .379 |
| Log interleukin 2 (n=94) | −.156 | .157 | .102 | .354 | −.267 | .014 | −.179 | .104 |
| Log interleukin 6 (n=160) | .011 | .924 | .233 | .033 | .005 | .966 | −.093 | .400 |
| Log interleukin 8 (n=165) | −.108 | .329 | .119 | .280 | −.017 | .879 | −.197 | .072 |
| Log TNF-alpha | .052 | .640 | .181 | .100 | −.035 | .749 | −.001 | .996 |
| Primarily anti-inflammatory factors | ||||||||
| Log interleukin 4 | .072 | .515 | .224 | .041 | .109 | .324 | −.036 | .743 |
| Log interleukin 10 (n=127) | −.051 | .644 | .110 | .321 | −.040 | .715 | −.108 | .328 |
|
| ||||||||
| MALES (n=49) | ||||||||
| Primarily pro-inflammatory factors | ||||||||
| Log c-reactive protein (CRP) | .050 | .829 | .122 | .597 | −.343 | .128 | .099 | .670 |
| Log interferon-gamma | .223 | .332 | .339 | .133 | .317 | .162 | .007 | .975 |
| Log interleukin 1 beta | −.123 | .596 | .300 | .186 | −.235 | .305 | −.258 | .259 |
| Log interleukin 2 (n=25) | −.199 | .388 | .270 | .237 | −.419 | .059 | −.286 | .209 |
| Log interleukin 6 (n=46) | .102 | .659 | .437 | .047 | −.054 | .817 | −.095 | .682 |
| Log interleukin 8 | −.135 | .559 | .201 | .383 | −.408 | .066 | −.167 | .468 |
| Log TNF-alpha | .057 | .806 | .369 | .100 | −.238 | .299 | −.061 | .791 |
| Primarily anti-inflammatory factors | ||||||||
| Log interleukin 4 | −.049 | .834 | .193 | .401 | .244 | .287 | −.244 | .286 |
| Log interleukin 10 (n=37) | −.036 | .877 | .273 | .232 | −.232 | .312 | −.131 | .572 |
|
| ||||||||
| FEMALES (n=117) | ||||||||
| Primarily pro-inflammatory factors | ||||||||
| Log c-reactive protein (CRP) | −.055 | .671 | −.108 | .405 | −.100 | .441 | .002 | .991 |
| Log interferon-gamma | .264 | .038 | .298 | .019 | −.046 | .723 | .253 | .048 |
| Log interleukin 1 beta | .053 | .685 | .214 | .095 | .044 | .736 | −.034 | .795 |
| Log interleukin 2 (n=69) | −.110 | .394 | .033 | .802 | −.227 | .076 | −.097 | .454 |
| Log interleukin 6 (n=114) | −.029 | .825 | .155 | .228 | −.008 | .951 | −.025 | .845 |
| Log interleukin 8 (n=116) | −.084 | .516 | .114 | .376 | .059 | .647 | −.186 | .148 |
| Log TNF-alpha | .067 | .603 | .083 | .519 | .010 | .936 | .054 | .674 |
| Primarily anti-inflammatory factors | ||||||||
| Log interleukin 4 | .168 | .192 | .285 | .025 | .057 | .660 | .092 | .475 |
| Log interleukin 10 (n=90) | −.036 | .781 | .017 | .894 | .029 | .826 | −.067 | .606 |
Pearson’s partial correlation coefficient and controlled p = scores controlled for age.
Bold = statistically significant (p < .05) after controlling for age. CRP measured in mg/L, other immunomarkers measured in pg/ml. IDS30 = Inventory of Depressive Symptomatology - Clinician Version. IDS22 = IDS30 excluding positive valence symptoms and negative valence symptoms items.
After controlling for age, the pro-inflammatory factors IFN-γ and, IL-6 showed the strongest associations with PV symptom scores (IFN-γ Pearson’s partial correlation coefficient = .293, p = .007; IL-6 Pearson’s partial correlation coefficient = .233, p = .033). In contrast, the pro-inflammatory IL-2 was the only immunomarker that had a significant correlation with NV symptom score (Pearson’s correlation coefficient = −.267, p = .014).
4. Discussion
We found that we could operationalize a proxy for the concepts of PV and NV systems function in a concise, clinician-friendly manner by using selected symptoms that potentially reflect the function of each system. Results revealed that PV and NV symptom scores were substantially associated with different clinical features. PV symptoms were associated with female gender, older age, and higher cognitive and physical impairment, while NV symptoms were associated with younger age, more anxious comorbidities, and higher rates of other commonly associated non criterion symptoms. Further, NV symptoms were less responsive to antidepressants than PV symptoms, while both PV and NV symptoms responded less to antidepressants than other MDD symptoms (i.e. non-PV, non-NV symptoms). Finally, at baseline, PV (but not NV) symptom scores were significantly and positively correlated with several immunological factors (both pro-inflammatory and anti-inflammatory).
The emotional regulation and reward processes form the essence of the depressive syndrome and are reflected in the PV and NV system function as expressed symptomatically. Based on observable and reported symptoms, we developed a proxy for PV and NV systems (named PV and NV symptom scores) using a common depression questionnaire (IDS30). These scores were associated with different clinical features, response to antidepressants and relationship with immunomarkers, suggesting that symptom scores might represent two systems that have different circuits and pathophysiology. This study is an initial attempt to conduct specific evaluations of PV and NV symptoms. In the future, assessment of PV and NV symptom scores in clinical practice may help treatment selection or prognostication.
NV symptoms were less responsive to antidepressants than PV symptoms - a finding that is consistent with previous findings that depression with significant NV symptoms, sometimes referred to as anxious depression, is associated with poorer response to antidepressants (Domschke et al., 2010; Fava et al., 2008). Anxious depression is usually defined as having a score of 7 or higher in the anxiety/somatization factor score of HAM-D17, and is associated with lower remission rates when compared to non-anxious depression (Domschke et al., 2010; Fava et al., 2008). Although, PV was more responsive to antidepressants than NV, PV was less responsive to antidepressants than other MDD symptoms, which agrees with previous symptoms that anhedonia is a difficult-to-treat symptom (Lally et al., 2014; Papakostas & Ionescu, 2015). Similarly, Uher and colleagues (2012) analyzed remission rates as a function of severity of specific symptom dimensions in two large clinical trials. They found that worse severity in the interest-activity symptom dimension, which reflected “low interest, reduced activity, indecisiveness and lack of enjoyment”, predicted poor outcomes to treatment to monotherapy with three different antidepressants (citalopram, escitalopram and nortriptyline). Although different scales were used, the definition and operationalization of PV symptom dimension in our study was similar to their interest-activity dimension (Uher et al., 2012)., The use of dopaminergic and/or glutamatergic medications such as ketamine, memantine or pramipexol might better treat PV symptoms (Lally et al., 2014; Lemke, Brecht, Koester, Kraus, & Reichmann, 2005; Reus et al., 2012). Non-pharmacological interventions such as behavioral activation, CBT focused on positive affect, and exercise have showed positive results (Craske et al., 2019; Hopko, Lejuez, Ruggiero, & Eifert, 2003; Toups et al., 2017).
The correlation between baseline immunomarkers and PV (but not NV) symptom scores and multiple positive associations with baseline levels of pro- and anti-inflammatory factors is consistent with the notion that hedonic and motivational capacity may in part reflect immunological issues (De La Garza, 2005; Eisenberger et al., 2010; Felger et al., 2016). Primate studies found that exogenous administration of interferon-α, a pro-inflammatory cytokine, decreased dopamine release in areas largely associated with reward such as the striatum (Felger et al., 2013). Similarly, Eisenberger et al., (2010) found that administration of low doses of bacterial endotoxin was associated with decreased ventral striatum activity to reward cues in humans (Eisenberger et al., 2010). Felger and colleagues (2016) observed that systemic inflammation (reflected by concentration of CRP, IL-1β and other immunomarkers) was correlated with clinical anhedonia and lower connectivity between the striatum and the ventromedial prefrontal cortex, a key reward circuit (Felger et al., 2016). Taken together, these studies suggest that PV symptoms may be connected to inflammation by impaired connectivity and decreased dopamine release induced by pro-inflammatory cytokines in reward-related circuits. Since PV symptoms had a significant relationship with immunological changes, the use of medications with anti-inflammatory properties may also specifically benefit those depressed patients with prominent PV symptoms. Some previous studies observed that medications that inhibit or antagonize inflammatory cytokines may help selected subjects with pro-inflammatory status (Raison et al., 2013; Savitz et al., 2018) or specific PV symptoms such as fatigue (Tyring et al., 2006).
PV and NV symptom scores were associated with distinct clinical and immunological features, although the scores were also modestly positively related to each other. PV and NV systems are relatively independent but have some degree of connection or common regulating structures. These systems seem to overlap in areas such as the amygdala and the striatum (Correia, McGrath, Lee, Graybiel, & Goosens, 2016; Janak & Tye, 2015). Another possible link between PV and NV systems is the common regulation by other brain structures such as the ventromedial pre-frontal cortex and the anterior cingulate cortex (Bogdan & Pizzagalli, 2006; Delgado et al., 2016; Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015; Stevens, Hurley, & Taber, 2011). The ultimate result of the complex relationships between PV and NV systems are elaborated emotional responses and behaviors, and heterogeneous psychiatric disorders such as MDD (Hyman, 2007; Insel et al., 2010).
Limitations
First, the systems which underlie PV and NV are very complex and were derived from IDS30, an instrument that was primarily developed to assessed overall depression severity, not specifically PV and NV symptom dimensions. Therefore, the clinical symptoms chosen to represent these systems may not fully capture all their functions. Second, the internal consistency for NV symptom score was relatively low. However, it must be considered that Cronbach’s alpha is greatly influenced by the number of items and that, given that there are only 3 items in the scale, it is acceptable to use the NV symptoms score in this pilot study. Future research on PV and NV symptoms should develop more comprehensive measures. Third, this was a secondary analysis of a sample of opportunity from the CO-MED study that did not use emotional or biological measures such as functional neuroimaging, reward-tasks or electroencephalogram, which could further characterize PV and NV systems and correlate the symptom scores with actual system function. Despite our interesting findings, there is a clear need for further investigations on PV and NV symptoms using functional biological modalities. Fourth, the biomarkers component was part of an add-on study and thus only a subset of the CO-MED participants had blood available to evaluate immunomarkers. In addition, our conclusions are limited by not having some variables that can impact levels of immunomarkers such as smoking, fasting status, and time of the blood collection. Fifth, we had to exclude individuals with baseline symptom score of zero in a specific dimension (n = 17, 2.6%), and those who came just for the baseline visit (n = 31, 4.7%). Despite the total number of excluded participants was relatively small (n = 48, 7.2%), this might affect the generalizability of our findings. Finally, CO-MED was a trial of outpatients with recurrent or chronic nonpsychotic MDD, thus, additional caution is warranted in generalizing from this sample and replication of these findings would strengthen its utility. Despite the limitations, this study has several strengths such as the conceptualization of a clinical proxy of PV and NV systems in a large clinical trial using a common depression questionnaire (IDS30). The two dimensions obtained showed differential clinical and immunological associations, as well as distinct responsiveness to antidepressants.
Conclusions
PV and NV system function appears to be reflected in selected clinical symptoms that differentially relate to other clinical features, treatment outcomes and immunological function.
Supplementary Material
Supplementary Figure 1: Relationship between positive valence and negative valence symptoms.
Acknowledgments
We thank Mr. Jeremy A. Kee for his editorial and administrative support. We also thank the trial participants and the clinical staff at each clinical site. Without them the project would not have been possible. Finally, the authors appreciate the support given by the Jordan Elizabeth Harris Foundation and the Rees-Jones Foundation.
Funding
The CO-MED trial (NCT00590863) was funded by NIMH (N01 MH-90003), and in part by the Hersh Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals provided medications for this trial at no cost. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R25MH101078.
Footnotes
Conflict of Interest Statement
Dr. Rush has received consulting fees from Akili, Brain Resource Inc., Compass Inc., Curbstone Consultant LLC., Emmes Corp, Holmusk, Inc., Liva-Nova, Sunovion, Takeda USA, Taj Medical; speaking fees from Liva-Nova; royalties from Guilford Press and the University of Texas Southwestern Medical Center, Dallas, TX. (for the Inventory of Depressive Symptoms and its derivatives). He is also named co-inventor on two patents: U.S. Patent No. 7,795,033: Methods to Predict the Outcome of Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S, Wilson AS and U.S. Patent No. 7,906,283: Methods to Identify Patients at Risk of Developing Adverse Events During Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S. Dr. Jha has received contract research grant from Acadia Pharmaceutical and Janssen Research. Dr. Trivedi has received research support from NIMH, NIDA, J&J, Janssen Research and Development LLC; has served as a consultant for Alkermes Inc., Allergan, Arcadia Pharmaceuticals Inc., AstraZeneca, Lundbeck, Medscape, MSI Methylation Sciences Inc., Merck, Otsuka America Pharmaceuticals Inc., and Takeda Pharmaceuticals Inc. Dr. Carmody has received an honorarium from the University of Texas San Antonio. Dr. Trombello currently owns stock in Merck and Gilead Sciences and within the past 36 months previously owned stock in Johnson & Johnson. Drs. Medeiros, Furman, Czysz, and Cooper have no conflicts to report.
Clinical Trials Registration: Combining Medications to Enhance Depression Outcomes (CO-MED) NCT00590863 - https://clinicaltrials.gov/ct2/show/NCT00590863
Data availability
CO-MED study data may be requested through the NIMH Data Archive.
References
- Afshan G, Afzal N, & Qureshi S (2012). CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin Lab, 58(5–6), 567–571. [PubMed] [Google Scholar]
- Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, … Rush AJ (2015). Depression Subtypes in Predicting Antidepressant Response: A Report From the iSPOT-D Trial. Am J Psychiatry, 172(8), 743–750. doi: 10.1176/appi.ajp.2015.14020181 [DOI] [PubMed] [Google Scholar]
- Bio-Rad Laboratories. (2016). Analyte Guide, Bio-Plex Multiplex Immunoassays [Google Scholar]
- Birur B, Amrock EM, Shelton RC, & Li L (2017). Sex Differences in the Peripheral Immune System in Patients with Depression. Front Psychiatry, 8, 108. doi: 10.3389/fpsyt.2017.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, & Pizzagalli DA (2006). Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry, 60(10), 1147–1154. doi: 10.1016/j.biopsych.2006.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K, Covinsky KE, Friedman MB, & Bruce ML (2010). High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry, 67(5), 489–496. doi: 10.1001/archgenpsychiatry.2010.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchese JM, Evans S, Hult C, Joslyn LR, Wessler T, Millar JA, … Kirschner DE (2018). Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev, 285(1), 147–167. doi: 10.1111/imr.12671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, McGrath AG, Lee A, Graybiel AM, & Goosens KA (2016). Amygdala-ventral striatum circuit activation decreases long-term fear. Elife, 5. doi: 10.7554/eLife.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol, 87(5), 457–471. doi: 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for Anhedonia: A Neuroscience Driven Approach. Depress Anxiety, 33(10), 927–938. doi: 10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med, 11, 126. doi: 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd. (2005). Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev, 29(4–5), 761–770. doi: 10.1016/j.neubiorev.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Beer JS, Fellows LK, Huettel SA, Platt ML, Quirk GJ, & Schiller D (2016). Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex. Nat Neurosci, 19(12), 1545–1552. doi: 10.1038/nn.4438 [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, & Pizzagalli DA (2014). Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety, 31(3), 233–249. doi: 10.1002/da.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, … Baune BT (2010). Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol, 20(5), 301–309. doi: 10.1016/j.euroneuro.2009.09.006 [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctot KL (2010). A meta-analysis of cytokines in major depression. Biol Psychiatry, 67(5), 446–457. doi: 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry, 68(8), 748–754. doi: 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Iosifescu DV, Pedrelli P, & Baer L (2009). Reliability and validity of the Massachusetts general hospital cognitive and physical functioning questionnaire. Psychother Psychosom, 78(2), 91–97. doi: 10.1159/000201934 [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, … Trivedi MH (2008). Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry, 165(3), 342–351. doi: 10.1176/appi.ajp.2007.06111868 [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, & Miller AH (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry, 21(10), 1358–1365. doi: 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, … Miller AH (2013). Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology, 38(11), 2179–2187. doi: 10.1038/npp.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, & Rush AJ (2009). What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv, 60(11), 1439–1445. doi: 10.1176/ps.2009.60.11.1439 [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, & Kivimaki M (2015). Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun, 49, 206–215. doi: 10.1016/j.bbi.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, & Gotlib IH (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry, 169(7), 693–703. doi: 10.1176/appi.ajp.2012.11071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry, 23, 56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Ruggiero KJ, & Eifert GH (2003). Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev, 23(5), 699–717. [DOI] [PubMed] [Google Scholar]
- Hyman SE (2007). Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci, 8(9), 725–732. doi: 10.1038/nrn2218 [DOI] [PubMed] [Google Scholar]
- IDS-QIDS. (2018). Inventory Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS) [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. In: Am Psychiatric Assoc [DOI] [PubMed] [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. doi: 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Miller AH, Minhajuddin A, & Trivedi MH (2018). Association of T and non-T cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology, 95, 1–7. doi: 10.1016/j.psyneuen.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, … Trivedi MH (2017). Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology, 78, 105–113. doi: 10.1016/j.psyneuen.2017.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, & Trivedi MH (2017). Platelet-Derived Growth Factor as an Antidepressant Treatment Selection Biomarker: Higher Levels Selectively Predict Better Outcomes with Bupropion-SSRI Combination. Int J Neuropsychopharmacol, 20(11), 919–927. doi: 10.1093/ijnp/pyx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Walters EE (1998). Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depress Anxiety, 7(1), 3–14. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci, 6(9), 691–702. doi: 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, & Zarate CA (2014). Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry, 4, e469. doi: 10.1038/tp.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke MR, Brecht HM, Koester J, Kraus PH, & Reichmann H (2005). Anhedonia, depression, and motor functioning in Parkinson’s disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci, 17(2), 214–220. doi: 10.1176/jnp.17.2.214 [DOI] [PubMed] [Google Scholar]
- Little TD (2013). Longitudinal structural equation modeling [Google Scholar]
- Mapi Research Trust. (2018). Inventory of Depressive Symptomatology (IDS-SR and IDS-C) [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, & Jovanovic T (2017). Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology, 42(1), 254–270. doi: 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol, 16(1), 22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, & Cuthbert BN (2012). Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci, 14(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry, 77(3), 276–284. doi: 10.1016/j.biopsych.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, & Greist JH (2002). The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry, 180, 461–464. [DOI] [PubMed] [Google Scholar]
- Nusslock R, & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord, 216, 3–16. doi: 10.1016/j.jad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, … Schatzberg AF (2016). Major depressive disorder. Nat Rev Dis Primers, 2, 16065. doi: 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, & Ionescu DF (2015). Towards new mechanisms: an update on therapeutics for treatment-resistant major depressive disorder. Mol Psychiatry, 20(10), 1142–1150. doi: 10.1038/mp.2015.92 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol, 10, 393–423. doi: 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, … Miller AH (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry, 70(1), 31–41. doi: 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Abelaira HM, Stringari RB, Fries GR, Kapczinski F, & Quevedo J (2012). Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis, 27(2), 175–182. doi: 10.1007/s11011-012-9281-2 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, & Trivedi MH (1996). The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med, 26(3), 477–486. [DOI] [PubMed] [Google Scholar]
- Rush AJ, & Ibrahim HM (2018). Speculations on the Future of Psychiatric Diagnosis. J Nerv Ment Dis, 206(6), 481–487. doi: 10.1097/NMD.0000000000000821 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, … Keller MB (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry, 54(5), 573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, … Wisniewski SR (2011). Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry, 168(7), 689–701. doi: 10.1176/appi.ajp.2011.10111645 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, & Nierenberg AA (2009). STAR*D: revising conventional wisdom. CNS Drugs, 23(8), 627–647. doi: 10.2165/00023210-200923080-00001 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, … Trivedi MH (2005). Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord, 87(1), 43–55. doi: 10.1016/j.jad.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Russo SJ, & Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci, 14(9), 609–625. doi: 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha O, Stucki G, Liang MH, Fossel AH, & Katz JN (2003). The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum, 49(2), 156–163. doi: 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, … Preskorn SH (2018). Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2x2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry, 8(1), 27. doi: 10.1038/s41398-017-0073-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, & Taber KH (2011). Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci, 23(2), 121–125. doi: 10.1176/appi.neuropsych.23.2.121,10.1176/jnp.23.2.jnp121 [DOI] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, & Melamed S (2005). The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J Occup Health Psychol, 10(4), 344–362. doi: 10.1037/1076-8998.10.4.344 [DOI] [PubMed] [Google Scholar]
- Toups M, Carmody T, Greer T, Rethorst C, Grannemann B, & Trivedi MH (2017). Exercise is an effective treatment for positive valence symptoms in major depression. J Affect Disord, 209, 188–194. doi: 10.1016/j.jad.2016.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, … Weissman MM (2016). Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J Psychiatr Res, 78, 11–23. doi: 10.1016/j.jpsychires.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Wisniewski SR, Morris DW, Fava M, Kurian BT, Gollan JK, … Rush AJ (2011). Concise Associated Symptoms Tracking scale: a brief self-report and clinician rating of symptoms associated with suicidality. J Clin Psychiatry, 72(6), 765–774. doi: 10.4088/JCP.11m06840 [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, … Krishnan R (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet, 367(9504), 29–35. doi: 10.1016/S0140-6736(05)67763-X [DOI] [PubMed] [Google Scholar]
- Uher R, Dernovsek MZ, Mors O, Hauser J, Souery D, Zobel A, … Farmer A (2011). Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J Affect Disord, 132(1–2), 112–120. doi: 10.1016/j.jad.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, … McGuffin P (2012). Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med, 42(5), 967–980. doi: 10.1017/S0033291711001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, … McGuffin P (2014). An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry, 171(12), 1278–1286. doi: 10.1176/appi.ajp.2014.14010094 [DOI] [PubMed] [Google Scholar]
- Vichaya EG, & Dantzer R (2018). Inflammation-induced motivational changes: Perspective gained by evaluating positive and negative valence systems. Curr Opin Behav Sci, 22, 90–95. doi: 10.1016/j.cobeha.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, & Gibb BE (2015). Integrating NIMH Research Domain Criteria (RDoC) into Depression Research. Curr Opin Psychol, 4, 6–12. doi: 10.1016/j.copsyc.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, … Wu Y (2011). Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res, 225(1), 135–141. doi: 10.1016/j.bbr.2011.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Relationship between positive valence and negative valence symptoms.
Data Availability Statement
CO-MED study data may be requested through the NIMH Data Archive.


