Abstract
Background
Polypharmacy and associated potentially inappropriate prescribing (PIP) place a considerable burden on patients and represent a challenge for general practitioners (GPs). Integration of pharmacists within general practice (herein ‘pharmacist integration’) may improve medications management and patient outcomes. This systematic review assessed the effectiveness and costs of pharmacist integration.
Methods
A systematic search of ten databases from inception to January 2021 was conducted. Studies that evaluated the effectiveness or cost of pharmacist integration were included. Eligible interventions were those that targeted medications optimization compared to usual GP care without pharmacist integration (herein ‘usual care’). Primary outcomes were PIP (as measured by PIP screening tools) and number of prescribed medications. Secondary outcomes included health-related quality of life, health service utilization, clinical outcomes, and costs. Randomised controlled trials (RCTs), non-RCTs, interrupted-time-series, controlled before-after trials and health-economic studies were included.
Screening and risk of bias using Cochrane EPOC criteria were conducted by two reviewers independently. A narrative synthesis and meta-analysis of outcomes where possible, were conducted; the certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation approach.
Results
In total, 23 studies (28 full text articles) met the inclusion criteria. In ten of 11 studies, pharmacist integration probably reduced PIP in comparison to usual care (moderate certainty evidence). A meta-analysis of number of medications in seven studies reported a mean difference of -0.80 [-1.17, -0.43], which indicated pharmacist integration probably reduced number of medicines (moderate certainty evidence). It was uncertain whether pharmacist integration improved health-related quality of life because the certainty of evidence was very low. Twelve health-economic studies were included; three investigated cost effectiveness. The outcome measured differed across studies limiting comparisons and making it difficult to make conclusions on cost effectiveness.
Conclusions
Pharmacist integration probably reduced PIP and number of medications however, there was no clear effect on other patient outcomes; and while interventions in a small number of studies appeared to be cost-effective, further robust, well-designed cluster RCTs with economic evaluations are required to determine cost-effectiveness of pharmacist integration.
Trial registration
CRD42019139679.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12875-022-01952-z.
Keywords: Polypharmacy, Potentially inappropriate prescribing, Primary care, Systematic review, Clinical pharmacist, Medication review
Background
Polypharmacy places a considerable burden on both patients and health care providers through an increased risk of PIP, increased treatment burden, adverse outcomes, and medication-related hospitalizations [1]. Polypharmacy is typically defined as using five or more regular medications [2]. A recent systematic review estimated PIP prevalence in primary care to be 33%, with 7% to 17% of all adverse outcomes related to older persons in primary care [3]. Various interventions have been trialled to improve medications optimization, including addressing polypharmacy, PIP and deprescribing (the process of withdrawal, including dose reduction, of an inappropriate medication, supervised by a healthcare professional [4]) with mixed effects being reported [5–7]. Interventions with organizational (pharmacist supported interventions), professional and multifaceted approaches may provide modest benefits [5].
While strategies for pharmacist interventions have been found to have a positive effect on medication-related problems in hospital and nursing home settings [8, 9], the evidence base for pharmacist interventions within the general practice or primary care settings is varied. Barriers have been identified that reduce the ability of community pharmacists to deliver the most effective care to patients and support GPs; these barriers include lack of integration with the general practice team, time restrictions, poor interprofessional communication, lack of access to patients’ medical histories and health policies which discourage collaborative agreements within primary care settings [10]. Therefore, one strategy to address these issues may be pharmacist integration within the general practice team either by co-location (herein ‘co-located integration’) or remotely. The pharmacist may not be present in the same geographical location as the GP but based in a community pharmacy and integrated in terms of a formal pathway for communication of medication review issues with the GP (herein ‘remote integration’).
Co-located integration of pharmacists has been shown to deliver a range of non-dispensing interventions, with medication management reviews being a primary activity [11]. Systematic reviews have reported mixed effects for these interventions on medications optimization outcomes such as level of PIP and deprescribing of inappropriate medications [12, 13]. However, the PINCER trial in the UK demonstrated that such interventions were effective at reducing medication-related errors [14]. Pharmacist integration may also reduce GP workload directly through supports for medication-related administration and management, medications reconciliation following hospital discharge and indirectly though reducing medication related adverse events leading to emergency department attendance and hospitalizations [15]. Issues surrounding heterogeneity, study quality and missing data, make conclusions about the effectiveness of interventions difficult to draw [13].
The evidence base to determine whether such interventions are cost effective also requires further study [12]. The association between polypharmacy and adverse drug events (ADEs) gives rise to substantial costs to both the healthcare system/health service and patients [16]. An estimated 237 million medication errors occur annually in England, with approximately 38% occurring in primary care. Avoidable ADEs resulted in an estimated £96,462,582 cost to the National Health Service (NHS) in 2018 [17]. Where interventions in hospital settings involving pharmacist and physician collaboration can result in cost-avoidance [18], there is little evidence regarding cost-effectiveness within general practice and the primary care setting.
Previous reviews of pharmacist interventions focused solely on co-located integration [12, 13, 15, 19]. This paper systematically updated this evidence and reviewed the literature on the effectiveness and cost of pharmacist integration, to improve prescribing practices and health outcomes for adult patients with polypharmacy in the primary care setting. A secondary aim was to explore and report the domains of integration for these interventions.
Methods
This systematic review was conducted using Cochrane methodology [20] and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. The review was registered on Prospero and a peer-reviewed protocol was published [22].
Data sources and search strategy
An electronic database search was conducted in 10 databases (PubMed, Cochrane Library, Cochrane Central Register of Controlled Trials, EMBASE, Web of Science, SCOPUS, Lilacs and CINAHL) from inception to end of January 2021 using a combination of free text terms, keywords and Medical Subject Headings (MeSH). No language or date restrictions were applied (see Additional file 1).
The systematic literature search for the health-economic studies was conducted in NHS Economic Evaluations Database (NHS EED) and the Health Technology Assessment (HTA) database, and an economic filter was applied to both PubMed and EMBASE. A combination of free text terms, keywords and MeSH terms were applied as above.
Eligibility criteria
Studies were included if they met the following inclusion criteria:
Participants
Community dwelling patients aged 18 years and over in the primary care setting with polypharmacy. Studies had to have a majority of patients (≥ 80%) identified as having polypharmacy (using any definition). Only studies conducted in the primary care setting were included. The definition of primary care for this review was; “integrated, easy to access, health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained and continuous relationship with patients, and practicing in the context of family and community” [23].
Pharmacists involved in medications optimization roles and co-located or remotely integrated. Pharmacist interventions in a nursing home, secondary or tertiary care setting were excluded. Domains of integration were adapted from the framework defined by Walshe and Smith [24], with definitions drawing on a previous systematic review [19], as shown in Table 1. These agreed definitions were that four to six domains of integration indicate robust integration, two to three domains indicate moderate integration, and one domain of integration indicates the minimum level of integration.
Table 1.
Description of integration domains by Walshe and Smith [19]
| Dimension | Definition |
|---|---|
| Organizational | Pharmacist is physically co-located with the GP or, the intervention is remote but encompassed within the same network |
| Informational | Integration and access to clinical patient systems |
| Clinical | Care delivery to patients and communication with GPs |
| Functional | Capture of other actions taken by pharmacists integrated within GP settings such as medications education or administrative support |
| Normative | Design of intervention in terms of shared goals and visions of activities involved and desired outcomes |
| Financial | Financial implications from internally funded pharmacist interventions |
Intervention
‘Pharmacist integration’ defined as all types of interventions targeted at patient or prescriber behaviours involving a pharmacist aiming to optimize medications for patients in a primary care setting were considered for inclusion. The relationship between the pharmacist and the GP could be conducted by co-located integration or by remote integration providing the relationship continued for the duration of the intervention.
Control
Usual GP care that did not include pharmacist integration.
Study design
As per the Cochrane Effective Practice and Organisation of Care (EPOC) study design criteria for effects of interventions [25], we included randomised controlled trials (RCTs), cluster RCTs (cRCTs) non-randomised controlled trials (nRCTs), controlled before-after studies (CBA) and interrupted time series (ITS) studies. Health-economic studies including comparative resource use studies and health-economic evaluations (cost-effectiveness analysis, cost-utility analysis, cost-minimization analysis, and cost–benefit analysis) were also eligible for inclusion.
Outcomes
The primary outcomes for this review included:
PIP or high risk prescriptions as reported by included studies. Studies reported potentially inappropriate or high risk prescriptions using screening tools such as; Screening Tool of Older Person’s Prescriptions / Screening Tool to Alert doctors to Right Treatment (STOPP/START) and Beers criteria (explicit criteria), or the Medications Appropriateness Index (MAI), Prescribing Appropriateness Index and Drug Burden Index (DBI) (implicit criteria).
The per-patient number of medications prescribed and change in the number of medications prescribed as reported by included studies. The definition varied across studies (e.g. some may use the number of repeat medications), however where possible we used the number of medications including acute and repeat prescribed medications.
Secondary outcomes included:
Health-related quality of life (HRQoL)
Adverse events or harms
Health service utilization
Clinical physical outcomes
Mental health outcomes
Comparative resource use, costs and cost-effectiveness
Study selection and data extraction
Citations were downloaded to Endnote [26] and duplicates removed. Titles were screened for clearly ineligible studies by one researcher (AC). Remaining titles and abstracts were independently screened using Rayyan SoFtware [27], by at least two of the three members of the review panel (AC, OJ and KC). Full text suitability for inclusion was independently determined by two researchers (AC and KC). Disagreement was managed by consulting a third reviewer (FM).
Data were extracted by two reviewers (AC and KC) on name of first author, year of publication, country of publication, study setting; study population and participant demographics, intervention details and design including framework of integration elements, control, setting details, and outcomes.
Data synthesis
Interventions were assessed for the six dimensions of integration dichotomously (yes/no).
Due to the heterogeneity relating to the wide variation in participants, interventions and outcomes assessed, the main synthesis of the results is presented narratively. A meta‐analysis using inverse variance with random effects statistical models for continuous variables with mean difference effect measures was conducted for one of the primary outcomes, number of medications, using data from eligible RCTs only. Heterogeneity was assessed using the I2 statistic, the percentage of variability in the estimates due to heterogeneity, and interpreted as per the Cochrane Handbook, 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity [20].
Subgroup analysis was based on location of intervention (co-located vs remote integration) and degree of polypharmacy. It was not possible to conduct subgroup analysis based on age of patients given the data presented in studies.
The costs for health-economic studies were inflated to 2021 prices using the Consumer Price Index (CPI) for each individual country and converted to euro (where appropriate) using the purchasing power parity (PPP) indices by the Organization for Economic Co-operation and Development (OECD).
Sensitivity analyses for estimates of effect size and determinants were not assessed owing to limitations in the data reported for studies.
Risk of bias
The risk of bias in all included effectiveness studies was assessed by two reviewers (AC and KC) using standard EPOC criteria [25] including the following domains: allocation (sequence generation and concealment); baseline characteristics; incomplete outcome data; contamination; blinding; selective outcome reporting; and other potential sources of bias. Robvis online SoFtware was used to generate risk of bias Figs. [28]. The health-economic studies were assessed for methodological quality using the Consensus on Health Economic Criteria (CHEC) [29] list by one reviewer (AC).
Assessing quality of included studies
The certainty of evidence for five critical and important outcomes was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria and GRADEPro SoFtware and judgements are presented in a 'Summary of findings' (SOF) table [30]. The five outcomes assessed were PIP, number of medications, ADEs, HRQoL and mortality. These outcomes were selected in accordance with the Core Outcome Set (COS) for Trials Aimed at Improving the Appropriateness of Polypharmacy in Older People in Primary Care [31].
Results
Search results
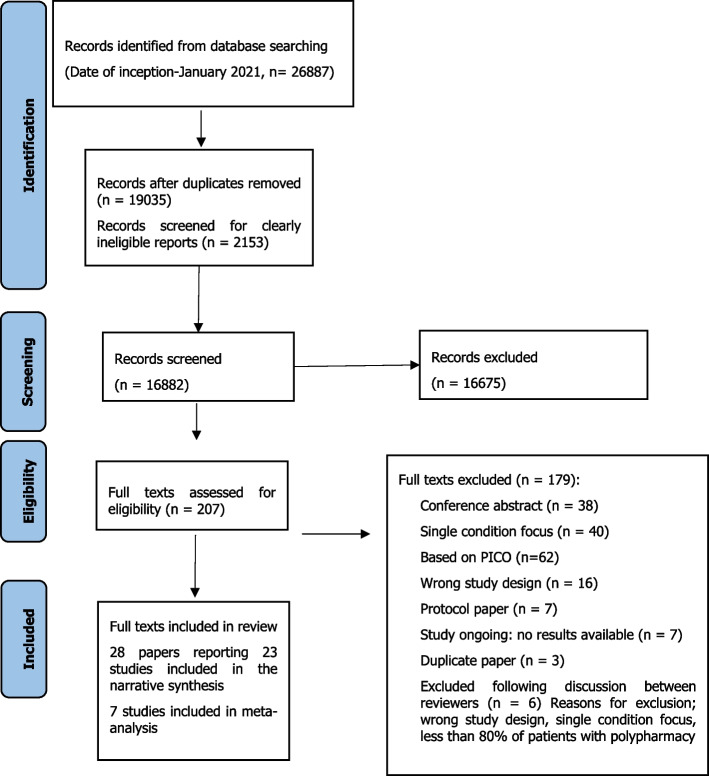
A total of 26,887 articles were retrieved up to the end of January 2021. Full texts of 207 articles were assessed for eligibility and 28 full texts were included in the systematic review (Fig. 1).
Fig. 1.
Prisma flow chart for included studies
Characteristics of included studies
Included studies, participants and outcomes reported
A total of 23 studies were reported across 28 articles. Seven studies were conducted in North America [32–38], three in the United Kingdom (UK) [39–41], ten in other European countries [42–51], and three in New Zealand or Australia [52–54] (Table 2). The age range of the 23,516 included participants was 1 to 102 years of age (one study reported ages from 1 to 102, however median age in that study was 65 years so study was included [39]) and the number of medications prescribed per person ranged from 3 to 27. In addition to the broad review inclusion criteria of polypharmacy, three studies had further inclusion criteria relating to high frequency of daily dosing (≥ 12 doses per day) and drugs that required monitoring [33, 37, 53]. Two studies included participants with more than three current disease states [33, 37] and one study included patients with 50 or more prescriptions filled in the previous year (100% correlation to 5 or more medications) [35].
Table 2.
Characteristics of included effectiveness studies
| Author (year, Country) |
Participants (Patients and healthcare professionals (HCPs)) |
Intervention type, Control group, Domains of integration, Duration of intervention |
Outcome measures | Results primary outcome |
|---|---|---|---|---|
| Randomised Controlled Trials | ||||
|
Britton [32] (1991, USA) |
Total, n: 572 Age: NR Number of medications: Intervention: 8.72 3.54 Control: 8.52 3.47 HCPs: NR |
Intervention type: Pharmacist reviewed patient treatment and cost. Medication profile review form attached to each patient file for review by GP. Post GP consult, the pharmacist reviewed files for no. meds, meds changes and compliance Control group: usual GP care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 3 months |
Primary Change in number of medications Change in cost due to no. of medications Secondary Discontinued medications no. and cost Medications added Dose change Cost |
Number of medications at follow-up (mean standard deviation (SD)): Intervention: -0.21 (1.43) Control: + 0.48 (1.18) P < 0.001 |
| Bryant [52] (2011, New Zealand) |
Total, n: 493 Age (range): Intervention, 75.9 (64–92) Control, 74.9 (60–91) Number of medications: Intervention: NR Control: NR HCPs: 44 pharmacists |
Intervention type: Patients had pharmaceutical care consultation with community pharmacist. Recommendations made to GP. The study pharmacist followed up with the patient clinically at 3, 6 and 12 months, updating of the pharmaceutical care plan as needed Control group: Wait-list control Domains of integration: Informational and clinical domains (moderate) Duration of intervention: 12 months |
Primary MAI HRQoL, SF-36 Secondary No. of inappropriate medications Change in medications Recommendation implementation by GP |
Mean MAI at 6-month follow-up: Intervention, 3.1 Control, 4.2 Mean difference at 6 months, 1.1 (95% CI -1.78 to -0.42, p = 0.003) SF-36: Emotional role: 13.4-unit difference, P = 0.024 Favouring control Social functioning: 7.7-unit difference, P = 0.019 Favouring control Change in social functioning not clinically significant change |
| Campins [42] (2017, Spain) |
Total, n: 503 Age, mean (SD): Intervention, 79.16 (5.5) Control, 78.78 (5.46) Number of medications: Intervention: 10.79 (2.52) Control: 10.91 (2.65) HCPs: NR |
Intervention type: Pharmacist performed chart review. Pharmacist discussed recommendations with GP and therapeutic plan made. Recommendations were discussed with the patient, and a final decision was agreed by physicians and their patients in a face-to-face visit Control group: Usual GP care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 12 months |
Primary No. medications Secondary Medication appropriateness Intervention Effectiveness Change in medications Adherence (Morisky-Green) HRQoL, EQ5D Healthcare utilization Mortality |
Mean number of medications at follow up: Intervention,10.03 Control, 10.91 P = 0.001 |
| Geurts [43] (2016, The Netherlands) |
Total, n: 512 Age (mean, SD): Intervention: 72.5 (7.735), Control: 73.1 (7.797) Number of medications: Intervention: 8.3 (2.721) Control: 7.9 (2.926) HCPs: 8 pharmacies/primary care sites |
Intervention type: Community pharmacists had clinical medication review with patients. Recommendations made to GP. Implemented care intervention Control group: usual care Domains of integration: Organizational, informational, clinical, and normative domains (robust) Duration of intervention: 18 months |
Primary Decrease in potential DRPs and PCIs Secondary Biomarkers for: Blood pressure, dyslipidaemia, BMI, diabetes and renal function |
Total number of DRPs and PCIs at follow-up: Intervention, 208 Control not reported |
| Graffen [53] (2004, Australia) |
Total, n: 402 Age (median, range): 77.7 (66–102) Number of medications (mean): 8.4 HCPs: 8 general practices |
Intervention type: Pharmacist reviewed medication profiles of rural patients in practice and made recommendations to GP. Changes were made by agreement between patient and GP Control group: usual care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 18 months |
Primary HRQoL SF-36 Secondary Recommendation uptake Hospitalizations |
SF-36 Significant difference in vitality (p < 0.009) and mental health (p < 0.0001) in favour of intervention post-intervention |
| Granas [39] (1998, UK) |
Total, n: 285 Age (median, range): 65 (1—102): Number of medications (median, range): 5 (3–27) HCPs: 1 community pharmacist 2 GPs |
Intervention type: Pharmacist performed chart-based medication review in practice. Recommendations made to GP. GP followed up with patient Control group: Usual care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 24 months |
Primary DRPs Secondary Recommendation uptake |
DRPs at follow-up Intervention, 7.8% Control, 11.6% Statistically significant difference (p < 0.001) Adjusted ARR, 26% |
| Hanlon [34] (1996, USA) |
Total, n: 208 Age (mean, SD): Intervention, 69.7 (3.5) Control, 69.9 (4.1) Number of medications (mean (SD)): Intervention: 7.6 (2.8) Control: 8.2 (2.7) HCPs: 2 pharmacists |
Intervention type: Comprehensive medication review with the pharmacist in practice and follow-up at 11.5–13 months Control group: usual care with closeout interview by second pharmacist blinded to allocation Domains of integration: Organizational, informational, clinical, and financial domains (robust) Duration of intervention: 13 months |
Primary Prescribing appropriateness; MAI Secondary Improvement in inappropriate prescribing Medication compliance Medication knowledge Number of medications HrQoL, SF-36 |
Adjusted MAI at 12 months (mean, SD) Intervention, 12.8 (0.7) Control, 16.7 (0.7) Inappropriate prescribing scores declined more in intervention than control at 12 months: 28% vs 5% (p = 0.0002) |
| Jameson [35] (2001, USA) |
Total, n: 168 Age: Intervention: 51.4 (10.1) Control: 52.5 (10.6) Number of medications: NR HCPs: 1 pharmacist 133 physicians (in 4 practices) |
Intervention type: Medication review with patient in practice. Discussed DRPs with GP. Care plan developed with GP. Changes discussed with patient Control group: Usual care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 9 months |
Primary Medical/drug costs Secondary Adverse effects |
Medication cost at follow-up $ Intervention: 1657 (1068) Control: 1602 (1202) No significant change |
| Krska [40] (2001, UK) |
Total, n: 381 Age (mean, range): Intervention: 74.8 (65–90), Control: 75.2 (65–93) Number of medications (mean, SD): Intervention: 7.4 ± 2.7 Control: 7.7 ± 2.8 HCPs: NR |
Intervention type: Pharmacist performed chart review followed by medication review with patient in their own home. Pharmaceutical care plan developed in practice. Notes forwarded to GP who indicated level of agreement. Implemented actions where appropriate Control group: Usual care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 3 months |
Primary Pharmaceutical care issues Secondary Medication costs HRQoL, SF-36 Healthcare utilization |
PCIs at 3 months Intervention: 256 (21.2%) Control: 856 (60.7%) Crude ARR: 39.5% |
| Kwint [44] (2011, The Netherlands) |
Total, n: 118 Age (mean ± SD): Intervention: 78.7 (6.8) Control: 80.0 (7.2) Number of medications (mean ± SD): Intervention: 10.3 (3.1) Control: 9.8 (3.6) HCPs: 6 community pharmacists; each community pharmacist recruited two GPs |
Intervention type: Community pharmacist collated information from pharmacy and GP. Data reviewed by 2 pharmacists. Results sent to community pharmacist to discuss with GP Control group: Wait-list control Domains of integration: Informational and clinical domains (moderate) Duration of intervention: 6 months |
Primary DRPs Secondary Medication changes Recommendation’s uptake |
DRPs at baseline Intervention: 4.5 Control: 4.4 DRPs at 6 months Intervention: 3.2 Control: 4.2 |
| Lenaghan [41] (2007, UK) |
Total, n: 136 Age: 84.3 Number of medications: 9.45 HCPs: 1 community pharmacist 9 GPs based in one practice |
Intervention type: Comprehensive medication review in patient’s home. Community pharmacist had notes from both pharmacy and medical notes. Recommendations discussed and implemented with GP and discussed with patients. Further follow-up at 6–8 weeks Control group: Wait-list control Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 6 months |
Primary Unplanned hospital admissions Secondary Mortality HRQoL, EQ5D No. of medications |
Hospitalizations at follow-up: Intervention, 21 Control, 20 P = 0.8 |
| Sellors [36] (2003, Canada) |
Total, n: 889 Age (mean, SD): Intervention: 74.0 (6.1) Control group: 74.0 (6.0) Number of medications (mean): Intervention: 12.4 Control: 12.2 HCPs: n = 24 pharmacists n = 48 physicians |
Intervention type: Community pharmacists (who had additional post-university training in the prevention, identification, and resolution of drug-related problems) conducted face-to-face medication reviews with the patients and then gave written recommendations to the physicians to resolve any drug-related problems Control group: Usual care Domains of integration: Organizational, informational, clinical, and financial domains (robust) Duration of intervention: 5 months |
Primary DRPs Secondary Uptake of recommendations Length of physician meeting Reasons for not implementing recommendations Cost HRQoL, SF-36 |
Recommendations uptake at follow-up: Fully implemented: 46.3% (506/1093) Partially implemented: 9.3% (102/1093) Unsuccessful implementation: 16.7% (182/1093) |
| Taylor [37] (2003, USA) |
Total, n: 69 Age (mean, SD): Intervention: 64.4 (13.7) Control: 66.7 (12.3) Number of medications (mean, SD): Intervention: 6.3 (2.2) Control: 5.7 (1.7) HCPs: 4 pharmacists |
Intervention type: Patients in the intervention group received usual care plus pharmacotherapeutic interventions by a pharmacist during regularly scheduled office visits before seeing a physician. Pharmacist developed education packs for patients with diabetes, hypertension, dyslipidaemia and anti-coagulation services Control group: Medical records review at baseline and 12 months later performed by pharmacist, no advice, or recommendations to patient/physician Domains of integration: Organizational, informational, clinical, and financial domains (robust) Duration of intervention: 12 months |
Primary Prescribing appropriateness (MAI) and ADRs Secondary Hospitalizations and ED visits Hypertension outcomes Biomarkers for: Diabetes, dyslipidaemia, blood pressure and anticoagulation services HRQoL, SF-36 |
Inappropriate prescriptions at baseline (MAI) Intervention: 210 Control: 207 Inappropriate prescriptions at follow-up Intervention: 155 Control: 224 Ratings for inappropriate prescribing improved in all 10 domains evaluated in the intervention group but worsened in 5 domains in the control group |
| VanDerMeer [45] (2018, The Netherlands) |
Total, n: 157 Age (mean, SD): Intervention: 75.7 (6.9) Control: 76.6 (6.7) Number of medications (mean, SD): Intervention: 8.4 (2.4) Control: 9.3 (3.2) HCPs: 15 community pharmacies |
Intervention type: Pharmacotherapeutic review with community pharmacist. Written recommendations forwarded to GP followed by an MDT meeting where an action plan was decided. This was discussed with the patient and decisions taken were followed up Control group: Wait-list control Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 3 months |
Primary Change in DBI Secondary Presence of anticholinergic/sedative side effects Falls Cognitive function Activities of daily living HRQoL, EQ5D-3L Hospital admission Mortality |
Proportion of patients with a decrease of DBI ≥ 0.5 at follow up Intervention: 17.3% Control: 15.9% OR 1.04, CI 0.47 to 2.64, p = 0.927) |
| Verdoorn [46] (2019, The Netherlands) |
Total, n: 629 Age (range): Intervention: 80 (76–83) Control: 78 (74–82) Number of medications (median, IQR): 9.0 (7.5–10.5) HCPs: 43 pharmacists 113 GPs |
Intervention type: Patients received a clinical medical review from community pharmacist who had both pharmacy data and medical history. Potential DRPs reported and discussed face-to-face with GP. PCP proposed and actions agreed. Two follow-up appointments to review changes made Control group: Wait-list control Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 6 months |
Primary EQ5D-5L EQ5D-VAS No. of health problems Secondary Number of medications Medications commenced or discontinued |
EQ5D-5L at follow up: Intervention, 0.73 (0.20) Control, 0.74 (0.18) EQ5D-VAS at follow up: Intervention, 70 (16) Control, 69 (15) HR-QoL measured with EQ-VAS increased by 3.4 points (95% confidence interval [CI] 0.94 to 5.8; p = 0.006), Number of health problems with impact on daily life: Decreased by 12% in intervention (difference at 6 months − 0.34; 95% CI − 0.62 to − 0.044; p = 0.024) as compared with the control group |
| Vinks [47] (2009, The Netherlands) |
Total, n: 174 Age (mean, SD): Intervention: 76.6 (6.5) Control: 76.6 (6.4) Number of medications (mean, SD): Intervention: 8.8 (2.5) Control: 8.5 (2.3) HCPs: 16 pharmacies |
Intervention type: Community pharmacists reviewed patients’ medications and compiled a list of recommended changes in medication was compiled by the pharmacist for the patients in the intervention group. Recommendations for medication change were discussed with the general practitioner (GP). Repeat screening conducted at follow-up Control group: Usual care Domains of integration: Informational and clinical domains (moderate) Duration of intervention: 12 months |
Primary DRPs Secondary No. of medications Recommendations and uptake |
Mean DRPs at follow up Intervention: 3.29 Control: 3.62 Mean difference –16.3%; 95% CI –24.3, –8.3) |
| Zillich [38] (2014, USA) |
Total, n: 961 Age (mean, SD): 73 (13) Number of medications: Intervention: 14 (11) Control: 13 (8) HCPs: NR |
Intervention type: Nurse completed admission procedures and forwarded meds info to MTM intervention provider. Pharmacy technician called to verify all meds. Community pharmacist then rang and completed comprehensive med review with patient/carer. Developed medication related action plan. Pharmacist provided follow-up phone call on day 7, and again at day 30 and 60 as needed Control group: Usual home health care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 60 days |
Primary 60-day hospitalizations Secondary MRPs Recommendation uptake |
Hospitalizations at follow up: Intervention: 83 Control: 112 Adjusted OR: 1.26, 95 percent CI: 0.89–1.77, p = 0.19) |
| Cluster Randomised Controlled Trials | ||||
|
Bernsten (2001) [48] Multiple sites: (seven EU countries) |
Total, n: 2454 Age (median, IQR): 74 (8) Number of medications: Intervention: 7.05 (2.51) Control: 6.97 (2.51) HCPs: 190 community pharmacies |
Intervention type: Pharmacists completed study training and informed local GPs and formed formal links. Patient received pharmaceutical care intervention in collaboration with GPs Control group: Usual care Domains of integration: Clinical domain (minimum) Duration of intervention: 18 months |
Primary HRQoL; SF-36 Secondary: Hospitalizations Cost Compliance Recommendation acceptance |
HRQoL: A general decline in health-related quality of life over time was observed in the pooled data; however, improvements were achieved in patients involved in the pharmaceutical care programme in some countries |
| Sorensen [54] (2004, Australia) |
Total, n: 400 Age (mean, range): Intervention 72.3 (37–100), control 71.4 (25–99) Number of medications: Intervention: 9.7 (9.1–10.3) Control: 8.9 (8.3–9.4) HCPs: n = 53 pharmacists n = 92 GPs |
Intervention type: GPs were the units of randomization. GPs made referrals to the community pharmacist who conducted medication review based on pharmacy data and medical records. Prepared report for GP, recommendations discussed at MDT meeting and action plan developed. GP implemented plan with patient agreement Control group: Usual care Domains of integration: Organizational and clinical domains (moderate) Duration of intervention: 6 months |
Primary DUSOI-A Secondary Problems and recommendations HRQoL, SF-36 ADEs Costs |
Change in DUSOI-A at follow up: Intervention, reduced by 4.92 Control, reduced by 1.34 |
| Varas-Doval [49] (2020, Spain) |
Total, n: 1403 Age (mean, SD): Intervention: 75.34 (6.46) Control: 74.92 (6.59) Number of medications (mean, SD): Intervention: 7.74 (2.5) Control: 7.39 (2.37) HCPs: n = 178 pharmacies, n = 250 pharmacists |
Intervention type: Community pharmacists provided Medication review with follow up (MRF). Pharmacist communicated with GPs via face to face or telephone. Follow up on a monthly basis for duration of the intervention Control group: Usual care Domains of integration: Organizational and clinical domains (moderate) Duration of intervention: 8 months |
Primary Uncontrolled health problems Secondary DRPs Interventions made by pharmacists |
Uncontrolled health problems at follow up [mean (95% CI)]: Intervention: 0.65 (0.43, 0.88) Control: 0.69 (0.47, 0.91) Reduction in the number of uncontrolled health problems: Intervention: -0.72 (95% CI: -0.80, -0.65) Control: -0.03 (95%CI: -0.10, 0.04) |
| Non-Randomised Controlled Trials | ||||
| Leendertse [50] (2013, The Netherlands) |
Total, n: 674 Age (95% CI): Intervention: 75.8* (74.9–76.4) Control: 75.7* (75.1–76.7) Number of medications: Intervention: 7.8* (7.7- 8.2) Control: 7.9* (7.5 – 8.2) HCPs: 42 primary health care settings. 1 intervention and 1 control GP. Number of GPs and pharmacists not reported * Values are results of the linear mixed-effects model |
Intervention type: In each practice, patients were recruited from community pharmacy, Community pharmacist conducted structured pharmacotherapeutic review with patient based on pharmacy history and medical notes. Pharmacist and GP met to discuss PCP. Agreed changes implemented and monitored by GP/practice nurse Control group: Usual care Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 12 months |
Primary Medication-related hospitalizations Secondary Survival HRQoL, EQ5D ADEs Drug therapy problems and care issues Interventions recommended |
Hospitalizations at follow-up: Intervention: 6 Control: 10 Reduction in hospitalizations in intervention 1.6% vs 3.2% (HR 0.50, 95%CI 0.12–1.59) |
| Controlled Before-After | ||||
| Sloeserwij [51] (2019, The Netherlands) |
Total, n: 11,928 Age (mean, SD): 75 (8) Number of medications: 6 (5–8) HCPs: 9 pharmacists 25 general practices |
Intervention type: Pharmacists embedded in general practices for three months prior to intervention. Pharmaceutical care for high-risk patients- pharmacist performed medication reviews with patients and medications reconciliation amongst other practice-related activities Control group: Usual care was normal GP review. Usual care plus was medication review conducted by accredited community pharmacist Domains of integration: Organizational, informational, and clinical domains (moderate) Duration of intervention: 12 months |
Primary Medication-related hospitalizations Secondary DBI Costs |
Medication-related hospitalizations at follow up: Intervention: 230 Usual care: 355 Usual care plus: 237 Rate ratio of medication‐related hospitalizations in the intervention group compared to usual care was 0.68 (95% CI: 0.57–0.82) and 1.05 (95% CI: 0.73–1.52) compared to usual care plus |
Text highlighted in bold indicate main column headings. Text highlighted in italics are subheadings located within a column
Key; HCP healthcare professional, RCT randomised controlled trial, CBA controlled before-after trial, HRQoL health related quality of life, SF-36 short form 36, MAI medications appropriateness index, EQ5D EuroQoL 5 domains, HbA1c haemoglobin A1C, LDL (low-density lipoprotein), DRP (drug related problem), ATC (Anatomical Therapeutic Chemical), PCI (pharmaceutical care issue), ARR (absolute risk reduction), NNT (number needed to treat), NR, not reported; HR (hazard ratio), CI (confidence interval), ITS (interrupted time series), PCT (primary care trust) DBI (drug burden index), MDT (multi-disciplinary team), DUSOI-A (Duke’s Severity of Illness Visual Analogue Scale), MRP (medication-related problem), VAS (visual analogue scale), MTM (medications therapeutic management), PDTP (potential drug therapy problem), CBA (controlled before-after)
Formal training qualifications or requirements for the pharmacists were outlined in five studies [32, 41, 46, 51, 52], one study detailed training provided to GPs and pharmacists by the study team [54] and two studies stated prior experience of clinical training of pharmacist(s) was required [40, 42].
Three of the 23 studies had cluster randomised designs [48, 49, 54], 18 studies had an individual patient randomised design, one study had a non-randomised design, and one adopted a controlled before-after design. Eleven studies recorded PIP outcomes [34, 37, 39, 40, 42–45, 47, 51, 52], and nine studies reported on differences in number of medications between pharmacist integration and usual care groups [32, 34, 37, 41, 42, 46–48, 55]. Review secondary outcomes included 15 studies (16 articles) which reported on HRQoL; nine studies reported Short Form 36 Health Survey Questionnaire (SF36) [33, 34, 36, 37, 40, 48, 52–54] and six studies (seven articles) reported EuroQol-5D (EQ5D) [41, 42, 45, 46, 49, 50, 55]. Five studies reported on ADEs [35, 37, 45, 50, 54], 10 studies reported on health service utilization [37, 38, 40–42, 45, 48, 50, 51, 53] and three studies reported on clinical physical outcomes [33, 37, 43]. No study reported mental health outcomes. Government bodies, university departments, or professional bodies funded all studies.
Interventions and comparators
All studies reported a pharmacist conducting a medication review with patients to optimize prescribing, nine reported co-located integration [32–35, 37, 39, 40, 51, 53] and 14 studies reported remote integration [36, 38, 41–50, 52, 54]. Intervention duration ranged from 60 days to 24 months.
Eighteen studies compared pharmacist integration with a comparator described as ‘usual care’ [32–40, 42, 43, 47–51, 53, 54]. In all, ‘usual care’ was considered to be standard best practice with no pharmacist integration. Of the 23 included studies, five studies adopted a wait-list control [41, 44–46, 52].
Some of the included studies took place in health systems in which pharmacists have prescribing rights, for example, in North America and the UK, others did not (New Zealand, the Netherlands). In terms of activities undertaken by the pharmacist, eight studies (five were co-located [32, 39, 40, 42, 53] and three were remotely integrated [44, 47, 54]) involved a chart-based patient review, the results of which were forwarded to the GP. One of the eight studies involved both chart-based review and face-to-face medication review with the patient [40]. One study looked at the impact of a medication review conducted by a remotely integrated pharmacist over the telephone [38]. The remaining 13 studies all involved a face-to-face medication review with the pharmacist (four were co-located [34, 35, 37, 51] and nine were remotely integrated [36, 41, 43, 45, 46, 48–50, 52]).
Characteristics of included health-economic studies
Twelve health-economic studies were included in the review, four were conducted in the US [32, 35, 56, 57], three were conducted in Spain [55, 58, 59], one was conducted in multiple EU countries [48], one in the UK [40], one in Canada [36], one in the Netherlands [51] and one in Australia [54]. All health-economic studies were further analyses of 11 primary studies already included in the systematic review (Table 3). Three studies presented cost-effectiveness analyses; two were cost-effectiveness analyses (CEA) [54, 56] and one was a cost-utility analysis (CUA) [55]. One study presented a cost–benefit analysis [58] and nine studies detailed cost [32, 35, 36, 40, 48, 51, 57–59].
Table 3.
Characteristics of included economic studies
| Author (year, study) | Country perspective | Analysis type | Follow-up/time horizon | Control | Intervention | Results |
|---|---|---|---|---|---|---|
| Campins (2019, Campins study) | Third party payer, public, Spain | CBA | 12 months post-intervention | Usual GP care | Pharmacist performed chart review. Pharmacist discussed recommendations with GP and therapeutic plan made. Recommendations were discussed with the patient, and a final decision was agreed by physicians and their patients in a face-to-face visit |
Drug expenditure savings per patient/year attributable to intervention: €88.42 Drug costs reduction: Intervention: €321.43 (CI = 233.77–409.79) Control: €232.94 (CI = 141.64–323.15) Intervention cost: €37.17 The estimated return per Euro invested in the programme: €3.27 per patient a year on average |
| Cowper (1998, Hanlon study) | Third party payer, public, US | CEA | 12 months | Usual care with closeout interview by second pharmacist blinded to allocation | Comprehensive medication review with the pharmacist in practice and follow-up at 11.5–13 months |
Cost-effectiveness as reported by MAI score: €1122.64/one unit change (estimated by researchers) |
| Jodar-Sanchez (2015, Varas-Doval study) | Third party payer, public, Spain | CUA | 12 months | Usual care | Community pharmacists provided Medication review with follow up (MRF). Pharmacist communicated with GPs via face to face or telephone. Follow up on a monthly basis for duration of the intervention |
Incremental effectiveness (intervention vs control) 0.0156 QALYs (SD = 0.004) (95% CI 0.008– 0.023) Mean incremental total cost of intervention -€321.88 ± 190.95 (95% CI -696.14 to 52.37) |
| Noain (2017, Varas-Doval study) | Third party payer, public, Spain | Micro-costing, cost analysis | 12 months | Usual care | Community pharmacists provided medication review to patients with follow-up with GPs and patients. Same study as Jodar-Sanchez above |
Service provider cost per patient ranged from €251.72 (SD 90.5) to €398.12 (SD 164.4) The mean initial investment per pharmacy was €5899.92 and the mean annual maintenance costs €3940.13 The potential service price ranged from €304.37 to €806.52 per patient a year |
| Malone (2000, Carter study) | Third party payer, public, US | Cost analysis | Not reported, costs calculated for 12 months prior to trial and 12 months of trial | Usual GP care | Patients were assessed by pharmacists at least three times (baseline, 6 & 12 months) but could see the patients as often as was medically indicated. Documentation via a standardised form was used for all patient encounters. Pharmacists saw patients in clinic or spoke on the phone. Discussed recommendations with GP | Mean increases in total health care costs were €1553.89 for the intervention group and €2000.25 for the control group (p = 0.06) |
| Effectiveness studies with cost and cost-effectiveness data | ||||||
| Bernsten (2001) | Third party payer, pan-European study, reported from Sweden | Cost analysis | 18-month trial period | Usual care | Pharmacists completed study training and informed local GPs and formed formal links. Patient received pharmaceutical care intervention in collaboration with GPs |
Total cost: Between-group analysis indicated that there were no significant differences between the total cost for control and intervention patients in any country (Mann–Whitney, p > 0.05) Hospitalisations Germany, intervention patients had significantly lower costs associated with hospitalisations and contacts with specialists compared with control patients in the second 6-month period (Mann–Whitney test, p < 0.05) Cost savings Cost savings were achieved in most of the countries at each assessment period |
| Britton (1991) | Third party payer, US | Cost analysis | 3-month trial period | Usual care | Pharmacist reviewed patient treatment and cost. Medication profile review form attached to each patient file for review by GP. Post GP consult, the pharmacist reviewed files for no. meds, meds changes and compliance |
Intervention total cost savings: €287.93 Control total cost savings: costs increased by €1295.93 Intervention total cost avoidance: €1588.39 |
| Jameson (2001) | Third party payer, US | Cost analysis | 9-month trial period | Usual care | Medication review with patient in practice. Discussed DRPs with GP. Care plan developed with GP. Changes discussed with patient |
No significant differences were demonstrated in the changes in medical or drug costs between the consult and the control groups Drug Changes at follow-up Intervention: Change €73.87 (904.06) Control: Change €23.45 (11,257.98) Medical changes at follow-up Intervention: Change €632.02 (11,716.46) Control: Change €367.02 (12,231.22) |
| Krska (2001) | Third party payer, UK | Cost analysis | 3-month trial period | Usual care | Pharmacist performed chart review followed by medication review with patient in their own home. Pharmaceutical care plan developed in practice. Notes forwarded to GP who indicated level of agreement. Implemented actions where appropriate |
No significant difference at baseline or follow-up Intervention Medication Costs (mean, SD) Baseline: €76.27 (56.52) Follow-up: €75.49 (57.55) Control Medication Costs Baseline: €83.21 (65.13) Follow-up: €82.84 (61.90) |
| Sellors (2003) | Third party payer, Canada | Cost analysis | 5-month trial period | Usual care | Community pharmacists (who had additional post-university training in the prevention, identification, and resolution of drug-related problems) conducted face-to-face medication reviews with the patients and then gave written recommendations to the physicians to resolve any drug-related problems |
Mean daily cost of medications per patient (intervention vs control) €4.10 vs €3.94 (p = 0.72) Mean total cost of medications per patient (intervention vs control) €2.92 vs €3.08 (p = 0.78) Mean cost of healthcare resources per patient (intervention vs control) €1047.97 vs €1062.78 (p = 0.45) |
| Sloeserwij (2019) | Third party payer, Netherlands | Cost analysis | 12-month trial period | Usual care was normal GP review. Usual care plus was medication review conducted by accredited community pharmacist | Pharmacists embedded in general practices for three months prior to intervention. Pharmaceutical care for high-risk patients- pharmacist performed medication reviews with patients and medications reconciliation amongst other practice-related activities |
Intervention vs usual care only. No difference in healthcare or medication costs reported Ratio of healthcare costs in intervention group vs usual care group (95% CI) Primary care costs: 1.08 (0.99–1.17) p = 0.073 Secondary care costs: 0.92 (0.65–1.29) p = 0.622 Medication costs: 1.04 (0.98–1.10) p = 0.172 Secondary healthcare costs related to hospitalisations: No differences: adjusted ratio 0.82 (95% CI 0.64–1.06) |
| Sorensen (2004) | Third party payer, Australia | CEA | 6-month trial period | Usual care | GPs were the units of randomization. GPs made referrals to the community pharmacist who conducted medication review based on pharmacy data and medical records. Prepared report for GP, recommendations discussed at MDT meeting and action plan developed. GP implemented plan with patient agreement |
The cost–effectiveness ratio for the intervention based on cost savings, reduced adverse events and improved health outcomes was small Cumulative cost/patient over the 8 months from enrolment Intervention: €5806.45 Control: €6160.15 Net cost saving per intervention patient (marginal cost benefit) was €58.05 per patient relative to controls Incremental cost–effectiveness ratio in reducing ADEs and in improving DUSOI-A for the groups ADEs: €74.18 DUSOI-A: €69.88 |
Text highlighted in bold indicate main column headings. Text highlighted in italics are subheadings located within a column
Key: CBA (cost benefit analysis), CI (confidence interval), CEA (cost-effectiveness analysis), CUA (cost utility analysis), MRF (medication review with follow up), MAI (medications appropriateness index), QALY (quality adjusted life years), SD (standard deviation), DRPs (drug related problems), ADEs (adverse drug events), DUSOI-A (Duke’s Severity of Illness Visual Analogue Scale)
The age of participants ranged from 25 to 100 years. Six studies outlined co-located integration [35, 40, 51, 56–58] and five studies (six articles) investigated remote integration [32, 36, 48, 54, 55, 59]. All studies adopted a third-party payer perspective. Four studies adopted a 12-month time horizon [55, 56, 58, 59] and one study detailed costs for 12 months before and after the intervention [57]. Seven studies did not state a specific time horizon but outlined that data was collected in line with intervention duration [32, 35, 36, 40, 48, 51, 54].
Domains of integration in included effectiveness studies
Five studies had robust integration (organizational, informational, clinical, and financial) [33, 34, 36, 37, 43]. Seventeen studies had moderate integration [32, 35, 38–42, 44–47, 49–54]. One study had a minimum level of integration [48]. Details of domains of integration associated with different studies are outlined in Table 2 and summarized in Additional file 2.
Risk-of-Bias Summary
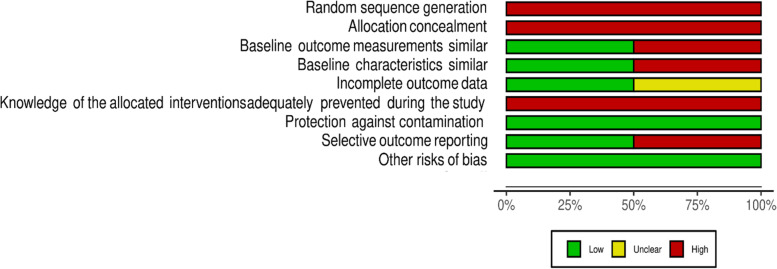
For included RCTs, there was low or unclear risk of bias (Fig. 2) across the majority of domains. Most studies however had a high risk of bias in relation to protection against contamination. The most common issue leading to a judgement of unclear risk of bias was lack of clarity around blinding of participants. There was high risk of bias for all nRCTs due to limitations in randomization and allocation concealment and a high risk of bias due to knowledge of allocation across all studies (Fig. 3). The full risk of bias assessment for all outcomes is available in Additional file 3.
Fig. 2.
EPOC Risk of Bias assessment for RCTs
Fig. 3.
EPOC Risk of Bias assessment for nRCTs
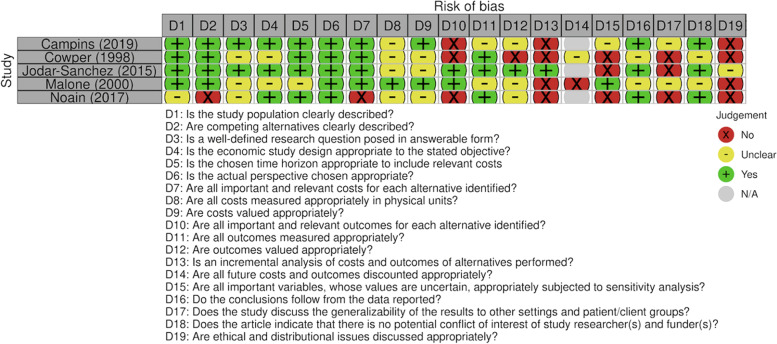
The full text articles related to health-economic studies were assessed for risk of bias. The quality was varied across the included health-economic studies (Fig. 4). Missing data was an issue across all studies which did not allow for an estimation of risk of bias in this review. Uncertainty about the rigour of outcomes reporting and sensitivity analyses were also noted.
Fig. 4.
CHEC List for assessing methodological quality of health-economic studies
Certainty of the evidence
The outcomes included in the SoF Table include the review primary outcomes, and important outcomes identified in the COS which were aligned with the outcomes of this review (See Table 4). In general, the majority of included studies were RCTs and as such, GRADE assessment for certainty of evidence was limited to that study design [30]. Of the 23 included studies, 21 were based on an RCT design.
Table 4.
Summary of Findings table
| The effectiveness and cost of integrating pharmacists within general practice to optimize prescribing and health outcomes in primary care patients with polypharmacy | |||
|---|---|---|---|
|
Patients or population: Patients over the age of 65 on five or more medications Settings: Primary care Intervention: Pharmacist integration to optimise medications and improve patient outcomes Comparison: Usual care | |||
| Outcomes | Impact |
Number of participants (Studies) |
Certainty of the evidence (GRADE) |
| Potentially inappropriate prescribing | Ten studies favoured pharmacist integration, eight of which demonstrated signficant changes in favour of the pharmacist integration group |
1486 participants (10 studies) |
⊕⊕⊕Ɵa Moderate |
| Number of medications | Mean difference -0.80 [-1.17, -0.43]. Direction of effect of four of the seven studies favoured pharmacist integration in reducing the number of medications prescribed. Confidence intervals for three studies included zero |
1176 participants (7 studies) |
⊕⊕⊕Ɵa Moderate |
| Health-related quality of life | Unclear effect, the direction of results could not be determined due to the heterogeneity in reported results |
4535 participants (15 studies) |
⊕ƟƟƟa, b, c Very low |
| Adverse drug events | Unclear effect, pharmacist integration tended to reduce the risk of ADEs, two studies reported significant results and two studies did not |
409 participants (4 studies) |
⊕⊕ƟƟa, c Low |
| Mortality | No clear effect on mortality |
327 participants (2 studies) |
⊕⊕ƟƟd Low |
Text highlighted in bold indicate main headings
GRADE Working Group grades of evidence
High = This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low
Moderate = This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate
Low = This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high
Very low = This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high
a downgrade by one level due to serious concerns relating to risk of bias
b downgrade by one level due to serious concerns relating to inconsistency of results
c downgrade by one level due to serious concerns relating to imprecision of results
d downgrade by two levels due to very serious concerns relating to imprecision of results
The certainty of evidence relating to the impact of pharmacist integration on PIP was moderate. Studies were downgraded for serious concerns relating to risk of bias. The certainty of evidence for number of medications was moderate due to serious concerns relating to risk of bias and limited to the seven studies included in the meta-analysis. The certainty of evidence for HRQoL was very low due to serious concerns relating to risk of bias, inconsistency of results and imprecision of results. Certainty of evidence for ADEs and mortality was low due to serious concerns relating to risk of bias and imprecision of results and very serious concerns relating to imprecision of results respectively. Economic outcomes were not included in the SoF table.
Primary Outcomes
Potentially inappropriate prescribing
Eleven of the 23 studies evaluated effects of pharmacist integration on a range of PIP indicators. Ten were RCTs with moderate certainty of evidence [34, 37, 40, 42–45, 47, 52]. Heterogeneity in terms of reported outcomes dictated a narrative synthesis of results. Six of the 11 studies utilised validated screening tools. Three studies used the MAI [34, 37, 52], two of which [34, 52] reported significant changes favouring pharmacist integration (Additional file 4). Two studies reported the Drug Burden Index (DBI) [45, 51] and reported a reduction in the DBI favouring pharmacist integration, significance not reported. One study used the STOPP/START criteria and reported significant improvements in PIP favouring pharmacist integration [42].
Of the remaining five studies, two studies used a structural assessment by Cipolle et al. [60] which is an assessment according to a rational order of indication, effectiveness, safety and compliance [43, 44], and three studies used locally defined drug related problems (DRPs) [39, 40, 47] (Additional file 4). Overall, four of these five studies reported an improvement in PIP for pharmacist integration [39, 40, 44, 47] with one study reporting significantly less pharmaceutical care issues (PCIs) for pharmacist integration groups in comparison to usual care (21.2% and 60.7% respectively, RR, 0.35 (95% CI 0.31 – 0.39)) [40], three studies did not report significance [39, 44, 47] and one study favoured usual care [43].
Number of medications
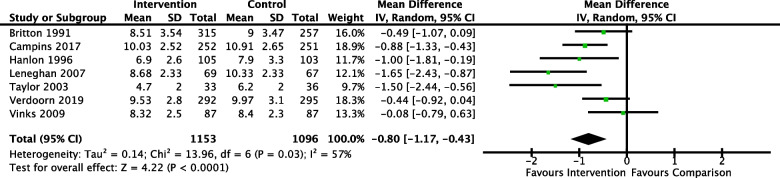
Nine studies reported on per-patient differences in number of medications at study endpoint (Additional file 5) [32, 34, 37, 41, 42, 46–48, 55]. Direction of effect favoured pharmacist integration in all but one of the studies. Seven of these studies could be included in a meta-analysis (Fig. 5) which indicated pharmacist integration probably reduced mean number of medications in comparison to usual care (mean difference -0.80 [95% CI -1.17 to -0.43]). There was moderate heterogeneity in the reported results as indicated by the I2 statistic of 57%.
Fig. 5.
Meta-analysis of number of medications
Secondary outcomes
HRQoL
Fifteen studies (16 articles) reported on HRQoL, two studies (three articles) reported some improvement [49, 53, 55], three reported mixed effects [33, 46, 52] and 10 studies reported no difference between groups (Additional file 6) [34, 36, 37, 40–42, 45, 48, 50, 54]. Of the two that reported an improvement, one study (two articles) reported a mean difference in utility score (SD) of 0.0550 (0.01) (95% CI 0.0306 to 0.0794) in favour of pharmacist integration [49, 55]; and the other reported significant improvements in mental health and vitality favouring pharmacist integration but did not provide data on other domains [53]. Of the three with mixed effects, one study favoured usual care with reported improvements in HRQoL in two domains; emotional role and social functioning but provided no further data [52]. One study only reported an improvement in the VAS score [46] and the other study did not report usual care group data thus an estimate could not be made [33].
Six of nine studies using the SF36 [34, 36, 37, 40, 48, 54] showed no significant difference between pharmacist integration and usual care across all eight domains. Two studies reported some mixed effects across the different SF36 domains [33, 52]. The remaining study reported an improvement [53]. Four of six studies used the EQ5D and reported no significant difference between groups at follow-up [41, 42, 45, 50]. The other two studies (three articles) reported significant differences though in Verdoorn et al. this was only in the EQ5D VAS and not in the index score [46, 49, 55].
ADEs
Five studies reported on ADEs (Additional file 7). [35, 37, 45, 50, 54] Overall three studies reported a decrease in ADEs in the pharmacist integration group versus usual care, though these were not significant differences. Of the remaining two studies, one reported improved adverse effect scores and symptoms in the pharmacist integration group (p = 0.024) [35], and the remaining study reported no significant difference between groups [50].
Health service utilization & mortality
Ten studies reported on health service utilization (Additional file 8). [37, 38, 40–42, 45, 48, 50, 51, 53]. Seven studies reported no significant difference between groups at follow-up [38, 41, 42, 45, 48, 50, 53]. Of the three studies which reported a reduction in hospitalizations associated with pharmacist integration, one reported a reduction of hospitalizations of 47% in emergency admissions however the reported numbers were deemed too small for meaningful statistical analysis [40]. The other two studies reported a reduction in hospitalizations; one study reported the adjusted rate ratio for medication‐related hospitalizations in the pharmacist integration group compared to usual care as 0.68 (95% CI 0.57–0.82) [51]. The remaining study reported a significant difference in reported hospitalizations over the course of the intervention (p = 0.003) which favoured pharmacist integration.
Two studies reported on mortality and no effect was found in either study. [42, 45].
Clinical physical outcomes
Three studies reported on clinical physical outcomes [33, 37, 43]. No significant changes were noted in body mass index (BMI) or renal function [43]. In one study, all patients in the pharmacist integration group had international normalised ratios (INRs) within the targeted range, compared with 25% of usual care patients [37] (Additional file 9). Two studies reported significant improvements in blood pressure (BP) management which favoured pharmacist integration [37, 43]. Three studies reported mixed results for glycaemic control, two reported no effect on glycosylated haemoglobin (HbA1c) levels [33, 43] and one reported significantly more patients in the pharmacist integration group had achieved their therapeutic goal [37]. These three studies all reported significant improvement in lipid profiles in the pharmacist integration group versus the usual care group [33, 37, 43].
Results of economic studies
Twelve health-economic studies were included. Two studies were CEAs [56]; one was a CUA [55]. The CUA (cost year 2014, Spanish jurisdiction) reported that the probability of the medication review with follow-up (MRF) service being cost effective, compared with usual care, was 100% when the willingness to pay threshold ranged from €30,000 to €45,000 per quality adjusted life year (QALY) (Additional file 10) [55]. One CEA (cost year 1991, US jurisdiction) reported a cost-effectiveness incremental ratio of €9.55 per one unit change in MAI [56]. The second CEA was based on analysis on cost savings. The incremental cost per ADE avoided was €74.18, the incremental cost per case of improvement in severity of illness (as measured by the Duke’s Severity of Illness Visual Analogue Scale) was €69.88 [54].
Nine studies considered costs [32, 35, 36, 40, 48, 51, 57–59]. Two articles reported on the same study [55, 59], the CUA [55] as outlined above and the other study (cost year 2014, Spanish jurisdiction) detailed the costs and potential price of a (MRF) service and found that mean initial investment per pharmacy was €5899.92 and mean annual maintenance costs of €3940.13 [59]. The potential service price ranged from €304.37 to €806.52 per patient per year [59]. One study (cost year 2012, Spanish jurisdiction) reported a non-significant reduction in drug expenditure in the pharmacist integration group at follow-up of €321.43 (95% CI 233.77– 409.79) in comparison to usual care €232.94 (95% CI 141.64 – 323.15) (p = 0.171) [58]. The estimated return on investment of pharmacist integration (control €0 per scenario) ranged from €2.34 to €3.27 per patient per year based on sensitivity analysis (basal, optimistic, and conservative scenarios) [58]. A cost analysis study (cost year 1991, US jurisdiction) reported a non-significant difference in change of mean total costs (cost of clinic visits, medications, hospitalizations, and laboratory tests) of -€446.36, p = 0.06 [57]. Five further cost analysis studies reported no difference in costs between pharmacist integration and usual care groups at follow-up [35, 36, 40, 48, 51]. Two studies reported no significant difference between pharmacist integration and usual care in relation to healthcare utilization costs [36, 51]. One study reported that total cost savings in the pharmacist integration group of €287.93 was significantly higher than the increase of €1295.93 cost observed in the usual care group (pharmacist integration total cost avoidance €1588.39) [32].
Sub-group analysis of primary outcomes
Subgroup analysis was based on location of intervention (remote vs co-located integration) and degree of polypharmacy. For PIP, six of the studies investigated remote integration and five investigated co-located integration. All of the studies that examined remote integration favoured pharmacist integration [42–45, 47, 52]; one reported a non-significant improvement in the pharmacist integration group [45], the other five studies reported significant changes favouring pharmacist integration. Of the co-located integration studies, 80% favoured the pharmacist integration group [34, 37, 39, 40] and 20% reported mixed results [51]. Overall, studies that investigated remote integration favoured pharmacist integration more than those that investigated co-located integration.
Subgroup analysis based on the degree of polypharmacy found that for per-patient number of medications, six studies investigated remote integration [41, 42, 46–48, 55] and three studies investigated co-located integration [32, 34, 37]. Of the six studies that investigated remote integration, 83.3% favoured pharmacist integration for reducing number of medications [41, 42, 46, 47, 55] and 16.7% found no difference between pharmacist integration and usual care groups [48]. All studies that investigated co-located integration favoured pharmacist integration group for reducing the number of medications. Both co-located and remote integration demonstrated a mix of significant and non-significant results which makes estimation of effect difficult.
Discussion
This review identified 23 studies of the effectiveness of pharmacist integration and eleven of the effectiveness studies reported a health-economic study, with four reported separately in five publications. Across the included studies, there was heterogeneity in terms of medications optimization interventions and health outcomes reported. Overall, ten of 11 studies reporting on PIP outcomes reported pharmacist integration probably reduced PIP (moderate certainty evidence), however one study favoured usual care. A meta-analysis of seven RCTs showed that pharmacist integration can probably reduce the number of medications a patient is prescribed in comparison to usual care (moderate certainty evidence).
Overall, our findings indicate that pharmacist integration may improve patient medications management, but it is uncertain if pharmacist integration improves HRQoL as the certainty of evidence is very low. The majority of included studies reported a decrease in PIP or drug-related problems, a small reduction in the number of medications and a potential reduction in ADEs. All interventions in the 23 included studies involved medication review, only one study included an additional intervention aimed at quality improvement in the practice [51]. Our stated inclusion criteria did not stipulate this, although it does logically follow that pharmacist interventions would follow a medication review model given their area of expertise. Patients with polypharmacy are at an increased risk of PIP and ADEs and medication review offers a structure by which these elements can be identified and addressed where appropriate. All studies demonstrated a degree of integration, the majority demonstrated moderate integration across three domains, the most common being organizational, informational, and clinical [32, 35, 38–42, 44–47, 49–54].
Our review findings are in keeping with previous broader systematic reviews, which suggest that pharmacist integration probably reduces the number of medication-related problems and improves appropriateness of prescribing [5, 13, 14, 61]. Previous studies have reported conflicting data in terms of the effect of pharmacist integration on the number of medications [13]. This current review found that pharmacist integration probably resulted in a reduction in the number of medications prescribed; this correlates with some published studies [62–64] and conflicts with others [13] which looked at co-located integration only. Medication count is often used to evaluate the effectiveness of prescribing interventions [65]. However, this measure may be insensitive to medication changes over time [66]. This is particularly the case where pharmacist interventions may reasonably increase, as well as decrease medication count. Included studies used a variety of screening tools, some were validated tools such as the MAI and STOPP/START criteria and some were locally defined DRPs and pharmaceutical care issues (PCIs). It is likely that many of the criteria used in validated screening tools overlapped with the locally defined protocols. PIP is identified using a variety of implicit and explicit screening tools with the aim of reducing medication harms. A retrospective cohort study identified a 20% reduction in potentially inappropriate medications (PIMs) following pharmacist intervention [64] and evidence from other reviews would suggest that pharmacist interventions targeting PIP may be associated with an improvement in prescribing appropriateness [67] which agrees with the positive trend found in this review.
We based our main outcomes as reported in the GRADE SoF tables, on the COS [31] designed by Rankin et al. The COS is a valuable tool in providing a structure for PIP to be measured in a more consistent manner across studies and enables more robust analysis of available evidence. Given the heterogeneity of outcomes and outcomes measures, reported in this review, more robust evidence is required, and though our review suggests a positive impact, this is based on moderate certainty evidence. Although COSs refer to the importance of assessment of appropriateness, using a screening tool like STOPP or MAI, there was heterogeneity found in terms of the screening tools used and reporting of results in this review which resulted in less robust evidence.
Consistent with current evidence [68, 69] it was uncertain whether pharmacist integration improved HRQoL as measured by the SF36 and EQ5D (very low certainty evidence). These measures might not be the most sensitive to the changes in HRQoL that improved medication management may produce. The length of follow-up of included studies may not have been sufficient for the effects of pharmacist integration on HRQoL to manifest. Nonetheless, HRQoL is an important outcome to consider, highlighted by its inclusion in the COS for interventions relating to polypharmacy [31] and it is also necessary to support economic evaluations. The quality of evidence in the trials reporting on HRQoL was very low with critical data needed for determining risk of bias often missing as has been previously reported [13, 15, 67].
Our review suggested that co-located or remote integration likely caused no harm. Interventions were shown to either decrease hospitalizations and emergency admissions or reported no differences between pharmacist integration and usual care and no effect was shown on mortality. However, most studies would have been underpowered to detect rarer adverse outcomes, one study reported ADEs as a primary outcome [37] but no power calculation was reported which likely meant that outcomes reported were underpowered. The findings of our review regarding ADEs and harm are consistent with evidence provided by previous studies [12]. One RCT examined the effect of a monitoring plan for medication-related ADEs, which resulted in a decrease in delirium, hospitalization and mortality in the care home setting [70]. Other studies have reported multifaceted approaches may reduce PIP [5] whilst others reported uncertainty surrounding reducing PIP [6]. There is a paucity of evidence in relation to the effect of pharmacist integration on ADEs in the general practice and primary care settings as most are conducted in the secondary or care home settings. While we found no evidence of harms as a result of pharmacist integration, this must be interpreted with a degree of caution.
Previous reviews have suggested that pharmacist integration can have positive impacts on clinical outcomes with one systematic review reporting a significant reduction in HbA1c between pharmacist integration and usual care (mean difference − 0.88%, 95% CI − 1.15% to − 0.62%, p < 0.001) [12]. The current review found mixed effects on HbA1c levels. Results for interventions on dyslipidaemias indicate significant improvements in lipid profiles which is in agreement with previous systematic reviews [71]. Similarly, for BP measurements, pharmacists can improve achievement of target levels, however a previous review found mixed results [72].
The majority of studies involved remote integration [36, 38, 41–50, 52, 54], although results were consistent with those that were co-located. Previous studies have found that the extent of pharmacist integration positively influences patient care, this review found that full integration is not required for positive patient outcomes, however this is likely influenced by the fact that even in the remote interventions there were clear structures for pharmacist and GP follow-up and face-to-face communications [19].
There was substantial heterogeneity in the types, results, and quality of included economic evaluations as shown in the risk of bias assessment as set out by the CHEC criteria. While nine studies considered costs and outcomes [32, 35, 36, 40, 48, 51, 57–59], two studies reported intervention cost-effectiveness [54, 56] and a third considered a CUA [55]. There was insufficient evidence in this review to determine whether pharmacist integration is cost-effective. Other studies have reported that interventions delivered at community pharmacies for adults with or at risk of developing acute illness and medical emergencies appear to be cost effective [73]. Six studies reported non-significant cost differences with pharmacist integration [35, 36, 40, 48, 51, 57], however costing studies are a useful tool when planning for service provision with one study reporting significant cost savings [32]. The CUA reported 100% willingness to pay between €30,000/QALY and €45,000/QALY, the upper limit of which is comparable to a threshold used by the health payer in Ireland within the drug-reimbursement decision making processes. However, transferability of this CUA to Ireland has not been investigated. Cost-effectiveness evaluations are generally not transferable across jurisdictions given differing methodological requirements and decision-making criteria [74].
Strengths and limitations
This systematic review involved a comprehensive search of databases with the search design being aided by expert librarians. We included remote and co-located interventions to get a comprehensive overview of interventions where the pharmacist and the GP work together to improve medications management and patient outcomes. No language limits were applied to ensure that all relevant studies were captured during the search process. While the analyses were predominantly narrative, there were sufficient data in the included studies to allow for a meta-analysis of the impact of pharmacist integration on the number of per-patient medications prescribed. The outputs of the meta-analysis should be interpreted with caution given the heterogeneity in interventions and health-care settings in the included studies.
This review included RCTs and other quasi-experimental designs in line with EPOC criteria, which ensured we only included robust study designs as smaller uncontrolled studies produce unreliable estimates of effectiveness. No contact was made with included study authors to resolve unclear information when judging risk of bias which may have led to some studies being downgraded. Baseline imbalances and a lack of allocation concealment could have had significant impacts on reported outcomes.
Implications for practice and future research
This review provides further evidence to inform policy in this area. Co-located or remotely integrated pharmacists probably improve PIP and reduce the number of per-patient medications. The heterogeneity of roles reported in this review outline how flexible the pharmacist role can be within practice. Results highlighted that pharmacists conducting medication reviews can have an impact by identifying PIP and reducing treatment burden through the reduction in the number of regular medications. This review suggests that pharmacist integration can also involve practice audits and patient and prescriber education. Pharmacists may help ease the time burden on other clinicians in relation to chronic disease management by modifying patient medication regimens as appropriate and ordering required laboratory tests for monitoring.
In line with the findings of other studies, we concluded that further high-quality economic evaluations should be conducted alongside interventional trials. There is some existing evidence to suggest that pharmacist interventions are cost effective in the primary care setting [73] but we did not find sufficient economic analyses to support this. The lack of certainty around cost effectiveness poses a barrier for policy maker making decisions on implementing such roles. Evidence suggests that pharmacist integration may positively impact clinical outcomes but this is based on a small number of studies.
A cluster RCT study design should be considered to reduce the risk of bias in future studies where interventions are targeting health professionals providing care for both pharmacist integration and usual care patients [75]. Future studies involving pharmacist integration should be powered to assess patient-reported and clinical outcomes, particularly for adverse events and harms. To reduce heterogeneity future studies should report on standardised outcomes, using the COS developed by Rankin et al. [31]. Currently, as there are no standardised approaches to outcome measurement, synthesising the evidence is challenging owing to the heterogeneity of reporting.
Conclusions
This review found that pharmacist integration probably reduces PIP and number of medications (moderate certainty evidence). Pharmacist integration may reduce ADEs (low certainty evidence) and make little or no difference to mortality (low certainty evidence) and reported uncertainty whether HRQoL improves (very low certainty evidence). Larger and longer term studies may be needed to explore the impact of pharmacist integration on patient health outcomes, healthcare utilization and costs.
Supplementary Information
Additional file 1. PubMed search strategy; sample search
Additional file 2. Domains of integration; summary table
Additional file 3. RoB Assessment for individual outcomes and health-economic studies; 1. PIP, 2. Number of medications, 3. HRQoL, 4. ADRs, 5. Mortality, 6. CHEC list for health-economic studies
Additional file 4. Medication related problems; summary of studies which evaluated PIP
Additional file 5. Difference in number of medications; summary of studies that evaluated number of medications
Additional file 6. HRQoL; summary of studies that evaluated HRQoL using SF36 and EQ5D
Additional file 7. Change in reported ADEs; summary of studies that evaluated ADEs
Additional file 8. Health service utilization; summary of studies that evaluated health service utilisation
Additional file 9. Clinical physical outcomes; summary of studies that evaluated clinical physical outcomes
Additional file 10. Health-economic studies; summary of results of included health-economic studies
Acknowledgements
Paul J Murphy MLIS, Information Specialist, Royal College of Surgeons Ireland Library, 26 York Street, D02 YN77. Advised on search strategies.
Grainne McCabe, Scholarly Communications & Research Support Officer, Royal College of Surgeons in Ireland Library, 26 York Street, D02 YN77. Advised on search strategies. Oscar James, Research Assistant. Completed title and abstract review.
Abbreviations
- ARR
Absolute risk reduction
- ADEs
Adverse drug events
- ATC
Anatomical therapeutic chemical
- BP
Blood pressure
- BMI
Body mass index
- EPOC
Cochrane effective practice and organisation of care
- CI
Confidence intervals
- CHEC
Consensus on health economic criteria
- CPI
Consumer price index
- CBA
Controlled before-after trials
- COS
Core outcome set
- CEA
Cost-effectiveness analysis
- CUA
Cost-utility analysis
- DBI
Drug burden index
- DRPs
Drug related problems
- DUSOI-A
Duke’s severity of illness visual analogue scale
- EQ5D
EuroQol-5D
- GPs
General practitioners
- GRADE
Grading of recommendations assessment, development and evaluation
- HBa1C
Haemoglobin A1c
- HR
Hazard ratio
- HTA
Health technology assessment
- HRQoL
Health-related quality of life
- INRs
International normalised ratios
- ITS
Interrupted-time-series
- LDL
Low-density lipoprotein
- MeSH
Medical subject headings
- MRF
Medication review with follow-up
- MAI
Medications appropriateness index
- MRP
Medication-related problem
- MTM
Medications therapeutic management
- MDT
Multi-disciplinary team
- NHS
National health service
- NHS EED
NHS economic evaluations database
- NHS EED
Non-randomised controlled trials
- NNT
Number needed to treat
- OECD
Organization for economic co-operation and development
- PROMs
Patient reported outcome measures
- PCIs
Pharmaceutical care issues
- PDTP
Potential drug therapy problem
- PIMs
Potentially inappropriate medications
- PIP
Potentially inappropriate prescribing
- PRISMA
Preferred reporting items for systematic reviews and meta-Analyses
- PCT
Primary care trust
- PPP
Purchasing power parity
- QALY
Quality adjusted life year
- RCTs
Randomised controlled trials
- STOPP/START
Screening tool of older person’s prescriptions / Screening tool to alert doctors to right treatment
- SF36
Short form 36 health survey questionnaire
- SOF
Summary of findings’
- UK
United Kingdom
- VAS
Visual analogue scale
Author’s contributions
AC conceived of the idea for the systematic review, designed the review, conducted the review, drafted the work, approved the submitted version and agreed to be personally accountable for their own contributions. KC conducted acquisition and analysis of data, approved the submitted version and agreed to be personally accountable for their own contributions. BC was involved in review design, interpretation of data, revised the work, approved the submitted version and agreed to be personally accountable for their own contributions. FM was involved in review design, interpretation of data, revised the work, approved the submitted version and agreed to be personally accountable for their own contributions. LMcC interpretated data, revised the work, approved the submitted version and agreed to be personally accountable for their own contributions. SMS was involved in review design, interpretation of data, revised the work, approved the submitted version and agreed to be personally accountable for their own contributions.
Funding
This research was funded by the Health Research Board Ireland (Grant reference HRB CDA 2018 Reference CDA-2018–003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included in its additional files and are available in the Open Science Framework repository, The effectiveness and cost-effectiveness of integrating pharmacists within general practice to optimise prescribing and health outcomes in primary care patients with polypharmacy: A systematic review. Extended Data. [DOI 10.17605/OSF.IO/RSWJT, https://doi.org/10.17605/OSF.IO/RSWJT]. This project contains the following extended data: PubMed Search Strategy for Effectiveness of integrating pharmacists within general practice systematic review.docx (PubMed search strategy) Data extraction pilot template, Risk of Bias tables, GRADE Assessment Sheets, Data extraction sheets, PRISMA checklist for “The effectiveness and cost-effectiveness of integrating pharmacists within general practice to optimise prescribing and health outcomes in primary care patients with polypharmacy: A systematic review.” Data are available under the terms of the Creative Commons Attribution 4.0 International Public License (CC-By Attribution 4.0 International).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez T, Moriarty F, Wallace E, McDowell R, Redmond P, Fahey T. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ. 2018;363:k4524. doi: 10.1136/bmj.k4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liew TM, Lee CS, Goh SKL, Chang ZY. The prevalence and impact of potentially inappropriate prescribing among older persons in primary care settings: multilevel meta-analysis. Age Ageing. 2020;49(4):570–579. doi: 10.1093/ageing/afaa057. [DOI] [PubMed] [Google Scholar]
- 4.Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging de fi nition of 'deprescribing' with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254–1268. doi: 10.1111/bcp.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clyne B, Fitzgerald C, Quinlan A, Hardy C, Galvin R, Fahey T, et al. Interventions to Address Potentially Inappropriate Prescribing in Community-Dwelling Older Adults: A Systematic Review of Randomized Controlled Trials. J Am Geriatr Soc. 2016;64(6):1210–1222. doi: 10.1111/jgs.14133. [DOI] [PubMed] [Google Scholar]
- 6.Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, Ryan C, Hughes C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018 Sep 3;9(9):CD008165. [DOI] [PMC free article] [PubMed]
- 7.Smith SM, Wallace E, Clyne B, Boland F, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst Rev. 2021;10(1):271. doi: 10.1186/s13643-021-01817-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skjot-Arkil H, Lundby C, Kjeldsen LJ, Skovgards DM, Almarsdottir AB, Kjolhede T, et al. Multifaceted Pharmacist-led Interventions in the Hospital Setting: A Systematic Review. Basic Clin Pharmacol Toxicol. 2018;123(4):363–379. doi: 10.1111/bcpt.13030. [DOI] [PubMed] [Google Scholar]
- 9.Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016 Feb 12;2(2):CD009095. [DOI] [PMC free article] [PubMed]
- 10.Edmunds J, Calnan MW. The reprofessionalisation of community pharmacy? An exploration of attitudes to extended roles for community pharmacists amongst pharmacists and General Practioners in the United Kingdom. Soc Sci Med. 2001;53(7):943–955. doi: 10.1016/s0277-9536(00)00393-2. [DOI] [PubMed] [Google Scholar]
- 11.Sudeshika T, Naunton M, Deeks LS, Thomas J, Peterson GM, Kosari S. General practice pharmacists in Australia: A systematic review. PLoS ONE. 2021;16(10):e0258674. doi: 10.1371/journal.pone.0258674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan EC, Stewart K, Elliott RA, George J. Pharmacist services provided in general practice clinics: a systematic review and meta-analysis. Res Social Adm Pharm. 2014;10(4):608–622. doi: 10.1016/j.sapharm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Hasan Ibrahim AS, Barry HE, Hughes CM. A systematic review of general practice-based pharmacists' services to optimize medicines management in older people with multimorbidity and polypharmacy. Fam Pract. 2021;38(4):509–523. doi: 10.1093/fampra/cmaa146. [DOI] [PubMed] [Google Scholar]
- 14.Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Cresswell K, Eden M, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379(9823):1310–1319. doi: 10.1016/S0140-6736(11)61817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayhoe B, Cespedes JA, Foley K, Majeed A, Ruzangi J, Greenfield G. Impact of integrating pharmacists into primary care teams on health systems indicators: a systematic review. Br J Gen Pract. 2019;69(687):e665–e674. doi: 10.3399/bjgp19X705461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedna K, Hakkarainen KM, Gyllensten H, Jonsson AK, Petzold M, Hagg S. Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol. 2015;71(12):1525–1533. doi: 10.1007/s00228-015-1950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott RA, Camacho E, Jankovic D, Sculpher MJ, Faria R. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual Saf. 2021;30(2):96–105. doi: 10.1136/bmjqs-2019-010206. [DOI] [PubMed] [Google Scholar]
- 18.Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37–46. doi: 10.2147/IPRP.S108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazen ACM, de Bont AA, Boelman L, Zwart DLM, de Gier JJ, de Wit NJ, et al. The degree of integration of non-dispensing pharmacists in primary care practice and the impact on health outcomes: A systematic review. Res Social Adm Pharm. 2018;14(3):228–240. doi: 10.1016/j.sapharm.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT GSe. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration2011. Available from: http://handbook.cochrane.org.
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. BMJ. 2009;339:b2535. [PMC free article] [PubMed]
- 22.Croke A, James O, Clyne B, Moriarty F, Cardwell K, Smith SM. The effectiveness of integrating clinical pharmacists within general practice to optimise prescribing and health outcomes in primary care patients with polypharmacy: A protocol for a systematic review. HRB Open Res. 2019;2:32. doi: 10.12688/hrbopenres.12966.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. doi: 10.1002/14651858.CD006560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walshe, Smith. Chronic disease and integrated care. Healthcare Management (second ed.), McGraw-Hill Education (2011).
- 25.Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors, 2017. Available at: epoc.cochrane.org/epoc-resources-review-authors.
- 26.The EndNote Team. EndNote. EndNote X9 ed. Philadelphia: Clarivate Analytics; 2013.
- 27.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuinness, LA, Higgins, JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing riskof-bias assessments. Res Syn Meth. 2020;1–7. [DOI] [PubMed]
- 29.Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240–245. [PubMed] [Google Scholar]
- 30.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
- 31.Rankin A, Cadogan CA, In Ryan C, Clyne B, Smith SM, Hughes CM. Core Outcome Set for Trials Aimed at Improving the Appropriateness of Polypharmacy in Older People in Primary Care. J Am Geriatr Soc. 2018;66(6):1206–1212. doi: 10.1111/jgs.15245. [DOI] [PubMed] [Google Scholar]
- 32.Britton ML, Lurvey PL. Impact of medication profile review on prescribing in a general medicine clinic. Am J Hosp Pharm. 1991;48(2):265–270. [PubMed] [Google Scholar]
- 33.Carter BL, Malone DC, Billups SJ, Valuck RJ, Barnette DJ, Sintek CD, et al. Interpreting the findings of the IMPROVE study. Am J Health Syst Pharm. 2001;58(14):1330–1337. doi: 10.1093/ajhp/58.14.1330. [DOI] [PubMed] [Google Scholar]
- 34.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 35.Jameson JP, VanNoord GR. Pharmacotherapy consultation on polypharmacy patients in ambulatory care. Ann Pharmacother. 2001;35(7):835–840. doi: 10.1345/aph.10259. [DOI] [PubMed] [Google Scholar]
- 36.Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ : Canadian Medical Association journal. 2003;169(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm. 2003;60(11):1123–9. [DOI] [PubMed]
- 38.Zillich AJ, Snyder ME, Frail CK, Lewis JL, Deshotels D, Dunham P, et al. A randomized, controlled pragmatic trial of telephonic medication therapy management to reduce hospitalization in home health patients. Health Serv Res. 2014;49(5):1537–1554. doi: 10.1111/1475-6773.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granås AG, Bates I. The effect of pharmaceutical review of repeat prescriptions in general practice. Int J Pharm Pract. 1999;7(4):264–275. [Google Scholar]
- 40.Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PRS, et al. Pharmacist-led medication review in patients over 65: A randomized, controlled trial in primary care. Age Ageing. 2001;30(3):205–211. doi: 10.1093/ageing/30.3.205. [DOI] [PubMed] [Google Scholar]
- 41.Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care - The POLYMED randomised controlled trial. Age Ageing. 2007;36(3):292–297. doi: 10.1093/ageing/afm036. [DOI] [PubMed] [Google Scholar]
- 42.Campins L, Serra-Prat M, Gózalo I, López D, Palomera E, Agustí C, et al. Randomized controlled trial of an intervention to improve drug appropriateness in communitydwelling polymedicated elderly people. Fam Pract. 2017;34(1):36–42. doi: 10.1093/fampra/cmw073. [DOI] [PubMed] [Google Scholar]
- 43.Geurts MM, Stewart RE, Brouwers JR, de Graeff PA, de Gier JJ. Implications of a clinical medication review and a pharmaceutical care plan of polypharmacy patients with a cardiovascular disorder. Int J Clin Pharm. 2016;38(4):808–15. [DOI] [PMC free article] [PubMed]
- 44.Kwint HF, Faber A, Gussekloo J, Bouvy ML. Medication review in patients using automated drug dispensing systems reduces drug related problems. Int J Clin Pharm. 2011;33(2):412. doi: 10.2165/11586850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open. 2018;8(7):e019042. [DOI] [PMC free article] [PubMed]
- 46.Verdoorn S, Kwint HF, Blom JW, Gussekloo J, Bouvy ML. Effects of a clinical medication review focused on personal goals, quality of life, and health problems in older persons with polypharmacy: a randomised controlled trial (DREAMeR-study) Plos medicine. 2019;16(5):e1002798. doi: 10.1371/journal.pmed.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinks TH, Egberts TC, de Lange TM, de Koning FH. Pharmacist-based medication review reduces potential drug-related problems in the elderly: the SMOG controlled trial. Drugs Aging. 2009;26(2):123–33. [DOI] [PubMed]
- 48.Bernsten C, Björkman I, Caramona M, Crealey G, Frøkjaer B, Grundberger E, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs Aging. 2001;18(1):63–77. doi: 10.2165/00002512-200118010-00005. [DOI] [PubMed] [Google Scholar]
- 49.Varas-Doval R, Gastelurrutia MA, Benrimoj SI, Garcia-Cardenas V, Saez-Benito L, Martinez-Martinez F. Clinical impact of a pharmacist-led medication review with follow up for aged polypharmacy patients: A cluster randomized controlled trial. Pharm Pract (Granada) 2020;18(4):2133. doi: 10.18549/PharmPract.2020.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leendertse AJ, de Koning GH, Goudswaard AN, Belitser SV, Verhoef M, de Gier HJ, et al. Preventing hospital admissions by reviewing medication (PHARM) in primary care: an open controlled study in an elderly population. J Clin Pharm Ther. 2013;38(5):379–387. doi: 10.1111/jcpt.12069. [DOI] [PubMed] [Google Scholar]
- 51.Sloeserwij VM, Hazen ACM, Zwart DLM, Leendertse AJ, Poldervaart JM, de Bont AA, de Gier JJ, Bouvy ML, de Wit NJ. Effects of non-dispensing pharmacists integrated in general practice on medication-related hospitalisations. Br J Clin Pharmacol. 2019;85(10):2321–31. [DOI] [PMC free article] [PubMed]
- 52.Bryant LJ, Coster G, Gamble GD, McCormick RN. The General Practitioner-Pharmacist Collaboration (GPPC) study: a randomised controlled trial of clinical medication reviews in community pharmacy. Int J Pharm Pract. 2011;19(2):94–105. doi: 10.1111/j.2042-7174.2010.00079.x. [DOI] [PubMed] [Google Scholar]
- 53.Graffen M, Kennedy D, Simpson M. Quality use of medicines in the rural ambulant elderly: a pilot study. Rural Remote Health. 2004;4(3):184. [PubMed]
- 54.Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott R, Roberts MS. Medication reviews in the community: results of a randomized, controlled effectiveness trial. Br J Clin Pharmacol. 2004;58(6):648–664. doi: 10.1111/j.1365-2125.2004.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jodar-Sanchez F, Malet-Larrea A, Martin JJ, Garcia-Mochon L, Lopez Del Amo MP, Martinez-Martinez F, et al. Cost-utility analysis of a medication review with follow-up service for older adults with polypharmacy in community pharmacies in Spain: the conSIGUE program. Pharmacoeconomics. 2015;33(6):599–610. doi: 10.1007/s40273-015-0270-2. [DOI] [PubMed] [Google Scholar]
- 56.Cowper PA, Weinberger M, Hanlon JT, Landsman PB, Samsa GP, Uttech KM, et al. The cost-effectiveness of a clinical pharmacist intervention among elderly outpatients. Pharmacotherapy. 1998;18(2):327–332. [PubMed] [Google Scholar]
- 57.Malone DC, Carter BL, Billups SJ, Valuck RJ, Barnette DJ, Sintek CD, et al. An economic analysis of a randomized, controlled, multicenter study of clinical pharmacist interventions for high-risk veterans: the IMPROVE study. Pharmacotherapy. 2000;20(10):1149–58. doi: 10.1592/phco.20.15.1149.34590. [DOI] [PubMed] [Google Scholar]
- 58.Campins L, Serra-Prat M, Palomera E, Bolibar I, Martinez MA, Gallo P. Reduction of pharmaceutical expenditure by a drug appropriateness intervention in polymedicated elderly subjects in Catalonia (Spain) Gac Sanit. 2019;33(2):106–111. doi: 10.1016/j.gaceta.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Noain A, Garcia-Cardenas V, Gastelurrutia MA, Malet-Larrea A, Martinez-Martinez F, Sabater-Hernandez D, et al. Cost analysis for the implementation of a medication review with follow-up service in Spain. Int J Clin Pharm. 2017;39(4):750–758. doi: 10.1007/s11096-017-0454-2. [DOI] [PubMed] [Google Scholar]
- 60.Cipolle RJSL, Morley PC. Pharmaceutical care practice. United States of America: The McGraw-Hill Companies Inc; 1998. [Google Scholar]
- 61.Dolovich L, Pottie K, Kaczorowski J, Farrell B, Austin Z, Rodriguez C, et al. Integrating family medicine and pharmacy to advance primary care therapeutics. Clin Pharmacol Ther. 2008;83(6):913–917. doi: 10.1038/clpt.2008.29. [DOI] [PubMed] [Google Scholar]
- 62.Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ (Clinical research ed) 2001;323(7325):1340–1343. doi: 10.1136/bmj.323.7325.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan EC, Stewart K, Elliott RA, George J. Pharmacist consultations in general practice clinics: the Pharmacists in Practice Study (PIPS) Research in social & administrative pharmacy : RSAP. 2014;10(4):623–632. doi: 10.1016/j.sapharm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Stuhec M, Gorenc K, Zelko E. Evaluation of a collaborative care approach between general practitioners and clinical pharmacists in primary care community settings in elderly patients on polypharmacy in Slovenia: a cohort retrospective study reveals positive evidence for implementation. BMC Health Serv Res. 2019;19(1):118. doi: 10.1186/s12913-019-3942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gnjidic D, Le Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28(2):237–253. doi: 10.1016/j.cger.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy C, Flood M, Clyne B, Smith SM, Wallace E, Boland F, Moriarty F. Medication changes and potentially inappropriate prescribing in older patients with significant polypharmacy. Int J Clin Pharm. 2022. 10.1007/s11096-022-01497-2. Epub ahead of print. [DOI] [PubMed]
- 67.Riordan DO, Walsh KA, Galvin R, Sinnott C, Kearney PM, Byrne S. The effect of pharmacist-led interventions in optimising prescribing in older adults in primary care: A systematic review. SAGE Open Med. 2016;4:2050312116652568. doi: 10.1177/2050312116652568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen TA, Gilmartin-Thomas J, Tan ECK, Kalisch-Ellett L, Eshetie T, Gillam M, et al. The Impact of Pharmacist Interventions on Quality Use of Medicines, Quality of Life, and Health Outcomes in People with Dementia and/or Cognitive Impairment: A Systematic Review. J Alzheimers Dis. 2019;71(1):83–96. doi: 10.3233/JAD-190162. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed A, Saqlain M, Tanveer M, Blebil AQ, Dujaili JA, Hasan SS. The impact of clinical pharmacist services on patient health outcomes in Pakistan: a systematic review. BMC Health Serv Res. 2021;21(1):859. doi: 10.1186/s12913-021-06897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc. 2011;59(7):1238–1245. doi: 10.1111/j.1532-5415.2011.03418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charrois TL, Zolezzi M, Koshman SL, Pearson G, Makowsky M, Durec T, et al. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacotherapy. 2012;32(3):222–233. doi: 10.1002/j.1875-9114.2012.01022.x. [DOI] [PubMed] [Google Scholar]
- 72.Reeves L, Robinson K, McClelland T, Adedoyin CA, Broeseker A, Adunlin G. Pharmacist Interventions in the Management of Blood Pressure Control and Adherence to Antihypertensive Medications: A Systematic Review of Randomized Controlled Trials. J Pharm Pract. 2021;34(3):480–492. doi: 10.1177/0897190020903573. [DOI] [PubMed] [Google Scholar]
- 73.Dawoud DM, Haines A, Wonderling D, Ashe J, Hill J, Varia M, et al. Cost Effectiveness of Advanced Pharmacy Services Provided in the Community and Primary Care Settings: A Systematic Review. Pharmacoeconomics. 2019;37(10):1241–1260. doi: 10.1007/s40273-019-00814-4. [DOI] [PubMed] [Google Scholar]
- 74.Gorry C, McCullagh L, Barry M. Transferability of Economic Evaluations of Treatments for Advanced Melanoma. Pharmacoeconomics. 2020;38(2):217–231. doi: 10.1007/s40273-019-00860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Croke A, Moriarty F, Boland F, McCullagh L, Cardwell K, Smith SM, et al. Integrating clinical pharmacists within general practice: protocol for a pilot cluster randomised controlled trial. BMJ Open. 2021;11(3):e041541. doi: 10.1136/bmjopen-2020-041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PubMed search strategy; sample search
Additional file 2. Domains of integration; summary table
Additional file 3. RoB Assessment for individual outcomes and health-economic studies; 1. PIP, 2. Number of medications, 3. HRQoL, 4. ADRs, 5. Mortality, 6. CHEC list for health-economic studies
Additional file 4. Medication related problems; summary of studies which evaluated PIP
Additional file 5. Difference in number of medications; summary of studies that evaluated number of medications
Additional file 6. HRQoL; summary of studies that evaluated HRQoL using SF36 and EQ5D
Additional file 7. Change in reported ADEs; summary of studies that evaluated ADEs
Additional file 8. Health service utilization; summary of studies that evaluated health service utilisation
Additional file 9. Clinical physical outcomes; summary of studies that evaluated clinical physical outcomes
Additional file 10. Health-economic studies; summary of results of included health-economic studies
Data Availability Statement
The datasets supporting the conclusions of this article are included in its additional files and are available in the Open Science Framework repository, The effectiveness and cost-effectiveness of integrating pharmacists within general practice to optimise prescribing and health outcomes in primary care patients with polypharmacy: A systematic review. Extended Data. [DOI 10.17605/OSF.IO/RSWJT, https://doi.org/10.17605/OSF.IO/RSWJT]. This project contains the following extended data: PubMed Search Strategy for Effectiveness of integrating pharmacists within general practice systematic review.docx (PubMed search strategy) Data extraction pilot template, Risk of Bias tables, GRADE Assessment Sheets, Data extraction sheets, PRISMA checklist for “The effectiveness and cost-effectiveness of integrating pharmacists within general practice to optimise prescribing and health outcomes in primary care patients with polypharmacy: A systematic review.” Data are available under the terms of the Creative Commons Attribution 4.0 International Public License (CC-By Attribution 4.0 International).