Abstract
Background
Medication nonadherence is a significant public health problem as it contributes to poor clinical outcomes and increased healthcare costs. Older patients with multimorbidity and polypharmacy often have low medication adherence. These patients also have a high prevalence of potentially inappropriate medication (PIM) use.
Aim
To explore risk factors related to medication nonadherence in older patients with multimorbidity and polypharmacy and examine the association between medication nonadherence and PIM use.
Method
A multicenter cross-sectional study was conducted from May to December 2019 in 16 tertiary hospitals from 12 provinces and cities in China. Data were collected from outpatients 65 years or older with multimorbidity and polypharmacy. The PIMs were evaluated using the 2019 Beers Criteria. Self-reported medication adherence was assessed using the Visual Analog Scale (VAS).
Results
A total of 773 outpatients were recruited. The prevalence of medication nonadherence was 31.8%. In the univariate analysis, nonadherence was significantly associated with sex, cognitive impairment, stroke, visiting the same physicians, self-administration of medication, the percentage of drug costs ≥ 10% of the medical expenses, and PIMs for the alimentary tract and metabolism. In the multivariate analysis, the results almost paralleled those of the univariate associations. Notably, the use of PIM was significantly associated with medication adherence.
Conclusion
Several factors that influence medication adherence were identified. Targeted interventions can be implemented to improve medication adherence, such as encouraging self-administering medications and reducing medication expenses.
Keywords: Medication adherence, Potentially inappropriate medication, Multimorbidity, Polypharmacy, Older adults
Introduction
According to the World Health Organization (WHO), the world population of people aged 60 years and older will reach 2.1 billion in 2050 [1]. China is currently experiencing an accelerated period of population aging. There will be 400 million Chinese citizens 65 years or older by 2030 [2]. With an aging population, the prevalence of multimorbidity (defined as having two or more chronic medical conditions) is likely to increase rapidly [3]. Older adults with multimorbidity require long-term treatment. Multiple medications are often prescribed simultaneously to these patients, leading to polypharmacy [4, 5]. Polypharmacy is usually defined as taking five or more medications simultaneously, although no consensus has been reached on the exact cutoff number [6, 7]. In the United States, 39% of adults over 65 take five or more daily medications [8]. A retrospective study in China reported that 15.9% of people aged over 65 years of age had multimorbidities [9]. Another Chinese study reported that 65.2% of patients aged over 60 years of age took five or more daily medications [10].
Medication adherence refers to the ability of patients to follow their medication schedule as prescribed by their healthcare provider. Medication adherence remains a significant challenge in older patients with chronic diseases [11, 12]. Nonadherence can lead to treatment failure, increased hospital readmissions related to medications, additional medical/surgical procedures, and excess healthcare costs [11, 13]. It can also result in mortality [14].
The risk factors for medication nonadherence are complex and relate to patients and drugs. Patient-related factors include sex [15], age [13], physical inactivity [16], cognitive function [5, 17, 18], marital status [13, 16], education level [19], caregivers [20], alcohol consumption [16], and multimorbidity [16]. Drug-related factors include the number of medications [18], copays [18], drug costs [5, 13], and adverse drug reactions [5, 17, 18]. The use of potentially inappropriate medications (PIM) in older patients is widespread. PIM use is associated with adverse drug events (ADE) and affects medication adherence [5, 21, 22].
Few studies have been conducted on medication nonadherence in older patients with multimorbidity and polypharmacy [23, 24]. This study aimed to evaluate the associations between patient characteristics, drug-related factors, and medication adherence in community-dwelling older patients with polypharmacy and chronic diseases. Furthermore, this study also examined the association between medication non-adherence and PIM use.
Methods
Study design and participants
The study was a multicenter cross-sectional study conducted from May to December 2019 in 16 tertiary hospitals in 12 provinces and cities in China. A convenience sampling method was used to recruit study participants. The inclusion criteria were patients: (1) 65 years or older, (2) diagnosed with two or more diseases, and at least one of the following five chronic diseases (hypertension, diabetes mellitus, coronary heart disease, dyslipidemia, and stroke), (3) taking five or more medications (traditional Chinese medicines were not included) for a period of ≥ 6 months. Patients who could not participate or did not complete the interview were excluded.
The project was approved by the Research Ethics Committee of the Xuanwu Hospital of Capital Medical University (approval number [2018] 022, June 25, 2018). It was registered by the Research Ethics Committees of the participating hospitals.
Sample size calculation
Based on the previous studies, we assumed that the proportion (P) of polypharmacy was 65.3% [9]. A minimum of 710 participants were required (the width of the 95% confidence interval of the proportion of polypharmacy < 8%, a dropout rate of 20%). The sample size was calculated using PASS 16. Fifty participants were recruited from each hospital, as 16 tertiary hospitals participated in this study. Finally, 800 participants were included in the study.
Medication adherence
Medication adherence was assessed using the visual analog scale (VAS), a self-reported measure of medication adherence. VAS asks individuals to mark a line at the point along a continuum (0–100), showing how well they followed physician orders in the last four weeks [25]. For example, 0 refers to the patient not following the physician’s instructions, and 100 means that the patient strictly follows physician orders. A score below 80 is considered medication nonadherence [26]. The interviews with cognitively impaired patients were conducted in the presence of their caregivers whenever possible to minimize the misreporting of VAS.
Data collection and standardization
Data were collected from face-to-face interviews using a standardized questionnaire. The following data were collected: age, sex, marital status, living condition, educational attainment, height, weight, smoking and alcohol consumption, medication, ability to self-administer medications, medical insurance, comorbidities, history of ADE, physician visits, hospital admissions, and percentage of drug costs within medical expenses. Height and weight were used to calculate Body Mass Index (BMI, kg/m2). Educational attainment was divided into low (below the middle school, < 9 years) and high (≥ 9 years) ranges. The Mini-Cog test was used to detect cognitive impairment [27]. Cognitive impairment was when the Mini-Cog scores ≤ 2.
The use of PIM was evaluated using the 2019 Beers criteria developed by the American Geriatrics Society [28]. The following five categories (tables) are in the criteria: Category A: Medications that are potentially inappropriate in most older adults; Category B: Medications that are potentially inappropriate in older adults with certain conditions; Category C: Medications that should be used with caution; Category D: Potentially clinically important drug-drug interaction and Category E: Medications that should be avoided or have their dosage reduced based on kidney function. Since serum creatinine values were not available, the PIMs listed in Category E were not evaluated. Two researchers assessed PIM use, and a third researcher was invited to resolve the discrepancy through discussions.
Diagnoses were recorded by the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD 10). Medication was classified using the WHO Anatomical Therapeutic Chemical (ATC) classification (2016 version).
Statistical analysis
Descriptive statistics are expressed as medians and interquartile ranges (IQR) for quantitative variables and as frequencies and proportions for categorical variables. Univariate analysis was performed with Student’s t-test for normally distributed quantitative variables. The Wilcoxon rank-sum test was used for nonnormally distributed quantitative variables. Chi-square tests were used for categorical variables in the univariate analysis. Adjusted odds ratios (aOR) and 95% confidence intervals (95%CI) were estimated using a multivariate logistic regression model. P < 0.05 was considered statistically significant. Significant risk factors associated with medication nonadherence in univariate analysis were analyzed in the multivariable logistic regression analyses. Given the exploratory purpose of this study, the type-I error was not adjusted for multiple tests. Data were analyzed using SAS9.4 (Cary, NC, USA).
Results
Patients’ demographic and clinical characteristics
A total of 800 patients completed face-to-face interviews, and 773 were included in the analysis. Fifteen had missing information and 12 did not meet the requirements of comorbidity and polypharmacy. The median age of the participants was 73.7 (69.1, 79.6) years, and 58.9% (455/773) were men. Most of the patients were married and lived with family members. A total of 164 patients (21.2%) had cognitive impairment. The median number of comorbidities was 5 (4, 6). Patients were taking a median number of 6 (5, 7) medications. Hypertension was the most prevalent chronic disease, at 88.4% (683/773). This corresponded to the highest percentage of patients taking medication for the cardiovascular system, 98.7% (763/773). Detailed information is shown in Table 1.
Table 1.
Patients’ characteristics, medication information, PIMs prevalence, medication with PIM use, and VAS scale for medication adherence
| Variable (n = 773) | Item | Total, n (%) |
|---|---|---|
| Age, years | Median (IQR) | 73.7(69.1, 79.6) |
| 65–74 | 431(55.8%) | |
| ≥ 75 | 342(44.2%) | |
| Gender | Male | 455(58.9%) |
| Female | 318(41.1%) | |
| Marital status | Married | 653(84.5%) |
| Single/divorced/widowed | 120(15.5%) | |
| Living condition | Living with family members | 665(86.0%) |
| Alone | 108(14.0%) | |
| Education attainment | Lowa | 602(77.9%) |
| Highb | 171(22.1%) | |
| BMI (kg/m2) | Median (IQR) | 24.3(22.0, 26.7) |
| Smoking | Yes | 256(33.1%) |
| No | 517(66.9%) | |
| Alcohol drinking | Yes | 82(10.6%) |
| No | 691(89.4%) | |
| Cognitive impairment | Yes | 164(21.2%) |
| No | 609(78.8%) | |
| Hospital admissions within the last year | Yes | 448(58.0%) |
| No | 325(42.0%) | |
| Comorbidities | Number, median (IQR) | 5.0(4.0, 6.0) |
| Hypertension | 683(88.4%) | |
| Coronary heart disease | 492(63.7%) | |
| Diabetes mellitus | 422(54.6%) | |
| Dyslipidemia | 372(48.1%) | |
| Stroke | 273(35.3%) | |
| Visiting the same physicians | Yes | 388(50.2%) |
| No | 385(49.8%) | |
| Number of prescribers | 1–2 | 607(78.5%) |
| 3 and above | 166(21.5%) | |
| Medications | Number, Median (IQR) | 6.0(5.0, 7.0) |
| 5–7 | 660(85.4%) | |
| 8–9 | 92(11.9%) | |
| > 10 | 21(2.7%) | |
| ATC Classificationc | Cardiovascular system | 763(98.7%) |
| Alimentary tract and metabolism | 679(87.8%) | |
| Blood and blood-forming organs | 316(40.9%) | |
| Nervous system | 182(23.5%) | |
| Ability to self-administer medications | Yes | 683(88.4%) |
| No | 90(11.6%) | |
| History of ADE | Yes | 140(18.1%) |
| No | 633(81.9%) | |
| Medical insurance | Yes | 733(94.8%) |
| No | 40(5.2%) | |
| % of drug costs of medical expenses | < 10% | 321(41.5%) |
| ≥ 10% | 452(58.5%) | |
| PIM use | 479(62.0%) | |
| Medications category with PIMs | Blood and blood-forming organs | 214(27.7%) |
| Cardiovascular system | 183(23.7%) | |
| Alimentary tract and metabolism | 86(11.1%) | |
| Nervous system | 83(10.7%) | |
| Genito urinary system and sex hormones | 13(1.7%) | |
| Musculo-skeletal system | 6(0.8%) | |
| Systemic hormonal preparations, excl. sex hormones and insulins | 4(0.5%) | |
| Antineoplastic and immunomodulating agents | 1(0.1%) | |
| Medication adherence (VAS scale) | Median (IQR) | 88.0(65.0, 100.0) |
| 0–19 | 5(0.7%) | |
| 20–39 | 34(4.4%) | |
| 40–59 | 114(14.7%) | |
| 60–79 | 88(11.4%) | |
| 80–100 | 532(68.8%) |
IQR Interquartile range, BMI Body mass index, ADE Adverse drug event, PIM Potentially inappropriate medication, VAS Visual analogue scale, ATC Anatomical Therapeutic Chemical
aPrimary school and below were included
bMiddle school and above were included
cOnly the categories of medications used more than 10% were listed
The prevalence and analysis of PIM use
The prevalence of PIMs was 62.0% (479/773). Patients mainly took PIMs from the ATC medication categories of blood and blood-forming organs (27.7%, 214/773), cardiovascular system (23.7%, 183/773), and the alimentary tract and metabolism (11.1%, 83/773).
Analysis of medication adherence and factors associated with medication adherence
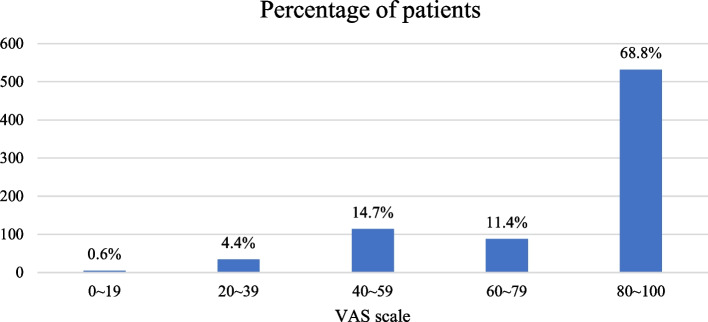
Among study participants, 532 (68.8%) patients had a VAS score above 80, indicating medication adherence (Table 1). The median VAS score was 88.0 (65.0, 100.0). Figure 1 shows the VAS score distribution stratified by 20%.
Fig. 1.
Histogram of the percentage of patients stratified by 20% of the VAS
Table 2 shows the univariate analysis comparing patient demographic and clinical characteristics between the medication adherent and nonadherent groups. There were significant differences in sex (P = 0.002), cognitive impairment (P < 0.001), stroke (P = 0.003), visiting the same physicians (P = 0.013), self-administering medications (P < 0.001), the percentage of drug costs ≥ 10% of the medical expenses (P = 0.002), and taking PIMs from the alimentary tract and metabolism ATC category (P = 0.02) between the two groups.
Table 2.
Univariate analysis for association of adherence with patients’ characteristics and medication information (n = 773)
| Variables | Item | VAS Score < 80 (n = 241) | VAS Score ≥ 80 (n = 532) | P |
|---|---|---|---|---|
| Age, years | Median (IQR) | 73.3(68.6,80.0) | 74.0(69.1,79.6) | 0.455 |
| 65–74 | 137(56.9) | 294(55.3) | 0.681 | |
| ≥ 75 | 104(43.2) | 238(44.7) | ||
| Gender | Male | 122(50.6) | 333(62.6) | 0.002 |
| Female | 119(49.4) | 199(37.4) | ||
| Marital status | Married | 198(82.2) | 455(85.5) | 0.231 |
| Single/divorced/widowed | 43(17.8) | 77(14.5) | ||
| Living condition | Living with family members | 200(83.0) | 465(87.4) | 0.101 |
| Alone | 41(17.0) | 67(12.6) | ||
| Education attainment | Low | 196(81.3) | 406(76.3) | 0.120 |
| High | 45(18.7) | 126(23.7) | ||
| BMI (kg/m2) | Median (IQR) | 24.5(22.3,26.7) | 24.3(22.0,26.7) | 0.651 |
| Smoking | Yes | 69(28.6) | 187(35.2) | 0.074 |
| No | 172(71.4) | 345(64.9) | ||
| Alcohol drinking | Yes | 27(11.2) | 55(10.3) | 0.718 |
| No | 214(88.8) | 477(89.7) | ||
| Cognitive impairment | Yes | 73(30.3) | 91(17.1) | < 0.001 |
| No | 168(69.7) | 441(82.9) | ||
| Hospital admissions within the last year | Yes | 148(61.4) | 300(56.4) | 0.190 |
| No | 93(38.6) | 232(43.6) | ||
| Comorbidities | Number, Median (IQR) | 5.0(4.0,6.0) | 5.0(4.0,6.0) | 0.311 |
| Hypertension | 211(87.6) | 472(88.7) | 0.639 | |
| Coronary heart disease | 146(60.6) | 346(65.0) | 0.233 | |
| Diabetes mellitus | 129(53.5) | 293(55.1) | 0.689 | |
| Dyslipidemia | 123(51.0) | 249(46.8) | 0.275 | |
| Stroke | 67(27.8) | 206(38.7) | 0.003 | |
| Visiting the same physicians | Yes | 105(43.6) | 283(53.2) | 0.013 |
| No | 136(56.4) | 249(46.8) | ||
| Number of prescribers | 1–2 physicians | 189(78.4) | 418(78.6) | 0.963 |
| 3 and above physicians | 52(21.6) | 114(21.4) | ||
| Medications | Number, Median (IQR) | 6.0(5.0,7.0) | 6.0(5.0,7.0) | 0.617 |
| 5–7 | 207(85.9) | 453(85.2) | 0.076 | |
| 8–9 | 32(13.3) | 60(11.3) | ||
| > 10 | 2(0.8) | 19(3.6) | ||
| Cardiovascular system | 235(97.5) | 528(99.3) | 0.079 | |
| Alimentary tract and metabolism | 212(88.0) | 467(87.8) | 0.942 | |
| Blood and blood forming organs | 97(40.3) | 219(41.2) | 0.810 | |
| Nervous system | 61(25.3) | 121(22.7) | 0.436 | |
| Ability to self-administer medications | Yes | 199(82.6) | 484(91.0) | < 0.001 |
| No | 42(17.4) | 48(9.0) | ||
| History of ADE | Yes | 48(19.9) | 92(17.3) | 0.380 |
| No | 193(80.1) | 440(82.7) | ||
| Medical insurance | Yes | 231(95.9) | 502(94.4) | 0.386 |
| No | 10(4.2) | 30(5.6) | ||
| % of drug costs of medical expenses | < 10% | 80(33.2) | 241(45.3) | 0.002 |
| ≥ 10% | 161(66.8) | 291(54.7) | ||
| PIM use | 140(58.1) | 339(63.7) | 0.135 | |
| Medications category with PIMs | Blood and blood forming organs | 6(2.5) | 7(1.3) | 0.241 |
| Cardiovascular system | 44(18.3) | 101(19.0) | 0.810 | |
| Alimentary tract and metabolism | 63(26.1) | 184(34.6) | 0.02 | |
| Nervous system | 32(13.3) | 48(9.0) | 0.072 | |
| Genito urinary system and sex hormones | 1(0.4) | 6(1.1) | 0.445 | |
| Musculo-skeletal system | 2(0.8) | 4(0.8) | 1.000 | |
| Systemic hormonal preparations, excl. sex hormones and insulins | 2(0.8) | 0(0.0) | 0.097 | |
| Antineoplastic and immunomodulating agents | 1(0.4) | 0(0.0) | 0.312 |
IQR Interquartile ranges, BMI Body mass index, ADE adverse drug event, PIMs Potentially inappropriate medications, VAS visual analogue scale
Table 3 shows the results of the multivariate analysis of factors of medication nonadherence. Medication nonadherence was significantly associated with sex (aOR0.65, 95%CI 0.46–0.92, P = 0.015), cognitive impairment (aOR1.84, 95%CI 1.26–2.69, P = 0.002), the number of comorbidities (aOR1.13 95%CI 1.03–1.24, P = 0.011), stroke (aOR 0.44, 95%CI 0.30–0.65, P < 0.0001), the % of drug costs ≥ 10% of the medical expenses (OR1.51 95%CI 1.07–2.14 p = 0.020), ability to self-administer medications (aOR2.28, 95%CI 1.40–3.71, P = 0.001), and PIM use (aOR 0.71, 95%CI 0.50–0.99, P = 0.041).
Table 3.
Multivariate analysis for the association of medication nonadherence with patients' characteristics and medication information (n = 773)
| Variable | Item | aOR (95%CI) | P |
|---|---|---|---|
| Age, years | 65–74 vs. ≥ 75 | 1.06(0.76,1.49) | 0.731 |
| Gender | Male vs. Female | 0.65(0.46,0.92) | 0.015 |
| Living condition | Living with family members vs. Alone | 0.66(0.42,1.04) | 0.074 |
| Alcohol drinking | Yes vs. No | 1.39(0.81,2.39) | 0.230 |
| Cognitive impairment | Yes vs. No | 1.84(1.26,2.69) | 0.002 |
| Comorbidities, number | 1.13(1.03,1.24) | 0.011 | |
| Hypertension | Yes vs. No | 0.79(0.47,1.31) | 0.354 |
| Coronary heart disease | Yes vs. No | 0.75(0.52,1.08) | 0.125 |
| Diabetes mellitus | Yes vs. No | 0.79(0.55,1.12) | 0.187 |
| Dyslipidemia | Yes vs. No | 1.05(0.74,1.50) | 0.780 |
| Stroke | Yes vs. No | 0.44(0.30,0.65) | < 0.0001 |
| Visiting the same physicians | Yes vs. No | 0.74(0.53,1.04) | 0.079 |
| % of drug costs of medical expenses | ≥ 10% vs. < 10% | 1.51(1.07,2.14) | 0.020 |
| Medications, number | 0.99(0.86,1.13) | 0.844 | |
| Ability to self-administer medication | No vs. Yes | 2.28(1.40,3.71) | 0.001 |
| History of ADE | Yes vs. No | 1.13(0.74,1.73) | 0.563 |
| PIM use | Yes vs. No | 0.71(0.50,0.99) | 0.041 |
aOR Adjusted odds ratio, CI Confidence interval, ADE Adverse drug event, PIMs Potentially inappropriate medications
Discussion
The study is one of the few studies that investigate the prevalence and risk factors of medication adherence in community-dwelling older adults with comorbidity and polypharmacy. Nonadherence to medications is common throughout the world. Only 50% of older patients who suffered from chronic diseases followed the treatment recommendations, and the rate was even lower in Asia, according to the 2013 WHO report [29]. The medication adherence rate in our study was 68.82%, higher than in similar studies conducted in China and other Asian countries, with reported rates of 32.6–45% [13, 23, 30, 31]. The differences are related to the study population and the adherence assessment tools. The present study population was older adults who lived in cities and were more likely to receive high-quality medical services from tertiary hospitals. In this study, we used the VAS scale to assess medication adherence. As a widely accepted self-reporting measure in medication adherence, the VAS scale has high levels of concordance with other adherence measures [32]. It has been validated in patients with chronic diseases or who need long-term medication therapies [26, 33]. The VAS scale is simple and easy to implement into the workflow to capture adherence information in a large population cohort. However, self-reporting has disadvantages, as patients are known to overestimate their level of adherence [34].
PIMs are medications whose potential risks outweigh their benefits [21, 35]. Several screening tools and criteria help identify PIM in older adults, and the American Geriatrics Society (AGS) Beers criteria are the most widely used and cited tool [5, 21, 36]. The prevalence of PIMs varies between 14.1–64.8% in the older adult population in China [37–39]. The prevalence of PIM use in this study was 62.0%. PIM prescribing increases significantly among older patients with polypharmacy [40]. Study subjects had a median of five comorbidities and were prescribed a median of six medications, which contributed to the higher rate of PIM use. The lack of awareness among physicians about PIM could be another contributing factor.
In the univariate analysis, medication adherence was not associated with age and educational status. However, in other studies, age and higher education status had better adherence [19, 41]. More female patients reported nonadherence in our research. Women generally play an essential role with the primary responsibility to care for other family members in the household. They may neglect to care for themselves and take their medications [42]. Cognitive impairment was associated with nonadherence, supported by previous studies [16, 17]. Patients with cognitive impairment have difficulty understanding medication instructions and remembering routes of administration [5, 18]. Visiting the same physicians regularly was associated with medication adherence. When patients saw the same physicians regularly, the physicians were updated about the changes in their condition. Medications could be appropriately adjusted, and ADE could be promptly managed, leading to improved medication adherence [43].
Stroke patients reported higher medication adherence than patients with other chronic diseases in this study, consistent with a previous study [44]. Stroke often leads to reduced cognitive function and self-care ability [42]. Patients or their family members may have paid more attention to medications due to the presence of stroke disabilities compared to patients with hypertension or diabetes, who usually do not have severe symptoms or disabilities [45]. The inability to take medications independently adversely affected medication adherence in this study. The support of a family caregiver is strongly correlated with medication adherence [45, 46]. Most of the patients in this study had health insurance. However, more than 50% of the patients had a percentage of drug costs that exceeded 10% of medical expenses, suggesting that the burden of the cost of medication was heavy [47]. Like other studies, medication costs negatively affected medication adherence [13, 48].
The results of the multivariate regression analysis almost paralleled those of the univariate associations. However, it should be noted that the number of comorbidities was linked to non-adherence. Previous studies demonstrate that complex disease conditions, polypharmacy, and PIM prescribing negatively impact medication adherence [32, 48, 49]. We hypothesized that PIM use could negatively affect medication adherence in our population. However, the results of this study indicate the opposite. A Japanese study showed that the use of PIM by older patients was significantly associated with self-reported medication adherence [23]. The reason may be related to the study population. Almost 90% of the study subjects could self-administer their medications. Patients who can self-administer their medications may have been educated and proactively monitored by physicians or pharmacists to prevent ADE or worsening outcomes [23]. Furthermore, the study subjects were motivated to take medications, as shown by the high median VAS score of 88. Moreover, the history of ADE, which was not strictly defined as events caused by PIM, was not associated with adherence in this study. Although some factors, such as sex, cognitive status, and comorbidities, cannot be changed, other factors, such as medication expenses and self-administering medications can improve medication adherence [50].
Limitations
Our study has several limitations. First, the study population was older patients who visited tertiary hospitals in cities. The study sample may not represent older patients living in rural areas or visiting community hospitals. Further studies are needed to recruit older patients from different regions and hospitals. Second, it is common for adults in China to use traditional Chinese medicines. However, we did not assess these medications as they cannot be standardized, and the Beers criteria do not cover them. Finally, VAS is a self-reported method used to assess medication adherence. VAS overestimates adherence compared to direct measures such as pill counting or blood drug concentration testing. In contrast, lower VAS scores could be obtained from cognitively impaired individuals due to misreporting.
Conclusion
Older adults with comorbidity and polypharmacy had good medication adherence and a high frequency of PIM use. Several factors influenced medication adherence, including cognitive status, medication expenses, ability to self-administer medications, and PIM use. Targeted interventions based on this study can be implemented to improve medication adherence.
Acknowledgements
We appreciated staff who recruited participants and input information for this study from Chui Yang Liu Hospital Affiliated to Tsinghua University, The PLA Navy Anqing Hospital, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Hefei First People’s Hospital, Hwa Mei Hospital, University of Chinese Academy of Sciences (Ningbo No 2 Hospital), Chinese Medicine Hospital of Puyang, Zhongshan Hospital of Xiamen University, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, The Second Hospital of Shanxi Medical University, Xinyang Central Hospital, The First Affiliated Hospital, Zhejiang University School of Medicine, The First Affiliated Hospital of University of Science and Technology of China, The First Affiliated Hospital of University of Science and Technology of China west district, Shengjing Hospital of China Medical University, The First Affiliated Hospital of Xinjiang Medical University, People’s Hospital of Tibet Autonomous Region.
Abbreviations
- PIM
Potentially inappropriate medication
- VAS
Visual Analog Scale
- WHO
World Health Organization
- ADE
Adverse drug events
- BMI
Body Mass Index
- ICD
International Statistical Classification of Diseases and Related Health Problems
- ATC
Anatomical Therapeutic Chemical
- IQR
Interquartile ranges
- aOR
Adjusted odds ratios
- CI
Confidence intervals
- AGS
American Geriatrics Society
Authors’ contributions
J.L. collected and analysed the data, and wrote the manuscript. Y.Y. analysed the data, and reviewed the manuscript. S.Y. supervised the study. Y.Z. and S.S. provided support when writing the manuscript. T.H. and Z.W. maintained the data. Q.D., R.Z. and W.L. annotated, scrubbed the data. X.W. and L.Z. provided critical feedback and helped shape the analysis and manuscript. X.Y. was the Principal Investigator, designed the study and acts as guarantor for the study.
Funding
The study was funded by the Beijing Science and Technology Commission Funded Project (No. D181100000218002) and Beijing Municipal Health Commission Funded Project (No. 11000022T000000444688).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The project was approved by the Research Ethics Committee of the Xuanwu Hospital of Capital Medical University (approval number [2018] 022, June 25, 2018). It was registered by the Research Ethics Committees of the participating hospitals. Written informed consent of the study participants was obtained before enrollment. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaming Liu and Yongpei Yu contributed equally to this work.
References
- 1.World Health Organization. Ageing and health. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed 20 Sep 2022.
- 2.Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo H, et al. A research agenda for aging in China in the 21st century. Ageing Res Rev. 2015;24(Pt B):197–205. doi: 10.1016/j.arr.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancet T. Making more of multimorbidity: an emerging priority. Lancet. 2018;391(10131):1637. doi: 10.1016/S0140-6736(18)30941-3. [DOI] [PubMed] [Google Scholar]
- 4.Mair A, Wilson M, Dreischulte T. Addressing the Challenge of Polypharmacy. Annu Rev Pharmacol Toxicol. 2020;60:661–681. doi: 10.1146/annurev-pharmtox-010919-023508. [DOI] [PubMed] [Google Scholar]
- 5.Koren G, Nordon G, Radinsky K, Shalev V. Clinical pharmacology of old age. Expert Rev Clin Pharmacol. 2019;12(8):749–755. doi: 10.1080/17512433.2019.1632188. [DOI] [PubMed] [Google Scholar]
- 6.Halli-Tierney AD, Scarbrough C, Carroll D. Polypharmacy: Evaluating Risks and Deprescribing. Am Fam Physician. 2019;100(1):32–38. [PubMed] [Google Scholar]
- 7.Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9:CD008165. doi: 10.1002/14651858.CD008165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1830. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Atun R, Oldenburg B, McPake B, Tang S, Mercer SW, et al. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population-based panel data. Lancet Glob Health. 2020;8(6):e840–e849. doi: 10.1016/S2214-109X(20)30127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su BB, Ma JX, Song W, Yuan J, Dong XY, Wan J. Analysis of comorbidity and polypharmacy in middle-aged and elderly patients(in Chinese) Zhonghua Yi Xue Za Zhi. 2020;100(25):1983–1987. doi: 10.3760/cma.j.cn112137-20200403-01066. [DOI] [PubMed] [Google Scholar]
- 11.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 12.Cross AJ, Elliott RA, Petrie K, Kuruvilla L, George J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020;5(5):CD012419. doi: 10.1002/14651858.CD012419.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai X, Zhu H, Huo X, Li Z. Polypharmacy in the oldest old (≥80 years of age) patients in China: a cross-sectional study. BMC Geriatr. 2018;18(1):64. doi: 10.1186/s12877-018-0754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kini V, Ho PM. Interventions to Improve Medication Adherence: A Review. JAMA. 2018;320(23):2461–2473. doi: 10.1001/jama.2018.19271. [DOI] [PubMed] [Google Scholar]
- 15.Franchi C, Ardoino I, Ludergnani M, Cukay G, Merlino L, Nobili A. Medication adherence in community-dwelling older people exposed to chronic polypharmacy. J Epidemiol Community Health. 2021;75(9):854–859. doi: 10.1136/jech-2020-214238. [DOI] [PubMed] [Google Scholar]
- 16.Reading SR, Black MH, Singer DE, Go AS, Fang MC, Udaltsova N, et al. Risk factors for medication non-adherence among atrial fibrillation patients. BMC Cardiovasc Disord. 2019;19(1):38. doi: 10.1186/s12872-019-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodford HJ, Fisher J. New horizons in deprescribing for older people. Age Ageing. 2019;48(6):768–775. doi: 10.1093/ageing/afz109. [DOI] [PubMed] [Google Scholar]
- 18.Bazargan M, Smith J, Yazdanshenas H, Movassaghi M, Martins D, Orum G. Non-adherence to medication regimens among older African-American adults. BMC Geriatr. 2017;17(1):163. doi: 10.1186/s12877-017-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gast A, Mathes T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Syst Rev. 2019;8(1):112. doi: 10.1186/s13643-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson CH, Dey S, Kumar V, Monsen KA, Westra BL. Clustering of elderly patient subgroups to identify medication-related readmission risks. Int J Med Inform. 2016;85(1):43–52. doi: 10.1016/j.ijmedinf.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Fralick M, Bartsch E, Ritchie CS, Sacks CA. Estimating the Use of Potentially Inappropriate Medications Among Older Adults in the United States. J Am Geriatr Soc. 2020;68(12):2927–2930. doi: 10.1111/jgs.16779. [DOI] [PubMed] [Google Scholar]
- 22.Clark CM, Shaver AL, Aurelio LA, Feuerstein S, Wahler RG, Jr, Daly CJ, et al. Potentially Inappropriate Medications Are Associated with Increased Healthcare Utilization and Costs. J Am Geriatr Soc. 2020;68(11):2542–2550. doi: 10.1111/jgs.16743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki M, Uchiyama M, Nakamura Y, Matsuo K, Ono C, Goto M, et al. Association of Self-Reported Medication Adherence with Potentially Inappropriate Medications in Elderly Patients: A Cross-Sectional Pilot Study. Int J Environ Res Public Health. 2020;17(16):5940. doi: 10.3390/ijerph17165940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pradhan S, Panda A. Effect of Potentially Inappropriate Medication on Treatment Adherence in Elderly with Chronic Illness. Biomed Pharmacol J. 2018;11(2):935–43. [Google Scholar]
- 25.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 26.Hachulla E, Le Gouellec N, Launay D, Balquet MH, Maillard H, Azar R, et al. Adherence to hydroxychloroquine in patients with systemic lupus: Contrasting results and weak correlation between assessment tools. Joint Bone Spine. 2020;87(6):603–610. doi: 10.1016/j.jbspin.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–94. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Switzerland. Adherence to long-term therapies: evidence for action. Switzerland: World Health Organization; 2003.
- 30.Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(4):e5641. doi: 10.1097/MD.0000000000005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang CT, Ang JY, Islam MA, Chan HK, Cheah WK, Gan SH. Prevalence of Drug-Related Problems and Complementary and Alternative Medicine Use in Malaysia: A Systematic Review and Meta-Analysis of 37,249 Older Adults. Pharmaceuticals (Basel) 2021;14(3):187. doi: 10.3390/ph14030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finitsis DJ, Pellowski JA, Huedo-Medina TB, Fox MC, Kalichman SC. Visual analogue scale (VAS) measurement of antiretroviral adherence in people living with HIV (PLWH): a meta-analysis. J Behav Med. 2016;39(6):1043–1055. doi: 10.1007/s10865-016-9770-6. [DOI] [PubMed] [Google Scholar]
- 33.Poltronieri NVG, Moreira RSL, Schirmer J, Roza BA. Medication non-adherence in heart transplant patients. Rev Esc Enferm USP. 2020;54:e03644. doi: 10.1590/S1980-220X2019009203644. [DOI] [PubMed] [Google Scholar]
- 34.Tedla YG, Bautista LE. Factors associated with false-positive self-reported adherence to antihypertensive drugs. J Hum Hypertens. 2017;31(5):320–326. doi: 10.1038/jhh.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguiar JP, Brito AM, Martins AP, Leufkens HGM, Alves da Costa F. Potentially inappropriate medications with risk of in Beijing, China adverse events in the elderly: A systematic review of tools addressing inappropriate prescribing. J Clin Pharm Ther. 2019;44(3):349–60. doi: 10.1111/jcpt.12811. [DOI] [PubMed] [Google Scholar]
- 36.Curtin D, Gallagher PF, O’Mahony D. Explicit criteria as clinical tools to minimize inappropriate medication use and its consequences. Ther Adv Drug Saf. 2019;10:2042098619829431. doi: 10.1177/2042098619829431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo L, Yang X, He J, Dong B. Evaluation of potentially inappropriate medications in older inpatients in China. J Am Geriatr Soc. 2014;62(11):2216–2218. doi: 10.1111/jgs.13118. [DOI] [PubMed] [Google Scholar]
- 38.Fu M, Wushouer H, Nie X, Shi L, Guan X, Ross-Degnan D. Potentially inappropriate medications among elderly patients in community healthcare institutions in Beijing. China Pharmacoepidemiol Drug Saf. 2020;29(8):923–930. doi: 10.1002/pds.5064. [DOI] [PubMed] [Google Scholar]
- 39.He D, Zhu H, Zhou H, Dong N, Zhang H. Potentially inappropriate medications in Chinese older adults: a comparison of two updated Beers criteria. Int J Clin Pharm. 2021;43(1):229–235. doi: 10.1007/s11096-020-01139-5. [DOI] [PubMed] [Google Scholar]
- 40.Fujie K, Kamei R, Araki R, Hashimoto K. Prescription of potentially inappropriate medications in elderly outpatients: a survey using 2015 Japanese Guidelines. Int J Clin Pharm. 2020;42(2):579–587. doi: 10.1007/s11096-020-00967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horii T, Momo K, Yasu T, Kabeya Y, Atsuda K. Determination of factors affecting medication adherence in type 2 diabetes mellitus patients using a nationwide claim-based database in Japan. PLoS ONE. 2019;14(10):e0223431. doi: 10.1371/journal.pone.0223431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manteuffel M, Williams S, Chen W, Verbrugge RR, Pittman DG, Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt) 2014;23(2):112–119. doi: 10.1089/jwh.2012.3972. [DOI] [PubMed] [Google Scholar]
- 43.Park HJ, Byun MK, Kim HJ, Ahn CM, Rhee CK, Kim K, et al. Regular follow-up visits reduce the risk for asthma exacerbation requiring admission in Korean adults with asthma. Allergy Asthma Clin Immunol. 2018;14:29. doi: 10.1186/s13223-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arif H, Aijaz B, Islam M, Aftab U, Kumar S, Shafqat S. Drug compliance after stroke and myocardial infarction: a comparative study. Neurol India. 2007;55(2):130–135. doi: 10.4103/0028-3886.32783. [DOI] [PubMed] [Google Scholar]
- 45.Punnapurath S, Vijayakumar P, Platty PL, Krishna S, Thomas T. A study of medication compliance in geriatric patients with chronic illness. J Family Med Prim Care. 2021;10(4):1644–1648. doi: 10.4103/jfmpc.jfmpc_1302_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal B, Liao M, Mosca L. Medication adherence is associated with having a caregiver among cardiac patients. Ann Behav Med. 2013;46(2):237–242. doi: 10.1007/s12160-013-9492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan K, Yang C, Zhang H, Ye D, Liu S, Chang J, et al. Impact of the zero-mark-up drug policy on drug-related expenditures and use in public hospitals, 2016–2018: an interrupted time series study in Shaanxi. BMJ Open. 2020;10(11):e037034. doi: 10.1136/bmjopen-2020-037034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araya EM, Gebrezgabiher HA, Tekulu GH, Alema NM, Getnet D, Gebru HT, et al. Medication Non-Adherence and Associated Factors Among Diabetic Patients Visiting General Hospitals in the Eastern Zone of Tigrai, Northern Ethiopia. Patient Prefer Adherence. 2020;14:2071–2083. doi: 10.2147/PPA.S278148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wimmer BC, Cross AJ, Jokanovic N. Clinical outcomes associated with medication regimen complexity in older people: a systematic review. J Am Geriatr Soc. 2017;65(4):747–753. doi: 10.1111/jgs.14682. [DOI] [PubMed] [Google Scholar]
- 50.Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.