Abstract
Background
ASEAN (Association of Southeast Asian Nations) is composed of ten Southeast Asian countries bound by socio-cultural ties that promote regional peace and stability. South Asia, located in the southern subregion of Asia, includes nine countries sharing similarities in geographical and ethno-cultural factors. Chikungunya is one of the most significant problems in Southeast and South Asian countries. Much of the current chikungunya epidemic in Southeast Asia is caused by the emergence of a virus strain that originated in Africa and spread to Southeast Asia. Meanwhile, in South Asia, three confirmed lineages are in circulation. Given the positive correlation between research activity and the improvement of the clinical framework of biomedical research, this article aimed to examine the growth of chikungunya virus-related research in ASEAN and South Asian countries.
Methods
The Scopus database was used for this bibliometric analysis. The retrieved publications were subjected to a number of analyses, including those for the most prolific countries, journals, authors, institutions, and articles. Co-occurrence mapping of terms and keywords was used to determine the current state, emerging topics, and future prospects of chikungunya virus-related research. Bibliometrix and VOSviewer were used to analyze the data and visualize the collaboration network mapping.
Results
The Scopus search engine identified 1280 chikungunya-related documents published by ASEAN and South Asian countries between 1967 and 2022. According to our findings, India was the most productive country in South Asia, and Thailand was the most productive country in Southeast Asia. In the early stages of the study, researchers investigated the vectors and outbreaks of the chikungunya virus. In recent years, the development of antivirus agents has emerged as a prominent topic.
Conclusions
Our study is the first to present the growth of chikungunya virus-related research in ASEAN and South Asian countries from 1967 to 2022. In this study, the evaluation of the comprehensive profile of research on chikungunya can serve as a guide for future studies. In addition, a bibliometric analysis may serve as a resource for healthcare policymakers.
Keywords: Chikungunya, ASEAN, South Asian, Arbovirus, Scopus, Bibliometric, VOSviewer
Background
Chikungunya virus, which was first isolated in Tanzania in 1953 [1, 2], has been identified in Asia, the Americas, the Pacific Islands, Europe, and Africa [3–8]. Chikungunya is part of the arbovirus (arthropod-borne) group, and it is considered the source of Chikungunya fever, an acute febrile sickness inciting millions of mortality cases worldwide [9–11]. The term “chikungunya”, which originated from the Makonde language of Tanzania, is attributed to the “bending posture” of patients infected with the chikungunya virus who experience excruciating pain in the joints, which might persist for months [1, 12–14]. Apart from severe muscular and joint pain, chikungunya virus infections are also accompanied by headaches, rashes, photophobia, and fever [15–18]. Prolonged chikungunya virus infection may trigger adverse effects, including brain inflammation, damage to optic nerves, injury to the spinal cord, Guillain–Barré syndrome, heart muscle inflammation, hepatitis, a renal lesion, or even death [19–24].
The transmission routes of the chikungunya virus mostly occur via urban and sylvatic cycles [25]. The sylvatic routes involve various types of organisms, including several Aedes species and non-human primate species, such as monkeys, baboons, and mandrills [25–30]. In recent years, Culex and Anopheles have also been recognized as vectors of the chikungunya virus [28]. Transmission via the urban route primarily occurs via Aedes albopictus and Aedes aegypti, which are reported as the primary vectors of chikungunya virus in Asia, Oceania, Europe, the Americas, and Africa [31–36]. Notably, A. aegypti also transmits other types of viruses, including dengue virus and zika virus, as supported by several studies reporting the occurrence of dengue/chikungunya/zika virus or dengue/chikungunya virus simultaneous infections [37–39]. At present, specific antiviral drugs for the chikungunya virus are not available; thus, treatment of the chikungunya virus-infected patients focuses on treating the observed symptoms [40, 41]. Recent developments in therapeutic strategies for treating chikungunya virus infection focus on antiviral drugs inhibiting viral adsorption, protein translation, genome replication, glycoprotein maturation, and activation of the immune system [42–48].
The primary goals of ASEAN, also known as the Association of Southeast Asian Nations, are to foster regional stability and economic progress among its members [49]. ASEAN consisted of ten nations, including Vietnam, Thailand, Singapore, the Philippines, Myanmar, Malaysia, Laos, Cambodia, Brunei Darussalam, and Indonesia. In recent years, the consumption of animal-based foods has rapidly increased in the Southeast Asia region, as a result of massive urbanization, rising incomes, and the expansion of industrialization [50, 51]. As a consequence, the Southeast Asian countries are prone to outbreaks of epizootic and zoonotic diseases [52, 53]. Threats to animal and human health are serious, since novel disease outbreaks have the potential to spread quickly across borders and may result in major socio-economic and public health casualties [54]. In addition, as a result of natural disasters, such as earthquakes and extreme weather, the Southeast Asia region might face food safety and public health crises [55, 56]. A number of zoonotic disease outbreaks in ASEAN countries, including chikungunya, caused serious consequences, including human deaths and economic losses [57–60]. In addition, significant numbers of chikungunya cases have been reported in Singapore, Malaysia, and Thailand [59]. Therefore, the shared vulnerability between ASEAN countries emphasizes the necessity of a collective response. By anticipating risks and taking prompt action in response to possible threats, the public and animal health systems in ASEAN countries will be more resilient and sustainable.
Among South Asian countries, India is estimated to contain the highest number of chikungunya virus infection cases [7, 61–63]. In addition, Bangladesh, Bhutan, Pakistan, Sri Lanka, Nepal, and the Maldives have also reported outbreaks of chikungunya [7, 62–64]. Multiple chikungunya virus lineages are confirmed to be circulating in South Asia, including the East-Central-South Africa (ECSA), Asian, and Indian Ocean lineages [62, 65–67]. The ECSA, which originated in Africa, has spread to South Asia and become predominant in recent years [63]. The ECSA lineage also gives rise to another unique lineage known as the Indian Ocean Lineage (IOL) [18, 65, 68, 69]. Meanwhile, the Asian Lineage (AL), another lineage detected in South Asia, was originally discovered during epidemics in Asian countries between 1958 and 1973 [62, 65]. South Asian countries are prone to chikungunya virus outbreaks and re-emergences. Because of the cyclical nature of chikungunya virus infection, outbreaks in endemic regions, such as South Asian countries, reoccur every few years. Furthermore, the persistent epidemics of chikungunya virus in other parts of the world, particularly Africa, have a substantial impact on its potential re-emergence in South Asian countries. Another urgent and serious concern for South Asian countries is the high probability of dengue and chikungunya viruses co-circulation [70, 71].
Bibliometric methodology has been established as a primary tool to evaluate research performance in various fields, including health [72, 73], sciences [74, 75], socials [76, 77], toxicology [78, 79], engineering [80, 81], and environmental studies [82, 83]. Nonetheless, bibliometric studies on chikungunya are limited [84, 85]. At present, chikungunya-related studies are receiving considerable attention from international scientific communities because of several outbreaks that have happened in recent years [63, 86–92]. Thus, the prevailing chikungunya-related literature from various perspectives must be evaluated and categorized thoroughly. The current study aims to adopt bibliometric techniques for assessing the scientific output of chikungunya-related studies by ASEAN and South Asian countries and identify areas of concern for future research. In the current study, the evaluation of chikungunya-related articles included authors, journals, institutions, countries of origin, and citation analysis. Identifying relevant topics and emerging trends might help researchers in ASEAN and South Asian countries working in the field of chikungunya. The findings in our study are also applicable to public leaders who strive to make well-informed decisions based on evidence-based policymaking.
Methods
Documents associated with the chikungunya virus from ASEAN and South Asian countries indexed in Scopus from 1967 to 2022, excluding erratum and retracted articles, were extracted for our bibliometric study. To avoid including duplicate or invalid documents in the analysis, erratum and retracted articles were excluded. In this study, the Scopus database served as our sole data source because it covered a large range of journals and a high number of articles relative to other libraries such as Web of Science or PubMed [93, 94]. Scopus has also been validated and recognized in other bibliometric analyses [75, 79, 95–97]. Data extraction was performed on December 17, 2022. The key term “chikungunya” was registered to identify chikungunya-related articles in Scopus. The search strategy for “chikungunya” was limited to titles only to improve accuracy. If additional search fields such as abstract or keywords were included, most of the extracted articles were false-positive data and not directly linked to chikungunya. Additional filtering was also established to exclusively retrieve documents published by ASEAN countries (Brunei Darussalam, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand, and Vietnam) and South Asian countries (Afghanistan, Bangladesh, Bhutan, the British Indian Ocean Territory, India, the Maldives, Nepal, Pakistan, and Sri Lanka). The following parameters were considered during the assessment of the extracted articles: the number of published articles per year, countries with prolific publication profiles, influential journals, institutions, and most cited articles. Based on the Scopus algorithm, countries and institutions were extracted from all authors associated with the published articles, regardless of the sequence or the position of the authors. Therefore, an article might be linked to several countries or institutions. In the current study, the global profile of chikungunya virus-related literature was extracted as well, irrespective of the countries of origin. The global profile data was used to measure the contribution of ASEAN and South Asian countries (Fig. 1b), and to calculate the global ranks of countries and institutions (Tables 2 and 4). Bibliometrix was used to analyze the data, and VOSviewer was used to construct maps of visual networks projecting collaborations and trending research topics [98, 99].
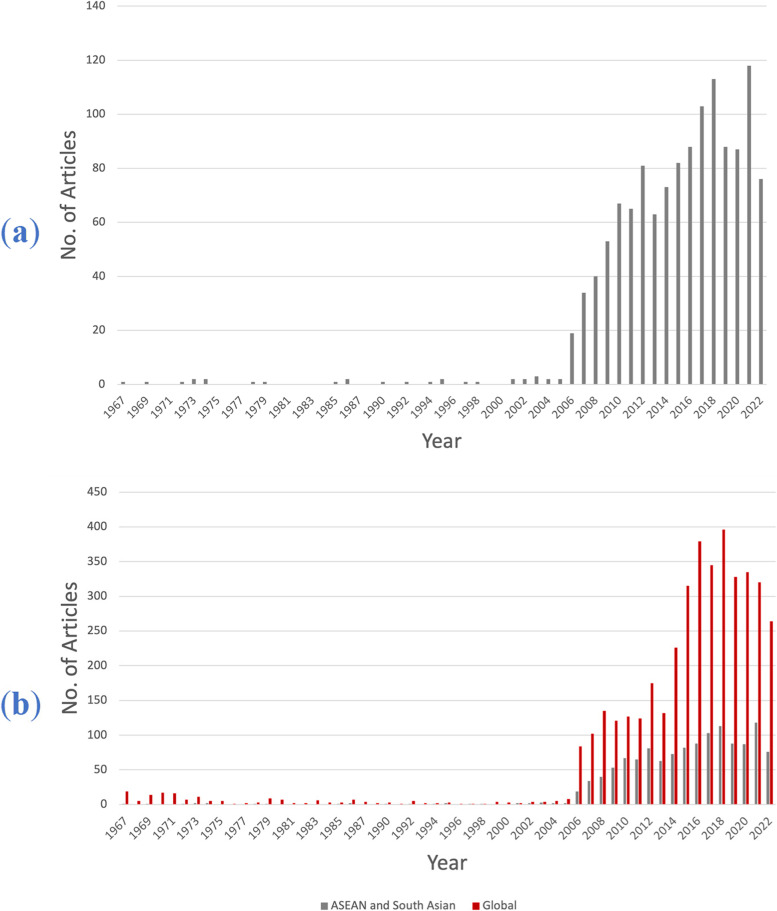
Fig. 1.
Trend of published articles on the chikungunya virus from 1967 to 2022 by ASEAN and South Asian countries. a Annual productivity of ASEAN and South Asian countries, totaling in1280 documents; b Contribution of ASEAN and South Asian countries to global productivity
Table 2.
Journals publishing articles related to the chikungunya virus produced by ASEAN and South Asian countries
| No. | Journal Title | No. of Articles (%) | Impact Factora | SNIPb |
|---|---|---|---|---|
| 1 | PLoS Neglected Tropical Diseases | 46 (3.59) | 4.41 | 1.640 |
| 2 | PLoS ONE | 36 (2.81) | 3.24 | 1.368 |
| 3 | Indian Journal of Medical Research | 33 (2.57) | 2.37 | |
| 4 | American Journal of Tropical Medicine and Hygiene | 30 (2.34) | 2.34 | 1.090 |
| 5 | Scientific Reports | 25 (1.95) | 4.37 | 1.389 |
| 6 | Emerging Infectious Diseases | 24 (1.87) | 6.88 | 2.771 |
| 7 | Transactions of the Royal Society of Tropical Medicine and Hygiene | 21 (1.64) | – | 0.801 |
| 8c | Antiviral Research | 18 (1.40) | 5.97 | 1.680 |
| 8c | Journal of Medical Virology | 18 (1.40) | 2.32 | 2.756 |
| 8c | Virology Journal | 18 (1.40) | 4.09 | 1.307 |
aImpact factors (IF), as reported in Journal Citation Reports (JCR) 2021 of Clarivate Analytics. bSNIP (Source Normalized Impact per Paper) 2021, obtained from Scopus at www.scopus.com/sources
cJournals with the same number of articles are listed with the same rank
Table 4.
Top ten institutions from ASEAN and South Asian countries with the highest performance in the field of chikungunya virus
| No. | Institutions | Country | No. of Articles (%) | Global Rank |
|---|---|---|---|---|
| 1 | National Institute of Virology | India | 79 (6.17) | 8 |
| 2 | Indian Council of Medical Research | India | 66 (5.15) | 10 |
| 3 | National University of Singapore (NUS) | Singapore | 65 (5.07) | 11 |
| 4a | Mahidol University | Thailand | 61 (4.76) | 13a |
| 4a | Agency for Science, Technology, and Research (A*STAR) | Singapore | 61 (4.76) | 13a |
| 5 | NUS Yong Loo Lin School of Medicine | Singapore | 57 (4.45) | 16 |
| 6 | Universiti Malaya | Malaysia | 47 (3.67) | 25 |
| 7 | Defence Research & Development Establishment (DRDE) | India | 42 (3.28) | 34 |
| 8 | Chulalongkorn University | Thailand | 33 (2.57) | 45 |
| 9 | Prince of Songkla University | Thailand | 27 (2.10) | 60 |
aInstitutions with the same number of articles are listed as having the same rank
Results
From 1967 to 2022, the Scopus algorithm identified 1280 documents published by ASEAN and South Asian countries in the chikungunya-related field, accounting for 31.11% of the global productivity (n = 4107). Our search results, focusing on ASEAN and South Asian countries, included 1011 (78.98%) articles, 103 (8.04%) letters, 92 (7.18%) reviews, 20 (1.56%) notes, 17 (1.32%) conference papers, and 39 (3.04%) other types of documents, including book chapters, editorials, notes, and short surveys. Among the published documents, English is the most common language, accounting for 1279 (99.92%) of the total documents.
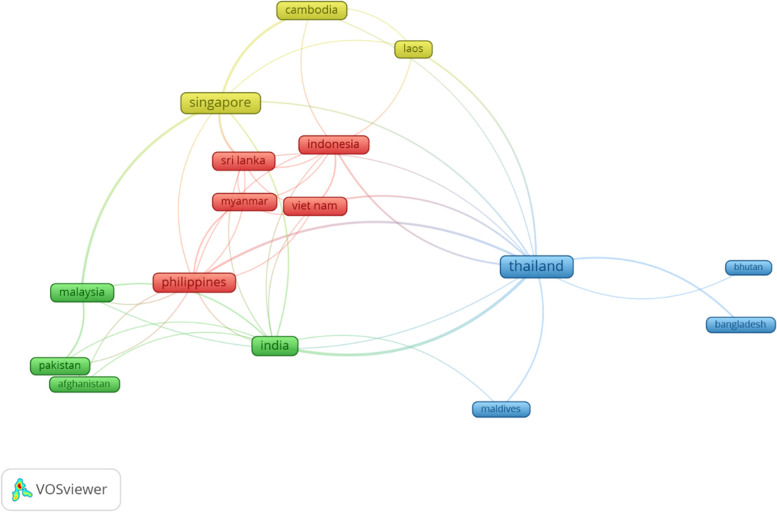
The chikungunya virus publishing trend in ASEAN and South Asian countries exhibited a gradual increase from 1967 to 2022 (Fig. 1a). In addition, ASEAN and South Asian countries showed a significant contribution to the global productivity of chikungunya virus-related publications (Fig. 1b). Fig. 2 displays the visualization of collaboration among ASEAN and South Asian countries. The relative size of the frames represents the number of collaborations. For example, the frame size of Thailand is relatively larger than that of the Maldives, indicating that Thailand (n = 12) builds collaborative studies with more countries compared to the Maldives (n = 2).
Fig. 2.
Mapping of country collaboration between ASEAN and South Asian countries. Countries assigned larger frames represent a relatively higher number of collaborations
The productivity among ASEAN and South Asian countries in research documents related to the chikungunya virus was also ranked, with India having the highest percentage (Table 1). The top ten journals publishing articles related to the chikungunya virus produced by ASEAN and South Asian countries were also listed (Table 2). The most documents were published in PLoS Neglected Tropical Diseases (n = 46; 3.59%), followed by PLoS ONE (n = 36; 2.81%), and the Indian Journal of Medical Research (n = 33; 2.57%). Also, the characteristics of papers published by ASEAN and South Asian countries with the highest number of citations in the last five decades were summarized (Table 3) [100–109].
Table 1.
Top ten productive ASEAN and South Asian countries in the field of chikungunya
| No. | Country | No. of Articles (%) | Global Rank |
|---|---|---|---|
| 1 | India | 743 (58.04) | 2 |
| 2 | Thailand | 151 (11.79) | 7 |
| 3 | Singapore | 134 (10.46) | 10 |
| 4 | Malaysia | 88 (6.87) | 14 |
| 5 | Pakistan | 54 (4.21) | 22 |
| 6 | Bangladesh | 46 (3.59) | 23 |
| 7 | Indonesia | 38 (2.96) | 26 |
| 8 | Sri Lanka | 24 (1.87) | 37 |
| 9a | The Phillipines | 15 (1.17) | 49a |
| 9a | Vietnam | 15 (1.17) | 49a |
aCountries with the same number of articles are listed as having the same rank
Table 3.
Most cited chikungunya virus-related papers published by ASEAN and South Asian countries
| No. | Authors | Title | No. of Citations | Journal Title | Year | Document Type |
|---|---|---|---|---|---|---|
| 1 | Pialoux et al. [100] | Chikungunya, an epidemic arbovirosis | 767 | Lancet Infectious Diseases | 2007 | Review |
| 2 | Musso et al. [101] | Zika virus: Following the path of dengue and chikungunya? | 346 | The Lancet | 2015 | Letter |
| 3 | Yergolkar et al. [102] | Chikungunya outbreaks caused by African genotype, India | 266 | Emerging Infectious Diseases | 2006 | Article |
| 4 | Chow et al. [103] | Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor | 254 | Journal of Infectious Diseases | 2011 | Article |
| 5 | Arankalle et al. [104] | Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic | 253 | Journal of General Virology | 2007 | Article |
| 6 | Ng et al. [105] | IL-1β, IL-6, and RANTES as biomarkers of Chikungunya severity | 218 | PLoS ONE | 2009 | Article |
| 7 | Burt et al. [106] | Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen | 208 | The Lancet Infectious Diseases | 2017 | Review |
| 8 | Laras et al. [107] | Tracking the re-emergence of epidemic chikungunya virus in Indonesia | 205 | Transactions of the Royal Society of Tropical Medicine and Hygiene | 2005 | Article |
| 9 | Her et al. [108] | Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response | 199 | Journal of Immunology | 2010 | Article |
| 10 | Mavalankar et al. [109] | Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India | 181 | Emerging Infectious Diseases | 2008 | Article |
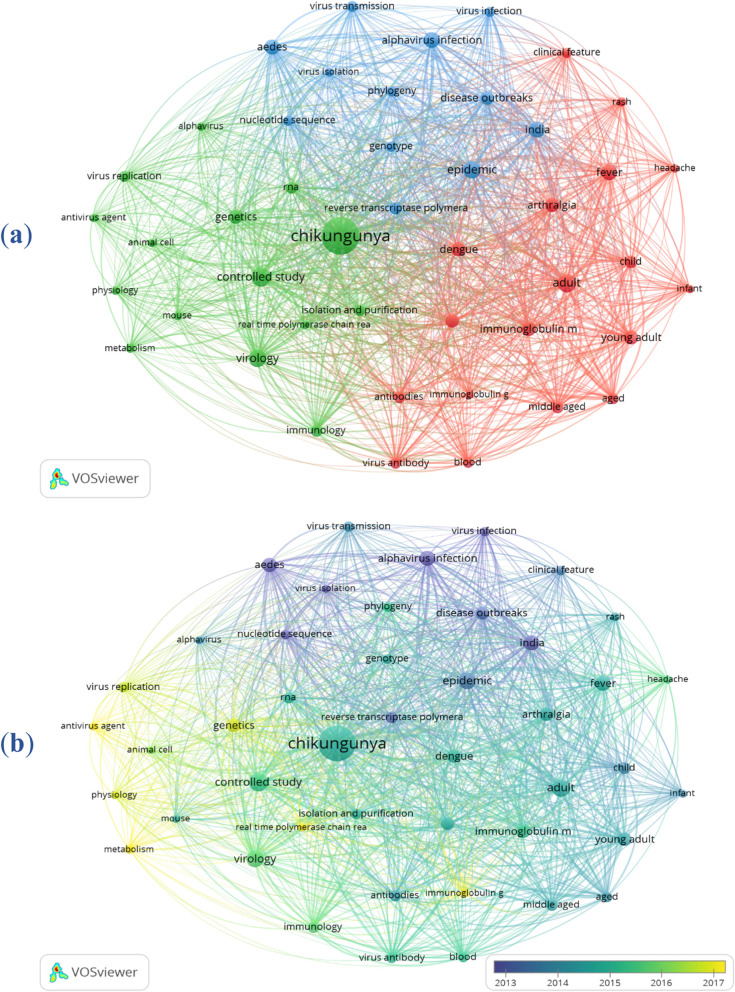
A network visualization of terms used by authors from ASEAN and South Asian countries in the 55-year period of chikungunya virus-related research was constructed (Fig. 3a). The extracted papers yielded a total of 7810 different terms, 56 of which occurred more than 100 times. The main three central clusters are assessed as follows: cluster 1 (in red) primarily consists of terms related to “dengue,” “arthralgia,” “immunoglobulin”; cluster 2 (in green) includes “genetics,” “virology,” “isolation and purification”; and cluster 3 (in blue) highlights the terms “epidemics,” “disease outbreaks,” “alphavirus infection”. VOSviewer also assigned colors to chikungunya-related terms based on publication years (Fig. 3b). Purple indicates that the terms appear in the early years, whereas the yellow color is designated for terms that emerge in recent years. The progress of research on Chikungunya virus shows a dynamic change over time, with a focus on “Aedes”, “disease outbreaks,” and “alphavirus infection” in 2013; “epidemic,” “reverse transcriptase polymerase,” and “antibodies” in 2014; “isolation and purification,” “arthralgia,” and “dengue” in 2015. In 2016, research on “virology,” “animal cells,” “mice,” and “immunology” gained a significant attention in the field of chikungunya studies. Importantly, from 2017 onwards, the published articles mainly reported on “virus replication,” “metabolism,” “physiology,” “antivirus agent,” and “genetics” chikungunya virus, indicating that these terms are emerging and gaining significant attention in the last few years. The institutions from ASEAN and South Asian countries with the highest productivity in the field of chikungunya from 1967 to 2022 were also listed, highlighting the prominent contributions of India, Singapore, and Thailand (Table 4).
Fig. 3.
Co-occurrence network of terms extracted from articles published by ASEAN and South Asian countries in chikungunya virus-related articles from 1967 to 2022. The minimum number of occurrences was set to 100 times. Of the 7810 terms, 56 were included. a Network visualization; b Overlay visualization. Terms highlighted in blue appeared earlier than those assigned in yellow
Discussion
Our research provided new perspectives on the scientific contribution of ASEAN and South Asian countries to chikungunya virus-related research. In general, ASEAN and South Asian countries contribute 31.11% of the global production of chikungunya virus-related studies. In 2006, the number of articles published by ASEAN and South Asian countries increased dramatically, from 2 documents in 2005 to 19 documents in 2006. After 2006, a gradual increase in chikungunya-related documents produced by ASEAN countries was observed, indicating that chikungunya virus-related topics are gaining prominence as a human health concern. A closer examination of the documents published in 2006 reveals that India, with 15 documents, contributed the most articles. Articles published in 2006 mostly discussed the re-emergence of the chikungunya virus in India and Malaysia [102, 110]. Meanwhile, in 2009, Tan Tock Seng Hospital, Singapore, and the Agency for Science, Technology and Research, Singapore were among the numerous organizations that reported the epidemiology of a chikungunya outbreak in Singapore [60, 111, 112]. Notably, 2021 has the highest productivity for ASEAN and South Asian countries, with 118 (9.21%) documents published. Consistent with the overall results shown in Table 1, India ranked first in 2021 with the highest output, publishing 63 (4.92%) documents. In accordance with other bibliometric studies analyzing the research productivity of ASEAN and South Asian countries [113–116], the current study also highlighted the prominent contributions of India, Thailand, and Singapore in scientific research. Recently, consecutive chikungunya epidemics have occurred in developing countries [60, 63, 86, 87, 89, 111, 112, 117–120], which may also explain the increasing number of ASEAN and South Asian countries participating in chikungunya-related studies.
In bibliometric analysis, directly evaluating the weight or effect of publications is difficult. However, a number of studies argued that important insights could be obtained by analyzing the relationship between article relevancy and journal significance [121, 122]. In addition, the top ten most cited articles may provide hints into the current trends, the changing landscape of research topics, and important directions for future research, as shown in Table 3.
After 2017, the topics of antiviral activity and neutralizing antibodies are emerging as the new focus on chikungunya-related studies in ASEAN and South Asian countries. Effective antiviral strategies are required to combat the rising prevalence of chikungunya virus infection and reduce mortality. Because there are no effective vaccines, significant research has been conducted to discover potent antivirals against the chikungunya virus (Table 5). In parallel, repurposing currently available drugs to treat chikungunya infections has been proposed and intensively investigated as an alternative [42, 43, 46, 123–129]. Determining novel compounds with anti-chikungunya virus properties has also been a focus of research in recent years [47, 130–143]. Additionally, in silico methods have been employed to find promising molecules against chikungunya virus [144–153].
Table 5.
Recent advances in the development of drugs for alternative treatment of chikungunya
| Repurposing commercially available drugs | |||
| No. | Drugs | Original purpose/disease target | References |
| 1 | Chloroquine | malaria | [42, 43, 123, 124] |
| 2 | Arbidol | influenza | [125, 126] |
| 3 | Imipramine | antidepressant drug | [46] |
| 4 | Ribavirin | Respiratory syncytial virus and hepatitis c virus | [127–129] |
| Novel compounds with antiviral properties | |||
| No. | Targeted pathway or process | Compounds | References |
| 1 | Entry and binding | Flavagline FL3, FL23, and sulfonyl amidine 1 m | [47] |
| 2 | Curcumin | [130] | |
| 3 | Replication | Andrographolide | [131] |
| 4 | Mycophenolic acid (MPA) | [132] | |
| 5 | 6-azauridine | [133] | |
| 6 | Suramin | [134] | |
| 7 | Harringtonine | [135] | |
| 8 | debromoaplysiatoxin and 3-methoxydebromoaplysiatoxin | [136] | |
| 9 | Phorbol-12, 13-didecanoate | [137] | |
| 10 | Salicylate-derived Bryostatin analogues | [138] | |
| 11 | Geldanamycin | [139] | |
| 12 | Jatrophane ester | [140] | |
| 13 | Trigocherrins A B, and F | [141] | |
| 14 | MBZM-N-IBT | [142] | |
| 15 | Abamectin, ivermectin, and berberine | [143] | |
| Promising molecules detected via in silico molecular docking | |||
| No. | Protein targets | Compounds | References |
| 1 | Non-structural Protein 2 (nsP2) | Astragaloside II-IV | [144] |
| 2 | ASN 01107557 and ASN 01541696 | [145] | |
| 3 | CID_5808891 | [146] | |
| 4 | Structural protein E3 | Arjungenin | [147] |
| 5 | Non-structural Protein 3 (nsP3) | CMPD178 | [148] |
| 6 | Hesperetin | [149] | |
| 7 | Baicalin, Rutaecarpine, Amentoflavone, Apigetrin, Luteoloside, and Baloxavir | [150] | |
| 8 | Baicalin | [151] | |
| 9 | Non-structural Protein 4 (nsP4) | Mitoxantrone hydrochloride | [152] |
| 10 | LabMol-309 | [153] | |
| 11 | Capsid protein | Catechin-5-O-gallate and Rosmarinic acid | [147] |
The majority of global efforts have been devoted to concentrating on treating symptoms and preventive measures, because there are no commercially available vaccines or medications to cure chikungunya infections [15]. The mitigation of mosquito bites, vector control, and disease containment are among the priorities of prevention efforts [154]. Meanwhile, to treat the infections, there are several pharmacologic and non-pharmacologic approaches have been developed. Systematic changes in lifestyle such as dietary adjustments, adequate fluid consumption, physiotherapy, and enough bed rest are examples of non-pharmacologic interventions, that are essential to supporting the work of the immune system in combating the virus [154–156]. On the other hand, pharmacologic therapies use medications to treat symptoms, such as joint pain and fever [106, 157]. Webb et al. reviewed the global Clinical Management Guidelines (CMGs) for chikungunya infection, reporting a lack of consistency in the classification of disease stages [158]. They also highlighted a variation in the prescriptions of corticosteroids and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [158], which are essential in symptomatic treatment for individuals infected with the chikungunya virus [159]. Despite having a low risk of mortality, the chikungunya virus can be lethal for children and the elderly [160, 161]. The current CMGs for these vulnerable population groups, on the other hand, were limited and varied [158]. Transmission of the chikungunya virus could also be prevented by cutting the reproductive cycles of the vectors, A. aegypti and A. albopictus [154, 162]. Effective interventions include using larvicides, removing larval breeding sites, and reducing contact between humans and vectors [154, 163, 164]. Additionally, special care for infected individuals is critical part of the preventive measures, since they may contribute to the cycle of transmission.
This study uses a bibliometric technique to assess the current state and trajectory of chikungunya virus-related research published by ASEAN and South Asian countries. However, a few limitations, which also appear in other bibliometric analyses, were identified. First, our study registered the key term “chikungunya” only for title searches. Our analysis may have overlooked any publications that utilized the keyword “chikungunya” in the abstract or within the publication. Second, this study focused on publications indexed in the Scopus library. Although Scopus is the most widely used and trusted database, a few outlier papers may have been left out. Despite these limitations, our bibliometric study is the first to provide a concise overview of the chikungunya virus-related research profile by ASEAN and South Asian countries. Our research also demonstrates how bibliometric analysis may be used to estimate research productivity in the field of chikungunya.
Conclusions
Our study is the first to present the contribution of ASEAN and South Asian countries to chikungunya virus-related research. By integrating literature review and bibliometric analysis, the current study aimed to provide an outline of the progress, current trend, and emerging topics in the field of chikungunya in ASEAN and South Asian countries. Over the past two decades, the research productivity on chikungunya virus-related topics in ASEAN and South Asian countries has increased remarkably, with a total of 1280 articles published in prominent journals. Previously, researchers prioritized studies related to the vector, infection, and nucleotide sequence of the chikungunya virus. However, in recent years, the research topics have shifted to the development of antiviral drugs and the genetics of chikungunya virus. Out of ten ASEAN countries, Thailand contributes the highest number of articles related to chikungunya, followed by Singapore. Meanwhile, India ranks first among South Asian countries in terms of productivity. The findings of this study can serve as a reference for ongoing chikungunya virus-related research and policymakers working in the field of healthcare, particularly in ASEAN and South Asian countries.
Acknowledgments
Not applicable.
Authors’ contributions
Conceptualization, F.S., N.W., and A.F.; methodology, F.S., D.S.P., and N.I.S.; validation, F.S., W.A.P., A.F., and N.W.; formal analysis, F.S., W.A.P., and N.W.; investigation, F.S., D.S.P., N.I.S., and W.A.P.; data curation, F.S., and D.S.P; writing—original draft preparation, F.S., D.S.P., W.A.P. and N.I.S.; visualization, F.S. and A.F., writing—review and editing, A.F., N.W., W.A.R., S.A.T., M.G., M.A., B.R.A., A.S.A., A.H.A., A. Alawfi, A. Ashengeti, M.H.A., S.A., A.A.R.; supervision, A.F. N.W., project administration, A.F., N.W., and A.A.R., funding acquisition, A.A.R, A.F., S.A.T., M.G., M.A., B.R.A., A.S.A., A.H.A., A. Alawfi, A. Ashengeti, M.H.A., and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Other datasets used during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
As this is a bibliometric study, without human involvement, there was no need for ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andri Frediansyah, Email: andri.frediansyah@brin.go.id.
Nastiti Wijayanti, Email: nastiti_wijayanti@ugm.ac.id.
Ali A. Rabaan, Email: arabaan@gmail.com
References
- 1.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952-53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 2.Ross RW. The newala epidemic: III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amraoui F, Failloux AB. Chikungunya: an unexpected emergence in Europe. Curr Opin Virol. 2016;21:146–150. doi: 10.1016/j.coviro.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Petersen LR, Powers AM. Chikungunya: epidemiology. F1000Res. 2016;5:1–9. Faculty of 1000 Ltd. [DOI] [PMC free article] [PubMed]
- 5.Pulmanausahakul R, Roytrakul S, Auewarakul P, Smith DR. Chikungunya in Southeast Asia: understanding the emergence and finding solutions. Int J Infect Dis. 2011;15:e671–e676. doi: 10.1016/j.ijid.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Russo G, Subissi L, Rezza G. Chikungunya fever in Africa: a systematic review. Pathog Glob Health. 2020;114:136–144. doi: 10.1080/20477724.2020.1748965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wimalasiri-Yapa BMCR, Stassen L, Huang X, Hafner LM, Hu W, Devine GJ, et al. Chikungunya virus in Asia–Pacific: a systematic review. Emerg Microbes Infect. 2019;8:70–79. doi: 10.1080/22221751.2018.1559708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yactayo S, Staples JE, Millot V, Cibrelus L, Ramon-Pardo P. Epidemiology of chikungunya in the Americas. J Infect Dis. 2016;214:S441–5. Oxford University Press. [DOI] [PMC free article] [PubMed]
- 9.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 10.Shragai T, Tesla B, Murdock C, Harrington LC. Zika and chikungunya: mosquito-borne viruses in a changing world. Ann N Y Acad Sci. 2017;1399:61–77. doi: 10.1111/nyas.13306. [DOI] [PubMed] [Google Scholar]
- 11.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 12.Consuegra-Rodríguez MP, Hidalgo-Zambrano DM, Vásquez-Serna H, Jimenez-Canizales CE, Parra-Valencia E, Rodriguez-Morales AJ. Post-chikungunya chronic inflammatory rheumatism: follow-up of cases after 1 year of infection in Tolima, Colombia. Travel Med Infect Dis. 2018;21:62–68. doi: 10.1016/j.tmaid.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Weinbren MP, Haddow AJ, Williams MC. The occurrence of chikungunya virus in Uganda I. isolation from mosquitoes. Trans R Soc Trop Med Hyg. 1958;52:253–262. doi: 10.1016/0035-9203(58)90084-1. [DOI] [PubMed] [Google Scholar]
- 14.Lumsden WH. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952-53. II. General description and epidemiology. Trans R Soc Trop Med Hyg. 1955;49:33–57. doi: 10.1016/0035-9203(55)90081-x. [DOI] [PubMed] [Google Scholar]
- 15.Khongwichit S, Chansaenroj J, Chirathaworn C, Poovorawan Y. Chikungunya virus infection: molecular biology, clinical characteristics, and epidemiology in Asian countries. J Biomed Sci. 2021;28:84. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 16.Mahendradas P, Avadhani K, Shetty R. Chikungunya and the eye: a review. J Ophthalmic Inflamm Infect. 2013;3:1–9. doi: 10.1186/1869-5760-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Pulgarín DF, Muñoz-Urbano DM, Zamora-de la Cruz D. The eye and the chikungunya virus. In: Current Topics in Chikungunya. London: IntechOpen; 2016. p. 49–66.
- 18.Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127:737–749. doi: 10.1172/JCI84417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balavoine S, Pircher M, Hoen B, Herrmann-Storck C, Najioullah F, Madeux B, et al. Guillain-Barré syndrome and chikungunya: description of all cases diagnosed during the 2014 outbreak in the French West Indies. Am J Trop Med Hyg. 2017;97:356–360. doi: 10.4269/ajtmh.15-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerny T, Schwarz M, Schwarz U, Lemant J, Gérardin P, Keller E. The range of neurological complications in Chikungunya fever. Neurocrit Care. 2017;27:447–457. doi: 10.1007/s12028-017-0413-8. [DOI] [PubMed] [Google Scholar]
- 21.de la Hoz JM, Bayona B, Viloria S, Accini JL, Juan-Vergara HS, Viasus D. Fatal cases of Chikungunya virus infection in Colombia: diagnostic and treatment challenges. J Clin Virol. 2015;69:27–29. doi: 10.1016/j.jcv.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Mehta R, Gerardin P, de Brito CAA, Soares CN, Ferreira MLB, Solomon T. The neurological complications of chikungunya virus: a systematic review. Rev Med Virol. 2018;28:e1978. John Wiley and Sons Ltd. [DOI] [PMC free article] [PubMed]
- 23.Mercado M, Acosta-Reyes J, Parra E, Guzmán L, Beltrán M, Gasque P, et al. Renal involvement in fatal cases of chikungunya virus infection. J Clin Virol. 2018;103:16–18. doi: 10.1016/j.jcv.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 24.van Aalst M, Nelen CM, Goorhuis A, Stijnis C, Grobusch MP. Long-term sequelae of chikungunya virus disease: a systematic review. Travel Med Infect Dis. 2017;15:8–22. doi: 10.1016/j.tmaid.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013;36:211–227. [PubMed] [Google Scholar]
- 26.Althouse BM, Guerbois M, Cummings DAT, Diop OM, Faye O, Faye A, et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat Commun. 2018;9:1046. Nature Publishing Group. [DOI] [PMC free article] [PubMed]
- 27.Chevillon C, Briant L, Renaud F, Devaux C. The Chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 2008;16:80–88. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 29.Kading RC, Borland EM, Cranfield M, Powers AM. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J Wildl Dis. 2013;49:587–599. doi: 10.7589/2012-08-212. [DOI] [PubMed] [Google Scholar]
- 30.Pruetz J, Socha A, Kante D. New range record for the lesser spot-nosed guenon (Cercopithecus petaurista) in southeastern Senegal. Afr Primates. 2010;7:64–6.
- 31.Horwood P, Bande G, Dagina R, Guillaumot L, Aaskov J, Pavlin B. The threat of chikungunya in Oceania. West Pacific Surveill Resp J WPSAR. 2013;4:8–10. doi: 10.5365/WPSAR.2013.4.2.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngoagouni C, Kamgang B, Kazanji M, Paupy C, Nakouné E. Potential of Aedes aegypti and Aedes albopictus populations in the Central African Republic to transmit enzootic chikungunya virus strains. Parasit Vectors. 2017;10:164. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 33.Vega-Rúa A, Zouache K, Girod R, Failloux A-B, Lourenço-de-Oliveira R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J Virol. 2014;88:6294–306. American Society for Microbiology. [DOI] [PMC free article] [PubMed]
- 34.Vega-Rúa A, Lourenço-De-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yébakima A, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9:e0003780. Public Library of Science. [DOI] [PMC free article] [PubMed]
- 35.Weaver SC. Evolutionary influences in arboviral disease. Curr Top Microbiol Immunol. 2006;299:285–314. doi: 10.1007/3-540-26397-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeller H, Van Bortel W, Sudre B. Chikungunya: its history in Africa and Asia and its spread to new regions in 2013-2014. J Infect Dis. 2016;214:S436–S440. doi: 10.1093/infdis/jiw391. [DOI] [PubMed] [Google Scholar]
- 37.Carrillo-Hernández MY, Ruiz-Saenz J, Villamizar LJ, Gómez-Rangel SY, 577 Martínez-Gutierrez M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect Dis. 2018;18:61. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 38.Le Coupanec A, Tchankouo-Nguetcheu S, Roux P, Khun H, Huerre M, Morales-Vargas R, et al. Co-infection of mosquitoes with chikungunya and dengue viruses reveals modulation of the replication of both viruses in midguts and salivary glands of Aedes aegypti mosquitoes. Int J Mol Sci. 2017;18:1708. MDPI AG. [DOI] [PMC free article] [PubMed]
- 39.Rückert C, Weger-Lucarelli J, Garcia-Luna SM, Young MC, Byas AD, Murrieta RA, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017;8:15412. Nature Publishing Group. [DOI] [PMC free article] [PubMed]
- 40.Jain M, Rai S, Chakravarti A. Chikungunya: a review. Trop Dr. 2008;38:70–72. doi: 10.1258/td.2007.070019. [DOI] [PubMed] [Google Scholar]
- 41.Kaur P, Chu JJH. Chikungunya virus: an update on antiviral development and challenges. Drug Discov Today. 2013;18:969–983. doi: 10.1016/j.drudis.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Lamballerie X, Boisson V, Reynier JC, Enault S, Charrel RN, Flahault A, et al. On chikungunya acute infection and chloroquine treatment. Vector-Borne Zoonotic Dis. 2008;8:837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 43.Khan M, Santhosh SR, Tiwari M, Lakshmana Rao PV, Parida M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in Vero cells. J Med Virol. 2010;82:817–824. doi: 10.1002/jmv.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pohjala L, Utt A, Varjak M, Lulla A, Merits A, Ahola T, et al. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS One. 2011;6:e28923. [DOI] [PMC free article] [PubMed]
- 45.Subudhi BB, Chattopadhyay S, Mishra P, Kumar A. Current strategies for inhibition of Chikungunya infection. Viruses. 2018;10:235. MDPI AG. [DOI] [PMC free article] [PubMed]
- 46.Wichit S, Hamel R, Bernard E, Talignani L, Diop F, Ferraris P, et al. Imipramine inhibits Chikungunya virus replication in human skin fibroblasts through interference with intracellular cholesterol trafficking. Sci Rep. 2017;7:3145. Nature Publishing Group. [DOI] [PMC free article] [PubMed]
- 47.Wintachai P, Thuaud F, Basmadjian C, Roytrakul S, Ubol S, Désaubry L, et al. Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol Immunol. 2015;59:129–141. doi: 10.1111/1348-0421.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wintachai P, Wikan N, Kuadkitkan A, Jaimipuk T, Ubol S, Pulmanausahakul R, et al. Identification of prohibitin as a Chikungunya virus receptor protein. J Med Virol. 2012;84:1757–1770. doi: 10.1002/jmv.23403. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa K. The ASEAN economic community and ASEAN economic integration. J Contemp East Asia Stud. 2021;10:24–41. [Google Scholar]
- 50.Hulshof P, Doets E, Seyha S, Bunthang T, Vonglokham M, Kounnavong S, et al. Food composition tables in Southeast Asia: the contribution of the SMILING project. Matern Child Health J. 2019;23:46–54. doi: 10.1007/s10995-018-2528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipoeto NI, Geok Lin K, Angeles-Agdeppa I. Food consumption patterns and nutrition transition in South-East Asia. Public Health Nutr. 2013;16:1637–1643. doi: 10.1017/S1368980012004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bordier M, Roger F. Zoonoses in South-East Asia: a regional burden, a global threat. Anim Health Res Rev. 2013;14:40–67. doi: 10.1017/S1466252313000017. [DOI] [PubMed] [Google Scholar]
- 53.Hassan L. Emerging Zoonoses in domesticated livestock of Southeast Asia. Encycl Agric Food Syst. 2014;3:68–81.
- 54.Clemmons EA, Alfson KJ, Dutton JW. Transboundary animal diseases, an overview of 17 diseases with potential for global spread and serious consequences. Animals. 2021;11:1–58. doi: 10.3390/ani11072039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in Southeast Asia: regional challenges to control. Lancet. 2011;377:599–609. doi: 10.1016/S0140-6736(10)62004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin HI, Yu YY, Wen FI, Liu PT. Status of food security in east and South-East Asia and challenges of climate change. Climate. 2022;10:40.
- 57.Chew LP, Chua HH. Outbreak of Chikungunya in Johor Bahru, Malaysia: clinical and laboratory features of hospitalized patients. Med J Malaysia. 2009;64:220–222. [PubMed] [Google Scholar]
- 58.Harapan H, Michie A, Mudatsir M, Nusa R, Yohan B, Wagner AL, et al. Chikungunya virus infection in Indonesia: a systematic review and evolutionary analysis. BMC Infect Dis. 2019;19:243. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 59.Khongwichit S, Chansaenroj J, Thongmee T, Benjamanukul S, Wanlapakorn N, Chirathaworn C, et al. Large-scale outbreak of Chikungunya virus infection in Thailand, 2018-2019. PLoS One. 2021;16:2018–2019. doi: 10.1371/journal.pone.0247314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leo YS, Chow AL, Tan LK, Lye DC, Lin L, Ng LC. Chikungunya Outbreak,Singapore, 2008. Emerg Infect Dis. 2009;15:836–837. doi: 10.3201/eid1505.081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravi V. Re-emergence of Chikungunya virus in India. Indian J Med Microbiol. 2006;24:83–84. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]
- 62.Powers AM, Logue CH. Changing patterns of chikunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 63.Sharif N, Sarkar MK, Ferdous RN, Ahmed SN, Billah MB, Talukder AA, et al. Molecular epidemiology, evolution and reemergence of Chikungunya virus in South Asia. Front Microbiol. 2021;12:1–14. doi: 10.3389/fmicb.2021.689979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of chikungunya and o’nyong-nyong viruses : evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 65.Lo Presti A, Cella E, Angeletti S, Ciccozzi M. Molecular epidemiology, evolution and phylogeny of Chikungunya virus: an updating review. Infect Genet Evol. 2016;41:270–278. doi: 10.1016/j.meegid.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Wahid B, Ali A, Rafique S, Idrees M. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017;58:69–76. doi: 10.1016/j.ijid.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Deeba F, Haider MSH, Ahmed A, Tazeen A, Faizan MI, Salam N, et al. Global transmission and evolutionary dynamics of the Chikungunya virus. Epidemiol Infect. 2020; 148:e63. [DOI] [PMC free article] [PubMed]
- 68.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney M-C, et al. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS med. Public library of. Science. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pyke AT, Moore PR, McMahon J. New insights into chikungunya virus emergence and spread from Southeast Asia. Emerg Microbes Infect. 2018;7:9–12. doi: 10.1038/s41426-018-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villamil-Gómez WE, Rodríguez-Morales AJ, Uribe-García AM, González-Arismendy E, Castellanos JE, Calvo EP, et al. Zika, dengue, and chikungunya co-infection in a pregnant woman from Colombia. Int J Infect Dis. 2016;51:135–138. doi: 10.1016/j.ijid.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 71.Furuya-Kanamori L, Liang S, Milinovich G, Soares Magalhaes RJ, Clements ACA, Hu W, et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16:84. [DOI] [PMC free article] [PubMed]
- 72.Al-Jabi SW. Global research trends in West Nile virus from 1943 to 2016: a bibliometric analysis. Glob Health. 2017;13:55. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 73.Li L, Ma X, Pandey S, Fan A, Deng X, Cui D. Bibliometric analysis of journals in the field of endoscopic Endonasal surgery for pituitary adenomas. J Craniofac Surg. 2018;29:e83–e87. doi: 10.1097/SCS.0000000000004133. [DOI] [PubMed] [Google Scholar]
- 74.Ekundayo TC, Okoh AI. A global bibliometric analysis of Plesiomonas- related research (1990–2017). PLoS One. 2018;13:e0207655. Public Library of Science. [DOI] [PMC free article] [PubMed]
- 75.Sofyantoro F, Yudha DS, Lischer K, Nuringtyas TR, Putri WA, Kusuma WA, et al. Bibliometric analysis of literature in Snake venom-related research worldwide (1933–2022) Animals. 2022;12:2058. doi: 10.3390/ani12162058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baier-Fuentes H, González-Serrano MH, Alonso-Dos Santos M, Inzunza- Mendoza W, Pozo-Estrada V. Emotions and sport management: a Bibliometric overview. Front Psychol. 2020;11:1512. Frontiers Media S.A. [DOI] [PMC free article] [PubMed]
- 77.Budler M, Župič I, Trkman P. The development of business model research: a bibliometric review. J Bus Res. 2021;135:480–495. [Google Scholar]
- 78.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM. Global cocaine intoxication research trends during 1975–2015: a bibliometric analysis of web of science publications. Subst Abus Treat Prev Policy. 2017;12:6. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 79.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM. Bibliometric profile of global scientific research on digoxin toxicity (1849–2015) Drug Chem Toxicol. 2020;43:553–559. doi: 10.1080/01480545.2018.1518453. [DOI] [PubMed] [Google Scholar]
- 80.Hamidah I, Pawinanto RE, Mulyanti B, Yunas J. A bibliometric analysis of micro electro mechanical system energy harvester research. Heliyon. 2021;7:e06406. Elsevier Ltd. [DOI] [PMC free article] [PubMed]
- 81.Su M, Peng H, Li S. A visualized bibliometric analysis of mapping research trends of machine learning in engineering (MLE). Expert Syst Appl. 2021;186:115728. Elsevier Ltd.
- 82.Hou Y, Wang Q. A bibliometric study about energy, environment, and climate change. Environ Sci Pollut Res. 2021;28:34187–34199. doi: 10.1007/s11356-021-14059-2. [DOI] [PubMed] [Google Scholar]
- 83.Srivastav AL, Kaur T, Rani L, Kumar A. Scientific research production of India and China in environmental chemistry: a bibliometric assessment. Int J Environ Sci Technol. 2019;16:4989–4996. [Google Scholar]
- 84.Ram S. A quantitative assessment of “chikungunya” research publications, 2004-2013. Trop J Med Res. 2016;19:52. [Google Scholar]
- 85.Vera-Polania F, Muñoz-Urbano M, Bañol-Giraldo AM, Jimenez-Rincón M, Granados-Álvarez S, Rodriguez-Morales AJ. Bibliometric assessment of scientific production of literature on chikungunya. J Infect Public Health. 2015;8:386–388. doi: 10.1016/j.jiph.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Amin MR, Hasan MJ, Khan MAS, Rafi MA, Islam R, Shams T, et al. Chikungunya outbreak in Bangladesh (2017): Sociodemographic and clinical characteristics of patients from three hotspots. Trop Med Health. 2022;50:9. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 87.Chan TC, Hsu YF, Huang SC, Chen RC. Rapidly containing the first indigenous outbreak of chikungunya in Taiwan—lessons learned. Trop Med Infect Dis. 2021;6:165. MDPI. [DOI] [PMC free article] [PubMed]
- 88.Intayot P, Phumee A, Kraivichian K, Sor-suwan S, Boonserm R, Siriyasatien P. Genetic characterization of chikungunya virus isolates from Aedes aegypti mosquitoes collected during a recent outbreak in Bangkok, Thailand. Arch Virol. 2021;166:3387–98. Springer. [DOI] [PubMed]
- 89.Kyaw AK, Ngwe Tun MM, Nabeshima T, Soe AM, Mon KM, Thida, et al. Chikungunya virus infection in blood donors and patients during outbreak, Mandalay, Myanmar, 2019. Emerg Infect Dis. 2020;26:2741–2745. doi: 10.3201/eid2611.201824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lutomiah J, Mulwa F, Mutisya J, Koskei E, Langat S, Nyunja A, et al. Probable contribution of Culex quinquefasciatus mosquitoes to the circulation of chikungunya virus during an outbreak in Mombasa County, Kenya, 2017–2018. Parasit Vectors. 2021;14. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 91.Prakoso D, Barr K, Imtiaz K, Farooqi J, Khan E, Long M. Chikungunya outbreak in Karachi Pakistan 2016-2017: an analysis of viral isolates. J Pak Med Assoc. 2021;71:1467–1471. doi: 10.47391/JPMA.1287. [DOI] [PubMed] [Google Scholar]
- 92.Rodríguez-Aguilar ED, Martínez-Barnetche J, González-Bonilla CR, Tellez-Sosa JM, Argotte-Ramos R, Rodríguez MH. Genetic diversity and spatiotemporal dynamics of Chikungunya infections in Mexico during the outbreak of 2014–2016. Viruses. 2022;14:70. MDPI. [DOI] [PMC free article] [PubMed]
- 93.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, web of science, and Google scholar: strengths and weaknesses. FASEB J. 2008;22:338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 94.Kulkarni AV, Aziz B, Busse JW. Comparisons of citations in web of science, Scopus, and Google scholar for articles published in general medical journals. JAMA. 2009;302:1092–1096. doi: 10.1001/jama.2009.1307. [DOI] [PubMed] [Google Scholar]
- 95.Al-Jabi SW. Arab world’s growing contribution to global leishmaniasis research (1998–2017): a bibliometric study. BMC Public Health. 2019;19:625. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 96.Zyoud SH, Sweileh WM, Awang R, Al-Jabi SW. Global trends in research related to social media in psychology: mapping and bibliometric analysis. Int J Ment Heal Syst. 2018;12:4. BioMed Central Ltd. [DOI] [PMC free article] [PubMed]
- 97.Sofyantoro F, Kusuma HI, Vento S, Rademaker M, Frediansyah A. Global research profile on monkeypox-related literature (1962–2022): a bibliometric analysis. Narra J. 2022;2:1–11. doi: 10.52225/narra.v2i3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Inf Secur. 2017;11:959–975. [Google Scholar]
- 99.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pialoux G, Gaüzère B-A, Jauréguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 101.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 102.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chow A, Her Z, Ong EKS, Chen JM, Dimatatac F, Kwek DJC, et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203:149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, Jadhav SM, et al. Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J Gen Virol. 2007;88:1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 105.Ng LFP, Chow A, Sun YJ, Kwek DJC, Lim PL, Dimatatac F, et al. IL-1β, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One. 2009;4:1–8. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burt FJ, Chen W, Miner JJ, Lenschow DJ, Merits A, Schnettler E, et al. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis. 2017;17:e107–e117. doi: 10.1016/S1473-3099(16)30385-1. [DOI] [PubMed] [Google Scholar]
- 107.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, Djauzi A, et al. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Her Z, Malleret B, Chan M, Ong EKS, Wong S-C, Kwek DJC, et al. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J Immunol. 2010;184:5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 109.Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14:412–415. doi: 10.3201/eid1403.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumarasamy V, Prathapa S, Zuridah H, Chem YK, Norizah I, Chua KB. Re-emergence of Chikungunya virus in Malaysia. Med J Malaysia. 2006;61:221–225. [PubMed] [Google Scholar]
- 111.Lim PL, May-Lin OH, Ooi EE. Chikungunya in Singapore: imported cases among travelers visiting friends and relatives. J Travel Med. 2009;16:289–291. doi: 10.1111/j.1708-8305.2009.00313.x. [DOI] [PubMed] [Google Scholar]
- 112.Ng KW, Chow ALP, Win MK, Dimatatac F, Neo HY, Lye DC, et al. Clinical features and epidemiology of chikungunya infection in Singapore. Singap Med J. 2009;50:785–790. [PubMed] [Google Scholar]
- 113.Tantengco OAG, Rojo RD. Bibliometric analysis of schistosomiasis research in Southeast Asia (1908–2020) Acta Trop. 2022;228:106322. doi: 10.1016/j.actatropica.2022.106322. [DOI] [PubMed] [Google Scholar]
- 114.Hew JJ, Lee VH, Ooi KB, Lin B. Computer science in ASEAN: a ten-year Bibliometric analysis (2009–2018) J Comput Inf Syst. 2021;61:247–255. [Google Scholar]
- 115.Ibrahim C, Hardiyati R, Ayunda WA, Fadhli R. Comparative study of Asean countries research productivity in library science. Webology. 2021;18:371–388. [Google Scholar]
- 116.Zhou YX, Cao XY, Peng C. A bibliometric analysis of the 100 most-cited articles on curcumin. Front Pharmacol. 2022;13:1–11. doi: 10.3389/fphar.2022.963032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jain J, Kaur N, Haller SL, Kumar A, Rossi SL, Narayanan V, et al. Chikungunya outbreaks in India: a prospective study comparing neutralization and sequelae during two outbreaks in 2010 and 2016. Am J Trop Med Hyg. 2020;102:857–868. doi: 10.4269/ajtmh.19-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sitepu FY, Suprayogi A, Pramono D, Harapan H, Mudatsir M. Epidemiological investigation of chikungunya outbreak, West Kalimantan, Indonesia. Clin Epidemiol Glob Heal. 2020;8:113–116. [Google Scholar]
- 119.Badar N, Salman M, Ansari J, Ikram A, Qazi J, Alam MM. Epidemiological trend of chikungunya outbreak in Pakistan: 2016-2018. PLoS Negl Trop Dis. 2019;13:2018–2019. doi: 10.1371/journal.pntd.0007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reller ME, Akoroda U, Nagahawatte A, Devasiri V, Kodikaarachchi W, Strouse JJ, et al. Chikungunya as a cause of acute febrile illness in southern Sri Lanka. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0082259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baldiotti ALP, Amaral-Freitas G, Barcelos JF, Freire-Maia J, De França PM, Freire-Maia FB, et al. The top 100 Most-cited papers in Cariology: a Bibliometric analysis. Caries Res. 2021;55:32–40. doi: 10.1159/000509862. [DOI] [PubMed] [Google Scholar]
- 122.Zhang S, Fan H, Zhang Y. The 100 top-cited studies on dyslexia research: a Bibliometric analysis. Front Psychiatry. 2021;12:714627. Frontiers Media SA. [DOI] [PMC free article] [PubMed]
- 123.Brighton SW. Chloroquine phosphate treatment of chronic Chikungunya arthritis. An open pilot study. South African Med J. 1984;66:217–218. [PubMed] [Google Scholar]
- 124.Roques P, Thiberville SD, Dupuis-Maguiraga L, Lum FM, Labadie K, Martinon F, et al. Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses. 2018;10:1–18. doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Delogu I, Pastorino B, Baronti C, Nougairède A, Bonnet E, de Lamballerie X. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antivir Res. 2011;90:99–107. doi: 10.1016/j.antiviral.2011.03.182. [DOI] [PubMed] [Google Scholar]
- 126.Di Mola A, Peduto A, La Gatta A, Delang L, Pastorino B, Neyts J, et al. Structure-activity relationship study of arbidol derivatives as inhibitors of chikungunya virus replication. Bioorg Med Chem. 2014;22:6014–6025. doi: 10.1016/j.bmc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 127.Gallegos KM, Drusano GL, D’Argenio DZ, Brown AN. Chikungunya virus: in vitro response to combination therapy with ribavirin and interferon Alfa 2a. J Infect Dis. 2016;214:1192–1197. doi: 10.1093/infdis/jiw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ravichandran R, Manian M. Ribavirin therapy for Chikungunya arthritis. J Infect Dev Ctries. 2008;2:140–142. [PubMed] [Google Scholar]
- 129.Rothan HA, Bahrani H, Mohamed Z, Teoh TC, Shankar EM, Rahman NA, et al. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 131.Wintachai P, Kaur P, Lee RCH, Ramphan S, Kuadkitkan A, Wikan N, et al. Activity of andrographolide against chikungunya virus infection. Sci Rep. 2015;5:1–14. doi: 10.1038/srep14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan M, Dhanwani R, Patro IK, Rao PVL, Parida MM. Cellular IMPDH enzyme activity is a potential target for the inhibition of Chikungunya virus replication and virus induced apoptosis in cultured mammalian cells. Antivir Res. 2011;89:1–8. doi: 10.1016/j.antiviral.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 133.Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-α and ribavirin combination. Antivir Res. 2004;61:111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 134.Albulescu IC, Van Hoolwerff M, Wolters LA, Bottaro E, Nastruzzi C, Yang SC, et al. Suramin inhibits chikungunya virus replication through multiple mechanisms. Antivir Res. 2015;121:39–46. doi: 10.1016/j.antiviral.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 135.Kaur P, Thiruchelvan M, Lee RCH, Chen H, Chen KC, Ng ML, et al. Inhibition of Chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother. 2013;57:155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gupta DK, Kaur P, Leong ST, Tan LT, Prinsep MR, Chu JJH. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar Drugs. 2014;12:115–27. [DOI] [PMC free article] [PubMed]
- 137.Nothias-Scaglia LF, Pannecouque C, Renucci F, Delang L, Neyts J, Roussi F, et al. Antiviral activity of Diterpene esters on Chikungunya virus and HIV replication. J Nat Prod. 2015;78:1277–1283. doi: 10.1021/acs.jnatprod.5b00073. [DOI] [PubMed] [Google Scholar]
- 138.Staveness D, Abdelnabi R, Near KE, Nakagawa Y, Neyts J, Delang L, et al. Inhibition of Chikungunya virus-induced cell death by salicylate-derived Bryostatin analogues provides additional evidence for a PKC-independent pathway. J Nat Prod. 2016;79:680–684. doi: 10.1021/acs.jnatprod.5b01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rathore APS, Haystead T, Das PK, Merits A, Ng ML, Vasudevan SG. Chikungunya virus nsP3 & nsP4 interacts with HSP-90 to promote virus replication: HSP-90 inhibitors reduce CHIKV infection and inflammation in vivo. Antivir Res. 2014;103:7–16. doi: 10.1016/j.antiviral.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 140.Nothias-Scaglia LF, Retailleau P, Paolini J, Pannecouque C, Neyts J, Dumontet V, et al. Jatrophane diterpenes as inhibitors of chikungunya virus replication: structure-activity relationship and discovery of a potent lead. J Nat Prod. 2014;77:1505–1512. doi: 10.1021/np500271u. [DOI] [PubMed] [Google Scholar]
- 141.Allard PM, Leyssen P, Martin MT, Bourjot M, Dumontet V, Eydoux C, et al. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry. 2012;84:160–8. Elsevier Ltd. [DOI] [PubMed]
- 142.Mishra P, Kumar A, Mamidi P, Kumar S, Basantray I, Saswat T, et al. Inhibition of Chikungunya virus replication by 1-[(2-Methylbenzimidazol-1-yl) methyl]-2-Oxo-Indolin-3-ylidene] amino] Thiourea (MBZM-N-IBT). Sci Rep. 2016;6:1–13. Nature Publishing Group. [DOI] [PMC free article] [PubMed]
- 143.Varghese FS, Kaukinen P, Gläsker S, Bespalov M, Hanski L, Wennerberg K, et al. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir Res. 2016;126:117–124. doi: 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 144.Indu P, Arunagirinathan N, Rameshkumar MR, Sangeetha K, Rajarajan S, Aljowaie RM, et al. Exploring potential anti-chikungunya virus activity of phytocompounds: computational docking and in vitro studies. J King Saud Univ - Sci. 2022;34:102157. [Google Scholar]
- 145.Singh KD, Kirubakaran P, Nagarajan S, Sakkiah S, Muthusamy K, Velmurgan D, et al. Homology modeling, molecular dynamics, e-pharmacophore mapping and docking study of Chikungunya virus nsP2 protease. J Mol Model. 2012;18:39–51. doi: 10.1007/s00894-011-1018-3. [DOI] [PubMed] [Google Scholar]
- 146.Agarwal T, Asthana S, Bissoyi A. Molecular modeling and docking study to elucidate novel chikungunya virus nsP2 protease inhibitors. Indian J Pharm Sci. 2015;77:453–460. doi: 10.4103/0250-474x.164769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jain J, Kumari A, Somvanshi P, Grover A, Pai S, Sunil S. In silico analysis of natural compounds targeting structural and nonstructural proteins of chikungunya virus. F1000Research. 2017;6:1601. doi: 10.12688/f1000research.12301.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kumar D, Meena MK, Kumari K, Patel R, Jayaraj A, Singh P. In-silico prediction of novel drug-target complex of nsp3 of CHIKV through molecular dynamic simulation. Heliyon. 2020;6:e04720. doi: 10.1016/j.heliyon.2020.e04720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oo A, Hassandarvish P, Chin SP, Lee VS, Bakar SA, Zandi K. In silico study on anti-Chikungunya virus activity of hesperetin. PeerJ. 2016;2016:1–23. doi: 10.7717/peerj.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chaudhary M, Sehgal D. In silico identification of natural antiviral compounds as a potential inhibitor of chikungunya virus non-structural protein 3 macrodomain. J Biomol Struct Dyn. 2021;0:1–11. doi: 10.1080/07391102.2021.1960195. [DOI] [PubMed] [Google Scholar]
- 151.Seyedi SS, Shukri M, Hassandarvish P, Oo A, Muthu SE, Abubakar S, et al. Computational approach towards exploring potential anti-Chikungunya activity of selected flavonoids. Sci Rep. 2016;6:1–8. doi: 10.1038/srep24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghildiyal R, Gupta S, Gabrani R, Joshi G, Gupta A, Chaudhary VK, et al. In silico study of chikungunya polymerase, a potential target for inhibitors. Virus Disease. 2019;30:394–402. doi: 10.1007/s13337-019-00547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Freire MCLC, Basso LGM, Mendes LFS, Mesquita NCMR, Mottin M, Fernandes RS, et al. Characterization of the RNA-dependent RNA polymerase from Chikungunya virus and discovery of a novel ligand as a potential drug candidate. Sci Rep. 2022;12:1–15. doi: 10.1038/s41598-022-14790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oqbazgi MD. Treatment and prevention of Chikungunya fever: current status and prospective. In: Engohang-Ndong J, editor. Chikungunya virus - a grow glob public heal threat. London: IntechOpen; 2021. [Google Scholar]
- 155.de Brito CAA, von Sohsten AKA, de Sá Leitão CC, de Brito R, de Azevedo Valadares LD, da Fonte CAM, et al. Pharmacologic management of pain in patients with Chikungunya: a guideline. Rev Soc Bras Med Trop. 2016;49:668–679. doi: 10.1590/0037-8682-0279-2016. [DOI] [PubMed] [Google Scholar]
- 156.Mourad O, Makhani L, Chen LH. Chikungunya: an emerging public health concern. Curr Infect Dis Rep. 2022;24:217–228. doi: 10.1007/s11908-022-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Millsapps EM, Underwood EC, Barr KL. Development and application of treatment for Chikungunya fever. Res Rep Trop Med. 2022;13:55–66. doi: 10.2147/RRTM.S370046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Webb E, Michelen M, Rigby I, Dagens A, Dahmash D, Cheng V, et al. An evaluation of global Chikungunya clinical management guidelines: a systematic review. eClinicalMedicine. 2022;54:101672. doi: 10.1016/j.eclinm.2022.101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parashar D, Cherian S. Antiviral perspectives for chikungunya virus. Biomed Res Int. 2014;2014:631642. Hindawi Publishing Corporation. [DOI] [PMC free article] [PubMed]
- 160.Peixoto VGMNP, Azevedo JP, Luz KG, Almondes KM. Cognitive dysfunction of Chikungunya virus infection in older adults. Front. Psychiatry. 2022;13:1–10. doi: 10.3389/fpsyt.2022.823218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sebastian MR, Lodha R, Kabra SK. Chikungunya infection in children. Indian J Pediatr. 2009;76:185–189. doi: 10.1007/s12098-009-0049-6. [DOI] [PubMed] [Google Scholar]
- 162.Higgs S, Vanlandingham D. Chikungunya virus and its mosquito vectors. Vector-Borne Zoonotic Dis. 2015;15:231–240. doi: 10.1089/vbz.2014.1745. [DOI] [PubMed] [Google Scholar]
- 163.Moulay D, Aziz-Alaoui MA, Kwon HD. Optimal control of chikungunya disease: larvae reduction, treatment and prevention. Math Biosci Eng. 2012;9:369–392. doi: 10.3934/mbe.2012.9.369. [DOI] [PubMed] [Google Scholar]
- 164.Guzzetta G, Trentini F, Poletti P, Baldacchino FA, Montarsi F, Capelli G, et al. Effectiveness and economic assessment of routine larviciding for prevention of chikungunya and dengue in temperate urban settings in Europe. PLoS Negl Trop Dis. 2017;11:e0005918. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Other datasets used during the current study are available from the corresponding authors on reasonable request.